Abstract
Introduction
Prophylactic cranial irradiation (PCI) improves survival for small cell lung cancer (SCLC). Evidence for PCI in limited-stage SCLC largely derives from studies requiring only chest x-ray (CXR) to determine remission status. We analyzed thoracic chemoradiation therapy (TCRT) outcomes by imaging modality to determine which patients benefitted most from PCI.
Methods
All limited-stage SCLC patients who received both TCRT and PCI at our institution were reviewed. Imaging between TCRT end and PCI start was characterized as complete (CR), partial (PR), or other response.
Results
38 consecutive patients were assessed for TCRT response prior to PCI with CXR (n=21), chest computed tomography (CT) (n=27), and/or positron emission tomography (PET)/CT (n=11). CR was identified on 71% of CXR, 41% of CT, and 18% of PET/CT. Median survival was 28.3 months for the entire cohort and did not differ for patients who had CXR alone vs. CT and/or PET/CT for restaging (p=0.78) or those with PR on any modality versus CR on all modalities (22.6 months vs. 45.5 months, p=0.22). CT CR patients had numerical but not statistically significant improved 2-year (p=0.18) and 3-year (p=0.13) survival compared to CT PR.
Conclusions
CXR remains an appropriate modality to assess TCRT response prior to PCI in limited-stage SCLC. Advanced imaging did not inform the decision to offer PCI in this study. Given similar excellent survival profiles independent of imaging modality and TCRT response, this analysis suggests limited-stage SCLC patients with PR on any modality should not be denied PCI, akin to standards for extensive-stage SCLC.
Keywords: Thoracic chemoradiation, partial response, chest x-ray, computed tomography, positron emission tomography
INTRODUCTION
Small cell lung cancer (SCLC) is considered a systemic disease with a high rate of distant dissemination, including to the brain. Approximately 10–15% of patients with SCLC are diagnosed with brain metastasis, and 30–60% develop intracranial metastasis during the course of their illness.1,2
At diagnosis, approximately one-third of patients with SCLC are found to have limited-stage disease without extrathoracic metastases. Radiation therapy administered concurrently with cisplatin and etoposide is standard treatment for patients with limited-stage SCLC.3–5 Thoracic chemoradiation therapy (TCRT) allows for up to a 90% response rate, with 50–80% of limited-stage SCLC patients achieving a complete response (CR) to therapy.6–8 However, platinum-based chemotherapy does not effectively cross the blood-brain barrier, leaving the central nervous system vulnerable to subsequent metastases. In fact, approximately 50–80% of patients who achieve a CR to initial therapy will develop brain metastasis within 2 years, with the risk increasing with a longer survival from diagnosis.9,10
Prophylactic cranial irradiation (PCI) has been shown to decrease the development of brain metastasis and improve both disease-free and overall survival in extensive-stage patients, but no similar survival benefit has been shown in a prospective study for limited-stage patients.1,11 An Institut Gustave-Roussy study randomized 300 patients with predominantly limited-stage SCLC who achieved a CR to chemoradiation to receive PCI vs. no PCI. Although PCI reduced the overall rate of brain metastasis (40% vs. 67%, p<0.00001), even an expansion analysis of 511 patients did not find a significant improvement in overall survival (18% vs. 15% at 5 years, p=0.06).1,12 Two meta-analyses have shown an overall survival benefits to PCI in limited-stage patients11,13, but an early presentation of a phase III randomized trial failed to show a benefit to PCI in this population.14
Unlike with extensive-stage SCLC, PCI trials for limited-stage SCLC have generally only included patients who have achieved a CR to initial therapy. However, prior published studies and meta-analyses, such as the Prophylactic Cranial Irradiation Overview Collaboration Group meta-analysis11, have often required only a chest x-ray (CXR) to assess response status. Consequently, many patients classified as having a CR on CXR may have been classified as having a more limited response to TCRT had they undergone more advanced imaging studies, such as computed tomography (CT) of the chest or positron emission tomography (PET)/CT15, and they accordingly would not have been offered PCI or been eligible for these prior trials despite the potential for PCI to improve survival in this population.11 Additionally, current practice guidelines dictate CT imaging to assess for thoracic treatment response prior to considering PCI.16
In this study, we analyzed and defined thoracic responses to TCRT according to imaging modality for patients with limited-stage SCLC to determine which imaging modality most correlates with overall survival and which patients benefit most from PCI. We hypothesized that fewer patients would have a CR on advanced imaging compared with CXR, and that patients with a CR on CXR or a PR or CR on advanced imaging would have prolonged survival that would warrant proceeding with PCI, whereas survival would be inferior in patients with a PR on CXR.
MATERIALS AND METHODS
In this Institutional Review Board-approved study, all patients with histologically-confirmed limited-stage SCLC who received both thoracic radiation therapy and concurrent chemotherapy followed by PCI at the University of Pennsylvania from 1998–2010 were reviewed to allow for adequate duration of follow-up to assess for survival outcomes. Patients who received any component of treatment of thoracic radiotherapy, chemotherapy, or PCI at another facility were excluded from this analysis. All patients were treated with definitive-intent thoracic radiotherapy delivered either once or twice daily with concurrent platinum-based chemotherapy. In all cases, radiation therapy began with either cycle 1 or cycle 2 of chemotherapy. PCI was delivered within 6 months of initiating chemotherapy and within 3–12 weeks after completing chemotherapy.
All patients underwent imaging with at least one radiologic modality (CXR, CT chest, and/or PET/CT) to assess thoracic disease response of both the primary tumor and sites of nodal metastases following the completion of TCRT but prior to beginning PCI. All CXRs were ordered and conducted with both a PA and lateral approach. All patients also underwent neuroimaging, most commonly with an MRI, but when not possible a CT with IV contrast, between the completion of TCRT and the start of PCI to confirm the absence of brain metastases. Each imaging study between the end of TCRT and the start of PCI was characterized as a CR, PR, stable disease, or disease progression. CXR and CT were characterized according to modified Response Evaluation Criteria in Solid Tumors (RECIST) criteria based on reports from board-certified radiologists who specialize in thoracic imaging.17 CR was defined as the total resolution of all parenchymal disease and a reduction of all lymph nodes to <1.0 cm in short axis. PR was defined as a ≥30% decrease in the sum of the diameters of all target lesions. Progressive disease was defined as a ≥20% increase in the sum of the diameters of lesions or the appearance of new lesions, whereas stable disease was defined as neither sufficient shrinkage to quality for PR nor sufficient increase to qualify for progressive disease.
PET was characterized according to Positron Emission Tomography Response Criteria in Solid Tumors (PERCIST) 1.018. CR was defined as resolution of FDG uptake, with FDG uptake less than the mean SUL of the liver, but designation of CR did not require the peak SUL to decrease to zero. PR was defined as a decrease of ≥30% and ≥0.8 SUL units in the most intense lesion between baseline and follow-up. Progressive disease was defined as an increase of ≥20% and of ≥0.8 SUL units in a target lesion or development of new lesions, whereas stable disease was defined as an increase or decrease of peak SUL of <30%.
Overall survival was calculated from the date of PCI start to the date of patient death or last contact. Two-way unpaired t-tests were used to determine significance between groups and statistical significance was defined as p<0.05.
RESULTS
Thirty-eight consecutive patients were included. Patients were predominantly female (76%) and Caucasian (84%), with a median age of 66 years at the time of PCI (Table 1). Patients received once (n=4, median 63 Gy /1.8 Gy) or twice (n=34, median 45 Gy/1.5 Gy) daily thoracic radiation therapy concurrent with platinum-based chemotherapy, most frequently for 4 cycles (89%). PCI began at a median of 11.4 weeks following the completion of thoracic radiation therapy and was most commonly administered to 25 Gy/2.5 Gy (n=17) (range 24–36 Gy/1.5–2.5 Gy). Following TCRT, patients underwent CXR (n=21), CT chest (n=27), and/or PET/CT (n=11) for restaging prior to PCI.
Table 1.
Patient Characteristics and Treatments
| Age at Time of PCI | |
| Median | 66 years (42 – 84 years) |
| Gender | |
| Female | 29 (76%) |
| Male | 9 (24%) |
| Race* | |
| Caucasian | 32 (84%) |
| African-American | 4 (11%) |
| Asian | 1 (3%) |
| Other | 1 (3%) |
| Thoracic Radiation Therapy | |
| Daily | 4 (11%) |
| BID | 34 (89%) |
| Median Daily Dose | 63 Gy (54 – 72 Gy) |
| Median BID Dose | 45 Gy (43.5 – 45 Gy) |
| Concurrent | 38 (100%) |
| Number of Chemotherapy Cycles | |
| 2 cycles | 2 (5%) |
| 4 cycles | 34 (89%) |
| 5 cycles | 1 (3%) |
| 6 cycles | 1 (3%) |
| Time Between TCRT Completion and PCI | |
| Median | 80 days (36 – 378 days) |
| PCI Dose/Dose per Fraction* | |
| 24 Gy/1.5 Gy BID | 2 (5%) |
| 25 Gy/2.5 Gy BID | 17 (45%) |
| 30 Gy/2 Gy | 9 (24%) |
| 31.5 Gy/1.5 Gy BID | 1 (3%) |
| 35 Gy/2.5 Gy | 3 (8%) |
| 36 Gy/2 Gy | 6 (16%) |
Abbreviations: PCI = prophylactic cranial irradiation, TCRT = thoracic chemoradiation therapy, BID = twice daily.
Does not sum to 100% due to rounding of percentages to whole numbers.
All patients had either a CR or PR to TCRT that was confirmed on all imaging modalities obtained, with no patient included in this analysis with identified disease progression or stable disease. Concordance in classifying treatment response in patients with more than one imaging modality was 65% (Table 2). All discordances were for patients classified as having CRs on chest x-ray who were found to have PRs on CT chest (overall CXR-CT chest concordance 57%, n=14) or PET/CT (overall CXR-PET/CT concordance 83%, n=6), and one patient with a CR on CT chest but PR on PET/CT (overall CT chest-PET/CT concordance 67%, n=3).
Table 2.
Concordances in Response Rates Between Imaging Studies
| Chest X-ray vs. CT Chest | |
| Overall | 8/14 (57%) |
| Chest X-ray CR | 5/11 (45%) |
| Chest X-ray PR | 3/3 (100%) |
| Chest X-ray vs. PET/CT | |
| Overall | 5/6 (83%) |
| Chest X-ray CR | 1/2 (50%) |
| Chest X-ray PR | 4/4 (100%) |
| CT Chest vs. PET/CT | |
| Overall | 2/3 (67%) |
| Chest X-ray CR | 0/1 (0%) |
| Chest X-ray PR | 2/2 (100%) |
| Total | 15/23 (65%) |
Abbreviations: CT = computed tomography, PET = positron-emission tomography, CR = complete response, PR = partial response.
A CR that was identified on reimaging prior to PCI was significantly more likely to be reported on chest x-ray (71%) than advanced imaging (34%; CT chest 41%, PET/CT 18%) (p=0.006). All remaining studies demonstrated a partial response to TCRT. Although only 18% (n=2/11) of patients were found to have a CR on PET/CT just prior to PCI, 60% (n=3/5) of the remaining patients who had a subsequent repeat PET/CT scan a median of 3 months following completion of TCRT that were classified as initially having a PR on the pre-PCI PET/CT were found to have a CR on subsequent PET/CT imaging without receiving any additional systemic or thoracic therapy.
Median survival was 28.3 months for the entire cohort and 45.1 months for patients alive at the time of censorship (n=17). Across modalities and responses, patients with PRs on chest x-ray (25.5 months) and PET/CT (22.6 months) had the lowest median survival (Table 3). Patients with a CR on CT chest had numerically but not statistically significant improvement in 1-yr (91% vs. 69%, p=0.19), 2-yr (82% vs. 56%, p=0.18), and 3-yr (55% vs. 25%, p=0.13) overall survival compared with those with a PR on CT chest. CR versus PR did not correlate with survival on either chest x-ray (median 30.0 months vs. 25.5 months) or PET/CT (median 25.5 months vs. 22.6 months) (p>0.05 for both). Survival did not differ based on if patients underwent CXR reimaging alone of if they underwent an advanced reimaging modality with CT and/or PET/CT (p=0.78). Furthermore, a CR on any one imaging modality did not show improved survival over a CR on any other imaging modality. Patients with a CR on all sampled modalities had numerically but not statistically significantly higher median survival (45.2 months vs. 22.6 months, p=0.22) and 2-yr overall survival (71% vs. 48%, p=0.17) compared with those found to have a PR on any modality, but this trend was lost with more extended follow-up (Figure 1).
Table 3.
Survival According to Imaging Modality and Thoracic Chemoradiation Therapy Response
| Number | Median Survival (months) | 1-year Overall Survival | 2-year Overall Survival | 3-year Overall Survival | |
|---|---|---|---|---|---|
| Entire Cohort | 38 | 28.3 | 70% | 57% | 35% |
| CXR CR | 15/21 | 30.0 | 80% | 67% | 40% |
| CXR PR | 6/21 | 25.5 | 67% | 50% | 33% |
| CT CR | 11/27 | 37.9 | 91% | 82% | 55% |
| CT PR | 16/27 | 26.6 | 69% | 56% | 25% |
| PET CR | 2/11 | 25.5 | 50% | 50% | 50% |
| PET PT | 9/11 | 22.6 | 67% | 33% | 33% |
| CR on All Modalities | 14/38 | 45.5 | 79% | 71% | 50% |
| PR on Any Modality | 24/38 | 22.6 | 65% | 48% | 26% |
Abbreviations: CXR = Chest X-ray, CT = computed tomography, PET = positron-emission tomography, CR = complete response, PR = partial response.
Figure 1.
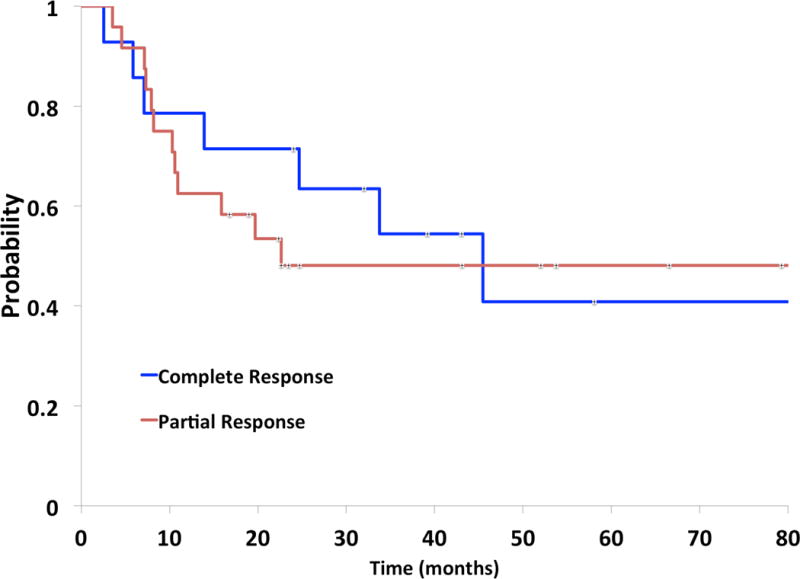
Kaplan-Meier analysis of overall survival by response. Survival is depicted in blue for patients with a complete response (CR) on all imaging modalities at the time of restaging prior to prophylactic cranial irradiation and in red for patients with a partial response (PR) on any imaging modality.
DISCUSSION
This study demonstrates similar overall survival for patients with limited-stage SCLC restaged prior to PCI with CXR, CT chest, or PET/CT. Significantly fewer patients were found to have a CR on advanced imaging (CT chest or PET/CT) than on CXR. This study also found that fluorodeoxyglucose (FDG) uptake can persist for greater than 12 weeks from TCRT completion, even in patients with a CR on CT chest who may ultimately achieve a CR on subsequent PET/CT. This study also demonstrated that although patients with CRs on all imaging modalities tended to live longer than those with a PR on any modality, high survival rates were achieved in this study even for patients with PRs on CXRs and advanced imaging studies.
A meta-analysis of nearly 2000 SCLC patients from 16 randomized trials from the last 30 years offers insight into which patients may benefit most from PCI.13 There was a 4.4% reduction in overall mortality in patients who received PCI compared with those randomized not to receive PCI, with the benefits of PCI greatest among patients obtaining a complete response after induction therapy (p=0.02) and no survival benefit among patients who failed to respond to therapy (p=0.29).
Whereas most studies have focused on patients receiving PCI following a complete response to chemotherapy, a recent Canadian meta-analysis investigated the effect of PCI on SCLC patients with incomplete responses to chemoradiation.19 Of the 93 patients who were incomplete responders, brain metastases occurred in 6.1% of patients who received PCI and 27.6% in those who did not receive PCI, indicating significant benefits of PCI even among incomplete responders. This analysis did allow for CT evaluation of response, however, so patients considered incomplete responders might have been classified as having a CR had they only been evaluated with a chest x-ray, which was generally the only reimaging modality used on previous trials. This benefit among patients not achieving a CR is in keeping with our findings that high rates of survival were seen in our cohort that were similar among CR and PR patients. As such, there is rationale for the consideration of PCI for all patients with limited-stage SCLC who respond to TCRT.
We acknowledge that the current study has several limitations. First, we had a limited patient sample size and not all patients received each of the different types of imaging modalities for assessment prior to PCI. These facts may have limited our ability to detect a difference in survival between patients achieving a CR and PR to TCRT, as well as limited our ability to definitively determine the concordance rates between imaging modalities for LS-SCLC. The findings of this retrospective study should be confirmed with data from larger, prospective series, such as the recently reported CONVERT trial20 or the currently accruing CALGB 30610/RTOG 0538 trial. Regarding our discordance rates, CRs were more likely to be reported on CXR than CT. We believe this to be a reflection of the decreased resolution and sensitivity of CXR compared to CT, as well as the potential for increased post-TCRT pulmonary changes to be interpreted as residual disease on CT or PET/CT.
Additionally, we only evaluated those patients who completed both definitive TCRT and PCI at our institution, which meant that we did not evaluate non-responders/patients who progressed on TCRT nor those found to have new brain metastases at the time of restaging neuroimaging. The survival numbers we present in this study are superior to most reports for LS-SCLC, which is likely in part due to selection bias. Our patient sample set also had a disproportionate number of women, who have better associated survival, leading to potential confounding gender disparities in our study cohort. Additionally, given the largely uniform timing of initiation of PCI following TCRT, our study is not able to assess if timing of these restaging studies and PCI delivery had an impact on outcome.
Prior phase II and III trials for LS-SCLC reported high CR plus PR overall response rates of approximately 80–95%,6,7,21 while our cohort only included patients who responded to TCRT. We are therefore not able to determine if or how PCI would have benefited patients who had either progressive or stable disease following TCRT and how advanced imaging response assessment should be used in these patients. As such, additional investigation is needed to compare patients responding versus not responding to initial therapy, although we hypothesize that the benefit of PCI is likely to be more limited in non-responders. Furthermore, our analysis was limited to patients treated with concurrent chemotherapy and standard fractionated radiation therapy. With the increasing use of stereotactic body radiation therapy (SBRT) in early stage SCLC,22 the current analysis cannot be generalized to assess the benefits of PCI in SBRT-treated patients, and such an analysis is best addressed as part of other multi-institutional analyses.23
CONCLUSIONS
Very little data are available assessing treatment response to TCRT for limited-stage SCLC based on advanced imaging modalities such as CT or PET/CT scans, and it is not currently known how a PR on advanced imaging influences overall survival nor how this should impact the decision to administer PCI. Provided patients achieved at least a PR to TCRT, advanced imaging did not add information to assist in the decision to offer PCI compared with CXR alone, and CXR remains an appropriate modality to assess response to TCRT prior to PCI. PET/CT restaging prior to PCI may be of limited utility since TCRT response on early PET/CT did not predict for survival. Furthermore, FDG uptake can persist for >12 weeks from the completion of TCRT, even in patients with a CR on CT chest who may ultimately achieve a CR on subsequent PET/CT imaging. Given the similar and high rates of survival independent of imaging modality and TCRT response, this study suggests that LS-SCLC patients with a PR on any modality should not be denied PCI, akin to current standards for patients with extensive-stage SCLC.
CLINICAL PRACTICE POINTS.
-
-
Prophylactic cranial irradiation (PCI) administered to small cell lung cancer (SCLC) patients without evidence of intracranial metastases has been shown to reduce the development of brain metastases and improve overall survival.
-
-
Previously published studies of PCI have generally required only a chest x-ray to assess response to thoracic chemoradiation (TCRT). Consequently, the response of patients who underwent more advanced imaging studies, such as computed tomography (CT) or positron emission tomography (PET)/CT, may have been classified as more limited than if they had undergone chest x-ray. They would then not have been offered PCI on those trials that required patients to have a thoracic complete response in order to be eligible for PCI.
-
-
In this study, we analyzed and defined responses to TCRT according to imaging modality for limited-stage SCLC patients.
-
-
Provided patients achieved at least a partial response to TCRT, we found that advanced imaging response neither impacted survival nor added information to assist in the decision to offer PCI.
-
-
Our findings suggest that limited-stage SCLC patients with a partial response on any imaging modality should not be denied PCI, akin to current standards for patients with extensive-stage SCLC.
Acknowledgments
This research was supported in part by the Intramural Research Program, Center for Cancer Research, National Cancer Institute, National Institutes of Health.
ABBREVIATIONS
- CR
complete response
- CT
computed tomography
- CXR
chest x-ray
- FDG
fluorodeoxyglucose
- PCI
prophylactic cranial irradiation
- PET
positron emission tomography
- PR
partial response
- RECIST
Response Evaluation Criteria in Solid Tumors
- SCLC
small-cell lung cancer
- TCRT
thoracic chemoradiation
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Arriagada R, Le Chevalier T, Riviere A, et al. Patterns of failure after prophylactic cranial irradiation in small-cell lung cancer: analysis of 505 randomized patients. Ann Oncol. 2002;13(5):748–754. doi: 10.1093/annonc/mdf123. [DOI] [PubMed] [Google Scholar]
- 2.Seute T, Leffers P, ten Velde GP, Twijnstra A. Neurologic disorders in 432 consecutive patients with small cell lung carcinoma. Cancer. 2004;100(4):801–806. doi: 10.1002/cncr.20043. [DOI] [PubMed] [Google Scholar]
- 3.Warde P, Payne D. Does thoracic irradiation improve survival and local control in limited-stage small-cell carcinoma of the lung? A meta-analysis. J Clin Oncol. 1992;10(6):890–895. doi: 10.1200/JCO.1992.10.6.890. [DOI] [PubMed] [Google Scholar]
- 4.Pignon JP, Arriagada R, Ihde DC, et al. A meta-analysis of thoracic radiotherapy for small-cell lung cancer. N Engl J Med. 1992;327(23):1618–1624. doi: 10.1056/NEJM199212033272302. [DOI] [PubMed] [Google Scholar]
- 5.Turrisi AT, 3rd, Kim K, Blum R, et al. Twice-daily compared with once-daily thoracic radiotherapy in limited small-cell lung cancer treated concurrently with cisplatin and etoposide. N Engl J Med. 1999;340(4):265–271. doi: 10.1056/NEJM199901283400403. [DOI] [PubMed] [Google Scholar]
- 6.Jeremic B, Shibamoto Y, Acimovic L, Milisavljevic S. Initial versus delayed accelerated hyperfractionated radiation therapy and concurrent chemotherapy in limited small-cell lung cancer: a randomized study. J Clin Oncol. 1997;15(3):893–900. doi: 10.1200/JCO.1997.15.3.893. [DOI] [PubMed] [Google Scholar]
- 7.Perry MC, Eaton WL, Propert KJ, et al. Chemotherapy with or without radiation therapy in limited small-cell carcinoma of the lung. N Engl J Med. 1987;316(15):912–918. doi: 10.1056/NEJM198704093161504. [DOI] [PubMed] [Google Scholar]
- 8.Murray N, Coy P, Pater JL, et al. Importance of timing for thoracic irradiation in the combined modality treatment of limited-stage small-cell lung cancer. The National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol. 1993;11(2):336–344. doi: 10.1200/JCO.1993.11.2.336. [DOI] [PubMed] [Google Scholar]
- 9.Komaki R, Cox JD, Whitson W. Risk of brain metastasis from small cell carcinoma of the lung related to length of survival and prophylactic irradiation. Cancer Treat Rep. 1981;65(9–10):811–814. [PubMed] [Google Scholar]
- 10.Nugent JL, Bunn PA, Jr, Matthews MJ, et al. CNS metastases in small cell bronchogenic carcinoma: increasing frequency and changing pattern with lengthening survival. Cancer. 1979;44(5):1885–1893. doi: 10.1002/1097-0142(197911)44:5<1885::aid-cncr2820440550>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 11.Auperin A, Arriagada R, Pignon JP, et al. Prophylactic cranial irradiation for patients with small-cell lung cancer in complete remission. N Engl J Med. 1999;341(7):476–484. doi: 10.1056/NEJM199908123410703. [DOI] [PubMed] [Google Scholar]
- 12.Arriagada R, Le Chevalier T, Borie F, et al. Prophylactic cranial irradiation for patients with small-cell lung cancer in complete remission. J Natl Cancer Inst. 1995;87(3):183–190. doi: 10.1093/jnci/87.3.183. [DOI] [PubMed] [Google Scholar]
- 13.Viani GA, Boin AC, Ikeda VY, Vianna BS, Silva RS, Santanella F. Thirty years of prophylactic cranial irradiation in patients with small cell lung cancer: a meta-analysis of randomized clinical trials. J Bras Pneumol. 2012;38(3):372–381. doi: 10.1590/s1806-37132012000300013. [DOI] [PubMed] [Google Scholar]
- 14.Seto T, Takahashi T, Yamanaka T, et al. Prophylactic cranial irradiation (PCI) has a detrimental effect on the overall survival (OS) of patients (pts) with extensive disease small cell lung cancer (ED-SCLC): Results of a Japanese randomized phase III trial. Journal of Clinical Oncology. 2014;32(15_suppl):7503–7503. [Google Scholar]
- 15.Xanthopoulos EP, Corradetti MN, Mitra N, et al. Impact of PET staging in limited-stage small-cell lung cancer. J Thorac Oncol. 2013;8(7):899–905. doi: 10.1097/JTO.0b013e31828e8996. [DOI] [PubMed] [Google Scholar]
- 16.The NCCN Clinical Practice Guidelines in Oncology. Small Cell Lung Cancer. Version 3. 2017 http://www.nccn.org/professionals/physician_gls/pdf/sclc.pdf. Accessed July 22, 2017.
- 17.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45(2):228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 18.O JH, Lodge MA, Wahl RL. Practical PERCIST: A Simplified Guide to PET Response Criteria in Solid Tumors 1.0. Radiology. 2016;280(2):576–584. doi: 10.1148/radiol.2016142043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tai P, Assouline A, Joseph K, Stitt L, Yu E. Prophylactic cranial irradiation for patients with limited-stage small-cell lung cancer with response to chemoradiation. Clin Lung Cancer. 2013;14(1):40–44. doi: 10.1016/j.cllc.2012.04.005. [DOI] [PubMed] [Google Scholar]
- 20.Faivre-Finn C, Snee M, Ashcroft L, et al. Concurrent once-daily versus twice-daily chemoradiotherapy in patients with limited-stage small-cell lung cancer (CONVERT): an openlabel, phase 3, randomised, superiority trial. The Lancet Oncology. 2017 doi: 10.1016/S1470-2045(17)30318-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bogart JA, Herndon JE, 2nd, Lyss AP, et al. 70 Gy thoracic radiotherapy is feasible concurrent with chemotherapy for limited-stage small-cell lung cancer: analysis of Cancer and Leukemia Group B study 39808. Int J Radiat Oncol Biol Phys. 2004;59(2):460–468. doi: 10.1016/j.ijrobp.2003.10.021. [DOI] [PubMed] [Google Scholar]
- 22.Stahl JM, Corso CD, Verma V, et al. Trends in stereotactic body radiation therapy for stage I small cell lung cancer. Lung Cancer. 2017;103:11–16. doi: 10.1016/j.lungcan.2016.11.009. [DOI] [PubMed] [Google Scholar]
- 23.Verma V, Simone CB, 2nd, Allen PK, et al. Multi-Institutional Experience of Stereotactic Ablative Radiation Therapy for Stage I Small Cell Lung Cancer. Int J Radiat Oncol Biol Phys. 2017;97(2):362–371. doi: 10.1016/j.ijrobp.2016.10.041. [DOI] [PMC free article] [PubMed] [Google Scholar]


