Abstract
Ethanol intoxication and withdrawal exact a devastating toll on the central nervous system. Abrupt ethanol withdrawal provokes massive release of the excitatory neurotransmitter glutamate, which over-activates its postsynaptic receptors, causing intense Ca2+ loading, p38 mitogen activated protein kinase activation and oxidative stress, culminating in ATP depletion, mitochondrial injury, amyloid β deposition and neuronal death. Collectively, these mechanisms produce neurocognitive and sensorimotor dysfunction that discourages continued abstinence. Although the brain is heavily dependent on blood-borne O2 to sustain its aerobic ATP production, brief, cyclic episodes of moderate hypoxia and reoxygenation, when judiciously applied over the course of days or weeks, evoke adaptations that protect the brain from ethanol withdrawal-induced glutamate excitotoxicity, mitochondrial damage, oxidative stress and amyloid β accumulation. This review summarizes evidence from ongoing preclinical research that demonstrates intermittent hypoxia training to be a potentially powerful yet non-invasive intervention capable of affording robust, sustained neuroprotection during ethanol withdrawal.
Keywords: amyloid β, ethanol intoxication, excitotoxicity, glutamate, heat shock protein 25, p38, presenilin 1, reactive oxygen species
1. Medical and socioeconomic impact of alcohol use disorder
Alcohol use disorder (AUD) is the destructive pattern of heavy drinking that often progresses to alcohol dependence, i.e. alcoholism [NIAAA, 2011: https://pubs.niaaa.nih.gov/publications/StrategicPlan/NIAAASTRATEGICPLAN.htm#Introduction]. Alcoholics experience difficulty curbing heavy drinking despite its adverse health and social consequences. AUD has reached epidemic proportions in the United States, where an estimated 15.1 million adults and nearly 700,000 youth or adolescents have experienced alcohol dependence [SAMHSA, 2015: https://www.samhsa.gov/data/sites/default/files/NSDUH-DetTabs-2015/NSDUH-DetTabs-2015/NSDUH-DetTabs-2015.htm#tab5–6a]. AUD is a worldwide problem; for example, an estimated 62 million people are alcoholics in India (Ganguly, 2008).
Many alcoholics die prematurely due to ethanol-inflicted organ damage, or from existing illnesses complicated by heavy ethanol consumption (Spies et al., 2001; Eggers et al., 2002). A 14-year longitudinal study in Germany identified a 2- to 3-fold higher mortality rate in individuals with AUD than the general population even after controlling for psychiatric comorbidity (John et al., 2013). From 1989 to 2006, AUD killed 88,000 per year in the United States and shortened the lifespan of 2.5 million people by one year per 10 years of alcohol abuse [CDC, 2016: https://www.cdc.gov/alcohol/onlinetools.htm], making AUD the fourth leading preventable cause of death in the United States (Mokdad et al., 2005). In 2012, the deaths of 7.6% of men and 4.0% of women were attributed to alcohol consumption [WHO, 2014: http://www.who.int/substance_abuse/publications/global_alcohol_report/msb_gsr_2014_1.pdf?ua=1], and in the United States, alcohol accounted for 10% of deaths of working age adults (Stahre et al., 2014). Although drug and/or behavioral therapies may lessen AUD’s devastating effects, only 20–25% of AUD sufferers receive detoxification or alcohol dependence treatment (Dawson et al., 2006), leaving most alcoholics at risk of mental and physical disorders and a shortened lifespan.
The adverse consequences of AUD extend beyond alcoholics and their families, and its socio-economic impact continues to mount. In the United States alone, AUD cost an estimated $249 billion in 2010 (Sacks et al., 2015). This amount approximates the combined cost of Alzheimer’s disease (AD) and all other dementias [ALZ, 2017: http://www.alz.org/facts/] and exceeds that of cancer ($124.5 billion in 2010) (Yabroff et al., 2011) or stroke ($33 billion in 2015) [CDC, 2016: https://www.cdc.gov/alcohol/onlinetools.htm]. AUD’s economic impact also includes productivity lost to disablement and premature death. These costs are largely based on self-reports, yet many alcohol dependent individuals under-report their alcohol consumption (Nelson et al., 2010). Thus, AUD’s true economic impact very likely exceeds the reported costs, ranking it among the costliest medical problems in the United States.
2. Ethanol withdrawal and its neurological consequences
Ethanol withdrawal (EW) syndromes, including tremor, seizure and other neurological sequelae, occur when alcoholics abruptly cease or sharply lower their alcohol consumption. Up to 25% of life-threatening seizures are due to EW (Hillbom et al., 2003; Hughes, 2009). EW severity varies with the amount and duration of ethanol consumption, and the presence of coexisting illnesses. In the United States, approximately 500,000 EW episodes per year require drug therapy (O'Connor and Schottenfeld, 1998), and EW kills 15% of untreated patients (Sander et al., 2006). Alcoholics often relapse into heavy drinking because they cannot tolerate EW (Barrett, 1985). EW symptoms, which begin within 6 – 24 h of drinking cessation, fall into three categories (Hall and Zador, 1997): (1) autonomic, primarily sympathetic, hyperactivities, manifested as tremor, sweating, nausea, and vomiting; (2) neuronal hyperexcitation, mediated by excessive glutamate release, manifests as anxiety, agitation, insomnia, and seizures; and (3) alcohol withdrawal delirium, i.e. delirium tremens, characterized by auditory and visual hallucinations, confusion, disorientation, clouded consciousness, and impaired attention, which may progress to permanent memory disorders, or in 5–25% of cases, death due to respiratory and cardiovascular collapse (O’Brien, 1996; Trevisan et al., 1998; Erwin et al., 1998). Thus, EW requires careful clinical management (Hall and Zador, 1997).
Ethanol withdrawal complicates coexisting conditions, and other illnesses may prolong or intensify EW (Monte Secades et al., 2010). EW syndromes occur in 25% of alcoholics after elective surgery (Eggers et al., 2002). 50–60% of trauma patients have AUD (Herve et al., 1986; Soderstrom et al., 1992; Ostrom and Eriksson, 1993); those admitted to intensive care, whose access to alcohol is interrupted, are likely to experience EW (Uusaro et al., 2005), especially upon awakening from sedation (Spies et al., 1996; Spies and Rommelspacher, 1999). EW exacerbates postoperative complications, e.g. pneumonia (Spies et al., 2001; Eggers et al., 2002), and may prolong intensive care (Spies et al., 1995, 1996). Thus, EW often challenges treatment of coexisting illnesses.
Many alcoholics make repeated attempts at abstinence but fail due to EW’s distressful sequelae, leading to a pattern of repeated drinking and withdrawal [NIH Publ. No. 95–3769] and more severe brain injury (Ballenger and Post, 1978; Brown et al., 1988). Alcoholics with multiple EW episodes experienced more frequent panic attacks (George et al., 1990), and heightened startle responses and anxiety (Krystal et al., 1997), and were less likely to seek treatment (Dawson et al., 2006) than those experiencing a single EW bout. Alcoholics with a history of EW displayed severe seizures and delirium tremens during detoxification, while those in their first EW had only mild tremors (Ballenger and Post, 1978). Even after long-term abstinence, persons with a history of EW were more likely to suffer seizures (Lechtenberg and Worner, 1991); thus, repeated EW may permanently lower the threshold for neuronal excitations, even in persons who eventually maintain abstinence. It is hardly surprising that alcoholics with repeated EW are less likely to achieve sustained abstinence and are at heightened risk of permanent alcohol-induced dementia.
Repeated EW may accelerate brain aging. Young adults who had experienced repeated EW were less able to associate a conditional stimulus with an aversive event, and showed reduced long-term neuronal potentiation in the hippocampus (Stephens et al., 2005), a center of learning and memory. Alcoholics, especially those with multiple EW episodes, showed impaired performance of maze and vigilance tests vs. healthy social drinkers (Duka et al, 2003). Repeated heavy drinking and EW impair cognitive functions (Weissenborn and Duka, 2003; Townshend and Duka, 2005) and increase vulnerability to AD and other age-related cognitive disorders (Zilkens et al., 2014; Venkataraman et al., 2017). Preclinical studies by the authors demonstrated the association between repeated EW and brain aging. Rats undergoing repeated EW showed earlier motoric impairment than their ethanol-free counterparts (Jung et al., 2011), and increased activity of presenilin-1 (PS1), an enzyme that generates AD’s pathological hallmark, amyloid β (Aβ) (Jung et al., 2016). Indeed, Aβ accumulated in the rat prefrontal cortex after repeated EW (Ryou et al., 2017).
3. Mechanisms of EW induced brain injury
A. Excessive glutamate release and NMDA receptor activation
The most abundant excitatory amino acid in mammalian brain (Lajtha et al., 1981), glutamate plays pivotal roles in inter-neuronal communication, brain development, learning and memory. After its Ca2+-dependent presynaptic release (Birnbaumer et al., 1994), glutamate activates postsynaptic metabotropic receptors as well as ionotropic 2-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA), N-methyl-D-aspartate (NMDA) and kainic acid receptor subtypes (Watkins et al., 1990; Nakanishi, 1992; Sommer et al., 1992). Glutamate signaling elicits long-term potentiation, a process essential for learning and memory (Oscar-Berman et al., 1997; Headley and Grillner, 1990).
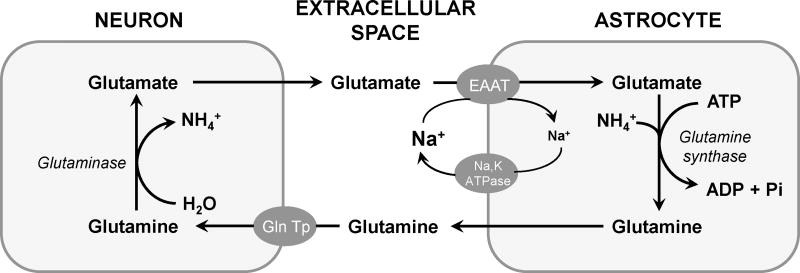
High extracellular glutamate concentrations can overactivate neurons to the point of death, the phenomenon of excitotoxicity. Astrocytes efficiently clear glutamate from the extracellular fluid (Danbolt, 2001) (Figure 1), thereby protecting neurons from excitotoxicity (Miguel-Hidalgo, 2009; Ayers-Ringler et al., 2016). Glutamate uptake is accomplished by excitatory amino acid transporters (EAATs) abundantly expressed in astrocyte plasma membranes (Amara and Fontana, 2002). Glutamate is aminated in astrocytes, released, taken up by adjacent neurons, and reconverted to glutamate (Figure 1) (Danbolt, 2001). Thus, astrocytes are indispensable for regulating extracellular glutamate and, thereby, preventing excitotoxic cell death.
Figure 1. Glutamate cycling between neurons and astrocytes.
The excessive level of glutamate in extracellular space is taken-up to astrocytes by excitatory amino acid transporters (EAAT). In astrocytes, glutamate is converted to glutamine by glutamine synthase in the presence of ATP. Non-excitatory glutamine is subsequently released from the astrocytes and taken up by neuronal glutamine transporters (Gln Tp). Inside neurons, glutamine is deaminated to regenerate glutamate.
While ethanol acts as a neurosuppressant by increasing inhibitory γ-aminobutyric acid (GABA) neurotransmission while dampening excitatory glutamatergic transmission (Valenzuela, 1997), EW precipitates intense glutamate release from presynaptic neurons (Chandler et al, 2006; De Witte et al, 2003). Synaptic glutamate concentrations then increase to the millimolar range, hyper-activating postsynaptic glutamate receptors and provoking excessive Ca2+ entry and ATP depletion culminating in neuronal injury (Figure 2). Hendricson et al. (2007) demonstrated increased NMDA receptor density and intensified excitatory postsynaptic currents and action potentials in rat hippocampal neurons during EW. NMDA receptor subunits were upregulated in mouse cortical neuronal cultures subjected to repeated ethanol exposure and EW (Qiang et al., 2007). NMDA receptor activation favors neuronal excitation, while GABA receptors are silenced to compensate for ethanol’s inhibitory effects. Cerebellar granular cells exposed to ethanol for 4 days and abrupt EW showed increased NMDA receptor activation that persisted for 2 days and was more intense than activation by continuous ethanol (Nath et al., 2012). Thus, glutamatergic NMDA receptors are activated during chronic ethanol exposure and even more so upon EW.
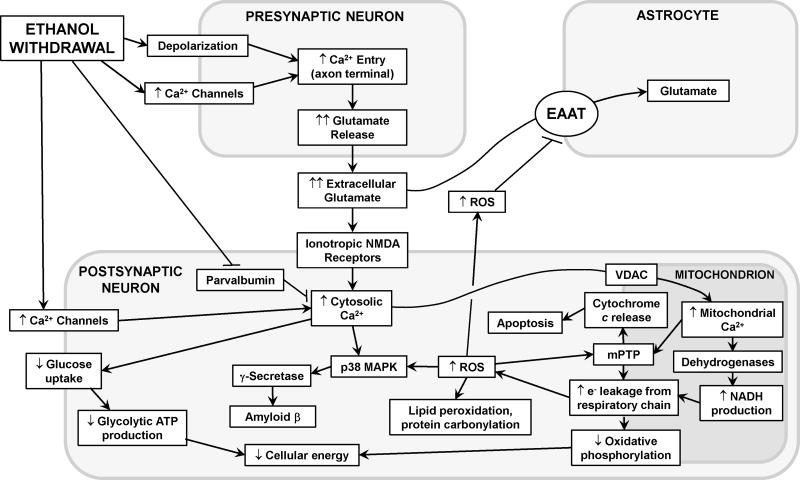
Figure 2. Neurotoxic signaling cascades initiated by ethanol withdrawal.
During EW, increased neuronal depolarization and axonal Ca2+ entry provoke excessive glutamate release, which activates ionotropic N-methyl-D-aspartate (NMDA) receptors, permitting massive Ca2+ influx into the postsynaptic neuron and, via voltage gated ion channels (VDAC), the mitochondria. Mitochondrial Ca2+ activates mitochondrial dehydrogenases to produce NADH which delivers electrons (e−) to the respiratory chain. e− leakage from complexes I and III partially reduces O2, generating reactive oxygen species (ROS). The resultant cytosolic and mitochondrial Ca2+ overload and ROS formation destabilize the mitochondrial membrane, opening mitochondrial permeability transition pores (mPTP) that compromise ATP production and initiate the mitochondrial apoptosis cascade. ROS and Ca2+ also activate p38 mitogen-activated protein kinase (p38 MAPK), which in turn activates γ-secretase and, thus, cytotoxic amyloid β formation. Also, ROS inflict oxidative damage on cellular phospholipids and proteins, and impair glutamate clearance by the astrocytic excitatory amino acid transporters (EAAT). See text for details.
Upregulation of glutamate neurotransmission during EW occurs in different brain regions and is manifested as various behavioral phenotypes. Glutamate release is increased in the hippocampus (Dahchour and De Witte, 2003), striatum (Rossetti and Carboni, 1995), and nucleus accumbens of ethanol-withdrawn rats (Seidemann et al., 2017). In the “kindling” phenomenon, neurons stimulated by EW are sensitized to the next EW episode, thereby lowering their activation threshold (Ballenger and Post, 1978). Neuronal kindling may underlie the behavioral manifestations of exacerbated and/or prolonged withdrawal after repeated EW episodes (Harper and Matsumoto, 2005). Studies in rat hippocampal neurons (Reynolds et al., 2015) suggested that kindling may be mediated by upregulated NMDA receptors.
Ethanol withdrawal increased NMDA receptor numbers and the frequency of spontaneous excitatory postsynaptic currents in rat basolateral amygdala, accompanied by anxiety behavior (Läck et al., 2007). The basolateral amygdala regulates the expression of fear and anxiety, and an increase in NMDA receptors in this region may mediate EW-induced anxiety (Läck et al., 2007). The periaqueductal grey area also is associated with anxiety. In rats subjected to chronic ethanol intake and abrupt EW, the increased synaptic glutamate concentration activated neuronal signaling to the basolateral amygdala or the dorsal periaqueductal grey matter (Christian et al, 2013). The increased synaptic glutamate concentration paralleled anxiety behavior (Christian et al, 2013), indicating that glutamate in the periaqueductal grey matter also mediates EW-associated anxiety. Bauer et al. (2013) demonstrated elevated glutamate in the ventral striatum, a region associated with drug craving and relapse, in alcoholics undergoing detoxification. Neuronal hyperexcitation in this region may provoke relapse.
Glutamate-induced neuronal hyperexcitation and consequent emotion and behaviors have been linked to neuronal damage in vulnerable brain areas. The hippocampal CA1 subregion of mice consuming a 10% ethanol diet for 2 weeks followed by two days EW showed intense apoptosis, particularly of excitatory glutamatergic neurons (Skrzypiec et al. 2009). Similarly, we demonstrated apoptosis in cerebellar granular cells of ethanol-withdrawn rats (Jung et al., 2003) and in cultured HT22 mouse hippocampal cells withdrawn from 100 mM ethanol (Prokai-Tatrai et al., 2009). Neuronal death following EW has also been reported in other animal and human studies (Charness, 1993; Lovinger, 1993; Harper and Matsumoto, 2005).
Antagonists of NMDA receptors enabled interrogation of the role of glutamatergic signaling in EW stress. Neramexane hydrochloride minimized seizures in ethanol withdrawn rats (Kotlinska et al., 2004) and memantine suppressed EW symptoms in humans (Krupitsky et al., 2007). Dizocilpine preserved eyeblink conditioning, a measure of learning ability, in ethanol withdrawn rats (Young et al., 2010). Amino-phosphonovaleric acid prevented the loss of hippocampal neurons subjected to multiple ethanol exposure-withdrawal cycles, implicating NMDA receptors in neuronal death associated with multiple EW episodes (Reynolds et al. 2015).
B. Astrocytic glutamate uptake and metabolism
Astrocytes effect rapid removal and recycling of glutamate for synaptic transmission. Two months of EW increased the prefrontal cortical content of astrocytic glutamine synthetase and the astrocyte marker, glial fibrillary acidic protein, vs. ethanol-free and ethanol-consuming rats (Miguel-Hidalgo, 2006). Brains of mice genetically susceptible to EW syndromes had higher glutamine synthetase activities than control mice (Sakellaridis et al., 1989). Smith (1997) found that glutamate uptake by neonatal rat cerebrocortical astrocytes was increased at 48 and 96 hours of ethanol exposure but gradually subsided by 3 days EW. These findings are concordant with a human study, where brains from alcoholics showed increased astrocytes in cingulate cortex and ventral tegmentum (He and Crews, 2008). Since astrocytes are responsible for management of extracellular glutamate, the EW-induced increases in astrocytes and glutamine synthetase may represent protective adaptations against excitotoxicity.
Ceftriaxone, which increases astrocytic expression and activity of the glutamate transporter, EAAT2 (Rothstein et al, 2005), attenuated tremor and startle responses in ethanol-withdrawn rats (Abulseoud et al. 2014) and ameliorated seizure activity in rats subjected to traumatic brain injury (Goodrich et al. 2013). Devaud et al. identified a sex-specific response of EAAT3; at 3 days of EW, only female rats showed increased expression of EAAT3 in cerebral cortex and hippocampus (Alele and Devaud, 2005); moreover, female rats largely recovered from EW symptoms within 3 days, while male rats continued to experience seizures (Devaud and Chadda, 2001). The earlier recovery of female rats may be attributed to increased astrocytic EAAT content and, thus, more effective clearance of extracellular glutamate.
C. Ionotropic Ca2+entry and intracellular Ca2+ overload during EW
Calcium ions enter neurons via membrane channels responsive to agonists and depolarization. Prolonged Ca2+ channel opening permits intracellular Ca2+ accumulation in the presynaptic axon terminus, triggering excessive glutamate release and hyperactivation of signaling proteins in the postsynaptic neuron (Figure 2) (Finn and Crabbe 1997; Lipton 1999). Moreover, excessive intracellular Ca2+ activates enzymes which provoke inflammation (Yoo et al., 2006) and generate reactive oxygen species, i.e. ROS (Finn and Crabbe 1997; Lipton 1999).
Ca2+ channels are upregulated during EW (Walter and Messing, 1999; N’Gouemo and Morad, 2003) and Ca2+ channel inhibitors are cerebroprotective against EW stress. In mice, EW increased expression of T-type Ca2+ channel genes (Graef et al., 2011), and the T-type Ca2+ channel blocker ethosuximide reduced seizures provoked by intermittent ethanol exposures and withdrawals (Riegle et al., 2014, 2015) and restored EW-disrupted sleep activity (Wiggins et al., 2013). Similarly, the Ca2+ channel antagonists nitrendipine and nimodipine attenuated EW-induced seizures and mortality in rats (Little et al., 1986).
Glutamate is a direct opener of neuronal Ca2+ channels. Upon EW, glutamate binding to its upregulated postsynaptic receptors provokes prolonged Ca2+ channel opening and massive Ca2+ entry (Figure 2). Kahlert et al. (2005) demonstrated increased Ca2+ influx in hippocampal cell cultures exposed to glutamate concentrations (100 µM) resembling those produced by excitatory neurotransmission (Meldrum, 2000). Overactivation of NMDA receptors by excess glutamate during EW-induced hyperexcitation may be intimately linked to Ca2+-induced cytotoxicity (Whittington et al., 1995), due in part to the high Ca2+ conductance of these receptors (Nagy, 2008). Further evidence of the glutamate-Ca2+ interaction is a study in primary cultures of cortical neurons (Nagy and László, 2002), where three cycles of 12 h ethanol exposure and 12 h EW potentiated intracellular Ca2+ overload evoked by NMDA. The glutamate-Ca2+ interaction likely plays a central role in EW-induced neuronal hyperexcitation.
Excessive Ca2+ may disrupt brain energy metabolism during EW. Cellular uptake of the glucose analog 2-deoxyglucose, a measure of glucose uptake, was decreased in hippocampal slices from ethanol withdrawn rats, and the Ca2+ channel antagonists nifedipine and flunarizine attenuated this effect (Shibata et al., 1995); thus, Ca2+ channel hyperactivation impedes glucose metabolism during EW. Excessive Ca2+ may harm mitochondria, too. Elevated cytosolic Ca2+ promotes mitochondrial Ca2+ uptake and Ca2+ overload, triggering mitochondrial membrane swelling, collapse of the electrochemical H+ gradient (Grant et al., 1990), and production of toxic ROS (Lafon-Cazal et al., 1993). Thus, intracellular Ca2+ management is critical, necessitating mechanisms to prevent Ca2+ overload, including Ca2+-binding proteins that limit mitochondrial Ca2+ uptake. Rewal et al. demonstrated decreased cerebellar and hippocampal contents of the Ca2+ binding protein parvalbumin in ethanol withdrawn vs. control rats (Rewal et al., 2005), suggesting that EW hampers defenses against intracellular Ca2+ overload (Figure 2). By opening Ca2+ channels and hindering Ca2+ buffering capacity, EW perturbs Ca2+ homeostasis in a manner that exacerbates excitotoxicity (Arundine and Tymianski 2003; Choi, 1992).
D. ROS overproduction during ethanol withdrawal
Reactive oxygen species are highly reactive O2 derivatives including superoxide (•O2−), hydrogen peroxide (H2O2), hydroxyl radical (•OH), and hydroperoxyl radical (HO2•). Most ROS possess unpaired electrons, rendering the molecules unstable and chemically aggressive. To balance their unpaired electrons, ROS extract electrons from other molecules, including proteins, phospholipids and DNA. ROS-induced lipid peroxidation generates thiobarbituric acid reactive substances (Wilbur et al., 1949; Wood and Watson, 1967), and protein oxidation leaves a footprint, protein carbonyls (Yan, 2009). Alcoholics undergoing EW had elevated circulating concentrations of the oxidized lipid products malondialdehyde and 4-hydroxynonenal, which gradually subsided over 6 months (Budzyński et al., 2016), showing the persistence of the lipid modifications. The plasma concentrations of •O2− and malondialdehyde were higher in ethanol withdrawn rats than control or ethanol-consuming rats (Gonzaga et al., 2015), showing that EW’s prooxidant effects may exceed those of the antecedent ethanol exposure (Jung, 2016). These findings are concordant with the authors’ findings of increased ROS, malondialdehyde and protein carbonyl contents in ethanol-withdrawn HT22 cells (Prokai-Tatrai et al., 2009) and rats (Jung et al., 2011) vs. the respective control and ethanol exposure values. These prooxidant effects may in part explain how EW inflicts more severe neuronal damage than ethanol exposure alone (Phillips and Cragg, 1984).
While ethanol metabolism directly yields ROS, EW indirectly generates ROS and oxidizes lipids and proteins through other mediators, including Ca2+ and glutamate (Jung, 2016). Ca2+ exposure provoked •OH generation in rat cerebrocortical and cerebellar mitochondria (Dykens, 1994). Ca2+ may perturb mitochondrial membranes in a manner that increases non-selective H+ efflux across the inner mitochondrial membranes (Kowaltowski et al., 1996; Grijalba et al., 1999), thereby generating ROS by increasing electron leakage from the respiratory chain (Figure 2). Ca2+ activation of mitochondrial dehydrogenases exacerbates ROS formation by increasing production of NADH (Chinopoulos and Adam-Vizi, 2006), which supplies electrons to the mitochondrial respiratory chain (Figure 2). The excess ROS modify critical components of the respiratory chain, thereby compromising ATP production (Nicholls et al. 2007). Similarly, glutamate increases ROS formation by elevating intracellular Ca2+; thus, glutamate activation of NMDA receptors increased Ca2+ influx into mouse hippocampal neurons, which activated ROS production (Hernández-Fonseca et al., 2008). ROS also may increase extracellular glutamate concentration by disabling astrocytic EAATs that manage extracellular glutamate (Sorg et al., 1997). Collectively, these studies suggest that the toxic interplay between ROS, Ca2+, and excess glutamate mediates cell death and behavioral hyperexcitation during EW.
The moderate amount of ROS that are normally produced during cellular ATP production are efficiently scavenged by antioxidant enzymes. On the other hand, Huang et al. (2009) reported decreased serum antioxidant enzyme activities in ethanol withdrawn human alcoholics. Serum malondialdehyde concentrations were higher in the alcoholics than in healthy subjects, and glutathione peroxidase and catalase activities remained depressed at 1 and 2 weeks of EW (Huang et al., 2009). Oxidative brain damage induced by EW may hence be attributed to a two-pronged attack from increased ROS production and compromised antioxidant defenses.
E. Mitochondrial permeability transition
Calcium ions enter mitochondria via the voltage-dependent anion channel (VDAC) within the outer mitochondrial membrane (Rizzuto et al., 2009). VDAC’s gating role in mitochondrial Ca2+ entry was demonstrated by Rapizzi et al. (2002) in the human HeLa cell line, where increased VDAC expression increased mitochondrial matrix Ca2+ concentrations, followed by apoptotic cell death. VDAC is a component of the mitochondrial permeability transition pore (mPTP), a multi-protein complex that permits passage of molecules < 1.5 kDa across mitochondrial membranes (Stuart 2002). The mPTP is minimally composed of adenine nucleotide translocase, mitochondrial creatine kinase, VDAC and cyclophilin D (Dolder et al., 2001; Halestrap and Brennerb 2003). These proteins interact to regulate mPTP opening and the passage of specific molecules (Beutner et al., 1998; Dolder et al., 2001). The functional coupling of these proteins is critical because of fluctuating energy requirements, especially under the stressful conditions of EW. Of particular significance, mPTP opening is greatly enhanced by oxidative stress. In an early study of hyperexcitability in rats, EW after 33 weeks ethanol exposure intensified the mitochondrial permeability response to a •O2− generator, phenazine methosulfate (French and Todoroff 1971). We demonstrated in rats that consumption of a 6.5% ethanol diet for 35 days, followed by 24 hours EW, increased •O2− and protein carbonyl contents and mPTP inducibility in isolated mitochondria from cerebral cortex, cerebellum and hippocampus (Jung et al., 2008). mPTP are embedded in mitochondrial membranes, so it is entirely possible that excessive mPTP opening changes the membrane structure and exacerbates oxidative damage.
Oxidative stress and mitochondrial Ca2+ loading can impede the regulatory function of mPTP proteins (Halestrap and Brennerb 2003). The malfunctioning mPTP permits random passage of molecules across the inner mitochondrial membrane, induces mitochondrial swelling, and dissipates the H+ electrochemical gradient for ATP production (Pepe 2000; Yan et al., 2002; Halestrap and Brennerb 2003). Skrzypiec et al. (2009) demonstrated mitochondrial membrane depolarization in hippocampal neurons upon EW, accompanied by a substantial increase in the fraction of cells displaying mitochondrial disruption and apoptosis. Cytochrome c oxidase, the terminal complex IV of the mitochondrial respiratory chain, reduces O2 to H2O to help generate ATP. Jaatinen et al. (2003) observed that repeated EW inactivated cytochrome c oxidase in cerebellar Purkinje neurons, a phenomenon that was more severe in 24- than 4-month-old male rats. Congruent with the effect of age on cytochrome c oxidase is our finding that repeated EW suppressed cytochrome c oxidase content and activity more severely in 15- than 8-month-old rats (Jung et al., 2012). Collectively, these studies support the proposal that factors associated with excessive mPTP opening contribute to brain injury upon EW.
F. EW activation of p38 MAPK
p38 is one of a large family of mitogen-activated protein kinases (MAPK) that regulate cellular responses to stresses by phosphorylating target proteins. p38 itself is phosphorylated and activated by MAPK kinase, i.e. MAPKK. Physiologically, p38 mediates signal transduction, synaptic plasticity, learning, and memory (Sweatt, 2001), but its over-activation is associated with apoptosis and inflammation. Stress-activated p38 phosphorylates a host of signaling proteins mediating apoptosis, inflammation, and oxidative stress (Moriguchi et al., 1996). For example, p38 mediated apoptosis of rat cerebrocortical astrocytes exposed to tumor necrosis factor (Barca et al., 2008) and neurotoxin-induced apoptosis of mouse cerebellar granular neurons (Giordano et al., 2008). Moriguchi et al. (1996) identified an intriguing relationship between p38 and ROS, wherein H2O2 activates MAPKK6 which, in turn, activates p38.
Tian et al. (2016) demonstrated p38 activation in prefrontal cortex of rats after 4 days binge drinking, suggesting that repeated EW, even short term, is capable of augmenting p38 activity. Further, a 7 week program of daily cycles of 17 hours ethanol intoxication and 7 hours withdrawal increased p38 gene expression by 3.3-fold in rat prefrontal cortex (Rimondini et al., 2002). We demonstrated in rats that two cycles of ethanol intoxication for 4 weeks and 3 weeks EW increased p38 expression in cerebellum (Ju et al., 2012) and prefrontal cortex (Jung et al., 2016). Similarly, repeated ethanol exposure and removal increased p38 expression in the rat cerebellar HT22 cell line (Jung et al., 2016). Of particular interest, these experiments demonstrated augmentation of EW-induced p38 by glutamate and suppression by the NMDA receptor antagonist dizocilpine (Jung et al., 2016), indicating that glutamate activates p38 expression via NMDA receptors. These findings are concordant with a report that the prototypical receptor agonist NMDA produced neuronal apoptosis in a manner dampened by p38 inhibition (Kim et al., 2011), implicating a p38-glutamate interaction in the neuronal death. Although we and others found p38 to be activated by multiple EW bouts, no studies have compared p38 activation by single vs. repeated EW, a matter that merits investigation.
G. Presenilin 1 activation by EW
Presenilin 1 (PS1) is the catalytic subunit of the γ-secretase enzyme complex that cleaves amyloid precursor protein (APP), yielding Aβ peptides. PS1 is encoded by PSEN genes, the mutation of which results in Aβ over-production and aggregation forming senile plaques, a histological hallmark of the devastating neurogenerative disorder, Alzheimer's disease.
Although Aβ overproduction by mutated PS1 is well known, the role of wild-type PS1 is more controversial. For example, protection of mouse cortical neurons by the proteolytic enzyme trypsin was disrupted in neurons lacking one or both PSEN alleles (Nikolakopoulou et al., 2016), suggesting PS1 was neuroprotective. Decreased PS1 expression in brains of 15 or 24 vs. 6 month-old mice (Thakur and Ghosh 2007; Kaja et al., 2015) implicated PS1 deficiency in brain aging. On the other hand, Kumar et al. (2009) reported PS1 accumulation in the hippocampus of senescent vs. younger mice, accompanied by memory loss. Furthermore, DeStrooper et al. (1999) demonstrated decreased γ-secretase cleavage of APP and a fivefold drop in Aβ production in cultured neurons from PS1-deficient vs. wild type mouse embryos. These studies indicate that even wild type PS1 in excess may contribute to brain aging and Aβ accumulation.
Recently, we demonstrated elevated PS1 expression in brains of young adult rats after repeated EW (Jung et al., 2016). Also, high dose glutamate induced PS1 over-expression in HT22 neuronal cells, placing PS1 in the molecular network associated with excitotoxicity. Although these findings seemed to contradict the neuroprotective effect of PS1 reported by Nikolakopoulou et al. (2016), that neuroprotection was independent of γ-secretase activity. EW toxicity in HT22 cells was ameliorated by γ-secretase inhibition (Jung et al., 2016), similar to the reported suppression of Ca2+ induced hippocampal cell death by γ-secretase inhibition (Choi et al. 2010). Li et al. (2011) demonstrated that PS1 over-expression is sufficient to enhance γ-secretase activity. The γ-secretase complex is minimally composed of PS1, the presenilin-binding protein nicastrin, and β-catenin, and also may contain Aph-1 and Pen-2 (Tandon and Fraser, 2002). These components may modulate PS1 in a manner which may vary depending upon cellular milieu or the nature of stress. Moreover, γ-secretase mediates two distinctive signaling events: APP cleavage that yields Aβ, and notch cleavage that produces notch intracellular domain, a transcription factor which enters nuclei and activates target genes. With such divergent molecular pathways, a given stressor may modulate PS1’s interactions with other γ-secretase components, culminating in beneficial or deleterious outcomes. In the case of peated neuronal hyperexcitation, excessive amounts of PS1 may contribute to neurotoxicity, not protection. Nevertheless, the possibility cannot be excluded that the increase in PS1 in ethanol withdrawn rats represents an adaptive response to prevent further brain damage.
4. Current EW treatments and their limitations
Benzodiazepines (BZDs), central nervous system depressants that enhance inhibitory GABA neurotransmission and are widely used for management of anxiety, agitation and seizures, currently are the primary treatments for clinical management of EW. The best-studied BZDs for EW include diazepam (Valium), lorazepam (Ativan), and chlordiazepoxide (Librium) (Mayo-Smith, 1997; Rosenbloom, 1988; Myrick and Anton, 2000). Other drug classes such as anticonvulsants, barbiturates, adrenergic drugs, and GABA agonists have been used to treat EW syndromes, but BZDs thus far have yielded the best outcomes (Sarff and Gold, 2010; Sachdeva et al., 2015). While BZDs have a wide therapeutic window, they also may impose untoward side effects that limit their clinical application. BZDs have dependence liability and are among the most abused prescription drugs [NIDA, 2016: https://www.drugabuse.gov/drugs-abuse/commonly-abused-drugs-charts]. Rebound effects can occur upon cessation of BZD treatment, increasing the risk of relapse into ethanol dependence (Malcolm, 2003). Alcoholics are particularly vulnerable to BZD dependency (Mueller et al., 2005). Both ethanol and BZD act at GABA-BZD receptor complexes and facilitate GABA-ergic neurotransmission. Because of this common mechanism, ethanol-tolerant alcoholics develop tolerance to BZDs and require higher doses than non-alcoholics (Book and Myrick, 2005; Gartenmaier et al., 2005). However, high BZD doses are heavily sedating and can suppress ventilation (Gillis et al., 1989; Megarbane et al., 2005) to an extent requiring emergency medical treatment (Hack et al., 2006). Chronic or repeated application of BZD, a prevalent pattern among alcoholics, raises major risk of complications. Upon repeated dosing with long-acting diazepine, its psychoactive, longer half-life metabolite desmethyldiazepam remains in the body for several days, which can exacerbate existing medical conditions, e.g. chronic obstructive pulmonary disease [NIAAA, 1989: https://pubs.niaaa.nih.gov/publications/aa05.htm].
The important limitations of BZD have prompted studies of alternative treatments for EW management with lower risks of adverse effects. Non-BZD compounds such as carbamazepine, gabapentin, valproic acid, topiramate, γ-hydroxybutyric acid, baclofen, and flumazenil have limited clinical efficacy (Leggio et al, 2008). The GABA analogue gabapentin (Eastes, 2010) and even intravenous ethanol (Weinberg et al., 2008) have been proposed to prevent EW stress, but the purported benefits weren’t achieved. The α2-adrenoreceptor agonist clonidine did not mitigate the agitation associated with EW, a potential disadvantage of treating hyper-excitatory syndromes (Etherington, 1996a, b). BZDs proved more effective against EW-induced seizure than γ-hydroxybutyric acid or anticonvulsants (Amato et al., 2011). Pharmacotherapies using disulfiram and naltrexone, aimed at alleviating EW symptoms and minimizing the risk of relapse, showed limited efficacy in large multicenter studies (Fuller et al., 1986; Krystal et al., 2003). As such, none of these alternative drug treatments has proven more effective than BZDs. Instead, non-pharmacological strategies may afford novel, non-invasive interventions against EW syndromes and the resultant neurodegeneration.
5. Intermittent hypoxia training: cerebroprotective intervention
A. Therapeutic vs. detrimental intermittent hypoxia in brain
The concept of brain protection by hypoxia may seem counterintuitive. The human brain consumes enormous amounts of ATP, and is heavily dependent on a steady supply of oxygen to sustain its energy demands. Indeed, the brain accounts for 15–20% of the total resting O2 consumption in adults (Clark & Sokoloff, 1999). Moreover, compromised O2 delivery is a core factor in brain ischemic syndromes, including stroke and cardiac arrest, which rank among the leading causes of death and permanent disability worldwide (Mozaffarian et al., 2016). Although severe hypoxia sufficient to impair ATP production is certainly detrimental to the brain, moderate and/or brief reductions in O2 supply may elicit adaptations that serve to increase the brain’s resistance to more severe insults, particularly ischemia and alcohol withdrawal.
It is important to distinguish cerebroprotective intermittent hypoxia training (IHT) from the intermittent hypoxia produced by obstructive or central sleep apnea, a major risk factor for cardiovascular morbidities including hypertension, stroke and coronary artery disease (Serebrovskaya et al., 2008; Mateika et al., 2015). Sleep apnea is characterized by interruptions in alveolar ventilation that produce brief yet intense episodes of hypoxia and hypercapnia. These apneic events may occur 30 times an hour, a rate associated with heightened risk of cardiovascular mortality (Marin et al., 2005). Preclinical models of sleep apnea utilize intense (e.g. 4–8% O2) hypoxic exposures, typically 60–90 s, followed by similarly brief exposures to 21% O2 (Fletcher, 2000; Serebrovskaya et al., 2008), repetitively for several hours per day. In contrast, therapeutic IHT utilizes somewhat longer hypoxia exposures, each several minutes duration, alternated with room air exposures also lasting several minutes, for a limited number of daily cycles over a period of several days to weeks (Serebrovskaya and Xi, 2016). The authors have utilized a 20 d program of 5–8 daily cycles of 5–10 min moderately intense hypoxia (9.5–10% O2) with intervening 4 min exposures to 21% O2, with each daily session totaling 45–98 min (Zong et al., 2004). This controlled hypoxia program has proven effective against myocardial infarction in dogs (Zong et al., 2004; Mallet et al., 2006; Estrada et al., 2016), hypertension in rats (Manukhina et al., 2013) and human patients (Lyamina et al., 2011), and alcohol withdrawal in rats (Jung et al., 2008; Ju et al., 2012; Ryou et al., 2017).
B. Mechanisms of IHT-induced cerebroprotection from alcohol withdrawal
The authors have demonstrated normobaric IHT programs can minimize brain injury and neurobehavioral deficits during EW following protracted ethanol diets. In rats consuming 6.5% ethanol for 5 weeks, the IHT regimen of 5–8 daily exposures to 9.5–10% O2 for 5–10 min followed by 4 min room air breathing (Zong et al., 2004; Mallet et al., 2006; Estrada et al., 2016), administered over the last 20 d of the ethanol diet, prevented the behavioral deficits seen at 24 hours EW (Jung et al., 2008). The IHT program altered neither ethanol intake, blood ethanol concentrations nor body weight. Ethanol intoxication increased •O2− and protein carbonyl contents in the cerebral cortex, cerebellum and hippocampus, and EW provoked further increases in these oxidative stress markers. IHT blunted •O2− formation in all 3 regions, and protein carbonyl formation in the cerebral cortex and cerebellum.
Cyclic hypoxia and reoxygenation provokes ROS formation (Prabhakar et al., 2007), primarily from the mitochondrial respiratory complexes (Selivanov et al., 2011). ROS generated during intermittent hypoxia-reoxygenation may mediate the evolving neuroprotection (Figure 3). Intraperitoneal injections of the broad-spectrum antioxidant, N-acetylcysteine, before each daily hypoxia session prevented the reductions in behavioral deficits. Paradoxically, the antioxidant treatment during IHT actually increased cerebellar •O2− content at 24 hours EW, a finding that implicates ROS in the development of the neuroprotection afforded by IHT. In response to ROS, the transcription factor, nuclear factor erythroid 2-related factor 2 (Nrf2), activates expression of an extensive gene program encoding numerous phase II defense enzymes that collectively afford powerful antioxidant and anti-inflammatory cytoprotection (Vargas & Johnson, 2009; Alfieri et al., 2013; Dringen et al., 2015). Induction of Nrf2’s gene program increases cellular resistance to ROS and carbonyl stress. Intermittent hypoxia could potentially be more neuroprotective than sustained, uninterrupted hypoxia exposures (Serebrovskaya et al., 2008) because the cyclic exposures to room air trigger modest, nontoxic ROS formation which activates Nrf2-driven expression of antioxidant genes (Figure 3) (Calabrese et al., 2008; Kaspar et al., 2009), increasing the brain’s antioxidant defenses against the more intense ROS formation provoked by EW. The impact of IHT on Nrf2 and its neuroprotective gene program in brain is unknown, but merits investigation.
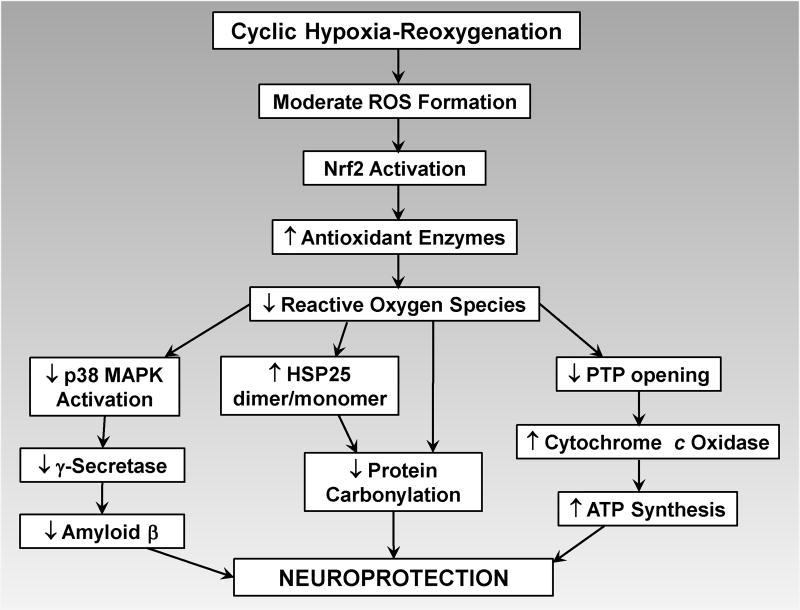
Figure 3. Reactive oxygen species are both the mediators and targets of intermittent hypoxia-induced neuroprotection.
Intermittent hypoxia induces moderate formation of ROS, which activate transcription factor Nrf2, initiating gene expression of antioxidant enzymes that protect neurons from the more intense ROS formation during ethanol withdrawal. See text for details.
Inactivation of cytochrome c oxidase causes electrons to accumulate within respiratory complexes I and III (Bailey et al., 1999) and monovalently reduce molecular oxygen to •O2−. Thus, the suppression of •O2− formation suggested IHT may have protected the mitochondrial respiratory complexes in vulnerable brain regions. Accordingly, Ju et al. (2012) examined the impacts of alcohol intoxication-withdrawal and IHT on cytochrome oxidase in cerebellum, a brain region vulnerable to EW. Male rats completed two cycles of 4 weeks consumption of a 6.5% ethanol diet and 3 weeks of an ethanol-free dextrin diet. IHT commenced 3 days before the first EW and continued for 17 days of the first but not second EW period. The rats exhibited marked EW signs 24 hours after completing the second ethanol diet which were partially mitigated by IHT completed 32 days earlier. Cerebellum-dependent motoric capacity was impaired at 10 days of the second EW in a manner prevented by the antecedent hypoxia training. Cerebellar cytochrome oxidase activity and content of cytochrome oxidase subunit IV fell by approximately 70% at 21 d of the second EW period, but IHT during the first EW partially maintained cytochrome oxidase activity and fully preserved subunit IV content at 21 days of the second EW, i.e. 52 days after hypoxia training. The two cycles of alcohol intoxication-withdrawal doubled protein carbonyl content in cerebellar mitochondria after the second EW cycle in a manner attenuated by IHT during the first EW cycle. When daily oral administration of the membrane-selective antioxidant vitamin E was substituted for IHT, cytochrome oxidase in cerebellar mitochondria was similarly preserved. Collectively, these findings suggested that IHT may have evoked antioxidant adaptations which rendered cerebellar mitochondria resistant to subsequent EW stress, and this robust mitochondrial protection persisted for several weeks.
Intermittent hypoxia training suppresses the cytotoxic signaling cascades activated by excess glutamate. In rats, two cycles of 28 days ethanol intoxication and 21 days EW sharply increased p38 activity in prefrontal cortex (Ryou et al., 2017) and cerebellum (Ju et al., 2012), but IHT during the first EW cycle prevented p38 activation during the second cycle. In the prefrontal cortex, EW and p38 activation were associated with increased PS1 content, and Aβ1–40 and Aβ1–42 accumulation paralleled that of PS1 at 21 days EW. On the other hand, IHT during the first EW cycle blunted subsequent accumulation of PS1, Aβ1–40 and Aβ1–42 during the second EW initiated after another 28 days of ethanol diet. Thus, a 20 day IHT regimen produced persistent protection against the neurotoxic signaling cascade unleashed by EW.
Two cycles of 28 days ethanol diet and 21 days EW produced appreciable protein carbonylation in the prefrontal cortex (Ryou et al., 2017), yet IHT during the first EW cycle prevented this increase, as it had in cerebellar mitochondria (Ju et al., 2012). The effects of EW vs. IHT on the molecular chaperone, heat shock protein 25 (HSP25), may have contributed to their respective pro- and antioxidant effects. HSP25 homodimers are effective chaperones that direct carbonylated proteins to degradation (Dudich et al., 1995), preventing cytotoxic accumulation of damaged proteins, but in its monomeric form, HSP 25 is ineffective (Diaz-Latoud et al., 2005). HSP25 dimer content fell by 60% at 21 days of the second EW cycle, while HSP25 monomers increased almost 30-fold, and the dimer/monomer ratio fell by 98%. On the other hand, the antecedent IHT regimen almost completely prevented accumulation of HSP25 monomers and partially restored the dimer/monomer ratio. Thus, IHT prevented disruption of functional HSP25 dimers, thereby preserving the chaperone’s cytoprotective capabilities.
C. IHT: potential intervention for alcoholic and Alzheimer’s dementias
The 6th leading cause of death in the United States (Feldstein, 2012), AD is a mounting pandemic. In the United States alone, 4.5 million people had AD in 2000 (Hebert et al., 2003), and this number is expected to increase to 13.2 million by 2050 (Hebert et al., 2003; Honjo et al., 2012). Forty percent of Americans aged 85 and above have cognitive impairment, and 75–80% of these are AD cases (Gandy & DeKosky, 2013). Worldwide, 36 million people had dementia, the majority of them AD, in 2010, a number expected to increase to 115 million by 2050 (Honjo et al., 2012; Li et al., 2015). AD risk factors include comorbidities of obesity: type 2 diabetes mellitus (Lu et al., 2009; Li et al., 2015), hypertension (Di Iorio et al., 1999; Qiu et al., 2005; Feldstein, 2012) and obstructive sleep apnea (Bu et al., 2015; Daulatzai, 2015). As the population ages and the obesity epidemic intensifies, the impact of AD will continue to surge.
Although alcohol-related impairments in executive function and memory are largely reversible with abstinence (Venkataraman et al., 2017), a history of alcohol dependence is a risk factor for AD (Panza et al., 2008; Ramesh et al., 2010; Zilkens et al., 2014). Glutamatergic signaling, responsible for EW excitotoxicity, also plays a central role in the pathogenesis of AD (Kocahan & Doğan, 2017). Indeed, it could be proposed that repeated bouts of ethanol intoxication and withdrawal inflict multiple excitotoxic insults that culminate in AD or AD-like dementia.
The pathogenesis of AD is extraordinarily complex (Manukhina et al., 2016), encompassing cerebrovascular lesions (Kalback et al., 2004; Roher et al., 2011), microvascular rarefaction, irreversible glycation of Aβ and other proteins (Gomes et al., 2005; Münch et al., 2003) in cerebrovascular endothelium (Mackic et al., 1998), neurons (Li et al., 2013) and astroglia (Lue et al., 2005), and oxido-nitrosative inactivation of metabolic (Tajes et al., 2013) and antioxidant (Kim et al., 2003) enzymes. This complex pathogenesis has defied pharmacological intervention. Despite extraordinary investment of research resources, no medication has been found capable of halting or reversing the neurocognitive decline in AD (Manukhina et al., 2016). On the other hand, preclinical evidence in a rat model of AD indicates controlled, intermittent hypoxia may delay or prevent neurocognitive impairment. In these rats, a 14 day program of daily 4 hour hypobaric (simulated altitude 4000 m) hypoxia exposures prevented memory loss, nitrosative stress and neuronal death produced by subsequent Aβ25–35 injection into the magnocellular nucleus (Goryacheva et al., 2010; Manukhina et al., 2010). A recent report has extended IHT to geriatric patients (Bayer et al., 2017). In this placebo-controlled trial, patients aged 64–92 years who completed a program of alternating intermittent hypoxia and hyperoxia demonstrated improved cognitive performance on dementia-detection and clock-drawing tests, and increased capacity for walking exercise. Importantly, the intermittent hypoxia-hyperoxia regimen was well tolerated by these patients.
Intermittent hypoxia’s powerful cerebroprotection against EW may have important implications for the pathogenesis and treatment of neurodegenerative disorders including AD. Repeated cycles of alcohol intoxication and withdrawal are known risk factors for neurodegenerative disorders, including AD, later in life. Aβ deposition in senile plaques is a histological hallmark of AD and a pivotal event in AD pathogenesis (Honjo et al., 2012). Accordingly, two cycles of alcohol intoxication-EW increased Aβ-generating γ-secretase, its catalytic subunit PS1, and the AD-associated peptides Aβ1–40 and Aβ1–42 in rat prefrontal cortex (Ryou et al., 2017). The ability of IHT to suppress glutamatergic signaling in the brain (Jung et al., 2016; Ryou et al., 2017) may minimize alcoholic dementia and possibly delay the development of AD in the face of alcohol dependency. Moreover, by minimizing EW-associated neurobehavioral deficits, IHT may enable alcoholics to avoid relapse and achieve sustained abstinence.
SUMMARY
Alcohol use disorder is a mounting worldwide epidemic, a leading cause of premature death and neurocognitive disablement, and a risk factor for Alzheimer’s disease and other neurodegenerative disorders. EW provokes excessive glutamatergic neurotransmission causing cytotoxic Ca2+ overload, oxidative stress, and amyloid β accumulation, culminating in injury and death of the postsynaptic neurons. These neurological sequelae discourage abstinence; moreover, the significant side effects and limited effectiveness of available pharmacotherapies underscore an unmet need for alternative treatments for EW. A noninvasive, non-pharmacological intervention, IHT has proven remarkably effective in preclinical studies against the deleterious signaling cascades initiated by EW and the resultant neurobehavioral deficits and neuronal death. IHT blunted oxidative stress, mitochondrial damage and amyloid β deposition, while preserving the cytoprotective molecular chaperone, HSP25, in EW-vulnerable brain regions. Importantly, the anti-EW neuroprotection persisted several weeks after completing the IHT program. Thus, IHT may offer a safe, facile and highly effective treatment to minimize EW-induced excitotoxicity and neurocognitive sequelae, thereby fostering sustained abstinence from ethanol intoxication. While BZDs are powerful interventions to attenuate EW syndromes, their therapeutic use is limited by dependence liability and other adverse effects such as movement and cognitive deficits that often develop after long-term BZD therapy, yet are not seen with IHT. Table 1 compares the advantages of IHT vs. BZDs for treating EW and for addressing both the clinical manifestations and underlying mechanisms of EW excitotoxicity discussed in this review.
Table 1.
Comparison of the effects of IHT and clinical benzodiazepines (BZDs) on ethanol withdrawal (EW) mediators and endpoints
| IHT | BZDs | |
|---|---|---|
|
| ||
| EW syndromes | Attenuates in rats (Jung et al., 2008) | Attenuates in humans (Sachdeva et al., 2015) |
|
| ||
| Physical dependence | No known IHT-related dependence | Occurs after long-term BZDs (Licata and Rowlett, 2008) |
|
| ||
| Cognition | Not reported in ethanol withdrawn subjects | Deficits occur in humans after long-term alprazolam (Vandesquille et al., 2012) |
|
| ||
| Motoric functions | Not reported in ethanol withdrawn subjects | Deficits occur in humans with lorazepam (Dawson et al., 2008) and in mice with diazepam and lorazepam (Stanley et al., 2005) |
|
| ||
| Glutamate concentration | Not reported in ethanol withdrawn subjects | Diazepam does not change extracellular glutamate in EW rats (Rossetti and Carboni, 1995) |
| Diazepam decreases extracellular glutamate in non-EW rat brain (Khan et al., 1999) | ||
|
| ||
| Ca2+ | Not reported in ethanol withdrawn subjects | Diazepam, brotizolam, and clobazam up-regulate Ca2+ channels in non-EW mouse cerebrocortical neurons (Katsura et al., 2007) |
Highlights.
Intermittent, normobaric hypoxia training (IHT) programs afford robust protection against the devastating effects of ethanol withdrawal in the brain.
This neuroprotection is manifest by near-prevention of ethanol withdrawal-induced neurological deficits and irreversible neuronal injury.
IHT’s neuroprotective actions stand in stark contrast to the detrimental effects of the more intense hypoxia imposed by sleep apnea.
The moderate amounts of reactive oxygen species produced in the brain during controlled, cyclic hypoxia and reoxygenation evoke molecular adaptations that increase the brain’s resistance to ethanol withdrawal-induced glutamate excitotoxicity.
IHT is a non-invasive, non-pharmacological intervention that overcomes the limitations of current treatments for ethanol withdrawal.
Acknowledgments
This work was funded by research grants from the U.S. National Institute of Alcohol Abuse and Alcoholism [AA015982], the National Institute of Aging [AG053974], the National Institute of Neurological Disorders and Stroke [NS-076975], UNTHSC Institute for Aging and Alzheimer’s Disease Research, and UNTHSC Institute for Cardiovascular and Metabolic Diseases.
Abbreviations
- Aβ
amyloid β
- AD
Alzheimer’s disease
- AMPA
2-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid
- APP
amyloid precursor protein
- AUD
alcohol use disorder
- BZDs
benzodiazepines
- EAATs
excitatory amino acid transporters
- EW
ethanol withdrawal
- GABA
γ-aminobutyric acid
- HSP25
heat shock protein 25
- IHT
intermittent hypoxia training
- MAPK
mitogen-activated protein kinase
- mPTP
mitochondrial permeability transition pore
- NMDA
N-methyl-D-aspartate
- Nrf2
nuclear factor erythroid 2-related factor 2
- PS1
presenilin-1
- ROS
reactive oxygen species
- VDAC
voltage-dependent anion channel
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of interests: The authors have no financial or personal relationships that could potentially bias this work.
References
- Abulseoud OA, Camsari UM, Ruby CL, Kasasbeh A, Choi S, Choi DS. Attenuation of ethanol withdrawal by ceftriaxone-induced upregulation of glutamate transporter EAAT2. Neuropsychopharmacology. 2014;39:1674–1684. doi: 10.1038/npp.2014.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alele PE, Devaud LL. Differential adaptations in GABAergic and glutamatergic systems during ethanol withdrawal in male and female rats. Alcohol Clin. Exp. Res. 2005;29:1027–1034. doi: 10.1097/01.alc.0000167743.96121.40. [DOI] [PubMed] [Google Scholar]
- Alfieri A, Srivastava S, Siow RC, Cash D, Modo M, Duchen MR, Fraser PA, Williams SC, Mann GE. Sulforaphane preconditioning of the Nrf2/HO-1 defense pathway protects the cerebral vasculature against blood-brain barrier disruption and neurological deficits in stroke. Free. Radic. Biol. Med. 2013;65:1012–1022. doi: 10.1016/j.freeradbiomed.2013.08.190. [DOI] [PubMed] [Google Scholar]
- Amara SG, Fontana AC. Excitatory amino acid transporters: keeping up with glutamate. Neurochem. Int. 2002;41:313–318. doi: 10.1016/s0197-0186(02)00018-9. [DOI] [PubMed] [Google Scholar]
- Amato L, Minozzi S, Davoli M. Efficacy and safety of pharmacological interventions for the treatment of the Alcohol Withdrawal Syndrome. Cochrane Database Syst. Rev. 2011 doi: 10.1002/14651858.CD008537.pub2. CD008537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arundine M, Tymianski M. Molecular mechanisms of calcium-dependent neurodegeneration in excitotoxicity. Cell Calcium. 2003;34:325–337. doi: 10.1016/s0143-4160(03)00141-6. [DOI] [PubMed] [Google Scholar]
- Ayers-Ringler JR, Jia YF, Qiu YY, Choi DS. Role of astrocytic glutamate transporter in alcohol use disorder. World J. Psychiatry. 2016;6:31–42. doi: 10.5498/wjp.v6.i1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey SM, Pietsch EC, Cunningham CC. Ethanol stimulates the production of reactive oxygen species at mitochondrial complexes I and III. Free Radic. Biol. Med. 1999;27:891–900. doi: 10.1016/s0891-5849(99)00138-0. [DOI] [PubMed] [Google Scholar]
- Ballenger JC, Post RM. Kindling as a model for alcohol withdrawal syndromes. Br. J. Psychiatry. 1978;133:1–14. doi: 10.1192/bjp.133.1.1. [DOI] [PubMed] [Google Scholar]
- Barca O, Costoya JA, Senaris RM, Arce VM. Interferon-beta protects astrocytes against tumour necrosis factor-induced apoptosis via activation of p38 mitogen-activated protein kinase. Exp. Cell Res. 2008;314:2231–2237. doi: 10.1016/j.yexcr.2008.04.005. [DOI] [PubMed] [Google Scholar]
- Barrett RJ. Behavioral approaches to individual differences in substance abuse. In: Galizio M, Maisto SA, editors. Determinants of Substance Abuse. Plenum Press; New York: 1985. pp. 125–175. [DOI] [Google Scholar]
- Bauer J, Pedersen A, Scherbaum N, Bening J, Patschke J, Kugel H, Heindel W, Arolt V, Ohrmann P. Craving in alcohol-dependent patients after detoxification is related to glutamatergic dysfunction in the nucleus accumbens and the anterior cingulate cortex. Neuropsychopharmacology. 2013;38:1401–1408. doi: 10.1038/npp.2013.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayer U, Glazachev OS, Likar R, Burtscher M, Kofler W, Pinter G, Stettner H, Demschar S, Trummer B, Neuwersch S. Adaptation to intermittent hypoxia-hyperoxia improves cognitive performance and exercise tolerance in elderly. Adv. Gerontol. 2017;30:255–261. [In Russian] [PubMed] [Google Scholar]
- Beutner G, Ruck A, Riede B, Brdiczka D. Complexes between porin, hexokinase,mitochondrial creatine kinase and adenylate translocator display properties of the permeability transition pore. Implication for regulation of permeability transition by the kinases. Biophys. Biochim. Acta. 1998;1368:7–18. doi: 10.1016/s0005-2736(97)00175-2. [DOI] [PubMed] [Google Scholar]
- Birnbaumer L, Campbell KP, Catterall WA, Harpold MM, Hofmann F, Horne WA, Mori Y, Schwartz A, Snutch TP, Tanabe T, et al. The naming of voltage-gated calcium channels. Neuron. 1994;13:505–506. doi: 10.1016/0896-6273(94)90021-3. [DOI] [PubMed] [Google Scholar]
- Book SW, Myrick H. Novel anticonvulsants in the treatment of alcoholism. Expert Opin. Investig. Drugs. 2005;14:371–376. doi: 10.1517/13543784.14.4.371. [DOI] [PubMed] [Google Scholar]
- Brown ME, Anton RF, Malcolm R, Ballenger JC. Alcohol detoxification and withdrawal seizures: clinical support for a kindling hypothesis. Biol. Psychiatry. 1988;23:507–514. doi: 10.1016/0006-3223(88)90023-6. [DOI] [PubMed] [Google Scholar]
- Bu XL, Liu YH, Wang QH, Jiao SS, Zeo XQ, Gao D, Chen JC, Wang YJ. Serum amyloid-beta levels are increased in patients with obstructive sleep apnea syndrome. Sci. Res. 2015;5:13917. doi: 10.1038/srep13917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budzynski J, Ziolkowski M, Klopocka M, Czarnecki D. Oxidoreductive homeostasis in alcohol-dependent male patients and the risk of alcohol drinking relapse in a 6-month follow-up. Alcohol. 2016;50:57–64. doi: 10.1016/j.alcohol.2015.10.004. [DOI] [PubMed] [Google Scholar]
- Calabrese V, Signorile A, Cornelius C, Mancuso C, Scapagnini G, Ventimiglia B, Ragusa N, Dinkova-Kostova A. Practical approaches to investigate redox regulation of heat shock protein expression and intracellular glutathione redox state. Methods Enzymol. 2008;441:83–110. doi: 10.1016/S0076-6879(08)01206-8. [DOI] [PubMed] [Google Scholar]
- Chandler LJ, Carpenter-Hyland E, Hendricson AW, Maldve RE, Morrisett RA, Zhou FC, Sari Y, Bell R, Szumlinski KK. Structural and functional modifications in glutamateric synapses following prolonged ethanol exposure. Alcohol Clin. Exp. Res. 2006;30:368–376. doi: 10.1097/01.ALC.0000167959.84516.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charness ME. Brain lesions in alcoholics. Alcohol Clin. Exp. Res. 1993;17:2–11. doi: 10.1111/j.1530-0277.1993.tb00718.x. [DOI] [PubMed] [Google Scholar]
- Chinopoulos C, Adam-Vizi V. Calcium, mitochondria and oxidative stress in neuronal pathology. FEBS J. 2006;273:433–450. doi: 10.1111/j.1742-4658.2005.05103.x. [DOI] [PubMed] [Google Scholar]
- Choi DW. Excitotoxic cell death. J. Neurobiol. 1992;23:1261–1276. doi: 10.1002/neu.480230915. [DOI] [PubMed] [Google Scholar]
- Choi YH, Gwon AR, Jeong HY, Park JS, Baik SH, Arumugam TV, Jo DG. Contribution of γ-secretase to calcium-mediated cell death. Neurosci. Lett. 2010;469:425–428. doi: 10.1016/j.neulet.2009.12.043. [DOI] [PubMed] [Google Scholar]
- Chung JK, Nakajima S, Shinagawa S, Plitman E, Chakravarty MM, Iwata Y, Caravaggio F, Pollock BG, Gerretsen P, Graff-Guerrero A. Benzodiazepine use attenuates cortical β-amyloid and is not associated with progressive cognitive decline in nondemented elderly adults: a pilot study using F18-florbetapir positron emission tomography Geriatr. Am. J. Psychiatry. 2016;24:1028–1039. doi: 10.1016/j.jagp.2016.04.013. [DOI] [PubMed] [Google Scholar]
- Christian DT, Alexander NJ, Diaz MR, McCool BA. Thalamic glutamatergic afferents into the rat basolateral amygdala exhibit increased presynaptic glutamate function following withdrawal from chronic intermittent ethanol. Neuropharmacology. 2013;65:134–142. doi: 10.1016/j.neuropharm.2012.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark DD, Sokoloff L. Circulation and energy metabolism of the brain. In: Siegel GJ, Agranoff BW, Albers RW, Fisher SK, Uhler MD, editors. Basic Neurochemistry: Molecular, Cellular and Medical Aspects. Philadelphia: Lippincott; 1999. pp. 637–670. [Google Scholar]
- Dahchour A, De Witte P. Excitatory and inhibitory amino acid changes during repeated episodes of ethanol withdrawal: an in vivo microdialysis study. Eur. J. Pharmacol. 2003;459:171–178. doi: 10.1016/s0014-2999(02)02851-0. [DOI] [PubMed] [Google Scholar]
- Danbolt NC. Glutamate uptake. Prog. Neurobiol. 2001;65:1–105. doi: 10.1016/s0301-0082(00)00067-8. [DOI] [PubMed] [Google Scholar]
- Daulatzai MA. Evidence of neurodegeneration in obstructive sleep apnea: relationship between obstructive sleep apnea and cognitive dysfunction in the elderly. J Neurosci Res. 2015;93:1778–1794. doi: 10.1002/jnr.23634. [DOI] [PubMed] [Google Scholar]
- Dawson DA, Grant BF, Stinson FS, Chou PS. Estimating the effect of help-seeking on achieving recovery from alcohol dependence. Addiction. 2006;101:824–834. doi: 10.1111/j.1360-0443.2006.01433.x. [DOI] [PubMed] [Google Scholar]
- Dawson J, Boyle J, Stanley N, Johnsen S, Hindmarch I, Skene DJ. Benzodiazepine-induced reduction in activity mirrors decrements in cognitive and psychomotor performance. Hum. Psychopharmacol. 2008;23:605–613. doi: 10.1002/hup.961. [DOI] [PubMed] [Google Scholar]
- De Strooper B, Annaert W, Cupers P, Saftig P, Craessaerts K, Mumm JS, Schroeter EH, Schrijvers V, Wolfe MS, Ray WJ, Goate A, Kopan R. A presenilin-1-dependent gamma-secretase-like protease mediates release of Notch intracellular domain. Nature. 1999;398:518–522. doi: 10.1038/19083. [DOI] [PubMed] [Google Scholar]
- Devaud LL, Chadda R. Sex differences in rats in the development of and recovery from ethanol dependence assessed by changes in seizure susceptibility. Alcohol Clin. Exp. Res. 2001;25:1689–1696. [PubMed] [Google Scholar]
- De Witte P, Pinto E, Ansseau M, Verbanck P. Alcohol and withdrawal: from animal research to clinical issues. Neurosci. Biobehav. Rev. 2003;27:189–197. doi: 10.1016/s0149-7634(03)00030-7. [DOI] [PubMed] [Google Scholar]
- Diaz-Latoud C, Buache E, Javouhey E, Arrigo AP. Substitution of the unique cysteine residue of murine Hsp25 interferes with the protective activity of this stress protein through inhibition of dimer formation. Antioxid Redox Signal. 2005;7:436–445. doi: 10.1089/ars.2005.7.436. [DOI] [PubMed] [Google Scholar]
- Di Iorio A, Zito M, Lupinetti M, Abate G. Are vascular factors involved in Alzheimer’s disease? Facts and theories. Aging Clin. Exp. Res. 1999;11:345–352. doi: 10.1007/BF03339811. [DOI] [PubMed] [Google Scholar]
- Dolder M, Wendt S, Wallimann T. Mitochondrial creatine kinase in contact sites: interaction with porin and adenine nucleotide translocase, role in permeability transition and sensitivity to oxidative damage. Biol. Signals Recept. 2001;10:93–111. doi: 10.1159/000046878. [DOI] [PubMed] [Google Scholar]
- Dringen R, Brandmann M, Hohnholt MC, Blumrich E-M. Glutathione-dependent detoxification processes in astrocytes. Neurochem. Res. 2015;40:561–571. doi: 10.1007/s11064-014-1481-1. [DOI] [PubMed] [Google Scholar]
- Dudich IV, Zav’yalov VP, Pfeil W, Gaestel M, Zav’yalova GA, Denesyuk AI, Korpela T. Dimer structure as a minimum cooperative subunit of small heat-shock proteins. Biochim. Biophys. Acta. 1995;1253:163–168. doi: 10.1016/0167-4838(95)00135-x. [DOI] [PubMed] [Google Scholar]
- Duka T, Townshend JM, Collier K, Stephens DN. Impairment of cognitive functions after multiple detoxifications in alcoholic inpatients. Alcohol Clin. Exp. Res. 2003;27:1563–1572. doi: 10.1097/01.ALC.0000090142.11260.D7. [DOI] [PubMed] [Google Scholar]
- Dykens JA. Isolated cerebral and cerebellar mitochondria produce free radicals when exposed to elevated Ca2+ and Na+: implications for neurodegeneration. J. Neurochem. 1994;63:584–591. doi: 10.1046/j.1471-4159.1994.63020584.x. [DOI] [PubMed] [Google Scholar]
- Eastes LE. Alcohol withdrawal syndrome in trauma patients: a review. J. Emerg. Nurs. 2010;36:507–509. doi: 10.1016/j.jen.2010.05.011. [DOI] [PubMed] [Google Scholar]
- Eggers V, Tio J, Neumann T, Pragst F, Muller C, Schmidt LG, Kox WJ, Spies CD. Blood alcohol concentration for monitoring ethanol treatment to prevent alcohol withdrawal in the intensive care unit. Intensive Care Med. 2002;28:1475–1482. doi: 10.1007/s00134-002-1413-4. [DOI] [PubMed] [Google Scholar]
- Erwin WE, Williams DB, Speir WA. Delirium tremens. South Med. J. 1998;91:425–432. doi: 10.1097/00007611-199805000-00003. [DOI] [PubMed] [Google Scholar]
- Estrada JA, Williams AG, Jr, Sun J, Gonzalez LA, Downey HF, Caffrey JA, Mallet RT. δ-Opioid receptor (DOR) signaling and reactive oxygen species (ROS) mediate intermittent hypoxia induced protection of canine myocardium. Basic Res. Cardiol. 2016;111:17. doi: 10.1007/s00395-016-0538-5. [DOI] [PubMed] [Google Scholar]
- Etherington JM. Emergency management of acute alcohol problems. Part 1: Uncomplicated withdrawal. Can. Fam. Physician. 1996a;42:2186–2190. [PMC free article] [PubMed] [Google Scholar]
- Etherington JM. Emergency management of acute alcohol problems. Part 2: Alcohol-related seizures, delirium tremens, and toxic alcohol ingestion. Can. Fam. Physician. 1996b;42:2423–2431. [PMC free article] [PubMed] [Google Scholar]
- Feldstein CA. Association between chronic blood pressure changes and development of Alzheimer’s disease. J. Alzheimer’s Dis. 2012;32:753–763. doi: 10.3233/JAD-2012-120613. [DOI] [PubMed] [Google Scholar]
- Finn DA, Crabbe JC. Exploring alcohol withdrawal syndrome. Alcohol Health Res. World. 1997;21:149–156. [PMC free article] [PubMed] [Google Scholar]
- Fletcher EC. Effect of episodic hypoxia on sympathetic activity and blood pressure. Respir. Physiol. 2000;119:189–197. doi: 10.1016/s0034-5687(99)00114-0. [DOI] [PubMed] [Google Scholar]
- French SW, Todoroff T. Effect of chronic ethanol ingestion and withdrawal on brain mitochondria. Res. Commun. Chem. Pathol. Pharmacol. 1971;2:206–215. [PubMed] [Google Scholar]
- Fuller RK, Branchey L, Brightwell DR, Derman RM, Emrick CD, Iber FL, James KE, Lacoursiere RB, Lee KK, Lowenstam I. Disulfiram treatment of alcoholism. A Veterans Administration cooperative study. JAMA. 1986;256:1449–1455. [PubMed] [Google Scholar]
- Gandy S, DeKosky ST. Toward the treatment and prevention of Alzheimer’s disease: rational strategies and recent progress. Annu. Rev. Med. 2013;64:367–383. doi: 10.1146/annurev-med-092611-084441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganguly KK. Pattern and process of drug and alcohol use in India. ICMR Bull. 2008;38:1–8. [Google Scholar]
- Gartenmaier A, Pelzer E, Soyka M. Treatment of alcohol withdrawal syndrome with combined carbamazepine and tiapride in a patient with probable sleep apnoea syndrome. Pharmacopsychiatry. 2005;38:96–98. doi: 10.1055/s-2005-837810. [DOI] [PubMed] [Google Scholar]
- George DT, Nutt DJ, Dwyer BA, Linnoila M. Alcoholism and panic disorder: is the comorbidity more than coincidence? Acta Psychiatr. Scand. 1990;81:97–107. doi: 10.1111/j.1600-0447.1990.tb06460.x. [DOI] [PubMed] [Google Scholar]
- Gillis RA, Namath IJ, Easington C, Abrahams TP, Guidotti A, Quest JA, Hamosh P, Taveira da Silva AM. Drug interaction with gamma-aminobutyric acid/benzodiazepine receptors at the ventral surface of the medulla results in pronounced changes in cardiorespiratory activity. J. Pharmacol. Exp. Ther. 1989;248:863–870. [PubMed] [Google Scholar]
- Giordano G, Klintworth HM, Kavanagh TJ, Costa LG. Apoptosis induced by domoic acid in mouse cerebellar granule neurons involves activation of p38 and JNK MAP kinases. Neurochem. Int. 2008;52:1100–1105. doi: 10.1016/j.neuint.2007.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes R, Sousa Silva M, Quintas A, Cordeiro C, Freire A, Pereira P, Martins A, Monteiro E, Barroso E, Ponces Freire A. Argpyrimidine, a methylglyoxal-derived advanced glycation end-product in familial amyloidotic polyneuropathy. Biochem. J. 2005;385:339–345. doi: 10.1042/BJ20040833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzaga NA, Mecawi AS, Antunes-Rodrigues J, De Martinis BS, Padovan CM, Tirapelli CR. Ethanol withdrawal increases oxidative stress and reduces nitric oxide bioavailability in the vasculature of rats. Alcohol. 2015;49:47–56. doi: 10.1016/j.alcohol.2014.12.001. [DOI] [PubMed] [Google Scholar]
- Goodrich GS, Kabakov AY, Hameed MQ, Dhamne SC, Rosenberg PA, Rotenberg A. Ceftriaxone treatment after traumatic brain injury restores expression of the glutamate transporter, GLT-1, reduces regional gliosis, and reduces post-traumatic seizures in the rat. J. Neurotrauma. 2013;30:1434–1441. doi: 10.1089/neu.2012.2712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goryacheva AV, Kruglov SV, Pshennikova MG, Smirin BV, Malyshev IY, Barskov IV, Viktorov IV, Downey HF, Manukhina EB. Adaptation to intermittent hypoxia restricts nitric oxide overproduction and prevents beta-amyloid toxicity in rat brain. Nitric Oxide. 2010;23:289–299. doi: 10.1016/j.niox.2010.08.005. [DOI] [PubMed] [Google Scholar]
- Graef JD, Huitt TW, Nordskog BK, Hammarback JH, Godwin DW. Disrupted thalamic T-type Ca2+ channel expression and function during ethanol exposure and withdrawal. J. Neurophysiol. 2011;105:528–540. doi: 10.1152/jn.00424.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant KA, Valverius P, Hudspith M, Tabakoff B. Ethanol withdrawal seizures and the NMDA receptor complex. Eur. J. Pharmacol. 1990;176:289–296. doi: 10.1016/0014-2999(90)90022-x. [DOI] [PubMed] [Google Scholar]
- Grijalba MT, Vercesi AE, Schreier S. Ca2+-induced increased lipid packing and domain formation in submitochondrial particles. Biochemistry. 1999;38:13279–13287. doi: 10.1021/bi9828674. [DOI] [PubMed] [Google Scholar]
- Hack JB, Hoffmann RS, Nelson LS. Resistant alcohol withdrawal: does an unexpectedly large sedative requirement identify these patients early? J Med Toxicol. 2006;2:55–60. doi: 10.1007/BF03161171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halestrap AP, Brennerb C. The adenine nucleotide translocase: a central component of the mitochondrial permeability transition pore and key player in cell death. Curr. Med. Chem. 2003;10:1507–1525. doi: 10.2174/0929867033457278. [DOI] [PubMed] [Google Scholar]
- Hall W, Zador D. The alcohol withdrawal syndrome. Lancet. 1997;349:1897–1900. doi: 10.1016/S0140-6736(97)04572-8. [DOI] [PubMed] [Google Scholar]
- Harper C, Matsumoto I. Ethanol and brain damage. Curr. Opin. Pharmacol. 2005;5:73–78. doi: 10.1016/j.coph.2004.06.011. [DOI] [PubMed] [Google Scholar]
- He J, Crews FT. Increased MCP-1 and microglia in various regions of the human alcoholic brain. Exp. Neurol. 2008;210:349–358. doi: 10.1016/j.expneurol.2007.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Headley PM, Grillner S. Excitatory amino acids and synaptic transmission: the evidence for a physiological function. Trends Pharmacol. Sci. 1990;11:205–211. doi: 10.1016/0165-6147(90)90116-p. [DOI] [PubMed] [Google Scholar]
- Hebert LE, Scherr PA, Bienias JL, Bennett DA, Evans DA. Alzheimer disease in the US population. Arch. Neurol. 2003;60:1119–1122. doi: 10.1001/archneur.60.8.1119. [DOI] [PubMed] [Google Scholar]
- Hendricson AW, Maldve RE, Salinas AG, Theile JW, Zhang TA, Diaz LM, Morrisett RA. Aberrant synaptic activation of N-methyl-D-aspartate receptors underlies ethanol withdrawal hyperexcitability. J. Pharmacol. Exp. Ther. 2007;321:60–72. doi: 10.1124/jpet.106.111419. [DOI] [PubMed] [Google Scholar]
- Hernandez-Fonseca K, Cardenas-Rodriguez N, Pedraza-Chaverri J, Massieu L. Calcium-dependent production of reactive oxygen species is involved in neuronal damage induced during glycolysis inhibition in cultured hippocampal neurons. J. Neurosci. Res. 2008;86:1768–1780. doi: 10.1002/jnr.21634. [DOI] [PubMed] [Google Scholar]
- Herve C, Gaillard M, Roujas F, Huguenard P. Alcoholism in polytrauma. J. Trauma. 1986;26:1123–1126. doi: 10.1097/00005373-198612000-00013. [DOI] [PubMed] [Google Scholar]
- Hillbom M, Pieninkeroinen I, Leone M. Seizures in alcohol-dependent patients: epidemiology, pathophysiology and management. CNS Drugs. 2003;17:1013–1030. doi: 10.2165/00023210-200317140-00002. [DOI] [PubMed] [Google Scholar]
- Honjo K, Black SE, Verhoeff NPLG. Alzheimer’s disease, cerebrovascular disease, and the β-amyloid cascade. Can. J. Neurol. Sci. 2012;39:712–728. doi: 10.1017/s0317167100015547. [DOI] [PubMed] [Google Scholar]
- Huang MC, Chen CH, Peng FC, Tang SH, Chen CC. Alterations in oxidative stress status during early alcohol withdrawal in alcoholic patients. J Formos. Med. Assoc. 2009;108:560–569. doi: 10.1016/S0929-6646(09)60374-0. [DOI] [PubMed] [Google Scholar]
- Hughes JR. Alcohol withdrawal seizures. Epilepsy Behav. 2009;15:92–97. doi: 10.1016/j.yebeh.2009.02.037. [DOI] [PubMed] [Google Scholar]
- Jaatinen P, Riikonen J, Riihioja P, Kajander O, Hervonen A. Interaction of aging and intermittent ethanol exposure on brain cytochrome c oxidase activity levels. Alcohol. 2003;29:91–100. doi: 10.1016/s0741-8329(03)00002-8. [DOI] [PubMed] [Google Scholar]
- Huopaniemi L, Keist R, Randolph A, Certa U, Rudolph U. Diazepam-induced adaptive plasticity revealed by α1 GABAa receptor-specific expression profiling. J. Neurochem. 2004;88:1059–1067. doi: 10.1046/j.1471-4159.2003.02216.x. [DOI] [PubMed] [Google Scholar]
- John U, Rumpf HJ, Bischof G, Hapke U, Hanke M, Meyer C. Excess mortality of alcohol-dependent individuals after 14 years and mortality predictors based on treatment participation and severity of alcohol dependence. Alcohol Clin. Exp. Res. 2013;37:156–163. doi: 10.1111/j.1530-0277.2012.01863.x. [DOI] [PubMed] [Google Scholar]
- Ju X, Mallet RT, Downey HF, Metzger DB, Jung ME. Intermittent hypoxia conditioning protects mitochondrial cytochrome c oxidase of rat cerebellum from ethanol withdrawal stress. J. Appl. Physiol. 2012;112:1706–1714. doi: 10.1152/japplphysiol.01428.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung ME. In: Alcohol withdrawal from the angle of oxidative stress, in: Drug Overdoses and Alcohol Withdrawal: Prevalence, Trends and Prevention. Morales DP, editor. Nova; New York: 2016. pp. 49–84. [Google Scholar]
- Jung ME, Ju X, Metzger DB, Simpkins JW. Ethanol withdrawal hastens the aging of cytochrome c oxidase. Neurobiol. Aging. 2012;33:618.e21–e32. doi: 10.1016/j.neurobiolaging.2011.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung ME, Ju X, Simpkins JW, Metzger DB, Yan LJ, Wen Y. Ethanol withdrawal acts as an age-specific stressor to activate cerebellar p38 kinase. Neurobiol. Aging. 2011;32:2266–2278. doi: 10.1016/j.neurobiolaging.2010.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung ME, Metzger DB, Das HK. The Role of Presenilin-1 in the Excitotoxicity of Ethanol Withdrawal. J. Pharmacol. Exp. Ther. 2016;358:516–526. doi: 10.1124/jpet.116.233361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung ME, Simpkins JW, Wilson AM, Downey HF, Mallet RT. Intermittent hypoxia conditioning prevents behavioral deficit and brain oxidative stress in ethanol-withdrawn rats. J. Appl. Physiol. 2008;105:510–517. doi: 10.1152/japplphysiol.90317.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung ME, Watson DG, Wen Y, Simpkins JW. Role of protein kinase C in estrogen protection against apoptotic cerebellar cell death in ethanol-withdrawn rats. Alcohol. 2003;31:39–48. doi: 10.1016/j.alcohol.2003.07.004. [DOI] [PubMed] [Google Scholar]
- Kahlert S, Zundorf G, Reiser G. Glutamate-mediated influx of extracellular Ca2+ is coupled with reactive oxygen species generation in cultured hippocampal neurons but not in astrocytes. J. Neurosci. Res. 2005;79:262–271. doi: 10.1002/jnr.20322. [DOI] [PubMed] [Google Scholar]
- Kaja S, Sumien N, Shah VV, Puthawala I, Maynard AN, Khullar N, Payne AJ, Forster MJ, Koulen P. Loss of spatial memory, learning, and motor function during normal aging is accompanied by changes in brain presenilin 1 and 2 expression levels. Mol. Neurobiol. 2015;52:545–554. doi: 10.1007/s12035-014-8877-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalback W, Esh C, Castaño EM, Rahman A, Kokjohn T, Luehrs DC, Sue L, Cisneros R, Gerber F, Richardson C, Bohrmann B, Walker DG, Beach TG, Roher AE. Atherosclerosis, vascular amyloidosis and brain hypoperfusion in the pathogenesis of sporadic Alzheimer’s disease. Neurol. Res. 2004;26:525–539. doi: 10.1179/016164104225017668. [DOI] [PubMed] [Google Scholar]
- Kaspar JW, Niture SK, Jaiswal AK. Nrf2:INrf2 (Keap1) signaling in oxidative stress. Free Radic. Biol. Med. 2009;47:1304–1309. doi: 10.1016/j.freeradbiomed.2009.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsura M, Shibasaki M, Kurokawa K, Tsujimura A, Ohkuma S. Up-regulation of L-type high voltage-gated calcium channel subunits by sustained exposure to 1,4- and 1,5-benzodiazepines in cerebrocortical neurons. J. Neurochem. 2007;103:2518–2528. doi: 10.1111/j.1471-4159.2007.04984.x. [DOI] [PubMed] [Google Scholar]
- Khan GM, Smolders I, Lindekens H, Manil J, Ebinger G, Michotte Y. Effects of diazepam on extracellular brain neurotransmitters in pilocarpine-induced seizures in rats. J. Eur. Pharmacol. 1999;373:153–161. doi: 10.1016/s0014-2999(99)00209-5. [DOI] [PubMed] [Google Scholar]
- Kim H-C, Yamada K, Nitta A, Olariu A, Tran MH, Mizuno M, Nakajima A, Nagai T, Kamei H, Jhoo W-K, Im D-H, Shin E-J, Hjelle OP, Ottersen OP, Park SC, Kato K, Mirault M-E, Nabeshima T. Immunocytochemical evidence that amyloid β (1–42) impairs endogenous antioxidant systems in vivo. Neuroscience. 2003;119:399–419. doi: 10.1016/s0306-4522(02)00993-4. [DOI] [PubMed] [Google Scholar]
- Kim SW, Lim CM, Kim JB, Shin JH, Lee S, Lee M, Lee JK. Extracellular HMGB1 released by NMDA treatment confers neuronal apoptosis via RAGE-p38 MAPK/ERK signaling pathway. Neurotox. Res. 2011;20:159–169. doi: 10.1007/s12640-010-9231-x. [DOI] [PubMed] [Google Scholar]
- Kocahan S, Doğan Z. Mechanisms of Alzheimer’s disease pathogenesis and prevention: the brain, neural pathology, N-methyl-D-aspartate receptors, tau protein and other risk factors. Clin. Psychopharmacol. Neurosci. 2017;15:1–8. doi: 10.9758/cpn.2017.15.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotlinska J, Biala G, Rafalski P, Bochenski M, Danysz W. Effect of neramexane on ethanol dependence and reinforcement. Eur. J. Pharmacol. 2004;503:95–98. doi: 10.1016/j.ejphar.2004.09.036. [DOI] [PubMed] [Google Scholar]
- Kowaltowski AJ, Castilho RF, Grijalba MT, Bechara EJ, Vercesi AE. Effect of inorganic phosphate concentration on the nature of inner mitochondrial membrane alterations mediated by Ca2+ ions. A proposed model for phosphate-stimulated lipid peroxidation. J. Biol. Chem. 1996;271:2929–2934. doi: 10.1074/jbc.271.6.2929. [DOI] [PubMed] [Google Scholar]
- Krupitsky EM, Neznanova O, Masalov D, Burakov AM, Didenko T, Romanova T, Tsoy M, Bespalov A, Slavina TY, Grinenko AA, Petrakis IL, Pittman B, Gueorguieva R, Zvartau EE, Krystal JH. Effect of memantine on cue-induced alcohol craving in recovering alcohol-dependent patients. Am. J. Psychiatry. 2007;164:519–523. doi: 10.1176/ajp.2007.164.3.519. [DOI] [PubMed] [Google Scholar]
- Krystal JH, Petrakis IL, Mason G, Trevisan L, D’Souza DC. N-methyl-D-aspartate glutamate receptors and alcoholism: reward, dependence, treatment, and vulnerability. Pharmacol. Ther. 2003;99:79–94. doi: 10.1016/s0163-7258(03)00054-8. [DOI] [PubMed] [Google Scholar]
- Krystal JH, Webb E, Grillon C, Cooney N, Casal L, Morgan CA, 3rd, Southwick SM, Davis M, Charney DS. Evidence of acoustic startle hyperreflexia in recently detoxified early onset male alcoholics: modulation by yohimbine and m-chlorophenylpiperazine (mCPP) Psychopharmacology (Berl.) 1997;131:207–215. doi: 10.1007/s002130050285. [DOI] [PubMed] [Google Scholar]
- Kumar VB, Franko M, Banks WA, Kasinadhuni P, Farr SA, Vyas K, Choudhuri V, Morley JE. Increase in presenilin 1 (PS1) levels in senescence-accelerated mice (SAMP8) may indirectly impair memory by affecting amyloid precursor protein (APP) processing. J. Exp. Biol. 2009;212:494–498. doi: 10.1242/jeb.022780. [DOI] [PubMed] [Google Scholar]
- Läck AK, Diaz MR, Chappell A, DuBois DW, McCool BA. Chronic ethanol and withdrawal differentially modulate pre- and postsynaptic function at glutamatergic synapses in rat basolateral amygdala. J. Neurophysiol. 2007;98:3185–3196. doi: 10.1152/jn.00189.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lafon-Cazal M, Pietri S, Culcasi M, Bockaert J. NMDA-dependent superoxide production and neurotoxicity. Nature. 1993;364:535–537. doi: 10.1038/364535a0. [DOI] [PubMed] [Google Scholar]
- Lajtha ABMHS, Clarke DD. Metabolism and transport of carbohydrates and amino acids. In: Siegel GJ, Albers RW, Agranoff BW, Katzman R, editors. Basic Neurochemistry. 3. Little, Brown and Company; Boston, MA, USA: 1981. pp. 329–353. [Google Scholar]
- Lechtenberg R, Worner TM. Relative kindling effect of detoxification and non-detoxification admissions in alcoholics. Alcohol Alcohol. 1991;26:221–225. doi: 10.1093/oxfordjournals.alcalc.a045104. [DOI] [PubMed] [Google Scholar]
- Leggio L, Kenna GA, Swift RM. New developments for the pharmacological treatment of alcohol withdrawal syndrome. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2008;32:1106–1117. doi: 10.1016/j.pnpbp.2007.09.021. [DOI] [PubMed] [Google Scholar]
- Li T, Li YM, Ahn K, Price DL, Sisodia SS, Wong PC. Increased expression of PS1 is sufficient to elevate the level and activity of gamma-secretase in vivo. PLoS One. 2011;6:e28179. doi: 10.1371/journal.pone.0028179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Song D, Leng SX. Link between type 2 diabetes and Alzheimer’s disease: from epidemiology to mechanism and treatment. Clin. Interv. Aging. 2015;10:549–560. doi: 10.2147/CIA.S74042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li XH, Du LL, Cheng XS, Jiang X, Zhang Y, Lv BL, Liu R, Wang JZ, Zhou XW. Glycation exacerbates the neuronal toxicity of β-amyloid. Cell Death Dis. 2013;4:e673. doi: 10.1038/cddis.2013.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Licata SC, Rowlett JK. Abuse and dependence liability of benzodiazepine-type drugs: GABAa receptor modulation and beyond. Pharmacol. Biochem. Behav. 2008;90:74–89. doi: 10.1016/j.pbb.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipton P. Ischemic cell death in brain neurons. Physiol. Rev. 1999;79:1431–1568. doi: 10.1152/physrev.1999.79.4.1431. [DOI] [PubMed] [Google Scholar]
- Little HJ, Dolin SJ, Halsey MJ. Calcium channel antagonists decrease the ethanol withdrawal syndrome. Life Sci. 1986;39:2059–2065. doi: 10.1016/0024-3205(86)90356-5. [DOI] [PubMed] [Google Scholar]
- Lovinger DM. Excitotoxicity and alcohol-related brain damage. Alcohol Clin. Exp. Res. 1993;17:19–27. doi: 10.1111/j.1530-0277.1993.tb00720.x. [DOI] [PubMed] [Google Scholar]
- Lu FP, Lin KP, Kuo HK. Diabetes and the risk of multi-system aging phenotypes: a systematic review and meta-analysis. PLoS ONE. 2009;4:e4144. doi: 10.1371/journal.pone.0004144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lue LF, Yan SD, Stern DM, Walker DG. Preventing activation of receptor for advanced glycation endproducts in Alzheimer’s disease. Curr. Drug Targets CNS Neurol. Disord. 2005;4:249–266. doi: 10.2174/1568007054038210. [DOI] [PubMed] [Google Scholar]
- Lyamina NP, Lyamina SV, Senchiknin VN, Mallet RT, Downey HF, Manukhina EB. Normobaric hypoxia conditioning reduces blood pressure and normalizes nitric oxide synthesis in patients with arterial hypertension. J. Hypertens. 2011;29:2265–2272. doi: 10.1097/HJH.0b013e32834b5846. [DOI] [PubMed] [Google Scholar]
- Mackic JB, Stins M, McComb JG, Calero M, Ghiso J, Kim KS, Yan SD, Stern D, Schmidt AM, Frangione B, Zlokovic BV. Human blood-brain barrier receptors for Alzheimer’s amyloid-beta 1–40. Asymmetrical binding, endocytosis, and transcytosis at the apical side of brain microvascular endothelial cell monolayer. J. Clin. Invest. 1998;102:734–743. doi: 10.1172/JCI2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malcolm RJ. GABA systems, benzodiazepines, and substance dependence. J. Clin. Psychiatry. 2003;64(Suppl. 3):36–40. [PubMed] [Google Scholar]
- Mallet RT, Ryou MG, Williams AG, Jr, Howard L, Downey HF. β1-Adrenergic receptor antagonism abrogates cardioprotective effects of intermittent hypoxia. Basic Res. Cardiol. 2006;101:436–446. doi: 10.1007/s00395-006-0599-y. [DOI] [PubMed] [Google Scholar]
- Manukhina EB, Belkina LM, Terekhina OL, Abramochkin DV, Smirnova EA, Budanova OP, Mallet RT, Downey HF. Normobaric, intermittent hypoxia conditioning is cardio- and vasoprotective in rats. Exp. Biol. Med. 2013;238:1413–1420. doi: 10.1177/1535370213508718. [DOI] [PubMed] [Google Scholar]
- Manukhina EB, Downey HF, Shi X, Mallet RT. Intermittent hypoxia training protects cerebrovascular function in Alzheimer’s disease. Exp. Biol. Med. 2016;241:1351–1363. doi: 10.1177/1535370216649060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manukhina EB, Goryacheva AV, Barskov IV, Viktorov IV, Guseva AA, Pshennikova MG, Khomenko IP, Mashina SY, Pokidyshev DA, Malyshev IY. Prevention of neurodegenerative damage to the brain in rats in experimental Alzheimer’s disease by adaptation to hypoxia. Neurosci. Behav. Physiol. 2010;40:737–743. doi: 10.1007/s11055-010-9320-6. [DOI] [PubMed] [Google Scholar]
- Marin JM, Carrizo SJ, Vicente E, Agusti AG. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet. 2005;365:1046–1053. doi: 10.1016/S0140-6736(05)71141-7. [DOI] [PubMed] [Google Scholar]
- Mateika JH, El-Chami M, Shaheen D, Ivers B. Intermittent hypoxia: a low-risk research tool with therapeutic value in humans. J. Appl. Physiol. 2015;118:520–532. doi: 10.1152/japplphysiol.00564.2014. [DOI] [PubMed] [Google Scholar]
- Mayo-Smith MF. Pharmacological management of alcohol withdrawal: a meta-analysis and evidence-based practice guideline. JAMA. 1997;278:144–151. doi: 10.1001/jama.278.2.144. [DOI] [PubMed] [Google Scholar]
- Megarbane B, Lesguillons N, Galliot-Guilley M, Borron SW, Trout H, Decleves X, Risede P, Monier C, Boschi G, Baud FJ. Cerebral and plasma kinetics of a high dose of midazolam and correlations with its respiratory effects in rats. Toxicol. Lett. 2005;159:22–31. doi: 10.1016/j.toxlet.2005.04.003. [DOI] [PubMed] [Google Scholar]
- Meldrum BS. Glutamate as a neurotransmitter in the brain: review of physiology and pathology. J. Nutr. 2000;130:1007S–1015S. doi: 10.1093/jn/130.4.1007S. [DOI] [PubMed] [Google Scholar]
- Miguel-Hidalgo JJ. Withdrawal from free-choice ethanol consumption results in increased packing density of glutamine synthetase-immunoreactive astrocytes in the prelimbic cortex of alcohol-preferring rats. Alcohol Alcohol. 2006;41:379–385. doi: 10.1093/alcalc/agl006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miguel-Hidalgo JJ. The role of glial cells in drug abuse. Curr. Drug Abuse Rev. 2009;2:72–82. [PubMed] [Google Scholar]
- Mokdad AH, Marks JS, Stroup DF, Gerberding JL. Actual causes of death in the United States, 2000. JAMA. 2004;291:1238–1245. doi: 10.1001/jama.291.10.1238. [DOI] [PubMed] [Google Scholar]
- Monte Secades R, Rabuñal Rey R, Paz Fuentes F, Pazos Ferro A, Pértega Díaz S, Bal Alvaredo M, Casariego Vales E. Course of alcohol withdrawal syndrome in a general hospital. Adicciones. 2010;22:141–146. [PubMed] [Google Scholar]
- Moriguchi T, Toyoshima F, Gotoh Y, Iwamatsu A, Irie K, Mori E, Kuroyanagi N, Hagiwara M, Matsumoto K, Nishida E. Purification and identification of a major activator for p38 from osmotically shocked cells. Activation of mitogen-activated protein kinase kinase 6 by osmotic shock, tumor necrosis factor-alpha, and H2O2. J. Biol. Chem. 1996;271:26981–26988. doi: 10.1074/jbc.271.43.26981. [DOI] [PubMed] [Google Scholar]
- Mozaffarian D, Benjamin EJ, Go AS, et al. Heart disease and stroke statistics - 2016 update: a report from the American Heart Association. Circulation. 2016;133:e38–e360. doi: 10.1161/CIR.0000000000000350. [DOI] [PubMed] [Google Scholar]
- Mueller TI, Pagano ME, Rodriguez BF, Bruce SE, Stout RL, Keller MB. Long-term use of benzodiazepines in participants with comorbid anxiety and alcohol use disorders. Alcohol Clin. Exp. Res. 2005;29:1411–1418. doi: 10.1097/01.alc.0000175016.01790.f1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Münch G, Apelt J, Rosemarie-Kientsch-Engel Stahl P, Lüth HJ, Schliebs R. Advanced glycation endproducts and pro-inflammatory cytokines in transgenic Tg2576 mice with amyloid plaque pathology. J. Neurochem. 2003;86:283–289. doi: 10.1046/j.1471-4159.2003.01837.x. [DOI] [PubMed] [Google Scholar]
- Myrick H, Anton RF. Clinical management of alcohol withdrawal. CNS Spectr. 2000;5:22–32. doi: 10.1017/s1092852900012797. [DOI] [PubMed] [Google Scholar]
- Nagy J. Alcohol related changes in regulation of NMDA receptor functions. Curr. Neuropharmacol. 2008;6:39–54. doi: 10.2174/157015908783769662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy J, Laszlo L. Increased sensitivity to NMDA is involved in alcohol-withdrawal induced cytotoxicity observed in primary cultures of cortical neurones chronically pre-treated with ethanol. Neurochem. Int. 2002;40:585–591. doi: 10.1016/s0197-0186(01)00131-0. [DOI] [PubMed] [Google Scholar]
- Nakanishi S. Molecular diversity of glutamate receptors and implications for brain function. Science. 1992;258:597–603. doi: 10.1126/science.1329206. [DOI] [PubMed] [Google Scholar]
- Nath V, Reneau JC, Dertien JS, Agrawal RG, Guerra I, Bhakta Y, Busari K, Neumann MK, Bergeson SE, Popp RL. An in vitro model for studying the effects of continuous ethanol exposure on N-methyl-D-aspartate receptor function. Alcohol. 2012;46:3–16. doi: 10.1016/j.alcohol.2011.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson DE, Naimi TS, Brewer RD, Roeber J. US state alcohol sales compared to survey data, 1993–2006. Addiction. 2010;105:1589–1596. doi: 10.1111/j.1360-0443.2010.03007.x. [DOI] [PubMed] [Google Scholar]
- N’Gouemo P, Morad M. Ethanol withdrawal seizure susceptibility is associated with upregulation of L- and P-type Ca2+ channel currents in rat inferior colliculus neurons. Neuropharmacology. 2003;45:429–437. doi: 10.1016/s0028-3908(03)00191-6. [DOI] [PubMed] [Google Scholar]
- Nicholls DG, Johnson-Cadwell L, Vesce S, Jekabsons M, Yadava N. Bioenergetics of mitochondria in cultured neurons and their role in glutamate excitotoxicity. J. Neurosci. Res. 2007;85:3206–3212. doi: 10.1002/jnr.21290. [DOI] [PubMed] [Google Scholar]
- Nikolakopoulou AM, Georgakopoulos A, Robakis NK. Presenilin 1 promotes trypsin-induced neuroprotection via the PAR2/ERK signaling pathway. Neurobiol. Aging. 2016;42:41–49. doi: 10.1016/j.neurobiolaging.2016.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Núñez MJ, Novío S, Amigo G, Freire-Garabal M. The antioxidant potential of alprazolam on the redox status of peripheral blood leukocytes in restraint-stressed mice. Life Sci. 2011;89:650–654. doi: 10.1016/j.lfs.2011.07.027. [DOI] [PubMed] [Google Scholar]
- O’Brien CP. Drug addiction and drug abuse. In: Hardman JG, Limbard LE, Gilman AG, editors. Goodman and Gilman’s the Pharmacological Basis of Therapeutics. 9. McGraw-Hill; New York: 1996. pp. 621–642. [Google Scholar]
- O’Connor PG, Schottenfeld RS. Patients with alcohol problems. N. Engl. J. Med. 1998;338:592–602. doi: 10.1056/NEJM199802263380907. [DOI] [PubMed] [Google Scholar]
- Oscar-Berman M, Shagrin B, Evert DL, Epstein C. Impairments of brain and behavior: the neurological effects of alcohol. Alcohol Health Res. World. 1997;21:65–75. [PMC free article] [PubMed] [Google Scholar]
- Ostrom M, Eriksson A. Single-vehicle crashes and alcohol: a retrospective study of passenger car fatalities in northern Sweden. Accid. Anal. Prev. 1993;25:171–176. doi: 10.1016/0001-4575(93)90057-4. [DOI] [PubMed] [Google Scholar]
- Panza F, Capurso C, Solfrizzi V. Alcohol use, thiamine deficiency, and cognitive impairment. JAMA. 2008;299:2853–2854. doi: 10.1001/jama.299.24.2853. [DOI] [PubMed] [Google Scholar]
- Parish JM, Somers VK. Obstructive sleep apnea and cardiovascular disease. Mayo Clin. Proc. 2004;79:1036–1046. doi: 10.4065/79.8.1036. [DOI] [PubMed] [Google Scholar]
- Pepe S. Mitochondrial function in ischaemia and reperfusion of the ageing heart. Clin. Exp. Pharmacol. Physiol. 2000;27:745–750. doi: 10.1046/j.1440-1681.2000.03326.x. [DOI] [PubMed] [Google Scholar]
- Phillips SC, Cragg BG. Alcohol withdrawal causes a loss of cerebellar Purkinje cells in mice. J. Stud. Alcohol. 1984;45:475–480. doi: 10.15288/jsa.1984.45.475. [DOI] [PubMed] [Google Scholar]
- Prabhakar NR, Kumar GK, Nanduri J, Semenza GL. ROS signaling in systemic and cellular responses to chronic intermittent hypoxia. Antioxid. Redox Signal. 2007;9:1397–1403. doi: 10.1089/ars.2007.1732. [DOI] [PubMed] [Google Scholar]
- Prokai-Tatrai K, Prokai L, Simpkins JW, Jung ME. Phenolic compounds protect cultured hippocampal neurons against ethanol-withdrawal induced oxidative stress. Int. J. Mol. Sci. 2009;10:1773–1787. doi: 10.3390/ijms10041773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiang M, Denny AD, Ticku MK. Chronic intermittent ethanol treatment selectively alters N-methyl-D-aspartate receptor subunit surface expression in cultured cortical neurons. Mol. Pharmacol. 2007;72:95–102. doi: 10.1124/mol.106.033043. [DOI] [PubMed] [Google Scholar]
- Qiu C, Winblad B, Fratiglioni L. The age-dependent relation of blood pressure to cognitive function and dementia. Lancet Neurol. 2005;4:487–499. doi: 10.1016/S1474-4422(05)70141-1. [DOI] [PubMed] [Google Scholar]
- Ramesh BN, Rao TS, Prakasam A, Sambamurti K, Rao KS. Neuronutrition and Alzheimer’s disease. J. Alzheimers Dis. 2010;19:1123–1139. doi: 10.3233/JAD-2010-1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapizzi E, Pinton P, Szabadkai G, Wieckowski MR, Vandecasteele G, Baird G, Tuft RA, Fogarty KE, Rizzuto R. Recombinant expression of the voltage-dependent anion channel enhances the transfer of Ca2+ microdomains to mitochondria. J. Cell Biol. 2002;159:613–624. doi: 10.1083/jcb.200205091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rewal M, Wen Y, Wilson A, Simpkins JW, Jung ME. Role of parvalbumin in estrogen protection from ethanol withdrawal syndrome. Alcohol Clin. Exp. Res. 2005;29:1837–1844. doi: 10.1097/01.alc.0000183013.64829.2e. [DOI] [PubMed] [Google Scholar]
- Reynolds AR, Berry JN, Sharrett-Field L, Prendergast MA. Ethanol withdrawal is required to produce persisting N-methyl-D-aspartate receptor-dependent hippocampal cytotoxicity during chronic intermittent ethanol exposure. Alcohol. 2015;49:219–227. doi: 10.1016/j.alcohol.2015.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riegle MA, Masicampo ML, Caulder EH, Godwin DW. Ethosuximide reduces electrographical and behavioral correlates of alcohol withdrawal seizure in DBA/2J mice. Alcohol. 2014;48:445–453. doi: 10.1016/j.alcohol.2014.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riegle MA, Masicampo ML, Shan HQ, Xu V, Godwin DW. Ethosuximide Reduces Mortality and Seizure Severity in Response to Pentylenetetrazole Treatment During Ethanol Withdrawal. Alcohol Alcohol. 2015;50:501–508. doi: 10.1093/alcalc/agv033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rimondini R, Arlinde C, Sommer W, Heilig M. Long-lasting increase in voluntary ethanol consumption and transcriptional regulation in the rat brain after intermittent exposure to alcohol. FASEB J. 2002;16:27–35. doi: 10.1096/fj.01-0593com. [DOI] [PubMed] [Google Scholar]
- Rizzuto R, Marchi S, Bonora M, Aguiari P, Bononi A, De Stefani D, Giorgi C, Leo S, Rimessi A, Siviero R, Zecchini E, Pinton P. Ca2+ transfer from the ER to mitochondria: when, how and why. Biochim Biophys Acta. 2009;1787:1342–1351. doi: 10.1016/j.bbabio.2009.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roher AE, Tyas SL, Maarouf CL, Daugs ID, Kokjohn TA, Emmerling MR, Garami Z, Belohlavek M, Sabbagh MN, Sue LI, Beach TG. Intracranial atherosclerosis as a contributing factor to Alzheimer’s disease dementia. Alzheimers Dement. 2011;7:436–444. doi: 10.1016/j.jalz.2010.08.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossetti ZL, Carboni S. Ethanol withdrawal is associated with increased extracellular glutamate in the rat striatum. Eur. J. Pharmacol. 1995;283:177–183. doi: 10.1016/0014-2999(95)00344-k. [DOI] [PubMed] [Google Scholar]
- Rosenbloom A. Emerging treatment options in the alcohol withdrawal syndrome. J. Clin. Psychiatry. 1988;49:28–32. [PubMed] [Google Scholar]
- Rossetti ZL, Carboni S. Ethanol withdrawal is associated with increased extracellular glutamate in the rat striatum. Eur. J. Pharmacol. 1995;283:177–183. doi: 10.1016/0014-2999(95)00344-k. [DOI] [PubMed] [Google Scholar]
- Rothstein JD, Patel S, Regan MR, Haenggeli C, Huang YH, Bergles DE, Jin L, Dykes Hoberg M, Vidensky S, Chung DS, Toan SV, Bruijn LI, Su ZZ, Gupta P, Fisher PB. β-lactam antibiotics offer neuroprotection by increasing glutamate transporter expression. Nature. 2005;433:73–77. doi: 10.1038/nature03180. [DOI] [PubMed] [Google Scholar]
- Ryou MG, Mallet RT, Metzger DB, Jung ME. Intermittent hypoxia training blunts cerebrocortical presenilin 1 overexpression and amyloid β accumulation in ethanol-withdrawn rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2017;313:R10–R18. doi: 10.1152/ajpregu.00050.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachdeva A, Choudhary M, Chandra M. Alcohol withdrawal syndrome: benzodiazepines and beyond. J. Clin. Diagn. Res. 2015;9:VE01–VE07. doi: 10.7860/JCDR/2015/13407.6538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacks JJ, Gonzales KR, Bouchery EE, Tomedi LE, Brewer RD. 2010 National and state costs of excessive alcohol consumption. Am. J. Prev. Med. 2015;49:e73–79. doi: 10.1016/j.amepre.2015.05.031. [DOI] [PubMed] [Google Scholar]
- Sakellaridis N, Mangoura D, Masserrano JM, Detsis V, Leoni CJ, Deitrich R, Vernadakis A. Developmental profile of glutamine synthetase in lines of mice bred for ethanol sensitivity. J. Neurosci. Res. 1989;24:391–397. doi: 10.1002/jnr.490240308. [DOI] [PubMed] [Google Scholar]
- Sander M, Neumann T, von Dossow V, Schonfeld H, Lau A, Eggers V, Spies C. Alcohol use disorder: risks in anesthesia and intensive care medicine. Internist (Berl.) 2006;47:332–338. doi: 10.1007/s00108-006-1588-9. [DOI] [PubMed] [Google Scholar]
- Sarff M, Gold JA. Alcohol withdrawal syndromes in the intensive care unit. Crit. Care Med. 2010;38:S494–S501. doi: 10.1097/CCM.0b013e3181ec5412. [DOI] [PubMed] [Google Scholar]
- Seidemann T, Spies C, Morgenstern R, Wernecke KD, Netzhammer N. Influence of volatile anesthesia on the release of glutamate and other amino acids in the nucleus accumbens in a rat model of alcohol withdrawal: a pilot study. PLoS One. 2017;12:e0169017. doi: 10.1371/journal.pone.0169017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selivanov VA, Votyakova TV, Pivtoraiko VN, Zeak J, Sukhomlin T, Trucco M, Roca J, Cascante M. Reactive oxygen species production by forward and reverse electron fluxes in the mitochondrial respiratory chain. PLoS Comput. Biol. 2011;7:e1001115. doi: 10.1371/journal.pcbi.1001115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serebrovskaya TV, Manukhina EB, Smith ML, Downey HF, Mallet RT. Intermittent hypoxia: cause of or therapy for systemic hypertension? Exp. Biol. Med. 2008;233:627–650. doi: 10.3181/0710-MR-267. [DOI] [PubMed] [Google Scholar]
- Serebrovskaya TV, Xi L. Intermittent hypoxia training as non-pharmacologic therapy for cardiovascular diseases: practical analysis on methods and equipment. Exp. Biol. Med. 2016;241:1708–1723. doi: 10.1177/1535370216657614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibata S, Shindou T, Tominaga K, Watanabe S. Calcium channel blockers improve hypoxia/hypoglycemia-induced impairment of rat hippocampal 2-deoxyglucose uptake in vitro after ethanol withdrawal. Brain Res. 1995;673:320–324. doi: 10.1016/0006-8993(94)01466-u. [DOI] [PubMed] [Google Scholar]
- Skrzypiec AE, Maiya R, Chen Z, Pawlak R, Strickland S. Plasmin-mediated degradation of laminin gamma-1 is critical for ethanol-induced neurodegeneration. Biol. Psychiatry. 2009;66:785–794. doi: 10.1016/j.biopsych.2009.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith TL. Regulation of glutamate uptake in astrocytes continuously exposed to ethanol. Life Sci. 1997;61:2499–2505. doi: 10.1016/s0024-3205(97)00985-5. [DOI] [PubMed] [Google Scholar]
- Soderstrom CA, Dischinger PC, Smith GS, McDuff DR, Hebel JR, Gorelick DA. Psychoactive substance dependence among trauma center patients. JAMA. 1992;267:2756–2759. [PubMed] [Google Scholar]
- Sommer B, Burnashev N, Verdoorn TA, Keinanen K, Sakmann B, Seeburg PH. A glutamate receptor channel with high affinity for domoate and kainate. EMBO J. 1992;11:1651–1656. doi: 10.1002/j.1460-2075.1992.tb05211.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorg O, Horn TF, Yu N, Gruol DL, Bloom FE. Inhibition of astrocyte glutamate uptake by reactive oxygen species: role of antioxidant enzymes. Mol. Med. 1997;3:431–440. [PMC free article] [PubMed] [Google Scholar]
- Spies C, Tonnesen H, Andreasson S, Helander A, Conigrave K. Perioperative morbidity and mortality in chronic alcoholic patients. Alcohol Clin. Exp. Res. 2001;25:164S–170S. doi: 10.1097/00000374-200105051-00028. [DOI] [PubMed] [Google Scholar]
- Spies CD, Dubisz N, Funk W, Blum S, Muller C, Rommelspacher H, Brummer G, Specht M, Hannemann L, Striebel HW, et al. Prophylaxis of alcohol withdrawal syndrome in alcohol-dependent patients admitted to the intensive care unit after tumour resection. Br. J. Anaesth. 1995;75:734–739. doi: 10.1093/bja/75.6.734. [DOI] [PubMed] [Google Scholar]
- Spies CD, Neuner B, Neumann T, Blum S, Muller C, Rommelspacher H, Rieger A, Sanft C, Specht M, Hannemann L, Striebel HW, Schaffartzik W. Intercurrent complications in chronic alcoholic men admitted to the intensive care unit following trauma. Intensive Care Med. 1996;22:286–293. doi: 10.1007/BF01700448. [DOI] [PubMed] [Google Scholar]
- Spies CD, Rommelspacher H. Alcohol withdrawal in the surgical patient: prevention and treatment. Anesth. Analg. 1999;88:946–954. doi: 10.1097/00000539-199904000-00050. [DOI] [PubMed] [Google Scholar]
- Stahre M, Roeber J, Kanny D, Brewer RD, Zhang X. Contribution of excessive alcohol consumption to deaths and years of potential life lost in the United States. Prev. Chronic Dis. 2014;11:E109. doi: 10.5888/pcd11.130293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley JL, Lincoln RJ, Brown TA, McDonald LM, Dawson GR, Reynolds DS. The mouse beam walking assay offers improved sensitivity over the mouse rotarod in determining motor coordination deficits induced by benzodiazepines. J. Psychopharmacol. 2005;19:221–227. doi: 10.1177/0269881105051524. [DOI] [PubMed] [Google Scholar]
- Stephens DN, Ripley TL, Borlikova G, Schubert M, Albrecht D, Hogarth L, Duka T. Repeated ethanol exposure and withdrawal impairs human fear conditioning and depresses long-term potentiation in rat amygdala and hippocampus. Biol. Psychiatry. 2005;58:392–400. doi: 10.1016/j.biopsych.2005.04.025. [DOI] [PubMed] [Google Scholar]
- Stuart R. Insertion of proteins into the inner membrane of mitochondria: the role of the Oxa1 complex. Biochim. Biophys. Acta. 2002;1592:79–87. doi: 10.1016/s0167-4889(02)00266-5. [DOI] [PubMed] [Google Scholar]
- Sweatt JD. The neuronal MAP kinase cascade: a biochemical signal integration system subserving synaptic plasticity and memory. J. Neurochem. 2001;76:1–10. doi: 10.1046/j.1471-4159.2001.00054.x. [DOI] [PubMed] [Google Scholar]
- Tajes M, Guivernau B, Ramos-Fernández E, Bosch-Morató M, Palomer E, Guix FX, Muñoz FJ. The pathophysiology of triose phosphate isomerase dysfunction in Alzheimer’s disease. Histol. Histopathol. 2013;28:43–51. doi: 10.14670/HH-28.43. [DOI] [PubMed] [Google Scholar]
- Tampellini D, Capetillo-Zarate E, Dumont M, Huang Z, Yu F, Lin MT, Gouras GK. Effects of synaptic modulation on beta-amyloid, synaptophysin, and memory performance in Alzheimer's disease transgenic mice. J. Neurosci. 2010;30:14299–14304. doi: 10.1523/JNEUROSCI.3383-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tandon A, Fraser P. The presenilins. Genome Biol. 2002;3 doi: 10.1186/gb-2002-3-11-reviews3014. reviews3014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thakur MK, Ghosh S. Age and sex dependent alteration in presenilin expression in mouse cerebral cortex. Cell Mol. Neurobiol. 2007;27:1059–1067. doi: 10.1007/s10571-007-9214-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian H, Ye X, Hou X, Yang X, Yang J, Wu C. SVCT2, a potential therapeutic target, protects against oxidative stress during ethanol-induced neurotoxicity via JNK/p38 MAPKs, NF-kappaB and miRNA125a-5p. Free Radic. Biol. Med. 2016;96:362–373. doi: 10.1016/j.freeradbiomed.2016.03.039. [DOI] [PubMed] [Google Scholar]
- Townshend JM, Duka T. Binge drinking, cognitive performance and mood in a population of young social drinkers. Alcohol Clin. Exp. Res. 2005;29:317–325. doi: 10.1097/01.alc.0000156453.05028.f5. [DOI] [PubMed] [Google Scholar]
- Trevisan LA, Boutros N, Petrakis IL, Krystal JH. Complications of alcohol withdrawal: pathophysiological insights. Alcohol Health Res. World. 1998;22:61–66. [PMC free article] [PubMed] [Google Scholar]
- Uusaro A, Parviainen I, Tenhunen JJ, Ruokonen E. The proportion of intensive care unit admissions related to alcohol use: a prospective cohort study. Acta Anaesthesiol. Scand. 2005;49:1236–1240. doi: 10.1111/j.1399-6576.2005.00839.x. [DOI] [PubMed] [Google Scholar]
- Valenzuela CF. Alcohol and neurotransmitter interactions. Alcohol Health Res. World. 1997;21:144–148. [PMC free article] [PubMed] [Google Scholar]
- Vandesquille M, Carrié I, Louis C, Beracochea D, Lestage P. Effects of positive modulators of α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA)-type glutamate receptors in a benzodiazepine-induced deficit of spatial discrimination in mice. J. Psychopharmacol. 2012;26:845–856. doi: 10.1177/0269881111416692. [DOI] [PubMed] [Google Scholar]
- Vargas MR, Johnson JA. The Nrf2-ARE cytoprotective pathway in astrocytes. Expert Rev. Mol. Med. 2009;11:e17. doi: 10.1017/S1462399409001094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkataraman A, Kalk N, Sewell G, Ritchie CW, Lingford-Hughes A. Alcohol and Alzheimer’s disease - does alcohol dependence contribute to β-amyloid deposition, neuroinflammation and neurodegeneration in Alzheimer’s disease? Alcohol Alcohol. 2017;52:151–158. doi: 10.1093/alcalc/agw101. [DOI] [PubMed] [Google Scholar]
- Vorobjev IA, Zorov DB. Diazepam inhibits cell respiration and induces fragmentation of mitochondrial reticulum. FEBS. Lett. 1983;163:311–314. doi: 10.1016/0014-5793(83)80842-4. [DOI] [PubMed] [Google Scholar]
- Walter HJ, Messing RO. Regulation of neuronal voltage-gated calcium channels by ethanol. Neurochem. Int. 1999;35:95–101. doi: 10.1016/s0197-0186(99)00050-9. [DOI] [PubMed] [Google Scholar]
- Watkins JC, Pook PC, Sunter DC, Davies J, Honore T. Experiments with kainate and quisqualate agonists and antagonists in relation to the sub-classification of 'non-NMDA' receptors. Adv. Exp. Med. Biol. 1990;268:49–55. doi: 10.1007/978-1-4684-5769-8_6. [DOI] [PubMed] [Google Scholar]
- Weinberg JA, Magnotti LJ, Fischer PE, Edwards NM, Schroeppel T, Fabian TC, Croce MA. Comparison of intravenous ethanol versus diazepam for alcohol withdrawal prophylaxis in the trauma ICU: results of a randomized trial. J. Trauma. 2008;64:99–104. doi: 10.1097/TA.0b013e31815eb12a. [DOI] [PubMed] [Google Scholar]
- Weissenborn R, Duka T. Acute alcohol effects on cognitive function in social drinkers: their relationship to drinking habits. Psychopharmacology (Berl.) 2003;165:306–312. doi: 10.1007/s00213-002-1281-1. [DOI] [PubMed] [Google Scholar]
- Whittington MA, Little HJ. Nitrendipine, given during drinking, decreases the electrophysiological changes in the isolated hippocampal slice, seen during ethanol withdrawal. Br. J. Pharmacol. 1991;103:1677–1684. doi: 10.1111/j.1476-5381.1991.tb09846.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiggins WF, Graef JD, Huitt TW, Godwin DW. Ethosuximide reduces ethanol withdrawal-mediated disruptions in sleep-related EEG patterns. Alcohol Clin. Exp. Res. 2013;37:372–382. doi: 10.1111/j.1530-0277.2012.01938.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilbur KM, Bernheim F, Shapiro OW. The thiobarbituric acid reagent as a test for the oxidation of unsaturated fatty acids by various agents. Arch. Biochem. Biophys. 1949;24:305–313. [PubMed] [Google Scholar]
- Wood JD, Watson WJ. Lipid peroxidation (thiobarbituric acid reacting material) and enzyme inhibition in rat brain homogenates exposed to oxygen at high pressure. Can. J. Physiol. Pharmacol. 1967;45:752–755. doi: 10.1139/y67-089. [DOI] [PubMed] [Google Scholar]
- Yabroff KR, Lund J, Kepka D, Mariotto A. Economic burden of cancer in the United States: estimates, projections, and future research. Cancer Epidemiol. Biomarkers Prev. 2011;20:2006–2014. doi: 10.1158/1055-9965.EPI-11-0650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan LJ. Analysis of oxidative modification of proteins. Curr. Protoc. Protein Sci. Chapter. 2009;14 doi: 10.1002/0471140864.ps1404s55. Unit14 14. [DOI] [PubMed] [Google Scholar]
- Yan LJ, Christians ES, Liu L, Xiao X, Sohal RS, Benjamin IJ. Mouse heat shock transcription factor 1 deficiency alters cardiac redox homeostasis and increases mitochondrial oxidative damage. EMBO J. 2002;21:5164–5172. doi: 10.1093/emboj/cdf528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo SAPB, Park GS, Koh HS, Lee MS, Ryu SH, Miyazawa K, Park SH, Cho CS, Kim WU. Calcineurin is expressed and plays a critical role in inflammatory arthritis. J. Immunol. 2006;177:2681–2690. doi: 10.4049/jimmunol.177.4.2681. [DOI] [PubMed] [Google Scholar]
- Young BW, Sengelaub DR, Steinmetz JE. MK-801 administration during neonatal ethanol withdrawal attenuates interpositus cell loss and juvenile eyeblink conditioning deficits. Alcohol. 2010;44:359–369. doi: 10.1016/j.alcohol.2009.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zilkens RR, Bruce DG, Duke J, Spilsbury K, Semmens JB. Severe psychiatric disorders in mid-life and risk of dementia in late-life (age 65–84 years): a population based case-control study. Curr. Alzheimers Res. 2014;11:681–693. doi: 10.2174/1567205011666140812115004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zong P, Setty S, Sun W, Martinez R, Tune JD, Ehrenburg IV, Tkatchouk EN, Mallet RT, Downey HF. Intermittent hypoxic training protects canine myocardium from infarction. Exp. Biol. Med. 2004;229:806–812. doi: 10.1177/153537020422900813. [DOI] [PubMed] [Google Scholar]