Abstract
Functional connectivity differences between children with autism spectrum disorder (ASD) and typically developing children have been described in multiple datasets. However, few studies examine the task-related changes in connectivity in disorder-relevant behavioral paradigms. In this paper, we examined the task-related changes in functional connectivity using EEG and a movement-based paradigm that has behavioral relevance to ASD. Resting-state studies motivated our hypothesis that children with ASD would show a decreased magnitude of functional connectivity during the performance of a motor-control task. Contrary to our initial hypothesis, however, we observed that task-related modulation of functional connectivity in children with ASD was in the direction opposite to that of TDs. The task-related connectivity changes were correlated with clinical symptom scores. Our results suggest that children with ASD may have differences in cortical segregation/integration during the performance of a task, and that part of the differences in connectivity modulation may serve as a compensatory mechanism.
Keywords: dyspraxia, autism, connectivity, functional connectivity, EEG, task-related
Introduction
Altered brain connectivity is an often-replicated finding of autism spectrum disorders (ASD) [Vasa, Mostofsky, & Ewen, 2016]. The disruption specifically of functional connectivity—defined as temporally coordinated activity of different brain regions—has been associated with a variety of symptoms of ASD, ranging from social-communicative to motor [Schipul, Keller, & Just, 2011]. Much of the evidence for altered connectivity in ASD has come from MRI-based studies of functional and structural connectivity [Schipul et al., 2011; Vasa et al., 2016; Wass, 2011]; comparatively fewer studies have been conducted using EEG and MEG [Vasa et al., 2016]. M/EEG’s greater time resolution than fMRI and direct indexing of neuronal electrical activity allow for better assessment of fast dynamic changes in brain connectivity and differential recording of different frequency bands of oscillatory activity [Siegel, Donner, & Engel, 2012; Varela, Lachaux, Rodriguez, & Martinerie, 2001]. Modulations of the power in these oscillations have been extensively linked to cognitive/behavioral function [Buzsáki & Draguhn, 2004; Klimesch, 1996, 1999; Kopell, Gritton, Whittington, & Kramer, 2014]. Even within the same cortical region, modulation of different frequencies can be differentially related to distinct tasks [Pfurtscheller, 2001].
Current accounts of ASD-connectivity suggest decreased long-range connectivity (“global under-connectivity”), with most EEG results coming primarily from resting state studies [O’Reilly et al., 2017; Schipul et al., 2011; Wass, 2011], showing decreased connectivity from delta (<4 Hz) [Coben, Clarke, Hudspeth, & Barry, 2008] to faster beta-band oscillations (14–30 Hz) [Duffy & Als, 2012; Murias, Webb, Greenson, & Dawson, 2007] (Table 1). Recent studies examining the fundamental nature of resting state networks [Deco et al., 2013, 2011; Hansen, Battaglia, Spiegler, Deco, & Jirsa, 2015] have shown that the networks involved in spontaneous activity form a “dynamical core” [Deco, Kringelbach, Jirsa, & Ritter, 2016] which facilitates behavioral output via state changes. Oscillations of different frequencies may facilitate tasks differentially within this context [Kopell et al., 2014]. Therefore, to relate state transitions more directly to behavioral function, we proposed to examine state transitions using task-related EEG recordings. By examining task-related changes in connectivity, we can more directly understand how online modulation of functional connectivity is relevant to function.
Table 1.
List of Studies That Examined Connectivity in ASD Using M/EEG
| Study | Method/population | Task | Relevant findings |
|---|---|---|---|
| Coben et al. [2008] | EEG on 20 children with ASD and 20 controls. Age range 6–11 years | Resting state. | Long-range under-connectivity found in delta (1.5–3.5Hz) and theta (3–7.5Hz) bands using coherence. |
| Murias et al. [2007] | EEG on 18 adults with ASD and 18 adult controls. | Resting state. | Long range under connectivity was also observed in alpha (8–10Hz) between frontal and temporal sites using coherence. |
| Duffy and Als [2012] | EEG analysis of large data sample. 1304 subjects with ASD (ages 1–18 years) and 463 controls. | Resting state. | Decreased beta band coherence found in frontal and temporal connections. |
| Catarino et al. [2013] | EEG on 15 adults with ASD and 15 controls. Age range 21–42 years. | Visual task. Identify whether the picture was a face or chair. | Reduced inter-hemispheric coherence for ASD for frequencies<13 Hz. No power spectra difference between groups. |
| Boersma et al. [2012] | EEG on 12 toddlers (age 3.5 +− 1.19) and 19 controls (3.3 +− .8) | Visual task. Passively watch picture of cars and faces. | Reduced whole brain connectivity in ASD base on phase lag index in alpha and beta bands. |
| Domínguez et al. [2013] | EEG on 72 toddlers with ASD and 31 controls. Age range 2–5 years. | Visual task. Passively view pictures of faces. | Increased connectivity based on imaginary coherence found in delta, theta and alpha bands. |
| Khan et al. [2013] | MEG on 17 subjects with ASD and 20 controls. Age range 14–20 years | Visual task. Passively view pictures of house and faces. | Reduced whole brain global and local coherence between fusiform face are and rest of brain in alpha band. |
| Kikuchi et al. [2013] | MEG on 26 children with ASD and 26 controls. Age range 5–7 years. | Visual and verbal reasoning task based on K-ABC. | Increased long range connectivity (temporooccipital coherence) for ASD group in gamma band. |
| Kikuchi et al. [2013] | MEG on 35 children with ASD and 35 controls. Age range 3–7 years. | Passive viewing of video presented on screen. | Increased right lateralized long range connectivity (coherence) for the ASD group in gamma band. |
| Kikuchi et al. [2015] | MEG on 50 children with ASD and 50 controls. Age range 3–7 years. | Passive viewing of video presented on screen. | Reduced long range connectivity (left-anterior to right posterior coherence) in ASD in theta band. |
In general, the studies tend to use data driven approaches to find group differences in connectivity and then relate it to symptom severity. These findings suggest that there is task and frequency band specific effects in long-range connectivity, but these effects are not general. Some studies find decreased long-range connectivity while others find increased connectivity in ASD
There are limited data assessing task-related EEG connectivity in ASD. Many task-based studies use passive paradigms (Table 1) and are limited in three ways. First, the absence of a required behavioral response means that there is uncertainty as to whether the two groups (ASD/control) are participating equally. Differences in degree of effort are known to affect EEG-derived measures [Smit, Eling, & Coenen, 2004; Ullsperger, Metz, & Gille, 1988] and could bias the results. Second, because many of the studies examine only the task-active condition without comparing it with the baseline data, they do not report on the task-related modulation of connectivity. Finally, many of these studies use data-driven approaches to search for differences in functional connectivity, rather than focusing in on brain regions known to be associated with the performance of the task.
In this article, we investigated task-related modulation of functional connectivity in children with ASD via EEG, using a task with known relevance to ASD and measuring a well characterized network. Praxis (tool-use pantomime) deficits have been extensively documented in ASD and have been shown to correlate with core social-communicative deficits [Dziuk et al., 2007; Mostofsky et al., 2006]. It is believed that parallel motor-skill and social-skill circuits account for this relationship [Denckla, 2005; Mostofsky & Ewen, 2011]. The cortical networks associated with praxis function have been extensively mapped out through over a century of neuropsychological [Goldenberg, 2013] and physiological study [Buxbaum et al., 2008; Wheaton & Hallett, 2007].
The praxis network, as established by lesion studies, consists of left-hemisphere premotor and inferior parietal regions [Wheaton & Hallett, 2007]. Physiological studies further demonstrate task-related activations of the homologous right-hemisphere regions [Bohlhalter et al., 2009; Ewen et al., 2015, 2016; Wheaton et al., 2008]. Previous EEG work has demonstrated that bilateral central (motor/premotor) and parietal scalp regions demonstrate event-related desynchronization (ERD) of alpha- and beta-band activity [Ewen et al., 2016; Wheaton & Hallett, 2007] and event-related synchronization (ERS) in the theta band during both praxis gesture production and imagining. ERD refers to task-related decreases in signal power within a particular frequency band; ERS refers to task-related increases. Since alpha and beta are generally understood to be actively inhibitory, or at least representative of a resting state [Jensen et al., 2005; Jensen & Mazaheri, 2010], alpha and beta ERD are indicative of cortical activation [Engel & Fries, 2010; Ewen et al., 2016; Neuper, Wörtz, & Pfurtscheller, 2006; Tuladhar et al., 2007].
In a previous report using EEG to study dyspraxia in children with ASD [Ewen et al., 2016], we observed decreased-magnitude of regional task-related power modulation (ERD) in alpha (7–13 Hz) and beta (18–23 Hz) bands. ASD-related decreases in alpha ERD were specific to posterior (parietal) scalp regions, and decreases in beta ERD were specific to central scalp regions. These changes can be interpreted in a connectivity framework as representing decreased local population-level connectivity. This decreased modulation of EEG oscillatory power was correlated with clinical measures of autism symptom severity (motor- and social-communicative symptoms).
These results, however, speak only to the individual action of each region. The aim of the current work was to assess long-range functional connectivity between the left parietal and premotor regions of the praxis network, and with their right-sided homologues. Based on the global underconnectivity account, we hypothesized that children with ASD would have a smaller magnitude of task-related increases in connectivity within the praxis network, during a tool-use pantomime task. Based on our ERD results showing group differences only in alpha and beta, we constrained our investigation to these bands. Given the premise that frontal-parietal network alterations drives not only praxis deficits but also impairments in social skills [Just, Keller, Malave, Kana, & Varma, 2012; Mostofsky et al., 2006; Mostofsky & Ewen, 2011], we performed a post hoc exploratory analysis of the correlations between task-related functional connectivity modulation and several behavioral tests of motor function and social/communicative symptom severity.
Materials & Methods
Subjects
The EEG dataset analyzed here is the same data used in the ERD analysis [Ewen et al., 2016]. The experimental protocol and consenting process were approved by the Johns Hopkins Medicine Institutional Review Board. Data were recorded from 46 children with ASD and 50 IQ- and age-matched typically developing (TD) participants. After exclusions, the two groups whose data were analyzed consisted of 25 children with ASD and 33 TD controls (24% female in each group; Fisher’s Exact P=1). All children were between the ages of 8 and 13 years (ASD, 10.7±1.4 years, TD, 10.52±1.3 years; P=0.62). All subjects were right handed. Handedness was assessed using the Edinburgh scale [Oldfield, 1971], between 0.5 and 1, when available; otherwise, using the PANESS scale [Denckla, 1985] (they had to do 10 out 11 gestures with right hand spontaneously). Other exclusion criteria for both groups included full-scale IQ<80 (Wechsler Intelligence Scale for Children—Fourth Edition (WISC-IV) [Wechsler, 2003]), visual impairment worse than 20/40 (corrected), pregnancy, or any other diagnosed medical or neurological condition. For IQ, if children showed a difference of greater than 12 points between Verbal Comprehension Index and Perceptual Reasoning Index, the lower score had to be >65.
In our sample, there was no difference seen in full scale IQ between the two groups (mean FSIQ for TD=115.6, and for ASD=109.3; P=0.095). In order to rule-out any group differences in IQ, we used Bayesian tests with two priors [Rouder, Speckman, Sun, Morey, & Iverson, 2009]. Neither test showed evidence for group differences (JZS Bayes Factor=1.5, and Scaled-Information Bayes Factor=0.825).
The ASD group consisted of children with high-functioning ASD (at least low-average IQ [Ghaziuddin & Mountain-Kimchi, 2004]). The diagnosis was made by a pediatric neurologist (SHM) experienced with ASD, based on DSM-IV criteria [American Psychiatric Association, 2000]. The children had diagnostic scores for both ADOS [Lord et al., 2000, 2012] and ADI-R [Lord, Rutter, & Le Couteur, 1994] and were assessed using Diagnostic Interview for Children and Adolescents (DICA-IV, [Reich, Welner, & Herjanic, 1997]) or Schedule for Affective Disorders and Schizophrenia for School-Age Children (K-SADS [Kaufman et al., 2013]) to examine for the presence of other psychiatric disorders. We allowed children with comorbid diagnoses of anxiety disorder and attention deficit/hyperactivity disorder (ADHD), consistent with much ASD research [Dowell, Mahone, & Mostofsky, 2009; Dziuk et al., 2007; MacNeil & Mostofsky, 2012; Mostofsky et al., 2006, 2009]. Both ADHD and anxiety disorder have high rates of comorbidity with ASD [Leyfer et al., 2006; Matson & Nebel-Schwalm, 2007; Simonoff et al., 2008], and exclusion of these diagnoses would have constricted recruitment considerably. Children with all other known neurological problems or etiologies associated with ASD were excluded.
Anxiety disorder, including obsessive compulsive disorder, generalized anxiety disorder and simple phobia were diagnosed via the DICA-IV or K-SADS in eight children. A total of 17 children with ASD were diagnosed with ADHD, as established by diagnostic scores on three of three instruments: the DICA-IV or K-SADS, the Connors Parent Rating Scale, and the DuPaul rating scale. For any subject who received a diagnostic score on one or two out of three of these scales, the final decision was made by a pediatric behavioral neurologists experienced in ADHD and ASD (SHM).
Eight children with ASD diagnosis were on medication. Stimulant medication was stopped 24 hr prior to testing. Two were on dextroamphetamine salts, five were on methylphenidate/dexmethylphenadate and one was on clonidine and “homeopathic lithium carbonate.”
The control group consisted of children who were free of ASD or other neurological and psychiatric disorders, including anxiety and ADHD, diagnosed by the same method as described above in the ASD group. In TD children, absence of ASD was determined using Social Responsiveness Scale [Constantino & Gruber, 2007]. Any TD subjects who had first-degree family members with ASD were excluded. TD subjects were also free of any DICA-IV diagnoses. Seven TD children were on second-generation histamine blockers, inhaled steroids and leukotriene inhibitors.
We excluded subjects who did not finish all 6 runs of the experiment. In addition, all subjects who had fewer than 50% of the trials remaining after behavioral scoring (see “Experimental Paradigm”) were excluded (18 ASD, 14 TD). The same threshold of 50% of remaining trials was applied after EEG artifact rejection and another 3 subjects with ASD, 3 TD subjects were excluded. The strict and conservative approach we took led to the exclusion of 38 subjects in total. It should be noted that all exclusions were made before any analyses were done on any data. After all rejections, the connectivity analyses were performed on a cohort of 25 children with ASD and 33 TD participants. See Supplementary Materials for details about the excluded subjects.
Experimental Paradigm
The paradigm was a modified version of the task from Wheaton, Fridman, Bohlhalter, Vorbach, and Hallett [2009], altered to minimize working memory and linguistic confounds; it was previously used in Ewen et al. [2015, 2016]. Subjects had to pantomime the use of 10 common tools (e.g., scissors, hammer, doorknob, pencil) after being cued by a picture of the tool. Before data collection, subjects had to demonstrate the proper usage of all the tools to ensure that they were familiar with them. Timeline of a single trial is shown in Figure 1. Each trial started with a 4 sec pre-stimulus period during which subjects had to focus on a fixation cross at the center of the dark screen. This was followed by a 3 sec Prepare (Prep) phase, during which subjects were shown the picture of a tool in the center of a monitor against and dark background. After 3 sec, a green square appeared around the tool for 3.5 sec, during which time the subject had to pantomime the use of the tool (Go). The word “Rest” then appeared on screen for 2 sec before moving on to the next trial. There were 120 trials (10 tools repeated 12 times each and presented in random order), grouped randomly into six runs of 20 trials each. The same pseudo-random order was used on all subjects.
Figure 1.
Shown is the structure of a single trial of the praxis task paradigm. The subjects had to fixate on a cross for 3 sec, after which a picture of a tool appeared. This stage (Prep) lasted for 3 sec. Next, a green box appeared around the tool, and the subject was required to start pantomiming the use of the shown tool. This stage (Go) of the trial lasted 6.5 sec. This interval was followed by the word Rest on the screen for 2 sec, and then the next trial.
All subjects were monitored by a psychological associate during task performance. The associate was trained and tested to verify research reliability [Mostofsky et al., 2006] in the administration and scoring of gesture assessment batteries, including the Florida Apraxia Battery (FAB) [Rothi, Raymer, & Heilman, 1997] in pediatric populations. If the subject moved earlier than cued or much later (>1000 ms), then the trial was marked via button press and excluded from all further analyses. Trials in which subjects did not finish pantomiming in the allotted time or did not move at all were also excluded. The movements were scored for accuracy, based on the pediatric FAB criteria which have been used in previous studies of praxis in ASD [Mostofsky et al., 2006]. The sessions were video recorded and scored by an independent rater afterwards. Only the trials marked correct by both scorers were used in the analyses.
Data Collection and Preprocessing
Data collection and preprocessing methods are similar to that reported in Ewen et al. [2016]. EEG data were recorded using Advanced NeuroTechnologies asa-lab system (Netherlands) and Waveguard cap, using a 47- channel equidistant Duke montage (see Fig. 2) at a sampling rate of 512 Hz. The signals were recorded using an averaged channel reference, with an impedance level of <5 kΩ. EEG data preprocessing and artifact correction was performed using asa-lab software. Preprocessing consisted of high-pass filtering (0.2 Hz cutoff, 24 dB/Oct slope), followed by PCA-based removal of eye blinks, lateral eye movements, and muscular or movement artifacts [Ille, Berg, & Scherg, 2002]. Artifacts for the PCA correction were visually identified in each subject by the brief monopolar potentials of eye blinks, longer duration bipolar increased amplitudes of lateral eye movements, and high amplitude, high frequency, muscle activity (verified using video). Using multiple instances of well-categorized artifact morphologies, the PCA-based algorithm removes the principal components which account for >90% variance of the noise subspace. Post-correction signals were visually inspected, and channels and trials with persistent artifact were removed. No channel was removed from final set of subjects. Only subjects that had more than 50% of the trials remaining were included.
Figure 2.

The figure shows the schematic diagram of the 47 channel electrode layout used. The channels that correspond to the known praxis regions are highlighted and labeled. The channels that correspond to the left hemispheric praxis regions are grouped into left-central (LC) and left-parietal (LP) regions with three channels each. The corresponding right hemispheric homologues were grouped into right-central (RC) and right-parietal (RP) groups as well. The arrows indicate the channel pairs that showed significant difference in task related connectivity between groups.
After all exclusion steps, TD group had on average 102.5±11.2 trials remaining, and ASD group had 86.12±14.9 trials. Since the number of trials were significantly different between groups (P<0.001), we used non-parametric tests which do not make assumptions about equal variance.
Connectivity Analysis
Spatial smoothing caused by volume conduction can lead to spurious connectivity results [Srinivasan, Winter, Ding, & Nunez, 2007]. To minimize the effects volume conduction, we converted the EEG signals to current source density (CSD) estimates using the CSD toolbox [Kayser & Tenke, 2006].
Connectivity analysis on the transformed data was carried out using the Fieldtrip toolbox [Oostenveld, Fries, Maris, & Schoffelen, 2011]. In high-temporal-precision signals like EEG and MEG, phase-based measures of functional connectivity are often used [Sauseng & Klimesch, 2008; Tognoli & Kelso, 2014; Varela et al., 2001]. We used phase locking value (PLV) [Lachaux, Rodriguez, Martinerie, & Varela, 1999] as our measure of functional connectivity. Compared with more traditional methods such as coherence, PLV is less influenced by power in the signal [Lachaux et al., 1999; Lowet, Roberts, Bonizzi, Karel, & Weerd, 2016]. This feature is critical in the current dataset, in which there are known group differences in task-related power modulation (ERD). Additionally, to assess the influence of power on PLV, we examined correlations between PLV values and ERD in corresponding channels (see Supplementary Materials). The channels, frequencies and time windows that went into our analyses were chosen based on the patterns of activation seen in our previous study [Ewen et al., 2016]. The frequency bands and time windows were chosen based on the averaged task-related spectro-grams from each group (see Fig. S1), which revealed well delineated regions of activity that were consistent with the traditional definitions of different frequency bands. Based on the task-related spectrograms, we chose 7–13 Hz (alpha) and 18–23 Hz (beta).
The channels were selected based on known literature about the praxis network. The praxis network is known to be lateralized consistently to the left parietal-central areas [Wheaton et al., 2008; Wheaton & Hallett, 2007]. We specifically investigated the functional connectivity of the left-hemisphere praxis network, which consists of left parietal (LP) and left central (LC) regions (3 channels covering each region; Fig. 2). Additionally, we also included the channels from the right hemispheric homologues of the left praxis network [Wheaton et al., 2009], since prior physiological studies have shown that those homologues activate consistently as well [Ewen et al., 2016; Wheaton et al., 2008]. Within each hemisphere, functional connectivity was estimated for every pair of channels between the regions of interests using PLV (9 pairs). We also estimated the functional connectivity between all channels in LP and LC regions to its corresponding right-sided homologues (6 pairs).
PLV values were computed separately for three time periods: the pre-stimulus phase (Rest, −2 to 0 sec, relative to the onset of the tool picture stimulus), and two active phases: the movement preparation phase (Prep, 0–2 sec), and the motor execution phase (Go, 3–5 sec), for each frequency band and channel pair.
To examine the task-related changes in functional connectivity we calculated the task-related PLV [Manganotti et al., 1998] changes (trPLV) as
Here the baseline is the Rest period, and task is either Prep or Go period. PLV is always positive number between 0 (no phase consistency) and 1 (perfectly consistent phase). trPLV then can range between −1 and 1. Positive trPLV means there was an increase in functional connectivity during task. Negative trPLV, on the other hand, means that there was a task-related decrease in functional connectivity compared with baseline.
PLV Statistical Analyses
To test our hypotheses, we conducted 3 series of comparisons.
To test our primary hypothesis that trPLV(ASD)< trPLV(TD), we performed between-group trPLV comparisons on each channel combination and frequency band (alpha, beta), independently, using non-parametric permutation tests. The permutation tests were based on surrogate data generated from 1000 random permutations in which the data were randomly partitioned into ASD and TD groups. The test makes no a priori assumptions [Lachaux et al., 1999; Maris, Schoffelen, & Fries, 2007], such as stationarity or equal variances or sample sizes. All subsequent analyses were done on channel pairs found in this analysis to be significantly different between groups. We used false discovery rate (FDR) procedure [Benjamini & Hochberg, 1995] for multiple comparison correction by grouping, channel pairs across frequency bands for each ROI and task phase.
To assess whether task-related increases and/or decreases from baseline were significant, we also conducted within-group permutation tests (Rest vs. Active (separately for Prep, Go)). The surrogate data for each within-group comparison was generated by randomly partitioning the corresponding rest and active epoch labels.
Finally, to validate that the task-related differences were not driven by baseline differences, we compared Rest PLV between groups, using non-parametric permutation tests. The surrogate data were generated by randomly partitioning the rest epoch between the two groups.
One possible confounding factor is the non-stationary nature of phase in biological signals. To assess the influence of non-stationarity we conducted separate analyses on 1s windows that made up our 2s window. These results are presented in Supplementary Materials.
Behavioral - PLV Correlations
We explored the relationship between functional connectivity and clinical/behavioral phenotype using test batteries that were chosen based on previous ERD work [Ewen et al., 2016]. In these exploratory analyses, we examined the correlation (Pearson’s r) between trPLV and the subscales/factors of the ADOS (ASD only), WISC-IV, SRS, and pediatric FAB [Mostofsky et al., 2006] (both groups).
Results
Between-Group PLV Comparisons
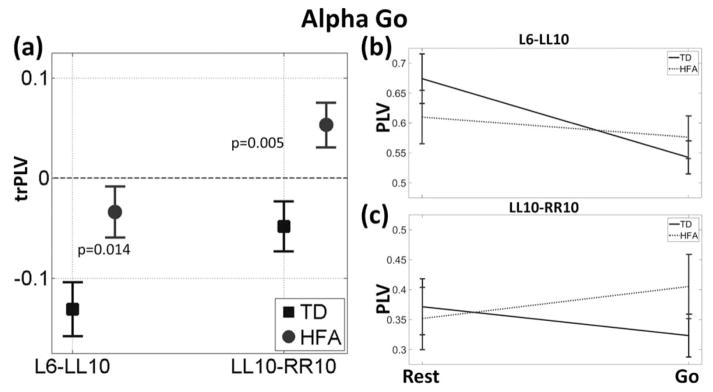
In the alpha band, within the left-hemisphere praxis network (left parietal–central comparisons), one channel pair (LL10-L6; Fig. 2), showed a significant difference in trPLV between the groups. This difference was seen during both Prep (P=0.031, ASD>TD) and Go (P=0.023) phases of the task. During both task phases, the controls showed negative trPLV (decrease from baseline). Children with ASD, by contrast, showed a positive trPLV (increase from baseline) during Prep. Comparing Go to Rest, ASD group showed a negative trPLV, similar to controls, but the magnitude was significantly smaller.
From the inter-hemispheric homologous connections, one parietal channel pair showed a statistically significant difference between the two groups. The alpha-band trPLV between LL10-RR10 (Fig. 2) was significantly different in both groups during both Prep (P=0.011) and Go (P=0.007) phases.
Only the inter-hemispheric trPLV connection survived the FDR correction in both task phases (LL10-RR10; FDR-adjusted P-values 0.03 and 0.018 for Prep and Go, respectively).
There were no significant group trPLV differences found in the beta band during either the Prep or Go phase, in any of the channel pairs.
Within-Group, Task-vs.-Rest PLV Comparisons
In controls, within-group comparisons (task-phases vs. Rest) showed that there was a decrease in connectivity from baseline during task (Prep and Go, Figs. 3a, 4a). During Go, PLV in both LL10-L6 (P=0.001) and LL10-RR10 (P=0.043) were significantly lower that Rest. The decrease in PLV (negative trPLV) in the same channel pairs from Rest to Prep approached significance (LL10-L6, P=0.079 and LL10-RR10, P=0.102).
Figure 3.
Alpha band group results during the Prep period of the praxis task. Panel (A) shows the trPLV results for the channels that showed significant differences between the two groups. Significant trPLV differences were seen in the L6-LL10 (left central-parietal) and LL10–RR10 (left-right parietal). Panels (B) and (C) show the PLV values for each group during that went into the trPLV calculations. The task related changes and interaction from the statistical test is evident from the slopes of the two lines.
Figure 4.
Alpha band group results during the Go period of the praxis task. As in Figure 3 panel (A) shows the group trPLV results for the significantly different channels and panels (B) and (C) show the PLV values that went into the trPLV calculations.
In children with ASD, on the other hand, in all but one channel pair, functional connectivity increased during the task (positive trPLV, Figs. 3a, 4a). This increase from baseline was significant for the interhemispheric connection (LL10-RR10) during Prep (P=0.034) and Go (P=0.026). The within-left-hemisphere connection was not significantly different in either task phase (Prep:P=0.167; Go:P=0.218).
Between-Group PLV Rest Comparisons
Comparisons of the baseline epochs (PLVbaseline) between groups revealed no significant differences for the two channel pairs that showed trPLV group differences. P-values for LL10-RR10 and LL10-L6 for alpha band were P=0.71 and P=0.34, respectively. Therefore, group differences in trPLV are not driven by baseline PLV group differences.
PLV-Behavioral Correlations
The exploratory analyses revealed no significant correlations between trPLV and any of the behavioral measures within the TD group. No significant correlations were found between IQ and trPLV in either group.
Within the ASD group, the inter-hemispheric parietal trPLV (Prep) was significantly correlated with FAB percentage total correct scores (r=0.42, P=0.035) and FAB imitation correct scores (r=0.47, P=0.019). The higher the FAB correct score, the less severe a participant’s dys-praxia. The positive correlation with trPLV therefore suggests that the increase from baseline (opposite direction to TD group), the better the motor performance is.
During the Go phase, symmetrical inter-hemispheric parietal trPLV was significantly correlated with the ADOS total score (r=−0.47, P=0.019) and with two domain sub-scores (Restricted Repetitive Behavior: r=−0.49, P=0.013; Communication: r=−0.42, P=0.038). In ADOS results, the higher the score, the more severe the ASD-related symptom. A negative correlation here indicates that the more positive (increase from baseline, opposite direction to TD group) trPLV gets, the less severe the symptoms are.
Also during the Go phase, the left parietal-central trPLV was significantly correlated with the ADOS total score (r=0.46, P=0.019) and the social interaction domain sub-score (r=0.41, P=0.042). In contrast to the inter-hemispheric connectivity, a positive correlation here suggests that lower the children with ASD’s trPLV is (similar direction of change to the mean of the TD participants), the less severe the ASD symptoms.
Discussion
In this study, we examined task-related changes in praxis network functional connectivity in children with ASD, as compared with controls. We predicted that the controls would show a task-related increase in connectivity, and the children with ASD would demonstrate lower magnitude of increase in PLV as compared to TD children. Counter to our prediction, the data did not show any significant increases in connectivity from baseline in controls. Rather, controls showed a task-related decrease in functional connectivity relative to baseline, which were significant during movement execution (Go) and approaching significance during movement planning (Prep). In contrast, children with ASD showed a task-related increase in PLV from baseline; this increase was significant in both task phases for the inter-hemispheric parietal connection.
Current theories of brain function posit that neural systems realize function as spatially distributed networks whose nodes are dynamically coordinated in a task-dependent manner [Sporns, 2002; Sporns, Chialvo, Kaiser, & Hilgetag, 2004; Tononi, Sporns, & Edelman, 1994]. One function may rely more on local processing (functional segregation), whereas another may be achieved in a more distributed manner (functional integration). Inter-regional communication and its modulation are necessary for both segregation and integration [Bastos & Schoffelen, 2016].
One interpretation of our results is that, in the TD group, the left parietal–central and the left–right parietal regions decouple during preparation and movement in the alpha band. In other words, in the alpha band, functional connectivity across the overall praxis network is reduced during task-related engagement of functionally segregated sub-networks in parietal and central regions. Previous reports of task-related decreases in EEG connectivity have interpreted this decrease as a mechanism that reduces distraction or assigns greater relative importance to incoming information [Classen, Gerloff, Honda, & Hallett, 1998].
In ASD group, on the other hand, central and parietal regions may not be as decoupled during praxis performance as they are in TD children. The within-group test showed that the intrahemispheric increase in ASD group was only numerical and was not significantly different from baseline. Hence it is possible that the measured task-related group difference is being driven by the changes in the TD group. However, communication between left and right parietal regions increased significantly for both task phases. Our previous work [Ewen et al., 2016] has shown relatively lower task-related decreases in alpha and beta power in similar regions in children with ASD, consistent with decreased local cortical activation. We interpreted this as a reflection of reduced localized task-related neural activation. Combining our ERD and PLV findings, it is possible that TD children use more localized processing (greater cortical activation, less large-scale connectivity) to accomplish the task than do children with ASD. Based on the EEG results alone, it is unclear as to whether the increased connectivity in the ASD group is a form of compensatory mechanism that the ASD brain uses to mitigate inadequate local activity or is part of more general alteration of functioning.
The exploratory correlation analyses with clinical measures may provide speculative answers as to whether these connectivity changes are pathogenic or compensatory, though these associations should be interpreted with caution. When children with ASD’s left hemispheric intra-hemispheric connectivity are more similar to that of TD subjects, they have less severe symptoms on the ADOS, consistent with the notion that TD-dissimilar function in the left-hemisphere frontal-parietal network is pathogenic [Denckla, 2005; Mostofsky & Ewen, 2011]. In contrast, when children with ASD have inter-hemispheric connectivity that moves in the opposite direction to that of the average of the TD group, they have less severe symptoms. Similarly, the FAB percent-correct scores also correlated positively with inter-hemispheric connectivity. This suggests that the inter-hemispheric connectivity increases may be a compensatory mechanism in children with ASD. The notion of decreased lateralization/increased activation of contralateral cortical regions in developmental disabilities has been raised in other contexts, and dyslexia in particular [(Simos, Breier, Fletcher, Bergman, & Papanicolaou, 2000].
Beta, the other frequency band we investigated, did not show any significant differences between groups. Similar to our results, Domínguez, Stieben, Velázquez, and Shanker [2013] also saw increased left-hemispheric alpha connectivity in ASD during their task and also did not observe beta band connectivity differences between the groups.
Published resting-state studies in ASD converge on the notion that the disorder is associated with decreases in functional connectivity. The goal of this study was to bridge the distance between resting-state physiology and the behaviors which are central to the disorder by studying the patterns of connectivity modulation associated with the performance of an ASD-relevant behavior (praxis). Our prediction, motivated by a “global underconnectivity” account of ASD, was that children with ASD, when performing a praxis task, would show a relative decrease in the task-related connectivity typically seen in the performance of this task. Instead, the data were more consistent with a scenario in which the connectivity pattern shift from rest to a task state has a qualitatively different pattern in ASD. As described below, statistical considerations demand replication of these patterns of results. It is possible that altered connectivity pattern might be task-specific. Another set of disorder-relevant tasks might reveal different mechanisms. The primary message of these results, in our view, is to call for a re-evaluation of the notion that altered behavioral performance in ASD is associated simply with a decreased long-range functional connectivity. Rather, altered connectivity may influence the production of diagnosis-relevant behavior in ASD through an altered ability to modulate task-dependent network states.
A few considerations limit the scope of our interpretations. Given the prevalence of ADHD in our sample and in general ASD population, the relative contribution of ASD and ADHD pathophysiology cannot be separated. Because the study included higher-functioning participants with ASD, and because those subjects excluded due to uninterpretable data (behavioral, EEG) had higher ADOS scores and lower IQ, it is conceivable that the pattern of results might be different in children who exhibit more severe symptoms. A few technical factors should also be considered. Even though PLV is relatively insensitive to power differences (compared to coherence), and though we saw no statistical relationship between ERD and PLV, there could theoretically be an influence of signal power on connectivity. Additionally, phase calculations make an assumption of stationarity which is not fully met in EEG. The analyses in Supplementary Materials speak against marked non-stationarity during the time-windows assessed, but there is no definitive way to remove this confound entirely. Also, scalp-level connectivity can be affected by volume conduction even though we tried to minimize this confound by using a CSD transformation. Finally, it should be noted that group differences in the intra-hemispheric connectivity are uncorrected for multiple comparisons and could be the result of a Type I error. Further task-based studies will be necessary to fully substantiate the pattern of altered task-related connectivity modulation found here.
Conclusion
We used a hypothesis-driven approach to examine task-related modulations of functional connectivity in children with ASD. Contrary to our hypotheses of increased task-related connectivity within the praxis network, the TD group showed decreased task-related connectivity. Functional connectivity changes during praxis were in the opposite direction in ASD group. These results suggest a fundamental deficit of task-related functional connectivity modulation in ASD. The exploratory correlations with clinical measures suggest that the altered connectivity pattern may be partly pathogenic and partly compensatory.
Supplementary Material
Figure S1. For selection of the task-relevant regions-of-interest (ROIs), we relied on the praxis-EEG literature [Wheaton et al., 2008; Wheaton et al., 2009; Wheaton & Hallett, 2007], as well as previously published results from the same dataset as considered here [Ewen et al., 2016]. For selection of the task-relevant time-frequency windows, we relied on the task-related ERD spectrograms as developed in Ewen et al. [2016]. Top row shows the ERD topoplots for alpha-band along with ROI selection schematic. Bottom row shows ERD spectrograms from the left hemisphere ROIs; the relevant time-frequency decomposition was similar on the right-sided homologues.
Figure S2. PLV data from the 1s-window analyses during Prep. Panels (a) and (b) show the data for left parietal-central channel pair (LL10-L6) and the parietal interhemispheric (LL10-RR10) channel pairs, respectively. In each panel, the first column shows the 1s rest window followed by the two consecutive 1s windows of the Prep phase. As can be seen in the plots, the two 1s-windows give similar PLV values to each other, suggesting that there is sufficient stationarity in the PLV measurements within the 2s windows from which the primary conclusions were derived.
Figure S3. PLV data from the 1s-window analyses during Go. Panels (a) and (b) show the data for left parietal-central channel pair (LL10-L6) and the parietal interhemispheric (LL10-RR10) channel pairs respectively. In each panel the first column shows the 1s rest window followed by the two consecutive 1s windows of the Go phase. As can be seen the two 1s windows give similar PLV values.
Figure S4. Mean percent change in PLV within an ROI. Given the specificity of the results from those channel pairs which show significant between-group differences (one channel pair each in the parietal inter-hemispheric and left parietal-central ROIs), we examined the nature of connectivity within an entire ROI-ROI comparison. During Go phase, the patterns of results are same as the trPLV results presented in the main text (compare to Figure 4 in main text). In the Prep phase, there is more variability in the data and numerical results do not exactly match the single channel pair trPLV results. The Prep phase results should be interpreted with particular caution.
Table S1. The results of the correlation analysis (Pearson’s correlation coefficient) between ERD and PLV. Each PLV value corresponding to a channel pair and task-phase was correlated with the ERD from each of the channels that was used for the PLV calculation. We did not find any significant correlations or consistent relationship between ERD and PLV. The only value approaching significance (p=0.09, rho=-0.35) was the correlation between ERD in RR10 and PLV between LL10-RR10 during Prep for ASD subjects. These results suggest that PLV results were not driven simply by group differences in ERD.
Lay Summary.
Decreased connectivity between brain regions is thought to cause the symptoms of autism. Because most of our knowledge comes from data in which children are at rest, we do not know how connectivity changes directly lead to autistic behaviors, such as impaired gestures. When typically developing children produced complex movements, connectivity decreased between brain regions. In children with autism, connectivity increased. It may be that behavior-related changes in brain connectivity are more important than absolute differences in connectivity in autism.
Acknowledgments
This work was supported by the National Institute of Neurological Disorders and Stroke (K23NS073626 and R21NS091569 to JBE; and R01NS048527 to SHM) grants.
Footnotes
Conflict of interest
The authors declare that there were no commercial or financial conflicts of interest.
Additional Supporting Information may be found in the online version of this article.
References
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders DSM-IV-TR. Washington, DC: American Psychiatric Association; 2000. [Google Scholar]
- Bastos AM, Schoffelen JM. A tutorial review of functional connectivity analysis methods and their interpretational pitfalls. Frontiers in Systems Neuroscience. 2016;9:175. doi: 10.3389/fnsys.2015.00175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boersma M, Kemner C, de Reus MA, Collin G, Snijders TM, Hofman D, … van den Heuvel MP. Disrupted functional brain networks in autistic toddlers. Brain Connectivity. 2012;3:41–49. doi: 10.1089/brain.2012.0127. [DOI] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. Journal of the Royal Statistical Society. Series B (Methodological) 1995;57:289–300. [Google Scholar]
- Bohlhalter S, Hattori N, Wheaton L, Fridman E, Shamim EA, Garraux G, Hallett M. Gesture subtype–dependent left lateralization of praxis planning: An eventrelated fMRI study. Cerebral Cortex. 2009;19:1256–1262. doi: 10.1093/cercor/bhn168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buxbaum LJ, Haaland KY, Hallett M, Wheaton L, Heilman KM, Rodriguez A, Gonzalez Rothi LJ. Treatment of limb apraxia: Moving forward to improved action. American Journal of Physical Medicine & Rehabilitation. 2008;87:149–161. doi: 10.1097/PHM.0b013e31815e6727. [DOI] [PubMed] [Google Scholar]
- Buzsáki G, Draguhn A. Neuronal oscillations in cortical networks. Science. 2004;304:1926–1929. doi: 10.1126/science.1099745. [DOI] [PubMed] [Google Scholar]
- Catarino A, Andrade A, Churches O, Wagner AP, Baron-Cohen S, Ring H. Task-related functional connectivity in autism spectrum conditions: An EEG study using wavelet transform coherence. Molecular Autism. 2013;4:1. doi: 10.1186/2040-2392-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Classen J, Gerloff C, Honda M, Hallett M. Integrative visuomotor behavior is associated with interregionally coherent oscillations in the human brain. Journal of Neurophysiology. 1998;79:1567–1573. doi: 10.1152/jn.1998.79.3.1567. [DOI] [PubMed] [Google Scholar]
- Coben R, Clarke AR, Hudspeth W, Barry RJ. EEG power and coherence in autistic spectrum disorder. Clinical Neurophysiology. 2008;119:1002–1009. doi: 10.1016/j.clinph.2008.01.013. [DOI] [PubMed] [Google Scholar]
- Constantino JN, Gruber CP. Social responsiveness scale (SRS) Los Angeles, CA: Western Psychological Services; 2007. [Google Scholar]
- Deco G, Jirsa VK, McIntosh AR. Emerging concepts for the dynamical organization of resting-state activity in the brain. Nature Reviews Neuroscience. 2011;12:43–56. doi: 10.1038/nrn2961. [DOI] [PubMed] [Google Scholar]
- Deco G, Kringelbach ML, Jirsa V, Ritter P. The dynamics of resting fluctuations in the brain: metastability and its dynamical cortical core. 2016 doi: 10.1038/s41598-017-03073-5. bioRxiv, 065284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deco G, Ponce-Alvarez A, Mantini D, Romani GL, Hagmann P, Corbetta M. Resting-state functional connectivity emerges from structurally and dynamically shaped slow linear fluctuations. Journal of Neuroscience. 2013;33:11239–11252. doi: 10.1523/JNEUROSCI.1091-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denckla MB. Revised neurological examination for subtle signs (1985) Psychopharmacology Bulletin. 1985;21:773–800. [PubMed] [Google Scholar]
- Denckla MB. Why assess motor functions “early and often?”. Mental Retardation and Developmental Disabilities Research Reviews. 2005;11:3. doi: 10.1002/mrdd.20054. [DOI] [PubMed] [Google Scholar]
- Domínguez LG, Stieben J, Velázquez JLP, Shanker S. The imaginary part of coherency in autism: Differences in cortical functional connectivity in preschool children. PloS One. 2013;8:e75941. doi: 10.1371/journal.pone.0075941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowell LR, Mahone EM, Mostofsky SH. Associations of postural knowledge and basic motor skill with dys-praxia in autism: Implication for abnormalities in distributed connectivity and motor learning. Neuropsychology. 2009;23:563–570. doi: 10.1037/a0015640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffy FH, Als H. A stable pattern of EEG spectral coherence distinguishes children with autism from neurotypical controls - a large case control study. BMC Medicine. 2012;10:64. doi: 10.1186/1741-7015-10-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dziuk MA, Larson JCG, Apostu A, Mahone EM, Denckla MB, Mostofsky SH. Dyspraxia in autism: Association with motor, social, and communicative deficits. Developmental Medicine & Child Neurology. 2007;49:734–739. doi: 10.1111/j.1469-8749.2007.00734.x. [DOI] [PubMed] [Google Scholar]
- Engel AK, Fries P. Beta-band oscillations — signalling the status quo? Current Opinion in Neurobiology. 2010;20:156–165. doi: 10.1016/j.conb.2010.02.015. [DOI] [PubMed] [Google Scholar]
- Ewen JB, Lakshmanan BM, Hallett M, Mostofsky SH, Crone NE, Korzeniewska A. Dynamics of functional and effective connectivity within human cortical motor control networks. Clinical Neurophysiology. 2015;126:987–996. doi: 10.1016/j.clinph.2014.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ewen JB, Lakshmanan BM, Pillai AS, McAuliffe D, Nettles C, Hallett M, … Mostofsky SH. Decreased modulation of EEG oscillations in high-functioning autism during a motor control task. Frontiers in Human Neuroscience. 2016;10:198. doi: 10.3389/fnhum.2016.00198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghaziuddin M, Mountain-Kimchi K. Defining the intellectual profile of Asperger syndrome: comparison with high-functioning autism. J Autism Dev Disord. 2004;34:279–284. doi: 10.1023/b:jadd.0000029550.19098.77. [DOI] [PubMed] [Google Scholar]
- Goldenberg G. Apraxia: The Cognitive side of motor control. 1. Oxford: Oxford University Press; 2013. [DOI] [PubMed] [Google Scholar]
- Hansen ECA, Battaglia D, Spiegler A, Deco G, Jirsa VK. Functional connectivity dynamics: Modeling the switching behavior of the resting state. NeuroImage. 2015;105:525–535. doi: 10.1016/j.neuroimage.2014.11.001. [DOI] [PubMed] [Google Scholar]
- Ille N, Berg P, Scherg M. Artifact correction of the ongoing EEG using spatial filters based on artifact and brain signal topographies. Journal of Clinical Neurophysiology. 2002;19:113–124. doi: 10.1097/00004691-200203000-00002. [DOI] [PubMed] [Google Scholar]
- Jensen O, Goel P, Kopell N, Pohja M, Hari R, Ermentrout B. On the human sensorimotor-cortex beta rhythm: Sources and modeling. NeuroImage. 2005;26:347–355. doi: 10.1016/j.neuroimage.2005.02.008. [DOI] [PubMed] [Google Scholar]
- Jensen O, Mazaheri A. Shaping functional architecture by oscillatory alpha activity: Gating by inhibition. Frontiers in Human Neuroscience. 2010;4:186. doi: 10.3389/fnhum.2010.00186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Just MA, Keller TA, Malave VL, Kana RK, Varma S. Autism as a neural systems disorder: A theory of frontal-posterior underconnectivity. Neuroscience & Biobehavioral Reviews. 2012;36:1292–1313. doi: 10.1016/j.neubiorev.2012.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman J, Birmaher B, Axelson D, Perepletchikova F, Brent D, Ryan N. Schedule for affective disorders and schizophrenia for school-age children-present and lifetime version (K-SADS-PL 2013 Working Draft) Pittsburgh, PA: Western Psychiatric Institute and Clinic and Yale UniversityWestern Psychiatric Institute and Clinic and Yale University; 2013. [Google Scholar]
- Kayser J, Tenke CE. Principal components analysis of Laplacian waveforms as a generic method for identifying ERP generator patterns: II. Adequacy of low-density estimates. Clinical Neurophysiology. 2006;117:369–380. doi: 10.1016/j.clinph.2005.08.033. [DOI] [PubMed] [Google Scholar]
- Khan S, Gramfort A, Shetty NR, Kitzbichler MG, Ganesan S, Moran JM, … Keten T. Local and long-range functional connectivity is reduced in concert in autism spectrum disorders. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:3107–3112. doi: 10.1073/pnas.1214533110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikuchi M, Shitamichi K, Yoshimura Y, Ueno S, Hiraishi H, Hirosawa T, … Minabe Y. Altered brain connectivity in 3-to 7-year-old children with autism spectrum disorder. NeuroImage: Clinical. 2013;2:394–401. doi: 10.1016/j.nicl.2013.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikuchi M, Yoshimura Y, Hiraishi H, Munesue T, Hashimoto T, Tsubokawa T, … Minabe Y. Reduced long-range functional connectivity in young children with autism spectrum disorder. Social Cognitive and Affective Neuroscience. 2015;10:248–254. doi: 10.1093/scan/nsu049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikuchi M, Yoshimura Y, Shitamichi K, Ueno S, Hirosawa T, Munesue T, … Minabe Y. A custom magneto-encephalography device reveals brain connectivity and high reading/decoding ability in children with autism. Scientific Reports. 2013;3:1139. doi: 10.1038/srep01139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klimesch W. Memory processes, brain oscillations and EEG synchronization. International Journal of Psychophysiology. 1996;24:61–100. doi: 10.1016/s0167-8760(96)00057-8. [DOI] [PubMed] [Google Scholar]
- Klimesch W. EEG alpha and theta oscillations reflect cognitive and memory performance: A review and analysis. Brain Research Brain Research Reviews. 1999;29:169–195. doi: 10.1016/s0165-0173(98)00056-3. [DOI] [PubMed] [Google Scholar]
- Kopell NJ, Gritton HJ, Whittington MA, Kramer MA. Beyond the Connectome: The Dynome. Neuron. 2014;83:1319–1328. doi: 10.1016/j.neuron.2014.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lachaux JP, Rodriguez E, Martinerie J, Varela FJ. Measuring phase synchrony in brain signals. Human Brain Mapping. 1999;8:194–208. doi: 10.1002/(SICI)1097-0193(1999)8:4<194::AID-HBM4>3.0.CO;2-C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leyfer OT, Folstein SE, Bacalman S, Davis NO, Dinh E, Morgan J, … Lainhart JE. Comorbid psychiatric disorders in children with autism: Interview development and rates of disorders. Journal of Autism and Developmental Disorders. 2006;36:849–861. doi: 10.1007/s10803-006-0123-0. [DOI] [PubMed] [Google Scholar]
- Lord C, Risi S, Lambrecht L, Cook EH, Jr, Leventhal BL, DiLavore PC, … Rutter M. The Autism Diagnostic Observation Schedule—Generic: A standard measure of social and communication deficits associated with the spectrum of autism. Journal of Autism and Developmental Disorders. 2000;30:205–223. [PubMed] [Google Scholar]
- Lord C, Rutter M, DiLavore P, Risi S, Gotham K, Bishop S. Autism diagnostic observation schedule–2nd edition (ADOS-2) Los Angeles, CA: Western Psychological Corporation; 2012. [Google Scholar]
- Lord C, Rutter M, Le Couteur A. Autism Diagnostic Interview-Revised: A revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. Journal of Autism and Developmental Disorders. 1994;24:659–685. doi: 10.1007/BF02172145. [DOI] [PubMed] [Google Scholar]
- Lowet E, Roberts MJ, Bonizzi P, Karel J, Weerd PD. Quantifying neural oscillatory synchronization: A comparison between spectral coherence and phase-locking value approaches. PloS One. 2016;11:e0146443. doi: 10.1371/journal.pone.0146443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacNeil LK, Mostofsky SH. Specificity of dys-praxia in children with autism. Neuropsychology. 2012;26:165–171. doi: 10.1037/a0026955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manganotti P, Gerloff C, Toro C, Katsuta H, Sadato N, Zhuang P, … Hallett M. Task-related coherence and task-related spectral power changes during sequential finger movements. Electroencephalography and Clinical Neurophysiology/Electromyography and Motor Control. 1998;109:50–62. doi: 10.1016/s0924-980x(97)00074-x. [DOI] [PubMed] [Google Scholar]
- Maris E, Schoffelen JM, Fries P. Nonparametric statistical testing of coherence differences. Journal of Neuroscience Methods. 2007;163:161–175. doi: 10.1016/j.jneumeth.2007.02.011. [DOI] [PubMed] [Google Scholar]
- Matson JL, Nebel-Schwalm MS. Comorbid psychopathology with autism spectrum disorder in children: An overview. Research in Developmental Disabilities. 2007;28:341–352. doi: 10.1016/j.ridd.2005.12.004. [DOI] [PubMed] [Google Scholar]
- Mostofsky SH, Dubey P, Jerath VK, Jansiewicz EM, Goldberg MC, Denckla MB. Developmental dyspraxia is not limited to imitation in children with autism spectrum disorders. Journal of the International Neuropsychological Society: JINS. 2006;12:314–326. doi: 10.1017/s1355617706060437. [DOI] [PubMed] [Google Scholar]
- Mostofsky SH, Ewen JB. Altered connectivity and action model formation in autism is autism. The Neuroscientist. 2011;17:437–448. doi: 10.1177/1073858410392381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mostofsky SH, Powell SK, Simmonds DJ, Goldberg MC, Caffo B, Pekar JJ. Decreased connectivity and cerebellar activity in autism during motor task performance. Brain. 2009;132:2413–2425. doi: 10.1093/brain/awp088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murias M, Webb SJ, Greenson J, Dawson G. Resting state cortical connectivity reflected in EEG coherence in individuals with autism. Biological Psychiatry. 2007;62:270–273. doi: 10.1016/j.biopsych.2006.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuper C, Wörtz M, Pfurtscheller G. ERD/ERS patterns reflecting sensorimotor activation and deactivation. Progress in Brain Research. 2006;159:211–222. doi: 10.1016/S0079-6123(06)59014-4. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Oostenveld R, Fries P, Maris E, Schoffelen J-M. FieldTrip: Open source software for advanced analysis of MEG, EEG, and invasive electrophysiological data. Computational Intelligence and Neuroscience. 2011:2011. doi: 10.1155/2011/156869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Reilly C, Lewis JD, Elsabbagh M. Is functional brain connectivity atypical in autism? A systematic review of EEG and MEG studies. PloS One. 2017;12:e0175870. doi: 10.1371/journal.pone.0175870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfurtscheller G. Functional brain imaging based on ERD/ERS. Vision Research. 2001;41:1257–1260. doi: 10.1016/s0042-6989(00)00235-2. [DOI] [PubMed] [Google Scholar]
- Reich W, Welner Z, Herjanic B. Diagnostic interview for children and adolescents-IV. North Tonawanda: Multi-Health System; 1997. [Google Scholar]
- Rothi LJG, Raymer AM, Heilman KM. Limb praxis assessment. In: Rothi LJG, Heilman KJ, editors. Apraxia: The neuropsychology of action. East Sussex, UK: Psychology Press; 1997. pp. 61–73. [Google Scholar]
- Rouder JN, Speckman PL, Sun D, Morey RD, Iverson G. Bayesian t tests for accepting and rejecting the null hypothesis. Psychonomic Bulletin & Review. 2009;16:225–237. doi: 10.3758/PBR.16.2.225. [DOI] [PubMed] [Google Scholar]
- Sauseng P, Klimesch W. What does phase information of oscillatory brain activity tell us about cognitive processes? Neuroscience & Biobehavioral Reviews. 2008;32:1001–1013. doi: 10.1016/j.neubiorev.2008.03.014. [DOI] [PubMed] [Google Scholar]
- Schipul SE, Keller TA, Just MA. Inter-regional brain communication and its disturbance in autism. Frontiers in Systems Neuroscience. 2011;5:10. doi: 10.3389/fnsys.2011.00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel M, Donner TH, Engel AK. Spectral finger-prints of large-scale neuronal interactions. Nature Reviews. Neuroscience. 2012;13:121–134. doi: 10.1038/nrn3137. [DOI] [PubMed] [Google Scholar]
- Simonoff E, Pickles A, Charman T, Chandler S, Loucas T, Baird G. Psychiatric disorders in children with autism spectrum disorders: Prevalence, comorbidity, and associated factors in a population-derived sample. Journal of the American Academy of Child & Adolescent Psychiatry. 2008;47:921–929. doi: 10.1097/CHI.0b013e318179964f. [DOI] [PubMed] [Google Scholar]
- Simos PG, Breier JI, Fletcher JM, Bergman E, Papanicolaou AC. Cerebral mechanisms involved in word reading in dyslexic children: A magnetic source imaging approach. Cerebral Cortex (New York, NY: 1991) 2000;10:809–816. doi: 10.1093/cercor/10.8.809. [DOI] [PubMed] [Google Scholar]
- Smit AS, Eling PATM, Coenen AML. Mental effort affects vigilance enduringly: After-effects in EEG and behavior. International Journal of Psychophysiology. 2004;53:239–243. doi: 10.1016/j.ijpsycho.2004.04.005. [DOI] [PubMed] [Google Scholar]
- Sporns O. Network analysis, complexity, and brain function. Complexity. 2002;8:56–60. [Google Scholar]
- Sporns O, Chialvo DR, Kaiser M, Hilgetag CC. Organization, development and function of complex brain networks. Trends in Cognitive Sciences. 2004;8:418–425. doi: 10.1016/j.tics.2004.07.008. [DOI] [PubMed] [Google Scholar]
- Srinivasan R, Winter WR, Ding J, Nunez PL. EEG and MEG coherence: Measures of functional connectivity at distinct spatial scales of neocortical dynamics. Journal of Neuroscience Methods. 2007;166:41–52. doi: 10.1016/j.jneumeth.2007.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tognoli E, Kelso JAS. The metastable brain. Neuron. 2014;81:35–48. doi: 10.1016/j.neuron.2013.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tononi G, Sporns O, Edelman GM. A measure for brain complexity: Relating functional segregation and integration in the nervous system. Proceedings of the National Academy of Sciences of the United States of America. 1994;91:5033–5037. doi: 10.1073/pnas.91.11.5033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuladhar AM, Huurne N, ter Schoffelen J-M, Maris E, Oostenveld R, Jensen O. Parieto-occipital sources account for the increase in alpha activity with working memory load. Human Brain Mapping. 2007;28:785–792. doi: 10.1002/hbm.20306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullsperger P, Metz AM, Gille HG. The P300 component of the event-related brain potential and mental effort. Ergonomics. 1988;31:1127–1137. doi: 10.1080/00140138808966752. [DOI] [PubMed] [Google Scholar]
- Varela F, Lachaux JP, Rodriguez E, Martinerie J. The brainweb: Phase synchronization and large-scale integration. Nature Reviews Neuroscience. 2001;2:229–239. doi: 10.1038/35067550. [DOI] [PubMed] [Google Scholar]
- Vasa RA, Mostofsky SH, Ewen JB. The disrupted connectivity hypothesis of autism spectrum disorders: Time for the next phase in research. Biological Psychiatry: Cognitive Neuroscience and Neuroimaging. 2016;1:245–252. doi: 10.1016/j.bpsc.2016.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wass S. Distortions and disconnections: Disrupted brain connectivity in autism. Brain and Cognition. 2011;75:18–28. doi: 10.1016/j.bandc.2010.10.005. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler intelligence scale for children–Fourth Edition (WISC-IV) San Antonio, TX: The Psychological Corporation; 2003. [Google Scholar]
- Wheaton LA, Bohlhalter S, Nolte G, Shibasaki H, Hattori N, Fridman E, … Hallett M. Cortico-cortical networks in patients with ideomotor apraxia as revealed by EEG coherence analysis. Neuroscience Letters. 2008;433:87–92. doi: 10.1016/j.neulet.2007.12.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheaton LA, Hallett M. Ideomotor apraxia: A review. Journal of the Neurological Sciences. 2007;260:1–10. doi: 10.1016/j.jns.2007.04.014. [DOI] [PubMed] [Google Scholar]
- Wheaton L, Fridman E, Bohlhalter S, Vorbach S, Hallett M. Left parietal activation related to planning, executing and suppressing praxis hand movements. Clinical Neurophysiology. 2009;120:980–986. doi: 10.1016/j.clinph.2009.02.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. For selection of the task-relevant regions-of-interest (ROIs), we relied on the praxis-EEG literature [Wheaton et al., 2008; Wheaton et al., 2009; Wheaton & Hallett, 2007], as well as previously published results from the same dataset as considered here [Ewen et al., 2016]. For selection of the task-relevant time-frequency windows, we relied on the task-related ERD spectrograms as developed in Ewen et al. [2016]. Top row shows the ERD topoplots for alpha-band along with ROI selection schematic. Bottom row shows ERD spectrograms from the left hemisphere ROIs; the relevant time-frequency decomposition was similar on the right-sided homologues.
Figure S2. PLV data from the 1s-window analyses during Prep. Panels (a) and (b) show the data for left parietal-central channel pair (LL10-L6) and the parietal interhemispheric (LL10-RR10) channel pairs, respectively. In each panel, the first column shows the 1s rest window followed by the two consecutive 1s windows of the Prep phase. As can be seen in the plots, the two 1s-windows give similar PLV values to each other, suggesting that there is sufficient stationarity in the PLV measurements within the 2s windows from which the primary conclusions were derived.
Figure S3. PLV data from the 1s-window analyses during Go. Panels (a) and (b) show the data for left parietal-central channel pair (LL10-L6) and the parietal interhemispheric (LL10-RR10) channel pairs respectively. In each panel the first column shows the 1s rest window followed by the two consecutive 1s windows of the Go phase. As can be seen the two 1s windows give similar PLV values.
Figure S4. Mean percent change in PLV within an ROI. Given the specificity of the results from those channel pairs which show significant between-group differences (one channel pair each in the parietal inter-hemispheric and left parietal-central ROIs), we examined the nature of connectivity within an entire ROI-ROI comparison. During Go phase, the patterns of results are same as the trPLV results presented in the main text (compare to Figure 4 in main text). In the Prep phase, there is more variability in the data and numerical results do not exactly match the single channel pair trPLV results. The Prep phase results should be interpreted with particular caution.
Table S1. The results of the correlation analysis (Pearson’s correlation coefficient) between ERD and PLV. Each PLV value corresponding to a channel pair and task-phase was correlated with the ERD from each of the channels that was used for the PLV calculation. We did not find any significant correlations or consistent relationship between ERD and PLV. The only value approaching significance (p=0.09, rho=-0.35) was the correlation between ERD in RR10 and PLV between LL10-RR10 during Prep for ASD subjects. These results suggest that PLV results were not driven simply by group differences in ERD.