Abstract
Lung cancer is the most common cause of cancer-related deaths in humans worldwide. There is strong evidence that the tobacco-specific nitrosamine 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK) and its metabolite 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol (NNAL), play an important role in carcinogenesis caused by tobacco products. NNK and racemic NNAL are reported to induce lung and pancreatic tumors in rats. The carcinogenicity in Fischer 344 (F344) rats of NNK, NNAL, and its enantiomers (R)-NNAL and (S)-NNAL has been studied recently and all test compounds induced significant numbers of lung tumors. We report here the detailed histopathological and immunohistochemical characterization of these tumors and their aggressive nature as shown by their metastasis locally and to the pancreas. The spectrum of treatment-related histopathological findings comprised pulmonary alveolar/bronchiolar (A/B) epithelial hyperplasia, A/B adenomas, and A/B carcinomas. Alveolar/bronchiolar carcinomas frequently exhibited local invasion/metastasis within the mediastinum and thoracic cavity and distant metastasis to the pancreas that was confirmed by immunohistochemistry using the lung-specific markers prosurfactant protein-C (SP-C) and club (Clara) cell-10 (CC-10). Our observation regarding metastasis to the pancreas was an important, and unexpected, finding in this study, both for the experimental animal model and potential human relevance.
Keywords: NNK, NNAL, smokeless tobacco, rats, pulmonary tumors, pancreatic metastasis, SP-C, CC10, CK-19
Introduction
Lung cancer is the most common cause of cancer-related deaths in humans worldwide, with average survival times measured in months. In the United States, lung cancer mortality is comparable to the combined cancer-related deaths due to breast, pancreas, prostate and colorectal cancers (United-States-Cancer-Statistics-Working-Group, 2013). Tobacco smoking is responsible for about 70% of lung cancer and 30% of pancreatic cancer-related deaths worldwide (Stewart and Wild, 2014). Therefore, it is important to understand the underlying mechanisms of carcinogenesis, cells of origin, and types of tumors caused by tobacco carcinogens in order to develop effective approaches to cancer prevention in smokers and smokeless tobacco users.
4-(Methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK) is present in all tobacco products – both combusted and smokeless - and is one of the key components of tobacco products that induces tumors of the lung in rats, mice, hamsters, and ferrets (Balbo 2014, Hecht 1998, Hecht, 2002, Akopyan and Bonavida, 2006). NNK and the related tobacco-specific nitrosamine N′-nitrosonornicotine (NNN) are considered “carcinogenic to humans (Group 1)” by the International Agency for Research on Cancer (IARC, 2012). NNK is metabolically converted to its metabolite 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol (NNAL) in all biological systems, both in vitro and in vivo, and is present in the urine of virtually all tobacco users as well as in people exposed to secondhand smoke (Hecht, 1998, Hecht 2016, Roethig et al., 2009). NNAL exists in enantiomeric forms, (S)-NNAL and (R)-NNAL due to the existence of a chiral center at its 1-position. (S)-NNAL is the major enantiomer formed in lung and liver microsomal fractions (Hecht et al., 1997), but studies in vivo demonstrate that both isomers are formed and are present in urine, including human urine, depending on the system investigated (summarized in Balbo et al., 2014). NNK has been reported to induce exocrine pancreatic tumors in rats and Syrian golden hamsters (Rivenson et al., 1988, Schuller et al., 1994). In a previous study of the carcinogenicity in rats of NNK and NNAL administered in the drinking water, the pancreatic tumors were characterized as exocrine ductular carcinomas based on histomorphology (Rivenson et al., 1988). More recently a carcinogenicity study of NNK and the enantiomers of NNAL administered in the drinking water to F344 rats has been performed (Balbo et al., 2014). In addition, carcinogenicity of tobacco specific N-nitrosamines and histopathological features of lung, liver and pancreatic tumors have been reported previously (Rivenson et al., 1991, Belinsky et al., 1990).
This study describes the detailed histopathology and immunohistochemical characterization of NNK- and NNAL enantiomer-induced pulmonary neoplasms and their isolated metastasis to the pancreas in male F344 rats.
Materials and Methods
Test compounds
Racemic NNAL, (S)-NNAL and (R)-NNAL were prepared as described previously and characterized by 1H-NMR and MS (Hecht et al., 1997, Hecht et al., 1980). NNK was purchased from Toronto Research Chemicals. Chemical purities, metabolism, and DNA adduct formation of these compounds were reported elsewhere (Balbo et al., 2014, Hecht et al., 1980).
Tumor study and histopathology
The tissues used in this study were from our recent 2-year carcinogenicity bioassay of NNK and the (R) and (S) enantiomers of its metabolite, NNAL in F344 rats described in detail by Balbo et al., 2014. Group of 15–24 male F344 rats were administered the test compound in drinking water as follows: NNK (24 rats, 5 ppm), (S)-NNAL (22 rats, 5 ppm), (R)-NNAL (24, 5 ppm), racemic NNAL (15 rats, 10 ppm) and negative control with no treatment. The method of preparation, purification, storage and dilution of the aqueous stock solutions of these test compounds were described previously (Balbo et al., 2014). Husbandry and experimental procedures were in compliance with requirements set forth by the University of Minnesota Institutional Animal Care and Use Committee (IACUC). After complete gross pathological examination, tissue sections from target organs including lungs, pancreas, nasal cavity and common sites for metastasis in the rat such as liver and kidney were reviewed by histopathological examination. The target organs were selected based on previous carcinogenicity studies (Rivenson et al., 1988). The National Toxicology Program (NTP) classification scheme for pulmonary epithelial neoplasms and International Harmonization of Nomenclature and Diagnostic criteria (INHAND) guidelines for proliferative lesions in the respiratory system of rat were used to classify pulmonary neoplasms in this study (Boorman and Eustis, 1990, Renne et al., 2009).
Immunohistochemistry
5-μm sections were prepared from paraffin embedded blocks of the target organs; lungs and pancreas, and organs of potential metastasis; liver and kidney, for staining with club (Clara) cell 10 (CC-10), prosurfactant protein C (SP-C) or cytokeratin 19 (CK19) antibodies. The tissue sections were deparaffinized in xylene and rehydrated through decreasing grades of alcohol followed by either Tris Buffered Saline or citrate buffer automation wash buffer (Biocare Medical, CA). Antigen retrieval was done by trypsin digestion. Endogenous peroxidase was quenched by incubating the tissue sections with 3% hydrogen peroxide. Subsequently, the sections were incubated with the primary antibody either club (Clara) cell-10 (CC-10, 1:500, goat Anti-CC10 polyclonal antibody, clone T-18, Santa Cruz Biotechnology, Inc. CA) or prosurfactant protein C (SP-C, 1:500, rabbit polyclonal Prosurfactant Protein-C antibody, Millipore, MA) or CK19 antibody (Santa Cruz Biotechnology, Inc. CA). For the negative controls, tissue sections were incubated with normal serum from the species in which the primary antibody was raised. The concentration of immunoglobulin in the negative control serum was normalized to that of primary antibody. Subsequently, the sections were incubated with a secondary antibody followed by incubation with EnVision+ system-HRP polymer based detection solution (Dako North America, Inc. CA) for 30 min. Antigen–antibody complexes were visualized using 3,3′-diaminobenzidine (DAB) chromogen. Finally, the slides were counterstained with hematoxylin, dehydrated through increasing grades of alcohol, cleared in xylene, and cover slipped. For CC-10 and SP-C staining, sections of normal rat lungs were used as positive control tissue. For CK-19, normal rat pancreas was used as a positive control. For all of these antibodies, intracytoplasmic immunoreactivity was analyzed in comparison to controls.
Results
In our recently reported carcinogenesis study (Balbo et al., 2014), all of the rats in various treatment groups had grossly visible neoplastic lesions in the lung lobes with no grossly discernible tumors in control rats. Representative gross pathology images of lung tumors are shown in Figure 1A. Within the NNK and racemic NNAL treated groups, there were several rats with intrathoracic invasion or mediastinal metastasis of pulmonary carcinomas (Figure 1B).
Figure 1.
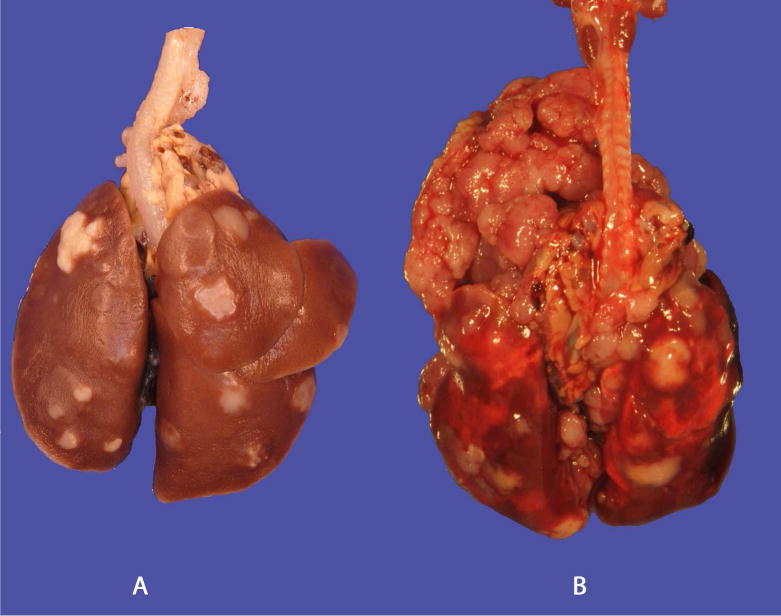
Gross images of lungs A: (R)-NNAL with pulmonary tumors, C: (S)-NNAL with intrathoracic invasion and mediastinal metastasis. Bar = 1 cm.
Histopathology
Treatment-related proliferative lesions were observed only in lungs and pancreas in all treated groups with no incidence of pulmonary tumors in control rats. Based on the morphological features, the pulmonary lesions were categorized as alveolar/bronchiolar (A/B) epithelial hyperplasia, A/B adenoma or A/B carcinoma as per the National Toxicology Program (NTP) scheme for primary pulmonary proliferative lesions of epithelial origin (Dixon and Maronpot, 1991) and International Harmonization of Nomenclature and Diagnostic Criteria (INHAND) guidelines on proliferative lesions of the respiratory tract of rat and mouse (Renne et al., 2009).
Alveolar/Bronchiolar Hyperplasia
The alveolar/bronchiolar hyperplastic lesions were focal or multifocal areas of hypercellularity, with indistinct peripheral margins and composed of round to oval or cuboidal to columnar often/mostly hypertrophic alveolar type II cells with abundant eosinophilic cytoplasm prominently outlining alveolar walls and club (Clara) cells with prominent apical protrusions; mitotic figures were not evident in any of the cell populations (Figure 2A, 3A). Areas of hyperplasia were often centered on terminal bronchioles and associated with influx of alveolar macrophages. Lung sections from all of the rats treated with NNK, racemic-NNAL, (S)-NNAL and (R)-NNAL had focal or multifocal areas of alveolar/bronchiolar hyperplasia.
Figure 2.
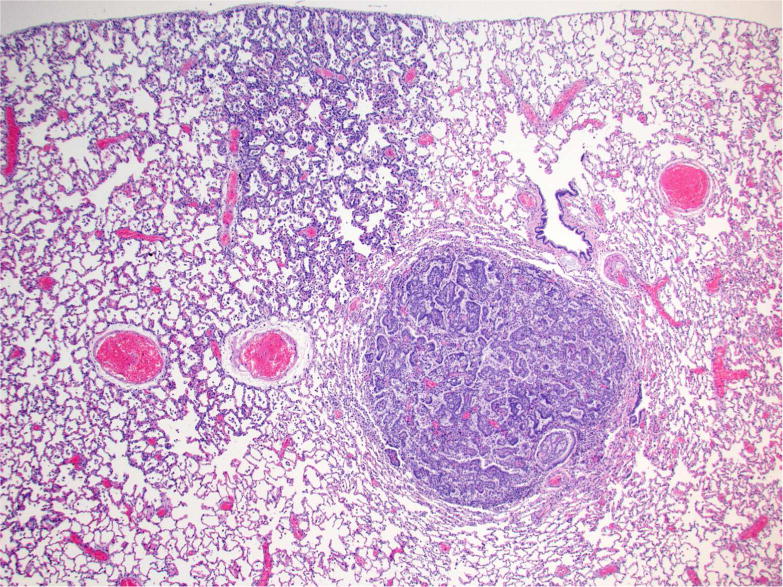
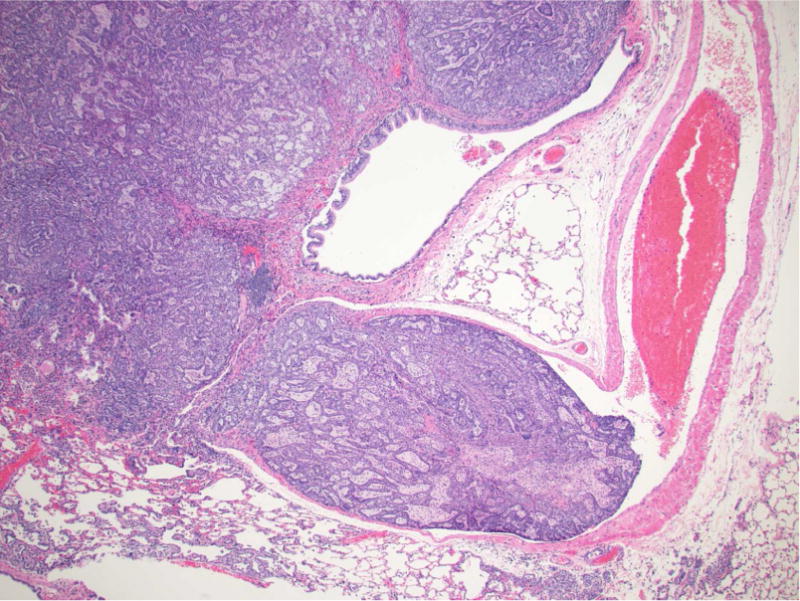
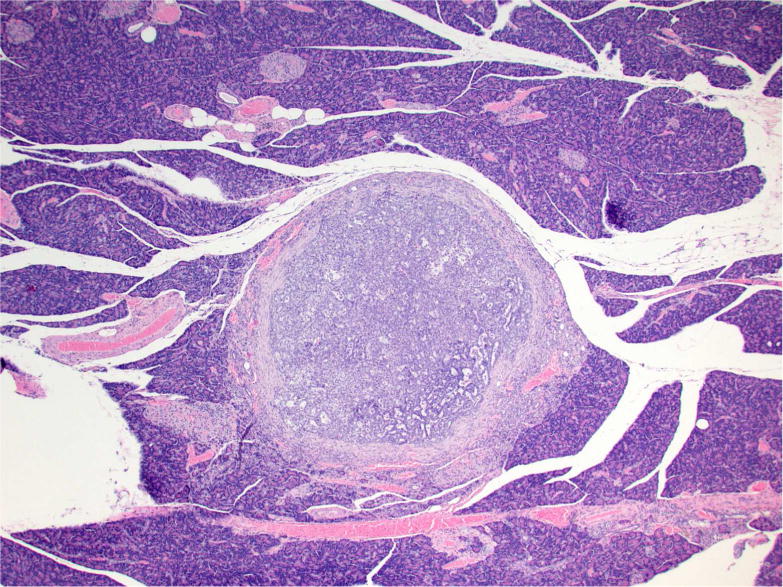
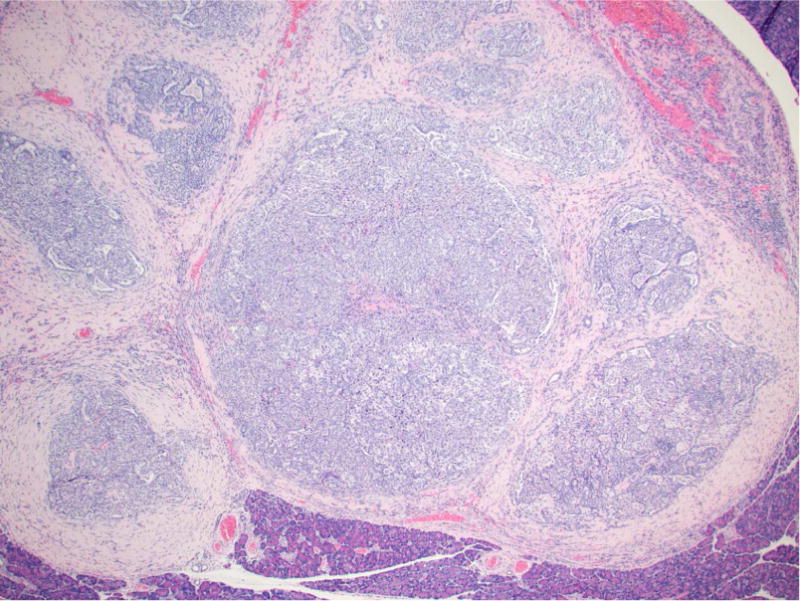
Photomicrographs of proliferative lesions in lung and pancreas. Lung: rac-NNAL treated rat, alveolar/bronchiolar (A/B) hyperplasia and A/B adenoma (A) and carcinoma with a tumor embolus within a medium caliber blood vessel (B). Pancreas: NNK treated rat, a focal carcinoma (C) and carcinoma with marked scirrhous reaction (D)
Figure 3.
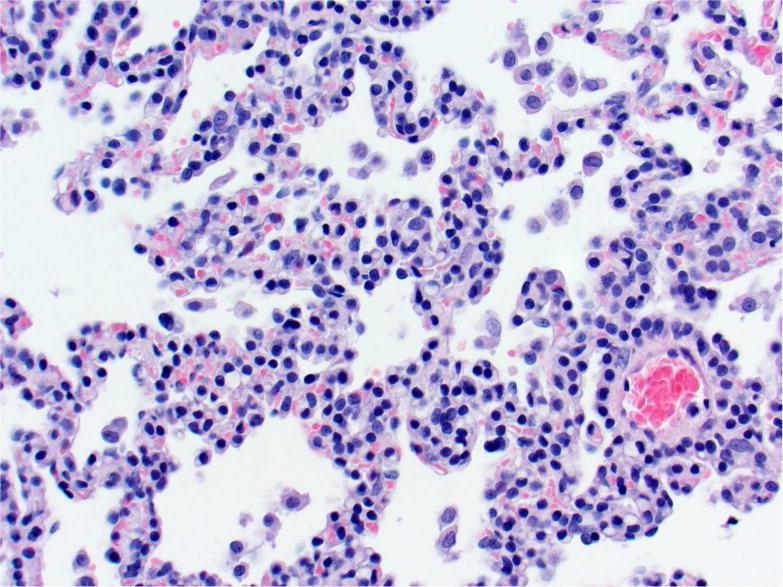
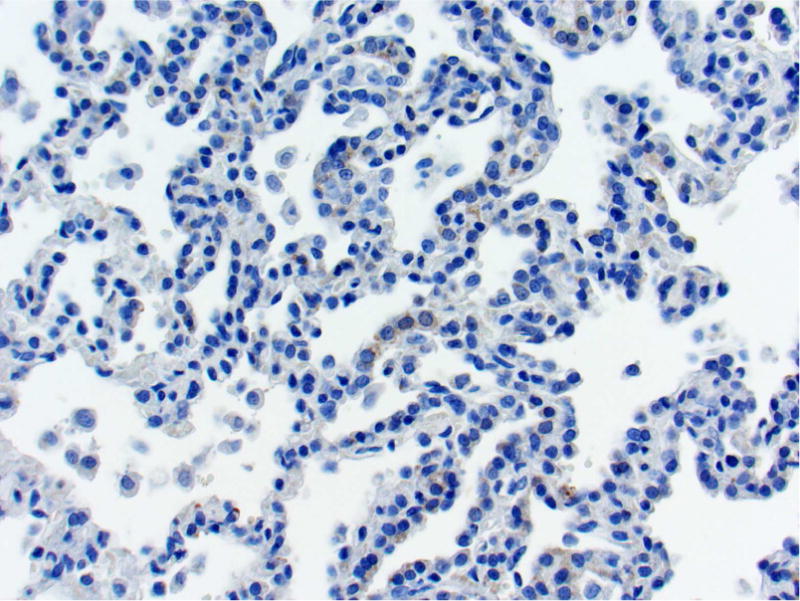
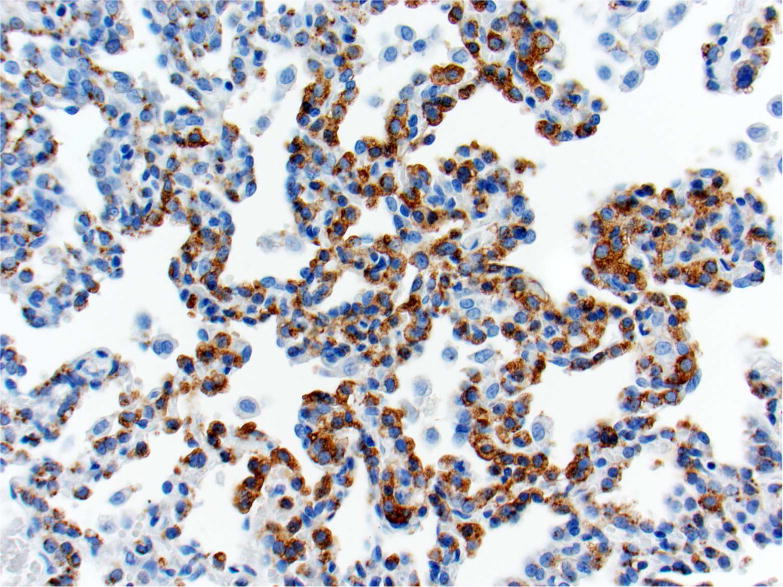
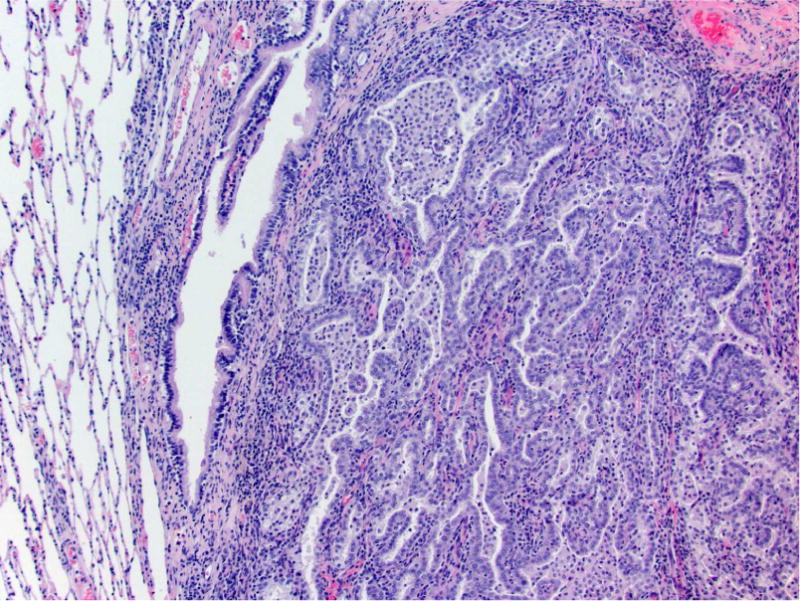
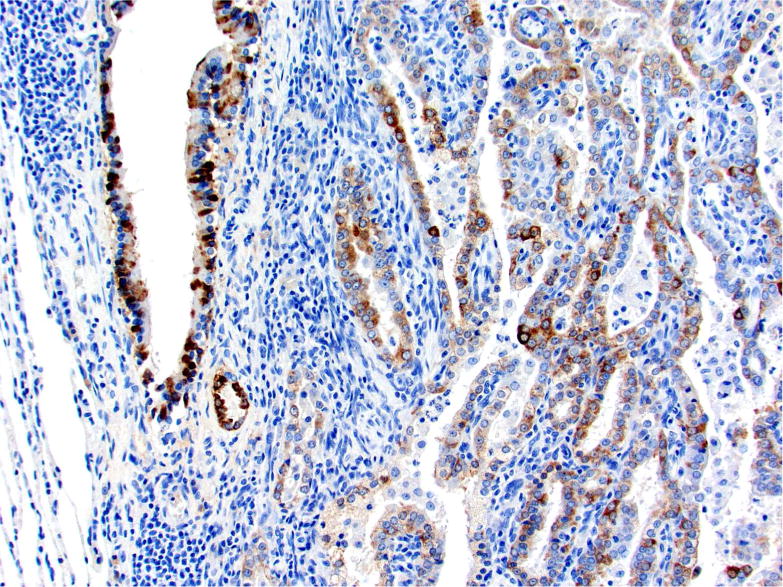
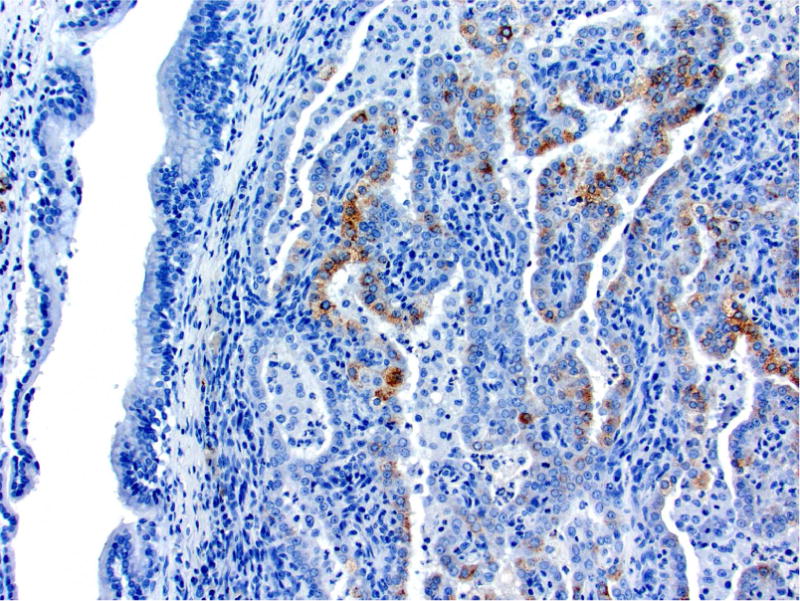
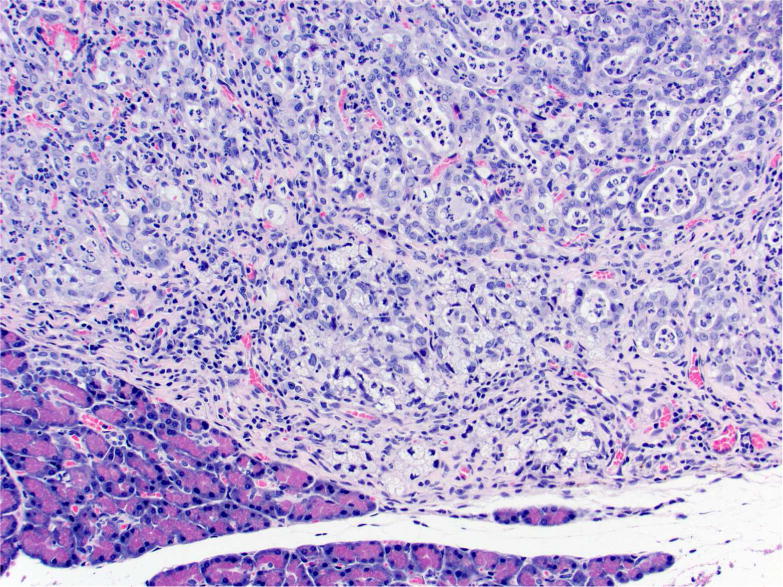
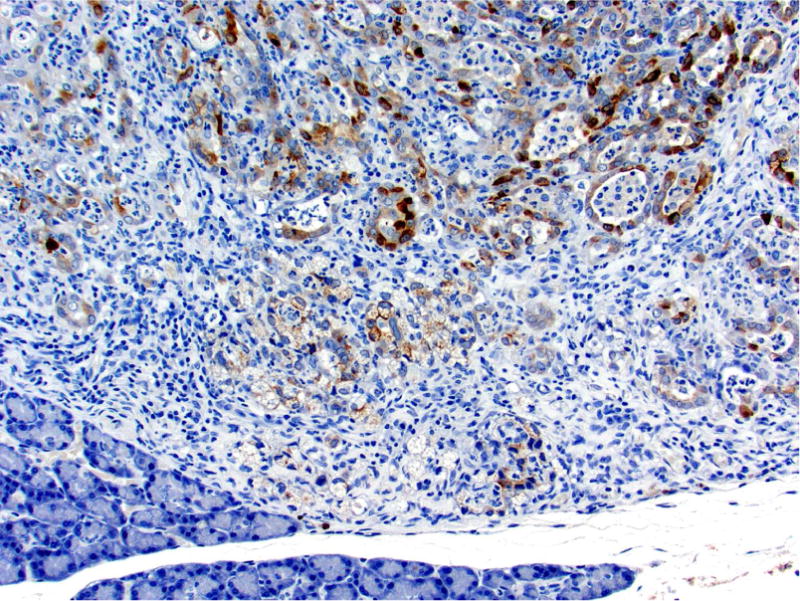
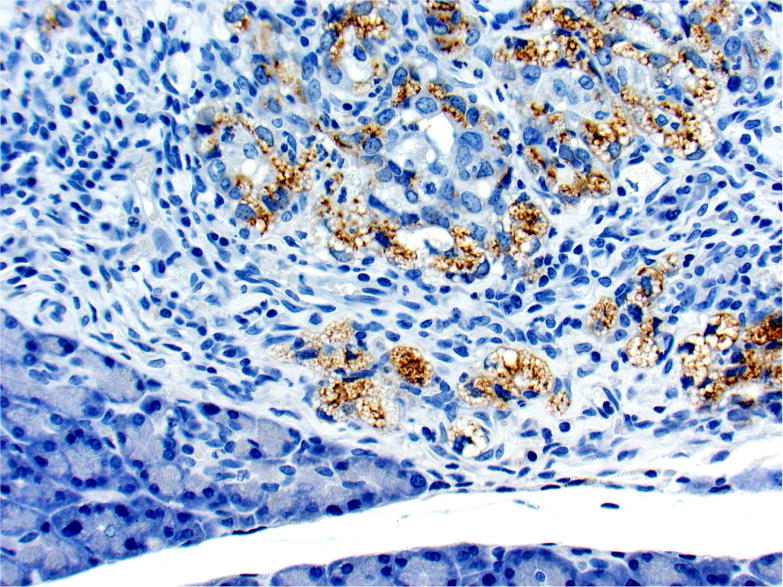
Photomicrographs of histology and immunohistochemistry of lung tumors, metastatic tumors in the pancreas and kidney*. Panel A–C: Lung. alveolar/bronchiolar (A/B) hyperplasia (A); Multifocally, a few hyperplastic pneumocytes exhibit weak intracytoplasmic immunoreactivity for CC10. (B); and diffusely the hyperplastic cells exhibit strong intracytoplasmic immunoreactivity for SP-C (C). *Panel D–F: Pulmonary carcinoma. The tumor cells exhibit strong immunoreactivity for CC10 and SP-C. Panels G–I and J–L: Pancreas and Kidney respectively. The tumor cells exhibit strong immunoreactivity for CC10 and moderate immunoreactivity for SP-C.
* Figures (3D–3I) “Proceedings of the 2015 National Toxicology Program Satellite Symposium: Elmore et al., 2016). Toxicol Pathol 45:1,11–51. Reprinted with permission.
Alveolar/Bronchiolar Adenoma
Alveolar/bronchiolar (A/B) adenomas were generally well demarcated, slightly compressed the adjacent non-neoplastic normal parenchyma with strongly convex border and had solid, tubulopapillary, alveolar or mixed histologic growth patterns. Adenomas of A/B origin were composed of monomorphic populations of well-differentiated epithelial cells with variably distinct cell borders, moderate to abundant amounts of eosinophilic cytoplasm, basally located nuclei with finely stippled chromatin and 0–1 prominent nucleolus. Mitotic figures were rare. There were low numbers of A/B adenomas with atypia characterized by a higher degree of pleomorphism, increased mitotic figures and occasional extension into adjacent bronchioles. All the rats treated with NNK, (R)-NNAL, (S)-NNAL, or racemic NNAL in this study had adenomas except one rat treated with (R)-NNAL (Fig 2A).
Alveolar/Bronchiolar Carcinoma
A/B carcinomas were generally large, poorly demarcated, irregular nodular growths that effaced the normal pulmonary architecture or often occupied an entire lobe. The neoplastic cells were predominantly arranged as tubulopapillary or alveolar or mixed growth patterns (Figure 2B, 3D). Neoplastic cells were cuboidal to columnar with variably distinct borders, moderate amounts of eosinophilic to foamy cytoplasm, centrally or basally located nuclei with finely stippled chromatin. Mitotic index was high ranging from 3–6 per 40× HPF with moderate to marked anisocytosis and anisokaryosis. There were multifocal areas of squamous metaplasia and marked necrosis within the larger A/B carcinomas. There was evidence of vascular invasion (Fig 2B, indicate by arrow) or complete occlusion of the bronchial lumen in some rats. As previously reported from this carcinogenicity study (Balbo et al., 2014), all the rats treated with racemic NNAL, and 17/24, 10/22, and 6/24 rats treated with NNK, (S)-NNAL, (R)-NNAL, respectively, had A/B carcinomas. In 6/24 of the NNK-treated rats, 9/15 of the rac-NNAL-treated rats, 2/22 of the (S)-NNAL-treated rats, and 2/24 of the (R)-NNAL-treated rats, there were multifocally extensive to diffuse mediastinal metastasis with intrathoracic invasion (Figure 1B. The histomorphology of these tumors were similar to A/B carcinomas within the pulmonary parenchyma. Proliferative lesions in lung and pancreas in different treatment groups in this study are tabulated in Table 1.
Table 1.
Lung and Pancreatic Proliferative Lesions in Male F-344 Rats Given (S)-NNAL, (R)-NNAL, NNK, or Racemic NNAL in Drinking Water.a
| Control | (R) NNAL 5 ppm |
(S) NNAL 5 ppm |
NNK 5 ppm |
racemic NNAL 10 ppm |
|
|---|---|---|---|---|---|
| No. of rats necropsied | 22 | 24 | 22 | 24 | 15 |
| No. of rats with lung lesions | |||||
| Hyperplasia | 0 | 24 | 22 | 24 | 15 |
| Adenoma | 0 | 23 | 22 | 24 | 15 |
| Carcinoma | 0 | 6* | 10* | 17* | 15* |
| Tumor Incidence | 0 | 23** | 22** | 24** | 15** |
| No. of rats with thoracic cavity tumors | 0 | 2 | 2 | 6 | 9 |
| No. of rats with metastasis to pancreas | 0 | 1 (4.2%) | 2 (9.0%) | 3 (12.5%) | 4*** (27.0%) |
Reproduced with modifications from Balbo et al., 2014).
p<.01 compared to control.
p<.0001 compared to control.
p<.05 compared to control (ψ2 test)
Malignant tumors in the pancreas and kidney
Tumors in the pancreas were only detected microscopically and ranged from 1–8 mm in diameter. Multifocally, expanding, compressing, and replacing the normal pancreatic tissue, there were nodular masses composed of cuboidal epithelium arranged in tubulopapillary growth patterns with moderate to marked scirrhous reaction (Figure 2C and 2D). Three out of 24 of the NNK-treated, 4/15 of the racemic NNAL-treated, 2/22 of the (S)-NNAL-treated rats, and 1/24 of the (R)-NNAL-treated rats had one or more (up to 6) discrete malignant tumors within the pancreas. Some of these tumors were close to the pancreatic islets. As previously reported (Balbo et al., 2014), a total of 22 pancreatic tumors were observed in treatment groups with no tumors in control rats. One rat treated with racemic NNAL had a single, large, malignant tumor within the kidney with tubulopapillary growth pattern (Figure 3J). Immunohistochemistry with lung-specific markers confirmed that all of these tumors were metastatic pulmonary carcinomas (see below).
Immunohistochemistry
Immunohistochemistry was performed on the tissue sections of all tumors in the pancreas, kidney and lung sections from selected paraffin blocks of corresponding H & E-stained tissue sections. Appropriate negative controls were used as detailed in Material and Methods. There was no nonspecific immunoreactivity in any of the species-specific antibodies in negative control sections.
Prosurfactant protein-C (SP-C)
The SP-C antibody specifically labelled type II pneumocytes in sections of the lung used as a positive control and unaffected pulmonary parenchyma within the treated groups. All sections of the lungs from treated groups with alveolar/bronchiolar (A/B) hyperplasia, A/B adenoma and A/B carcinoma showed strong positive immunoreactivity for prosurfactant protein-C (SP-C) (Figure 3C, 3F). The immunoreactivity was intracytoplasmic and punctate or finely granular. There was a weak and patchy immunoreactivity for SP-C in some of the pulmonary A/B adenomas and carcinomas. All the thoracic cavity and mediastinal tumors exhibited strong immunoreactivity for SP-C. Of the 22 pancreatic metastases and one renal metastasis, all exhibited strong intracytoplasmic immunoreactivity for prosurfactant protein-C (Figure 3I, 3L). Within the kidney and pancreas, there was no immunoreactivity for SP-C in any of the constituent cell types.
Club (Clara) cell-10 (CC-10)
In sections of the lung used as positive control tissue, the antibody specifically labelled the club (Clara) cells within the bronchioles. All A/B hyperplasia exhibited weak intracytoplasmic immunoreactivity (Figure 3B). Alveolar/bronchiolar adenomas and A/B carcinomas exhibited strong immunoreactivity for CC-10 (Figure 3E). The thoracic cavity and mediastinal tumors exhibited strong immunoreactivity similar to lung tumors. Metastatic tumors in the pancreas (22/22) and kidney (1/1) were strongly positive for CC-10 (Figure 3H, 3K). There was no positive immunoreactivity in any of the cell types within the pancreas and kidney.
Cytokeratin 19 (CK19)
In the sections of the pancreas used as positive control, the antibody weakly labelled the pancreatic ductular epithelium. Within the lung, which was used as a negative control, the antibody labelled type I pneumocytes, type II pneumoncytes, and bronchial epithelial cells. All the lung tumors, thoracic cavity tumors, metastatic tumors in the pancreas and kidney showed weak intracytoplasmic immunoreactivity (data not shown).
Discussion
Alveolar/bronchiolar adenomas and carcinomas are the most common spontaneous and chemically induced pulmonary neoplasms in B6C3F1 mice and F344 rats (Dixon and Maronpot, 1991). Based on experimental animal and human epidemiological studies, tobacco is causally linked with cancer of the lung, oral cavity, larynx, esophagus, pancreas, renal pelvis, nasal cavity, cervix and urinary bladder (IARC, 2012). Tobacco specific carcinogen NNK’s metabolite NNAL and its enantiomers are found in human urine samples of tobacco users and many people exposed to second hand smoke (Carmella et al., 2013, Hecht et al., 2002, Xia et al., 2011). Most risk factors for pancreatic cancer, such as genetic disposition, metabolic diseases or chronic pancreatitis cannot be easily influenced. By contrast, tobacco usage is one of the strongest risk factors for exocrine pancreatic cancer and it is a completely avoidable hazard. In rodents several tobacco-derived carcinogens including NNK and NNAL in rats (Rivenson et al., 1988), and N- nitroso-2,6-dimethylmorpholine (cis-NNDM) in rats and hamsters (Kokkinakis and Scarpelli, 1989, Lijinsky et al., 1982) have been reported to induce pancreatic ductal tumors. There are reports describing morphological features of lung and pancreatic tumors induced by tobacco- specific carcinogens (Belinsky et al., 1990, Rivenson et al., 1991, Rivenson et al., 1988), however in this study we report further immunohistochemical characterization of the lung tumors and their specific metastatic lesions to the pancreas.
The number and size of tumors, and extent of involvement of pulmonary parenchyma varies with different tobacco-specific carcinogens. Based on our recent study (Balbo et al., 2014), racemic NNK is the most potent carcinogen when compared to its enantiomers, (S)-NNAL and (R)-NNAL, or its metabolite, NNAL, in the male F344 rat. Treatment-related microscopic lesions in the lung from this study are in substantial agreement with the previous chronic bioassay of NNK and NNAL in rats (Rivenson et al., 1988); however, using immunohistochemistry, we were able to demonstrate that the pancreatic tumors observed were metastatic tumors rather than primary pancreatic exocrine ductular carcinomas as initially diagnosed based on histomorphology (Rivenson et al., 1988).
Our recent chronic bioassay (Balbo et al., 2014) resulted in a number of important observations including evidence for chemical induced multistep carcinogenesis (Barrett, 1993) with progression of lesions from alveolar/bronchiolar epithelial hyperplasia to atypical hyperplasia to A/B adenoma to A/B carcinoma in most of the lung sections from treatment groups. There was evidence of aggressive malignant behavior of A/B carcinomas including extensive necrosis, squamous metaplasia, marked anisocytosis and anisokaryosis, high mitotic index, intravascular invasion, and local invasion of the mediastinum and thoracic cavity. Our observation of the presence of extrapulmonary intrathoracic cavity and mediastinal carcinomas in several animals has not previously been reported from tumors induced by tobacco carcinogens. However, alveolar bronchiolar tumors that are purely or largely mediastinal with alveolarbronchiolar histological features are reported in F344 rats from several NTP chronic studies (Howroyd et al., 2009).
Pulmonary neoplasms can be diagnosed by their location and typical histomorphology. However, diagnosing isolated metastatic carcinomas in organs like kidney, pancreas or other parenchymatous organs is often difficult as the neoplastic cells may have similar morphology to primary neoplasm of these organs. In this study, besides a detailed histomorphological examination, immunohistochemical characterization of alveolar/bronchiolar neoplasms by using pulmonary epithelial specific markers including prosurfactant protein C (type II pneumocytes marker) and club (Clara) cell -10, and a previously reported pancreatic ductular epithelial marker, cytokeratin 19 (Kasper et al., 1991, Real et al., 1993) was performed. Based on the histomorphology and immunohistochemical characteristics, the intrathoracic and mediastinal tumors were interpreted to have originated from A/B carcinomas by either direct extension or by seeding the thoracic cavity, mediastinum and pleural surfaces. All of the malignant tumors in the pancreas and one tumor in the kidney exhibit strong immunoreactivity for SP-C and CC-10, indicating that all the malignant tumors in the pancreas and kidney are indeed metastatic pulmonary alveolar/bronchiolar adenocarcinomas. The morphology ofthe metastatic lesions in the pancreas and kidney closely resemble the primary lung A/B carcinomas. Another observation indicating that the pancreatic tumors are not primary pancreatic ductular neoplasms is the absence of a developmental progression of the lesions from adenoma to carcinoma within the pancreas, while in the lung progression of lesions was observed from alveolar/bronchiolar hyperplasia to A/B adenoma to A/B carcinomas. Moreover, all of the animals with pancreatic neoplasms also had A/B carcinomas, further reinforcing that the pancreatic tumors are indeed metastatic A/B carcinomas. The kidney is one of the common sites of metastasis of malignant pulmonary tumors in rodents (Boorman and Eustis, 1990). However, in this study only one rat treated with rac-NNAL had focal metastasis in one kidney. Immunoreactivity to cytokeratin 19 was equivocal as pancreatic ductular epithelium, alveolar epithelium and bronchial epithelium exhibit moderate immunoreactivity to CK19 and the expression of CK19 in rat lungs has been previously reported (Schlage et al., 1998). Therefore, CK19 may not be a specific pancreatic ductular epithelial marker in rats.
The most common sites of metastatic spread from primary lung cancer include the brain, bone marrow, liver, adrenal glands, and skin in humans and the liver and kidney in rats. Isolated metastasis to the pancreas is a rare event. In humans, about 2% of pancreatic cancers are metastatic from another primary site (Roland and van Heerden, 1989, Stankard and Karl, 1992, Z’Graggen et al., 1998). In humans, isolated pancreatic metastases are most often from renal cell carcinoma, lung cancer, breast, and colon cancer (Bonapasta et al., 2010, Niess et al., 2013, Showalter et al., 2008). There are no reports of isolated metastases to the pancreas in rats. The underlying mechanism of specific isolated metastasis of A/B carcinomas to pancreas in humans in rodents is not clear.
In conclusion, tobacco specific carcinogens, NNK, NNAL and its enantiomers induced a spectrum of histopathological lesions including alveolar/bronchiolar hyperplasia, A/B adenoma and A/B carcinomas in the lungs of male F344 rats, and isolated metastasis of A/B carcinomas to the pancreas. Immunohistochemical characterization of tumors in this study has demonstrated that pancreatic tumors in NNK and NNAL-treated rats are metastatic lesions arising from primary lung tumors, rather than primary pancreatic tumors as had been originally reported on the basis of histomorphology alone.
Acknowledgments
We sincerely thank Drs. Jerry Hardisty and colleagues at Experimental Pathology Laboratories Inc., and at the National Toxicology Program (NTP), Research Triangle Park, for reviewing lung and pancreatic tumors from this study; Paula Overn for preparation of all histology and immunohistochemistry slides, Josh Parker for gross pathology image preparation, and Dr. Janardhan Kyathanahalli, Integrated Laboratory Systems (ILS) and NTP for sharing CC10 and SPC IHC protocols.
Funding
This study was supported by United States National Cancer Institute grant (CA-81301).
Footnotes
Declaration of Conflicting Interests Statement
The authors declare no potential conflicts of interest with respect to the research, authorship, and publication of this article.
References
- Akopyan G, Bonavida B. Understanding tobacco smoke carcinogen NNK and lung tumorigenesis. International journal of oncology. 2006;29:745–752. [PubMed] [Google Scholar]
- Balbo S, Johnson CS, Kovi RC, James-Yi SA, O’Sullivan MG, Wang M, Le CT, Khariwala SS, Upadhyaya P, Hecht SS. Carcinogenicity and DNA adduct formation of 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone and enantiomers of its metabolite 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol in F-344 rats. Carcinogenesis. 2014;35:2798–2806. doi: 10.1093/carcin/bgu204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett JC. Mechanisms of multistep carcinogenesis and carcinogen risk assessment. Environmental health perspectives. 1993;100:9–20. doi: 10.1289/ehp.931009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belinsky SA, Foley JF, White CM, Anderson MW, Maronpot RR. Dose-response relationship between O6-methylguanine formation in Clara cells and induction of pulmonary neoplasia in the rat by 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone. Cancer Res. 1990;50:3772–3780. [PubMed] [Google Scholar]
- Bonapasta SA, Gregori M, Lanza R, Sangiorgi E, Menghi A, Scarpini M, Modesti M. Metastasis to the Pancreas from Breast Cancer: Difficulties in Diagnosis and Controversies in Treatment. Breast care (Basel, Switzerland) 2010;5:170–173. doi: 10.1159/000314249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boorman GA, Eustis SL. Lung. In: Boorman Ga, ESLEMRMCA. Mackenzie WF., editors. Pathology of the Fisher Rat. Academic Press Inc; New York: 1990. pp. 339–367. [Google Scholar]
- Carmella SG, Chen M, Zarth A, Hecht SS. High throughput liquid chromatography-tandem mass spectrometry assay for mercapturic acids of acrolein and crotonaldehyde in cigarette smokers’ urine. Journal of chromatography B, Analytical technologies in the biomedical and life sciences. 2013;935:36–40. doi: 10.1016/j.jchromb.2013.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon D, Maronpot RR. Histomorphologic features of spontaneous and chemically-induced pulmonary neoplasms in B6C3F1 mice and Fischer 344 rats. Toxicol Pathol. 1991;19:540–556. doi: 10.1177/019262339101900419. [DOI] [PubMed] [Google Scholar]
- Elmore SA, Farman CA, Hailey JR, Kovi RC, Malarkey DE, Morrison JP, Neel J, Pesavento PA, Porter BF, Szabo KA, Teixeira LB, Quist EM. Proceedings of the 2015 National Toxicology Program Satellite Symposium. Toxicol Pathol. 2016;44:502–535. doi: 10.1177/0192623316631844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hecht SS. Biochemistry, biology, and carcinogenicity of tobacco-specific N-nitrosamines. Chemical research in toxicology. 1998;11:559–603. doi: 10.1021/tx980005y. [DOI] [PubMed] [Google Scholar]
- Hecht SS. Cigarette smoking and lung cancer: chemical mechanisms and approaches to prevention. The Lancet Oncology. 2002;3:461–469. doi: 10.1016/s1470-2045(02)00815-x. [DOI] [PubMed] [Google Scholar]
- Hecht SS, Carmella SG, Ye M, Le KA, Jensen JA, Zimmerman CL, Hatsukami DK. Quantitation of metabolites of 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone after cessation of smokeless tobacco use. Cancer research. 2002;62:129–134. [PubMed] [Google Scholar]
- Hecht SS, Spratt TE, Trushin N. Absolute configuration of 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol formed metabolically from 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone. Carcinogenesis. 1997;18:1851–1854. doi: 10.1093/carcin/18.9.1851. [DOI] [PubMed] [Google Scholar]
- Hecht SS. Biochemistry, biology, and carcinogenicity of tobacco-specific N-nitrosamines. Chem Res Toxicol. 1998;11:559–603. doi: 10.1021/tx980005y. [DOI] [PubMed] [Google Scholar]
- Hecht SS, Stepanov I, Carmella SG. Exposure and metabolic activation biomarkers of carcinogenic tobacco-specific nitrosamines. Accounts Chem Res. 2016;49:106–114. doi: 10.1021/acs.accounts.5b00472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hecht SS, Young R, Chen CB. Metabolism in the F344 rat of 4-(N-methyl-N-nitrosamino)-1-(3-pyridyl)-1-butanone, a tobacco-specific carcinogen. Cancer research. 1980;40:4144–4150. [PubMed] [Google Scholar]
- Howroyd P, Allison N, Foley JF, Hardisty J. Apparent alveolar bronchiolar tumors arising in the mediastinum of F344 rats. Toxicol Pathol. 2009;37:351–358. doi: 10.1177/0192623309332988. [DOI] [PubMed] [Google Scholar]
- IARC, I.W.G.o.t.E.o.C.R.t.H. Personal habits and indoor combustions. Volume 100 E. A review of human carcinogens. IARC monographs on the evaluation of carcinogenic risks to humans / World Health Organization, International Agency for Research on Cancer. 2012;100:1–538. [PMC free article] [PubMed] [Google Scholar]
- Kasper M, von Dorsche H, Stosiek P. Changes in the distribution of intermediate filament proteins and collagen IV in fetal and adult human pancreas. I. Localization of cytokeratin polypeptides. Histochemistry. 1991;96:271–277. doi: 10.1007/BF00271547. [DOI] [PubMed] [Google Scholar]
- Kokkinakis DM, Scarpelli DG. Carcinogenicity of N-nitroso(2-hydroxypropyl)(2-oxopropyl)amine, N-nitrosobis(2-hydroxypropyl)amine and cis-N-nitroso-2,6-dimethylmorpholine administered continuously in the Syrian hamster, and the effect of dietary protein on N-nitroso(2-hydroxypropyl)(2-oxopropyl)amine carcinogenesis. Carcinogenesis. 1989;10:699–704. doi: 10.1093/carcin/10.4.699. [DOI] [PubMed] [Google Scholar]
- Lijinsky W, Reuber MD, Reznik-Schuller HM. Contrasting carcinogenic effects of nitroso-2,6-dimethylmorpholine given by gavage to F344 rats and Syrian golden hamsters. Cancer letters. 1982;16:281–286. doi: 10.1016/0304-3835(82)90008-8. [DOI] [PubMed] [Google Scholar]
- Niess H, Conrad C, Kleespies A, Haas F, Bao Q, Jauch KW, Graeb C, Bruns CJ. Surgery for metastasis to the pancreas: is it safe and effective? J Surg Oncol. 2013;107:859–864. doi: 10.1002/jso.23333. [DOI] [PubMed] [Google Scholar]
- Real FX, Vila MR, Skoudy A, Ramaekers FC, Corominas JM. Intermediate filaments as differentiation markers of exocrine pancreas. II. Expression of cytokeratins of complex and stratified epithelia in normal pancreas and in pancreas cancer. International journal of cancer. Journal international du cancer. 1993;54:720–727. doi: 10.1002/ijc.2910540503. [DOI] [PubMed] [Google Scholar]
- Renne R, Brix A, Harkema J, Herbert R, Kittel B, Lewis D, March T, Nagano K, Pino M, Rittinghausen S, Rosenbruch M, Tellier P, Wohrmann T. Proliferative and nonproliferative lesions of the rat and mouse respiratory tract. Toxicologic pathology. 2009;37:5S–73S. doi: 10.1177/0192623309353423. [DOI] [PubMed] [Google Scholar]
- Rivenson A, Hecht SS, Hoffmann D. Carcinogenicity of tobacco-specific N-nitrosamines (TSNA): the role of the vascular network in the selection of target organs. Crit Rev Toxicol. 1991;21:255–264. doi: 10.3109/10408449109017913. [DOI] [PubMed] [Google Scholar]
- Rivenson A, Hoffmann D, Prokopczyk B, Amin S, Hecht SS. Induction of lung and exocrine pancreas tumors in F344 rats by tobacco-specific and Areca-derived N-nitrosamines. Cancer research. 1988;48:6912–6917. [PubMed] [Google Scholar]
- Roethig HJ, Munjal S, Feng S, Liang Q, Sarkar M, Walk RA, Mendes PE. Population estimates for biomarkers of exposure to cigarette smoke in adult U.S. cigarette smokers. Nicotine Tob Res. 2009;11:1216–1225. doi: 10.1093/ntr/ntp126. [DOI] [PubMed] [Google Scholar]
- Roland CF, van Heerden JA. Nonpancreatic primary tumors with metastasis to the pancreas. Surg Gynecol Obstet. 1989;168:345–347. [PubMed] [Google Scholar]
- Schlage WK, Bulles H, Friedrichs D, Kuhn M, Teredesai A. Cytokeratin expression patterns in the rat respiratory tract as markers of epithelial differentiation in inhalation toxicology. I. Determination of normal cytokeratin expression patterns in nose, larynx, trachea, and lung. Toxicol Pathol. 1998;26:324–343. doi: 10.1177/019262339802600307. [DOI] [PubMed] [Google Scholar]
- Schuller HM, Jorquera R, Lu X, Riechert A, Castonguay A. Transplacental carcinogenicity of low doses of 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone administered subcutaneously or intratracheally to hamsters. Journal of cancer research and clinical oncology. 1994;120:200–203. doi: 10.1007/BF01372556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Showalter SL, Hager E, Yeo CJ. Metastatic disease to the pancreas and spleen. Seminars in oncology. 2008;35:160–171. doi: 10.1053/j.seminoncol.2007.12.008. [DOI] [PubMed] [Google Scholar]
- Stankard CE, Karl RC. The treatment of isolated pancreatic metastases from renal cell carcinoma: a surgical review. Am J Gastroenterol. 1992;87:1658–1660. [PubMed] [Google Scholar]
- United-States-Cancer-Statistics-Working-Group. United States Cancer Statistics Working Group: 1999–2010 Incidence and Mortality Web-based Report. US Cancer Statistics Working Group 2013 [Google Scholar]
- Xia Y, Bernert JT, Jain RB, Ashley DL, Pirkle JL. Tobacco-specific nitrosamine 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol (NNAL) in smokers in the United States: NHANES 2007–2008. Biomarkers : biochemical indicators of exposure, response, and susceptibility to chemicals. 2011;16:112–119. doi: 10.3109/1354750X.2010.533288. [DOI] [PubMed] [Google Scholar]
- Z’Graggen K, Fernandez-del Castillo C, Rattner DW, Sigala H, Warshaw AL. Metastases to the pancreas and their surgical extirpation. Arch Surg. 1998;133:413–417. doi: 10.1001/archsurg.133.4.413. discussion 418–419. [DOI] [PubMed] [Google Scholar]


