Abstract
Objective
We previously identified septic shock endotypes A and B based on 100 genes reflecting adaptive immunity and glucocorticoid receptor signaling. The endotypes differ with respect to outcome and corticosteroid responsiveness. We determined whether endotypes change during the initial three days of illness, and whether changes are associated with outcomes.
Design
Observational cohort study including existing and newly enrolled participants.
Setting
Multiple pediatric intensive care units.
Patients
Children with septic shock.
Interventions
None.
Measurements and Main Results
We measured the 100 endotyping genes at day 1 and day 3 of illness in 375 patients. We determined if endotype assignment changes over time, and whether changing endotype is associated with corticosteroid response and outcomes. We used multivariable logistic regression to adjust for illness severity, age, and comorbidity burden. Among 132 subjects assigned to endotype A on day 1, 56 (42%) transitioned to endotype B by day 3. Among 243 subjects assigned to endotype B on day 1, 77 (32%) transitioned to endotype A by day 3. Assignment to endotype A on day 1 was associated with increased odds of mortality. This risk was modified by the subsequent day 3 endotype assignment. Corticosteroids were associated with increased risk of mortality among subjects who persisted as endotype A.
Conclusions
A substantial proportion of children with septic shock transition endotypes during the acute phase of illness. The risk of poor outcome and the response to corticosteroids change with changes in endotype assignment. Patients persisting as endotype A are at highest risk of poor outcomes.
Keywords: sepsis, endotypes, gene expression, adaptive immunity, glucocorticoids
INTRODUCTION
Most forms of critical illness reflect heterogeneous syndromes rather than distinct diseases. Clinical care is challenging because not all therapies are appropriate for all patients. In the absence of differentiating what therapies are best for which patients, outcomes in many critical illnesses have changed incrementally over the last decade.
Endotypes are biologically defined subclasses of clinical syndromes that differentiate a heterogeneous cohort based on differing molecular pathobiology. Once differentiated, the underlying pathobiology can be more directly targeted. Current opinion in the field emphasizes the need to define endotypes [1, 2], and recent studies reported endotypes of adults with acute respiratory distress syndrome and in sepsis [3–5].
We previously identified pediatric septic shock endotypes A and B using discovery-oriented hierarchical clustering and transcriptomic data generated from whole blood-derived RNA [6–8]. The endotyping strategy was subsequently refined to a 100-gene expression mosaic reflecting adaptive immunity and glucocorticoid receptor signaling, two biological pathways highly relevant to septic shock pathobiology [9]. Patients assigned to endotype A are characterized by repression of the majority of these genes relative to patients assigned to endotype B. In previous studies, assignment to endotype A on day one of septic shock was independently associated with poor outcome. Corticosteroid prescription was also independently associated with poor outcome among endotype A subjects [9]. We subsequently combined this endotyping strategy with biomarker-based mortality risk stratification and found a subgroup of patients in whom corticosteroids might be beneficial [10]. Thus, endotyping septic shock based on transcriptional profiling has the potential to inform clinical decision-making.
Endotyping strategies reported to date have typically been cross-sectional, enrolling patients at a single time point [3, 4]. Our earlier studies focused on endotype assignment during the first 24 hours of septic shock [9]. Septic shock is a dynamic process; assigning an endotype at a single time point fails to consider this complexity. We hypothesized that patients can change endotype over time, and that such changes are associated with outcome, treatment response, or both. Here, we test this hypothesis by applying our endotyping strategy at days 1 and 3 of illness in a diverse cohort of children with septic shock.
METHODS
Study Protocol
Data were obtained from an ongoing study enrolling children admitted to pediatric intensive care units (PICU) across the United States. The protocol was approved by the Institutional Review Boards of each institution [11, 12]. Children ≤ 10 years of age meeting pediatric-specific criteria for septic shock [13] were enrolled after informed consent from parents or legal guardians. This age group is considered biologically distinct from adults and teenagers as they are pre-pubertal. Blood samples were obtained within 24 hours of a septic shock diagnosis, representing “day 1”. A second blood sample was obtained 48 hours later, representing “day 3”. Total RNA was isolated from whole blood using the PaxGene™ RNA System (Qiagen/Becton Dickson, Valencia, CA). Clinical and laboratory data were collected daily while in the PICU. Mortality and organ failure were tracked for 28 days after enrollment. Organ failure was defined using pediatric specific criteria [13]. Major co-morbidities were coded as being present or absent. The presence of malignancy, immune suppression, and bone marrow transplantation were specifically noted because the immune dysfunction associated with these comorbidities can affect outcome from septic shock. The procedures for coding corticosteroid exposure were previously detailed [14] and was modified for this study to focus on the initial three days of septic shock. Illness severity was measured using PRISM-III scores [15].
Subjects for the current study consisted of one group reported in our previous study focused on day 1 of septic shock [9], and another group of newly enrolled subjects. Among the 300 study subjects in our prior study, there were 247 (82%) with an available day 3 RNA sample. The new group consisted of 128 subjects, generating a final cohort of 375 subjects with complete day 1 and 3 endotyping data.
Public Data
As an initial test of the generalizability, we accessed publically available transcriptomic data representing six critically ill children with meningococcal sepsis who had four or more serial RNA samples during the first 72 hours of illness (ArrayExpress Accession #: E-MEXP-3850, and Gene Expression Omnibus Accession #: GSE11755) [16, 17]. From these data sets, we extracted expression data for the 100 endotyping genes and assigned endotypes at each time point as described below.
Multiplex mRNA Quantification and Endotype Assignment
The 100 endotype-defining genes and the 4 housekeeping genes were previously reported [9]. Gene expression was quantified using the NanoString nCounter™ platform (NanoString Technologies, Seattle, WA) [8, 9]. The endotype assignment procedure was also previously detailed [8, 9]. Briefly, gene expression data from unique subjects at each time point were uploaded to the Gene Expression Dynamics Inspector to generate individual gene expression mosaics [18, 19]. These were then compared to reference mosaics to allocate each subject to either endotype A or B, for both day 1 and 3, using computer-assisted image analysis. The reference mosaics represent the average expression patterns of study subjects assigned to endotype A and B, respectively, in our previous studies [6–8].
Gene Expression Score
As an alternative approach to endotype assignment, we used the gene expression score (GES) [9]. The GES quantifies the variability of the expression of the 100 endotype-defining genes within a subject; it reflects the sum of the squared differences between the expression levels of each gene and the geometric mean of all genes for a given patient. Endotype A subjects have a gene expression pattern dominated by decreased expression across a majority of endotyping genes, reflected as decreased variability between genes. Conversely, endotype B subjects have a mixture of increased and decreased expression of the endotyping genes, reflected as increased variability between genes. Consequently, the GES is a continuous variable reflecting how close each patient is to being characterized as endotype A or B.
Comparisons to Healthy Controls
Previous studies have not considered how the endotyping genes are expressed in healthy children. We therefore measured the endotyping genes in 47 healthy controls. The enrollment procedures for controls were previously reported [11, 12, 20, 21]. Briefly, they were recruited from the ambulatory departments of participating institutions using criteria that excluded subjects with any form of systemic inflammation. There were 24 males and 23 females in the control cohort with a median age (IQR) of 2.9 years (1.2 – 5.7). Control data represent a single time point based on the assumption that expression of the endotyping genes does not vary significantly in the healthy state.
We calculated the mean GES of controls as a baseline measure of variability of expression of the 100 endotyping genes. We then calculated the GES difference (GESD) relative to controls for each septic shock subject by subtracting the individual subjects’ GES from the mean GES of the control subjects. The mean GES of endotype A subjects is less than that of control subjects, while the GES of endotype B subjects is greater than that of control subjects. Therefore, higher GESD values reflect the variability associated with endotype A while lower values reflect the variability associated with endotype B.
Baseline Mortality Probability
Baseline mortality probability was estimated using the Pediatric Sepsis Biomarker Risk Model (PERSEVERE), which is calculated from the serum protein concentrations of five biomarkers [10, 22]. There were 356 subjects (95%) with available PERSEVERE data. Among these, 235 (66%) had PERSEVERE data previously reported [10], while the remainder had newly generated PERSEVERE data.
Data Analysis
Statistical procedures used SigmaStat Software (Systat Software, Inc., San Jose, CA). Comparisons between groups used the Mann-Whitney U-test, chi-square test, or Fisher’s exact tests as appropriate. The association between endotype allocation and outcome was modeled using multivariable logistic regression, adjusting for illness severity, age, and comorbidity burden. The primary outcome variable for the regression procedures was all cause 28-day mortality. We also modeled a composite variable, “complicated course”, defined as the persistence of two or more organ failures at day seven of septic shock or 28-day mortality [9, 10, 14, 23]. Since this was an exploratory study, a priori we planned to extend upon our initial analysis, guided by the findings. For ease of reference and to contextualize the choice of analytic approaches, we describe exploratory analyses in the results section.
RESULTS
Binary Temporal Endotype Assignment
Initially, subjects were allocated into one of four temporal endotypes, AA, AB, BB, or BA, where the first letter describes the day 1 endotype and the second describes the day 3 endotype. Table 1 shows the clinical characteristics and demographic data based on temporal endotype. Among 132 subjects allocated to endotype A on day 1, 56 (42%) transitioned to endotype B by day 3. Among 243 subjects allocated to endotype B on day 1, 77 (32%) transitioned to endotype A by day 3. The proportion of subjects with poor outcome, as measured by 28-day mortality and complicated course, was greater among the AA and AB groups when compared to the BB and BA groups. The groups also differed with respect to age, neutrophil counts, lymphocyte counts, the proportion of subjects prescribed corticosteroids, and the proportion of subjects with either a co-morbidity or immune suppression. No other differences were noted.
Table 1.
Clinical and demographic data based on temporal endotype groups.
| Variable | Temporal Endotype Group | |||
|---|---|---|---|---|
| AA | AB | BB | BA | |
| N | 76 | 56 | 166 | 77 |
| Median age, years (IQR)a | 0.8 (0.2 – 2.9) | 2.0 (0.8 – 4.3) | 4.3 (1.8 – 7.2) | 2.8 (1.1 – 7.3) |
| Males, n (%) | 44 (58) | 34 (61) | 98 (59) | 37 (48) |
| Median PRISM (IQR) | 12 (8 – 19) | 16 (9 – 20) | 12 (8 – 18) | 10 (6 – 16) |
| 28 day mortality, n (%)b | 12 (16) | 10 (18) | 8 (5) | 1 (1) |
| Complicated course, n (%)b | 37 (49) | 22 (39) | 36 (22) | 9 (12) |
| Gram positive bacteria, n (%) | 14 (18) | 11 (20) | 44 (27) | 15 (19) |
| Gram negative bacteria, n (%) | 18 (24) | 10 (18) | 37 (22) | 17 (22) |
| Other organism, n (%) | 9 (12) | 8 (14) | 23 (14) | 11 (14) |
| Culture negative, n (%) | 35 (46) | 27 (48) | 62 (37) | 34 (44) |
| Co-morbidity, n (%)b | 21 (28) | 16 (29) | 78 (47) | 44 (57) |
| Malignancy, n (%) | 2 (3) | 2 (4) | 16 (10) | 5 (6) |
| Immune suppression, n (%)b,c | 0 (0) | 6 (11) | 21 (13) | 8 (10) |
| Bone marrow transplantation, n (%) | 0 (0) | 1 (2) | 13 (8) | 3 (4) |
| Prescribed corticosteroids, n (%)b | 28 (37) | 28 (50) | 105 (63) | 34 (44) |
| Day 1 median WBC count ×103/mm3 (IQR)d | 8.3 (5.5 – 16.7) | 11.1 (4.6 – 17.0) | 12.7 (6.5 – 19.5) | 12.4 (8.1 – 16.9) |
| Day 1 neutrophil count ×103/mm3 (IQR)a | 4.9 (2.7 – 10.7) | 5.9 (2.3 – 10.4) | 9.3 (3.8 – 16.2) | 9.5 (5.1 – 13.6) |
| Day 1 lymphocyte count ×103/mm3 (IQR)a | 2.3 (1.2 – 3.9) | 2.5 (1.3 – 4.3) | 1.4 (0.6 – 2.4) | 1.5 (0.6 – 2.7) |
| Day 1 monocyte count ×103/mm3 (IQR) | 0.6 (0.2 – 1.4) | 0.6 (0.1 – 1.3) | 0.5 (0.2 – 1.1) | 0.5 (0.3 – 1.1) |
| Day 3 median WBC count ×103/mm3 (IQR) | 8.5 (6.2 – 16.5) | 11.3 (7.4 – 17.4) | 12.5 (8.3 – 19.6) | 10.5 (7.1 – 17.1) |
| Day 3 neutrophil count ×103/mm3 (IQR)a | 4.9 (2.7 – 10.5) | 7.1 (3.8 – 13.7) | 8.8 (5.4 – 15.3) | 7.6 (4.7 – 11.6) |
| Day 3 lymphocyte count ×103/mm3 (IQR)a | 3.2 (2.1 – 4.6) | 2.0 (1.1 – 3.4) | 1.6 (0.6 – 2.8) | 2.4 (1.3 – 3.8) |
| Day 3 monocyte count ×103/mm3 (IQR) | 0.5 (0.2 – 0.8) | 0.6 (0.2 – 1.1) | 0.6 (0.3 – 1.0) | 0.5 (0.3 – 0.9) |
p < 0.05, Kruskal-Wallis ANOVA on Ranks.
p < 0.05, chi-square, 3 degrees of freedom.
Refers to patients with immune suppression not related to malignancy (for example, those receiving immune suppressive medications for solid organ transplantation, or those with a primary immune deficiency).
Complete WBC data were not available for all subjects.
Table 2 shows the results of multivariable logistic regression exploring the association between temporal endotype assignment and outcomes. Assignment to temporal endotype AA or AB was associated with increased odds of mortality. Assignment to temporal endotype AA was also associated with increased odds of complicated course. Conversely, assignment to temporal endotype BB or BA was associated with decreased odds of mortality and complicated course.
Table 2.
Multivariable logistic regression to test for associations between temporal endotype group assignment and poor outcome.
| Mortality | Complicated Course | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| Independent Variable | O.R. | 95% C.I. | P value | O.R. | 95% C.I. | P value |
| Assignment to AA | 2.6 | 1.1 – 6.0 | 0.032 | 3.0 | 1.7 – 5.4 | <0.001 |
| PRISM | 1.1 | 1.0 – 1.1 | <0.001 | 1.1 | 1.1 – 1.1 | <0.001 |
| Age | 1.0 | 0.9 – 1.1 | 0.798 | 1.0 | 0.9 – 1.0 | 0.253 |
| Co-morbidity | 1.1 | 0.5 – 2.6 | 0.748 | 0.9 | 0.6 – 1.6 | 0.798 |
| Assignment to AB | 3.0 | 1.3 – 7.1 | 0.010 | 1.7 | 0.9 – 3.2 | 0.097 |
| PRISM | 1.1 | 1.0 – 1.1 | <0.001 | 1.1 | 1.1 – 1.1 | <0.001 |
| Age | 1.0 | 0.9 – 1.1 | 0.541 | 0.9 | 0.9 – 1.0 | 0.053 |
| Co-morbidity | 1.1 | 0.5 – 2.4 | 0.885 | 0.9 | 0.5 – 1.4 | 0.563 |
| Assignment to BB | 0.4 | 0.2 – 0.9 | 0.033 | 0.6 | 0.4 – 1.0 | 0.044 |
| PRISM | 1.1 | 1.0 – 1.1 | <0.001 | 1.1 | 1.1 – 1.1 | <0.001 |
| Age | 1.0 | 0.9 – 1.1 | 0.811 | 0.9 | 0.9 – 1.0 | 0.127 |
| Co-morbidity | 1.1 | 0.5 – 2.4 | 0.862 | 0.9 | 0.5 – 1.4 | 0.544 |
| Assignment to BAa | -- | -- | -- | 0.3 | 0.2 – 0.7 | 0.004 |
| PRISM | -- | -- | -- | 1.1 | 1.1 −1.1 | <0.001 |
| Age | -- | -- | -- | 0.9 | 0.9 – 1.0 | 0.039 |
| Co-morbidity | -- | -- | -- | 0.9 | 0.6 – 1.5 | 0.759 |
Analysis for mortality not performed because there was only one death among the subjects assigned to the BA group.
Using publically available gene expression data for six critically ill children with meningococcal sepsis [16, 17], we assigned endotypes at between four to six time points over the initial 72 hours of illness (Supplemental Table 1). The one subject who died in this group, was assigned to endotype A during the initial 4 hours, and subsequently transitioned to endotype B. Two other subjects were assigned endotype A at the initial time point and subsequently transitioned to endotype B. These subjects had longer stays in the ICU when compared to the three subjects who remained endotype B during the entire sampling period.
We previously noted that corticosteroid prescription was associated with increased odds of poor outcome among endotype A subjects [9]. We tested for an association between corticosteroid prescription and poor outcome within each temporal endotype group. Table 3 shows that corticosteroid prescription was associated with increased odds of mortality and complicated course among subjects in the AA temporal endotype group. Corticosteroid prescription was not associated with outcomes among the other temporal endotype groups.
Table 3.
Multivariable logistic regression to test for associations between corticosteroid prescription and poor outcome, within temporal endotype group. A separate model was fit for each temporal endotype group.
| Mortality | Complicated Course | ||||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Group | Variable | O.R. | 95% C.I. | P value | O.R. | 95% C.I. | P value |
| AA | Corticosteroids | 15.0 | 2.8 – 80.8 | 0.002 | 2.9 | 1.0 – 7.9 | 0.043 |
| PRISM | 1.1 | 1.0 – 1.1 | 0.219 | 1.1 | 1.0 – 1.1 | 0.039 | |
| Age | 0.8 | 0.6 – 1.1 | 0.243 | 0.9 | 0.7 – 1.1 | 0.226 | |
| Co-morbidity | 3.4 | 0.6 – 18.5 | 0.162 | 1.8 | 0.6 – 5.7 | 0.329 | |
| AB | Corticosteroids | 2.3 | 0.5 – 11.5 | 0.299 | 2.0 | 0.6 – 6.7 | 0.236 |
| PRISM | 1.1 | 1.0 – 1.2 | 0.059 | 1.1 | 1.0 – 1.2 | 0.019 | |
| Age | 1.2 | 1.0 – 1.5 | 0.109 | 1.0 | 0.9 – 1.3 | 0.599 | |
| Co-morbidity | 0.6 | 0.0 – 3.3 | 0.525 | 0.6 | 0.2 – 2.2 | 0.425 | |
| BB | Corticosteroids | 1.2 | 0.2 – 7.3 | 0.862 | 0.8 | 0.3 – 1.9 | 0.637 |
| PRISM | 1.1 | 1.0 – 1.2 | 0.008 | 1.1 | 1.0 – 1.1 | <0.001 | |
| Age | 1.0 | 0.8 – 1.3 | 0.946 | 1.0 | 0.8 – 1.1 | 0.422 | |
| Co-morbidity | 3.0 | 0.5 – 16.6 | 0.212 | 1.2 | 0.5 – 2.6 | 0.724 | |
| BAa | Corticosteroids | -- | -- | -- | 0.9 | 0.2 – 4.0 | 0.858 |
| PRISM | -- | -- | -- | 1.1 | 1.0 – 1.2 | 0.113 | |
| Age | -- | -- | -- | 1.0 | 0.7 – 1.2 | 0.714 | |
| Co-morbidity | -- | -- | -- | 1.0 | 0.2 – 4.5 | 0.966 | |
Analysis for mortality not performed because there was only one death among the subjects assigned to the BA group.
In a sensitivity analysis, we repeated the logistic regression modeling but only included the subjects without preexisting comorbidities (n = 216). This subset did not have corticosteroid exposure prior to the episode of septic shock and one can therefore assume that corticosteroid prescription was exclusively for the indication of septic shock. Among these subjects, corticosteroid prescription was independently associated with increased odds of mortality among subjects in the AA temporal endotype group (O.R. 16.6; 95% C.I. 1.8 to 154.7; p = 0.014), but not among the other temporal endotype groups.
Temporal Endotypes Based on the GES
As an alternative to binary endotype assignment based on gene expression mosaics we used the GES as a continuous measure of endotype. Endotype B subjects have a higher GES relative to endotype A subjects. As well as avoiding the information loss associated with dichotomizing a decision, the GES provides analytical opportunities not possible with binary classifications. Because the GES spanned a range of three logs we log transformed the GES values. We used multivariable logistic regression to test for an association between the GES and poor outcome, as shown in Table 4. On day 1, a higher GES was associated with decreased odds of mortality and complicated course. There was no association between the day 3 GES and outcome.
Table 4.
Multivariable logistic regression to test for associations between the day 1 and day 3 GES, and poor outcome.
| Mortality | Complicated Course | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| Independent Variable | O.R. | 95% C.I. | P value | O.R. | 95% C.I. | P value |
| Day 1 GES | 0.3 | 0.1 – 0.8 | 0.022 | 0.3 | 0.2 – 0.6 | <0.001 |
| Day 3 GES | 0.8 | 0.4 – 1.9 | 0.622 | 1.6 | 0.9 – 2.7 | 0.107 |
| PRISM | 1.1 | 1.0 – 1.1 | <0.001 | 1.1 | 1.1 – 1.1 | <0.001 |
| Age | 1.0 | 0.9 – 1.1 | 0.879 | 0.9 | 0.9 – 1.0 | 0.175 |
| Co-morbidity | 1.2 | 0.5 – 2.8 | 0.627 | 1.0 | 0.6 – 1.6 | 0.934 |
To quantify the degree of exposure to being endotype A or B over the three day period, we computed the sum of the raw GES day 1 and day 3 values. A low GES sum indicates a predominantly endotype A patient, characterized by consistent repression of the endotype-defining genes over the three day study period, while a high GES sum would indicate a subject with more variability in their gene expression. Figures 1A and 1B show that subjects in the lowest quartile of the GES sum, the most endotype A-like subjects, had the highest rates of mortality and complicated course, respectively. The inclusion of patients from all temporal endotypes in the lowest quartile of GES sum, including temporal endotype BB subjects with relatively lower GES scores, illustrates the benefits of using a continuous variable.
Figure 1. Graphical representations of the association between the sum of the raw day 1 and 3 GES values and poor outcome.
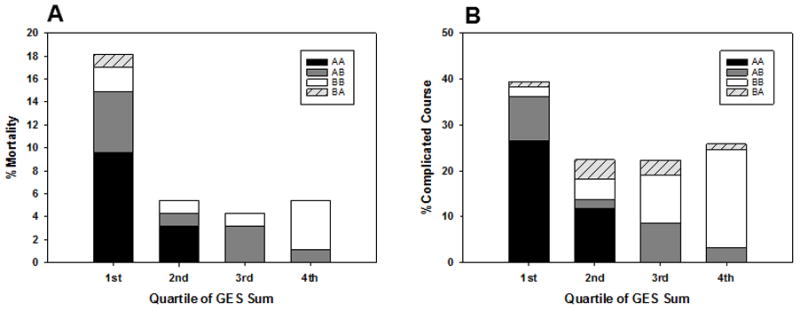
(A), Mortality rates according to quartiles of the sum of the day 1 and day 3 GES values. P = 0.001, Chi-square, 3 degrees of freedom. (B), complicated course rates according to quartiles of the sum of the day 1 and day 3 GES values. P = 0.015, Chi-square, 3 degrees of freedom. The bars are colored to show the distribution of temporal endotypes within the four quartiles.
Comparisons to Normal Healthy Controls
The GESD provides an opportunity to assess endotype changes relative to a reference, control state. Endotype A subjects have a greater GESD relative to endotype B subjects. When considering the interaction between GESD and the PERSEVERE-based mortality probability, we found that higher GESD for both day 1 and day 3 were associated with increased mortality (p= 0.027 and p=0.048, respectively). This suggests that the greater the exposure to the endotype A profile, the worse the outcome even after adjustment for baseline mortality risk.
Corticosteroids and Endotype Transitions
Temporal endotype BB subjects were more frequently prescribed corticosteroids than temporal endotype BA subjects (Table 1). Similarly, temporal endotype AB subjects were more frequently prescribed corticosteroids than temporal endotype AA subjects. This raises the possibility that corticosteroid prescription is associated with transitions to, or sustained assignment as endotype B. We used multivariable logistic regression to test this possibility. Among subjects who were endotype B on day 1, corticosteroids were associated with increased odds of being an endotype B on day 3 (O.R. 2.5, 95% C.I.: 1.3 to 5.0, p = 0.003). Among subjects who were endotype A on day 1, corticosteroids were not associated with transitioning to endotype B on day 3, although there was a trend (O.R. 1.7, 95% C.I.: 0·9 to 3·6, p = 0.126).
DISCUSSION
Our data show that endotyping based on transcriptional profiling has the potential to inform clinical decision making for pediatric septic shock. Our data support the need to consider the dynamic nature of septic shock and expand beyond cross-sectional assignment of endotypes in critical illness. Classification of temporal endotypes reveals that a substantial proportion of subjects transitioned endotypes from day 1 to day 3. Based on a binary classification strategy, subjects assigned to endotype A on day 1 had worse outcomes compared to subjects assigned to endotype B on day 1, consistent with previous findings [9]. We corroborated these observations, qualitatively, in a small independent cohort with greater temporal granularity.
Binary endotype classification is readily understood clinically, but fails to capture that each endotype exists on a spectrum of gene expression. As a continuous variable, the GES provides an opportunity to capture this spectrum and therefore may enable greater analytical granularity. The day 1 GES was independently associated with poor outcomes, but the day 3 GES was not. However, when we grouped the patients based on quartiles reflecting the sum of the day 1 and day 3 GES, those in the lowest quartile had higher rates of mortality and complicated course. Because a lower GES sum reflects being more closely associated with endotype A over the first three days of illness, this indicates that persistence of endotype A portends poor outcome from septic shock. This observation was further corroborated when analyzing the interaction between the GESD and baseline mortality probability.
Our analysis of temporal endotypes provides an opportunity to further explore the influence of corticosteroids. While we previously showed that corticosteroids were associated with increased odds of poor outcome among endotype A subjects [9], we now refine this observation by showing that corticosteroids were associated with poor outcome among subjects who persisted as an endotype A, but not among subjects who transitioned from endotype A to B. Further, our data suggest that corticosteroids might be associated with sustained assignment as endotype B. As in previous studies, these observations and their implications should be interpreted with caution because corticosteroid prescription was not standardized. Our data suggest a study designed to support causal inference is warranted.
The poor outcomes in our cohort are associated with persistent repression of genes corresponding to the adaptive immune system and glucocorticoid receptor signaling. Whether this represents a cause or an effect is currently unknown. It appears that this repression pattern is not simply a manifestation of baseline immune suppression because there were no subjects with this comorbidity in the AA group. We note that the biology and outcomes associated with the pediatric endotypes are analogous to the sepsis response signatures reported among adults with sepsis [4], although there is limited overlap between the sepsis response signatures and the endotype-defining expression pattern [5]. We posit that our findings reflect persistent immune suppression and an altered response to corticosteroids among patients who remain endotype A.
The clinical utility of molecular endotyping is not in prognostication per se. Rather, the primary clinical utility is in the identification of septic shock subgroups based on biological differences having the potential to inform therapeutic decisions beyond antibiotics and supportive care. Two potential therapies relevant to our endotyping strategy are corticosteroids [24] and immune modulation [25]. Our endotyping strategy is based on genes directly involved in the biological pathways targeted by corticosteroids and immune modulation. We note that the endotyping genes were identified through unsupervised analyses seeking to identify gene expression-based subgroups of pediatric septic shock, rather than pre-selection of genes. Once verified either in epidemiological studies or in stratified analyses of current studies, the utility of the endotyping strategy should be tested in clinical trials. For example, we hypothesize that patients who persist as endotype A are perhaps the best candidates for immune enhancing therapies and that corticosteroids should be avoided in such patients. Conversely, our previous studies indicate that endotype B patients who are at higher baseline risk of mortality, might derive the most benefit from adjunctive corticosteroids [10].
In summary, a substantial proportion of children with septic shock transition endotypes over the first three days of illness. The risk of mortality is most strongly associated with the day 1 endotype, but is modified by the day 3 endotype. We replicated this finding using publicly available data, although the sample was small. Corticosteroids are associated with poor outcomes among patients with a persistent endotype A, but not in those who transition from endotype A to B, nor in those initially assigned to endotype B. Given that the biology associated with the endotype-defining genes, the effects of these endotype transitions on septic shock outcomes and treatment responses warrant further studies. While the current study suggests a high degree of dynamic complexity, it is based only on two time points. Studies with greater temporal granularity will allow for disentangling the complexity of septic shock.
Supplementary Material
Acknowledgments
FUNDING SOURCE
Supported by National Institutes of Health Grants RO1GM099773 and R01GM108025.
Footnotes
AUTHOR CONTRIBUTIONS
Hector R. Wong: Conceived and developed the study, obtained funding for the study, conducted the analyses, and wrote the initial manuscript.
Natalie Z. Cvijanovich, Nick Anas, Geoffrey L. Allen, Neal J. Thomas, Michael T. Bigham, Scott L. Weiss, Julie C. Fitzgerald, Paul A. Checchia, Keith Meyer, Michael Quasney, Mark Hall, Rainer Gedeit, Robert J. Freishtat, Jeffrey Nowak, Shekhar S. Raj, Shira Gertz, and Jocelyn R. Grunwell: Enrolled subjects at the participating institutions, provided clinical data and biological samples, and provided critical review and approval of the manuscript.
Christopher J. Lindsell: Collaborated with Dr. Wong in conceiving the study, data analysis, and editing the manuscript.
Copyright form disclosure: Drs. Wong, Weiss, Fitzgerald, and Checchia’s institutions received funding from the National Institutes of Health (NIH). Drs. Wong, Cvijanovich, Allen, Fitzgerald, Checchia, Meyer, Lutfi, and Lindsell received support for article research from the NIH. Dr. Wong disclosed: The Cincinnati Children’s Hospital Research Foundation has submitted a provisional patent application for the temporal endotyping strategy reported in this manuscript. Drs. Wong and Lindsell are named as co-inventors on the patent application. Dr. Cvijanovich received funding from Cincinnati Children’s Hospital Medical Center and Boston Children’s Hospital. Dr. Allen’s institution received funding from a subcontract from Cincinnati Children’s from their NIH grant. Dr. Thomas’s institution received funding from the University of Cincinnati through an NIH subaward, and he received funding from Therabron, CareFusion, and GeneFluidics. Dr. Weiss received funding from ThermoFisher Scientific and Bristol-Meyers Squibb Company. Dr. Meyer’s institution received funding from Cincinnati Children’s Medical Center (funds paid to institution for specimen collection). Dr. Quasney received support for article research from the University of Michigan Medical School. Dr. Hall received funding from Bristol-Myers Squibb. Dr. Lutfi’s institution receive funding from NIH funding by principal investigator through Cincinnati Children Hospital Medical Center. Dr. Lindsell’s institution received funding from NIH/National Institute of General Medical Sciences, and he disclosed he is a co-inventor on invention disclosures and patents in the area of septic shock risk stratification, as disclosed by the lead author. The remaining authors have disclosed that they do not have any potential conflicts of interest.
COMPETING INTERESTS
The Cincinnati Children’s Hospital Research Foundation has submitted a provisional patent application for the temporal endotyping strategy reported in this manuscript. Drs. Wong and Lindsell are named as co-inventors on the patent application.
References
- 1.Cohen J, Vincent JL, Adhikari NK, et al. Sepsis: a roadmap for future research. Lancet Infect Dis. 2015;15(5):581–614. doi: 10.1016/S1473-3099(15)70112-X. [DOI] [PubMed] [Google Scholar]
- 2.Prescott HC, Calfee CS, Thompson BT, et al. Toward Smarter Lumping and Smarter Splitting: Rethinking Strategies for Sepsis and Acute Respiratory Distress Syndrome Clinical Trial Design. Am J Respir Crit Care Med. 2016;194(2):147–155. doi: 10.1164/rccm.201512-2544CP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Calfee CS, Delucchi K, Parsons PE, et al. Subphenotypes in acute respiratory distress syndrome: latent class analysis of data from two randomised controlled trials. Lancet Respir Med. 2014;2(8):611–620. doi: 10.1016/S2213-2600(14)70097-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Davenport EE, Burnham KL, Radhakrishnan J, et al. Genomic landscape of the individual host response and outcomes in sepsis: a prospective cohort study. Lancet Respir Med. 2016;4(4):259–271. doi: 10.1016/S2213-2600(16)00046-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burnham KL, Davenport EE, Radhakrishnan J, et al. Shared and Distinct Aspects of the Sepsis Transcriptomic Response to Fecal Peritonitis and Pneumonia. Am J Respir Crit Care Med. 2016;196(3):328–339. doi: 10.1164/rccm.201608-1685OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wong HR, Cvijanovich N, Lin R, et al. Identification of pediatric septic shock subclasses based on genome-wide expression profiling. BMC Med. 2009;7:34. doi: 10.1186/1741-7015-7-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wong HR, Wheeler DS, Tegtmeyer K, et al. Toward a clinically feasible gene expression-based subclassification strategy for septic shock: proof of concept. Crit Care Med. 2010;38(10):1955–1961. doi: 10.1097/CCM.0b013e3181eb924f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wong HR, Cvijanovich NZ, Allen GL, et al. Validation of a gene expression-based subclassification strategy for pediatric septic shock. Crit Care Med. 2011;39(11):2511–2517. doi: 10.1097/CCM.0b013e3182257675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wong HR, Cvijanovich NZ, Anas N, et al. Developing a clinically feasible personalized medicine approach to pediatric septic shock. Am J Respir Crit Care Med. 2015;191(3):309–315. doi: 10.1164/rccm.201410-1864OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wong HR, Atkinson SJ, Cvijanovich NZ, et al. Combining prognostic and predictive enrichment strategies to identify children with septic shock responsive to corticosteroids. Crit Care Med. 2016;44(10):e1000–1003. doi: 10.1097/CCM.0000000000001833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shanley TP, Cvijanovich N, Lin R, et al. Genome-level longitudinal expression of signaling pathways and gene networks in pediatric septic shock. Mol Med. 2007;13(9–10):495–508. doi: 10.2119/2007-00065.Shanley. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wong HR, Shanley TP, Sakthivel B, et al. Genome-level expression profiles in pediatric septic shock indicate a role for altered zinc homeostasis in poor outcome. Physiol Genomics. 2007;30(2):146–155. doi: 10.1152/physiolgenomics.00024.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goldstein B, Giroir B, Randolph A. International pediatric sepsis consensus conference: definitions for sepsis and organ dysfunction in pediatrics. Pediatr Crit Care Med. 2005;6(1):2–8. doi: 10.1097/01.PCC.0000149131.72248.E6. [DOI] [PubMed] [Google Scholar]
- 14.Atkinson SJ, Cvijanovich NZ, Thomas NJ, et al. Corticosteroids and pediatric septic shock outcomes: a risk stratified analysis. PLoS One. 2014;9(11):e112702. doi: 10.1371/journal.pone.0112702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pollack MM, Patel KM, Ruttimann UE. The Pediatric Risk of Mortality III--Acute Physiology Score (PRISM III-APS): a method of assessing physiologic instability for pediatric intensive care unit patients. J Pediatr. 1997;131(4):575–581. doi: 10.1016/s0022-3476(97)70065-9. [DOI] [PubMed] [Google Scholar]
- 16.Kwan A, Hubank M, Rashid A, et al. Transcriptional instability during evolving sepsis may limit biomarker based risk stratification. PLoS One. 2013;8(3):e60501. doi: 10.1371/journal.pone.0060501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Emonts M. Erasmus University Rotterdam. 2008. Thesis. [Google Scholar]
- 18.Eichler GS, Huang S, Ingber DE. Gene Expression Dynamics Inspector (GEDI): for integrative analysis of expression profiles. Bioinformatics. 2003;19(17):2321–2322. doi: 10.1093/bioinformatics/btg307. [DOI] [PubMed] [Google Scholar]
- 19.Guo Y, Eichler GS, Feng Y, et al. Towards a holistic, yet gene-centered analysis of gene expression profiles: a case study of human lung cancers. J Biomed Biotechnol. 2006;2006(5):69141. doi: 10.1155/JBB/2006/69141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cvijanovich N, Shanley TP, Lin R, et al. Validating the genomic signature of pediatric septic shock. Physiol Genomics. 2008;34(1):127–134. doi: 10.1152/physiolgenomics.00025.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wong HR, Cvijanovich N, Allen GL, et al. Genomic expression profiling across the pediatric systemic inflammatory response syndrome, sepsis, and septic shock spectrum. Crit Care Med. 2009;37(5):1558–1566. doi: 10.1097/CCM.0b013e31819fcc08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wong HR, Salisbury S, Xiao Q, et al. The pediatric sepsis biomarker risk model. Crit Care. 2012;16(5):R174. doi: 10.1186/cc11652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Abulebda K, Cvijanovich NZ, Thomas NJ, et al. Post-ICU admission fluid balance and pediatric septic shock outcomes: a risk-stratified analysis. Crit Care Med. 2014;42(2):397–403. doi: 10.1097/CCM.0b013e3182a64607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Patel GP, Balk RA. Systemic steroids in severe sepsis and septic shock. Am J Respir Crit Care Med. 2012;185(2):133–139. doi: 10.1164/rccm.201011-1897CI. [DOI] [PubMed] [Google Scholar]
- 25.Hotchkiss RS, Sherwood ER. Immunology. Getting sepsis therapy right. Science. 2015;347(6227):1201–1202. doi: 10.1126/science.aaa8334. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


