Abstract
Background
No randomized trial has directly compared the efficacy of prolonged infant antiretroviral prophylaxis versus maternal antiretroviral therapy (mART) for prevention of mother-to-child transmission throughout the breastfeeding period.
Setting
Fourteen sites in sub-Saharan Africa and India.
Methods
A randomized, open label strategy trial was conducted in HIV-1-infected women with CD4 counts ≥350 cells/mm3 (or ≥country-specific ART threshold if higher) and their breastfeeding HIV-1-uninfected newborns. Randomization at 6-14 days postpartum was to mART or infant nevirapine prophylaxis (iNVP) continued until 18 months post-delivery or breastfeeding cessation, infant HIV-1 infection, or toxicity, whichever occurred first. The primary efficacy outcome was confirmed infant HIV-1 infection. Efficacy analyses included all randomized mother-infant pairs except those with infant HIV-1 infection at entry.
Results
Between June 2011-October 2014, 2431 mother-infant pairs were enrolled; 97% of women were WHO Clinical Stage I, median screening CD4 count 686 cells/mm3. Median infant gestational age/birthweight were 39 weeks/2.9 kilograms. Seven of 1219 (0.57%) and seven of 1211 (0.58%) analyzed infants in the mART and iNVP arms, respectively, were HIV-infected (hazard ratio [HR] 1.0, 96% repeated confidence interval 0.3-3.1); infant HIV-free survival was high (97.1%, mART and 97.7%, iNVP, at 24 months). There were no significant differences between arms in median time to breastfeeding cessation (16 months) or incidence of severe, life-threatening or fatal adverse events for mothers or infants (14 and 42 per 100 person-years, respectively).
Conclusion
Both mART and iNVP prophylaxis strategies were safe and associated with very low breastfeeding HIV-1 transmission and high infant HIV-1-free survival at 24 months.
Keywords: Breastfeeding, HIV-1, prevention of perinatal HIV-1 transmission, antiretroviral therapy (ART), nevirapine
Introduction
Substantial progress has been made towards preventing perinatal transmission of HIV-1 in the developing world, but questions remain as to the relative safety and efficacy of various antiretroviral regimens. In the Antepartum Component of a large randomized multi-country clinical trial, Promoting Maternal Infant Survival Everywhere (PROMISE), we showed maternal antiretroviral therapy (ART) during pregnancy and intrapartum can reduce perinatal HIV-1 transmission to ∼0.5% in sub-Saharan African settings.1 However, breastfeeding-associated HIV-1 exposure and potential transmission continues in breastfed HIV-exposed infants living in resource-limited settings. While WHO guidelines recommend that all HIV-1-infected pregnant women initiate life-long ART, adherence to ART, particularly postpartum, has proven to be a major challenge; postpartum viral rebound has been observed in 31% of women starting ART during pregnancy who had initial viral suppression and 22% of women receiving preconception ART had detectable viremia at first antenatal visit.2-4 Thus, evaluation of the safety and efficacy of alternative strategies, such as infant prophylaxis, to reduce postnatal infection remains important.5
Based on prior clinical trial data, two strategies have been shown to be safe and effective in preventing postnatal HIV-1 transmission: 1)providing ART to the lactating woman, thereby reducing breastmilk viral load; or 2)providing daily single-drug antiretroviral (ARV) prophylaxis to the breastfeeding infant, maintaining prophylactic infant ARV blood levels throughout the period of HIV-1 transmission risk.6-13 Prior studies, however, focused on interventions only given through the first 6 to 12 months of breastfeeding and there were additional late infections related to breastfeeding transmission when breastfeeding continued after prophylaxis stopped. Because increased morbidity and mortality have been associated with weaning compared to continued breastfeeding through the second year of life in HIV-1-exposed infants, breastfeeding beyond 12 months, and interventions to reduce HIV-1 transmission during breastfeeding will be required to maximize infant HIV-1-free survival.14-16
A few studies have evaluated the safety and efficacy of prolonged maternal or infant prophylaxis, but only through 12 months.11, 12 No prior randomized trial has directly compared the efficacy and safety of prolonged infant ARV prophylaxis to maternal ART throughout the duration of breastfeeding into the second year of life. We report results from the PROMISE trial's Postpartum Component, which randomized breastfeeding HIV-1-infected women with high CD4 cell count and their HIV-1-uninfected infants at 6-14 days postpartum to either maternal ART (mART) or infant nevirapine (iNVP) prophylaxis to prevent HIV-1 transmission during breastfeeding.
Methods
Study design and participants
PROMISE was a randomized, open-label strategy trial sponsored by the U.S. National Institutes of Health conducted at 14 health-facility-based research sites in seven countries: India (1 site), Malawi (2), South Africa (5), Tanzania (1), Uganda (1), Zambia (1) and Zimbabwe (3). HIV-1-infected postpartum women were recruited from two sources: women completing the PROMISE Antepartum Component1 and women first identified as HIV-1-infected during active labor, and hence received no ARV during pregnancy (“late presenters”).
The PROMISE Postpartum Component was designed to compare the relative efficacy and safety of mART versus daily iNVP prophylaxis for prevention of breastmilk HIV-1 transmission. Women who intended to breastfeed and planned to remain in the study site area through 24 months postpartum were eligible. Randomization occurred at the postpartum week 1 (day 6-14 after delivery) visit. Inclusion criteria for women (within 30 days prior to enrollment in the Postpartum Component) were: CD4 count ≥ 350 cells/mm3 (or ≥ the country-specific ART initiation threshold if it was >350 cells/mm3); hemoglobin >7.0 grams/dL; white blood cell count (WBC) >1,500 cells/mm3;absolute neutrophil count (ANC) >750 cells/mm3; platelets >50,000 cells/mm3; alanine aminotransferase (ALT) ≤ 2.5 × upper limit of normal (ULN); and estimated creatinine clearance of ≥60 mL/min. Inclusion criteria for infants were: ≤14 days of age; birthweight ≥2.0 kilograms; uninfected (negative HIV-1 nucleic acid test [NAT] on a specimen drawn prior to the week 1 visit); hemoglobin ≥10 g/dL; WBC ≥1,500 cells/mm3; ANC ≥750 cells/mm3; platelets ≥50,000 cells/mm3; and ALT ≤2.5 × ULN. For multiple births, a mother-infant pair was enrolled only if all live infants could be enrolled. Exclusion criteria included positive infant HIV-1 NAT result on a specimen drawn prior to study entry or NAT result not available; or a life-threatening condition.
Prior to obtaining informed consent and during follow-up, women were regularly informed of the current and evolving country-specific guidelines for preventing perinatal HIV-1 transmission and for ART initiation and other options for prevention outside of PROMISE. The study was approved by local and collaborating institutional review boards and other relevant regulatory authorities; and was reviewed for safety and efficacy by an independent Data and Safety Monitoring Board (DSMB).
Randomization
Women enrolled in the PROMISE Antepartum Component1 continued their antepartum randomized regimen (zidovudine alone [ZDV] or ZDV/lamivudine [3TC]/lopinavir boosted with ritonavir [LPVr] or tenofovir [TDF]/emtricitabine [FTC]/LPVr)until assessment for the Postpartum Component at the postpartum week 1 visit (day 6-14 after delivery). Late-presenting women who had not been enrolled in the PROMISE Antepartum Component were enrolled in labor or within 5 days after delivery; those in labor received intrapartum single-dose nevirapine (sdNVP) with a TDF/FTC tail until the postpartum week 1 visit. All infants received daily NVP through age 6 weeks as recommended by World Health Organization (WHO) guidelines.17
Mother-infant pairs were randomized at the week 1 postpartum visit to one of two prophylaxis regimens (in a 1:1 ratio): mART or iNVP. TDF/FTC/LPVr was the study-preferred mART regimen. TDF/FTC fixed dose combination tablets containing 300 mg/200 mg orally daily plus LPV/r fixed dose combination tablets, two tablets of 200 mg/50 mg orally twice daily were recommended dosage. Women who experienced intolerance, toxicity, or clinical, immunologic or virologic failure were allowed to receive other three ARV regimens selected by their provider. Infant NVP was prescribed using age-based NVP dosing (Birth to 6 Weeks - birth weight ≥ 2500 gm, 15mg and for birth weight 2000–2499 gm, 10mg; >6 Weeks to 6 Months - 20mg; >6 Months to 9 Months - 30mg; >9 Months to cessation of BF or 18 months, whichever is first - 40mg). Randomized regimen was continued until 42 days after last breastmilk exposure (two weeks following breastfeeding cessation, defined as no breastmilk exposure for >28 days) or age 18 months, whichever came first. Randomized regimen was discontinued early if infant HIV-1 infection was diagnosed or for severe toxicity. A web-based, central computer randomization system used permuted block allocation with stratification by country and the antepartum/intrapartum maternal ARV prophylaxis [maternal ART versus ZDV/sdNVP versus ZDV/sdNVP (late presenter) vs. none (late presenter)].18
Procedures
Maternal visits occurred at weeks 1 (6-14 days postpartum, entry), 6 and 14 postpartum, and then every 12 weeks through week 74 postpartum. General medical history and limited physical examination were obtained at each visit. Complete blood count (CBC) was performed at all visits and chemistry safety labs (alanine aminotransferase [ALT], aspartate aminotransferase [AST], creatinine, alkaline phosphatase, total bilirubin and albumin) were also obtained except at week 62. CD4 cell counts were done at all visits except week 6 postpartum. Pregnancy tests were obtained if pregnancy was suspected. Infant visits occurred at postpartum weeks 1, 6, 10, 14, 18, 22 and 26, then every 12 weeks until week 98, with a final visit at week 104. History and physical evaluation were performed at all visits. HIV-1 NAT, CBC, and stored plasma were collected at every visit except week 10; ALT was obtained at weeks 1 and 6. For infants randomized to iNVP, ALT was done at week 26 and every 12 weeks while receiving NVP. An HIV-1 antibody test could be substituted for NAT if the infant was >18 months old and had ceased breastfeeding. Infants diagnosed with HIV infection were referred to the local treatment clinic to initiate ART; study follow–up continued regardless of infection status.
Outcomes
The primary efficacy outcome was confirmed infant HIV-1 infection, defined as positive HIV-1 NAT from a specimen drawn at any post-randomization visit, confirmed by a positive HIV-1 NAT on a second specimen drawn at a subsequent time point. Cases of uncertain infant HIV-1 infection status were reviewed, blinded to arm assignment, by an independent four-member committee who made the definitive determination of infant HIV-1 infection status and timing. Infant HIV-1-free survival (infant alive and not HIV-1-infected) and infant death were secondary efficacy outcome measures. All HIV-1 NATs were performed in laboratories certified by the Division of AIDS (DAIDS) Virology Quality Assurance Program.
The DAIDS Table for Grading the Severity of Adult and Pediatric Adverse Events, 2004 Version 1.0 (clarification August 2009) was used to grade adverse events.19 For women, the primary safety outcome was a composite of Grade 2 hematologic, renal, or hepatic abnormalities or Grade ≥3 adverse events or death, whichever occurred first. For infants, the primary safety outcome was a composite of Grade ≥3 adverse events or death, whichever occurred first. Secondary safety outcomes included the individual components of the primary composite outcomes.
Statistical analysis
The original target sample size was 4650 mother-infant pairs (approximately 3100 from the Antepartum Component and approximately 1550 late presenters), providing ≥90% power to detect a difference between groups in cumulative postnatal transmission at breastfeeding cessation of approximately 3% versus 5%, with 2-sided Type I error of 5% and allowing for loss-to-follow-up (10%) and interim efficacy monitoring (3%). These projected cumulative postnatal transmission rates were anticipated based on the following information and assumptions: postnatal transmission rates through 6 months postpartum would be approximately 1-3% among early presenters (based on the MITRA20 and MITRA-PLUS21 studies) and approximately 5-7% among late presenters (based on the SWEN7 and PEPI-Malawi8 studies); late presenters would represent 33% of all study participants; the postnatal transmission rate would increase by an average of 0.35% per month after 6 months postpartum (half the estimated rate reported in a meta-analysis22); and a projected average breastfeeding duration across all sites of approximately 9 months.
In July 2014, due to slow accrual, the sponsor (NIAID) decided to stop enrollment to all PROMISE Components when the Antepartum Component reached its accrual target for breastfeeding women or on October 1, 2014, whichever came first. The sponsor's decision was based in part on revised power calculations that used using the same approach as the original power calculations but with the anticipated postnatal transmission rates updated based on the smaller observed percentage of late presenters enrolled (6%) and the longer observed median duration of breastfeeding (15 months). The revised power calculations indicated that a projected total accrual of 2436 mother-infant pairs by October 1, 2014 would provide 85% power to detect a difference between groups in cumulative postnatal transmission at breastfeeding cessation of approximately 3.2% versus 6%.
All analyses used the intent-to-treat principle with one pre-specified exception: mother-infant pairs who had a positive infant HIV-1 NAT result on a specimen drawn prior to or on the randomization date were excluded from the efficacy analyses. The risk period for the primary efficacy and safety analyses was the time from randomization until 56 days after the last exposure to breastmilk or 18 months postpartum, whichever came first. The risk period was extended until 24 months postpartum in secondary efficacy and infant safety analyses. The a priori analysis plan specified that the primary efficacy and safety analyses would be censored at the recommended minimum duration of breastfeeding at each enrolling site (at the time of the study design, this was 6 or 12 months), due to concerns that the PROMISE post-breastfeeding maternal randomization of mART women to continue or stop mART could induce longer breastfeeding in the mART arm, which could introduce bias. Sensitivity analyses were also conducted which did not censor at the recommended minimum duration of breastfeeding.
Comparisons between randomization arms and estimation of effect sizes used time-to-event analyses with the Cox proportional hazards regression model. The distributions of time until the first event occurred were summarized using Kaplan-Meier plots. Statistical significance was defined as a two-sided p-value <0.05 for all analyses except efficacy; efficacy analyses used a group-sequential repeated confidence interval (RCI) for the hazard ratio in cumulative postnatal transmission using the Lan-DeMets approach with an O'Brien-Fleming Type 1 error spending function (for the final analysis, the confidence coefficient was 96%, to preserve an experiment-wise Type I error rate of 0.05). The RCI indicated the range of hazard ratio values that were consistent with the observed data; if the RCI excluded 1.0, this would indicate a statistically significant difference between arms and if the RCI included 1.0, the width of the RCI would indicate the ability of the study to rule out a difference between treatment arms (the narrower the RCI, the stronger the evidence of equivalence).Two interim efficacy analyses were performed during the conduct of the study.
On July 7, 2015, the sites were instructed to inform all maternal participants about the START trial results23, which demonstrated statistically significant benefits of starting ART early compared to delayed initiation, and offer ART to those who were not receiving it. In November 2015, the DSMB recommended that the primary PROMISE analyses should include only follow-up through July 6 to minimize bias if large numbers of women in the iNVP arm chose to start ART or the women who chose to take ART were systematically different than those who did not. Statistical analyses were performed using SAS software version 9.4 (SAS Institute, Cary, NC).
Results
Between June 6, 2011 and October 1, 2014, a total of 2431 mother-infant pairs (including 13 pairs of twins) were enrolled (1220 randomized to mART and 1211 to iNVP) from sites in Malawi (32%), South Africa (23%), Zimbabwe (22%), Uganda (16%), Zambia (2%), Tanzania (2%), and India (3%). Ninety-five percent of mother-infant pairs in each arm were previously enrolled in the PROMISE Antepartum Component (42% randomized to Maternal ZDV Arm and 53% randomized to a Maternal Triple ARV Arm) and 5% were late-presenting women enrolled at delivery or within 5 days after delivery. Nearly all (1207, 98.9%) of the mothers in the mART arm started the preferred study-supplied mART regimen and nearly all (1204, 98.9%) of the infants in the iNVP arm started on daily NVP.A patient flow diagram is shown in Supplemental Digital Content.
Table 1 summarizes selected baseline characteristics of mothers and infants at study entry. The median time to breastfeeding cessation was 16 months (70 weeks) with no statistically significant difference between arms (p=0.70).Kaplan-Meier probability estimates for continued breastfeeding at age 6, 9, 12, 18, and 24 months were 93.3%, 86.2%, 81.0%, 34.3%, and 12.5%, respectively.
Table 1. Baseline Characteristics of Women and Infants by Study Arm.
| Maternal Characteristic | Maternal ART (N=1,220) | Infant NVP Prophylaxis (N=1,211) | Total (N=2,431) | |
|---|---|---|---|---|
| Age (years) | Median (IQR) | 26 (23-30) | 26 (23-30) | 26 (23-30) |
| Race | Black African | 1,178 (97%) | 1,168 (96%) | 2,346 (97%) |
| Asiana | 41 (3%) | 42 (3%) | 83 (3%) | |
| Coloured | 1 (0%) | 1 (0%) | 2 (0%) | |
| Weight (kg) | # missing | 0 | 0 | 0 |
| Median (IQR) | 61.0 (54.9-69.8) | 61.0 (54.7-69.1) | 61.0 (54.8-69.4) | |
| Previous PROMISE Treatment Arm | Antepartum Component – LPV/r + ZDV/3TC | 508 (42%) | 497 (41%) | 1,005 (41%) |
| Antepartum Component – LPV/r + TDF/FTC | 140 (11%) | 149 (12%) | 289 (12%) | |
| Antepartum Component – ZDV +sdNVP | 506 (41%) | 503 (42%) | 1,009 (42%) | |
| Late Presenter Component - None | 42 (3%) | 39 (3%) | 81 (3%) | |
| Late Presenter Component – Intrapartum ZDV + sdNVP | 24 (2%) | 23 (2%) | 47 (2%) | |
| Gestational Age at Antepartum Randomization | # missing (includes late presenters) | 67 | 62 | 129 |
| Median (IQR) | 26.3 (21.4-30.7) | 26.3 (21.7-31.4) | 26.3 (21.6-31.1) | |
| HIV-1 Viral load (copies/ml) (obtained at Delivery or Entry Visit (Week 1 postpartum) | # missing | 2 | 3 | 5 |
| Median (IQR) | 220 (40-1029) | 400 (40-1960) | 322 (40-1422) | |
| Below assay limit of detection | 499 (41%) | 379 (31%) | 878 (36%) | |
| <400 | 276 (23%) | 296 (24%) | 572 (24%) | |
| 400 – 1,000 | 136 (11%) | 139 (11%) | 275 (11%) | |
| 1,000 - <10,000 | 196 (16%) | 278 (23%) | 474 (19%) | |
| 10,000 - <100,000 | 91 (7%) | 105 (9%) | 196 (8%) | |
| 100,000 - <200,000 | 12 (1%) | 9 (1%) | 21 (1%) | |
| >=200,000 | 10 (1%) | 5 (0%) | 15 (1%) | |
| Screening CD4 count (cells/mm3) | Median (IQR) | 682.5 (555-870) | 691 (550-868) | 686 (553-869) |
| 350 - < 400 | 37 (3%) | 49 (4%) | 86 (4%) | |
| 400 - < 450 | 70 (6%) | 57 (5%) | 127 (5%) | |
| 450 - < 500 | 88 (7%) | 90 (7%) | 178 (7%) | |
| 500 - < 750 | 550 (45%) | 522 (43%) | 1,072 (44%) | |
| ≥ 750 | 475 (39%) | 493 (41%) | 968 (40%) | |
| WHO Clinical Stageb | Clinical Stage I | 1,174 (96%) | 1182 (98%) | 2356 (97%) |
| Clinical Stage II | 45 (4%) | 28 (2%) | 73 (3%) | |
| Clinical Stage III | 1 (0%) | 1 (0%) | 2 (0%) | |
| # of live infants at entry | 1 | 1,213 (99%) | 1,205 (100%) | 2,418 (99%) |
| 2 | 7 (1%) | 6 (0%) | 13 (1%) | |
|
| ||||
| Infant Characteristic | Maternal ART (N=1,227) | Infant NVP Prophylaxis (N=1,217) | Total (N=2,444) | |
|
| ||||
| Gender | Male | 622 (51%) | 614 (50%) | 1,236 (51%) |
| Female | 605 (49%) | 603 (50%) | 1,208 (49%) | |
| Gestational age at birth (weeks) | # missing | 0 | 0 | 0 |
| Median (IQR) | 39 (38-40) | 39 (38-40) | 39 (38-40) | |
| < 34 | 10 (1%) | 8 (1%) | 18 (1%) | |
| 34 - < 37 | 113 (11%) | 135 (11%) | 268 (11%) | |
| ≥ 37 | 1,084 (88%) | 1,074 (88%) | 2,158 (88%) | |
| Birth weight (gm)c | # missing | 9 | 6 | 15 |
| Median (IQR) | 2,910 (2,600-3,230) | 2,900(2,600-3,200) | 2,900 (2,600-3,200) | |
| 2000 - <2500 | 149 (12%) | 183 (15%) | 332 (14%) | |
| ≥ 2,500 | 1,069 (88%) | 1,028 (85%) | 2,097 (86%) | |
Include women from the Indian subcontinent and natives of India
Two women had WHO Stage III at entry, but were tuberculosis-negative and had CD4 >350 copies/ml and therefore, did not require ART for their own health.
The birth weight is the infant weight closest to birth and within 0-5 days postpartum. Using the determination closest to birth (not restricting to 0-5 days postpartum) gave similar summary statistics. All infants had weight of at least 2,000 grams.
3TC: lamivudine; ART: antiretroviral therapy; ARV: antiretroviral; FTC: emtricitabine; LPV/r: lopinavir boosted with ritonavir; NVP: nevirapine; sdNVP: single-dose nevirapine; TDF: tenofovir disoproxil fumarate; ZDV: zidovudine
The efficacy analysis included 2430 of the 2431 enrolled mother-infant pairs (one infant born to a late-presenting mother was found to have been HIV-1-infected at time of randomization and was excluded from the efficacy analysis). Thirty-five infants (22 in the mART and 13 in the iNVP arm, 1.5%) had no post-randomization HIV-1 testing results and were censored at the date of randomization; 10 of the infants (6 mART and 4 iNVP) died of non-HIV (5 infants) or unknown causes (5 infants) without any record of HIV testing, 10 (7 mART and 3 iART) were not able to get to clinic, 9 (6 mART and 3 iNVP) had consent withdrawn, and 6 (3 mART and 3 iNVP) could not be contacted (Supplemental Digital Content). Maternal and infant baseline characteristics (maternal RNA and CD4, infant gestational age and birthweight) were similar for the 22 mART and 13 iNVP arm infants.
Eighteen infants were diagnosed with HIV-1 infection at a post-randomization visit; 14 were included in the primary analysis (4 were censored at the country recommended minimum duration of breastfeeding: 6 months for one and 12 months for three infants) and all were included in the secondary analysis through 24 months postpartum.
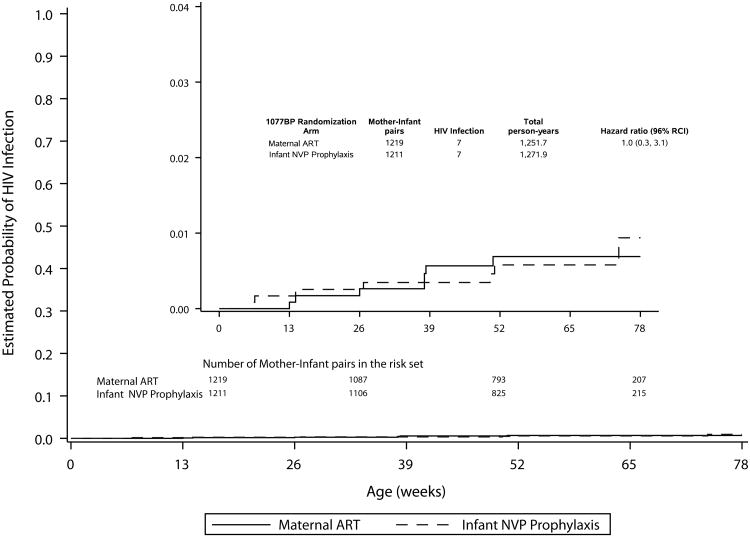
In the primary analysis, infant HIV-1 infection occurred in seven infant of 1219 mother-infant pairs randomized to mART and seven of 1211 randomized to iNVP. The HIV-1 transmission rates in both arms were much lower than anticipated and the 96% RCI was quite wide (0.3-3.1), indicating very limited ability to rule out differences between arms due to the small numbers of infants who were HIV-1 infected (Figure 1A, Table 2). Sensitivity analyses that did not censor at the minimum recommended duration of breastfeeding gave similar results (not shown).
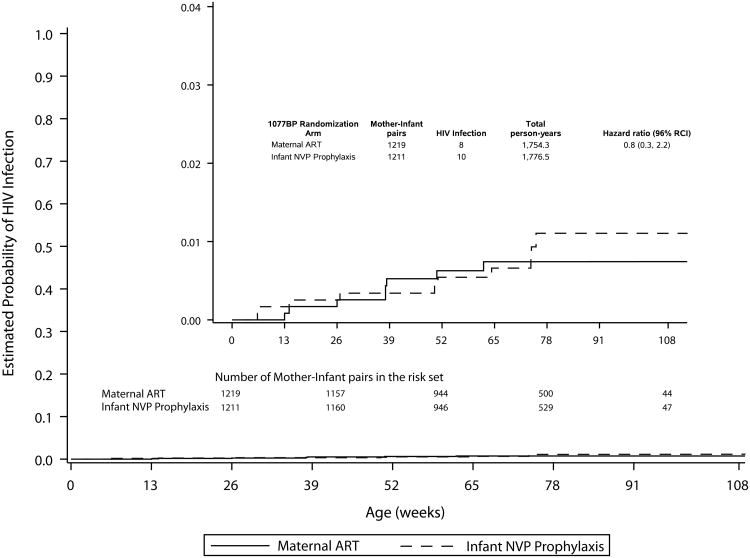
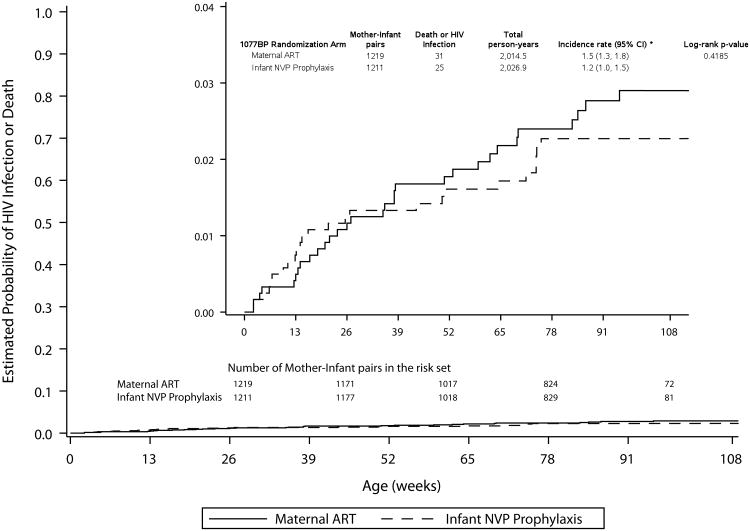
Figure. 1
Table 2. Probability of HIV-1 Infection or HIV-1 Infection/Death by Study Arm: Primary and Secondary Analyses.
| Age | Maternal ART | Infant NVP Prophylaxis | Overall |
|---|---|---|---|
| Probability of HIV-1 Infection % (95% Confidence Interval) | |||
|
| |||
| Primary analysis (Follow-up time is censored per planned analysis specifications)a | |||
|
| |||
| 6 months | 0.3% (0.1-0.8%) | 0.3% (0.1-0.8%) | 0.3% (0.1-0.6%) |
| 9 months | 0.6% (0.3%-1.3%) | 0.3% (0.1-0.9%) | 0.5% (0.2-0.8%) |
| 12 months | 0.7% (0.3-1.4%) | 0.6% (0.3-1.3%) | 0.6% (0.4-1.1%) |
| 18 months | 0.7% (0.3-1.4%) | 0.9% (0.4-2.3%) | 0.8% (0.4-1.5%) |
|
| |||
| Secondary analysis (Includes all infections and total duration follow-up)b | |||
|
| |||
| 24 months | 0.7% (0.4-1.5%) | 1.1% (0.6-2.1%) | 0.9% (0.6-1.5%) |
|
| |||
| Probability of HIV-1-Infection or Death % (95% Confidence Interval) | |||
|
| |||
| Secondary analysis (includes all infections and total duration follow-up)b | |||
| 6 months | 1.2% (0.7-2.0%) | 1.2% (0.8-2.1%) | 1.2% (0.8-1.7%) |
| 9 months | 1.7% (1.1-2.6%) | 1.3% (0.8-2.2%) | 1.5% (1.1-2.1%) |
| 12 months | 1.8% (1.2-2.7%) | 1.6% (1.0-2.5%) | 1.7% (1.2-2.3%) |
| 18 months | 2.4% (1.6-3.5%) | 2.3% (1.5-3.4%) | 2.3% (1.8-3.1%) |
| 24 months | 2.9% (2.0-4.1%) | 2.3% (1.5-3.4%) | 2.6% (2.0-3.4%) |
Primary analysis: post-randomization to end of transmission risk period, 18 months postpartum, or minimum recommended duration of breastfeeding at site (whichever occurs first)
Secondary analysis: post-randomization to 24 months postpartum
ART: antiretroviral therapy; NVP: nevirapine
In the secondary analysis, which included all follow-up data, there were eight cumulative infections among 1218 mother-infant pairs randomized to mART and 10 among the 1211 randomized to iNVP. For the 18 infected infants, the median infant age at HIV-1 infection was 38 weeks (range, 13 – 62 weeks) in the mART arm and 50.5 weeks (range, 6 – 75 weeks) in the iNVP arm. The cumulative probability of infection at 24 months was 0.9% (95%CI 0.6-1.5%) (Figure 1B, Table 2). The probability of infant HIV-1 infection/death at 24 months in the mART arm was 2.9% (95% CI 2.0-4.1%), and in the iNVP arm was 2.3% (95% CI 1.5-3.4%), resulting in HIV-1-free survival at 24 months of 97.1% in the mART and 97.7%in the iNVP arm (Figure 1C, Table 2).
All 2431 women are included in the safety analysis; 15 had no post-randomization visit (nine in mART and six in iNVP) and were censored at date of randomization. There was no significant difference in time to the first composite safety endpoint between arms (p=0.98 and p=0.61) and the incidence rates of adverse events were similar in the two arms (Table 3). Sensitivity analyses that did not censor at the minimum recommended duration of breastfeeding gave similar results (not shown). Three maternal deaths occurred (two in the mART and one in the iNVP arm) during the perinatal HIV-1 transmission risk period; no death was judged related to study intervention. Of the 1207 women in the mART arm who started on the study recommended regimen, 9 (<1%) stopped the recommended regimen because of toxicity.
Table 3. Numbers of Women and Infants who Experienced Adverse Events by Study Arm (Primary Analysis: Randomization to End of Transmission Risk Period, 18 Months Postpartum, or the Minimum Recommended Duration of Breastfeeding, Whichever came First).
| Adverse Event | Maternal ART | Infant NVP | Total |
|---|---|---|---|
| Women | N=1211 | N=1205 | N=2416 |
|
| |||
| Composite (Grade >3 adverse events or Grade 2 laboratory or death)a | 159 (13.1%) | 161(13.4%) | 320 (13.2%) |
| Composite (Grade >3 adverse events/laboratory or death)b | 59 (4.9%) | 66 (5.5%) | 125 (5.2%) |
| Death | 2 (0.2%) | 1 (0.1%) | 3 (0.1%) |
| Grade >3 signs/symptoms | 22 (1.8%) | 24 (2.0%) | 46 (1.9%) |
| Grade >2 laboratory | 141(11.6%) | 140 (11.6%) | 281 (11.6%) |
| Grade >2 hematology | 128 (10.6%) | 119 (9.9%) | 247 (10.2%) |
| Grade >2 chemistry | 18 (1.5%) | 27 (2.2%) | 45 (1.9%) |
| Grade >3 laboratory | 38 (3.1%) | 42 (3.5%) | 80 (3.3%) |
| Grade >3 hematology | 35 (2.9%) | 27 (2.2%) | 62 (2.6%) |
| Grade >3 chemistry | 3 (0.2%) | 15 (1.2%) | 18 (0.7%) |
|
| |||
| Infant | N=1225 | N=1215 | N=2440 |
|
| |||
| Composite (Grade >3 adverse events or death)c,d | 427 (34.9%) | 426 (35.1%) | 853 (35.0%) |
| Death | 16 (1.3%) | 14 (1.2%) | 30 (1.2%) |
| Grade >3 signs/symptoms | 61 (5.1%) | 44 (3.6%) | 105 (4.3%) |
| Grade >3 laboratoryd | 383 (31.3%) | 385 (31.7%) | 768 (31.5%) |
| Grade >3 hematology | 383 (31.3%) | 384 (31.6%) | 767(31.4%) |
| Grade >3 chemistryd | 0 (0%) | 2 (0.2%) | 2 (0.1%) |
| Grade ≥3 ALT (weeks 1 and 6 assessment) | 0 (0%) | 2 (0.2%) | 2 (0.1%) |
| Grade ≥3 ALT (week 26, 28, 50, 62, and 74 assessment) | NA | 5 (0.4%) | NA |
Incidence rates were 14.4 per 100 person-years (95% CI 12.4-16.6 per 100 person-years) in women in the mART arm and 14.1 per 100 person-years (95% CI 12.2-16.3 per 100 person-years) in women in the iNVP arm, and not significantly different between arms (p=0.98).
Incidence rates were 5.0 per 100 person-years (95% CI 4.2-5.9 per 100 person-years) in mART and 5·4 per 100 person-years (95% CI 4.6-6.4 per 100 person-years) in iNVP, and not significantly different between arms (p=0.61).
Incidence rates were 42.5 per 100 person-years (95% CI 37.9-47.5 per 100 person-years) for infants in the mART and 42.0 per 100 person-years (95% CI 37.5-47.0 per 100 person-years) in infants in the iNVP arm, and not significantly different between arms (p=0.99).
Includes ALT from week 1 and 6 visit only since infants randomized to the mART arm did not have ALT assessed at subsequent study visits.
ALT: alanine aminotransferase; ART: antiretroviral therapy; NVP: nevirapine
All 2444 infants are included in the safety analysis; 4 had no post-randomization visit information (2 in each arm) and were censored at date of randomization. There was no significant difference in time to the first composite safety endpoint between arms (p=0.99) and the incidence rates of adverse events were similar in the two arms (Table 3). Sensitivity analyses that did not censor at the minimum recommended duration of breastfeeding gave similar results (not shown). Additionally, among infants randomized to iNVP, safety assessments beyond week 6 did not demonstrate increased incidence of liver or skin toxicity compared to the mART arm (Table 3). Of the 1204 infants in the iNVP arm who started on the study recommended regimen, 20 (<2%) stopped the recommended regimen because of toxicity.
Thirty infant deaths occurred within the minimum recommended breastfeeding duration, and an additional eight thereafter (Table 3). Of the 30 deaths in the primary analysis, 16 were infants randomized to mART (incidence rate 1.2 per100 person-years; 95%CI 1.0-1.5 per 100 person-years) and 14 in infants randomized to iNVP (incidence rate 1.1 per 100 person-years; 95%CI 0.9-1.3 per 100 person-years), and not significantly different between arms (p=0.72). The Kaplan-Meier estimates of probabilities of infant death at 6, 9, 12, and 18 months postpartum were 1.0% (95% CI 0.6-1.5%), 1.1% (95% CI 0.7-1.6%), 1.1% (95% CI 0.7-1.6%), and 1.7% (95% CI 1.1-2.6%), respectively. The secondary analysis included all 38 infant deaths (23 in the mART and 15 in the iNVP arm); and the cumulative probability of infant death at 24 months was 1.7% (95% CI 1.3-2.4%).No infant death was attributed to study regimen and no HIV-1-infected infant died.
Discussion
The PROMISE study is the first randomized trial to conduct a head-to-head comparison of mART and iNVP for postnatal HIV-1 transmission through up to 18 months of breastfeeding in asymptomatic women with high CD4 counts who did not meet treatment criteria at the time the study was conducted. While the cumulative postnatal HIV-1 transmission rates in both the mART and iNVP arms were much lower than anticipated in sample size calculations, which greatly limited the study's statistical power to detect a difference in transmission risk between arms, the findings demonstrate that both mART and iNVP regimens were highly efficacious, with 12- and 24-month postnatal infection rates of 0.6% and 0.9% and high rates of HIV-1-free survival at 24 months (>97%). The high under-two year survival rates (1.7% infant mortality) was particularly noteworthy compared to prevailing rates in most of the settings where the study was conducted.24
Two contemporary studies, ANRS 12174 and the Uganda PROMOTE trial demonstrated similar survival rates but at 50 weeks of follow-up. ANRS 12174 randomized HIV-1-uninfected breastfed infants to either LPV/r or 3TC through cessation of breastfeeding or 50 weeks. HIV-1-free survival at 50 weeks was 96% in both study arms.11 HIV-1-infected women in the PROMOTE study were randomized between 12 and 28 weeks of pregnancy to either a LPV/r or efavirenz based regimen that was continued for 48 weeks of breastfeeding. HIV-1-free infant survival at 8 weeks postpartum was 92.9% in the LPVr arm and 97.2% in the efavirenz arm.12
HIV-1 transmission rates in these studies were also low. In the PROMOTE study, HIV-1 infection occurred during breastfeeding in one infant in the LPV/r arm. Infections rates after 50 weeks in the ANRS 12174 study were 1.4% in the LPV/r arm and 1.5% in the 3TC arm. To date, there is only one study that has compared the efficacy of mART and infant prophylaxis. The Breastfeeding, Antiretrovirals and Nutrition Study (BAN) compared a control regimen of intrapartum sdNVP plus 7 days of ZDV/3TC in all women followed by a randomization into one of three groups: extended mART prophylaxis or daily iNVP prophylaxis given for up to 7 months of exclusive breast feeding or no further ARV prophylaxis in mothers nor infants. Although not designed nor powered to directly compare the efficacy of mART and iNVP, study findings indicated that both mART and the iNVP arms were superior to the one-week control arm (3%, 1.8% and 6.4%, respectively) at 28 weeks post-delivery.10 It should be noted the women in BAN received only sdNVP and 7 days of ZDV/3TC as prophylaxis for perinatal transmission whereas 95% of the PROMISE cohort had been followed in the antepartum component and received either mART or ZDV. In addition, women in the BAN study were only enrolled if their CD4 count was greater than or equal to 200 or 250 cells/mm3 (depending on time of enrollment) whereas women enrolled in the PROMISE cohort had CD4 cell counts ≥ 350 cells/mm3 (or ≥ the country-specific ART initiation threshold if it was >350 cells/mm3). These underlying differences in subject selection likely explain the higher HIV transmission rates observed in the BAN study.
Additionally, no safety concerns were observed in both mART and iNVP arms of the study. Less than 1% of women and 2% of infants discontinued their study regimen because of toxicity. Concerns have been raised about the potential infant toxicity from ingestion of ARVs in breastmilk of mothers receiving ART. Our study found no evidence of increased rates of toxicity in breastfeeding infants of mothers receiving tenofovir-based ART compared to breastfeeding infants whose mothers were not receiving ART. These data are also reassuring regarding the safety of TDF/FTC pre-exposure prophylaxis by breastfeeding, uninfected women at risk of HIV and their infants.25-27 Similarly, the prolonged use of daily NVP prophylaxis by HIV-1-uninfected infants for up to 18 months was not associated with elevated infant toxicity, including skin and liver toxicity, compared to infants not receiving NVP.
The median infant age to breastfeeding cessation in the study was 16 months, with 86% of infants still not having achieved complete breastfeeding cessation by 9 months, decreasing to 34% by 18 months. This is longer than what was originally hypothesized during the design of the trial and likely reflects changing guidelines on breastfeeding by HIV-1-infected women as well as changing habits among sub-Saharan women during the trial. This prolonged duration of breastfeeding puts the HIV-1-exposed infant at risk of infection should there be suboptimal ART adherence by the mother. Approximately 67% of infections occurred after age 6 months, and 33% after age 12 months, with infections continuing to occur through 24 months.
During the conduct of the PROMISE randomized trial, the WHO guidelines for prevention of perinatal HIV-1 transmission were modified in 2013 to recommend maternal ART through at least the duration of breastfeeding; current guidelines recommend life-long ART for all HIV-1-infected individuals, including pregnant and breastfeeding women.17,28 Despite these recommendations, due to postpartum adherence problems, many women experience rebound viremia, resulting in continued postnatal transmission.3,4,29 The PROMISE data demonstrate mART and iNVP have similar efficacy and safety profiles through up to 24 months of breastfeeding, indicating that while treatment for breastfeeding women is a priority, prolonged use of iNVP is an effective and safe alternative, for example, for women who refuse or do not adhere to ART, have persistent viremia, or who temporarily stop ART for toxicity.5 However, for women who refuse or are not adherent to ART, similar barriers to iNVP administration may exist.
Our data underline the importance of providing postpartum support for women receiving ART, since we observed a continuing risk of infant postnatal infection for the duration of breastfeeding even when effective interventions were being provided. A variety of approaches will be needed to achieve an HIV-1-free generation, including interventions to support ART adherence and postpartum retention in care for women and ensuring the availability of equally effective and safe infant prophylaxis alternatives for situations in which maternal ART may be insufficient to protect the breastfeeding infant.
Supplementary Material
Acknowledgments
Overall support for the International Maternal Pediatric Adolescent AIDS Clinical Trials Group (IMPAACT) was provided by the National Institute of Allergy and Infectious Diseases (NIAID) of the National Institutes of Health (NIH) under Award Numbers UM1AI068632 (IMPAACT LOC), UM1AI068616 (IMPAACT SDMC) and UM1AI106716 (IMPAACT LC), with co-funding from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) and the National Institute of Mental Health (NIMH). The PROMISE team gratefully acknowledges the contributions of the mothers and their infants who participated in the study. The team also acknowledges the support and donation of study products of Gilead, GSK/Viiv/Healthcare, Abbvie, and Boehringer-Ingelheim pharmaceutical companies.
We gratefully acknowledge the contributions of the study staff, site investigators and site staff who conducted IMPAACT 1077BFstudy:
PROMISE Study Team Members: Judith Currier, Katherine Luzuriaga, Adriana Weinberg, James McIntyre, Tsungai Chipato, Karin Klingman, Renee Browning, Mireille Mpoudi-Ngole, Jennifer S. Read, George Siberry, Heather Watts, Lynette Purdue, Terrence Fenton, Linda Barlow-Mosha, Mary Pat Toye, Mark Mirochnick, William B. Kabat, Benjamin Chi, Marc Lallemant, Karin Nielsen; Statistical and Data Analysis Center, Harvard T.H. Chan School of Public Health: Kevin Butler MS, Konstantia Angelidou MS, and Sean Brummel Ph.D. FHI360: Melissa Allen, Anne Coletti, Megan Valentine, Kathleen George; Frontier Science Data Management Center: Michael Basar, Amy Jennings, Adam Manzella, Amanda Zadzilka; Retroviral Core Laboratory, University of North Carolina Virology Laboratory: Amy James
INDIA. Byramjee Jeejeebhoy Medical College: Ajay Sahebrao Chandanwale, MS; Pradip Wamanrao Sambarey, MD PhD; Uma Nitin Wankhede MD. MALAWI. Blantyre: Taha E. Taha MBBS, PhD; Bonus Makanani MBBS, FCOG; Rachel Chamanga MBBS; Newton Kumwenda MPH, PhD. Lilongwe/UNC: Cornelius Mukuzunga, MBChB; Godwin Chikopa, MBBS, MSC Paeds; Ezylia Makina, RNM. SOUTH AFRICA. Durban Paediatric: Sajeeda Mawlana MBChB, Post Graduate Diploma in Clinical HIV/AIDS Management Nozibusiso Rejoice Sikhosana BN; Kimesh Naidoo MBChB, DCH, FCPaed. Family Clinical Research Unit: Jeanne Louw MSc; Magdel Rossouw MNutr, MBChB; Lindi Rossouw MBChB. Shandukani Research: Janet Grab BPharm; Lee Fairlie MBChB, FCPaeds (SA), MMED (Paeds); Hermien Gous PharmD; Gurpreet Kindra MBBS. Soweto: Sylvia Dittmer, MD; Mandisa Nyati, MD; Nasreen Abrahams, MBA. Umlazi: Megeshinee Naidoo MBChB; Vani Chetty BscHon; Alicia Catherine Desmond BPharm, MPharm; TANZANIA. Kilimanjaro Christian Medical Centre Blandina Theophil Mmbaga MD, MMed, PhD; Boniface Njau, MPH; Julitha Kimbi RN. UGANDA. MU-JHU Research Collaboration: Moreen Kamateeka, MBChB, MPH; Dorothy Sebikari MBChB, MPH; Philippa Musoke, PhD. ZAMBIA. George Clinic: Mwangelwa Mubiana-Mbewe MBChB, MMed, MBA; Felistas M. Mbewe, RN, BSc; Bethany Freeman, MSPH, MSW. ZIMBABWE. Harare Family Care: Tapiwa Mbengeranwa MBChB; Tsungai Mhembere BPharm; Sukunena Maturure SRN MS; Tichaona Vhembo MBChB. Seke North: Lynda Stranix-Chibanda MBChB, MMED (Paeds); Teacler Nematadzira MBChB Msc; Suzen Maonera SRN MS; Vongai Chanaiwa BPharm. St. Mary's: Tsungai Chipato MB ChB FRCOG; Bangani Kusakara MBChB; Mercy Mutambanengwe BPharm; Emmie Marote SRN MA.
The PROMISE team dedicates this manuscript to Dr. Edward Handelsman, Division of AIDS, NIH, Dr. Stephen Lagakos from the Center for Biostatistics in AIDS Research, Harvard School of Public Health, and Mrs. Linda Millar from The Frontier Science & Technology Research Foundation, Amherst, NY in grateful memory of their many contributions to the PROMISE 1077 trial and HIV/AIDS research. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Funding: Funding for this study was provided by the National Institute of Allergy and Infectious Diseases (NIAID) of the National Institutes of Health (NIH) under Award Numbers UM1AI068632 (IMPAACT LOC), UM1AI068616 (IMPAACT SDMC) and UM1AI106716 (IMPAACT LC), with co-funding from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) and the National Institute of Mental Health (NIMH).Study drugs were provided by AbbVie, Boehringer-Ingelheim, Gilead Sciences, and ViiV/GlaxoSmithKline.
Footnotes
Prior presentation: Data was presented in part at the 21st International AIDS Conference, July 18-22, 2016, Durban, South Africa
Conflict of Interest: Dr. Flynn is a consultant for Merck. No other authors declare conflict of interest.
Registration: ClinicalTrials.gov: NCT01061151; closed to follow-up
References
- 1.Fowler MG, Qin M, Fiscus S, et al. Benefits and risks of Antiretroviral Therapy for Perinatal HIV Prevention. N Engl J Med. 2016;375(18):1726–1737. doi: 10.1056/NEJMoa1511691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Myer L, Dunning L, Lesosky M, et al. Frequency of viremic episodes in HIV-infected women initiating antiretroviral therapy during pregnancy: a cohort study. Clin Infect Dis. 2016;64(4):422–427. doi: 10.1093/cid/ciw792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Haas AD, Msukwa MT, Egger M, et al. Adherence to antiretroviral therapy during and after pregnancy: cohort study on women receiving care in Malawi's Option B+ program. Clin Infect Dis. 2016;63:1227–35. doi: 10.1093/cid/ciw500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gill MM, Hoffman HJ, Bobrow EA, et al. Detectable viral load in late pregnancy among women in the Rwanda Option B+ PMTCT program: enrollment results from the Kabeho Study. PLosOne. 2016;11:e0168671. doi: 10.1371/journal.pone.0168671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Van de Perre P, Kankasa C, Nagot N, et al. Pre-exposure prophylaxis for infants exposed to HIV through breastfeeding. BMJ. 2017;356:j1053. doi: 10.1136/bmj.j1053. [DOI] [PubMed] [Google Scholar]
- 6.Kesho Bora Study Group. de Vincenzi I. Triple antiretroviral compared with zidovudine and single-dose nevirapine prophylaxis during pregnancy and breastfeeding for prevention of mother-to-child transmission of HIV-1 (Kesho Bora study): a randomised controlled trial. Lancet Infect Dis. 2011 Mar;11(3):171–80. doi: 10.1016/S1473-3099(10)70288-7. [DOI] [PubMed] [Google Scholar]
- 7.Six Week Extended-Dose Nevirapine (SWEN) Study Team. Extended-dose nevirapine to 6 weeks of age for infants to prevent HIV transmission via breastfeeding in Ethiopia, India, and Uganda: an analysis of three randomised controlled trials. Lancet. 2008;372(9635):300–12. doi: 10.1016/S0140-6736(08)61114-9. [DOI] [PubMed] [Google Scholar]
- 8.Kumwenda NI, Hoover DR, Mofenson LM, et al. Extended antiretroviral prophylaxis to reduce breast-milk HIV transmission. N Engl J Med. 2008;359(2):119–29. doi: 10.1056/NEJMoa0801941. [DOI] [PubMed] [Google Scholar]
- 9.Coovadia HM, Brown ER, Fowler MG, et al. Efficacy and safety of an extended nevirapine regimen in infant children of breastfeeding mothers with HIV-1 infection for prevention of postnatal HIV-1 transmission (HPTN 046): a randomised, double-blind, placebo-controlled trial. Lancet. 2012 Jan 21;379(9812):221–8. doi: 10.1016/S0140-6736(11)61653-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chasela CS, Hudgens MG, Jamieson DJ, et al. Maternal or infant antiretroviral drugs to reduce HIV-1 transmission. N Engl J Med. 2010;362(24):2271–2281. doi: 10.1056/NEJMoa0911486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nagot N, Kankasa C, Tumwine JK, et al. Extended pre-exposure prophylaxis with lopinavir-ritonavir versus lamivudine to prevent HIV-1 transmission through breastfeeding up to 50 weeks in infants in Africa (ANRS 12174): a randomised controlled trial. Lancet. 2016;387:566–573. doi: 10.1016/S0140-6736(15)00984-8. [DOI] [PubMed] [Google Scholar]
- 12.Cohan D, Natureeba P, Koss CA, et al. Efficacy and safety of lopinavir-ritonavir versus efavirenz-based antiretroviral therapy in HIV-infected pregnant Ugandan women. AIDS. 2015;29:183–91. doi: 10.1097/QAD.0000000000000531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shapiro RL, Hughes MD, Ogwu A, et al. Antiretroviral regimens in pregnancy and breastfeeding in Botswana. N Engl J Med. 2010;362:2282–94. doi: 10.1056/NEJMoa0907736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Taha TE, Hoover DR, Chen S, et al. Effects of cessation of breastfeeding in HIV-1-exposed, uninfected children in Malawi. Clin Infect Dis. 2011;53:388–95. doi: 10.1093/cid/cir413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marquez C, Okiring J, Chamie G, et al. Increased morbidity in early childhood among HIV-exposed uninfected children in Uganda is associated with breastfeeding duration. J Trop Ped. 2014;60:434–441. doi: 10.1093/tropej/fmu045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kuhn L, Sinkala M, Semrau, et al. Elevations in mortality associated with weaning persist into the second year of life among uninfected infants bornt to HIV-infected mothers. Clin Infect Dis. 2010;50:437–44. doi: 10.1086/649886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.World Health organization. Geneva, Switzerland: 2013. [accessed August 15, 2016]. Consolidated Guidelines on the Use of Antiretroviral Drugs for Treating and Preventing HIV Infection. Available at: www.who.int/hiv/pub/guidelines/arv2013/en/ [Google Scholar]
- 18.Zelen M. The Randomization and Stratification of Subjects to Clinical Trials. Journal of Chronic Diseases. 1974 Sep;27(7-8):365–75. doi: 10.1016/0021-9681(74)90015-0. [DOI] [PubMed] [Google Scholar]
- 19.U.S. Department of Health and Human Services, National Institutes of Health, National Institute of Allergy and Infectious Diseases, Division of AIDS. Division of AIDS (DAIDS) Table for Grading the Severity of Adult and Pediatric Adverse Events, Version 2.0. [accessed October 23, 2016];2014 Nov; Available at: http://rsc.tech-res.com/docs/default-source/safety/daids_ae_grading_table_v2_nov2014.pdf.
- 20.Kilewo C, Karlsson K, Massawe A, et al. Mitra Study Team. Prevention of mother-to-child transmission of HIV-1 through breast-feeding by treating infants prophylactically with lamivudine in Dar es Salaam, Tanzania: the Mitra Study. J Acquir Immune Defic Syndr. 2008;48(3):315–23. doi: 10.1097/QAI.0b013e31816e395c. [DOI] [PubMed] [Google Scholar]
- 21.Kilewo C, Karlsson K, Ngarina M, et al. Mitra Plus Study Team. Prevention of mother-to-child transmission of HIV-1 through breastfeeding by treating mothers with triple antiretroviral therapy in Dar es Salaam, Tanzania: the Mitra Plus study. J Acquir Immune Defic Syndr. 2009;52(3):406–16. doi: 10.1097/QAI.0b013e3181b323ff. [DOI] [PubMed] [Google Scholar]
- 22.The Breastfeeding and HIV International Transmission Study Group. Late postnatal transmission of HIV in breast-fed children: an independent patient data meta-analysis. J Infect Dis. 2004;189:2154–66. doi: 10.1086/420834. [DOI] [PubMed] [Google Scholar]
- 23.Group ISS, Lundgren JD, Babiker AG, et al. Initiation of Antiretroviral Therapy in Early Asymptomatic HIV Infection. N Engl J Med. 2015;373(9):795–807. doi: 10.1056/NEJMoa1506816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shapiro RL, Lockman S. Mortality among HIV-exposed infants: the first and final frontier. Clin Infect Dis. 2010;50:445–447. doi: 10.1086/649887. [DOI] [PubMed] [Google Scholar]
- 25.Stranix-Chibanda L, Tierney C, Marr C, et al. HIV Pediatrics Workshop. Durban, South Africa: 2016. Jul, Impact of tenofovir-containing triple antiretroviral therapy (ART) on bone mineral density in HIV-infected breastfeeding women in sub-Saharan Africa; pp. 15–16. 2016. [Google Scholar]
- 26.Mofenson LM, Baggaley RC, Mameletzis J. Tenofovir disoproxil fumarate safety for women and their infants during pregnancy and breastfeeding. AIDS. 2017;31:213–32. doi: 10.1097/QAD.0000000000001313. [DOI] [PubMed] [Google Scholar]
- 27.Mofenson LM. Tenofovir pre-exposure prophylaxis for pregnant and breastfeeding women at risk of HIV infection: the time is now. PLosMed. 2016;13(9):e1002133. doi: 10.1371/journal.pmed.1002133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.World Health Organization. Antiretroviral therapy for HIV infection in adults and adolescents Recommendations for a public health appraoch 2010 revision. Geneva, Switzerland: 2010. [accessed August 15, 2016]. Available at: http://apps.who.int/iris/bitstream/10665/44379/1/9789241599764_eng.pdf. [PubMed] [Google Scholar]
- 29.Myer L, Phillips TK, Hsiao NY, et al. Plasma viremia in HIV-positive pregnant women entering antenatal care in South Africa. J Int AIDS Soc. 2015;18:2004. doi: 10.7448/IAS.18.1.20045. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.