Abstract
On average, patients with psychosis perform worse than controls on visual change-detection tasks, implying that psychosis is associated with reduced capacity of visual working memory (WM). In the present study, 79 patients diagnosed with various psychotic disorders and 166 controls, all African Americans, completed a change-detection task and several other neurocognitive measures. The aims of the study were to (1) determine whether we could observe a between-group difference in performance on the change-detection task in this sample; (2) establish whether such a difference could be specifically attributed to reduced WM capacity (k); and (3) estimate k in the context of the general cognitive deficit in psychosis. Consistent with previous studies, patients performed worse than controls on the change-detection task, on average. Bayesian hierarchical cognitive modeling of the data suggested that this between-group difference was driven by reduced k in patients, rather than differences in other psychologically meaningful model parameters (guessing behavior and lapse rate). Using the same modeling framework, we estimated the effect of psychosis on k while controlling for general intellectual ability (g, obtained from the other neurocognitive measures). The results suggested that reduced k in patients was stronger than predicted by the between-group difference in g. Moreover, a mediation analysis suggested that the relationship between psychosis and g (i.e., the general cognitive deficit) was mediated by k. The results were consistent with the idea that reduced k is a specific deficit in psychosis, which contributes to the general cognitive deficit.
Keywords: Psychosis, change detection, working memory, working memory capacity, Bayesian estimation, IQ
1. Introduction
Extensive prior research has investigated working-memory (WM) dysfunction in psychosis (for reviews, see Forbes et al. 2009; Lee and Park 2005; Piskulic et al. 2007). According to most definitions, WM encapsulates the storage and manipulation of temporary information (e.g., Miyake and Shah 1999). Numerous studies have reported differences in performance between patients and controls on simple visual change-detection tasks, or modifications thereof (e.g., Choi et al. 2012; Erickson et al. 2015; Glahn et al. 2003; Gold et al. 1997; Gold et al. 2010; Haenschel et al. 2007; Johnson et al. 2013; Leonard et al. 2013; Mayer et al. 2012). It is widely believed that performance on such tasks is limited by WM capacity (Cowan 2010; Luck and Vogel 2013). Therefore, reduced WM capacity may be a specific deficit in psychosis.
In the present study, patients with psychosis and controls completed a brief visual-change detection task, along with several other neurocognitive tests. The subject sample was unusual compared to those from previous studies (e.g., Johnson et al. 2013): all subjects were African Americans; the patient group comprised individuals with various diagnoses involving psychosis; and neither patients nor controls were excluded for having non-psychotic psychiatric disorders.
The first aim of the study was to determine whether there would be a between-group difference in performance on the change-detection task, given our unusual sample characteristics. African Americans are underserved by psychiatric research, and there is a particular need to redress this balance for psychotic disorders, which are more common in this community than others (Schwartz and Blankenship 2014). Based on the foregoing literature, we expected patients to perform worse than controls, although we could not find any previous studies addressing this question in African Americans specifically. Moreover, we expected WM dysfunction to be a feature of psychosis per se, rather than of a specific diagnostic category (e.g., schizophrenia). We anticipated, however, that because we chose to include patients with various diagnoses, the between-group difference might be smaller in this study than in previous one. Another reason why the between-group difference might be small is that our control group included people with non-psychiatric disorders. Comorbidities are common in psychotic disorders (Addington et al. 2017). If comorbid disorders influence WM (e.g., Potvin et al. 2014; Rock et al. 2014; Stavro et al. 2013), these effects might have been conflated with the effect of psychosis on WM per se in previous studies, which typically excluded controls with any psychiatric disorder (e.g., Johnson et al. 2013).
Our second aim was to characterize performance using cognitive models. Psychophysics has a long history of applying such models to change-detection tasks. A popular category of model assumes that WM capacity is “slots-based,” and allows researchers to directly estimate the number of slots, here denoted by k (e.g., Rouder et al. 2008). Typically, previous studies collected many trials per subject in order to yield highly accurate estimates of k. However, recent theoretical work has shown that it is possible to obtain reasonable estimates of k from relatively few trials, via Bayesian hierarchical inference (Morey 2011). Here, we used this framework to estimate k, as well as the effects of covariates on k (e.g., psychosis), with greater accuracy than via traditional approaches based on maximum-likelihood estimation. The framework also allowed us to estimate other psychologically meaningful parameters, and the effects of covariates on those parameters. Based on previous research, we expected psychosis to influence k, but we did not know whether psychosis would influence the other parameters.
Patients with psychosis tend to perform worse than controls on many tasks, including composite measures of general intellectual ability, suggesting that they experience a general cognitive deficit (e.g., Dickinson et al. 2008). Our third aim was to estimate k within the context of this general deficit. Previous work has shown that k correlates at least moderately with many other measures, in patients with psychosis and healthy individuals (Johnson et al. 2013; Fukuda et al. 2010). Based on these findings, it could be argued that k constrains higher-order cognition, and also that reduced k contributes to the general cognitive deficit in psychosis. Indeed, many researchers have assumed this to be true, treating WM as the key to understanding cognitive dysfunction in psychosis (e.g., Goldman-Rakic 1994). The present study aimed to provide support for this idea, by (a) estimating the magnitude of the between-group difference in k while controlling for general intellectual ability; and (b) performing a mediation analysis (MacKinnon 2008). If reduced k contributes to the general deficit, there should be a between-group difference in k after controlling for differences in general ability, and k should mediate the relationship between psychosis and general ability. On the other hand, if the difference in k merely reflects the general deficit, it should be no stronger than predicted by differences in general ability, and there should be no mediation.
2. Materials and methods
2.1. Subjects
Subjects provided informed consent, and the review boards at Hartford Hospital and Yale University approved the study. Data were available from 79 patients with psychosis and 166 unaffected individuals. All subjects were African Americans from the Hartford area. Patients had various diagnoses including substantial psychotic features, namely schizophrenia (N = 39), schizoaffective disorder (N = 21), psychotic bipolar disorder (N = 7), psychotic major depression (N = 4), and psychosis not otherwise specified (N = 8). Neither patients nor controls were excluded for having non-psychotic psychiatric disorders. DSM-IV diagnoses were made using structured clinical interviews (First et al. 2002) and a consensus process. Subjects were excluded for a history of major non-psychiatric medical disorders or FSIQ < 70. Table 1 provides additional information.
Table 1.
Subject information.
| Total | Patients | Controls | Test statistica | p | DOF | Effect sizeb | |
|---|---|---|---|---|---|---|---|
| Demographics | |||||||
| N | 245 | 79 | 166 | - | - | - | - |
| Age (SD) | 39.5 (13.8) | 40.3 (13.1) | 39.2 (14.1) | −0.645 | 0.52 | 164.0 | −0.0855 |
| Female (%) | 128 (52.2) | 41 (51.9) | 87 (52.4) | 0.98 | 1.0 | 1 | - |
| Right handedc (%) | 219 (89.8) | 72 (91.1) | 147 (89.1) | 1.26 | 0.822 | 1 | - |
| High school diploma or GED (%) | 214 (87.3) | 66 (83.5) | 148 (89.2) | 0.617 | 0.223 | 1 | - |
| Bachelors or higher degree (%) | 45 (18.4) | 8 (10.1) | 37 (22.3) | 0.393 | 0.0222d | 1 | - |
| Non-psychotic disorders | |||||||
| Anxiety disorders (%) | 25 (10.2) | 12 (15.2) | 13 (7.83) | 2.11 | 0.112 | 1 | - |
| Attention-deficit hyperactivity disorder (%) | 1 (0.408) | 0 (0) | 1 (0.602) | 0.0 | 1.0 | 1 | - |
| Major depressive disorder (%) | 13 (5.31) | 3 (3.8) | 10 (6.02) | 0.616 | 0.557 | 1 | - |
| Alcohole (%) | 72 (29.4) | 31 (39.2) | 41 (24.7) | 1.97 | 0.0244d | 1 | - |
| Cocainee (%) | 32 (13.1) | 12 (15.2) | 20 (12.0) | 1.31 | 0.544 | 1 | - |
| Cannabise (%) | 69 (28.2) | 31 (39.2) | 38 (22.9) | 2.18 | 0.00981d | 1 | - |
| Amphetaminee (%) | 2 (0.816) | 2 (2.53) | 0 (0) | ∞ | 0.103 | 1 | - |
| Opioide (%) | 11 (4.49) | 4 (5.06) | 7 (4.22) | 1.21 | 0.75 | 1 | - |
| Other/unknown substancee (%) | 11 (4.49) | 7 (8.86) | 4 (2.41) | 3.94 | 0.0415d | 1 | - |
| Medication | |||||||
| Antipsychotics (typical or atypical) (%) | 53 (21.6) | 52 (65.8) | 1 (0.602) | 318.0 | < 0.001d | 1 | - |
| Cognitive measures | |||||||
| Change detection (SD) | 32.1 (5.24) | 30.0 (5.2) | 33.2 (4.95) | 4.5 | < 0.001d | 147.0 | 0.624 |
| CVLT-II trials 1–4 (SD) | 43.7 (11.0) | 39.0 (10.5) | 45.9 (10.6) | 4.8 | < 0.001d | 155.0 | 0.651 |
| CVLT-II trial 5 (SD) | 9.35 (3.29) | 8.0 (3.3) | 9.99 (3.09) | 4.51 | < 0.001d | 145.0 | 0.629 |
| Forced-choice digit-symbol (SD) | 37.2 (10.6) | 32.3 (9.26) | 39.5 (10.5) | 5.46 | < 0.001d | 172.0 | 0.711 |
| WASI matrix reasoning (SD) | 18.6 (7.23) | 17.7 (6.94) | 19.1 (7.34) | 1.38 | 0.168 | 162.0 | 0.185 |
| WASI vocabulary (SD) | 49.6 (9.39) | 47.8 (9.19) | 50.5 (9.39) | 2.08 | 0.039d | 157.0 | 0.281 |
| COWAT fas (SD) | 40.3 (12.9) | 37.6 (11.3) | 41.5 (13.5) | 2.34 | 0.0203d | 180.0 | 0.3 |
| COWAT animal (SD) | 20.3 (5.51) | 19.4 (5.73) | 20.7 (5.37) | 1.72 | 0.0879 | 145.0 | 0.24 |
| Sequencing span (SD) | 4.21 (1.25) | 3.73 (1.19) | 4.43 (1.22) | 4.24 | < 0.001d | 152.0 | 0.578 |
| WTAR (SD) | 27.4 (11.1) | 25.3 (10.4) | 28.4 (11.3) | 2.15 | 0.0329d | 163.0 | 0.286 |
| FSIQ (SD) | 90.9 (12.8) | 88.6 (12.3) | 92.0 (12.9) | 1.97 | 0.0503 | 161.0 | 0.264 |
| g (SD) | 0.0473 (1.01) | −0.375 (0.92) | 0.249 (0.986) | 4.85 | < 0.001d | 163.0 | 0.644 |
DOF, degrees of freedom
SD, standard deviation
GED, general educational development
ADHD, Attention-deficit hyperactivity disorder
CVLT-II, California verbal learning test, version II
WASI, Wechsler Abbreviated Scale of Intelligence
COWAT, Conditional oral word association test
WTAR, Wechsler test of adult reading
FSIQ, Full-scale IQ
Welch’s t-test for continous variables, Fisher’s exact test for discrete variables
Hedges’ g * (continuous variables only)
Handedness information missing for one subject
Nominally significant at the 0.05 level
Abuse or dependency
2.2. Change-detection task
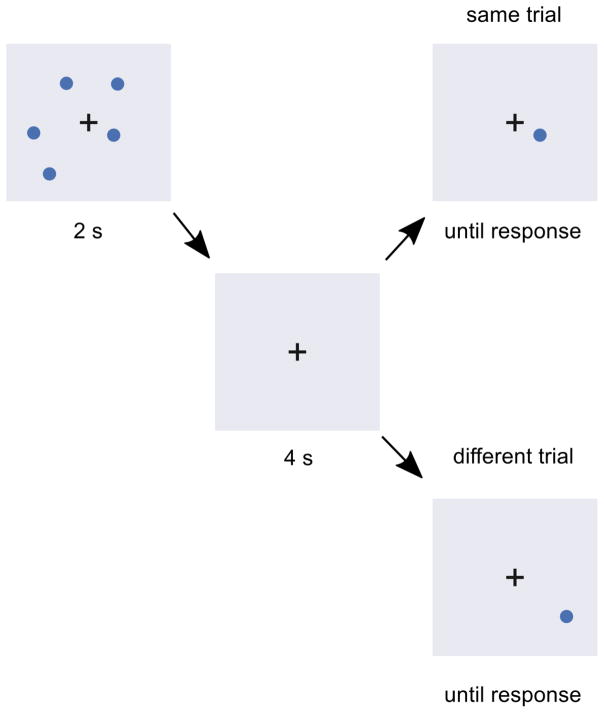
On each trial, subjects saw a sample array containing three, four, or five circles positioned randomly on the computer screen, for 2 s (Fig. 1). After a 4-s delay, during which only a fixation cross was shown, subjects saw a single circle, and indicated whether its position was the same as or different to one of the circles from the sample array. Feedback about response accuracy was given after responses on the first three (practice) trials, which were discarded from the analysis. No feedback was provided on the remaining trials. Responses were made using the left- and right-arrow keys on the keyboard, and response times were unlimited. After the practice trials, there were seven same trials and seven different trials per sample-array size, yielding 42 trials per subject.
Figure 1.
Timeline of an example trial in the visual change-detection task.
2.3. Other cognitive measures
Subjects completed a test battery (“Charlie”; https://github.com/sammosummo/Charlie) containing several other measures. We selected nine measures—which have all been used extensively in prior research, and whose psychometric properties and cognitive demands are well understood (see Table 1)—to derive a composite measure of general intellectual ability, g, via principal component analysis (see the Supplementary Text for details).
2.4. Bayesian hierarchical cognitive models
2.4.1. Motivation
Previous studies have often used simple formulae to calculate k from change-detection tasks (Cowan 2001; Pashler 1988). However, as discussed elsewhere (Rouder et al. 2011; Morey 2011), this approach has many limitations that can result in poor estimates of k, especially when there are relatively few trials per subject. Therefore, following Morey (Morey 2011), we analyzed the data using Bayesian hierarchical models. Here, “Bayesian” refers to the method of inference that uses Bayes’ theorem to update beliefs about parameter values given the data (Gelman et al. 2013; Kruschke 2014), and “hierarchical” models are those with multiple levels of free parameters, such as a subject level and a group level (Gelman 2006). This approach provides better estimates of k than traditional methods—in fact, when there are few trials but many subjects, as was the case here, the parameter-recovery improvements are quite impressive. For proof, interested readers are encouraged to consult Morey (2011).
Besides providing better estimates of k, the Bayesian approach held other advantages. The models provided estimates of other psychologically meaningful parameters (see next section). More generally, Bayesian statistics have the potential to resolve many of the deep-rooted problems of traditional null-hypothesis testing, and as a result are rapidly becoming mainstream in the psychological sciences. For contemporary discussions, see Kruschke and Liddell (2017), and Rouder et al. (2016).
2.4.2. Design
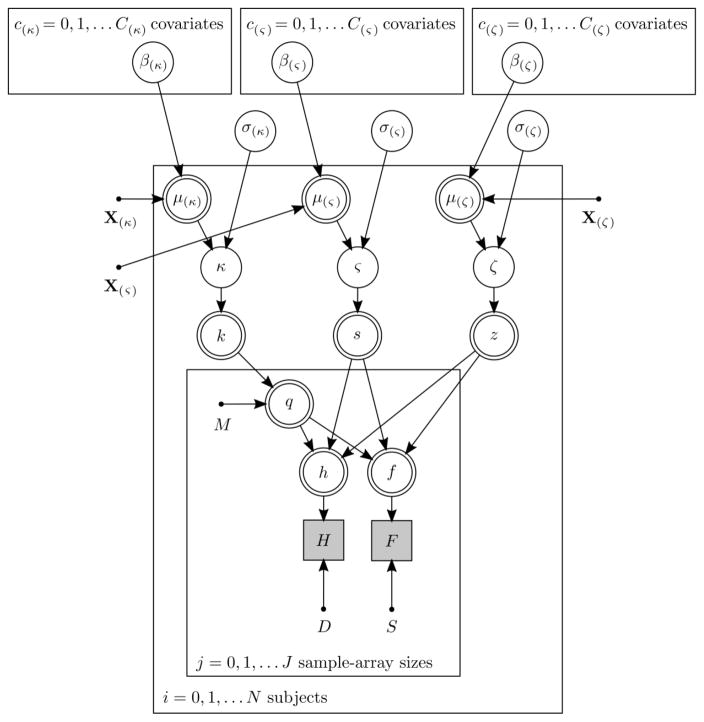
We assumed that responses in the change-detection task were made according to the decision process proposed by Rouder et al. (2008), which contains three variables: k, the number of slots; s, the probability of guessing “different” when the probed item was not remembered on a given trial; and z, one minus the probability that the subject suffered a “lapse in attention” on a given trial. This decision process formed the basis of several Bayesian models, which predicted the probabilities of hits (correct responses on different trials) and false alarms (incorrect response on same trials). The decision-process variables k, s, and z were transformations of subject-level free parameters, denoted by κ, ς, and ζ, respectively. The means of the prior distributions on these parameters (μ(κ), μ(ς), and μ(ζ)) differed between subjects according to linear functions whose coefficients (e.g., β(μ(κ))0) were group-level free parameters. The standard deviations of the prior distributions (e.g., σ(κ)) were also group-level free parameters. Several such models were fitted to the data, which differed from each other only in terms of the covariates and coefficients included in the linear functions. Figure 2 specifies this framework graphically, and the Supplementary Text provides the equations and further details about model fitting and checking.
Figure 2.
Directed acyclic graph of the structure of all the Bayesian hierarchical cognitive models. Panels represent iterations, unfilled single-lined circles represent continuous stochastic random variables, unfilled double-lined circles represent deterministic variables, shaded squares represent discrete observed random variables, and dots represent constants. Variables and constants not defined elsewhere are D, the number of different trials; S, the number of same trials; H, the number of hits; F, the number of false alarms; M, the sample-array size; Xκ), X(ς), and X(ζ), design matrices for μ(κ), μ(ς), and μ(ζ), respectively.
2.5. Mediation analysis
We performed a mediation analysis to determine whether the relationship between psychosis and g was mediated by k. The independent variable was psychosis, coded so that 0 indicated a control and 1 indicated a patient. The dependent variable was g. The mediator was the mean of the subject-specific posterior distributions on κ under a cognitive model with intercepts as the only covariates on μ(κ), μ(ς), and μ(ζ). The paths in the mediation model were estimated via Bayesian inference (Yuan and MacKinnon 2009) using “default” priors (Nuijten et al. 2015), which allowed the analytic computation of Bayes factors (BFs; see the Supplementary Text for details).
3. Results
3.1. Aim 1: Between-group differences in change detection
Table 1 and Figure 3 show subjects’ performance on the change-detection task. As expected, patients made fewer correct responses than controls, on average. However, the effect size (Hedge’s g* = 0.624) was somewhat smaller than observed in previous studies, which often reported effect sizes exceeding 1 (e.g., Johnson et al. 2013).
Figure 3.
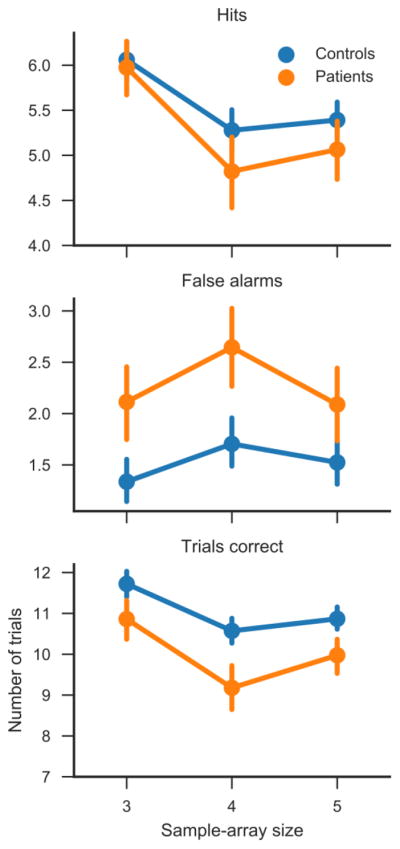
Hits, false alarms, and overall accuracy on the change-detection task. Ordinates are group means and error bars are 95% bootstrap confidence intervals.
We speculated that the relatively modest between-group difference might be related to the fact that the patient sample included people with various psychotic disorders. We therefore tested whether the two largest diagnostic subgroups, schizophrenia and schizoaffective disorder, differed in performance using a Welch’s t test, which was not significant [t(52.0) = −0.747; p = 0.458]. We did not perform similar tests on the other subgroups due to their small sizes. The difference between patients taking and not taking antipsychotic medication was also not significant [t(47.9) = 0.997; p = 0.324]. We further speculated that non-psychotic psychiatric disorders might have played a role. However, Welch’s t tests indicated a moderate, nominally significant difference between controls with and without a diagnosis of alcohol abuse or dependency [t(61.5) = 2.02; p = 0.048; Hedge’s g* = 0.386], but not differences between controls with and without any other disorders (p >= 0.574).
3.2. Aim 2: Effects of psychosis on k, s, and z
Our first cognitive model (Model 1) included intercepts and psychosis (coded so that 0 indicated a control and 1 indicated a patient) as covariates on μ(κ), μ(ς), and μ(ζ). Table 2 summarizes the posterior distributions under this and other models. Based on inspection of the posterior means 95% highest-density regions reported in the table, it appears that psychosis had a credibly non-zero effect on k, but not on either s or z. To more concretely test the idea that psychosis influenced k, but not s or z, we fitted a simplified model (Model 2), which included an intercept and psychosis as covariates μ(κ), but only intercepts on μ(ς), and μ(ζ). The deviance information criterion (DIC; Spiegelhalter et al. 2002) of this model was lower than that of the first model, suggesting that it was more parsimonious. We also fitted a third model (Model 3), which included only intercepts on all three means: this model had a higher DIC than Model 2, suggesting that the difference in DIC between Models 1 and 2 was not simply due to reduced model complexity.
Table 2.
Summary of the posterior distributions on the group-level free parameters under the various cognitive models.
| Description of parameter | Mean of posterior (95% HDR) | |
|---|---|---|
| Model 1 (DIC = 6457) | ||
| β(κ)c(κ)=0 | Intercept on μ(κ) | 3.06 (2.65, 3.51) |
| β(κ)c(κ)=1 | Psychosis on μ(κ) | −0.833 (−1.46, −0.193) |
| β(ς)c(ς)=0 | Intercept on μ(ς) | 0.0611 (−0.117, 0.24) |
| β(ς)c(ς)=1 | Psychosis on μ(ς) | 0.273 (−0.0264, 0.574) |
| β(ζ)c(ζ)=0 | Intercept on μ(ζ) | 1.46 (1.14, 1.78) |
| β(ζ)c(ζ)=1 | Psychosis on μ(ζ) | −0.184 (−0.903, 0.583) |
| σ(κ) | SD on κ | 1.39 (1.09, 1.71) |
| σ(ς) | SD on ς | 0.919 (0.78, 1.06) |
| σ(ζ) | SD on ζ | 0.367 (0.018, 0.705) |
| Model 2 (DIC = 6401) | ||
| β(κ)c(κ)=0 | Intercept on μ(κ) | 3.06 (2.67, 3.5) |
| β(κ)c(κ)=1 | Psychosis on μ(κ) | −0.971 (−1.44, −0.513) |
| β(ς)c(ς)=0 | Intercept on μ(ς) | 0.157 (0.0139, 0.302) |
| β(ζ)c(ζ)=0 | Intercept on μ(ζ) | 1.44 (1.13, 1.74) |
| σ(κ) | SD on κ | 1.37 (1.1, 1.66) |
| σ(ς) | SD on ς | 0.924 (0.785, 1.07) |
| σ(ζ) | SD on ζ | 0342 (0.00901, 0.661) |
| Model 3 (DIC = 6441) | ||
| β(κ)c(κ)=0 | Intercept on μ(κ) | 2.78 (2.41, 3.18) |
| β(ς)c(ς)=0 | Intercept on μ(ς) | 0.158 (0.0142, 0.302) |
| β(ζ)c(ζ)=0 | Intercept on μ(ζ) | 1.41 (1.08, 1.74) |
| σ(κ) | SD on κ | 1.45 (1.16, 1.77) |
| σ(ς) | SD on ς | 0.923 (0.783, 1.06) |
| σ(ζ) | SD on ζ | 0.364 (0.0081, 0.7) |
| Model 4 (DIC = 6350) | ||
| β(κ)c(κ)=0 | Intercept on μ(κ) | 2.88 (2.39, 3.4) |
| β(κ)c(κ)=1 | Psychosis on μ(κ) | −0.518 (−0.972, −0.0798) |
| β(κ)c(κ)=2 | Age on μ(κ) | 0.00447 (−0.15, 0.155) |
| β(κ)c(κ)=3 | Sex on μ(κ) | −0.28 (−0.674, 0.124) |
| β(κ)c(κ)=4 | g on μ(κ) | 0.711 (0.418, 1.02) |
| β(κ)c(κ)=5 | Psychosis-by-interaction g on μ(κ) | −0.00489 (−0.469, 0.469) |
| β(ς)c(ς)=0 | Intercept on μ(ς) | 0.158 (0.0155, 0.304) |
| β(ζ)c(ζ)=0 | Intercept on μ(ζ) | 1.42 (1.11, 1.74) |
| σ(κ) | SD on κ | 1.21 (0.963, 1.47) |
| σ(ς) | SD on ς | 0.922 (0.783, 1.06) |
| σ(ζ) | SD on ζ | 0.344 (0.00577, 0.671) |
DIC, deviance information criterion
SD, standard deviation
HDR, highest-density region
3.3. Aim 3(a): Controlling for general ability
Table 2 shows posterior distributions on the coefficients under Model 4, which contained an intercept, psychosis, age (in decades, minus 1.8), sex (−0.5 indicating male and 0.5 indicating female), g, and an interaction between psychosis and g as covariates on μ(κ). Thus, Model 4 estimated the influence of psychosis on k whilst controlling for the potential influences of other factors, including general intellectual ability. The influence of psychosis on k was lower under this model than previous models, but was still credibly non-zero—in other words, the influence of psychosis on k was larger than predicted by the general cognitive deficit. Neither age nor sex appeared to influence k. While there was a strong, credibly non-zero influence of g, the interaction term was negligible, suggesting that the relationship between g and k was similar across patients and controls.
3.4. Aim 3(b): Mediation
Figure 4 summarizes the results of the mediation analysis. Under a model without mediation, there was decisive evidence (BF = 2156; see Jeffreys, 1961) for a direct effect of psychosis on g (coefficient = −0.288). Under a model with mediation, there was decisive evidence for an indirect effect of psychosis on g via k (coefficient = −0.275 × 0.401 = −0.110; BF = 791), and moderate evidence for the direct effect (coefficient = −0.178; BF = 7.17). These results suggest partial mediation.
Figure 4.
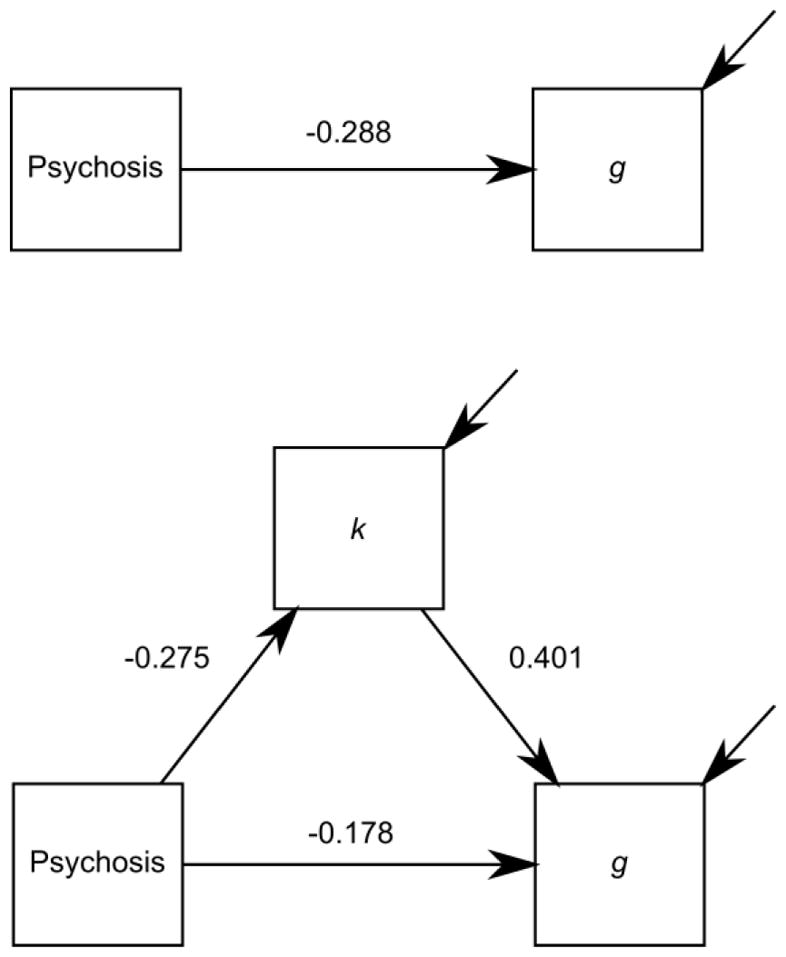
Results of the mediation analysis. Values are path strengths (posterior mean standardized regression coefficients) under a model without mediation (top figure) and a model with mediation (bottom figure).
4. Discussion
Patients with psychosis usually perform worse than controls on simple visual change-detection tasks (e.g., Choi et al. 2012; Erickson et al. 2015; Glahn et al. 2003; Gold et al. 1997; Gold et al. 2010; Haenschel et al. 2007; Johnson et al. 2013; Leonard et al. 2013; Mayer et al. 2012). Despite several differences in sample characteristics between the present study and previous ones—all of our subjects were African Americans, patients had various psychotic disorders, and controls were not excluded for other psychiatric disorders—we replicated this basic observation. The results suggest that previous findings concerning cognitive deficits in patients with psychosis from mixed or other groups generalize to African Americans, which is an important finding in our view, because African Americans are underserved by psychiatric research, despite being disproportionately affected by psychosis (Schwartz and Blankenship 2014).
However, a limitation of the present work is that the between-group difference was smaller than typically observed. We speculated that our heterogeneous patient group and inclusion of controls with other psychiatric disorders might have contributed to the modest effect size, but found no clear evidence that these features influenced the results. Since we were not able to identify the precise cause of this modest effect, it is difficult to gauge how generalizable our results might be to future studies.
Our cognitive models suggested that patients with psychosis had smaller k (the number of slots in visual WM) than controls. The same models suggested that there was little difference in either guessing or lapsing behavior between the groups. These findings were consistent with those of several previous studies. For example, Gold et al. (2010) found that k, but not the precision of WM representations, was smaller in 31 patients with psychosis and 26 controls when measured using a delayed-estimation task (Wilken and Ma 2004). Likewise, in the study by Johnson et al. (2013), 99 patients with psychosis had smaller k, on average, than 77 healthy controls, measured via a change-localization task (similar to change detection).
It is worth pointing out that the present study, like previous studies, assumed that visual WM is “slots based”—that is, that individuals can remember up to k items at once, without any loss in precision of their representations. While this view has been dominant for many years (Cowan 2010; Luck and Vogel 2013), more recently, some researchers have characterized WM capacity as a “flexible resource”, that can be used to remember more or fewer items with less or greater precision, depending on the context (Ma et al. 2014). This changing perspective on visual WM may have important implications for future psychiatric research. For instance, under the flexible-resource view, patients with psychosis could do poorly on change-detection tasks due to having reduced WM resource, or for more subtle reasons, such as resource inflexibility (i.e., a difficulty in appropriately allocating the resource to task-relevant features of the visual scene).
Patients with psychosis experience a general cognitive deficit, and it is sometimes unclear whether a between-group difference in performance on a particular task is driven by a specific or the general deficit (e.g., Reilly et al. 2016; Reilly and Sweeney 2014; Kristian Hill et al. 2015). Many previous studies have assumed that WM is specifically disrupted in psychosis (Forbes et al. 2009; Lee and Park 2005; Piskulic et al. 2007). Two findings from present study support for this assumption. First, we found that psychosis influenced k, while controlling for g. This finding complements those from the study by Johnson et al. (2013), which reported positive correlations between k and performance on many other tasks. The authors performed several detailed analyses of their data, including linear regressions which tested to what extent k could explain the between-group differences on the other tasks. Intriguingly, found that k explained about 40% of the variance in those differences, consistent with the idea that k constrains other aspects of cognition. However, they did not test the reverse of this relationship, which is also important for demonstrating that smaller k is a specific impairment in psychosis, as we did in the present study. Second, via mediation analysis, we found decisive evidence for an indirect (mediated) relationship between psychosis and general intellectual ability via k (MacKinnon 2008). However, the mediation effect was partial, not complete—in other words, psychosis appeared to influence additional aspects of cognition besides k, which also contributed to the general cognitive deficit.
Another limitation of the present study was that our change-detection task was quite brief, containing fewer trials per sample-array size and per subject that some previous studies. Naturally, fewer trials lead to poorer estimates of k, and could have contributed to the smaller between-group difference observed in the present study than in previous ones. However, the Bayesian hierarchical models meliorated this limitation (see Morey 2011). Moreover, our task was clearly sufficient to reveal a between-group difference in k while controlling for g, and a clear mediation by k on the relationship between psychosis and g. Here, as in a previous study from our laboratory (Mathias et al. 2017), we argue that the Bayesian approach allows complex cognitive models to be applied to the data from brief neuropsychological tests completed by groups of individuals with mental illness. While this approach has gained popularity in the basic sciences (Lee and Wagenmakers 2014), it remains underused in psychiatry, where it has arguably even stronger advantages, because it is generally more difficult to collect good quality data from patients than healthy individuals.
Cognitive abilities predict functional outcome in patients with psychosis (Green 1996; Green et al. 2000). Since WM plays a crucial role in daily life, WM dysfunction could lead to difficulties in everyday functioning, for example in forming relationships or maintaining employment. Indeed, previous studies have found specific correlations between performance on WM tasks and measures of functional outcome (Bowie et al. 2008; Shamsi et al. 2011; González-Ortega et al. 2013). Based on our conclusions from the present study—namely, that k is a specific deficit in psychosis, which contributes to the general cognitive deficit—we speculate that the frequently observed correlations between cognition and functional outcome might be at least partly driven by k, which could be tested in future studies.
Supplementary Material
Acknowledgments
This research was supported by National Institute of Mental Health grant R01MH106324-01 awarded to David Glahn, Russell Poldrack, and John Blangero. The authors report no biomedical financial interests or potential conflicts of interest.
Footnotes
Conflicts of interest
The authors report no biomedical financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Addington J, Piskulic D, Liu L, Lockwood J, Cadenhead KS, Cannon TD, Bowie CR, Leung WW, Reichenberg A, McClure MM, Patterson TL, Heaton RK, Harvey PD. Predicting schizophrenia patients’ real-world behavior with specific neuropsychological and functional capacity measures. Biol Psychiatry. 2008;63(5):505–511. doi: 10.1016/j.biopsych.2007.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornblatt BA, McGlashan TH, Perkins DO, Seidman LJ, Tsuang MT, Walker EF, Bearden CE, Mathalon DH, Woods SW. Comorbid diagnoses for youth at clinical high risk of psychosis. Schizophr Res. 2017 doi: 10.1016/j.schres.2017.03.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi JS, Park JY, Jung MH, Jang JH, Kang DH, Jung WH, Han JY, Choi CH, Hong KS, Kwon JS. Phase-specific brain change of spatial working memory processing in genetic and ultra-high risk groups of schizophrenia. Schizophr Bull. 2012;38(6):1189–1199. doi: 10.1093/schbul/sbr038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowan N. The Magical Mystery Four: How is Working Memory Capacity Limited, and Why? Curr. Dir Psychol Sci. 2010;19(1):51–57. doi: 10.1177/0963721409359277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowan N. The magical number 4 in short-term memory: a reconsideration of mental storage capacity. Behav Brain Sci. 2001;24(1):87–114. doi: 10.1017/s0140525x01003922. discussion 114–85. [DOI] [PubMed] [Google Scholar]
- Dickinson D, Ragland JD, Gold JM, Gur RC. General and specific cognitive deficits in schizophrenia: Goliath defeats David? Biol. Psychiatry. 2008;64(9):823–827. doi: 10.1016/j.biopsych.2008.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson MA, Hahn B, Leonard CJ, Robinson B, Gray B, Luck SJ, Gold J. Impaired working memory capacity is not caused by failures of selective attention in schizophrenia. Schizophr Bull. 2015;41(2):366–373. doi: 10.1093/schbul/sbu101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV-TR Axis I Disorders. Biometrics Research, New York State Psychiatric Institute; New York: 2002. [Google Scholar]
- Forbes NF, Carrick LA, McIntosh AM, Lawrie SM. Working memory in schizophrenia: a meta-analysis. Psychol Med. 2009;39(6):889–905. doi: 10.1017/S0033291708004558. [DOI] [PubMed] [Google Scholar]
- Fukuda K, Vogel E, Mayr U, Awh E. Quantity, not quality: the relationship between fluid intelligence and working memory capacity. Psychon Bull Rev. 2010;17(5):673–679. doi: 10.3758/17.5.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelman AHJ. Data Analysis Using Regression and Multilevel/Hierarchical Models. Cambridge University Press; UK: 2006. [Google Scholar]
- Gelman A, Carlin JB, Stern HS, Dunson D, Vehtari A, Rubin DB. Bayesian Data Analysis. 3. Chapman & Hall/CRC; Boca Raton: 2013. [Google Scholar]
- Glahn DC, Therman S, Manninen M, Huttunen M, Kaprio J, Lonnqvist J, Cannon TD. Spatial working memory as an endophenotype for schizophrenia. Biol Psychiatry. 2003;53(7):624–626. doi: 10.1016/s0006-3223(02)01641-4. [DOI] [PubMed] [Google Scholar]
- Green MF. What are the functional consequences of neurocognitive deficits in schizophrenia? Am. J Psychiatry. 1996;153(3):321–330. doi: 10.1176/ajp.153.3.321. [DOI] [PubMed] [Google Scholar]
- Green MF, Kern RS, Braff DL, Mintz J. Neurocognitive deficits and functional outcome in schizophrenia: are we measuring the “right stuff”? Schizophr. Bull. 2000;26(1):119–136. doi: 10.1093/oxfordjournals.schbul.a033430. [DOI] [PubMed] [Google Scholar]
- Gold JM, Carpenter C, Randolph C, Goldberg TE, Weinberger DR. Auditory working memory and Wisconsin Card Sorting Test performance in schizophrenia. Arch Gen Psychiatry. 1997;54(2):159–165. doi: 10.1001/archpsyc.1997.01830140071013. [DOI] [PubMed] [Google Scholar]
- Gold JM, Hahn B, Zhang WW, Robinson BM, Kappenman ES, Beck VM, Luck SJ. Reduced capacity but spared precision and maintenance of working memory representations in schizophrenia. Arch Gen Psychiatry. 2010;67(6):570–577. doi: 10.1001/archgenpsychiatry.2010.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman-Rakic PS. Working memory dysfunction in schizophrenia. J Neuropsychiatry Clin Neurosci. 1994;6(4):348–357. doi: 10.1176/jnp.6.4.348. [DOI] [PubMed] [Google Scholar]
- González-Ortega I, de Los Mozos V, Echeburúa E, Mezo M, Besga A, Ruiz de Azúa S, González-Pinto A, Gutierrez M, Zorrilla I, González-Pinto A. Working memory as a predictor of negative symptoms and functional outcome in first episode psychosis. 2013;206(1):8–16. doi: 10.1016/j.psychres.2012.08.025. [DOI] [PubMed] [Google Scholar]
- Haenschel C, Bittner RA, Haertling F, Rotarska-Jagiela A, Maurer K, Singer W, Linden DE. Contribution of impaired early-stage visual processing to working memory dysfunction in adolescents with schizophrenia: a study with event-related potentials and functional magnetic resonance imaging. Arch Gen Psychiatry. 2007;64(11):1229–1240. doi: 10.1001/archpsyc.64.11.1229. [DOI] [PubMed] [Google Scholar]
- Jeffreys H. Theory of probability. 3. Clarendon Press; Oxford: 1961. [Google Scholar]
- Johnson MK, McMahon RP, Robinson BM, Harvey AN, Hahn B, Leonard CJ, Luck SJ, Gold JM. The relationship between working memory capacity and broad measures of cognitive ability in healthy adults and people with schizophrenia. Neuropsychology. 2013;27(2):220–229. doi: 10.1037/a0032060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kristian Hill S, Buchholz A, Amsbaugh H, Reilly JL, Rubin LH, Gold JM, Keefe RS, Pearlson GD, Keshavan MS, Tamminga CA, Sweeney JA. Working memory impairment in probands with schizoaffective disorder and first degree relatives of schizophrenia probands extend beyond deficits predicted by generalized neuropsychological impairment. Schizophr Res. 2015;166(1–3):310–315. doi: 10.1016/j.schres.2015.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruschke JK. Doing Bayesian data analysis: A tutorial with R, JAGS, and Stan. 2. Academic Press; Burlington, VT: 2014. [Google Scholar]
- Kruschke JK, Liddell TM. The Bayesian New Statistics: Hypothesis testing, estimation, meta-analysis, and power analysis from a Bayesian perspective. Psychon Bull Rev. 2017 doi: 10.3758/s13423-016-1221-4. [DOI] [PubMed] [Google Scholar]
- Lee J, Park S. Working memory impairments in schizophrenia: a meta-analysis. J Abnorm Psychol. 2005;114(4):599–611. doi: 10.1037/0021-843X.114.4.599. [DOI] [PubMed] [Google Scholar]
- Lee MD, Wagenmakers E. Bayesian cognitive modeling: a practical course. Cambridge University Press; Cambridge, UK: 2014. [Google Scholar]
- Leonard CJ, Kaiser ST, Robinson BM, Kappenman ES, Hahn B, Gold JM, Luck SJ. Toward the neural mechanisms of reduced working memory capacity in schizophrenia. Cereb Cortex. 2013;23(7):1582–1592. doi: 10.1093/cercor/bhs148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luck SJ, Vogel EK. Visual working memory capacity: from psychophysics and neurobiology to individual differences. Trends Cogn Sci. 2013;17(8):391–400. doi: 10.1016/j.tics.2013.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma WJ, Husain M, Bays PM. Changing concepts of working memory. NatNeurosci. 2014;17(3):347–356. doi: 10.1038/nn.3655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKinnon DP. Introduction to statistical mediation analysis. Lawrence Erlbaum Associates; Hove: 2008. [Google Scholar]
- Mathias SR, Knowles EE, Barrett J, Leach O, Buccheri S, Beetham T, Blangero J, Poldrack RA, Glahn DC. The Processing-Speed Impairment in Psychosis Is More Than Just Accelerated Aging. Schizophr Bull. 2017 doi: 10.1093/schbul/sbw168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer JS, Fukuda K, Vogel EK, Park S. Impaired contingent attentional capture predicts reduced working memory capacity in schizophrenia. PLoS One. 2012;7(11):e48586. doi: 10.1371/journal.pone.0048586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyake A, Shah P. Models of working memory: Mechanisms of active maintenance and executive control. 1999. [Google Scholar]
- Morey RD. A Bayesian hierarchical model for the measurement of working memory capacity. J Math Psychol. 2011;55(1):8–24. [Google Scholar]
- Nuijten MB, Wetzels R, Matzke D, Dolan CV, Wagenmakers EJ. A default Bayesian hypothesis test for mediation. Behav Res Methods. 2015;47(1):85–97. doi: 10.3758/s13428-014-0470-2. [DOI] [PubMed] [Google Scholar]
- Pashler H. Familiarity and visual change detection. Percept Psychophys. 1988;44(4):369–378. doi: 10.3758/bf03210419. [DOI] [PubMed] [Google Scholar]
- Piskulic D, Olver JS, Norman TR, Maruff P. Behavioural studies of spatial working memory dysfunction in schizophrenia: a quantitative literature review. Psychiatry Res. 2007;150(2):111–121. doi: 10.1016/j.psychres.2006.03.018. [DOI] [PubMed] [Google Scholar]
- Potvin S, Stavro K, Rizkallah E, Pelletier J. Cocaine and cognition: a systematic quantitative review. J Addict Med. 2014;8(5):368–376. doi: 10.1097/ADM.0000000000000066. [DOI] [PubMed] [Google Scholar]
- Reilly JL, Hill SK, Gold JM, Keefe RS, Clementz BA, Gershon E, Keshavan MS, Pearlson G, Tamminga CA, Sweeney JA. Impaired Context Processing is Attributable to Global Neuropsychological Impairment in Schizophrenia and Psychotic Bipolar Disorder. Schizophr Bull. 2016 doi: 10.1093/schbul/sbw081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reilly JL, Sweeney JA. Generalized and specific neurocognitive deficits in psychotic disorders: utility for evaluating pharmacological treatment effects and as intermediate phenotypes for gene discovery. Schizophr Bull. 2014;40(3):516–522. doi: 10.1093/schbul/sbu013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rock PL, Roiser JP, Riedel WJ, Blackwell AD. Cognitive impairment in depression: a systematic review and meta-analysis. Psychol Med. 2014;44(10):2029–2040. doi: 10.1017/S0033291713002535. [DOI] [PubMed] [Google Scholar]
- Rouder JN, Morey RD, Cowan N, Zwilling CE, Morey CC, Pratte MS. An assessment of fixed-capacity models of visual working memory. Proc Natl Acad Sci U S A. 2008;105(16):5975–5979. doi: 10.1073/pnas.0711295105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouder JN, Morey RD, Verhagen J, Province JM, Wagenmakers EJ. Is There a Free Lunch in Inference? Top. Cogn Sci. 2016;8(3):520–547. doi: 10.1111/tops.12214. [DOI] [PubMed] [Google Scholar]
- Schwartz RC, Blankenship DM. Racial disparities in psychotic disorder diagnosis: A review of empirical literature. World J Psychiatry. 2014;4(4):133–140. doi: 10.5498/wjp.v4.i4.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shamsi S, Lau A, Lencz T, Burdick KE, DeRosse P, Brenner R, Lindenmayer JP, Malhotra AK. Cognitive and symptomatic predictors of functional disability in schizophrenia. 2011;126(1–3):257–164. doi: 10.1016/j.schres.2010.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiegelhalter DJ, Best NG, Carlin BP, van der Linde A. Bayesian measures of model complexity and fit (with discussion) Journal of the Royal Statistical Society, Series B. 2002;64(4):583–639. [Google Scholar]
- Stavro K, Pelletier J, Potvin S. Widespread and sustained cognitive deficits in alcoholism: a meta-analysis. Addict Biol. 2013;18(2):203–213. doi: 10.1111/j.1369-1600.2011.00418.x. [DOI] [PubMed] [Google Scholar]
- Wilken P, Ma WJ. A detection theory account of change detection. J Vis. 2004;4(12):1120–1135. doi: 10.1167/4.12.11. [DOI] [PubMed] [Google Scholar]
- Yuan Y, MacKinnon DP. Bayesian mediation analysis. Psychol Methods. 2009;14(4):301–322. doi: 10.1037/a0016972. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.