Abstract
Background & Aims
Joubert Syndrome (JS) is a rare, inherited, ciliopathy defined by cerebellar and brainstem malformations and is variably associated with liver, kidney, and ocular dysfunction. This study characterizes the hepatic findings in JS and identifies factors associated with probable portal hypertension.
Methods
100 individuals with JS were prospectively evaluated at the National Institutes of Health Clinical Center. Laboratory tests, imaging, and DNA sequencing were performed. Patients were stratified based on the spleen length/patient height (SL/H) ratio as a marker of splenomegaly, used as a surrogate for probable portal hypertension.
Results
Forty-three patients (43 %) had liver involvement based on elevated liver enzymes and/or liver hyperechogenicity and/or splenomegaly. None of the patients had macroscopic liver cysts or bile duct dilatation. Based on the SL/H ratio, 13 patients were stratified into a probable portal hypertension group. We observed significant elevations in Alkaline Phosphatase (269 vs. 169 U/L, P = <0.001), ALT (92 vs. 42 U/L, P = 0.004), AST (77 vs. 40 U/L, P = 0.002), and GGT (226 vs. 51 U/L, P = <0.001) in the probable portal hypertension group. Platelets were lower in the probable portal hypertension cohort (229 vs. 299 x 103/μL, P = 0.008), while synthetic function was intact in both groups. Probable portal hypertension was also more prevalent in patients with kidney disease (P = 0.001) and colobomas (P = 0.02), as well as mutations in the TMEM67 gene (P = 0.001).
Conclusions
In JS, probable portal hypertension is associated with abnormal hepatic enzymes, as well as presence of kidney disease, coloboma, and/or mutation in TMEM67. These findings may allow early identification of JS patients who have or are more likely to develop liver disease.
Keywords: Joubert syndrome, COACH syndrome, ciliopathy, congenital hepatic fibrosis, portal hypertension
Introduction
Joubert syndrome (JS) is a rare, autosomal-recessive condition primarily characterized by cerebellar and brainstem malformations. It was first described in 1969 by a pediatric neurologist, Dr. Marie Joubert, in four siblings with cerebellar agenesis, along with hyperpnea, abnormal eye movements, intellectual disability, and ataxia.1 Diagnosis is based on the brain magnetic resonance imaging (MRI) finding of the “molar tooth sign,” caused by cerebellar vermis hypoplasia, thickened and horizontally oriented superior cerebellar peduncles and a deep interpeduncular fossa (Supplementary Figure 1).2
The disease is classified as a ciliopathy, because mutations in JS genes result in dysfunction of primary, non-motile cilia that protrude from the surface of most differentiated cells. Primary cilia are involved in transduction of various signaling pathways and play critical roles in cell growth, proliferation, and polarity.3 Given the roles of cilia in the function of multiple cell types, individuals with ciliopathies tend to have multi-organ involvement. JS, in particular, is often associated with ocular features of retinal dystrophy and colobomas, renal disease such as nephronophthisis and polycystic kidneys, skeletal abnormalities including polydactyly, and hepatic abnormalities, generally manifesting as congenital hepatic fibrosis (CHF).4 This clinical heterogeneity led to the use of the term Joubert syndrome and related disorders (JSRD), which includes Senior-Løken syndrome (juvenile-onset nephronophthisis and retinal dystrophy) and COACH (colobomas, “oligophrenia” for cognitive impairment, ataxia, cerebellar vermis hypoplasia, and hepatic fibrosis) syndrome5. Recently, the term JS is recommended to refer to all individuals with the “molar tooth sign” including those with or without extra-neurological system involvement.6 In this article, for simplicity, we will use JS to include Senior-Løken and COACH syndromes.
CHF, the typical liver disease in various ciliopathies such as autosomal recessive polycystic kidney disease, JS and Meckel-Gruber syndromes, results from embryonic malformation of ductal plates due to dysfunctional cilia.8 It is characterized by persistent embryonic ductal plate structures, progressive fibrosis of portal tracts and cystic dilation of intrahepatic biliary ducts; its complications include non-cirrhotic portal hypertension and/or recurrent cholangitis.7,8 The exact frequency of CHF in patients with JS is uncertain, because CHF does not manifest at birth, but tends to develop later in life; most patients remain asymptomatic for many years. In addition, liver disease in JS may be underdiagnosed, since most physicians are unfamiliar with the multisystem nature of JS, and formal clinical management guidelines have not been published. Two recent studies reported liver disease in 9% and 14 % of patients with JS9,10 Ciliopathy patients with CHF can have variable signs and symptoms, ranging from asymptomatic elevations of liver enzymes to portal hypertension and its sequelae including esophageal varices and thrombocytopenia. Notably, CHF typically causes non-cirrhotic portal hypertension, and patients generally have intact synthetic function and do not progress to cirrhosis11,12 Ultrasonography and MRI may reveal increased liver echogenicity, liver cysts, and intrahepatic biliary ductal dilation.13 Liver biopsy, which typically shows ductal plate malformation, abnormal branching of intrahepatic portal veins, and fibrosis of portal tracts, is usually not required because a clinical diagnosis of CHF can be made in the context of other features typical for a ciliopathy.7
Several studies and case reports have presented data on the hepatic manifestations of JS.10,14,15 However, no study has characterized liver disease directly in a large JS cohort and attempted to identify liver abnormalities associated with progression to portal hypertension. Since portal hypertension is associated with significant morbidity, it is important to determine which individuals are at risk of developing this complication. Therefore, the goal of this study is to characterize hepatic manifestations of JS and identify parameters that predict development of probable portal hypertension.
Methods
Patients
All subjects were prospectively evaluated at the National Institutes of Health (NIH) Clinical Center, between 2003 and 2014, under the protocol, “Clinical and Molecular Investigations Into Ciliopathies” (www.clinicaltrials.gov, trial NCT00068224), approved by the National Human Genome Research Institute Institutional Review Board. For patient recruitment, the study was advertised to patients and families by The Joubert Syndrome & Related Disorders Foundation as a natural history study aiming to describe the individual organ system involvement in JS, including kidney and liver disease. Patients and/or their parents gave written, informed consent. The only enrollment criterion was clinical diagnosis of JS, including Senior-Løken and COACH syndromes.
Initial phone interviews were conducted (MGA) on 120 JS individuals from 105 families. Fifteen families could not travel to the NIH Clinical Center in Bethesda Maryland; in some cases this was due to the severity of their child’s neurological and/or respiratory condition requiring intensive care. The remaining 105 JS patients from 90 families all underwent week-long clinical evaluations at the NIH Clinical Center. Based on our review of brain MRI images, 8 subjects from 6 families did not have the “molar tooth sign”, including 3 individuals with classical Senior-Løken syndrome.16 In this paper, we describe 100 subjects from 86 families, including 3 with Senior-Løken syndrome without molar tooth sign (with typical clinical features of severe retinal degeneration and nephronophthisis) and excluding the 5 patients without molar tooth sign, whose clinical features were non-specific. NIH evaluations included review of past medical history, family history, brain MRI images and medical records; physical examination; high resolution abdominal ultrasonography; formal neurocognitive evaluations; electroencephalogram; complete ophthalmologic examination; and comprehensive blood and urine chemistries. Blood samples for DNA were collected from all patients and parents, when available.
The patients were classified as having chorioretinal coloboma, retinal degeneration, kidney disease and liver disease based on clinical and laboratory evaluations performed at the NIH Clinical Center. Retinal degeneration was diagnosed based on typical findings on NIH retinal examination after dilation of pupils; when available, electroretinography findings and findings on past eye examinations were also used for decision making. Kidney disease was defined based on decreased glomerular filtration rate (GFR) and/or abnormal findings on renal ultrasonography including loss of corticomedullary differentiation, increased or decreased kidney size and presence of cystic changes. All available brain MR images were qualitatively evaluated by a team of five physicians, including two pediatric neuroradiologists, one adult neuroradiologist, one pediatric neurologist and one pediatric clinical geneticist (MGA), as a group to achieve consensus.16 Twelve families contributed more than 1 affected individual.
Patients were classified as having liver disease if they had elevation in liver enzymes and/or abnormal hepatic parenchyma (moderately or severely increased echogenicity) on ultrasonography. As per Schneider et al, presence of portal hypertension was described as “definite”, if there was a history of portal hypertension-related complications or clinical findings of thrombocytopenia and splenomegaly; “probable”, if there was presence of only one of the two clinic findings; or, “absent,” if neither of the clinical findings were present.32 In this study, splenomegaly was determined based on increased spleen length/height (SL/H) ratio, as explained in the imaging section below.
DNA Sequencing
Molecular Inversion Probes (MIP)
The coding exons of 27 genes associated with JS (AHI1, ARL13B, B9D1, B9D2, C2CD3, C5orf42, CC2D2A, CEP290, CEP41, CSPP1, IFT172, INPP5E, KIF7, MKS1, NPHP1, OFD1, RPGRIP1L, TCTN1, TCTN2, TCTN3, TMEM138, TMEM216, TMEM231, TMEM237, TMEM67, TTC12B and ZNF423) were sequenced by combining a MIP capture method and next-generation sequencing. For this approached, performed at the University of Washington, we used 100ng of genomic DNA and the captured DNA was amplified by PCR and sequenced on either an Illumina HiSeq or a MiSeq platform.9 Sequence reads were mapped using the Burrows-Wheeler Aligner (V.0.5.9). Variants were called using the Genome Analysis Toolkit (V.2.5–2) and annotated with SeattleSeq (http://snp.gs.washington.edu/SeattleSeqAnnotation138/).
Whole exome sequencing (WES)
Genomic DNA was obtained from leukocytes using standard protocols. For exome sequencing we used the HiSeq2000 (Illumina, San Diego, CA) that employed 101-bp paired-end read sequencing. Image analysis and base calling were performed using Illumina Genome Analyzer Pipeline software (V 1.13.48.0) with default parameters.17 Reads were aligned to a human reference sequence (UCSC assembly hg19, NCBI build 37) using a package called Efficient Large-scale Alignment of Nucleotide Databases (Illumina, San Diego, CA). Genotypes were called at all positions where there were high-quality sequence bases using a Bayesian algorithm called the Most Probable Genotype and variants were filtered using the graphical software tool VarSifter v1.18,19 The database dbSNP (http://www.ncbi.nlm.nih.gov/snp/) covers the 1.22% of the human genome corresponding to the Consensus Conserved Domain Sequences.
gDNA and cDNA sequencing
Genomic DNA was obtained from leukocytes using standard protocols. For dideoxy sequencing of gDNA, primers were designed to cover areas of variants identified by WES. Direct sequencing of the PCR amplification products was carried out using BigDye 3.1 Terminator chemistry (Applied Biosystems, Austin, TX) and separated on an ABI 3130xl genetic analyzer (Applied Biosystems Austin, TX). Data were evaluated using Sequencher v5.0 software (Gene Codes Corporation, Ann Arbor, MI).
Biochemical Testing
All patients underwent comprehensive bloodwork, including platelet count, alkaline phosphatase (AP), alanine aminotransferase (ALT), aspartate aminotransferase (AST), total and direct bilirubin, gamma-glutamyl transferase (GGT), albumin, and prothrombin time (PT). For normal ranges for the laboratory values standard references adjusted for age and sex were used. The GFR was calculated using a formula based on the creatinine, cystatin C level, blood urea nitrogen (BUN), patient height, and sex.20,21 Additionally, the AST to platelet ratio index (APRI), used for predicting significant hepatic fibrosis or cirrhosis, was calculated for each patient.29 (In the calculation, the AST was adjusted for the patient’s age and sex). An APRI > 1.0 was considered to be consistent with probable portal hypertension.29
Imaging Studies
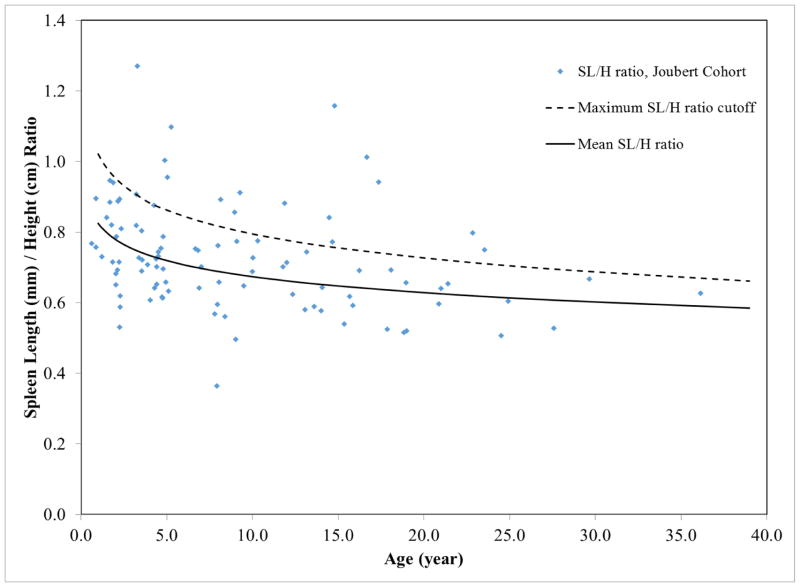
Complete abdominal ultrasonography (USG) evaluations and color Doppler studies were performed on all patients by a single technologist (K.D.) using standard (4-MHz) and high-resolution (HR) (7-MHz) USG probes (AVI Sequoia Inc, Mountain View, CA). We calculated the SL/H ratio by dividing spleen length (mm) on USG by the patient’s height (cm). For controls, we combined normative data on 819 children from 2 references.22,23 These references provided data on children under 17 years of age; for older patients we extrapolated the normative SL/H curve. Patients with SL/H ratios greater than the upper limit of normal defined in these 2 references were classified as having splenomegaly (Figure 1). Dilatation of the common bile duct (CBD) was based on published normal values of <3.3 mm (1.27 ± 0.67 mm), obtained by performing USG on 173 children.24 Adults with a CBD diameter more than 5 mm on USG were considered to have a dilated CBD. MRI, including magnetic resonance cholangiopancreatography (MRCP), was performed on patients who were old enough to tolerate the study without sedation. MRI was performed using a 1.5- or 3-Tesla machine (Philips Medical Systems, NA, Bothell, WA; General Electric Healthcare, Waukesha, WI) without intravenous contrast media. All USG, MRI, and MRCP images were interpreted in consensus by 2 radiologists (P.C and B.T.), a hepatologist (T.H) and pediatrician geneticist (M.G.A). Liver echogenicity was judged to be normal or mildly, moderately, or severely increased based on the score card presented (Supplementary Figure 2).
Figure 1.
Spleen Length/Height (SL/H) ratios of the Joubert syndrome cohort plotted against age. Blue dots represent SL/H ratios of Joubert syndrome patients. Patients with SL/H ratios above the dotted line (upper limit of normal SL/H ratio) were considered as having splenomegaly.
Statistics
Descriptive statistics were calculated for the entire cohort. Continuous variables were expressed in terms of mean and standard deviation (SD), while categorical variables were expressed in terms of ratios and percentages.
The cohort was subsequently stratified based on presence or absence of splenomegaly (increased SL/H ratio), which was used as a surrogate for probable portal hypertension. Statistical analysis of the data stratified by SL/H ratio was performed to determine which parameters were associated with probable portal hypertension. Parameters that were evaluated included: AST, ALT, AP, GGT, platelet count, albumin, PT, liver imaging findings, kidney disease, retinal dystrophy, coloboma, polydactyly and the causative gene.
Each categorical variable was examined using a simple exact logistic regression, while each continuous variable was examined using a simple logistic regression. Data that did not fit a normal curve were transformed using the Box-Cox transformation.
Analyses were performed in SAS 9.3 (SAS Institute, Cary NC) and a significance level of 0.05 was used for both univariate and final models.
Results
Patient Cohort
The cohort included 100 JS patients from 86 independent families. Twelve families contributed more than one child; 11 families had 2 and 2 families had 3 affected siblings. There were 43 females and 57 males. Ages ranged from 0.6 to 36 years (mean 9.1 + 7.5 years) (Table 1). We identified the molecular genetic cause in 95 subjects; all 5 patients in whom no genetic cause was identified had the molar tooth sign on brain MRI (Supplementary Figure 1).25 Retinal degeneration was detected in 20%, coloboma in 30%, polydactyly in 13%, and fibrocystic kidney disease in 32%. Twenty-seven percent of patients had no extra-neurological organ system abnormalities.
Table 1.
Patient Demographics
| Sex, n (%) | 100 (100) |
| Male | 57 (57) |
| Female | 43 (43) |
|
| |
| Age, y (range, SD) | 9.1 (0.6–36.2, 7.5) |
|
| |
| Race, n (%) | 100 |
| Caucasian | 90 (90) |
| Black | 3 (3) |
| Asian | 1 (1) |
| Hispanic | 2 (2) |
| Indian | 1 (1) |
| Middle Eastern | 3 (3) |
|
| |
| GFR, n1 | 92 |
| Average (SD) | 102 (33) |
|
| |
| Growth, n | 100 |
| Height Z-score, average (SD) | −1.42 (1.39) |
|
| |
| Associated disease, n (%) | 100 (100) |
| Kidney disease | 32 (32) |
| Polydactyly | 13 (13) |
| Coloboma | 30 (30) |
| Retinal Dystrophy | 20 (20) |
7 patients had renal transplantation; data not available for 1 patient
Presence of liver disease
Signs, Symptoms, and Diagnostic Evaluation
Among the cohort, 43/100 patients (43%) had at least one piece of evidence for liver involvement based on elevated liver enzymes and/or abnormal hepatic parenchyma with moderate /severe hyperechogenicity and/or splenomegaly (Supplementary Table 1). In 15 of these 43 patients with liver involvement (35%), liver enzymes were elevated without splenomegaly and without definitely abnormal liver echogenicity (normal in 5 and mild hyperechogenicity in 10). In 11 of these 43 patients (26%), liver hyperechogenicity was the only abnormal finding (moderate in 9 and severe in 2).
Among the individuals with liver disease, only 4 had related signs and/or symptoms (Supplementary Table 1, patients #8, 9, 11, 15). One had recurrent variceal bleeding (patient 11); one had a history of easy bruising and pruritus; one had hepatomegaly on physical examination, but no symptoms; and one had a history of 1 episode of cholangitis. No patients had evidence of splenomegaly on physical exam.
No patient with liver disease underwent endoscopy at the NIH, in the absence of clinical indication. However, 5 patients had upper endoscopies performed at outside institutions prior to their NIH visit (Supplementary Table 1, patients #9, 11, 12, 21, 22). In only one of these individuals (patient 11), endoscopy was performed for hematemesis and esophageal varices were identified and treated with sclerotherapy. This patient had multiple subsequent variceal bleeds, ultimately requiring liver transplantation. The remaining 4 patients had endoscopies for other GI symptoms; none had evidence of esophageal varices.
Finally, liver biopsies were performed in 12 patients with liver disease. Biopsies from 5 of the patients were reviewed by the NIH Clinical Center Department of Pathology. All had findings consistent with congenital hepatic fibrosis, which included ductal plate malformation and portal fibrosis.
Laboratory Data
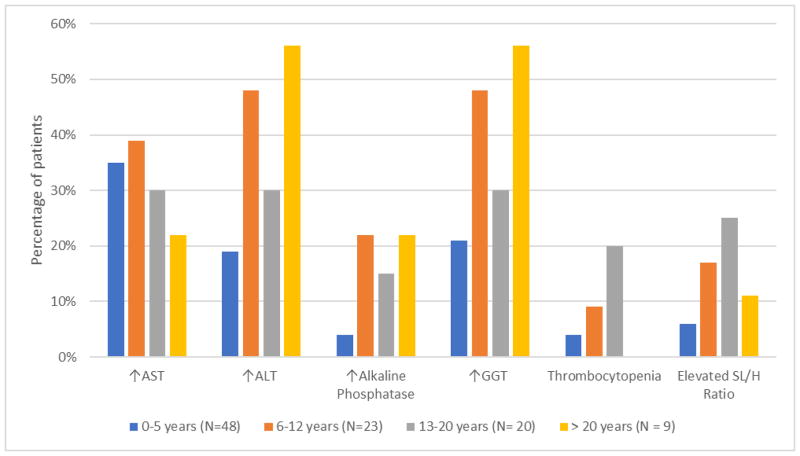
Thrombocytopenia was identified in 4/43 (9%) patients with liver disease; 2 of these patients had evidence of splenomegaly, as defined by the SL/H ratio. AP, ALT, AST, and GGT were elevated in 12/43 (28%), 28/43 (65%), 30/43 (70%), and 28/43 (65%), respectively. When stratified into age categories, the percentage of the patient cohort with abnormal liver enzymes and thrombocytopenia increased with age and was most pronounced in patients over the age of 20 (Figure 2).
Figure 2.
Percentages of Joubert syndrome patients with abnormalities of hepatic enzymes (AST, ALT, alkaline phosphatase and GGT) and markers of portal hypertension (thrombocytopenia and elevated SL/H ratio) shown in age groups.
Total and direct bilirubin was elevated in only 2 patients. In terms of synthetic function, the albumin level was low in only one patient, while 2 patients had an elevated PT. Notably, the patient with hypoalbuminemia had normal coagulation markers.
Hepatic Findings on Imaging
The entire cohort underwent abdominal USG and 15 patients, who were able to tolerate the study without sedation, had MRI and MRCP to evaluate the liver and the hepato-biliary tract and to look for stigmata of portal hypertension including splenomegaly, direction of portal flow and collateral blood vessels (Table 2). Liver echogenicity was mildly, moderately, or severely increased in 53% (52/99), 20% (20/99), and 2% (2/99) of the cohort, respectively; the remaining 25% had normal liver echogenicity. Liver echotexture was normal in 47% of the patients, with mild, moderate, or severe granularity in 35%, 13%, and 2%, respectively.
Table 2.
Abdominal Ultrasonography Findings
| Liver Echogenicity, n* (%) | 99 |
| Normal | 25 (25) |
| Mildly increased | 52 (53) |
| Moderately increased | 20 (20) |
| Severely increased | 2 (2) |
| Liver Echotexture, n* (%) | 99 |
| Normal | 44 (44) |
| Mildly granular | 35 (35) |
| Moderately granular | 14 (14) |
| Severely granular | 6 (6) |
| Common bile duct size, n | 75 |
| Average, mm (SD) | 2.36 (1.0) |
| Gallbladder, n** | 99 |
| Normal | 95 |
| Abnormal | 4*** |
One patient excluded, because he was post-liver transplant.
Gallbladder not visualized in 1 patient
Enlarged gallbladder (2 patients); cholelithiasis (1 patient); gallbladder polyps (1 patient)
None of the patients had evidence of biliary ductal dilation, and none had evidence of small or large hepatic cysts. In terms of the stigmata of portal hypertension, the direction of portal flow was normal in the entire cohort; no patient had recanalization of the umbilical vein, and collateral vessels were present in 2 of the 100 patients.
Comparison of cohorts with and without liver involvement
The age of patients with liver involvement ranged from 0.9 to 36.2 years (11.4 ± 8.6) and was higher than that of patients with normal liver function, i.e., 0.6 y to 23.5 y (7.2 ± 6.1, P = 0.005). Similarly, the average age of patients in the elevated ALT (11.9 vs. 7.9, P = 0.011) and GGT (11.3 vs. 8.1, P = 0.045) groups were higher in comparison to those without elevations in these enzymes. Although the average age of patients with elevated AP was also higher than that of patients with a normal AP, this difference did not reach statistical significance (12.5 vs. 8.7, P = 0.10). There was no statistically significant difference in the average age of patients with and without elevations in AST or with and without thrombocytopenia.
When data on subgroups of patients based on individual genes were analyzed, 21 of 22 patients with TMEM67 mutations had evidence of liver disease (P = <0.001). Notably, 6 of 7 patients with the CEP290 gene mutation had no hepatic involvement (P = 0.044). No apparent differences were found in the frequency of JS patients with mutations in the remaining 18 genes when the groups with and without liver involvement were compared; for the majority of these genes, the small number of patients limited interpretation.
In terms of other organ involvement associated with liver disease, colobomas were significantly more common in patients with liver disease (58% vs. 19%, P = 0.03) and retinal dystrophy was significantly less prevalent among patients with liver involvement (39% vs. 17%, P = 0.03). There was no statistically significant difference in the frequency of kidney disease or polydactyly between the groups with and without liver involvement.
Presence of probable portal hypertension as determined by SL/H ratio
The SL/H ratio was elevated in 13 of 100 patients (13%). Two out of these thirteen patients (15%) could be characterized as having “definite” portal hypertension, as both had thrombocytopenia and one of them also had a history of esophageal varices complicated by bleeding. The remaining 11 patients were characterized as having “probable” portal hypertension, in the absence of thrombocytopenia or clinical stigmata. Given the small number of patients in the “definite” portal hypertension group, the “probable” and “definite” portal hypertension groups were analyzed together in a single cohort.
The average age of JS patients with probable portal hypertension was higher, although the difference did not reach statistical significance (11.7 vs. 8.8 years, P = 0.18). When the data were stratified into age groups, both thrombocytopenia and probable portal hypertension was most prevalent in patients 13–20 years of age (Figure 2).
On laboratory analysis, AP (269 vs. 169 U/L, P = <0.001), ALT (92 vs. 42 U/L, P = 0.004), AST (77 vs. 40 U/L, P = 0.002), and GGT (226 vs. 51 U/L, P = <0.001) were all significantly more elevated in the probable portal hypertension group (Table 3). Although there was a statistically significant difference in the total bilirubin (0.52 vs. 0.36 mg/dL, P = 0.009) and direct bilirubin (0.14 vs. 0.11 mg/dL, P = 0.003), these two laboratory tests remained within normal limits in the entire cohort. Platelet counts were lower in the probable portal hypertension cohort (229 vs. 299 x 103/μL, P = 0.008), although the mean was within normal limits in both groups.
Table 3.
Comparison of laboratory data between portal hypertension and non-portal hypertension groups.
| Portal Hypertension, Mean (SD) | No portal hypertension, Mean (SD) | P-value | |
|---|---|---|---|
|
| |||
| Alkaline Phosphatase (U/L) | 269 (103) | 169 (84) | <0.001 |
| ALT (U/L) | 92 (75) | 42 (55) | 0.004 |
| AST (U/L) | 77 (48) | 40 (37) | 0.002 |
| GGT (U/L) | 226 (305) | 51 (94) | <0.001 |
| Total Bilrubin (mg/dL) | 0.52 (0.26) | 0.36 (0.17) | 0.009 |
| Direct Bilirubin (mg/dL) | 0.14 (0.07) | 0.11 (0.02) | 0.003 |
| Platelets (x 103/μL) | 229 (98) | 299 (85) | 0.008 |
| Albumin (g/dL) | 4.0 (0.4) | 4.0 (0.3) | 0.76 |
| Prothrombin Time (s) | 13.9 (1.0) | 13.5 (0.6) | 0.04 |
In terms of synthetic liver function, the average PT was higher, though still within normal limits, in the probable portal hypertension group (13.9 vs. 13.5, P = 0.04). Albumin levels were similar in both groups (P = 0.76).
The APRI, an index used for predicting significant hepatic fibrosis or cirrhosis, was also compared between the two cohorts and the score was significantly higher in the probable portal hypertension group (1.2 ±1.3 vs. 0.3 ± 0.3, P = <0.001). Overall, 8 patients in the entire cohort had an APRI score > 1.0 and of those, 6 patients also had an elevated SL/H ratio.
The USG findings of liver echotexture and echogenicity were compared in patients with and without probable portal hypertension (Table 4). Echogenicity was divided into 2 groups: normal or mildly increased versus moderately or severely increased. The echotexture was also split into 2 groups: normal or mildly granular versus moderately or severely granular. When the two groups were compared, the frequency of increased echogenicity was higher in the probable portal hypertension group(P = 0.004). However, there was no statistically significant difference in the severity of granularity (P = 0.7) between the two groups.
Table 4.
Comparison of abnormal liver echogenicity and echotexture between JS patients with and without portal hypertension
| Portal Hypertension | No portal Hypertension | P- value | |
|---|---|---|---|
|
| |||
| Echogenicity, n | 12* | 87 | 0.004 |
| Normal + mildly increased | 5 | 72 | |
| Moderate + severely increased | 7 | 15 | |
|
| |||
| Echotexture, n | 12* | 87 | 0.72 |
| Normal + mildly granular | 9 | 70 | |
| Moderate + severely granular | 3 | 17 | |
One patient in the portal hypertension cohort was excluded, because he was post-liver transplant.
The frequency of patients with each JS gene mutation was compared between the two cohorts. Of the 20 genes, TMEM67-related JS was found to be significantly more common among patients with an increased SL/H ratio (56% vs. 16%, P = 0.001). There was no statistically significant difference in the frequencies of other genes between the two groups. CEP290-related JS was only represented among patients without portal hypertension, although this did not reach statistical significance due to small numbers (0% vs. 88%, P=0.6).
The entire cohort was evaluated for correlations between liver involvement and presence of other organ dysfunction, including kidney disease, retinal dystrophy, coloboma, and polydactyly. Kidney disease (77% versus 25%, P = 0.001) and colobomas (62% versus 25%, P = 0.02) were more prevalent in the probable portal hypertension group (Table 5).
Table 5.
Frequency of other organ involvement in patients with and without portal hypertension as determined by SL/H ratio
| Portal Hypertension | No portal Hypertension | P value | |
|---|---|---|---|
|
| |||
| Polydactyly | 1 (8%) | 12 (14%) | 0.29 |
| Kidney Disease | 10 (77%) | 22 (25%) | 0.001 |
| Coloboma | 8 (62%) | 22 (25%) | 0.02 |
| Retinal Dystrophy | 1 (8%) | 19 (22%) | 0.46 |
| Total* | 13 | 87 | |
Total represents patients in portal hypertension and non-portal hypertension cohorts. Each patient may have more than one extra-hepatic organ involvement.
Discussion
The rarity of JS makes it difficult to perform studies on a sufficiently large group of patients for statistically meaningful characterization of liver involvement in the context of other organ disease and specific JS genes. Studies published to date estimate the incidence of liver disease in JS at approximately 10–15 %, likely an underestimate, as signs and symptoms of hepatic disease become noticeable later in life.7 This study describes the first large cohort of JS patients who prospectively underwent comprehensive multisystem evaluations at a single center. Hence, we are able to present liver-related findings in JS in the context of other extra-neurological manifestations as well as molecular genetic findings. In addition, the comprehensive nature of our data allowed us to identify parameters that may predict development of portal hypertension in JS.
Patients with liver involvement, as well as those with probable portal hypertension, were older than patients without liver involvement, underscoring the fact that liver disease in JS is progressive and symptomatic disease requires time to develop.
Our analysis of the laboratory biomarkers revealed significantly lower platelet counts, as well as elevated ALT, AST, AP and GGT, levels in JS patients with probable portal hypertension. All patients with elevated AP also had increased GGT levels, confirming hepatic origin for alkaline phosphatase. In fact, the elevation in GGT was much higher than that of the other enzymes. This finding is likely related to bile duct injury or proliferation, which is common in patients with congenital hepatic fibrosis in association with other ciliopathies.8 PT and albumin levels were normal in all patients including the probable portal hypertension group, suggesting intact synthetic function of the liver, consistent with the pathophysiology of congenital hepatic fibrosis, which is a form of non-cirrhotic portal hypertension.
APRI, an index used for predicting significant hepatic fibrosis and cirrhosis, was significantly higher among JS patients deemed to have probable portal hypertension based on the SL/H ratio. The APRI has been used as a non-invasive tool for predicting cirrhosis in patients with hepatitis C, and subsequently validated in several other liver diseases.26,27,28 The APRI score correlates with the hepatic venous pressure gradient29 but, prior to this study, the APRI has not been applied to patients with non-cirrhotic portal hypertension. This study suggests that there may be a role for this scoring system in determining severity of liver disease in patients with non-cirrhotic portal hypertension, as in JS.
Liver USG in CHF shows diffusely increased echogenicity and echotexture as well as grossly heterogeneous liver parenchyma with hyperechoic areas representing sites of fibrosis.8 In addition, some CHF patients may have macroscopic cystic dilatations of the medium sized intrahepatic bile ducts, referred to as Caroli’s syndrome.8,11 None of the patients in this JS cohort had evidence of macrocystic disease in the liver, as seen in Caroli’s Syndrome, and the common bile duct was normal size in all patients. This finding suggests that the risk for cholangitis in JS may be lower than other ciliopathies with biliary abnormalities. In our cohort, we found that JS patients with probable portal hypertension were more likely to have increased liver echogenicity on ultrasound, although there was no difference in the frequency of abnormal echotexture between the two groups. This suggests that liver ultrasonography can aid in the diagnosis of probable portal hypertension, but is best used in conjunction with other clinical findings.
Genotype-phenotype correlations in JS are hampered by the extreme genetic heterogeneity of JS, with more than 30 causative genes identified to date. Even in a large cohort such as ours, many genes are represented by only a few patients, limiting statistical power for correlations. The most commonly mutated genes in our JS cohort included TMEM67 (20%), C5orf42 (14%), CC2D2A (11%), CEP290 (8%), AHI1 (7%) and KIAA0586 (7%). We identified a strong association between liver disease in JS and the TMEM67 gene. This is consistent with data from previous studies, which reported similar associations.8,10,30,31 Only 1 of the 7 CEP290-related JS patients had liver disease, based on abnormal-appearing liver parenchyma seen on ultrasound, and none had enlarged spleen, suggesting that patients with CEP290-related JS are less likely to develop hepatic involvement and may not require rigorous surveillance for liver disease. Bachmann-Gagescu et. al (2015) also reported similarly low risk of liver disease in CEP290-related JS.9 However, to validate this finding a larger number of CEP290-related JS patients needs to be phenotyped for liver disease.
Finally, our data revealed that coloboma as well as kidney disease were more prevalent among patients with probable portal hypertension. The liver disease in JS is known to be associated with coloboma; this subset of JS patients has been referred to as having COACH syndrome.7–9,21 The relatively high frequency of kidney disease among JS patients with liver disease, an association recently reported in another large study, has implications on management of these patients.9
Under the same protocol, we previously performed a similar analysis in a large cohort of patients with autosomal recessive polycystic kidney disease (ARPKD), another ciliopathy that by definition is associated with CHF.11 In our ARPKD cohort, the prevalence of portal hypertension was 65%, much higher than the 12 % in our JS cohort. Furthermore, the severity of portal hypertension in ARPKD was much worse in comparison to JS, based on much larger spleen sizes, and higher number of patients with collateral vessels.11 We also noted other differences in the nature of liver disease when comparing our cohorts with JS and ARPKD. Differing from our findings on JS, the ARPKD cohort demonstrated no significant difference in hepatic enzymes between the portal hypertension and non-portal hypertension groups; patients with ARPKD without portal hypertension also had elevated liver enzymes. This is explainable by the fact that ARPKD is caused by mutations in a single gene (PKHD1) and hence results in some degree of CHF in all patients. In contrast, JS is caused by mutations in one of over 30 genes, many of which do not have a known role in bile duct remodeling and, hence, not associated with ductal plate malformation/CHF. In addition, in ARPKD, we found a higher frequency of moderately/severely increased liver echogenicity on ultrasonography among those with portal hypertension. These differences between the two ciliopathies are potentially explainable by the more severe nature of CHF in ARPKD. Finally, the ARPKD study also showed that the APRI score was elevated in patients with portal hypertension.
This study does have several limitations. First, the size of the cohort is small, although it represents the largest number of subjects with JS evaluated at a single center. This reflects the rarity of JS. Second, due to the extreme genetic heterogeneity of JS, many genes were represented by only a few patients. As a result, genotype-phenotype correlations were not possible to draw except for the most common genes. Third, our cohort may be enriched with JS patients with liver disease such as those with TMEM67-related JS because of ascertainment bias; such patients may have a greater interest in enrolling in research studies. Given that this study also evaluated other organ involvement, such as kidney and retina disease, there may also be a bias towards JS patients with other extra-neurological disease. Lastly, use of the SL/H ratio to classify patients as having probable portal hypertension could have been confounded if the splenomegaly was caused by a disease process not related to hepatic dysfunction and we recognize that the majority of our patients could only be categorized as having “probable” portal hypertension. However, given the pediatric cohort, it was not feasible to look for more definitive stigmata of portal hypertension, such as esophageal varices or elevated portal pressures, without clinical indication for EGD or transjugular liver biopsy, respectively.
In conclusion, this study characterized liver disease in a large cohort of patients with JS. Patients with liver disease were relatively older underscoring the progressive nature of CHF in JS that may not be recognized early on, and hence, requires regular monitoring. Significant elevations in liver enzymes, especially GGT, as well as spleen size and thrombocytopenia, may be useful in predicting probable portal hypertension. APRI may also be useful in predicting disease severity in non-cirrhotic portal hypertension. Differing from ARPKD, JS patients do not have cystic dilatations of the biliary system (Caroli’s syndrome), suggesting that JS patients may not be at increased risk for cholangitis. Patients with TMEM67-related JS are at highest risk for liver disease complicated by probable portal hypertension; hence, retinal colobomas that are present at birth and associated with TMEM67-related JS, increase the risk for significant liver disease. This information can be useful for clinicians to identify patients with JS at risk of developing advanced liver disease, and to devise optimal surveillance for those individuals.
Supplementary Material
What is current knowledge
Joubert syndrome is a ciliopathy associated with liver disease
Liver disease in this syndrome usually occurs in the form of congenital hepatic fibrosis, a form of non-cirrhotic portal hypertension
What is new here
In JS, the cohort of patients with probable portal hypertension was found to have intact synthetic liver function, but elevated AST, ALT, AP and GGT
Differing from other ciliopathies, JS did not carry an increased cholangitis risk
The JS patient cohort with probable portal hypertension was found to have an increased prevalence of mutations in TMEM67; those individuals are also more likely to have colobomas
References
- 1.Joubert M, Eisenring JJ, Robb JP, Andermann F. Familial agenesis of the cerebellar vermis: a syndrome of episodic hyperpnea, abnormal eye movements, ataxia, and retardation. 1969. Neurology. 1969 Sep;19(9):813–825. doi: 10.1212/wnl.19.9.813. [DOI] [PubMed] [Google Scholar]
- 2.Maria BL, Quisling RG, Rosainz LC, Yachnis AT, Gitten J, Dede D, Fennell E. Molar tooth sign in Joubert syndrome: clinical, radiologic, and pathologic significance. J Child Neurol. 1999 Jun;14(6):368–76. doi: 10.1177/088307389901400605. [DOI] [PubMed] [Google Scholar]
- 3.Goetz SC, Anderson KV. The primary cilium: a signalling centre during vertebrate development. Nat Rev Genet. 2010;11(5):331–44. doi: 10.1038/nrg2774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Parisi MA. Clinical and molecular features of Joubert syndrome and related disorders. Am J Med Genet C Semin Med Genet. 2009;151C(4):326–40. doi: 10.1002/ajmg.c.30229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gleeson JG, Keeler LC, Parisi MA, et al. Molar tooth sign of the midbrain-hindbrain junction: occurrence in multiple distinct syndromes. Am J Med Genet A. 2004;125A(2):125–134. doi: 10.1002/ajmg.a.20437. discussion 117. [DOI] [PubMed] [Google Scholar]
- 6.Romani M, Micalizzi A, Valente EM. Joubert syndrome: congenital cerebellar ataxia with the molar tooth. Lancet Neurology Sep. 2013;12(9):894–905. doi: 10.1016/S1474-4422(13)70136-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Desmet VJ. Congenital diseases of intrahepatic bile ducts: variations on the theme “ductal plate malformation”. Hepatology. 1992;16:1069–1083. doi: 10.1002/hep.1840160434. [DOI] [PubMed] [Google Scholar]
- 8.Rock N, McLin V. Liver involvement in children with ciliopathies. Clin Res Hepatol Gastroenterol. 2014 Sep;38(4):407–14. doi: 10.1016/j.clinre.2014.04.001. [DOI] [PubMed] [Google Scholar]
- 9.Bachmann-Gagescu R, Dempsey JC, Phelps IG, O’Roak BJ, Knutzen DM, Rue TC, Ishak GE, Isabella CR, Gorden N, Adkins J, Boyle EA, de Lacy N, O’Day D, Alswaid A, Ramadevi AR, Lingappa L, Lourenço C, Martorell L, Garcia-Cazorla Ă, Ozyürek H, Haliloğlu G, Tuysuz B, Topçu M, Chance P, Parisi MA, Glass IA, Shendure J, Doherty D University of Washington Center for Mendelian Genomics. Joubert syndrome: a model for untangling recessive disorders with extreme genetic heterogeneity. J Med Genet. 2015 Aug;52(8):514–22. doi: 10.1136/jmedgenet-2015-103087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Doherty D, Parisi MA, Finn LS, Gunay-Aygun M, Al-Mateen M, Bates D, Clericuzio C, Demir H, Dorschner M, van Essen AJ, Gahl WA, Gentile M, Gorden NT, Hikida A, Knutzen D, Ozyurek H, Phelps I, Rosenthal P, Verloes A, Weigand H, Chance PF, Dobyns WB, Glass IA. Mutations in 3 genes (MKS3, CC2D2A and RPGRIP1L) cause COACH syndrome (Joubert syndrome with congenital hepatic fibrosis) J Med Genet. 2010;47(1):8–21. doi: 10.1136/jmg.2009.067249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gunay-Aygun Liver and Kidney Disease in Cilopathies. Am J Med Genet C Semin Med Genet. 2009;151C(4):296–306. doi: 10.1002/ajmg.c.30225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gunay-Aygun M, Font-Montgomery E, Lukose L, Tuchman Gerstein M, Piwnica-Worms K, Choyke P, Daryanani KT, Turkbey B, Fischer R, Bernardini I, Sincan M, Zhao X, Sandler NG, Roque A, Douek DC, Graf J, Huizing M, Bryant JC, Mohan P, Gahl WA, Heller T. Characteristics of congenital hepatic fibrosis in a large cohort of patients with autosomal recessive polycystic kidney disease. Gastroenterology. 2013 Jan;144(1):112–121. doi: 10.1053/j.gastro.2012.09.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Turkbey B, Ocak I, Daryanani K, Font-Montgomery E, Lukose L, Bryant J, Tuchman M, Mohan P, Heller T, Gahl WA, Choyke PL, Gunay-Aygun Autosomal recessive polycystic kidney disease and congenital hepatic fibrosis (ARPKD/CHF) M Pediatr Radiol. 2009 Feb;39(2):100–11. doi: 10.1007/s00247-008-1064-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brancati F, Iannicelli M, Travaglini L, Mazzotta A, Bertini E, Boltshauser E, D’Arrigo S, Emma F, Fazzi E, Gallizzi R, Gentile M, Loncarevic D, Mejaski-Bosnjak V, Pantaleoni C, Rigoli L, Salpietro CD, Signorini S, Stringini GR, Verloes A, Zabloka D, Dallapiccola B, Gleeson JG, Valente EM International JSRD Study Group. MKS3/TMEM67 mutations are a major cause of COACH Syndrome, a Joubert Syndrome related disorder with liver involvement. Hum Mutat. 2009;30(2):432–42. doi: 10.1002/humu.20924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gentile M, Di Carlo A, Susca F, Gambotto A, Caruso ML, Panella C, Vajro P, Guanti G. COACH syndrome: report of two brothers with congenital hepatic fibrosis, cerebellar vermis hypoplasia, oligophrenia, ataxia, and mental retardation. Am J of Med Genet. 1996;64(3):514–20. doi: 10.1002/(SICI)1096-8628(19960823)64:3<514::AID-AJMG13>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 16.Poretti Andrea, Snow Joseph, Summer Angela, Tekes Aylin, Huisman Thierry, Aygun Nafi, Carson Kathryn A, Parisi Melissa A, Toro Camilo, Yildirimli Deniz, Vemulapalli Meghana, Mullikin Jim C, Cullinane Andrew, Vilboux Thierry, Gahl William A, Gunay-Aygun Meral NISC Comparative Sequencing Program. Joubert syndrome: neuroimaging findings in 110 patients in correlation with cognitive function and genetic cause. J Med Genet. 2017 Jan 13; doi: 10.1136/jmedgenet-2016-104425. pii: jmedgenet-2016-104425. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 17.Bentley DR, Balasubramanian S, Swerdlow HP, et al. Accurate whole human genome sequencing using reversible terminator chemistry. Nature. 2008 Nov 6;456(7218):53–59. doi: 10.1038/nature07517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Teer JK, Mullikin JC. Exome sequencing: the sweet spot before whole genomes. Hum Mol Genet. 2010 Oct 15;19(R2):R145–151. doi: 10.1093/hmg/ddq333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Teer JK, Green ED, Mullikin JC, Biesecker LG. VarSifter: visualizing and analyzing exome-scale sequence variation data on a desktop computer. Bioinformatics. 2012 Feb 15;28(4):599–600. doi: 10.1093/bioinformatics/btr711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schwartz GJ, Muñoz A, Schneider MF, Mak RH, Kaskel F, Warady BA, Furth SL. New equations to estimate GFR in children with CKD. J Am Soc Nephrol. 2009;20(3):629–37. doi: 10.1681/ASN.2008030287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grubb A, Björk J, Lindström V, Sterner G, Bondesson P, Nyman U. A cystatin C-based formula without anthropometric variables estimates glomerular filtration rate better than creatinine clearance using the Cockcroft-Gault formula. Scand J Clin Lab Invest. 2005;65(2):153–62. doi: 10.1080/00365510510013596. [DOI] [PubMed] [Google Scholar]
- 22.Megremis SD, Vlachonikolis IG, Tsilimigaki AM. Spleen length in childhood with US: normal values based on age, sex, and somatometric parameters. Radiology. 2004;231:129–134. doi: 10.1148/radiol.2311020963. [DOI] [PubMed] [Google Scholar]
- 23.Konus OL, Ozdemir A, Akkaya A, et al. Normal liver, spleen, and kidney dimensions in neonates, infants, and children: evaluation with sonography. AJR Am J Roentgenol. 1998;171:1693–1698. doi: 10.2214/ajr.171.6.9843315. [DOI] [PubMed] [Google Scholar]
- 24.Hernanz-Schulman M, Ambrosino MM, Freeman PC, et al. Common bile duct in children: sonographic dimensions. Radiology. 1995;195:193–195. doi: 10.1148/radiology.195.1.7892467. [DOI] [PubMed] [Google Scholar]
- 25.Vilboux Thierry, Doherty Daniel A, Glass Ian A, Parisi Melissa A, Malicdan May Christine V, Phelps Ian G, Cullinane Andrew R, Zein Wadih, Brooks Brian P, Heller Theo, Soldatos Ariane, Oden Neal L, Yildirimli Deniz, Vemulapalli Meghana, Mullikin James C, Gahl William A, Gunay-Aygun Meral NISC Comparative Sequencing Program. Molecular Genetic Findings and Clinical Correlations in 100 Patients with Joubert Syndrome and Related Disorders Patients Prospectively Evaluated at a Single Center. Genet Med. 2017 Jan 26; doi: 10.1038/gim.2016.204. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lin ZH, Xin YN, Dong QJ, et al. Performance of the aspartate aminotransferase-to-platelet ratio index for the staging of hepatitis C-related fibrosis: an updated meta-analysis. Hepatology. 2011;53:726–36. doi: 10.1002/hep.24105. [DOI] [PubMed] [Google Scholar]
- 27.Shin WG, Park SH, Jun S-Y, et al. Simple tests to predict hepatic fibrosis in nonalcoholic chronic liver diseases. Gut Liver. 2007;1:145–150. doi: 10.5009/gnl.2007.1.2.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Forestier J, Dumortier J, Guillaud O, Ecochard M, Roman S, Boillot O, et al. Noninvasive diagnosis and prognosis of liver cirrhosis: a comparison of biological scores, elastometry, and metabolic liver function tests. Eur J Gastroenterol Hepatol. 2010;22:532–40. doi: 10.1097/MEG.0b013e3283343f58. [DOI] [PubMed] [Google Scholar]
- 29.Verma V, Sarin SK, Sharma P, Kumar A. Correlation of aspartate aminotransferase/platelet ratio index with hepatic venous pressure gradient in cirrhosis. United European Gastroenterol J. 2014 Jun;2(3):226–31. doi: 10.1177/2050640614527084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bachmann-Gagescu R, Dempsey JC, Phelps IG, O’Roak BJ, Knutzen DM, Rue TC, Ishak GE, Isabella CR, Gorden N, Adkins J, Boyle EA, de Lacy N, O’Day D, Alswaid A, Radha Ramadevi A, Lingappa L, Lourenço C, Martorell L, Garcia-Cazorla Ă, Ozyürek H, Haliloğlu G, Tuysuz B, Topçu M, Chance P, Parisi MA, Glass IA, Shendure J, Doherty D University of Washington Center for Mendelian Genomics. Joubert syndrome: a model for untangling recessive disorders with extreme genetic heterogeneity. J Med Genet. 2015 Aug;52(8):514–22. doi: 10.1136/jmedgenet-2015-103087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kroes HY, Monroe GR, van der Zwaag B, Duran KJ, de Kovel CG, van Roosmalen MJ, Harakalova M, Nijman IJ, Kloosterman WP, Giles RH, Knoers NV, van Haaften G. Joubert syndrome: genotyping a Northern European patient cohort. Eur J Hum Genet. 2016 Feb;24(2):214–20. doi: 10.1038/ejhg.2015.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shneider BL, Abel B, Haber B, Karpen SJ, Magee JC, Romero R, Schwarz K, Bass LM, Kerkar N, Miethke AG, Rosenthal P, Turmelle Y, Robuck PR, Sokol RJ. Childhood Liver Disease Research and Education Network. Portal hypertension in children and young adults with biliary atresia. J Pediatr Gastroenterol Nutr. 2012 Nov;55(5):567–73. doi: 10.1097/MPG.0b013e31826eb0cf. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.