Abstract
Objectives
Emerging data suggest that early deep sedation may negatively impact clinical outcomes. This systematic review and meta-analysis defines and quantifies the impact of deep sedation within 48 hours of initiation of mechanical ventilation, as described in the world’s literature. The primary outcome was mortality. Secondary outcomes included hospital and intensive care unit lengths of stay, mechanical ventilation duration, and delirium and tracheostomy incidence.
Data Sources
The following data sources were searched: MEDLINE, EMBASE, Scopus, Cochrane Central Register of Controlled Trials, Database of Abstracts of Reviews and Effects, Cochrane Database of Systematic Reviews databases, ClinicalTrials.gov, conference proceedings, and reference lists.
Study Selection
Randomized controlled trials (RCTs), and non-randomized studies were included.
Data Extraction
Two reviewers independently screened abstracts of identified studies for eligibility.
Data Synthesis
Nine studies (n= 4521 patients) published between 2012 and 2017 were included. A random effects meta-analytic model revealed that early light sedation was associated with lower mortality (9.2%) versus deep sedation (27.6%) [OR, 0.34 (0.21 – 0.54)]. Light sedation was associated with fewer mechanical ventilation (mean difference −2.1, 95% CI −3.6 to −0.5) and ICU days (mean difference −3.0, (95% CI −5.4 to −0.6). Delirium incidence was 28.7% in the light sedation group and 48.5% in the deep sedation group, OR 0.50 (0.22 – 1.16).
Conclusion
Deep sedation in mechanically ventilated patients, as evaluated in a small number of qualifying heterogeneous RCTs and observational studies, was associated with increased mortality and lengths of stay. Interventions targeting early sedation depth assessment, starting in the ED and subsequent ICU admission, deserve further investigation and could improve outcome.
Registration Details
This study is registered in the PROSPERO international prospective register of systematic reviews (#CRD42017057264).
Keywords: Mechanical ventilation, sedation, analgesia, Meta-analysis
INTRODUCTION
Sedation is often used in the care of mechanically ventilated patients and there is increasing recognition that the management of such non-ventilator aspects of care influences outcome(1). Present guidelines recommend titrating analgesics and sedatives to achieve light levels of sedation depth (1). Despite these recommendations, deep sedation in the intensive care unit (ICU) is common, and is associated with adverse outcomes such as increased mortality, lengths of stay, and delirium incidence(2).
Sedation during the initial period of mechanical ventilation appears especially impactful on clinical outcome(3, 4). Observational data shows that deep sedation within the first 48 hours following initiation of mechanical ventilation occurs in over 70% of patients, and is associated with increased mortality(2, 4). Despite this, the great majority of prior sedation research has not addressed this early period(2). As such, there is a knowledge gap regarding the impact of early sedation depth on clinically relevant outcomes.
The objectives of this study were to: 1) describe the global literature focused on sedation practices within 48 hours of initiating mechanical ventilation; and 2) quantify the impact of early sedation depth on clinical outcomes. We hypothesized that deep sedation in the 48-hour period following initiation of mechanical ventilation would be associated with increased mortality, longer mechanical ventilation duration, and increased hospital and ICU lengths of stay.
METHODS AND ANALYSIS
Protocol and registration
A systematic review protocol was prepared and published in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analysis Protocol (PRISMA-P) statement (5–8). These final results are reported according to PRISMA and the Meta-analysis of Observational Studies in Epidemiology (MOOSE) guidelines(5, 9) (Supplemental Digital Content 1). This systematic review was registered in the PROSPERO international prospective register of systematic reviews (#CRD42017057264) prior to protocol publication. Ethical approval was not required for this study.
Study Identification
An electronic search included the following databases: MEDLINE, EMBASE, Scopus, Cochrane Central Register of Controlled Trials (CENTRAL), Database of Abstracts of Reviews and Effects (DARE), and Cochrane Database of Systematic Reviews. Each database was searched from the earliest available date (i.e. the beginning of the database) through October 2016 (Supplemental Digital Content 2). The search was designed in cooperation with a trained medical librarian (SAF), experienced in systematic reviews, who performed the electronic search. Details of this search
Reference lists of included articles were manually screened to identify additional studies. Manual search of abstracts from the following meetings (2010 to 2017) identified unpublished data: Society of Critical Care Medicine, European Society of Intensive Care Medicine, International Symposium on Intensive Care and Emergency Medicine, American Thoracic Society, Society for Academic Emergency Medicine, Pharmacotherapy, American Society of Anesthesiologists; European Society of Anaesthesiology; International Anesthesia Research Society; Trauma, Critical Care & Acute Care Surgery; American Association for the Surgery of Trauma, and Eastern Association for the Surgery of Trauma. An online search for details of clinical trials registration (ClinicalTrials.gov) was conducted to identify completed, but not yet published, clinical studies. The principal investigators were contacted via electronic mail for clarification of data as needed.
Inclusion criteria
Studies were eligible regardless of language, and included adult patients receiving invasive positive pressure ventilation. Randomized controlled trials (RCT), as well as non-randomized studies (prospective and retrospective cohort analyses, cross-sectional studies, before-after trials) were included. Reviews, correspondences, editorials, and non-human studies were excluded. Eligible studies had to report some objective measure of sedation depth, such as the Richmond Agitation-Sedation Scale (RASS) or the Glasgow Coma Scale (GCS).
We compared outcomes between patients undergoing deep versus light sedation during the first 48 hours of mechanical ventilation. The primary outcome was hospital mortality. Secondary outcomes included: delirium, duration of ventilation, hospital and ICU stay, and tracheostomy incidence.
Study selection and data abstraction
Two independent reviewers (RJS and MRD) screened abstracts of identified studies for eligibility. In the case of disagreement, a third reviewer (BMF) arbitrated consensus. Manuscripts were reviewed for potential inclusion.
Data was extracted using standardized forms. Study characteristics, including author, publication year, study design, number of patients included, sedation data, study quality or risk of bias, and outcomes were collected.
Study quality assessment
Clinical trial quality was assessed using the Cochrane Collaboration’s tool for assessing the risk of bias in clinical trials, and a summary assessment for the risk of bias for each studied outcome was reported(10). Observational studies were assessed with the Newcastle Ottawa Scale, assigning up to nine points. Five or fewer points indicated poor quality(11)
Data analysis
A meta-analytic approach was used to analyze the data, using Review Manager (RevMan, Version 5.3, Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014). A random effects model calculated pooled effect sizes and corresponding 95% confidence intervals [CI] between deep and light sedation groups. Odds ratios were calculated for binary outcomes, continuous variables were reported as mean differences, and overall effect estimates were generated using a Z test. Heterogeneity between studies was assessed using the I2 statistic (12). Publication bias was assessed using a funnel plot of the size of the treatment effect against the precision of the trial. The kappa statistic was used to evaluate inter-rater agreement in study selection. The drugs, dosages, and study locations (i.e. ICU, emergency department) were reported qualitatively.
Deviations from previously published protocol
Studies of patients mechanically ventilated in the operating room (OR) and then admitted to the ICU were included; we continued to exclude studies focused on OR sedation practices and perioperative outcomes.
To account for study heterogeneity, the following four post hoc subgroup analyses were conducted, combining data: 1) from studies originally designed to study early sedation, 2) studies measured sedation depth with RASS, 3) prospective studies, and 4) retrospective studies. A subgroup analysis was not performed on the results from the randomized controlled trials due to the small number of patients enrolled (n=97).
RESULTS
Search and Selection
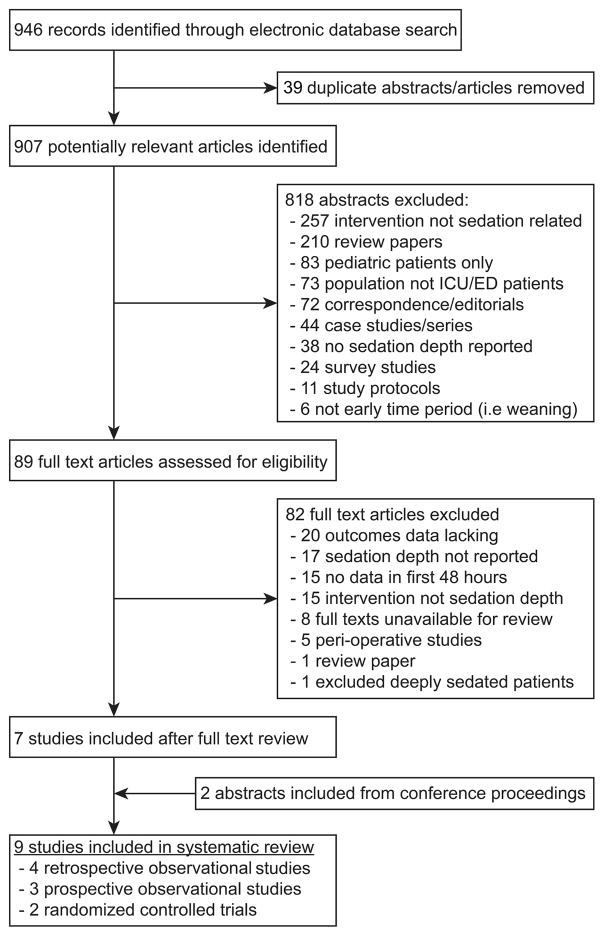
Comprehensive digital search resulted in 946 potentially relevant publications, of which 89 were selected for full-text review. Thirty-nine duplicate studies were eliminated. The kappa statistic following abstract review was 0.77 (95%CI: 0.70 – 0.84), indicating substantial agreement between reviewers. In final analysis, nine studies were included(2–4, 13–18). Figure 1 displays the details of study selection and exclusion at each stage of review.
Figure 1.
Study inclusion flow diagram
Study Characteristics
The nine included studies were published between 2012 and 2017. Two were randomized control trials and seven were observational. Seven were published in peer-reviewed journals and 2 presented as conference abstracts. The total number of patients across studies was 4521.
Table 1 displays each included study, its characteristics, and bias and quality assessments. Both RCTs were rated as low risk of bias in five out of seven domains by the Cochrane collaboration tool for assessing risk of bias. All nonrandomized studies rated as high quality on the nine-point Newcastle-Ottawa Scale. Bias assessment details for randomized and non-randomized studies can be found in Supplemental Digital Content 3 and 4 respectively.
Table 1.
Included study characteristics
| Randomized Studies | ||||||||
|---|---|---|---|---|---|---|---|---|
| Author, Year | N | Primary Outcome Assessed |
Secondary Outcomes Assessed |
Bias Assessment a |
Randomization allocations |
Deep sedation n (%) |
Definition of deep sedation |
Comments |
| Shehabi, 2013 | 60 | Number of RASS measurements between −2 and −3 in 48 hours | 1) Time to randomization 2) Dose and duration of rescue sedatives and opioids |
Low risk of bias in 5/7 domains | EGDS + Dexmedetomidine vs. EGDS + Other sedatives | 29 (48.3) | RASS ≤ −3 for first 48 hours | Abstract only Malaysian Cohort |
| Shehabi, 2013 | 37 | Number of RASS measurements between −2 and −3 in 48 hours | 1) Time to randomization 2) Dose and duration of rescue sedatives and opioids |
Low risk of bias in 5/7 domains | EGDS vs. Standard Sedation | 16 (43.2) | RASS ≤ −3 for first 48 hours | Australia/New Zealand Cohort |
| Non-Randomized Studies | ||||||||
| Author/Year | N | Primary Outcomes Assessed |
Secondary Outcomes Assessed |
Quality Assessmentb |
Study Design | Deep sedation n (%) |
Definition of deep sedation |
Comments |
| Shehabi, 2012 | 251 | Time to extubation | 1) Time to delirium 2) Time to hospital mortality 3) Mortality (180 day) |
High Quality | Prospective cohort | 171 (68.1) | RASS ≤ −3 for first 48 hours | |
| Van den Boogaard, 2012 | 1266 c | Duration of mechanical ventilation | 1) Reintubation 2) Incidence of unplanned tube or catheter removal 3) ILOS 4) Mortality (hospital) |
High Quality | Prospective cohort | 249 (19.7) | RASS −4 or −5 for first 24 hours | |
| Shehabi, 2013 | 254 | Time to extubation | 1) Mortality (180 day) 2) Delirium after 48 h 3) Mortality (hospital) 4) ILOS 5) HLOS |
High Quality | Prospective cohort | 209 (80.6) | RASS ≤ −3 for first 48 hours | |
| Samarin, 2014 | 66 | Duration of mechanical ventilation | 1) Delirium 2) ILOS 3) HLOS 4) Discharge facility |
High Quality | Retrospective cohort | 27 (40.8) | RASS ≤ −3 at ICU arrival | Abstract only |
| Tanaka, 2014 | 322 | Mortality (hospital) | 1) Tracheostomy 2) Hemodialysis 3) Ventilation duration 4) Vasopressor use 5) ARDS 6) Extubation failure 7) HLOS 8) ILOS 9) Mortality (ICU) |
High Quality | Retrospective cohort | 113 (35.1) | GCS<9 for first 48 hours | |
| Balzer, 2015 | 1884 | Mortality (ICU) | 1) Mortality (hospital) 2) Mortality (2 year) 3) ILOS 4) HLOS 5) Time to Extubation 6) Delirium 7) Hemodialysis in first 48 hours |
High Quality | Retrospective cohort | 513 (27.2) | 85% of RASS measurements during first 48 hours ≤ −3 | |
| Stephens, 2017 | 381 | Mortality (hospital) | 1) Duration mechanical ventilation 2) HLOS 3) ILOS |
High Quality | Retrospective cohort | 244 (64.0) | Median RASS ≤ −3 in ED or at presentation to ICU | |
Randomized Trials assessed for bias using Cochrane collaboration tool. Full assessment is available in Supplemental Digital Content 3.
Nonrandomized studies assessed for quality using Newcastle-Ottawa Scale. Full assessment is available in Supplemental Digital Content 4.
van den Boogaard, et al collected data on both mechanically ventilated and non-mechanically ventilated patients. Only the mechanically ventilated cohort was included in this systematic review and meta-analysis.
RASS=Richmond Agitation Sedation Scale; ILOS=ICU Length of Stay; HLOS = Hospital Length of Stay; ICU=Intensive Care Unit; EGDS = Early Goal Directed Sedation
The RASS was used to define deep sedation in 8 studies, with a RASS of ≤ −3 as the cutoff for deep sedation in 7 of those studies. The remaining study defined deep sedation as a GCS of < 9 (Table 1). All included studies used the Confusion Assessment Method for the Intensive Care Unit (CAM-ICU) to assess for delirium.
The incidence of early deep sedation was 34.7% and ranged from 19.6% to 80.6%. In reporting outcomes associated with deep sedation, two studies included data from the ED, while seven studies focused exclusively on early sedation in the ICU(14). Overall, descriptive statistics regarding analgesics and sedatives were variably reported. Six studies (n= 1305) had analgesic medication data available (2–4, 13, 16, 17). Six studies (n= 3129) had sedative medication data available (2–4, 13, 17, 18). Fentanyl was used in 841 (64.2%) patients and morphine in 349 (26.7%) patients. Propofol [(n= 2020 (64.6%)], midazolam [n= 1253 (40.0)], and dexmedetomidine [n= 101 (3.2%)] were the most commonly used sedative medications.
Meta-analysis
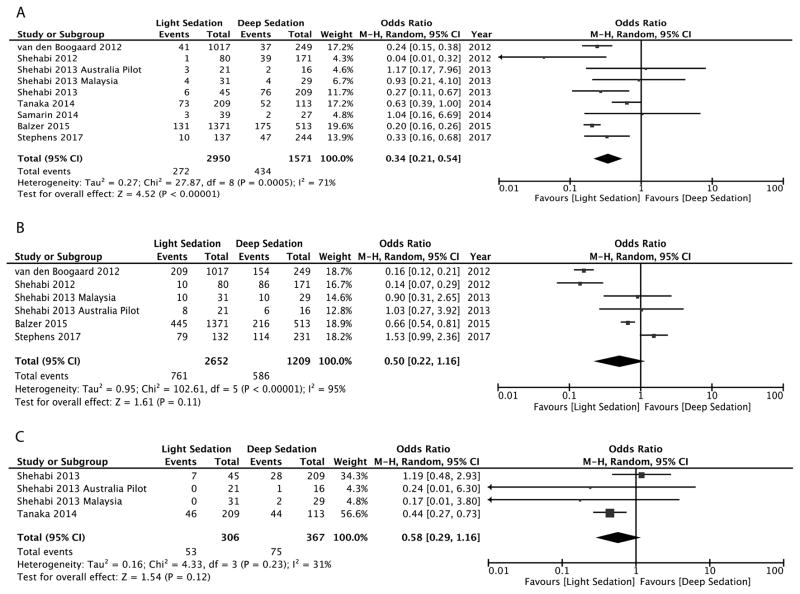
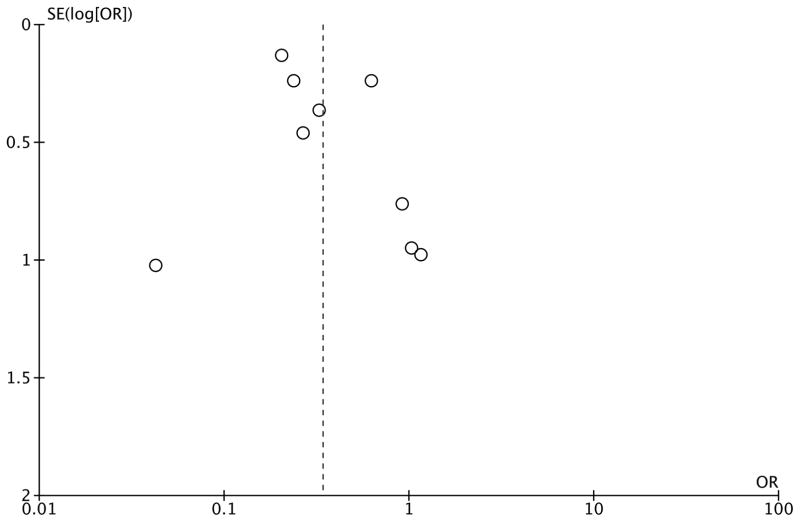
The aggregate meta-analysis for binary clinical outcomes is presented in Figure 2. All nine studies reported hospital mortality, including all patients (n=4521). Early light sedation was associated with a lower hospital mortality rate (9.2%) versus deep sedation (27.6%) [OR, 0.34 (0.21 – 0.54), P<0.001]. Delirium incidence was reported in six studies (n=3861). The incidence of delirium was 28.7% in the light sedation group and was 48.4% in the deep sedation group, which was not statistically significant, OR 0.50 (0.22 – 1.16), P=0.11. Tracheostomy incidence was reported in four studies, including 673 patients. There was a 17.3% incidence of tracheostomy in the light sedation group versus 20.4% in the deep sedation group, which was not statistically significant, OR 0.58 (0.29 – 1.16), P=0.12. Funnel-plot analysis for mortality (Figure 3) revealed a symmetrical distribution of odds ratios for mortality, indicating low publication bias risk.
Figure 2.
Figure 2A–C. Forest plots displaying the impact of early sedation depth on mortality (A), and incidence of delirium (B) and tracheostomy (C).
Figure 3.
Funnel plot for mortality outcome across studies.
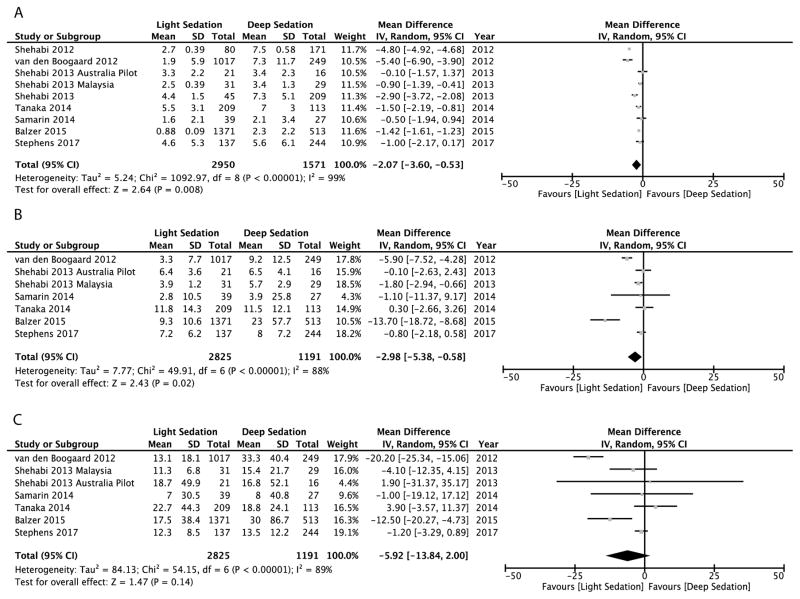
Results for the continuous outcomes are represented in Figure 4. All nine studies reported mechanical ventilation duration (n=4521). Seven studies (n=4016) reported ICU and hospital lengths of stay. Early light sedation was associated with significantly fewer mechanical ventilator days [mean difference −2.1 (95%CI −3.6 to −0.5), P=0.008] and shorter ICU lengths of stay [mean difference −3.0 days (95%CI −5.4 to −0.6), P=0.02]. Hospital length of stay was not significantly different in the light sedation cohort when compared to the deep sedation cohort [mean difference −5.9 (95%CI −13.8 to 2.0), P=0.14].
Figure 4.
Figure 4A–C. Forest plots displaying impact of early sedation depth on duration (days) of (A) mechanical ventilation, (B) intensive care unit length of stay, and (C) hospital length of stay.
Statistical heterogeneity, as described by the I2 test, was high across all outcomes (30.8 – 99.3%), and is displayed in Supplemental Digital Content 5, along with corresponding 95% confidence intervals.
Subgroup meta-analysis on studies originally designed to examine early sedation
Results of the subgroup analysis focused on the studies originally designed to examine early sedation were similar to the primary analysis (Supplemental Digital Content 6). Eight studies were originally designed to examine early sedation, including 3255 patients. Early light sedation was associated with lower hospital mortality rate (12.0%) versus deep sedation (30.0%), [OR, 0.37 (0.21 – 0.67), P <0.001]. Delirium incidence was reported in five of eight studies, including 2595 patients. The incidence of delirium was 33.8% in the light sedation group and 45.0% in the deep sedation group, which was not statistically significant, OR 0.66 (0.32 – 1.36), P=0.26. All eight of these studies (n=3255) reported mechanical ventilation duration and six of these studies (n=2750) reported ICU and hospital length of stay. Early light sedation was associated with significantly decreased mechanical ventilation duration [mean difference −1.7 (95%CI −3.3 to −0.1), P=0.04], but not ICU length of stay [mean difference −2.2 (95%CI −4.5 to 0.1), P=0.06] or hospital length of stay [mean difference −2.8 (95%CI −7.4 to 1.9), P=0.25].
Subgroup meta-analysis of studies using RASS to measure sedation depth
Eight studies (n=4199) used RASS to measure sedation depth. Results of the subgroup analysis of these studies were similar to the primary analysis (Supplemental Digital Content 7). Early light sedation was associated with lower hospital mortality rate (7.3%) versus deep sedation (26.2%), [OR, 0.28 (0.19 – 0.42), P<0.001], including all studies. Six of these studies (n=3861) reported delirium incidence. Delirium incidence was 28.6% in the light sedation group and 48.5% in the deep sedation group, which was not statistically significant, OR 0.50 (0.22 – 1.16), P=0.11. All eight of these studies (n=4199) reported mechanical ventilation duration and six of these studies (n=3694) reported ICU and hospital lengths of stay. Early light sedation was associated with significantly decreased ICU length of stay [mean difference −3.6 days (95%CI −6.2 to −0.9), P=0.008] and mechanical ventilation duration [mean difference −2.1 days (95%CI −3.8 to −0.5), P=0.01]. Hospital length of stay [mean difference −7.8 (95%CI −16.9 to 1.3), P=0.09] was not significantly different between light and deep sedation groups.
Subgroup meta-analysis of prospective studies
Five studies (n=1868) with a prospective design were analyzed. Results of this subgroup analysis were similar to the primary analysis (Supplemental Digital Content 8). Early light sedation was associated with lower hospital mortality rate (4.6%) versus deep sedation (23.4%), [OR, 0.31 (0.14 – 0.65), P=0.002]. Four studies (n=1614) reported delirium incidence, with significantly decreased incidence in the light sedation group (20.6%) compared to the deep sedation group, (55.1%), OR 0.32 (0.13 – 0.78), P=0.01. All five of these studies (n=1868) reported mechanical ventilation duration and three of these studies (n=1363) reported ICU length of stay and hospital length of stay. Early light sedation was associated with significantly decreased mechanical ventilation duration [mean difference −2.8 days (95%CI −5.0 to −0.7), P=0.01]. Neither ICU length of stay [mean difference −2.7 days (95%CI −5.9 to 0.5), P=0.10] nor hospital length of stay [mean difference −10.6 (95%CI −24.9 to 3.7), P=0.15] was significantly different between light and deep sedation groups.
Subgroup meta-analysis of retrospective studies
There were four retrospective studies, including 2653 patients. Results of the subgroup analysis of retrospective studies remained similar to the primary analysis (Supplemental Digital Content 9). All four studies reported hospital mortality rates. Early light sedation was associated with lower hospital mortality rate (12.4%) versus deep sedation (30.8%), [OR, 0.39 (0.18 – 0.81), P=0.01]. Only two studies (n=2247) reported delirium incidence. Delirium incidence was 34.9% in the light sedation group and 44.4% in the deep sedation group [OR, 0.98 (0.43 – 2.24), P=0.97]. Early light sedation was associated with significantly shorter mechanical ventilation duration [mean difference −1.4 (95%CI −1.6 to −1.2), P<0.001]. ICU length of stay [mean difference −3.7 (95%CI −8.9 to 1.5), P=0.16] and hospital length of stay [mean difference −2.7 (95%CI −9.1 to 3.7), P=0.40] were not significantly different between light and deep sedation groups.
DISCUSSION
The literature examining the impact of sedation in mechanically ventilated patients has expanded over the past decade. The majority of sedation RCTs either enrolled patients after 48 to 96 hours of mechanical ventilation, while observational studies have typically focused on sedation across an entire ICU stay(2). By comparison, little data exists on early sedation and its impact on outcome. This meta-analysis and systematic review was undertaken to characterize the literature on early sedation and assess the potential impact of early sedation depth on clinical outcomes. In this process, we found several important results.
The main finding was a significant relationship between early sedation depth and clinical outcomes. Early light sedation was associated with decreased hospital mortality, mechanical ventilation duration, and ICU length of stay compared to early deep sedation. There was also a mean difference of 5.9 days in hospital length of stay, though this was not statistically significant. Similarly, despite a statistically non-significant difference, delirium incidence was almost two-fold higher in deeply sedated patients. Early delivery of critical care interventions is especially impactful on outcome in many disease states, such as shock resuscitation, sepsis, mechanical ventilation, and acute lung injury(19–23). While there is a relative paucity of literature examining early sedation depth in mechanical ventilation, our results suggest that early sedation could be a modifiable treatment variable to improve outcome.
Second, although our systematic review demonstrates the importance of early sedation depth, it revealed a small literature base in this domain, finding only nine studies. This fact, combined with the limitations detailed below, indicates that the certainty of the evidence is very low. The two randomized studies, totaling 97 patients, were pilot studies for two RCTs being conducted in Malaysia, Australia, and New Zealand(3, 16). The remaining data is from observational studies, one of which did not originally focus on early sedation care (i.e. pertinent data for this meta-analysis directly obtained from principal investigators). The majority of prospective trials regarding sedation have enrolled patients several days into their ICU stay. Our results suggest that the impact of early sedation on outcome may have been missed in prior studies.
Finally, our results provide descriptive data on early sedation practice. Deep sedation appears to be common during the early time period of mechanical ventilation. Fentanyl, midazolam, and propofol were the most commonly used medications for analgesia and sedation during early mechanical ventilation. Dexmedetomidine use was rarely reported. Dexmedetomidine is being used with increasing frequency and its use is associated with reduced ICU sedation depth and improved outcomes (24, 25). Early dexmedetomidine use is the topic of an ongoing RCT, which should provide further data in this arena (26). Only two studies focused on patients in the ED, a location of frequent initiation of mechanical ventilation. This is another knowledge gap that our study highlights as a future direction for investigation.
This systematic review has several limitations. Due to a lack of RCTs, we included observational studies in our analyses. Including these studies carries an increased risk of bias, though we sought to control for bias by systematically assessing and transparently reporting study quality. It is important to note that high-quality observational studies do not provide the same strength of evidence as high quality RCTs, and the findings should not be viewed as equivalent. Additionally, sub-group analyses attempted to control for this bias, and provided similar results as the aggregate meta-analysis. Non-randomized trials introduce increased risk of confounding. It is possible that more severely ill patients received early deep sedation or that early deep sedation reflects severely depressed mental status. This was difficult to evaluate, as only two studies reported illness severity by depth of sedation and different measures of illness severity (i.e. APACHE, SOFA) were used between studies. Additionally, whether sicker patients who received deeper sedation have worse than predicted outcomes cannot be answered with these data. Likewise, delirium, one of the outcomes of this study, is often attributed to disease process, medications administered, or a combination of these effects. Though our results point toward increased delirium incidence in patients deeply sedated in the first 48 hours of mechanical ventilation, it is important to remember that this delirium may have been unrelated to sedation depth.
Statistical heterogeneity was high, reflecting few RCTs, inclusion of non-randomized studies, and use of differing definitions for deep sedation between studies. Given the clinical heterogeneity that exists among mechanically ventilated patients, statistical heterogeneity is not surprising and should not limit the ability to collate these data. GCS was used to assess sedation depth in one study, while RASS was used in the remaining studies, adding further heterogeneity. This heterogeneity is also an important result of this study, as it indicates inconsistent methods used to study the clinical implications of early sedation practices. It demonstrates that randomized studies with consistent measures of sedation depth are needed to better evaluate this relationship.
Furthermore, while the inclusion of observational studies reflects associations and not causation, it reflects real-world clinical practice and enhances external validity. It is possible that studies investigating early sedation were missed in the literature search. Our search strategy was exhaustive, included detailed electronic search developed in consultation with a trained medical librarian, and an extensive review of references and conference proceedings. The results represent a diverse patient population from an international domain. Though this large, diverse cohort increases statistical power and clinical generalizability of our results, it creates potential for confounding due to local practice variation in regard to different sedative strategies, including sedation holidays. These variable practices may be reflected in the wide range of deep sedation incidence between studies. Regardless, this systematic review has uncovered the largest amount of published data on the topic thus far.
CONCLUSIONS
This systemic review aimed to characterize and quantify the impact of early sedation depth on outcome. Deep sedation in mechanically ventilated patients, as evaluated in a small number of qualifying heterogeneous RCTs and observational studies, was associated with increased mortality and lengths of stay. Interventions targeting early sedation depth assessment, starting in the ED and subsequent ICU admission, deserve further investigation and could improve outcomes.
Supplementary Material
Acknowledgments
For providing data:
We would like to thank the authors of several of the manuscripts that were included and excluded in this review. Their time and generosity in responding to our inquiries is very much appreciated.
SOURCES OF FUNDING: RJS received funding from Washington University Institute of Clinical and Translational Sciences grant UL1TR000448, sub-award TL1TR000449, from the National Center for Advancing Translational Sciences (NCATS) of the National Institutes of Health (NIH). BWR was supported by a grant from the National Institutes of Health/National Heart, Lung, and Blood Institute (K23HL126979). EA was supported by the Washington University School of Medicine Faculty Scholars grant and the Foundation for Barnes-Jewish Hospital. BMF was funded by the KL2 Career Development Award, and this research was supported by the Washington University Institute of Clinical and Translational Sciences (Grants UL1 TR000448 and KL2 TR000450) from the National Center for Advancing Translational Sciences (NCATS). BMF was also funded by the Foundation for Barnes-Jewish Hospital Clinical and Translational Sciences Research Program (Grant # 8041-88). MHK is supported by the Foundation for Barnes-Jewish Hospital.
Footnotes
CONFLICTS OF INTEREST: The Authors have no financial conflicts of interest to disclose.
REGISTRATION: This systematic review has been registered with the PROSPERO international prospective register of systematic reviews (#CRD42017057264)
Copyright form disclosure: Mr. Stephens and Dr. Fuller received support for article research from the National Institute of Health (NIH). Dr. Ablordeppey’s institution received funding from Washington University School of Medicine and the BJH Foundation. Dr. Fuller’s indisclosed that research reported in this publication was supported by the Washington University Institute of Clinical and Translational Sciences grant UL1TR000448, sub-award KL2TR000450, from the National Center for Advancing Translational Sciences (NCATS) of the NIH. The content is solely the responsibility of the authors and does not necessarily represent the official view of the NIH. The remaining authors have disclosed that they do not have any potential conflicts of interest.
Contributor Information
Robert J. Stephens, Washington University School of Medicine in St. Louis, 660 S Euclid Avenue, St. Louis, MO 63110.
Matthew R. Dettmer, Emergency Services Institute, Respiratory Institute, Cleveland Clinic Foundation, 9500 Euclid Avenue E19, Cleveland, OH 44106.
Brian W. Roberts, Department of Emergency Medicine, Cooper University Hospital, One Cooper Plaza, K152, Camden, NJ 08103.
Enyo Ablordeppey, Departments of Emergency Medicine and Anesthesiology, Division of Critical Care, Washington University School of Medicine in St. Louis, St. Louis, MO 63110.
Susan A. Fowler, Bernard Becker Medical Library, Washington University in St. Louis, 660 S Euclid Avenue, St. Louis, MO 63110.
Marin H. Kollef, Department of Medicine, Division of Pulmonary and Critical Care Medicine, Washington University School of Medicine in St. Louis.
Brian M. Fuller, Departments of Emergency Medicine and Anesthesiology, Division of Critical Care Medicine, Washington University School of Medicine in St. Louis, 660 S Euclid Avenue, St. Louis, MO 63110.
References
- 1.Barr J, Fraser GL, Puntillo K, et al. Clinical practice guidelines for the management of pain, agitation, and delirium in adult patients in the intensive care unit. Crit Care Med. 2013;41(1):263–306. doi: 10.1097/CCM.0b013e3182783b72. [DOI] [PubMed] [Google Scholar]
- 2.Shehabi Y, Chan L, Kadiman S, et al. Sedation depth and long-term mortality in mechanically ventilated critically ill adults: a prospective longitudinal multicentre cohort study. Intensive Care Med. 2013;39(5):910–918. doi: 10.1007/s00134-013-2830-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shehabi Y, Bellomo R, Reade MC, et al. Early goal-directed sedation versus standard sedation in mechanically ventilated critically ill patients: a pilot study*. Crit Care Med. 2013;41(8):1983–1991. doi: 10.1097/CCM.0b013e31828a437d. [DOI] [PubMed] [Google Scholar]
- 4.Shehabi Y, Bellomo R, Reade MC, et al. Early intensive care sedation predicts long-term mortality in ventilated critically ill patients. Am J Respir Crit Care Med. 2012;186:724–731. doi: 10.1164/rccm.201203-0522OC. [DOI] [PubMed] [Google Scholar]
- 5.Foy R. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. Ann Intern Med. 2010;151:264–269. doi: 10.7326/0003-4819-151-4-200908180-00135. [DOI] [PubMed] [Google Scholar]
- 6.Moher D, Shamseer L, Clarke M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. 2015;4:1. doi: 10.1186/2046-4053-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shamseer L, Moher D, Clarke M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation. BMJ. 2015;349:g7647. doi: 10.1136/bmj.g7647. [DOI] [PubMed] [Google Scholar]
- 8.Stephens RJ, Dettmer MR, Roberts BW, et al. Practice patterns and outcomes associated with early sedation depth in mechanically ventilated patients: a systematic review protocol. BMJ Open. 2017;7(6):e016437. doi: 10.1136/bmjopen-2017-016437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stroup D, Berlin J, Morton S, et al. Meta-analysis of Observational Studies in Epidemiology: A proposal for reporting. JAMA. 2000;283:2010–2012. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 10.Higgins J, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions. The Cochrane Collaboration; 2011. www.handbook.cochrane.org. [Google Scholar]
- 11.Wells G, Shea B, O’Connell D, et al. The Newcaste-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. 2008 [cited 2017 02/02]Available from: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp.
- 12.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21(11):1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 13.Stephens RJ, Ablordeppey E, Drewry AM, et al. Analgosedation practices and the impact of sedation depth on clinical outcomes among patients requiring mechanical ventilation in the emergency department: a cohort study. Chest. 2017 doi: 10.1016/j.chest.2017.05.041. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Samarin MSJ, Olsen K, Peitz G. The impact of emergency room sedation on ICU outcomes. Critical Care Medicine. 2014;42(12):A1493. [Google Scholar]
- 15.van den Boogaard M, Schoonhoven L, van der Hoeven JG, et al. Incidence and short-term consequences of delirium in critically ill patients: A prospective observational cohort study. International Journal of Nursing Studies. 2012;49(7):775–783. doi: 10.1016/j.ijnurstu.2011.11.016. [DOI] [PubMed] [Google Scholar]
- 16.Shehabi YCL, Ismail WN, Saman MA, Yong CY, Kadiman SB, Alias A, Howe B. Early goal directed sedation with dexmedetomidine vs standard sedatives, randomized control trial. Critical Care Medicine. 2013;41(12):A217. [Google Scholar]
- 17.Tanaka LM, Azevedo LC, Park M, et al. Early sedation and clinical outcomes of mechanically ventilated patients: a prospective multicenter cohort study. Crit Care. 2014;18(4):R156. doi: 10.1186/cc13995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Balzer FWB, Kumpf O, Treskatsch S, Spies C, Wernecke KD, Krannich A, Kastrup M. Early deep sedation is associated with decreased in-hospital and two-year follow-up survival. Critical Care. 2015;19(1) doi: 10.1186/s13054-015-0929-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jansen TC, van Bommel J, Schoonderbeek FJ, et al. Early lactate-guided therapy in intensive care unit patients: a multicenter, open-label, randomized controlled trial. Am J Respir Crit Care Med. 2010;182(6):752–761. doi: 10.1164/rccm.200912-1918OC. [DOI] [PubMed] [Google Scholar]
- 20.Jones AE, Shapiro NI, Trzeciak S, et al. Lactate clearance vs central venous oxygen saturation as goals of early sepsis therapy: a randomized clinical trial. JAMA. 2010;303(8):739–746. doi: 10.1001/jama.2010.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fuller BM, Ferguson IT, Mohr NM, et al. Lung-Protective Ventilation Initiated in the Emergency Department (LOV-ED): A Quasi-Experimental, Before-After Trial. Ann Emerg Med. 2017;70(3):406–418. e404. doi: 10.1016/j.annemergmed.2017.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rivers E, Nguyen B, Havstad S, et al. Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med. 2001;345(19):1368–1377. doi: 10.1056/NEJMoa010307. [DOI] [PubMed] [Google Scholar]
- 23.Needham DM, Yang T, Dinglas VD, et al. Timing of low tidal volume ventilation and intensive care unit mortality in acute respiratory distress syndrome. A prospective cohort study. Am J Respir Crit Care Med. 2015;191(2):177–185. doi: 10.1164/rccm.201409-1598OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jiang YKWS, Lam TS, Hanna A, DeMuro JP, Calixte R, Brathwaite CE. Prevalence of Delirium and Coma In Mechanically Ventilated Patients Sedated With Dexmedetomidine or Propofol. Pharmacology & Therapeutics. 2016;41(7):442–445. [PMC free article] [PubMed] [Google Scholar]
- 25.Riker RRSY, Bokesch PM, Ceraso D, Wisemandle W, Koura F, Whitten P, Margolis BD, Byrne DW, Wesley Ely E, Rocha MG. Dexmedetomidine vs midazolam for sedation of critically Ill patients A randomized trial. JAMA. 2009;301(5):489–499. doi: 10.1001/jama.2009.56. [DOI] [PubMed] [Google Scholar]
- 26.ClinicalTrials.gov[Internet] Early Goal-Directed Sedation Compared With Standard Care in Mechanically Ventilated Critically Ill Patients (SPICE III) 2012 [cited 2017 April 26] Identifier NCT01728558]. Available from: https://clinicaltrials.gov/ct2/show/record/NCT01728558.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.