Abstract
The ability of neurons to modify or remodel their synaptic connectivity is critical for the function of neural circuitry throughout the life of an animal. Understanding the mechanisms underlying neuronal structural changes is central to our knowledge of how the nervous system is shaped for complex behaviors and how it further adapts to developmental and environmental demands. Caenorhabditis elegans provides a powerful model for examining developmental processes and for discovering mechanisms controlling neural plasticity. Recent findings have identified conserved themes underlying neural plasticity in development and under environmental stress.
Keywords: synapse remodeling, locomotor circuit, MYRF, ER-bound transcriptional factor, neuronal remodeling, dendritic remodeling, circuit rewiring, trans-differentiation
Introduction
The nematode C. elegans is well-known for its invariant lineage and precise connectome, both of which can be viewed in living animals at single cell and single synapse resolution [1, 6]. Decades of research on C. elegans have profoundly affected our understanding of the development and function of the nervous system. Recent technological advance has also enabled automation of in vivo visualization and quantitative analyses of neuronal connections and activity [2–5]. Defying assumptions on stereotypic development, these findings have revealed a surprising degree of structural plasticity that is critical for the establishment and maintenance of stereotypic connections in the C. elegans nervous system at different life stages and under harsh environments. Here, we review mechanistic insights from selected examples.
Developmental plasticity in the locomotor circuit
Following embryogenesis, C. elegans goes through four consecutive larval (L) stages to become fertile adult hermaphrodites, increasing body size by fivefold. An adult hermaphrodite consists of 302 neurons and 56 glial cells [6]. Locomotion of C. elegans is propelled by coordinated contraction and relaxation of ventral and dorsal body wall muscles along the anterior-posterior axis. The body wall muscles are innervated by eight distinct classes of motor neurons, cell bodies of which are intermingled and lined up primarily in the ventral midline of the body. In young L1 (larval stage 1) animals the ventral nerve cord contains only 22 motor neurons in three classes [7]. A series of cell divisions at the end of the L1 stage produces five new classes of motor neurons, increasing the total number of motor neurons to 75 in adults [8]. These post-embryonically-born neurons differentiate and integrate into the juvenile motor circuit to ensure sinusoidal locomotion in adults.
Complete reversal of axo-dendro polarity of Dorsal-type D GABAergic motor neurons
A striking example of circuit plasticity in C. elegans concerns six Dorsal D (DD) motor neurons (Figure 1). DD neurons are so named because in adults they form GABAergic neuromuscular junctions (NMJ) to dorsal body wall muscles, with their ventral processes receiving inputs from the cholinergic motor neurons [6]. About 40 years ago, using ultrastructural analysis, White et al. revealed that in L1 animals DD neurons form NMJs to ventral body wall muscles, receiving cholinergic inputs in their dorsal processes (Figure 1A, left panel) [9]. This dramatic re-organization of DD connectivity, known as “DD remodeling”, in the developing motor circuit has provided a unique model of neural plasticity.
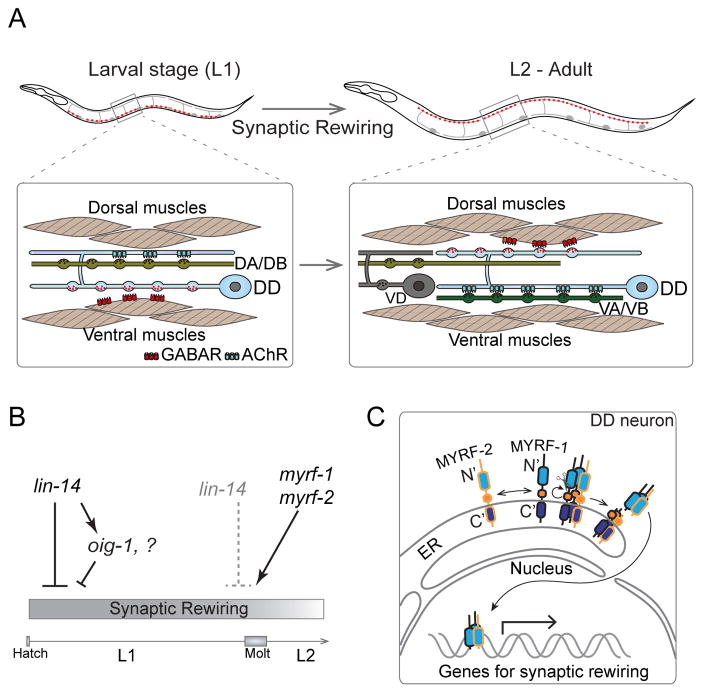
Figure 1. Synaptic rewiring of DD motor neurons in L1 stage.
A. Illustration of position and morphology of six DD-type GABA motor neurons, which also shows remodeling of pre- and post-synaptic components. Synapses (red puncta) of DDs are localized on the ventral processes in L1 animals, but appear on dorsal processes in L2 and older animals, while the ventral juvenile synapses are eliminated. At L1, DDs receive input from DA/DB on the dorsal process, and innervate ventral muscles. Rewired DDs receive inputs from VA/VB ventrally, and innervate dorsal muscles from L2 to adult.
B. Genetic regulation of DD synaptic rewiring. lin-14 activity is high at L1 and acts to repress DD rewiring. One downstream transcriptional target of lin-14, the Ig-domain containing factor oig-1, functions to maintain L1-connectivity of DDs. lin-14 activity is downregulated at the end of L1, and meanwhile myrf-1 and myrf-2 are both upregulated and promote DD rewiring.
C. Full length MYRF-1 and MYRF-2 localize on the ER membrane in DDs. They form trimers on the ER and undergo cleavage, releasing N-terminal fragments. The N-MYRFs translocate into the nucleus and regulate synaptic rewiring.
Mechanistic understanding of temporal and spatial sequences in DD remodeling was made possible following the discovery of GFP [1], which enables in vivo visualization of subcellular compartments. Presynaptic terminals in DD axons can be visualized with fluorescent reporters for synaptic vesicles [10,11], and dendritic specializations can be labeled with an ionotropic acetylcholine receptor [12]. Additionally, GABAA receptors expressed in body wall muscles represent the postsynaptic compartments at GABAergic NMJs [13]. Using these markers, three features of DD remodeling have been characterized. First, in young L1, presynaptic components are exclusively localized in the ventral neurites of DDs (Figure 1A). The emergence of new presynaptic terminals in the dorsal neurites of DD neurons starts around 10 hours after hatching, spreading from the anterior to the entire dorsal cord within a few hours [14]. As the new dorsal synapses increase in size and number, old synapses in the ventral neurites of DD neurons are eliminated in an orderly manner, with some components reused at new sites [15].
Second, the ionotropic acetylcholine receptor ACR-12 is localized to the dorsal neurites of L1 DDs, consistent with a function in receiving cholinergic inputs (Figure 1A). Remodeling of ACR-12-GFP foci from the dorsal to ventral neurites of DD neurons follows a similar time course as that for the presynaptic terminals [16]. Third, in early L1, clusters of the post-synaptic UNC-49 GABAAR are present only in ventral muscles [17]. Concurrent with remodeling of DD presynaptic terminals, UNC-49 clusters appear in dorsal muscles by late L1. Moreover, upon stimulation of a GABA agonist, the muscle response changes from contraction at early L1 to relaxation by mid-late L1, coinciding with DD remodeling [18]. Thus, a complete re-organization of DD neuron connectivity occurs without major changes in cellular morphology.
Transcriptional control of DD remodeling
The first clue to the genetic control of DD remodeling was identifying that the developmental timing (“heterochronic”) gene lin-14 regulates initiation of DD remodeling [14,19]. LIN-14 proteins are expressed in nuclei of DD neurons and are down-regulated by the end of L1. Loss of function (lf) in lin-14 causes precocious remodeling of DD neurons in the L1 stage (Figure 1B). Specific expression of wild type lin-14 in DDs rescues the precocious remodeling in lin-14(lf) mutants, indicating that lin-14 normally suppresses an intrinsic transcription program that initiates DD synaptic remodeling. Recent studies have shed light on some targets of LIN-14. The secreted immunoglobulin domain containing protein OIG-1 is detected at the ventral presynaptic sites of DD neurons at early L1, and its expression is downregulated at the end of L1 [20] (Figure 1B). In lin-14(lf) mutants, oig-1 expression is reduced early in the L1. oig-1(lf) mutants exhibit precocious, though partial, defects in DD remodeling. oig-1(lf) mutants also display premature disassembly of ACR-12 clusters from the dorsal neurites of DD neurons, suggesting an additional role of OIG-1 in maintaining the ACR-12 clusters in the dorsal neurites of L1 DDs [16,20]. However, prolonged expression of lin-14 does not block DD remodeling, suggesting that other factors may act independently of lin-14 to promote DD rewiring.
The myelin gene regulatory factor (Myrf) family proteins are newly identified membrane bound transcription factors [21–23]. They contain an NDT80/PhoG-like DNA-binding domain at the N-terminus, and a centrally located Intramolecular Chaperone of Endosialidase (ICE) domain, with its C-terminal tail residing inside the lumen of the endoplasmic reticulum (ER). The ICE domain can trimerize, resulting in an intra-molecular cleavage and release of N-terminal DNA binding domain of MYRF, which can then enter the nucleus to mediate transcription [21,22]. A recent study has identified two C. elegans homologs, MYRF-1 and MYRF-2, as the first factors essential to promote DD remodeling (Figure 1B–C) [24]. C. elegans myrf-1 and myrf-2 are broadly expressed in many neurons including DDs, and are up-regulated in DDs at late L1; myrf-1 is essential for larval development [24,25]. In a genetic screen for mutants showing temporal misregulation of DD remodeling, a missense mutation of myrf-1 that caused a complete failure of DD remodeling was identified (Meng et al., 2017). This mutation alters the DNA binding domain of MYRF-1, and acts in a dominant negative manner to inhibit the function of both MYRF-1 and MYRF-2. Simultaneous loss of myrf-1 and myrf-2 blocks DD remodeling, whereas over-expression of the N-terminal DNA binding domain of either MYRF-1 or MYRF-2 in DD neurons is sufficient to accelerate DD remodeling. Thus, nuclear translocation of MYRF promotes DD remodeling, likely by activating a transcriptional program (Figure 1C). Studies on mouse MYRF/GM98 have revealed its important role in inducing terminal differentiation of oligodendrocytes [26]. At present, little is known about the function of MYRF proteins in other species in the development and maintenance of neuronal circuitry, although the absence of MYRF generally leads to developmental lethality [26,27]. The findings in C. elegans suggest that MYRF proteins have broad roles in cellular differentiation in different developmental contexts.
Multiple intracellular events coordinate DD remodeling
The orientation of microtubule (MT) polymerization is often considered to be an instructive signal for polarized delivery and transport of axonal and dendritic compartments [28]. DD remodeling entails a complete reversal of neuronal axonal and dendritic polarity. However, microtubule orientation, visualized by a marker for growing MT plus ends, remains unchanged during DD remodeling [29]. Instead, the number of dynamic microtubules increases significantly at the onset of DD synaptic remodeling. This increase in dynamic microtubules requires temporal activation of the conserved MAPKKK DLK-1, in conjunction with changes in the microtubule cytoskeleton [29].
The increased microtubule dynamics during DD remodeling facilitates synaptic vesicle transport mediated by plus-end directed kinesin motors and minus-end directed dynein [29,30]. The cyclin-dependent kinase CDK-5 also promotes new synapse formation in dorsal neurites by modulating UNC-104/Kinesin-3 activity [15]. Concomitant with formation of new synapses, elimination of ventral synapses requires the classical apoptotic factors caspase CED-3 and Apaf-1/CED-4. CED-3 causes cleavage of gelsolin to facilitate actin filament assembly and disassembly [31].
In the developing vertebrate brain extensive studies have shown that GABA first induces depolarization of the postsynaptic membrane, and then switches to hyperpolarization, largely due to developmentally-regulated expression of chloride ion exchangers and pumps [32]. In C. elegans DD remodeling, eliminating GABA neurotransmission per se has few effects on the remodeling of presynaptic synaptic terminals [33], or on the appearance of UNC-49 GABAAR clusters in dorsal body muscles [17]. Nonetheless, several studies have shown that the strength of synaptic transmission can affect DD remodeling. Increasing or decreasing circuit activity causes precocious or delayed DD remodeling, respectively [34]. Reduced activity of the voltage-gated calcium channel UNC-2 impairs synapse elimination [35]. Moreover, the Degenerin/Epithelial Sodium Channel (DEG/ENaC) UNC-8 promotes DD synapse elimination, likely by elevating intracellular calcium, which in turn, via the calcium-activated phosphatase TAX-6/calcineurin, initiates a ced-3/caspase-dependent mechanism to disassemble the ventral synapses of DD neurons [35].
Integration of postembryonic Ventral-type D neurons into the mature locomotor circuit
The mature locomotor circuit in adult C. elegans has 75 motor neurons, 13 of which are in the class VD (Ventral D) GABAergic motor neurons. VD neurons are born post-embryonically and have axonal morphology similar to DDs, but innervate ventral body wall muscles, therefore resembling L1 DDs [6] (Figure 1A, right panel). Early studies identified the COUP-TF like nuclear receptor UNC-55 as a regulator of synapse specificity of VD neurons; loss of unc-55 function causes VDs to form ectopic synapses in dorsal neurites, dubbed “ectopic VD rewiring” [36]. Recent studies using transcriptional profiling have identified numerous transcriptional targets of UNC-55 (Petersen et al., 2011). UNC-55 represses expression of the Iroquois-like homeodomain protein IRX-1 and the Hunchback-like transcription factor HBL-1 to maintain normal neuronal polarity of VDs; elevated activity of IRX-1 and HBL-1 causes ectopic rewiring of VD [34,37]. The DEG/ENaC protein UNC-8 and the secreted protein OIG-1 are also targets of UNC-55, and loss of unc-8 or oig-1 suppresses ectopic rewiring of VDs in unc-55 mutants [16,35]. The similarities in morphology and synaptogenesis pathways between DD and VD neurons suggest that differential use of common molecular signaling mechanisms underlies the distinct structural plasticity of these two classes of neurons in temporal development of the locomotor circuit.
A fascinating question is how the post-embryonically born motor neurons are integrated into the juvenile motor circuit. A recent study has shed light on the roles of cholinergic motor neurons in sculpting the synaptic pattern of VD GABAergic neurons in the motor circuit [38]. Genetic ablation of cholinergic motor neurons or selective inhibition of cholinergic transmission alters the size and distribution of VD neuron synaptic outputs to muscles. This effect depends on acute reduction of cholinergic activity during the integration of VD neurons into the motor circuit. These observations give a glimpse of activity-dependent processes in shaping the synaptic pattern of mature GABAergic neurons.
Sex-specific cellular and synapse plasticity
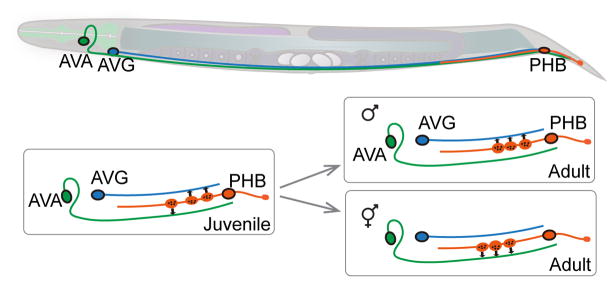
C. elegans reproduce mostly through hermaphrodites, and males arise spontaneously or under conditions of increasing chromosomal non-disjunction. There are 294 neurons present in both hermaphrodites and males. These neurons exhibit similar lineage history, positions, patterns, and molecular identities [8,39]. Hermaphrodites have eight additional sex-specific neurons and males have 91 [40,41]. Sex-specific neurons are born and differentiate in the L4 stage, leading to innervation of sex-specific muscles [8]. Several neurons, although common in both males and hermaphrodites, display sexually dimorphic wiring patterns [42], raising questions of when and how seemingly similar neurons develop dimorphic characters in different sexes. For example, in hermaphrodites, PHB phasmid sensory neurons synapse onto three pre-motor interneurons. In males, PHBs synapse onto the interneuron AVG, which in turn connects to male tail motor neurons (Figure 2) [42,43]. The function of hermaphrodite PHBs is to provide an antagonistic input to pre-motor interneurons, avoiding the noxious chemical Sodium Dodecyl Sulfate (SDS). In males, PHBs are not required for SDS-avoidance behaviors; instead, PHB and AVG function to transduce mating-specific contact signals [43]. This sex-specific connectivity is thus consistent with the sex-specific behaviors. Analyses of synaptic connections further indicate that the sex-dimorphic connectivity between these neurons is mainly established via a pruning or ‘repurposing’ mechanism, through which the primitive connections are selectively eliminated [43]. By manipulating global sex-identity-determining genes, cells can be masculinized in hermaphrodites, or feminized in males in a cell-specific manner. Sex-specific wiring can be altered by sex-reversal of either presynaptic or postsynaptic neurons, even in non-contacting neurons within the circuit, implying that sex-dimorphic connectivity is instructed by both genetic sex-maturing programs and complex interactions within the circuit [43].
Figure 2. Establishing sex-specific connectivity.
Interneurons AVA, AVG, and sensory neuron PHB are common to both hermaphrodites (shown in the drawing) and males. PHB synapses onto both AVA and AVG in early larvae. During sex maturation, sex-specific synapse pruning occurs, producing sex-dimorphic connectivity. Illustration is based on Ref [43].
Neuronal remodeling induced by dauer formation
Under starvation or overcrowding C. elegans L2 larvae enter a diapause, or “dauer” (for ‘enduring’), stage [44,45]. Upon encountering fresh environments with food, dauer animals re-enter the developmental cycle as L4 larvae. Dauers exhibit behaviors distinctive from L3 larvae, a normal developmental stage equivalent to dauer. The nervous system or behaviors of dauers has not been extensively characterized; however, studies have started to reveal dauer-induced morphological changes that may reflect adaptation of the nervous system to harsh conditions [46,47].
Dauer-induced remodeling of sensory dendritic arbors
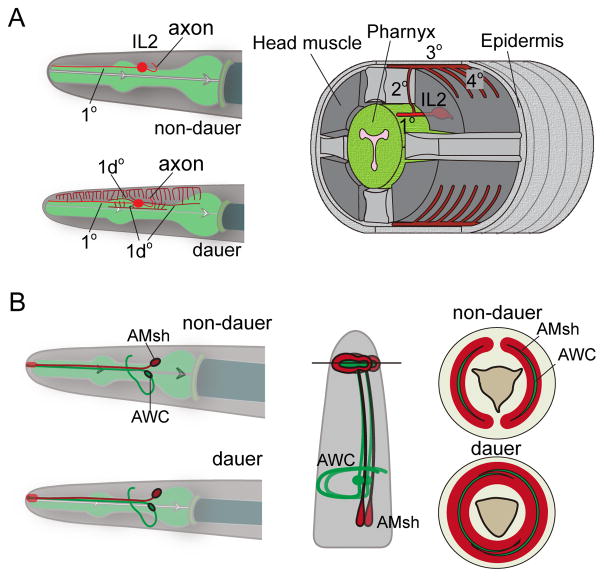
Dauers exhibit a dispersal and survival strategy known as nictation, in which a dauer worm stands on its tail and waves its head in three dimensions, enabling dauers to be transported to new environments [44]. The nictation behavior depends on IL2 sensory neurons located in the head [48]. In non-dauer animals IL2 neurons have single un-branched primary 1° dendrites with exposed cilia endings [49]. In dauer larvae, dendrites of four IL2 neurons undergo extensive branching and growth (Figure 3A) [46]. Upon dauer larvae re-entering the reproductive L4 stage, the dauer-induced dendritic arbors disappear within a few hours, often leaving behind detached remnant branches. The POU homeodomain transcription factor UNC-86 is required for the cell identity of IL2, and the intact function of unc-86 is also necessary for IL2 dendrite remodeling [46]. Additionally, the proprotein convertase kpc-1 is upregulated in IL2 during dauer formation, and is necessary for dauer-induced dendrite branching, but not retraction, in dauer recovery. Dauers of kpc-1 mutants also exhibit defective nictation behavior, suggesting IL2 dendritic arborization supports nictation behavior in dauers.
Figure 3. Structural plasticity in sensory neurons.
A. IL2 neuron (red) extends one simple dendrite to the nose in non-dauer animals (upper left panel), while the IL2 of dauer extends high-order dendritic branches (lower left panel), and also generates a 1d° dendrite directed posteriorly from the cell body. Right: 3-D schematic of a transverse segment of a dauer animal showing IL2 dendritic branches. Illustration is redrawn from Ref [46].
B. In dauers, two amphid sheaths (red) at the left and right sides expand and fuse into each other. The cilia endings of AWC (green), wrapped inside the amphid sheath, also expand and become overlapped. Middle: sagittal section view. Right: cross section view of non-dauer and dauer animals, respectively. Illustration is redrawn from Ref [47].
Dauer-induced IL2 dendrite arbor elaboration is reminiscent of PVD arborization in respect to both morphogenesis and mechanisms [46,50,51]. These studies suggest that the core cellular inner workings underlying structural plasticity are conserved, and yet can be initiated by diverse developmental or sensory signaling. Insights from studying these plastic processes in C. elegans likely enhance our understanding of general principles underlying neural plasticity.
Dauer-induced glia plasticity
Like glia in the vertebrate nervous system, C. elegans glia are tightly associated with neurons, providing structural and functional support [52]. A dedicated program regulates glia plasticity in dauers. The amphid sheath consists of cellular processes from amphid sheath glia cells (AMsh), and the sheath envelops the wing-like cilium endings of amphid sensory neurons, e.g. AWC (Figure 3B). In dauers, distal swellings of the amphid sheath extend, and the two wings then fuse into each other [47]. Each AWC cilium also extends and encloses the nose circumferentially (Figure 3B). Fusion of AMsh glia depends on cell autonomous function of the OTC/OTX transcriptional factor ttx-1 [47]. ttx-1 is not expressed in AWC neurons; however, in ttx-1 mutants, AWC cilia fail to expand and instead fold back aberrantly, suggesting that remodeling of AWC cilia depends on proper fusion of AMsh glia. The VER-1 receptor tyrosine kinase, a transcriptional target of TTX-1, is weakly expressed in non-dauer larvae and strongly up-regulated in AMsh glia of dauers [47]. ver-1 mutants show reduced AMsh fusion. ver-1 induction can also be regulated by ztf-16, a C2H2 zinc-finger factor, and AMsh glial fusion was greatly reduced in ztf-16 mutant dauers [53]. These studies of dauer-induced structural plasticity likely represent the tip of iceberg.
Neurogenesis via trans-differentiation from epithelial and glial cells
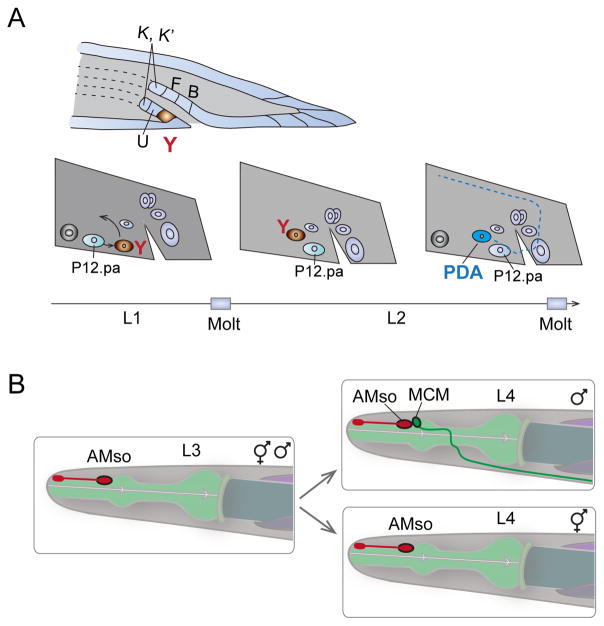
Trans-differentiation occurs when mature somatic cells transform into a different type of cell [54]. Modification of chromatin states or transcriptional programs can induce trans-differentiation. Studies from C. elegans have revealed naturally occurring trans-differentiation events [55]. For example, in early L1 animals, the rectal cell Y cells have epithelial characteristics, including junctions at the apical side with the surrounding epithelial cells and fibrous organelles with the cuticle. During L2, Y cells trans-differentiate into PDA motor neurons (Figure 4A) [56]. This reprogramming is under transcriptional regulation involving a Node-like (Nanog and Oct4-associated deacetylase) complex and stepwise histone-modifying activities [57–59].
Figure 4. Trans-differentiation in cell fate plasticity.
A. In hermaphrodites, epithelial cell Y (brown) retracts from the rectum and migrates anteriodorsally, transdifferentiating into a PDA motor neuron. Another cell, P12.pa, replaces the Y cell at the rectum. The time line (bottom) denotes the stage of larval development when the transdifferentiation occurs. Illustration is redrawn from Ref [56].
B. Amphid socket cell (AMso), a fully differentiated glia cell, divides and gives rise to an interneuron MCM only in males during sex maturation in L4. The second daughter cell from the division remains an AMso glia cell. Illustration is redrawn from Ref [41].
A class of glial cells known as amphid socket cells (AMso) also undergoes developmentally programmed trans-differentiation to generate male specific interneuron MCMs (Mystery Cells of the Male) [41]. MCMs are located in the head and send projections posteriorly into the nerve ring in males (Figure 4B). MCMs are required for a male-specific switch in chemosensory behavior induced by sexual conditioning [60]. C. elegans is normally attracted to salt, but when salt has been previously associated with aversive signals such as starvation, both in males and their mates, hermaphrodites can learn to avoid salt. However, if mates are present along with males during preconditioning of salt-starvation, males can become attracted to salt, overriding the aversive association with starvation. This behavioral switch is mediated by the MCMs in males, but not in hermaphrodites [41].
In both hermaphrodites and males, AMso arise from the same lineages during embryogenesis, and are fully differentiated as glia [41]. During sexual maturation of males, AMso cells re-enter the cell cycle such that one daughter cell retained the AMso identity and the other acquired the MCM neuronal identity. Masculinization of the AMso cells in hermaphrodites results in AMso cell division and MCM neuronal differentiation in otherwise hermaphrodite animals. Conversely, feminization of AMso cells in males results in a lack of AMso cell division and absence of MCM cells. Thus, the competence of AMso glia cells to become neural progenitors is determined intrinsically by the sex identity of the cells.
Conclusion
The nervous system of C. elegans exhibits a broad range of plastic processes. In addition to the examples described here, numerous studies have revealed other modifications of synapses, dendrites, and cilia, depending on cell-type, developmental stage, and sensory input (e.g. reviewed in [51]). Technological advances in imaging power and resolution will continue to uncover additional forms of neural plasticity in C. elegans [5]. Together with systematic efforts to define gene expression at the single-cell level [61], future studies will expand our understanding of the integrative networks linking transcriptional programs, physiological activity, and cellular mechanisms.
Highlights.
Mechanistic insights to drastic circuit rewiring
MRFY ER to nuclear translocation promote developmental circuit rewiring
Neuronal remodeling induced by dauer formation
Trans-differentiation from glial or epithelial to neurons
Acknowledgments
We gratefully acknowledge funding supports to our labs’ work: Y. J. (from NIH NS R01 035546 and HHMI), and Y. B. Q. (from National Natural Science Foundation of China (NFSC) #31571272 and #31171197).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
(* with annotation for significance)
- 1.Chalfie M, Tu Y, Euskirchen G, Ward WW, Prasher DC. Green fluorescent protein as a marker for gene expression. Science. 1994;263:802–805. doi: 10.1126/science.8303295. [DOI] [PubMed] [Google Scholar]
- 2.Moore JL, Du Z, Bao Z. Systematic quantification of developmental phenotypes at single-cell resolution during embryogenesis. Development. 2013;140:3266–3274. doi: 10.1242/dev.096040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Venkatachalam V, Ji N, Wang X, Clark C, Mitchell JK, Klein M, Tabone CJ, Florman J, Ji H, Greenwood J, et al. Pan-neuronal imaging in roaming Caenorhabditis elegans. Proc Natl Acad Sci U S A. 2016;113:E1082–1088. doi: 10.1073/pnas.1507109113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chung K, Crane MM, Lu H. Automated on-chip rapid microscopy, phenotyping and sorting of C. elegans. Nat Methods. 2008;5:637–643. doi: 10.1038/nmeth.1227. [DOI] [PubMed] [Google Scholar]
- 5*.Keil W, Kutscher LM, Shaham S, Siggia ED. Long-Term High-Resolution Imaging of Developing C. elegans Larvae with Microfluidics. Dev Cell. 2017;40:202–214. doi: 10.1016/j.devcel.2016.11.022. A technological advane enabling long-term imaging with single-cell resolution. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.White JG, Southgate E, Thomson JN, Brenner S. The Structure of the Nervous System of the Nematode Caenorhabditis elegans. Philos Trans R Soc Lond B Biol Sci. 1986;314:1–340. doi: 10.1098/rstb.1986.0056. [DOI] [PubMed] [Google Scholar]
- 7.Sulston JE. Post-embryonic development in the ventral cord of Caenorhabditis elegans. Philos Trans R Soc Lond B Biol Sci. 1976;275:287–297. doi: 10.1098/rstb.1976.0084. [DOI] [PubMed] [Google Scholar]
- 8.Sulston JE, Horvitz HR. Post-embryonic cell lineages of the nematode, Caenorhabditis elegans. Dev Biol. 1977;56:110–156. doi: 10.1016/0012-1606(77)90158-0. [DOI] [PubMed] [Google Scholar]
- 9.White JG, Albertson DG, Anness MA. Connectivity changes in a class of motoneurone during the development of a nematode. Nature. 1978;271:764–766. doi: 10.1038/271764a0. [DOI] [PubMed] [Google Scholar]
- 10.Jorgensen EM, Hartwieg E, Schuske K, Nonet ML, Jin Y, Horvitz HR. Defective recycling of synaptic vesicles in synaptotagmin mutants of Caenorhabditis elegans. Nature. 1995;378:196–199. doi: 10.1038/378196a0. [DOI] [PubMed] [Google Scholar]
- 11.Nonet ML. Visualization of synaptic specializations in live C. elegans with synaptic vesicle protein-GFP fusions. J Neurosci Methods. 1999;89:33–40. doi: 10.1016/s0165-0270(99)00031-x. [DOI] [PubMed] [Google Scholar]
- 12.Petrash HA, Philbrook A, Haburcak M, Barbagallo B, Francis MM. ACR-12 Ionotropic Acetylcholine Receptor Complexes Regulate Inhibitory Motor Neuron Activity in Caenorhabditis elegans. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2013;33:5524–5532. doi: 10.1523/JNEUROSCI.4384-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bamber BA, Beg AA, Twyman RE, Jorgensen EM. The Caenorhabditis elegans unc-49 locus encodes multiple subunits of a heteromultimeric GABA receptor. J Neurosci. 1999;19:5348–5359. doi: 10.1523/JNEUROSCI.19-13-05348.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hallam SJ, Jin Y. lin-14 regulates the timing of synaptic remodelling in Caenorhabditis elegans. Nature. 1998;395:78–82. doi: 10.1038/25757. [DOI] [PubMed] [Google Scholar]
- 15.Park M, Watanabe S, Poon VY, Ou CY, Jorgensen EM, Shen K. CYY-1/cyclin Y and CDK-5 differentially regulate synapse elimination and formation for rewiring neural circuits. Neuron. 2011;70:742–757. doi: 10.1016/j.neuron.2011.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16**.He S, Philbrook A, McWhirter R, Gabel CV, Taub DG, Carter MH, Hanna IM, Francis MM, Miller DM., 3rd Transcriptional Control of Synaptic Remodeling through Regulated Expression of an Immunoglobulin Superfamily Protein. Curr Biol. 2015;25:2541–2548. doi: 10.1016/j.cub.2015.08.022. Together with the work in Howell et al. [ref 20], these two studies reveal roles of OIG-1, an immunoglobulin-protein, in organizing pre-synaptic and dendrite changes in DD remdeling. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gally C, Bessereau JL. GABA is dispensable for the formation of junctional GABA receptor clusters in Caenorhabditis elegans. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2003;23:2591–2599. doi: 10.1523/JNEUROSCI.23-07-02591.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18*.Han B, Bellemer A, Koelle MR. An evolutionarily conserved switch in response to GABA affects development and behavior of the locomotor circuit of Caenorhabditis elegans. Genetics. 2015;199:1159–1172. doi: 10.1534/genetics.114.173963. This is a thorough analysis of postsynaptic changes in muscles during DD remodeling. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ambros V, Horvitz HR. Heterochronic mutants of the nematode Caenorhabditis elegans. Science. 1984;226:409–416. doi: 10.1126/science.6494891. [DOI] [PubMed] [Google Scholar]
- 20**.Howell K, White JG, Hobert O. Spatiotemporal control of a novel synaptic organizer molecule. Nature. 2015;523:83–87. doi: 10.1038/nature14545. Together with the work in He et al. [ref 16], these two studies reveal roles of OIG-1, an immunoglobulin-protein, in organizing pre-synaptic and dendrite changes in DD remdeling. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bujalka H, Koenning M, Jackson S, Perreau VM, Pope B, Hay CM, Mitew S, Hill AF, Lu QR, Wegner M, et al. MYRF is a membrane-associated transcription factor that autoproteolytically cleaves to directly activate myelin genes. PLoS biology. 2013;11:e1001625. doi: 10.1371/journal.pbio.1001625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li Z, Park Y, Marcotte EM. A Bacteriophage tailspike domain promotes self-cleavage of a human membrane-bound transcription factor, the myelin regulatory factor MYRF. PLoS biology. 2013;11:e1001624. doi: 10.1371/journal.pbio.1001624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li H, Richardson WD. Evolution of the CNS myelin gene regulatory program. Brain Res. 2016;1641:111–121. doi: 10.1016/j.brainres.2015.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24**.Meng J, Ma X, Tao H, Jin X, Witvliet D, Mitchell J, Zhu M, Dong MQ, Zhen M, Jin Y, et al. Myrf ER-Bound Transcription Factors Drive C. elegans Synaptic Plasticity via Cleavage-Dependent Nuclear Translocation. Dev Cell. 2017;41:180–194. e187. doi: 10.1016/j.devcel.2017.03.022. This study reports the identification of a unique mutation affecting Myrf proteins, from an elegant genetic screen for mis-regulation of DD synaptic remodeling. The work has uncovered an unexpected genetic redundancy of two MRYF homologs in promoting DD remodeling through ICE-mediated proteocleavage and nuclear translocation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Russel S, Frand AR, Ruvkun G. Regulation of the C. elegans molt by pqn-47. Developmental biology. 2011;360:297–309. doi: 10.1016/j.ydbio.2011.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Emery B, Agalliu D, Cahoy JD, Watkins TA, Dugas JC, Mulinyawe SB, Ibrahim A, Ligon KL, Rowitch DH, Barres BA. Myelin gene regulatory factor is a critical transcriptional regulator required for CNS myelination. Cell. 2009;138:172–185. doi: 10.1016/j.cell.2009.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Senoo H, Wang HY, Araki T, Williams JG, Fukuzawa M. An orthologue of the Myelin-gene Regulatory Transcription Factor regulates Dictyostelium prestalk differentiation. The International journal of developmental biology. 2012;56:325–332. doi: 10.1387/ijdb.120030jw. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Conde C, Caceres A. Microtubule assembly, organization and dynamics in axons and dendrites. Nat Rev Neurosci. 2009;10:319–332. doi: 10.1038/nrn2631. [DOI] [PubMed] [Google Scholar]
- 29**.Kurup N, Yan D, Goncharov A, Jin Y. Dynamic Microtubules Drive Circuit Rewiring in the Absence of Neurite Remodeling. Current biology : CB. 2015 doi: 10.1016/j.cub.2015.04.061. This study reports the first analysis of microtubule polarity and dynamics during DD remodeling. In combination with genetic mutant studies it reveals a role of microtubule dynamics in regulating kinesin-mediated directional transport. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30*.Kurup N, Yan D, Kono K, Jin Y. Differential regulation of polarized synaptic vesicle trafficking and synapse stability in neural circuit rewiring in Caenorhabditis elegans. PLoS Genet. 2017;13:e1006844. doi: 10.1371/journal.pgen.1006844. This study reports a set of novel genetic mutations in kinesin and dynein that specifically regulate vesicular transport during DD remodeling. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31*.Meng L, Mulcahy B, Cook SJ, Neubauer M, Wan A, Jin Y, Yan D. The Cell Death Pathway Regulates Synapse Elimination through Cleavage of Gelsolin in Caenorhabditis elegans Neurons. Cell Rep. 2015;11:1737–1748. doi: 10.1016/j.celrep.2015.05.031. This study uncovers synapse elimination mediated by caspase CED-3 and its target gelsolin in DD remodeling. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Represa A, Ben-Ari Y. Trophic actions of GABA on neuronal development. Trends Neurosci. 2005;28:278–283. doi: 10.1016/j.tins.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 33.Jin Y, Jorgensen E, Hartwieg E, Horvitz HR. The Caenorhabditis elegans gene unc-25 encodes glutamic acid decarboxylase and is required for synaptic transmission but not synaptic development. J Neurosci. 1999;19:539–548. doi: 10.1523/JNEUROSCI.19-02-00539.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Thompson-Peer KL, Bai J, Hu Z, Kaplan JM. HBL-1 patterns synaptic remodeling in C. elegans. Neuron. 2012;73:453–465. doi: 10.1016/j.neuron.2011.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35**.Miller-Fleming TW, Petersen SC, Manning L, Matthewman C, Gornet M, Beers A, Hori S, Mitani S, Bianchi L, Richmond J, et al. The DEG/ENaC cation channel protein UNC-8 drives activity-dependent synapse removal in remodeling GABAergic neurons. Elife. 2016:5. doi: 10.7554/eLife.14599. This study reports a series of analysis linking the UNC-8 DEG/ENac channel to a calcium- and caspase-mediate synapse elimination in DD remodeling. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhou HM, Walthall WW. UNC-55, an orphan nuclear hormone receptor, orchestrates synaptic specificity among two classes of motor neurons in Caenorhabditis elegans. J Neurosci. 1998;18:10438–10444. doi: 10.1523/JNEUROSCI.18-24-10438.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Petersen SC, Watson JD, Richmond JE, Sarov M, Walthall WW, Miller DM., 3rd A transcriptional program promotes remodeling of GABAergic synapses in Caenorhabditis elegans. J Neurosci. 2011;31:15362–15375. doi: 10.1523/JNEUROSCI.3181-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38*.Barbagallo B, Philbrook A, Touroutine D, Banerjee N, Oliver D, Lambert CM, Francis MM. Excitatory neurons sculpt GABAergic neuronal connectivity in the C. elegans motor circuit. Development. 2017;144:1807–1819. doi: 10.1242/dev.141911. This study examines how synaptic inputs affect integration of the GABAergic VD motor neurons in the formation of mature locomotor circuit. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sulston JE, Schierenberg E, White JG, Thomson JN. The embryonic cell lineage of the nematode Caenorhabditis elegans. Dev Biol. 1983;100:64–119. doi: 10.1016/0012-1606(83)90201-4. [DOI] [PubMed] [Google Scholar]
- 40.Sulston JE, Albertson DG, Thomson JN. The Caenorhabditis elegans male: postembryonic development of nongonadal structures. Dev Biol. 1980;78:542–576. doi: 10.1016/0012-1606(80)90352-8. [DOI] [PubMed] [Google Scholar]
- 41**.Sammut M, Cook SJ, Nguyen KC, Felton T, Hall DH, Emmons SW, Poole RJ, Barrios A. Glia-derived neurons are required for sex-specific learning in C. elegans. Nature. 2015;526:385–390. doi: 10.1038/nature15700. This report reveals for the first time transdifferentiation from glia to neurons in C. elegans, and further elaborates a cellular mechanism depending on sex-determination identity. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jarrell TA, Wang Y, Bloniarz AE, Brittin CA, Xu M, Thomson JN, Albertson DG, Hall DH, Emmons SW. The connectome of a decision-making neural network. Science. 2012;337:437–444. doi: 10.1126/science.1221762. [DOI] [PubMed] [Google Scholar]
- 43**.Oren-Suissa M, Bayer EA, Hobert O. Sex-specific pruning of neuronal synapses in Caenorhabditis elegans. Nature. 2016;533:206–211. doi: 10.1038/nature17977. This study examines sex-dimorphoic synaptic connection, revealing distinct patterns of same neurons utilizing mechanisms involving prunning in target selection. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cassada RC, Russell RL. The dauerlarva, a post-embryonic developmental variant of the nematode Caenorhabditis elegans. Dev Biol. 1975;46:326–342. doi: 10.1016/0012-1606(75)90109-8. [DOI] [PubMed] [Google Scholar]
- 45.Golden JW, Riddle DL. A pheromone influences larval development in the nematode Caenorhabditis elegans. Science. 1982;218:578–580. doi: 10.1126/science.6896933. [DOI] [PubMed] [Google Scholar]
- 46.Schroeder NE, Androwski RJ, Rashid A, Lee H, Lee J, Barr MM. Dauer-specific dendrite arborization in C. elegans is regulated by KPC-1/Furin. Curr Biol. 2013;23:1527–1535. doi: 10.1016/j.cub.2013.06.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Procko C, Lu Y, Shaham S. Glia delimit shape changes of sensory neuron receptive endings in C. elegans. Development. 2011;138:1371–1381. doi: 10.1242/dev.058305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lee H, Choi MK, Lee D, Kim HS, Hwang H, Kim H, Park S, Paik YK, Lee J. Nictation, a dispersal behavior of the nematode Caenorhabditis elegans, is regulated by IL2 neurons. Nat Neurosci. 2011;15:107–112. doi: 10.1038/nn.2975. [DOI] [PubMed] [Google Scholar]
- 49.Ward S, Thomson N, White JG, Brenner S. Electron microscopical reconstruction of the anterior sensory anatomy of the nematode Caenorhabditis elegans?2UU. J Comp Neurol. 1975;160:313–337. doi: 10.1002/cne.901600305. [DOI] [PubMed] [Google Scholar]
- 50.Salzberg Y, Ramirez-Suarez NJ, Bulow HE. The proprotein convertase KPC-1/furin controls branching and self-avoidance of sensory dendrites in Caenorhabditis elegans. PLoS Genet. 2014;10:e1004657. doi: 10.1371/journal.pgen.1004657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dong X, Shen K, Bulow HE. Intrinsic and extrinsic mechanisms of dendritic morphogenesis. Annu Rev Physiol. 2015;77:271–300. doi: 10.1146/annurev-physiol-021014-071746. [DOI] [PubMed] [Google Scholar]
- 52.Oikonomou G, Shaham S. The glia of Caenorhabditis elegans. Glia. 2011;59:1253–1263. doi: 10.1002/glia.21084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Procko C, Lu Y, Shaham S. Sensory organ remodeling in Caenorhabditis elegans requires the zinc-finger protein ZTF-16. Genetics. 2012;190:1405–1415. doi: 10.1534/genetics.111.137786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jopling C, Boue S, Izpisua Belmonte JC. Dedifferentiation, transdifferentiation and reprogramming: three routes to regeneration. Nat Rev Mol Cell Biol. 2011;12:79–89. doi: 10.1038/nrm3043. [DOI] [PubMed] [Google Scholar]
- 55.Becker SF, Jarriault S. Natural and induced direct reprogramming: mechanisms, concepts and general principles-from the worm to vertebrates. Curr Opin Genet Dev. 2016;40:154–163. doi: 10.1016/j.gde.2016.06.014. [DOI] [PubMed] [Google Scholar]
- 56.Jarriault S, Schwab Y, Greenwald I. A Caenorhabditis elegans model for epithelial-neuronal transdifferentiation. Proc Natl Acad Sci U S A. 2008;105:3790–3795. doi: 10.1073/pnas.0712159105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57**.Zuryn S, Ahier A, Portoso M, White ER, Morin MC, Margueron R, Jarriault S. Transdifferentiation. Sequential histone-modifying activities determine the robustness of transdifferentiation. Science. 2014;345:826–829. doi: 10.1126/science.1255885. This study demonstrates a mechanism involving both sequential and redundant action of multiple chromatin remodeling regulators in epithelial trans-differentiation into neurons. [DOI] [PubMed] [Google Scholar]
- 58.Kagias K, Ahier A, Fischer N, Jarriault S. Members of the NODE (Nanog and Oct4-associated deacetylase) complex and SOX-2 promote the initiation of a natural cellular reprogramming event in vivo. Proc Natl Acad Sci U S A. 2012;109:6596–6601. doi: 10.1073/pnas.1117031109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Richard JP, Zuryn S, Fischer N, Pavet V, Vaucamps N, Jarriault S. Direct in vivo cellular reprogramming involves transition through discrete, non-pluripotent steps. Development. 2011;138:1483–1492. doi: 10.1242/dev.063115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sakai N, Iwata R, Yokoi S, Butcher RA, Clardy J, Tomioka M, Iino Y. A sexually conditioned switch of chemosensory behavior in C. elegans. PLoS One. 2013;8:e68676. doi: 10.1371/journal.pone.0068676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61*.Cao J, Packer JS, Ramani V, Cusanovich DA, Huynh C, Daza R, Qiu X, Lee C, Furlan SN, Steemers FJ, et al. Comprehensive single-cell transcriptional profiling of a multicellular organism. Science. 2017;357:661–667. doi: 10.1126/science.aam8940. This study reveals a promising technological advance in defining specific neuronal cell type. [DOI] [PMC free article] [PubMed] [Google Scholar]