Abstract
Objective
Disruption of satiety signaling may lead to increased caloric intake and obesity. Uroguanylin, the intestinal hormone, travels as a precursor to the central nervous system where it activates guanylyl cyclase C (GUCY2C) and stimulates pro-satiety neurons. Rodent studies have demonstrated that (1) GUCY2C-knockout mice over-eat and have increased weight gain vs. wild-type mice; and (2) hyper-caloric obesity diminishes uroguanylin expression. We measured circulating plasma pro-uroguanylin, along with other gastrointestinal peptides and inflammatory markers, in human adolescents with and without obesity, as a pilot study. We hypothesized that adolescents with obesity would have less circulating pro-uroguanylin than adolescents without obesity have.
Methods
We recruited 24 adolescents (age 14–17) with and without obesity (BMI >95% or BMI <95%) and measured plasma pro-uroguanylin at fasting and successive time points after a meal. We measured three other satiety hormones and two inflammatory markers to characterize overall satiety signaling and highlight any link between uroguanylin and inflammation.
Results
Female adolescents with obesity had lower circulating pro-uroguanylin levels than female adolescents without obesity; we observed no difference in males. Other measured gastrointestinal peptides varied in their differences between cohorts. Inflammatory markers were higher in female participants with obesity.
Conclusions
In adolescents with and without obesity, we can measure circulating pro-uroguanylin levels. In female adolescents without obesity, levels are particularly higher. Pro-uroguanylin secretion patterns differ from other circulating gastrointestinal peptides. In female adolescents with obesity, inflammation correlates with decreased pro-uroguanylin levels.
Keywords: Food-intake regulation, appetite, gastrointestinal hormones, guanylyl cyclase C, pro-uroguanylin
Introduction
Early nutrition and feeding behaviors influence eating patterns (food intake regulation).1–7 One measurable8 index of food intake regulation is satiety responsiveness9 but the mechanisms of satiety control remain incompletely identified. The gastrointestinal tract secretes small peptides that act centrally on the nervous system to affect the development and maintenance of food intake regulation in animal and human studies.10 Peptide tyrosine-tyrosine (PYY) has pro-satiety secretory patterns in pediatric patients with obesity, including a different response depending on meal content.11–15 Glucagon-like-peptide 1 (GLP-1) is implicated in obesity pathogenesis and type-2 diabetes.16, 17 Meal consumption in pediatric patients with and without obesity affects ghrelin, an orexigenic hormone.12, 14, 15 Obesity is a health care crisis18, 19 and the lack of effective treatments highlights the importance of prevention.20–24 Understanding gastrointestinal signaling pathways may provide targets for intervention in order to prevent obesity.
Uroguanylin has a critical role in weight gain and satiety.25–27 Secreted basolaterally from enterochromaffin-like cells in the duodenum, uroguanylin binds guanylyl cyclase 2C (GUCY2C), a membrane-bound protein expressed primarily in the mucosa of the mammalian intestinal tract. GUCY2C activation produces intracellular cyclic guanosine-3', 5’-monophosphate (cGMP). GUCY2C knockout mice exhibited overeating and developed obesity highlighting an extra-intestinal role for the uroguanylin-GUCY2C-cGMP pathway.28–30 Uroguanylin enters the circulation as an 86 amino acid form, pro-uroguanylin.31 Cleavage in the hypothalamus to active uroguanylin (16 amino acids) stimulates local GUCY2C. In wild-type mice, parenteral administration of the GUCY2C activating enterotoxin, STa, suppressed appetite.28 In obesity, uroguanylin expression is suppressed or lost,32 altering satiety; thus the uroguanylin-GUCY2C system is at the intersection of obesity pathogenesis and sequelae.33–35
Our objective was to measure pro-uroguanylin in pediatric patients with and without obesity. Leveraging in vivo rodent studies,28 we hypothesized that pro-uroguanylin levels would increase after a meal and have a lower baseline in obesity. We measured fasting levels and post-meal levels in adolescents with and without obesity. We measured PYY, GLP-1, and ghrelin in the same participants as well as Interleukin-6 (IL-6) and C-reactive protein (CRP).36, 37 IL-6 secretion from adipocytes and immune system cells acutely increases C-reactive protein, a hepatic protein that activates the complement system for cell degradation. We expected to see an inverse correlation between obesity’s pro-uroguanylin levels and inflammatory marker levels. We predicted that pro-uroguanylin and other hormone and inflammatory marker data in adolescents would help demonstrate a link between gastrointestinal hormones, systemic signaling, and obesity phenotype. Data on pro-uroguanylin in adolescents would suggest that obesity-triggered satiety suppression begins early in life and would support the hypothesis that gut environment alterations in obesity can influence subsequent morbidity.
Methods
Study Subjects
The Nemours Institutional Review Board approved the study (No. 657386). We recruited volunteers from outpatient clinics. Participants were ages 14–17 years, with or without obesity. The CDC defines obesity as BMI for age percentile (BMIFA) greater than the 95th percentile (>95%). Participants had BMIFA >95% or <95%, without any gastrointestinal tract infection, any condition affecting the bowel mucosa integrity, or any condition precluding fasting. Research staff recorded anthropometric data (BMIFA38, 39 and BMI z-score40 [for very high BMI participants, z-score was calculated using www.peditools.org]); medical history; allergies; medications; grams of food consumed; and pregnancy status. Females in the study were post-menarche and not pregnant; the sexual maturity rating of all participants was four or five. We collected subjective data on hunger and fasting from all participants before the protocol began; we tracked subjective fullness during the protocol using a 5-point Likert scale.
Study Protocol
After confirming a minimum 8-hour fast and reviewing consent/assent, we obtained participants’ vital signs and applied topical anesthetic to antecubital fossae and dorsal hands. After 30 minutes, via an intravenous catheter (IV), we collected an initial blood sample (ti). We provided participants a standardized,41 calorically dense meal; we recorded elapsed time of consumption. Research nurses weighed meals and packaging before and after consumption on a Valor™ 7000 scale (USDA Accepted, Model V71P3T; OHAUS, Parsippany, NJ) to determine the weight of consumed food (grams) and nurses collected blood draws at 0, 15, 30, 45, 60, 75, and 90 min intervals after consumption.28 We collected samples for pro-uroguanylin and other gut hormone assays into BD-P800™ vials via 12-inch tubing (Becton, Dickinson and Company, Franklin Lakes, NJ) to preserve hormone integrity. Samples for inflammatory marker assays were collected into EDTA-treated collection vials (Becton, Dickinson and Company, Franklin Lakes, NJ) at ti, t0, t15, and t90. Repeat vital signs were obtained prior to discharge.
Meal and Caloric Intake
The study site’s food service vendor prepared meals delivered the morning of the protocol. Each participant was challenged with an estimated 1/3 of their total energy expenditure (TEE) per day. We estimated the TEE for participants by using 50% height-for-age and either 50% weight-for-age or 95% weight-for-age41 (Table, Supplemental Digital Content 1). Meals were standardized to three options (Table, Supplemental Digital Content 2) with two different meals for males, and an isocaloric meal for females, using nutritional information provided by the vendor. Participants chose one of three isocaloric beverages. To determine calories consumed, the post-meal weight of food was divided by the pre-meal weight of food and the ratio was used to estimate caloric intake (ratio × total calories offered, column 5, Table, Supplemental Digital Content 3). After all participants had completed the study, we calculated participants’ actual 1/3 TEE (column 6, Table, Supplemental Digital Content 3) for comparison to estimated caloric intake (column 7).
Sample Processing and Assay Methods
All blood samples were immediately inverted several times for protease inhibition then placed on ice. Samples were centrifuged at ~1300 RCF at four degrees Celsius for 10 minutes. Supernatant plasma was removed to a fresh, pre-cooled labeled tube for aliquotting to cryovials and storage at −80 degrees Celsius. Human pro-uroguanylin levels in freshly thawed plasma, assayed in duplicate, were measured by ELISA using a quantitative sandwich enzyme immunoassay kit from BioVendor (Brno, Czech Republic). Briefly, standards and samples are added to microplate wells coated with polyclonal antihuman pro-uroguanylin antibody. After incubation (60 minutes) and washing, a biotin-labeled polyclonal anti-human pro-uroguanylin antibody is added to the wells (60 minutes) followed by another washing step then by incubation (30 minutes) with streptavidin-HRP conjugate and final washing step. The addition of the substrate 3,3’,5,5’-tetramethylbenzidine (TMB) allows the enzyme activity proportional to the concentration of pro-uroguanylin to be measured spectrophotometrically.
Total human GLP-1 and human IL-6 levels were determined using ELISA kits from EMD-Millipore (St. Charles, MO). Human PYY and total human ghrelin level in plasma samples were measured by ELISA using kits from EMD Millipore (Darmstadt, Germany). C-reactive protein (CRP) level in plasma samples was measured by ELISA using Human CRP kit (EMD Millipore, Darmstadt, Germany). Absorbance readings were measured in a tunable BIOTEK Epoch microplate reader; concentrations were calculated from standard curves and reported in pg/mL except for CRP (µg/mL) and GLP-1 (pM). GLP-1 concentrations were converted to pg/mL (pM × 3.2976 = pg/mL). Samples were batched for assays within 6 months of collection.
Statistics
The proposed study was a pilot study, with no direct precedent in the literature for comparison of pro-uroguanylin levels between different weight cohorts of pediatric participants. Human plasma sampling of pro-uroguanylin was completed by Valentino et al. only in a control population of normal BMI adults.28 Rodent circulation pro-uroguanylin levels were only measured in wild-type mice. As proof of concept, we based our a priori power calculations on rodent studies comparing weight gain in mice with and without the GUCY2C receptor.28 We estimated that with 11–13 participants in each cohort, a significant difference in pro-uroguanylin concentration between cohorts could be detected at 5% significance level with 90% power and an effect size of d= 1.3964.
Descriptive statistics were used to summarize the distribution of participants’ demographic and clinical variables. Pearson’s correlation was used to determine any effect of age on baseline hormone concentration. Chi-square test was used to examine differences in subjective categorical values between cohorts. A linear mixed effects model with random intercepts was fitted to quantify the association between sex and each of the gastrointestinal hormones and inflammatory markers and to account for multiple variables between participants, including assay times and trends. The model was implemented using the lme4 package and R Statistical Software (Foundation for Statistical Computing, Vienna, Austria).
Results
Twenty-four participants completed the protocol: 13 in the <95% BMIFA cohort and 11 in the >95% BMIFA cohort. Table 1 shows anthropometric differences and similarities between cohorts overall and when segregated by sex. All participants had similar sensation of hunger upon waking and at start of the protocol (Table, Supplemental Digital Content 4). At the end of the protocol, the <95% BMIFA cohort had 12 of 13 participants who were full at or before the end of the meal while the >95% BMIFA cohort had 9 of 11 participants who were full. The participants in the >95% BMIFA cohort were more likely to eat their whole meal and still be hungry; the <95% BMIFA participants were more likely not to finish the meal due to being full more quickly (Table, Supplemental Digital Content 4). For females and males, post-protocol calculated 1/3 TEE differed between cohorts (females, p<0.04; males, p<0.01) and weight of food consumed (g) differed for males (p<0.02, Table, Supplemental Digital Content 3). The ratio of consumed to calculated calories for either sex, in each cohort, was not statistically significant. One female participant who did not complete the meal was included in the analysis and another female participant did not eat the cheese due to lactose intolerance.
Table 1.
Demographics, BMI, and vital signs of participants
| OVERALL | FEMALES | MALES | |||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Category | <95% BMIFA (n=13) |
>95% BMIFA (n=11) |
p value* | <95% BMIFA (n=7) |
>95% BMIFA (n=5) |
p value* | <95% BMIFA (n=6) |
>95% BMIFA (n=6) |
p value* |
| Age (years) | 15.1, 1.1 | 15.6, 1.3 | NS | 14.3, 0.49 | 15.8, 0.84 | <0.002 | 14.8, 1.2 | 16.7, 0.52 | <0.003 |
| Sex (female, %) | 53.8 | 45.5 | NS | ||||||
| Race (white, %) | 100 | 63.6 | NS | 100 | 100 | NS | 50 | 80 | NS |
| Ethnicity (non-Hispanic, %) | 100 | 90.9 | NS | 100 | 100 | NS | 80 | 100 | NS |
|
| |||||||||
| BMI (kg/m2) | 21.7, 2.9 | 36.5, 7.77 | <0.0001 | 22.5, 3.1 | 39.3, 7.3 | <0.0001 | 20.8, 2.7 | 34.1, 8.0 | <0.002 |
| BMIFA (%) | 63.3, 18.8 | 98.3, 7.7 | <0.0001 | 70.2, 14.8 | 98.6, 1.3 | <0.001 | 55.2, 21.0 | 98.0, 2.2 | <0.0003 |
| BMIFA (%; median, IQR) | 62, 17.8 | 99, 2.3 | 68.1, 13.0 | 99.0, 1.6 | 54.0, 20.8 | 98.8, 3.2 | |||
| BMI Z-score | 0.40, 0.58 | 2.3, 0.5 | <0.0001 | 0.59, 0.50 | 2.34, 0.45 | <0.0001 | 0.18, 0.64 | 2.26, 0.56 | <0.0001 |
| BMI Z-score (median, IQR) | 0.31, 0.48 | 2.2, 0.8 | 0.47, 0.40 | 2.31, 0.50 | 0.10, 0.53 | 2.20, 0.89 | |||
|
| |||||||||
| Weight (kg) | 59.1, 10.5 | 102.0, 24.3 | <0.0001 | 58.3, 9.6 | 101.5, 21.8 | <0.0004 | 60.2, 12.2 | 102.4, 28.3 | <0.004 |
| Height (cm) | 164.7, 7.6 | 166.9, 7.4 | NS | 161.8, 6.9 | 170.1, 6.3 | <0.04 | 166.1, 7.8 | 168.0, 7.7 | NS |
| Systolic BP (mm Hg) | 111.5, 12.3 | 125.9, 9.9 | <0.002 | 107.9, 9.6 | 126,0, 7.6 | <0.003 | 115.7, 14.6 | 125.8, 12.2 | NS |
| Diastolic BP (mm Hg) | 66.4, 8.3 | 72.9, 7.6 | <0.05 | 67.3, 9.8 | 74.2, 9.4 | NS | 65.3, 6.8 | 71.7, 6.5 | NS |
| Heart Rate (beats per min.) | 77.2, 16.3 | 76.8, 8.9 | NS | 81.1, 18.1 | 68.4, 10.8 | NS | 76.7, 9.5 | 79.7, 10.5 | NS |
| Temperature (°C) | 36.4, 0.22 | 36.4, 0.34 | NS | 36.5, 0.21 | 36.4, 0.25 | NS | 36.2, 0.28 | 36.5, 0.26 | <0.02 |
Table 1. Clinical data was collected for participants in the two cohorts, including demographics, anthropometrics, and vital signs. Columns 5–7 and 8–10 show segregated (by sex) data for all participants. Unless otherwise noted, values expressed are mean and standard deviation;
two-tailed Student’s t-Test for the mean. Interquartile range (IQR), Body mass index (BMI), BMI for age percentile (BMIFA), blood pressure (BP), not significant (NS).
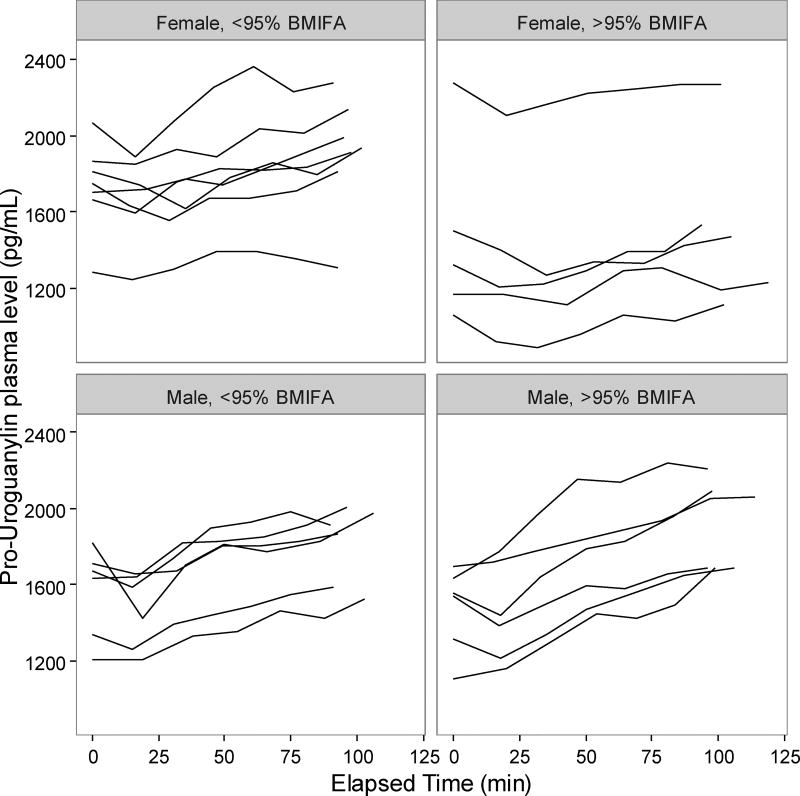
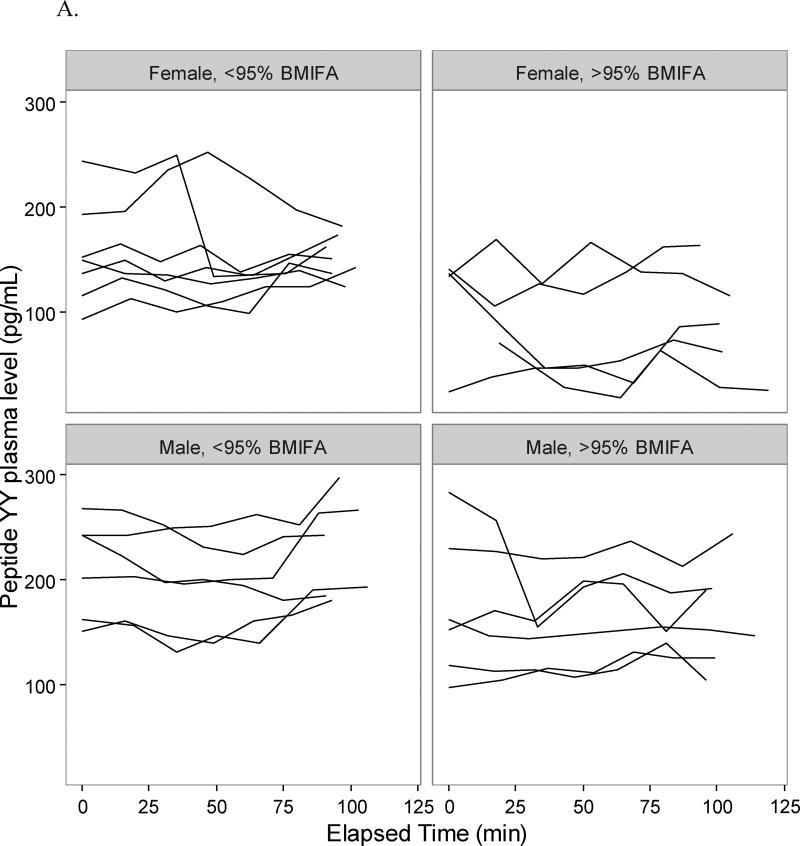
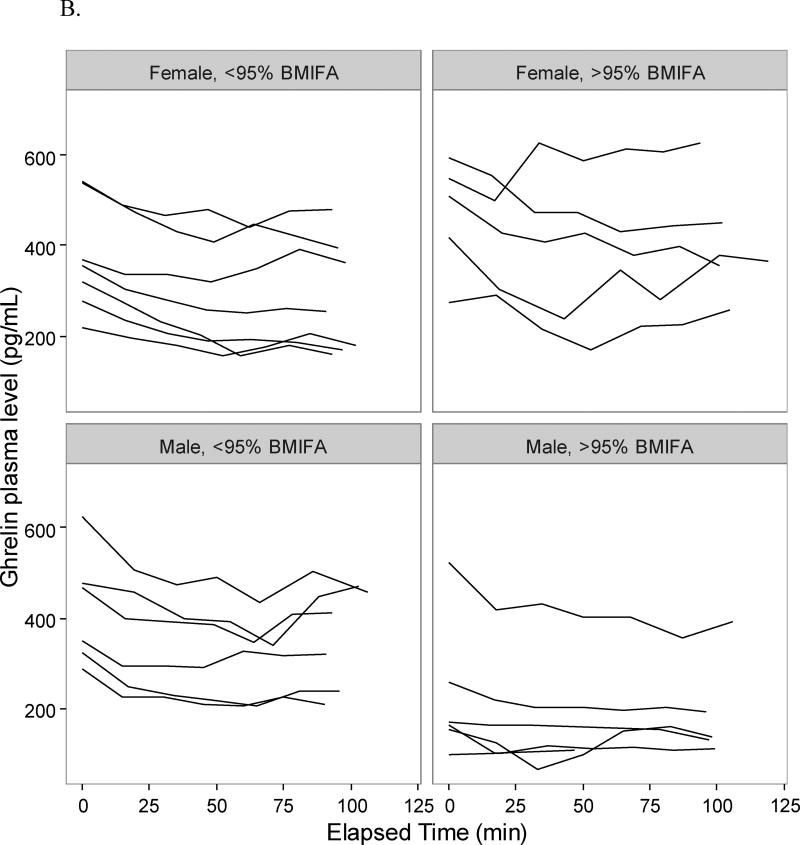
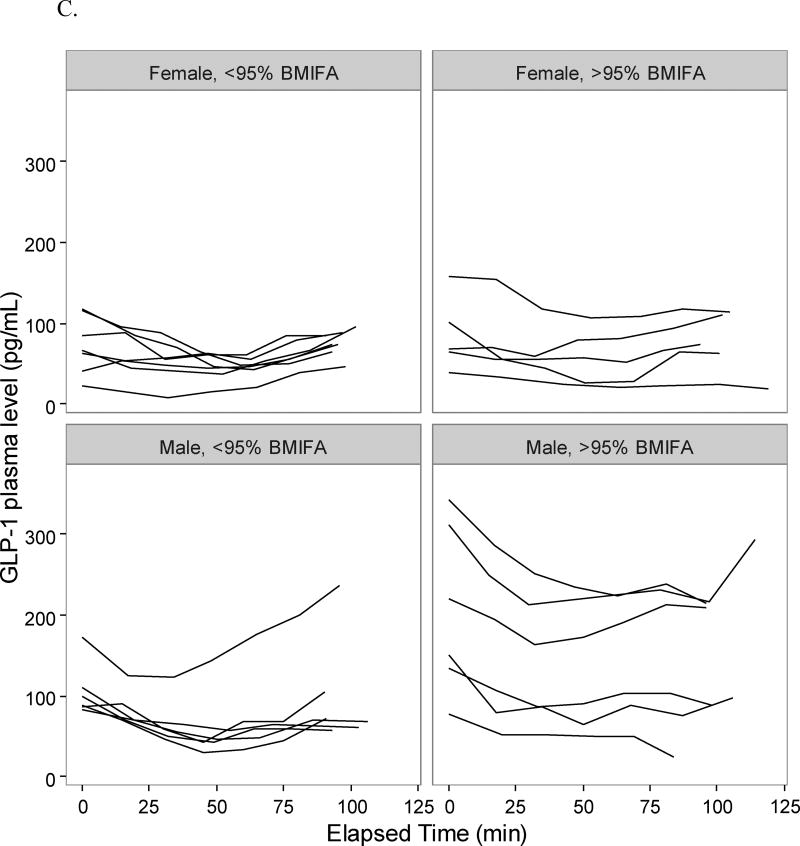
Adolescents in the >95% BMIFA cohort had lower pro-uroguanylin levels compared to <95% BMIFA adolescents (Table 2, p<0.05). When stratified by sex, we observed that the difference was driven by female adolescents in the >95% BMIFA cohort (Figure 1, Table 2, p<0.006). All participants’ pro-uroguanylin levels rose after the meal at comparable rates (Figure 1). Adolescents with >95% BMIFA had lower levels of PYY than <95% BMIFA adolescents (Table 2, p<0.05), but we did not observe a difference when stratified by sex (Figure 2A, Table 2). The rise in PYY over time for all groups was not as notable as it was for pro-uroguanylin. Total ghrelin levels trended down after the meal for all participants, but less consistently so in females with >95% BMIFA (Figure 2B, Table 2, p<0.04). GLP-1 levels were not different between cohorts overall or when stratified by sex and did not rise uniformly after the meal (Figure 2C, Table 2). In males, most participants had a similar trend of GLP-1, but a broader spread of concentrations in the >95% BMIFA group. Hormone concentrations at baseline did not correlate with age: pro-uroguanylin, r = −1.05; PYY, r = −0.012; ghrelin, r = 0.002; and GLP-1, r = 0.004. Concentrations of circulating pro-uroguanylin were 5-fold to 10-fold higher than PYY, GLP-1, or ghrelin. Concentrations of IL-6 differed between cohorts (p<0.02, Table 2) and the difference was driven by female participants (p<0.03, Table 2). Concentrations of CRP were higher in the >95% BMIFA group (p<0.004, Table 2) but were not significantly different for males or females in each cohort.
Table 2.
Gastrointestinal hormone and inflammatory marker concentrations
| Entire cohort (n=24) | Matched subset (n=16) | |||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Hormone or Marker |
Group | Difference from <95% BMIFA |
95% C.I. |
p value |
Difference from <95% BMIFA |
95% C.I. |
p value |
|
| Pro-uroguanylin | All >95% BMIFA | −400.4, (185.3) | −763.6, −37.1 | <0.05 | −694.1, (147.9) | −999.9, −398.3 | <0.001 | |
| >95% BMIFA | M | 5.2, (180.8) | −349.1, 359.5 | NS | −21.2, (182.0) | −385.2, 342.8 | NS | |
| >95% BMIFA | F | −586.4, (158.3) | −896.6, −276.1 | <0.006 | −744.1, (123.2) | −990.5, −497.7 | <0.004 | |
|
| ||||||||
| Peptide YY | All >95% BMIFA | −61.7, (28.3) | −117.2, −6.2 | <0.05 | −90.9, (30.0) | −150.9, −30.9 | <0.02 | |
| >95% BMIFA | M | −24.5, (39.1) | −101.1, 52.0 | NS | −21.5, (32.7) | −86.9, 43.9 | NS | |
| >95% BMIFA | F | −64.3, (30.6) | −124.2, −4.3 | NS | −92.0, (28.9) | −149.8, −34.2 | <0.04 | |
|
| ||||||||
| Glucagon-like peptide 1 | All >95% BMIFA | 12.7, (31.1) | −48.3, 73.7 | NS | 1.4, (28.2) | −55.0, 57.8 | NS | |
| >95% BMIFA | M | 44.5, (51.8) | −57.1, 146.1 | NS | 64.7, (42.0) | −19.3, 148.7 | NS | |
| >95% BMIFA | F | 12.1, (19.3) | −25.8, 49.9 | NS | −4.9, (21.4) | −47.7, 37.9 | NS | |
|
| ||||||||
| Ghrelin | All >95% BMIFA | 122.3, (65.8) | −6.7, 251.3 | NS | 176.2, (81.0) | 14.2, 338.2 | NS | |
| >95% BMIFA | M | −117.7, (94.8) | −303.5, 68.1 | NS | −66.6, (111.8) | −290.2, 157.0 | NS | |
| >95% BMIFA | F | 150.5, (60.5) | 32, 269.1 | <0.04 | 199.5, (47.8) | 103.9, 295.1 | <0.02 | |
|
| ||||||||
| Interleukin-6 | All >95% BMIFA | 7.3, (2.6) | 2.2, 12.4 | <0.02 | 7.76, (3.31) | 1.14, 14.38 | <0.04 | |
| >95% BMIFA | M | 6.7, (3.1) | 0.6, 12.8 | NS | 7.04, (2.86) | 1.32, 12.76 | NS | |
| >95% BMIFA | F | 5.9, (2.1) | 1.7, 10.1 | <0.03 | 7.94, (3.19) | 1.56, 14.32 | NS | |
|
| ||||||||
| C-reactive protein | All >95% BMIFA | 6.3, (1.9) | 2.6, 10.0 | <0.004 | 8.09, (2.61) | 2.87, 13.31 | <0.02 | |
| >95% BMIFA | M | 2.1, (1.4) | −0.6, 4.8 | NS | 8.84, (3.19) | 2.46, 15.22 | <0.05 | |
| >95% BMIFA | F | 12.1, (19.3) | −25.8, 49.9 | NS | 3.67, (1.33) | 1.01, 6.33 | NS | |
Table 2. By linear effects model, differences in mean concentration for >95% BMIFA cohorts, as compared to the <95% BMIFA cohort, is reported for each of the four measured gastrointestinal hormones or the two measured inflammatory markers. Shaded columns: Subset of patients matched by race and ethnicity. By linear effects model, differences in mean concentration for >95% BMIFA cohorts, as compared to the <95% BMIFA cohort, is reported for each of the four measured gastrointestinal hormones or the two measured inflammatory markers. Values are expressed as mean and standard error, 95% confidence intervals (C.I.), and p value. Concentrations are pg/mL except for CRP which is µg/mL. Body mass index for age percentile (BMIFA), not significant (NS).
Figure 1.
Pro-uroguanylin concentrations pre- and post-meal in adolescents. Plasma concentrations of pro-uroguanylin for each participant at each time point were plotted individually, after sorting by group (>95% BMIFA, n=11; <95% BMIFA, n = 13) and by sex. Pro-uroguanylin concentration is reported in pg/mL vs. elapsed time in minutes. Body mass index for age percentile, BMIFA.
Figure 2.
Gastrointestinal hormone concentrations pre- and post-meal in adolescents. Plasma concentrations of each participant at each time point were plotted individually, after sorting by group (>95% BMIFA; <95% BMIFA) and by sex. A. Peptide YY is reported in pg/mL vs. elapsed time in minutes. B. Ghrelin is reported in pg/mL vs. elapsed time in minutes. C. GLP-1 is reported in pg/mL vs. elapsed time in minutes. Body mass index for age percentile, BMIFA.
A subsequent analysis of the gastrointestinal peptides and inflammatory markers, controlling for race and ethnicity, demonstrated that non-Hispanic, Caucasian females without obesity had almost 750 pg/mL greater concentrations of pro-uroguanylin than non-Hispanic, Caucasian females with obesity (Table 2, shaded columns, p<0.004; Figure, Supplemental Digital Content 1). Table 2 and Figure, Supplemental Digital Content 2, show comparative concentration data for the other gastrointestinal peptides and inflammatory markers.
Discussion
This pilot study presents the first data on pro-uroguanylin in adolescents. We measure serum pro-uroguanylin pre- and post-meal in adolescents of dichotomous BMI alongside other known satiety peptides and inflammatory markers. Pro-uroguanylin trends down, initially, after divergent baseline fasting levels then rebounds with meal ingestion. In female adolescents with obesity, compared to female peers without obesity, pro-uroguanylin concentration differences over time are statistically significant.
In our study, the >95% BMIFA group, compared with <95% BMIFA controls, has no statistically significant higher proportional food intake or hunger scores (Table, Supplemental Digital Content 4), despite lower pro-uroguanylin levels. However, we observe a trend to a more rapid sense of fullness in the <95% BMIFA group, and the drive to eat more in the >95% BMFA cohort. These data suggest a smaller direct feedback effect of consuming a calorically dense meal on satiety peptides. A participant’s overall subjective sense of hunger or fullness may be affected less after a meal, reflecting transit through the intestine, entero-endocrine sensing, and secretion to the circulation. We postulate that high BMIFA female adolescents have a disruption in the pro-uroguanylin-GUCY2C-cGMP signaling pathway affecting regulation of satiety baseline over time. The role of sex hormones in secretion of pro-uroguanylin in female adolescents with obesity remains unclear.
For the other measured gastrointestinal hormones, we observe unanticipated results. The distal small bowel secretes peptide YY (PYY) in response to nutrients in the intestinal lumen. PYY then travels via the circulation to interact with neuropeptide Y receptors in the arcuate nucleus of the hypothalamus.11, 13 Levels peak after 15 minutes and plateau by 90 minutes as noted in animal studies11; in human studies 1–2 hours is noted as peak rise.11 This time-course pattern is not observed in our participants (Figure 2A). We do observe greater concentrations of PYY in the plasma of <95% BMIFA females than in >95% BMIFA females mirroring the pro-uroguanylin observation, but it is not statistically significant, likely due to different study populations (age), different food challenge caloric densities, and shorter time courses of plasma collection. Ghrelin, an orexigenic hormone secreted by the stomach, decreases over time in most of our participants as expected after a meal challenge. Females with >95% BMIFA as compared to females with <95% BMIFA have higher measured ghrelin (Figure 2B, Table 2, p<0.04), suggesting a pro-appetite drive despite the meal challenge. Our cohort does not report significantly higher hunger ratings; these data conflict with other published data on ghrelin in children.12, 14 Glucagon-like peptide 1 (GLP-1) is derived from the pro-glucagon gene, acts to lower blood sugar, inhibit glucagon release, and increase satiety in the central nervous system. In our cohorts, GLP-1 appears to decrease after a caloric challenge, and then increase with time, but we observe no significant difference between cohorts (Figure 2C, Table 2). Our data appear to agree with published literature on GLP-1 in children.12, 16 IL-6 and CRP levels are higher in the high BMIFA groups (Table 2), reflecting the inflammatory nature of obesity.36, 37, 42 Pre-protocol glucose and insulin levels are similar among all participants.
Our study is limited by small cohort size and lack of racial heterogeneity, the latter an unplanned consequence of recruitment. The time commitment required on the part of the participants and their caregivers may have played a role in limiting recruitment. We believe the observations to be valid, and they warrant reproduction with a larger sample size and with a more diverse population. Reviewing the literature on other gut hormone studies in children or adolescents, we note cohort sizes and demographics similar to ours.12, 14–16 Our data suggest that interruption of pro-uroguanylin signaling may contribute to the pathogenesis of obesity in children, but the findings were noted only in female participants; a larger sample size may be needed to detect differences in male participants. Varying amounts of macronutrient consumption and lack of individualized calorie targets for each participant prevent us from ascertaining the effect of a proportional calorie load in a given individual on pro-uroguanylin. We attempt to account for this by using the linear mixed effects model, which also allowed adjustment for sex and age and post-hoc adjustment of p-values for multiple tests. Peak and trough levels may have been missed due to long consumption times between sampling and an abbreviated time course. We were unable to control for social desirability bias in our subjective surveys of hunger and fullness for all participants (Table, Supplemental Digital Content 4).
Satiety signaling drives food intake self-regulation, develops in early childhood, and contributes to obesity when disrupted.3, 4, 6, 7, 9 Environmental factors, genetic traits, epigenetic influences, intestinal microbiota, and gastrointestinal hormones, including pro-uroguanylin, regulate and direct food intake (appetite and satiety). Prior studies on gastrointestinal hormones imply that obesity alters or suppresses these neuropeptide satiety signals.12, 27, 43–45
We sought to obtain data in adolescents on a novel satiety hormone, pro-uroguanylin. While the mechanisms of any one hormone-receptor signaling pathway may be insufficient for understanding food-intake self-regulation, in mice the absence of the uroguanylin-GUCY2C pathway was enough to alter appetite and affect weight gain.28 The pathway is an example of a gut-brain endocrine axis that integrates calorie consumption, secretion of a peptide (pro-uroguanylin), hypothalamic receptor signaling (GUCY2C-cGMP), and a pro-satiety neuropeptide (pro-opiomelanocortin, POMC).28 POMC is elevated in wild-type mice when an analog to uroguanylin is administered intravenously.28 A more recent study reports lower plasma pro-uroguanylin levels in adults with obesity,25 and another demonstrates that obesity disrupts the expression of ligands for GUCY2C in mice.34 Moreover, uroguanylin suppression and loss was observed in a high-calorie, 'obesogenic' diet, due to increased endoplasmic reticulum (ER) stress.32 Other studies have demonstrated the critical role of ER stress in obesity.46, 47 Additional studies suggest a role for uroguanylin in satiety regulation and the development of obesity.26, 27 Our data suggest that uroguanylin may play a role in adolescent satiety, and may be disrupted by obesity.
The challenge of pediatric obesity is primarily in prevention, as treatment options require significant efforts of patient, family, and clinician but often have limited success. Infusion of pro-uroguanylin in rodents followed by a high-calorie diet demonstrated satiation effect.28, 32 Pro-uroguanylin hormone replacement is under study for other gastrointestinal disorders48 and colorectal cancer,49 but the clinical ambition to use pro-uroguanylin for appetite and satiety augmentation in a therapeutic manner is appealing. Pro-uroguanylin may not serve as an acute response to a meal. The hormone may serve a more chronic regulatory feedback function: continued caloric intake exposure leads to decreased expression and decreased thermostatic control of appetite. Future experiments with larger, more diverse cohorts will validate our observations and help to clarify this hypothesis.
Supplementary Material
Figure, Supplemental Digital Content 1. Pro-uroguanylin concentrations pre- and post-meal in race- and ethnicity-matched subset of >95% and <95% BMIFA adolescents. Plasma concentrations of pro-uroguanylin for each participant at each time point were plotted individually, after sorting by group (>95% BMIFA, n=6; <95% BMIFA, n = 10) and by sex (50:50). Pro-uroguanylin concentration is reported in pg/mL vs. elapsed time (min). Body mass index for age percentile, BMIFA.
Figure, Supplemental Digital Content 2. Gastrointestinal hormone concentrations pre- and post-meal in race- and ethnicity-matched subset of >95% and <95% BMIFA adolescents. Plasma concentrations of each participant at each time point were plotted individually, after sorting by group (>95% BMIFA, n=6; <95% BMIFA, n=10) and by sex (50:50). A. Peptide YY, pg/mL; B. Ghrelin, pg/mL; C. GLP-1, pg/ mL vs. elapsed time (min). Body mass index for age percentile, BMIFA.
What is known
Uroguanylin is a gastrointestinal hormone that activates guanylyl cyclase C (GUCY2C) in the small intestine to drive fluid homeostasis.
Gastrointestinal hormones like peptide-YY and ghrelin are part of a gut-brain hormonal signaling axis regulating appetite.
Uroguanylin is secreted from the gastrointestinal tract into the bloodstream as pro-uroguanylin to signal GUCY2C in the brain and affect satiety.
What is new
We measured circulating pro-uroguanylin in adolescents with and without obesity.
Pro-uroguanylin levels differ between adolescents of different body-mass-index, particularly female adolescents.
Satiety regulation in adolescents may involve a cGMP-mediated mechanism.
Acknowledgments
Stephanie Yeager for recruiting efforts; Christina Russell and Maria Haubrich for protocol; Julie Vannicola, Jennifer Spera, and Andrea Hamill for scheduling; Lore Noyes for dietetics; Leslie Stewart and Yanping Wang for sample processing; Li Xie for statistics; Dr. Scott Waldman for scientific advisory; Erik Blomain for manuscript review.
Source of Funding
Work for the study was supported by an Institutional Development Award from the National Institute of General Medical Sciences of the National Institutes of Health under grant number U54-GM104941 (PI: Binder-Macleod).
Footnotes
Conflicts of Interest
The authors declare no conflicts of interest or financial disclosures.
Author contributions
MD, ZH, AA, and KVG conceived experiments, analyzed data, and interpreted the results. DT carried out the experiments, interpreted the data, and interpreted the results. MD generated figures with assistance from the study statistician. All authors were involved in writing the manuscript and had final approval of the submitted version.
References
- 1.Birch LL, Fisher JO. Development of eating behaviors among children and adolescents. Pediatrics. 1998;101:539–49. [PubMed] [Google Scholar]
- 2.Dewey KG. Nutrition, growth, and complementary feeding of the breastfed infant. Pediatr Clin North Am. 2001;48:87–104. doi: 10.1016/s0031-3955(05)70287-x. [DOI] [PubMed] [Google Scholar]
- 3.Scaglioni S, Arrizza C, Vecchi F, et al. Determinants of children's eating behavior. Am J Clin Nutr. 2011;94:2006s–11s. doi: 10.3945/ajcn.110.001685. [DOI] [PubMed] [Google Scholar]
- 4.Smeets PA, Charbonnier L, van Meer F, et al. Food-induced brain responses and eating behaviour. Proc Nutr Soc. 2012;71:511–20. doi: 10.1017/S0029665112000808. [DOI] [PubMed] [Google Scholar]
- 5.Carnell S, Benson L, Pryor K, et al. Appetitive traits from infancy to adolescence: using behavioral and neural measures to investigate obesity risk. Physiol Behav. 2013;121:79–88. doi: 10.1016/j.physbeh.2013.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Birch LL, Doub AE. Learning to eat: birth to age 2 y. Am J Clin Nutr. 2014;99:723s–8s. doi: 10.3945/ajcn.113.069047. [DOI] [PubMed] [Google Scholar]
- 7.Taut D, Baban A, Giese H, et al. Developmental trends in eating self-regulation and dietary intake in adolescents. Appl Psychol Health Well Being. 2015;7:4–21. doi: 10.1111/aphw.12035. [DOI] [PubMed] [Google Scholar]
- 8.Wardle J, Guthrie CA, Sanderson S, et al. Development of the Children's Eating Behaviour Questionnaire. J Child Psychol Psychiatry. 2001;42:963–70. doi: 10.1111/1469-7610.00792. [DOI] [PubMed] [Google Scholar]
- 9.Faith MS, Carnell S, Kral TV. Genetics of food intake self-regulation in childhood: literature review and research opportunities. Hum Hered. 2013;75:80–9. doi: 10.1159/000353879. [DOI] [PubMed] [Google Scholar]
- 10.Kim GW, Lin JE, Blomain ES, et al. Antiobesity pharmacotherapy: new drugs and emerging targets. Clin Pharmacol Ther. 2014;95:53–66. doi: 10.1038/clpt.2013.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Batterham RL, Cohen MA, Ellis SM, et al. Inhibition of food intake in obese subjects by peptide YY3–36. N Engl J Med. 2003;349:941–8. doi: 10.1056/NEJMoa030204. [DOI] [PubMed] [Google Scholar]
- 12.Stock S, Leichner P, Wong AC, et al. Ghrelin, peptide YY, glucose-dependent insulinotropic polypeptide, and hunger responses to a mixed meal in anorexic, obese, and control female adolescents. J Clin Endocrinol Metab. 2005;90:2161–8. doi: 10.1210/jc.2004-1251. [DOI] [PubMed] [Google Scholar]
- 13.le Roux CW, Bloom SR. Peptide YY, appetite and food intake. Proc Nutr Soc. 2005;64:213–6. doi: 10.1079/pns2005427. [DOI] [PubMed] [Google Scholar]
- 14.Lomenick JP, Clasey JL, Anderson JW. Meal-related changes in ghrelin, peptide YY, appetite in normal weight and overweight children. Obesity (Silver Spring) 2008;16:547–52. doi: 10.1038/oby.2007.129. [DOI] [PubMed] [Google Scholar]
- 15.Lomenick JP, Melguizo MS, Mitchell SL, et al. Effects of meals high in carbohydrate, protein, and fat on ghrelin and peptide YY secretion in prepubertal children. J Clin Endocrinol Metab. 2009;94:4463–71. doi: 10.1210/jc.2009-0949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lomenick JP, White JR, Smart EJ, et al. Glucagon-like peptide 1 and pancreatic polypeptide responses to feeding in normal weight and overweight children. J Pediatr Endocrinol Metab. 2009;22:493–500. doi: 10.1515/jpem.2009.22.6.493. [DOI] [PubMed] [Google Scholar]
- 17.Kelly AS. Glucagon-like peptide-1 receptor agonist treatment for pediatric obesity. Endocr Dev. 2016;30:23–8. doi: 10.1159/000439323. [DOI] [PubMed] [Google Scholar]
- 18.Spieker EA, Pyzocha N. Economic Impact of obesity. Prim Care. 2016;43:83–95. doi: 10.1016/j.pop.2015.08.013. [DOI] [PubMed] [Google Scholar]
- 19.Ogden CL, Carroll MD, Fryar CD, et al. Prevalence of obesity among adults and youth: United States, 2011–2014. NCHS Data Brief. 2015:1–8. [PubMed] [Google Scholar]
- 20.Gortmaker SL, Taveras EM. Who becomes obese during childhood--clues to prevention. N Engl J Med. 2014;370:475–6. doi: 10.1056/NEJMe1315169. [DOI] [PubMed] [Google Scholar]
- 21.Lumeng JC, Taveras EM, Birch L, et al. Prevention of obesity in infancy and early childhood: a National Institutes of Health Workshop. JAMA Pediatr. 2015;169:484–90. doi: 10.1001/jamapediatrics.2014.3554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Daniels SR, Hassink SG. The role of the pediatrician in primary prevention of obesity. Pediatrics. 2015;136:e275–92. doi: 10.1542/peds.2015-1558. [DOI] [PubMed] [Google Scholar]
- 23.Brown CL, Halvorson EE, Cohen GM, et al. Addressing childhood obesity: opportunities for prevention. Pediatr Clin North Am. 2015;62:1241–61. doi: 10.1016/j.pcl.2015.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brown CL, Skelton JA, Perrin EM, et al. Behaviors and motivations for weight loss in children and adolescents. Obesity (Silver Spring) 2016;24:446–52. doi: 10.1002/oby.21370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rodriguez A, Gomez-Ambrosi J, Catalan V, et al. Guanylin and uroguanylin stimulate lipolysis in human visceral adipocytes. Int J Obes (Lond) 2016;40:1405–15. doi: 10.1038/ijo.2016.66. [DOI] [PubMed] [Google Scholar]
- 26.Folgueira C, Sanchez-Rebordelo E, Barja-Fernandez S, et al. Uroguanylin levels in intestine and plasma are regulated by nutritional status in a leptin-dependent manner. Eur J Nutr. 2016;55:529–36. doi: 10.1007/s00394-015-0869-2. [DOI] [PubMed] [Google Scholar]
- 27.Folgueira C, Beiroa D, Callon A, et al. Uroguanylin action in the brain reduces weight gain in obese mice via different efferent autonomic pathways. Diabetes. 2016;65:421–32. doi: 10.2337/db15-0889. [DOI] [PubMed] [Google Scholar]
- 28.Valentino MA, Lin JE, Snook AE, et al. A uroguanylin-GUCY2C endocrine axis regulates feeding in mice. J Clin Invest. 2011;121:3578–88. doi: 10.1172/JCI57925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Seeley RJ, Tschöp MH. Uroguanylin: how the gut got another satiety hormone. J Clin Invest. 2011;121:3384–6. doi: 10.1172/JCI58297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fruhbeck G. Gastrointestinal hormones: uroguanylin-a new gut-derived weapon against obesity? Nat Rev Endocrinol. 2012;8:5–6. doi: 10.1038/nrendo.2011.206. [DOI] [PubMed] [Google Scholar]
- 31.Hidaka Y, Shimono C, Ohno M, et al. Dual function of the propeptide of prouroguanylin in the folding of the mature peptide: disulfide-coupled folding and dimerization. J Biol Chem. 2000;275:25155–62. doi: 10.1074/jbc.M000543200. [DOI] [PubMed] [Google Scholar]
- 32.Kim GW, Lin JE, Snook AE, et al. Calorie-induced ER stress suppresses uroguanylin satiety signaling in diet-induced obesity. Nutr Diabetes. 2016;6:e211. doi: 10.1038/nutd.2016.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim GW, Lin JE, Waldman SA. GUCY2C: at the intersection of obesity and cancer. Trends Endocrinol Metab. 2013;24:165–73. doi: 10.1016/j.tem.2013.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lin JE, Colon-Gonzalez F, Blomain E, et al. Obesity-induced colorectal cancer is driven by caloric silencing of the Guanylin-GUCY2C paracrine signaling axis. Cancer Res. 2016;76:339–46. doi: 10.1158/0008-5472.CAN-15-1467-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Blomain ES, Merlino DJ, Pattison AM, et al. GUCY2C hormone axis at the intersection of obesity and colorectal cancer. Mol Pharmacol. 2016;90:199–204. doi: 10.1124/mol.115.103192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Galcheva SV, Iotova VM, Yotov YT, et al. Circulating proinflammatory peptides related to abdominal adiposity and cardiometabolic risk factors in healthy prepubertal children. Eur J Endocrinol. 2011;164:553–8. doi: 10.1530/EJE-10-1124. [DOI] [PubMed] [Google Scholar]
- 37.Lumeng CN, Saltiel AR. Inflammatory links between obesity and metabolic disease. J Clin Invest. 2011;121:2111–7. doi: 10.1172/JCI57132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang Y. Epidemiology of childhood obesity--methodological aspects and guidelines: what is new? Int J Obes Relat Metab Disord. 2004;28(Suppl 3):S21–8. doi: 10.1038/sj.ijo.0802801. [DOI] [PubMed] [Google Scholar]
- 39.Flegal KM, Wei R, Ogden CL, et al. Characterizing extreme values of body mass index-for-age by using the 2000 Centers for Disease Control and Prevention growth charts. Am J Clin Nutr. 2009;90:1314–20. doi: 10.3945/ajcn.2009.28335. [DOI] [PubMed] [Google Scholar]
- 40.Wang Y, Moreno LA, Caballero B, et al. Limitations of the current world health organization growth references for children and adolescents. Food Nutr Bull. 2006;27:S175–88. doi: 10.1177/15648265060274S502. [DOI] [PubMed] [Google Scholar]
- 41.Henes ST, Cummings DM, Hickner RC, et al. Comparison of predictive equations and measured resting energy expenditure among obese youth attending a pediatric healthy weight clinic: one size does not fit all. Nutr Clin Pract. 2013;28:617–24. doi: 10.1177/0884533613497237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Aygun AD, Gungor S, Ustundag B, et al. Proinflammatory cytokines and leptin are increased in serum of prepubertal obese children. Mediators Inflamm. 2005;2005:180–3. doi: 10.1155/MI.2005.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Oyama LM, do Nascimento CM, Carnier J, et al. The role of anorexigenic and orexigenic neuropeptides and peripheral signals on quartiles of weight loss in obese adolescents. Neuropeptides. 2010;44:467–74. doi: 10.1016/j.npep.2010.07.002. [DOI] [PubMed] [Google Scholar]
- 44.Bascietto C, Giannini C, D'Adamo E, et al. Implications of gastrointestinal hormones in the pathogenesis of obesity in prepubertal children. J Pediatr Endocrinol Metab. 2012;25:255–60. doi: 10.1515/jpem-2011-0478. [DOI] [PubMed] [Google Scholar]
- 45.Horner K, Lee S. Appetite-related peptides in childhood and adolescence: role of ghrelin, PYY, and GLP-1. Appl Physiol Nutr Metab. 2015;40:1089–99. doi: 10.1139/apnm-2015-0050. [DOI] [PubMed] [Google Scholar]
- 46.Kawasaki N, Asada R, Saito A, et al. Obesity-induced endoplasmic reticulum stress causes chronic inflammation in adipose tissue. Sci Rep. 2012;2:799. doi: 10.1038/srep00799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lionetti L, Mollica MP, Lombardi A, et al. From chronic overnutrition to insulin resistance: the role of fat-storing capacity and inflammation. Nutr Metab Cardiovasc Dis. 2009;19:146–52. doi: 10.1016/j.numecd.2008.10.010. [DOI] [PubMed] [Google Scholar]
- 48.Foxx-Orenstein AE. New and emerging therapies for the treatment of irritable bowel syndrome: an update for gastroenterologists. Therap Adv Gastroenterol. 2016;9:354–75. doi: 10.1177/1756283X16633050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Blomain ES, Pattison AM, Waldman SA. GUCY2C ligand replacement to prevent colorectal cancer. Cancer Biol Ther. 2016;17:713–8. doi: 10.1080/15384047.2016.1178429. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure, Supplemental Digital Content 1. Pro-uroguanylin concentrations pre- and post-meal in race- and ethnicity-matched subset of >95% and <95% BMIFA adolescents. Plasma concentrations of pro-uroguanylin for each participant at each time point were plotted individually, after sorting by group (>95% BMIFA, n=6; <95% BMIFA, n = 10) and by sex (50:50). Pro-uroguanylin concentration is reported in pg/mL vs. elapsed time (min). Body mass index for age percentile, BMIFA.
Figure, Supplemental Digital Content 2. Gastrointestinal hormone concentrations pre- and post-meal in race- and ethnicity-matched subset of >95% and <95% BMIFA adolescents. Plasma concentrations of each participant at each time point were plotted individually, after sorting by group (>95% BMIFA, n=6; <95% BMIFA, n=10) and by sex (50:50). A. Peptide YY, pg/mL; B. Ghrelin, pg/mL; C. GLP-1, pg/ mL vs. elapsed time (min). Body mass index for age percentile, BMIFA.