Abstract
In response to the explosion of the Deepwater Horizon and the massive release of oil that followed, we conducted three annual research voyages to investigate how the oil spill would impact the marine offshore environment. Most investigations into the ecological and toxicological impacts of the Deepwater Horizon Oil crisis have mainly focused on the fate of the oil and dispersants, but few have considered the release of metals into the environent. From studies of previous oil spills, other marine oil industries, and analyses of oil compositions, it is evident that metals are frequently encountered. Several metals have been reported in the MC252 oil from the Deepwater Horizon oil spill, including the nonessential metals aluminum, arsenic, chromium, nickel, and lead; genotoxic metals, such as these are able to damage DNA and can bioaccumulate in organisms resulting in persistent exposure. In the Gulf of Mexico, whales are the apex species; hence we collected skin biopsies from sperm whales (Physeter macrocephalus), short-finned pilot whales (Globicephala macrorhynchus), and Bryde’s whales (Balaenoptera edeni). The results from our three-year study of monitoring metal levels in whale skin show (1) genotoxic metals at concentrations higher than global averages previously reported and (2) patterns for MC252-relevant metal concentrations decreasing with time from the oil spill.
Keywords: Chromium, Deepwater Horizon, Gulf of Mexico, Metals, Nickel, Oil spill, Whales
1. Introduction
After the Deepwater Horizon exploded, over 4.9 million barrels (> 779 million liters) of MC252 light sweet crude oil were spilled into the Gulf of Mexico and over 200 million liters of dispersants were applied on the surface and at the well head (McNutt et al., 2012). The size of such a spill in an offshore marine environment is unprecedented; as are the volume and methods of dispersant application. In response to this incident, a large proportion of the literature has focused on figuring out the final size of the spill, the environmental fate of the oil, or approximating the environmental impact – while few have assessed the health impacts on the environment.
Metals are known to be present in crude oil, elevated in the environments surrounding oil industries, and elevated after oil spills (Adedara et al., 2013; Benson and Etesin, 2008; Efe, 2010; Fowler et al., 1993; Gondal et al., 2006; Kuhuhawar et al., 2012; Osuji and Onojake, 2004). However, for this crisis, only a few reports assessing the environmental and health effects of the oil have considered metals; which reported detection of aluminum (Al), chromium (Cr), cobalt (Co), copper (Cu), iron (Fe), lead (Pb), magnesium (Mg), manganese (Mn), molybdenum (Mo), nickel (Ni), vanadium (V), and zinc (Zn) (Carmichael et al., 2012; Liu et al., 2012; Joung and Shiller, 2013; Steffy et al., 2013; Fitzgerald and Gohlke, 2014; Wise Jr. et al., 2014; Botello et al., 2015; Granneman et al., 2017). In particular, Mg, Al, Mn, Fe, Ni, and Pb were observed to increase in concentration as the sea mousse became more weathered (Liu et al., 2012). Here, we present data on metal levels in three whale species from the Gulf of Mexico: Bryde’s, pilot, and sperm whales.
Bryde’s whales (Balaenoptera edeni) are mysticetes that primarily feed on very small species of fish (e.g. sardines or herring) and generally live solitary lives except to mate and raise calves (Tershy, 1992; Tershy et al., 1993; Waring et al., 2013a). While Bryde’s whales live in all oceans across the globe, the specific population in the Gulf of Mexico is isolated and was recently identified as a distinct subspecies (Rosel and Wilcox, 2014). Furthermore, this population is the only residential population of mysticetes in the Gulf of Mexico (Wursig, 2017). With a population size of approximately 33 individuals, this subspecies is one of the rarest of the great whales (Rosel and Wilcox, 2014).
Sperm whales (Physeter macrocephalus) are odontocetes that primarily feed on squids and fishes found in the deep abyss; they also have a cosmopolitan distribution, but the population in the Gulf of Mexico is a residential population with few individuals (primarily males) leaving or entering the area each year (O’Hern and Biggs, 2009; Waring et al., 2013c; Wursig, 2017). Their population size is currently estimated at 763 individuals; the global population of sperm whales is currently classified as vulnerable and has an unknown status as to whether it is declining or improving (Waring et al., 2013c). A recent report showed the matriarchal sperm whale pods remained in waters about 200–3500 m deep, south and southwest of the Mississippi/Atchafalaya river mouths (Ortega-Ortiz et al., 2012). Sperm whales have a relatively large residential population in the northern Gulf of Mexico, are apex predators at the highest trophic level, and are known to use echolocation to communicate and hunt their prey (Waring et al., 2013c). This population of whales is critical to the ecological health and stability of the Gulf of Mexico, and thus they are an important species to study.
Short-finned pilot whales (Globicephala macrorhynchus) are also odontocetes, but prey on mesopelagic fishes and squids not nearly at the same depths as sperm whales (Waring et al., 2013b; Wursig, 2017). It is currently unknown if the population in the Gulf of Mexico is distinct from the Atlantic stock, but it is currently classified as distinct for management purposes (Waring et al., 2013b). This northern Gulf of Mexico stock is estimated to be 2415 individuals (Wursig, 2017). These are an important species to study, as they share a similar trophic level and habitat to sperm whales. Despite the vast size of these animals and the critical ecological role they play in the ocean ecosystem, (both at the surface and the ocean floor), our knowledge of their lives and health is very limited. It is clear that both Bryde’s and sperm whale populations reside in areas of the Gulf that were most heavily affected by the oil.
To better understand the health of these whales and the potential toxic effects of the oil spill, our group conducted three research voyages in the summers of 2010, 2011, and 2012 to collect biopsies of skin and blubber from these whales to analyze their contaminant loads. Our initial report observed high concentrations of nickel and chromium in these Gulf whales in the immediate aftermath of the spill (August–November) following capping of the riser; with a mean Ni concentration of 15.9 ± 3.5 ppm (range 1.7–4.6 ppm wet weight) and a mean Cr concentrations of 12.8 ± 2.6 ppm (range 2.0–73.6 ppm wet weight), which were significantly elevated when compared to global means (2.4 ± 0.4 ppm Ni, 9.3 ± 1.0 ppm Cr) (Wise Sr. et al., 2009; Wise Jr. et al., 2014). In this study, we report our findings regarding the concentrations of 26 metals in Gulf whale skin over a three-year period after the spill, with a focus on metals also found in the MC252 oil. The precise route and duration of exposure cannot be determined, because the oil was burned, metal exposure from the spill could have occurred through oral, dermal or inhalation routes and may have lasted for a short time to several months. Since metals accumulate, the exposure may last for years inside the animal.
2. Materials and methods
2.1. Sample collection
Skin biopsies were collected from free-ranging adult Bryde’s, pilot, and sperm whales in the northern Gulf of Mexico in the summers of 2010, 2011, and 2012 (Wise Jr. et al., 2014) (see also Table 2). Our platform was the research vessel Odyssey, a 93-ft motor-sailer ketch. The Odyssey was specially equipped to acoustically track echolocating whales, using an underwater hydrophone array and RainbowClick software. This equipment was used 24 h per day while we were in open sea in conjunction with visual efforts from various observation platforms above the deck from sunrise to sunset. These platforms are on top of the pilot house (approximately 10 ft above the deck), halfway up the main mast (approximately 30 ft above the deck), and the crow’s nest near the top of the main mast (approximately 50 ft off the deck); visual efforts were taken in 1–2 h shifts from one of the platforms, weather permitting. Upon encountering a whale, one whale biopsier would take a position on the Odyssey’s “whale boom”, a 30-ft pole on the starboard bow with a deer stand attached to the end; a second, backup biopsier would be positioned in the bowsprit. This “whale boom” allowed the primary biopsier to get closer to the whale while keeping the research vessel at a respectful distance. The backup biopsier was positioned to only release an arrow if the primary biopsier missed, did not make an attempt, or was incapable of making an attempt (e.g. if the whale moved too close). As much detail about the whale and the biopsy was recorded as possible, including suspected age (adult or subadult), location where the biopsy was collected, whale’s reaction (e.g. tail flick), any identifying markings (e.g. scars and flukes), GPS coordinates of the encounter, and number of individuals present.
Table 2.
Number of whale biopsies analyzed from each year (by Species).
| 2010
|
2011
|
2012
|
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Male | Female | Total | Male | Female | Total | Male | Female | Total | |
| Sperm whales | 18 | 22 | 40 | 25 | 28 | 53 | 21 | 26 | 47 |
| Bryde’s whales | 0 | 1 | 1 | 0 | 1 | 1 | 0 | 0 | 0 |
| Pilot whales | 0 | 0 | 0 | 2 | 1 | 3 | 9 | 2 | 11 |
2.2. Biopsies
Biopsies were consistently collected from the left flank of the whale’s back, approximately 1 m caudal to the dorsal, in order to avoid hitting any critical body parts (e.g. blowhole or eyes). The biopsy dart was a modified crossbow bolt, constructed of a hydrostatic buoy behind a stainless steel tip approximately 20 mm in length and 6 mm in diameter. The hydrostatic buoy doubled as a means to keep the arrow afloat, and to prevent the arrow from penetrating the whale beyond the 20 mm tip or getting stuck in the whale’s flank. After the biopsy arrow was retrieved, the sample was removed from the tip and processed in a sterile biosafety cabinet (generously donated by the Baker Company). Processing of the biopsy sample consisted of separating the skin and blubber using a ceramic knife and glass petri dish and finally isolating a section between the skin and blubber (where living, dividing skin cells reside) for tissue culture. Hence, for our purposes, skin refers to all physiological layers above the blubber. The skin samples were then further divided each into two pieces; one for metals analysis and one for genotyping analysis. Previously, we demonstrated that metals are not released from the biopsy darts into the samples (Wise Sr. et al., 2009). All animal procedures complied with approved institutional animal care protocols.
2.3. Genotyping
Gender was determined by genotyping based on published methods (Yang and Miyazaki, 2003). DNA was extracted from a piece of whale skin using standard methods (Carvalho et al., 2002; Wise Jr. et al., 2014). Gender was determined by PCR amplification reactions by amplifying the SRY (male determining factor) gene (Yang and Miyazaki, 2003). The keratin gene was used as an amplification control for all samples; hence, male samples showed both the keratin band (~311 bp) and SRY band (~152 bp), whereas females only showed the keratin band. Primer sequences were as follows:
SryPMF: 5′-CATTGTGTGTGGTCTCGTGATC-3′
SryPMR: 5′-AGTCTCTGTGCCTCCTCGAA-3′
KF: 5′-AGATCAGGGGTTCATGTTTCTTTGC-3′
KR: 5′-TTTACAGAGGTACCCAAGCCTAAG-3′
2.4. Inductively coupled plasma mass spectrometry
Samples were analyzed for total metal concentration using inductively coupled plasma mass spectrometry (ICPMS) according to our published methods using a Perkin-Elmer/Sciex ELAM ICPMS (Wise Sr. et al., 2009). Interference check solutions were analyzed with all sample runs to compensate for any matrix effects which might interfere with sample analysis. Standard quality assurance procedures were employed (Table 1). Instrument response was evaluated initially, after every 10 samples, as well as at the end of each analytical run using calibration verification standard and blank. Whale skin samples were analyzed for 25 metals and selenium and measured as μg metal per g tissue wet weight.
Table 1.
Quality assurance and quality control data for analysis of tissue samples.
| Element | LODa | Duplicate | LCSc | Spike | SRMd | |
|---|---|---|---|---|---|---|
|
|
|
|||||
| [μg/g(ppm)] | Blank | RPDb (%) | % Recovery | % Recovery | % Recovery | |
| Ag | 0.04 | BDLe | 8.5 | 103.7 | 102.9 | 99.7 |
| Al | 1.90 | BDL | 8.2 | 97.8 | 100.4 | N/A |
| As | 0.04 | BDL | 14.8 | 99.6 | 97.8 | 94.5 |
| Au | 0.19 | BDL | * | 95.8 | 108.0 | N/A |
| Ba | 0.04 | BDL | * | 93.8 | 94.0 | N/A |
| Be | 0.04 | BDL | * | 99.0 | 103.6 | N/A |
| Cd | 0.04 | BDL | 6.1 | 98.3 | 99.3 | 104.1 |
| Co | 0.04 | BDL | 8.0 | 107.1 | 106.7 | N/A |
| Cr | 0.10 | BDL | 8.6 | 103.4 | 95.9 | 111.4 |
| Cu | 0.19 | BDL | 15.4 | 104.1 | 105.5 | 97.7 |
| Fe | 3.74 | BDL | 6.1 | 111.3 | 110.2 | 102.7 |
| Li | 0.09 | BDL | * | 100.1 | 101.2 | N/A |
| Mg | 1.90 | BDL | 4.0 | 100.9 | 106.6 | N/A |
| Mn | 0.04 | BDL | 10.0 | 101.6 | 99.1 | N/A |
| Mo | 0.09 | BDL | 14.7 | 104.3 | 102.1 | N/A |
| Ni | 0.09 | BDL | 8.5 | 102.0 | 95.5 | 70.3 |
| Pb | 0.09 | BDL | * | 98.7 | 104.5 | 95.7 |
| Sb | 0.19 | BDL | * | 102.6 | 107.5 | N/A |
| Se | 0.09 | BDL | 7.1 | 97.4 | 96.1 | 111.2 |
| Sr | 0.04 | BDL | 2.8 | 93.6 | 89.5 | N/A |
| Ti | 0.09 | BDL | 5.9 | 107.9 | 100.6 | N/A |
| U | 0.04 | BDL | * | 101.2 | 109.2 | N/A |
| V | 0.04 | BDL | * | 101.3 | 105.9 | N/A |
| Zn | 0.19 | BDL | 13.1 | 106.6 | 117.0 | 108.3 |
| Hg | 0.05 | BDL | 4.8 | 100.8 | 104.3 | 97.8 |
LOD = Limit of detection.
RPD = Relative percent difference.
LCS = Laboratory control sample.
SRM = Standard reference material (DOLT-4; DORM-3).
BDL = Below detection limit.
All duplicate measurements were below the project quantitation limit.
2.5. Statistics
Means and standard error were calculated for all groups and subgroups. Mean values were compared using t-tests. Because the distributions of values were skewed, a normalizing logarithmic transformation was used for statistical testing. P-values less than 0.05 were regarded as statistically significant, and no adjustment was made for multiple comparisons. The statistical analyses were all conducted in SAS 9.2 (SAS Institute, Cary, NC).
3. Results
We measured the concentrations of 26 metals in skin biopsies from three species of free-ranging whales in the Gulf of Mexico in 2010, 2011, and 2012 (descriptive statistics in Supplementary Table 1). Here we report metal levels for all whales biopsied in 2010, all pilot and Bryde’s whales biopsied in 2011 and 2012, and cohorts of adult sperm whales biopsied in 2011 and 2012. Sperm whale cohorts were selected for adult individuals from each gender. Fig. 1 shows the average metal concentration for all whales pooled by year. Interestingly, these data show some MC252-associated metals decreased with time; e.g. Co, Al, Mn, Cu, Ni, and Cr all show decreased average levels in the skin in 2012 when compared to 2010 or 2011. To better examine metal concentrations present in these whales, we next compare each species individually and by gender.
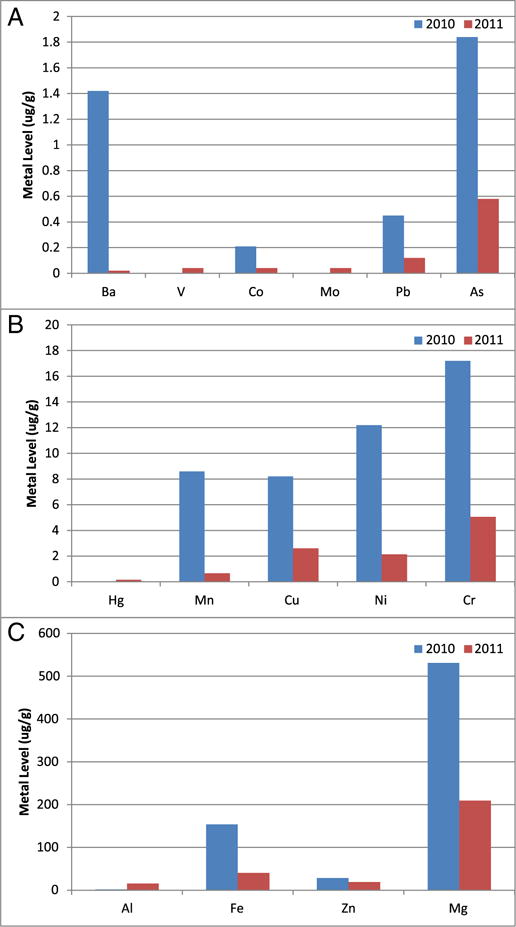
Fig. 1.
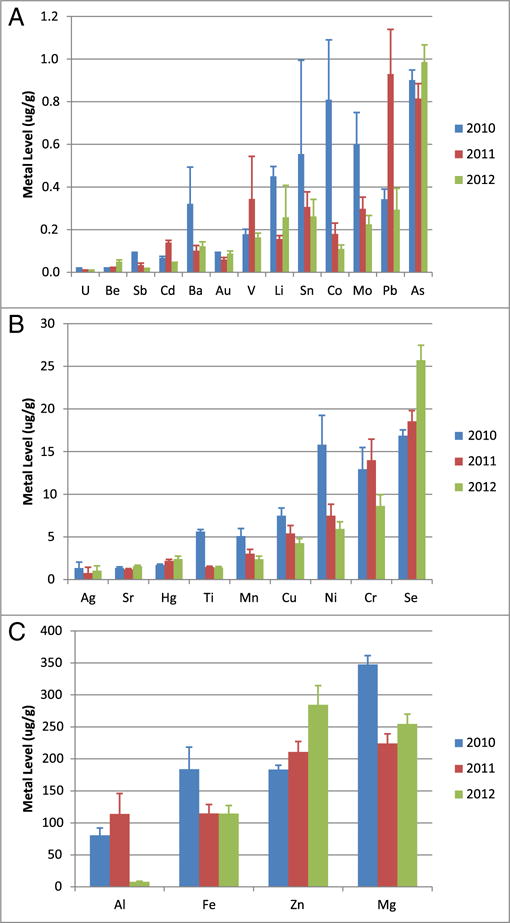
Average metal concentrations in all whales biopsied in the Northern Gulf of Mexico in 2010, 2011, and 2012. We measured 26 metals in the whale tissues. The average concentrations of Ba, Sn, Co, Mo, Ti, Mn, Cu, Ni, Cr, Sb, Al, Fe and Mg decreased from 2010 to 2012. Meanwhile, Pb and Zn increased. Sample sizes were 41, 57, and 58 for 2010, 2011, and 2012 respectively. Data represented as mean ± standard deviation in units of μg/g tissue. (A) Metals with mean skin concentrations less than 1 μg/g; (B) Metals with mean skin concentrations between 1 and 25 μg/g and selenium; (C) Metals with mean skin concentrations greater than 50 μg/g.
3.1. Sperm whales
Here, we analyze metal concentrations in skin samples from each year (Fig. 2), then by gender separately (Fig. 3). We conducted glm analysis for MC252 relevant metals (Al, arsenic (As), barium (Ba), Co, Cr, Cu, Fe, Mg, Mn, Ni, Pb, V, Zn) without interaction between gender and year (Suppl. Table 2). We found statistically significant decreasing levels across years (p < 0.05) for Al, Co, Cu, Mn, and Ni, but statistically significant increasing levels for Pb (Fig. 2). We found statistical significance between genders (p < 0.05) for Al. We found interactions between gender and year for As, Ba and Mg, with statistically significant different levels for As in 2010; As, Ba, and Mg in 2011 (Suppl. Table 3). Specifically, we found statistically significant differences in As for females between 2010 and 2011 (0.95 ± 0.04 and 0.74 ± 0.12 μg/g, respectively); Ba for females across all years (0.45 ± 0.33 μg/g in 2010, 0.06 ± 0.02 in 20,122, 0.09 ± 0.01 μg/g); Mg for males across all years (320.88 ± 12.78 μg/g in 2010, 155.05 ± 15.64 μg/g in 2011, 265.13 ± 27.89 μg/g) and for females between 2010 and 2011 and 2010–2012 (361.67 ± 19.36 μg/g in 2010, 278.59 ± 21.30 μg/g in 2011, 260.86 ± 23.16 in 2012) (Fig. 3).
Fig. 2.
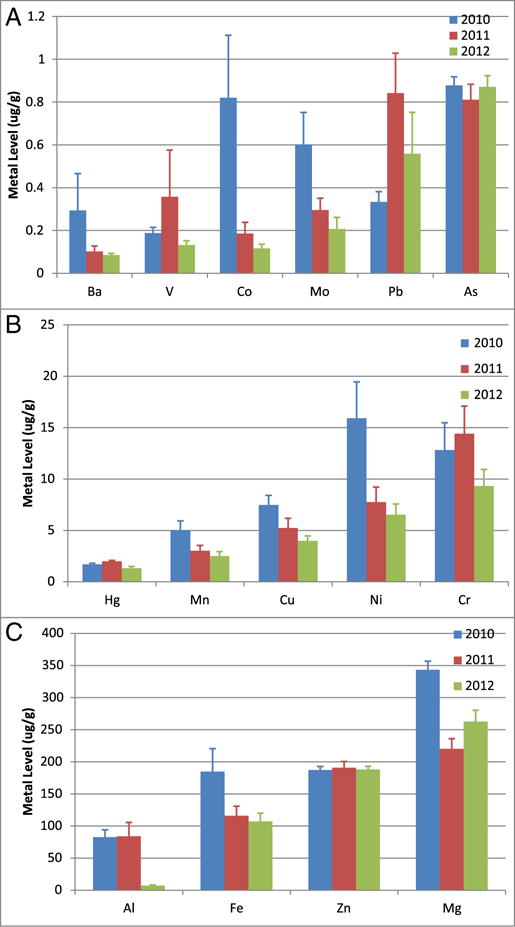
Metal concentrations in all sperm whales biopsied in the Gulf of Mexico. Average concentrations of Ba, Co, Mo, Mn, Cu, Ni, Cr, Al, Fe, and Mg decreased over time. Meanwhile, V and Pb showed increased concentrations across years. Sample sizes were 40, 53, and 47 for 2010, 2011, and 2012 respectively. Data represented as mean ± standard deviation in units of μg/g tissue. Data are divided into panels solely for optimal visualization. (A) Metals with mean skin concentrations less than 1 μg/g; (B) Metals with mean skin concentrations between 1 and 30 μg/g; (C) Metals with mean skin concentrations greater than 50 μg/g.
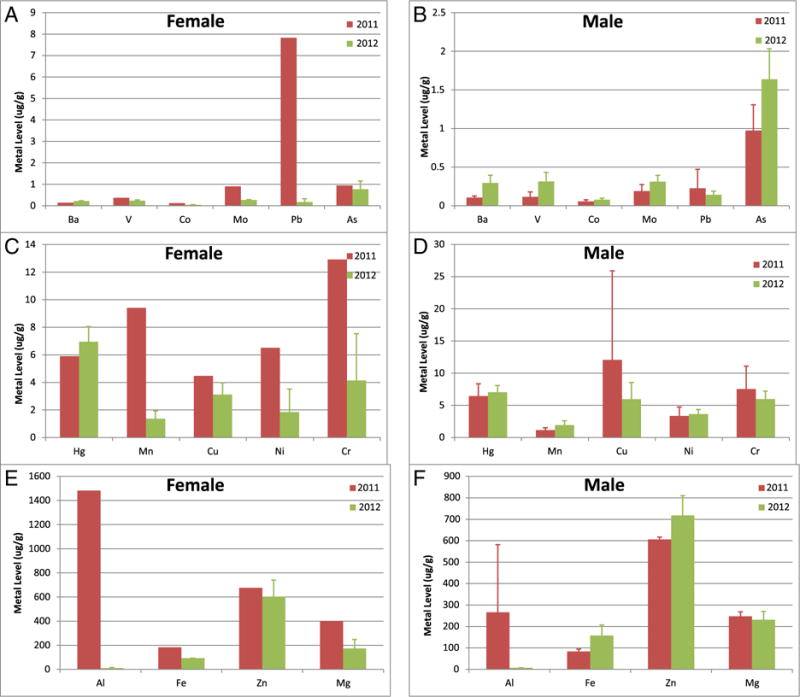
Fig. 3.
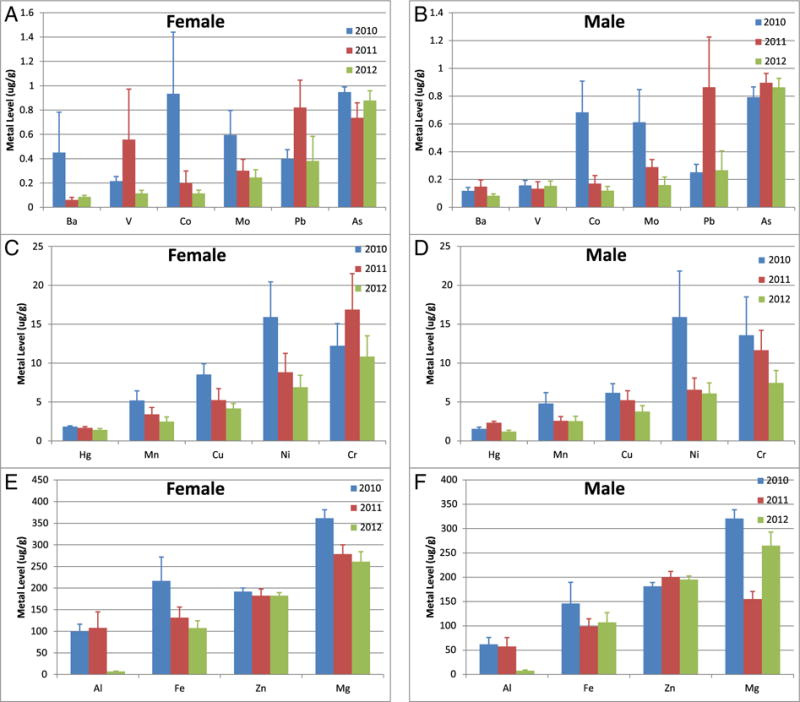
Metal concentrations in female and male sperm whales biopsied in the Gulf of Mexico. Sample sizes were as follows, for 2010: 18 males, 22 females; for 2011: 25 males, and 28 females; for 2012: 21 males, and 26 females. Data represented as mean ± standard deviation in units of μg/g tissue. Data are divided into panels solely for optimal visualization. (A,B) Metals with mean skin concentrations less than 1 μg/g for females (A) and males (B), respectively; (C,D) Metals with mean skin concentrations between 1 and 20 μg/g for females (C) and males (D), respectively; (E,F) Metals with mean skin concentrations greater than 50 μg/g for females (E) and males (F), respectively.
3.2. Bryde’s and pilot whales
Bryde’s whales have a much smaller residential population than sperm whales, and do not echolocate while traveling or hunting, making them very difficult to find and limiting search mechanisms to visual spotting (Waring et al., 2013a). We had four distinct encounters with individual Bryde’s whales across all three years, and were only able to collect two biopsies from adult females. While this is a very small sample size, and is difficult to draw conclusions from – when one considers the best estimate of the residential population size is 33, these two samples potentially represent 6% of the entire population (Waring et al., 2013a). As with the sperm whales, we observed a decrease in some MC252 metals with time: As, Ba, Co, Cr, Cu, Fe, Mn, Ni, Pb and Zn. Although we only have an n of 1 per year, we can hope that these data represent this small population and conclude that the overall load of metals is decreasing with time (Fig. 4).
Fig. 4.
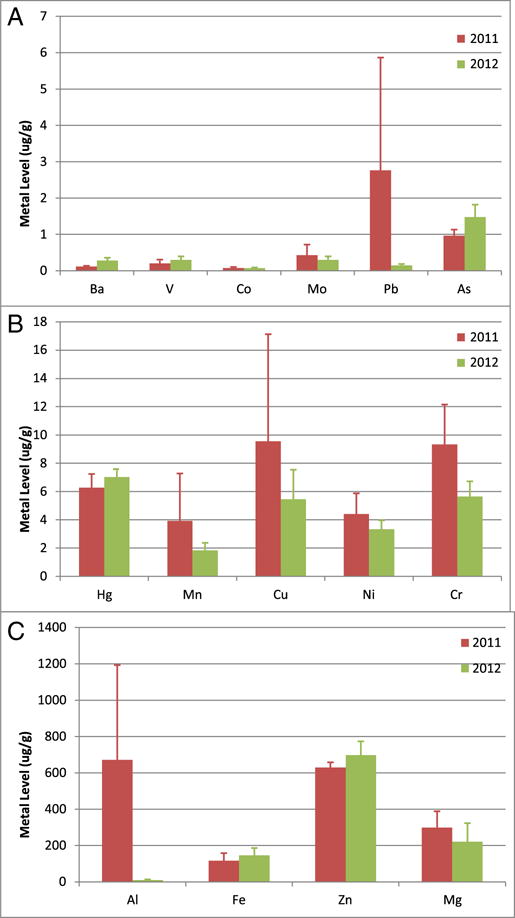
Metal Concentrations in All Bryde’s Whales Biopsied in the Gulf of Mexico. Sample size for 2010 and 2011 were one whale each year. Data represented as mean in units of μg/g tissue. Data are divided into panels solely for optimal visualization. (A) Metals with mean skin concentrations less than 2 μg/g; (B) Metals with mean skin concentrations between 2 and 20 μg/g; (C) Metals with mean skin concentrations greater than 20 μg/g.
Pilot whales, like sperm whales, are odontocetes and primarily prey on squid and fish and also required visual spotting (Waring et al., 2013b). They are much smaller in size than sperm whales, and tend to travel in larger familial groups; further, they do not dive as deep as sperm whales to hunt their prey. We were not able to collect any pilot whale tissue samples in 2010 because we did not encounter any that year. Again, due to our small sample size it is difficult to draw conclusions (two adult males and one adult female in 2011; 9 adult males and 2 adult females in 2012). The best current estimate for the residential pilot whale population in the Gulf of Mexico is 2415 individuals (Waring et al., 2013b). Fig. 5 compares the average metal concentrations in all pilot whales sampled in 2011 versus 2012; here, we again see decreasing levels of Al, Cu, Cr, Mg, Mn, Ni, and Pb. The large variability in our 2011 data set is especially due to the single female we biopsied, as she had a much higher metal load than the other two whales (Fig. 6). Fig. 6 compares pilot whale data by gender and by year; unlike our sperm whale data, many of the MC252-relevant metals appear to increase between 2011 and 2012 (e.g. As, Ba, Mo, V). We found statistically significant decreases for Al and Pb across years when adjusted for gender (p < 0.0001 and 0.010, respectively). Mn and Pb were the only MC252-relevant metals with statistically significant interactions between gender and year (p = 0.033 and 0.017, respectively); however, further statistical analyses for stratification by year or gender did not reveal significance (see Suppl. Table 6).
Fig. 5.
Metal concentrations in all pilot whales biopsied in the Gulf of Mexico. Sample sizes were 3 and 11 for 2011 and 2012 respectively. Data represented as mean ± standard deviation in units of μg/g tissue. Data are divided into panels solely for optimal visualization. (A) Metals with mean skin concentrations less than 2 μg/g; (B) metals with mean skin concentrations between 2 and 20 μg/g; (C) metals with mean skin concentrations greater than 20 μg/g.
Fig. 6.
Metal concentrations in female and male pilot whales biopsied in the Gulf of Mexico. Sample sizes were as follows, for 2011: 2 males and 1 female; for 2012: 9 males and 2 females. Data represented as mean ± standard deviation in units of μg/g tissue. (A,B) Metals with mean skin concentrations less than 2 μg/g for females (A) and males (B), respectively; (C,D) metals with mean skin concentrations between 2 and 20 μg/g for females (C) and males (D), respectively; (E,F) metals with mean skin concentrations greater than 50 μg/g for females (E) and males (F), respectively.
3.3. MC252 metals in whale skin
Here, we consider the concentrations of MC252-relevant metals across species by year. Given that Cu, Fe, Mg, Mn, and Zn are essential elements for mammals, it is difficult to determine if the levels we observed in the whales are abnormal (though excess of any metal can have multiple pathogenic effects). This section will analyze the essential and nonessential metals separately, as these metals were detected in all whales analyzed.
We measured the concentrations of the essential metals Cu, Fe, Mg, Mn, and Zn in whale skin samples (see Fig. 7). For Cu, Fe, Mg, and Mn we observed a gradual decrease in dermal metal level with time; this pattern was observed for all three species and both genders. The dermal concentrations of Zn did not appear to change within any species over time. Interestingly, we observed a dramatic difference in dermal Zn between species (see Fig. 7E). Bryde’s whales had the lowest concentrations of dermal Zn (the two females had 28.72 and 19.4 μg Zn/g tissue), while pilot whales had the highest (females had 675.9 μg Zn/g tissue in 2011, 506.5 and 699.4 μg Zn/g tissue in 2012).
Fig. 7.

Comparison of MC252-relevant essential metal concentrations in all whales biopsied in the Gulf of Mexico. Data represented as mean ± standard deviation in units of μg/g tissue. Several essential metals found in MC252 oil were observed at elevated concentrations in these whales, including: Cu (panel A), Fe (panel B), Mg (panel C), Mn (panel D), and Zn (panel E).
We considered the concentrations of nonessential metals Al, As, Ba, Cr, Ni, Pb, and V in our whale skin samples (see Fig. 8). Here, we observed decreasing levels of Al, Cr, and Ni with time for all species; whereas As, Ba, Pb, and V skin concentrations did not follow a pattern across species. Pilot whales had dramatically higher levels of Al than either Bryde’s (2011 female mean = 94.7 ×) or sperm whales (2011 female mean = 13.7 ×; 2011 male mean = 4.6 ×); and showed the most dramatic decrease in skin Al load with time (133 × and 37 × higher means in 2011 than 2012 for females and males, respectively). Sperm whales showed a prominent drop in Al levels after 2011 (107.89 to 6.96 μg/g tissue and 57.72 to 7.78 μg/g tissue in females and males, respectively). Average Cr concentrations generally decreased with time for all species and genders; however, female sperm whales showed the smallest decreases in average Cr concentration. Average Ni concentrations showed the clearest decrease with time of the nonessential metals detected in oil and whale skin samples, but appeared to level off in males near 6 and 3 μg/g tissue for sperm whale and pilot whales, respectively, between 2011 and 2012, whereas average female Ni concentrations did not appear to level off during these years. Average Pb concentrations in male and female sperm whales showed a similar pattern; Pb concentrations increased from 2010 to 2011, then decreased from 2011 to 2012. Average As concentrations showed the least changes across years and were the most consistent across species, with most skin levels observed near 1 μg/g. Average Ba skin concentrations were highest in female Bryde’s and sperm whales biopsied in 2010, and not much change between 2011 and 2012. Average V skin concentrations were highest in female whales biopsied in 2011 pilot and sperm whales, with average levels not changing much in male whales across years. Considering these data altogether and given that (1) both metals are found at relatively low levels in sea water, (2) have poor dermal uptake, (3) whale skin is continuously sloughed, and (4) both metals were detected in MC252 oil, it is rational to deduce these metal levels likely came from heavy exposure to the oil or the airborne particulates from oil burning activities.
Fig. 8.
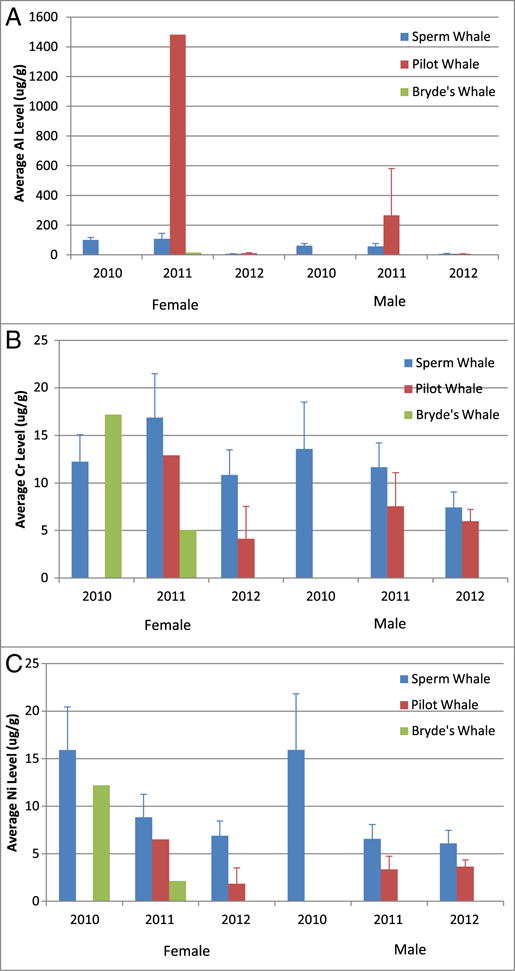
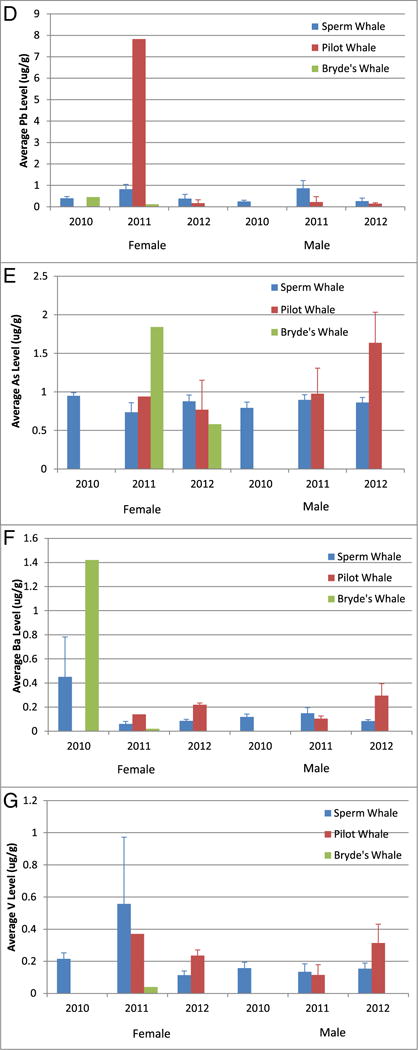
Comparison of MC252-relevant nonessential metal concentrations in whales from the Gulf of Mexico. Data represented as mean ± standard deviation in units of μg/g tissue. Four nonessential metals found in MC252 oil were observed at elevated concentrations in these whales: Al (panel A), Cr (panel B), Ni (panel C), Pb (panel D), As (panel E), Ba (panel F), and V (panel G).
4. Discussion
As we have seen from the Exxon Valdez accident and others before, the harmful effects of an accidental release of crude oils are widespread and continue for decades (Freeman et al., 1985; Cohen, 1995; Peterson et al., 2003). The Deepwater Horizon oil spill was, to date, the largest release of crude oil into a marine environment in U.S. history. While the precise amount of oil released may never be determined, the amount is currently estimated at 779 million liters ± 10% of crude oil (or 627,000 t) with a similar amount of released methane gas (Joung and Shiller, 2013). Most of the oil and methane gas are believed to have been removed by natural processes, such as evaporation to the atmosphere, dissolution and dispersion in the seawater, or degradation by methanogens and oil-metabolizing bacteria. These processes combined with the relief efforts (skimming, burning, recovery at the wellhead, or chemical dispersion) are estimated to account for approximately 75% of the entire spill, leaving 25% unaccounted for and likely still in the environment (Ramseur, 2010).
In the wake of this crisis, most toxicological studies have focused on the effects of PAHs in the oil organics and dispersants. While these aspects are indeed important to understand, few health effects have been linked to PAH levels from this crisis (Carmichael et al., 2012; Fitzgerald and Gohlke, 2014; Schwacke et al., 2014; Lane et al., 2015; Millemann et al., 2015; Sammarco et al., 2015). This outcome may reflect the unique ecosystem of the Gulf of Mexico as one with some resilience to oil, due to the large amounts of oil that naturally seeps from the sea floor. There are over 350 constant seeps in the Gulf of Mexico that produce perennial oil slicks; annual seepage estimates from satellite or astronaut imaging of the Gulf provide a range from 4000 to 17,000 t per year (MacDonald et al., 1994; MacDonald et al., 1996; MacDonald et al., 2002). Another study estimated an additional 3571 t per year from the oil and gas industry (Council, 2003). Taken together, these annual inputs of oil into the Gulf of Mexico marine environment make up only 3% (20,571 t annually estimated out of 627,000 t during Deepwater Horizon) of the total amount spilled during the Deepwater Horizon oil spill. These aspects may have enhanced PAHs degradation and reduce their environmental health impact, or improved assessments of environmental impact are still needed.
By contrast, metals from the oil cannot be degraded by enzymes or environmental conditions, and generally are not bioactivated or metabolized for toxic effects. They can easily enter the food web by inhalation, ingestion or dermal exposure to animals at any trophic level and many metals will bioaccumulate. Many metals are also sequestered into tissue and can remain there for months to years with the potential for re-exposure during physiological stress. Metal exposure has been known to be an important component of oil spills for many years (Fowler et al., 1993; Osuji and Onojake, 2004; Gondal et al., 2006; Edema, 2012; Kuhuhawar et al., 2012). As the residual oil is degraded or weathered, the relative concentrations of the metals in the oil are likely to increase or be released into the environment and incorporated into some other component (living or non-living) of the ecosystem. Therefore, it seems likely that metal exposures from this crisis are more likely to have chronic effects on animals or the ecosystem from continuing exposure as it is released from residual oil or from environmental or biological sinks that have accumulated metals.
Indeed, studies of this spill show elevated metals in the oil and sediment, and in some animals immediately following the oil spill (Carmichael et al., 2012; Joung and Shiller, 2013; Steffy et al., 2013; Fitzgerald and Gohlke, 2014; Wise Jr. et al., 2014). These previous studies were limited in scope to a few metals or limited time periods. Liu et al. (2012) reported increasing metal concentrations as oil mousse aged and weathered, with Mg, Al, and Fe at the highest levels. Carmichael et al. (2012) measured metal levels in oyster shells that were transplanted into affected areas for the months immediately following the Deepwater Horizon explosion. Here, they report notable differences for Pb and V only, but at very low concentrations (0.01–0.02 and 0.03–0.08 mg/L, respectively). Joung and Shiller (2013) reported concentrations of metals in the water column near the epicenter from May and October 2010 and October 2011; water metal concentrations were highest for Mo, Ba, and V (112, 61, 31 nmol/kg). Finally, Steffy et al. (2013) reported changes in concentrations of Cr, Ni and Pb in the sediments east of the Deepwater Horizon, in the waters most heavily affected; their results included pre-spill data from 2009 and showed elevated concentrations in 2010 and 2011. For example, Ni concentrations increased from 1.94 mg/kg in 2009 to 6.09 mg/kg in 2010 and 2.36 mg/kg in 2011. Our report considers three years, is the first to broadly consider metal concentrations in affected mammals from the Gulf of Mexico and to consider an offshore species. The data in this study confirm these previous reports and extends the findings over three years.
Our results suggest whales were exposed to metals during the Deepwater Horizon oil spill. In support of this, nonessential metals also found in the MC252 oil (Al, Cr, Ni, Pb), decreased over the three years following the Deepwater Horizon oil spill. This pattern was observed when we considered all species together (Fig. 1), each species individually (Figs. 2, 4, 5), and by gender (Figs. 3, 6). While metals alone cannot be traced to their original source and we unfortunately have no pre-spill data from these whale populations, the results are consistent with a significant exposure to metals in oil from the Deepwater Horizon accident. Metal concentrations in skin are typically lower than many other critical organs, including lungs, liver, brain, and reproductive organs (Aubail et al., 2013; Lemos et al., 2013), suggesting a significant concern for whale health at these levels.
4.1. Considerations for chronic metal exposure from gas flares
Although the oil from the accident, MC252, contains a number of metals (Al, As, Ba, Co, Cr, Cu, Fe, Mg, Mn, Ni, Pb, V, Zn), many of these metals are also known to be elevated in environments and animals surrounding regions of heavy oil industries (Osuji and Onojake, 2004; Benson and Etesin, 2008; Efe, 2010; Adedara et al., 2013). A normal process of oil drilling is the regular burning of natural gases extracted with the oil which can release metals into the air; furthermore, metals can be released into the environment from drilling muds associated with oil extraction (Pozebon et al., 2005). Given that offshore oil is an established industry in the Gulf of Mexico, it is probable that some of the metals we observed in the whales came from these various sources. However, our data show a general pattern of MC252-associated metals decreasing over the course of our three-year study; during this time, the number of offshore oil rigs steadily increased (Stowers, 2013). If these unusually high metal concentrations in the whales’ skin (when compared to means observed in sperm whales across the globe) were from these background sources of oil industry, we would expect the concentrations to remain constant or possibly increase during these years and we would not expect to see metal concentrations decreasing (Wise Sr. et al., 2009).
4.2. Oil burned during Deepwater Horizon crisis
Given that a significant amount of oil was cleared by in situ burns (approximately 5%, or over 41 million liters) in a short period of time (April 28-July 19, 2010) and all the burns were conducted in a relatively small area (4–24 km from the epicenter), it is likely that a significant amount of inhalation exposure was possible for air breathing animals nearby (Aurell and Gullett, 2010; Schaum et al., 2010; Allen et al., 2011; Ryerson et al., 2011). A previous study considered the dispersion of dioxin released from these burns and found dioxin could spread as far as 125 km away from the burn site (Schaum et al., 2010). Efforts were taken to prevent incidental exposure of animals to the flames or the plumes of smoke; however, based on the report by Schaum et al. (2010), these efforts may have been ineffective as high concentrations of sperm whales were observed within 50 km of the epicenter and smoke plumes were visible from space for very long distances (Ackleh et al., 2012). Thus far, only one paper has considered metal concentrations in the particulate emissions from these burns; here, the authors reported finding the metals: Mg, Al, Fe, Ba, Sr, Ti, Pb, Mn, Zn, Ni and Cu, in order of descending concentrations (Gullett et al., 2016). A follow up study was published by the same group characterizing emissions and residues from simulations of the Deepwater Horizon oil burns but the metals were detected at much lower concentrations, perhaps due to changing their detection method from ICPMS to energy dispersive X-ray fluorescence spectrometry (ED-XRF) (Gullett et al., 2017).
4.3. Respiratory effects of exposure to Deepwater Horizon oil spill
Consistent with inhalation as a route of exposure and with the respiratory toxicity of metals in this crisis, several reports documented respiratory effects from the crisis in humans working in relief areas around the oil and in bottlenose dolphins from heavily polluted bays (McCoy and Salerno, 2010; D’Andrea and Reddy, 2013; Schwacke et al., 2014). A follow-up study on this bottlenose dolphin population inicated several deaths of individuals and poor reproductive success, also consistent with metal exposure (Lane et al., 2015). While these observations were from a single population from an area heavily polluted by oil (Barataria Bay, LA), it still raises concerns for other cetacean populations residing within the affected waters, especially those typically residing far offshore where such deaths and health assessments are less likely to be recorded. Also, consistent with these outcomes were observations that spill-workers frequently had lower respiratory tract issues that lasted three or more days and these outcomes were more frequently observed in workers associated with the in situ burns (the soot of which was noted to have detectable levels of transition metals) (Jaligama et al., 2015).
4.4. Other considerations for respiratory exposure risk
The route of exposure for the whales to the metals is uncertain. Several of the metals associated with the oil and found in the whales are poorly absorbed via ingestion (Al, Ba, Cr, Ni, and V) (ATSDR, 2005, ATSDR, 2007, ATSDR, 2008, ATSDR, 2012a , 2012b). Dermal uptake is unlikely for whales, as their skin is extremely thick, they continuously slough their skin, and the metals would have to be in constant contact with the animals’ skin for long periods of time for uptake to occur. Both Cr and Ni are known lung toxicants and are readily absorbed through alveoli into the bloodstream; Al is not well absorbed through the lungs, but Al particulates can get lodged in the alveoli and release Al over time which contributes to body burden (ATSDR, 2005, ATSDR, 2008, ATSDR, 2012a , 2012b).
Further support of inhalation exposure concern associated with this crisis comes from a study analyzing the air quality over the years following the Deepwater Horizon explosion. That report found the particulates in the air maintain significantly higher genotoxic responses than control areas (Singleton et al., 2016). We have previously shown both soluble and particulate metals are genotoxic to sperm whale skin cells consistent with this exposure route (Wise Sr. et al., 2011; Li Chen et al., 2012; Pabuwal et al., 2013). Fitzgerald and Gohlke (2014) observed a black substance in the body cavity and inner organs of a significant proportion of Gulf menhaden (a pelagic filter-feeding fish) following the burns, and found the substance had high levels of PAHs from a “pyrogenic source”, further indicating the reach of the smoke. Another important factor to consider regarding metal release from burning oil, is that metal levels in the oil tend to increase as oil is weathered or degraded, which would likely increase the levels of metals in particulate matter released from burning the oil (Liu et al., 2012).
Given that whales are exposed to fewer sources of metal particulates than most humans (e.g. from urban air) and reside in the waters most heavily affected, they provide a valuable species to understand the health effects that could be observed in both humans and the environment. To further evaluate the toxic legacy of the Deepwater Horizon oil spill, our future directions will include analyzing concentrations of petroleum products and chemical dispersants in the blubber of these whales, assessing their genetic health, and determining the effects of observed contaminants on their genetic health.
Supplementary Material
Acknowledgments
The content is solely the responsibility of the presenters and does not necessarily represent the official views of the National Institute of Environmental Health Sciences, the National Institutes of Health, the Army Research Office or the Department of Defense. Work was conducted under National Marine Fisheries Service permit #1008-1637-03 (J. Wise Sr., PI) and permit #751-1614 (Iain Kerr, PI). We would like to thank Amie Holmes, Shouping Huang, Selma Botman, Timothy Stevens, W. Douglas Thompson, and all the Wise Laboratory/Odyssey science team volunteers for their help with technical support, whale spotting and sample collection. We thank Iain Kerr, Bob Wallace, Ian Glass, Jeffrey Kunz, John Atkinson, Roger Payne, Nathan Farr and all of the Odyssey boat crew for their help with sample collection and logistics. We thank Alana Alexander and Scott Baker for technical assistance with the early genotyping results. Finally, we thank all the volunteers who support us in this project.
Funding
This work was supported by the National Institute of Environmental Health Sciences [ES016893 (J. Wise Sr., PI)]; Army Research Office [W911NF-09-1-0296 (J. Wise Sr., PI)]; University of Southern Maine, the Maine Center for Toxicology and Environmental Health; The Albemarle Corporation; the Campbell Foundation; the Ocean Foundation; Quiznos; Ocean Alliance; and the many individual and anonymous Wise Laboratory donors.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.cbpc.2017.12.003.
Footnotes
The authors declare no competing financial interests.
References
- Ackleh AS, Ioup GE, Ioup JW, Ma B, Newcomb JJ, Pal N, Sidorovskaia NA, Tiemann C. Assessing the Deepwater Horizon oil spill impact on marine mammal population through acoustics: endangered sperm whales. J Acoust Soc Am. 2012;131(3):2306–2314. doi: 10.1121/1.3682042. [DOI] [PubMed] [Google Scholar]
- Adedara IA, Ebokaiwe AP, Farombi EO. Tissues distribution of heavy metals and erythrocytes antioxidant status in rats exposed to Nigerian bonny light crude oil. Toxicol Ind Health. 2013;29(2):162–168. doi: 10.1177/0748233711427049. [DOI] [PubMed] [Google Scholar]
- Allen AA, Mabile NJ, Jaeger D, Costanzo D. The use of controlled burning during the Gulf of Mexico Deepwater Horizon MC-252 oil spill response. 2011 International Oil Spill Conference. 2011:2011–2194. [Google Scholar]
- ATSDR (Agency for Toxic Substances and Disease Registry) Toxicological Profile for Nickel. U.S. Department of Health and Human Services, Public Health Service; Atlanta, GA: p. 2005. [Google Scholar]
- ATSDR (Agency for Toxic Substances and Disease Registry) Toxicological Profile for Barium. U.S. Department of Health and Human Services, Public Health Service; Atlanta, GA: p. 2007. [Google Scholar]
- ATSDR (Agency for Toxic Substances and Disease Registry) Toxicological Profile for Aluminum. U.S. Department of Health and Human Services, Public Health Service; Atlanta, GA: p. 2008. [Google Scholar]
- ATSDR (Agency for Toxic Substances and Disease Registry) Toxicological Profile for Chromium. U.S. Department of Health and Human Services, Public Health Service; Atlanta, GA: 2012a. [Google Scholar]
- ATSDR (Agency for Toxic Substances and Disease Registry) Toxicological Profile for Vanadium. U.S. Department of Health and Human Services, Public Health Service; Atlanta, GA: 2012b. [Google Scholar]
- Aubail A, Mendez-Fernandez P, Bustamante P, Churlaud C, Ferreira M, Vingada JV, Caurant F. Use of skin and blubber tissues of small cetaceans to assess the trace element content of internal organs. Mar Pollut Bull. 2013;76(1–2):158–169. doi: 10.1016/j.marpolbul.2013.09.008. [DOI] [PubMed] [Google Scholar]
- Aurell J, Gullett BK. Aerostat sampling of PCDD/PCDF emissions from the Gulf oil spill in situ burns. Environ Sci Technol. 2010;44(24):9431–9437. doi: 10.1021/es103554y. [DOI] [PubMed] [Google Scholar]
- Benson NU, Etesin UM. Metal contamination of surface water, sediment, and Tympanotonus fuscatus varradula of Iko River and environmental impact due to Utapete gas flare station, Nigeria. Environmentalist. 2008;28:195–202. [Google Scholar]
- Botello AV, Soto LA, Ponce-Velez G, Villanueva FS. Baseline for PAHs and metals in NW Gulf of Mexico related to the Deepwater Horizon oil spill. Estuar Coast Shelf Sci. 2015;156:124–133. [Google Scholar]
- Carmichael RH, Jones AL, Patterson HK, Walton WC, Perez-Huerta A, Overton EB, Dailey M, Willett KL. Assimilation of oil-derived elements by oysters due to the Deepwater Horizon oil spill. Environ Sci Technol. 2012;46(23):12787–12795. doi: 10.1021/es302369h. [DOI] [PubMed] [Google Scholar]
- Carvalho ML, Pereira RA, Brito J. Heavy metals in soft tissues of Tursiops truncatus and Delphinus delphis from West Atlantic Ocean by X-ray spectrometry. Sci Total Environ. 2002;292(3):247–254. doi: 10.1016/s0048-9697(01)01131-7. [DOI] [PubMed] [Google Scholar]
- Cohen MJ. Technological disasters and natural resource damage assessment: an evaluation of the Exxon Valdez oil spill. Land Econ. 1995;71(1):65–82. [Google Scholar]
- Council NR. Oil in the Sea III: Inputs, Fates, and Effects. The National Academic Press; Washington, D.C: 2003. [PubMed] [Google Scholar]
- D’Andrea MA, Reddy GK. Health consequences among subjects involved in gulf oil spill clean-up activities. Am J Med. 2013;126(11):966–974. doi: 10.1016/j.amjmed.2013.05.014. [DOI] [PubMed] [Google Scholar]
- Edema N. Effects of crude oil contaminated water on the environment. In: Abdul-Raof ME-S, editor. Crude Oil Emulsions- Composition Stability and Characterization. InTech; 2012. [Google Scholar]
- Efe SI. Spatial variation in acid and some heavy metal composition of rainwater harvesting in the oil-producing region of Nigeria. Nat Hazards. 2010;55:307–319. [Google Scholar]
- Fitzgerald TP, Gohlke JM. Contaminant levels in Gulf of Mexico reef fish after the Deepwater Horizon oil spill as measured by a fishermen-led testing program. Environ Sci Technol. 2014;48(3):1993–2000. doi: 10.1021/es4051555. [DOI] [PubMed] [Google Scholar]
- Fowler SW, Readman JW, Oregioni B, Villeneuve JP, McKay K. Petroleum hydrocarbons and trace metals in nearshore gulf sediments and biota before and after the 1991 war: an assessment of temporal and spatial trends. Mar Pollut Bull. 1993;27:171–182. [Google Scholar]
- Freeman RL, Holland SM, Ditton RB. Measuring the impact of the Ixtoc I oil spill on visitation at three Texas public coastal parks. J Coast Zone Manag. 1985;13(2):177–201. [Google Scholar]
- Gondal MA, Hussain T, Yamani ZH, Baig MA. Detection of heavy metals in Arabian crude oil residue using laser induced breakdown spectroscopy. Talanta. 2006;69(5):1072–1078. doi: 10.1016/j.talanta.2005.11.023. [DOI] [PubMed] [Google Scholar]
- Granneman JE, Jones DL, Peebles EB. Associations between metal exposure and lesion formation in offshore Gulf of Mexico fishes collected after the Deepwater Horizon oil spill. Mar Pollut Bull. 2017;117(1–2):462–477. doi: 10.1016/j.marpolbul.2017.01.066. [DOI] [PubMed] [Google Scholar]
- Gullett BK, Hays MD, Tabor D, Wal RV. Characterization of the particulate emissions from the BP Deepwater Horizon surface oil burns. Mar Pollut Bull. 2016;107(1):216–223. doi: 10.1016/j.marpolbul.2016.03.069. [DOI] [PubMed] [Google Scholar]
- Gullett BK, Aurell J, Holder A, Mitchell W, Greenwell D, Hays M, Conmy R, Tabor D, Preston W, George I, Abrahamson JP, Vander Wal R, Holder E. Characterization of emissions and residues from simulations of the Deepwater Horizon surface oil burns. Mar Pollut Bull. 2017;117(1–2):392–405. doi: 10.1016/j.marpolbul.2017.01.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaligama S, Chen Z, Saravia J, Yadav N, Lomnicki SM, Dugas TR, Cormier SA. Exposure to Deepwater Horizon crude oil Burnoff particulate matter induces pulmonary inflammation and alters adaptive immune response. Environ Sci Technol. 2015;49(14):8769–8776. doi: 10.1021/acs.est.5b01439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joung D, Shiller AM. Trace element distributions in the water column near the Deepwater Horizon well blowout. Environ Sci Technol. 2013;47(5):2161–2168. doi: 10.1021/es303167p. [DOI] [PubMed] [Google Scholar]
- Kuhuhawar MY, Mirza MA, Jahangir TM. Determination of Metal Ions in Crude Oil. In: Abdul-Raof ME-S, editor. Crude Oil Emulsions- Composition Stability and Characterization. InTech; 2012. [Google Scholar]
- Lane SM, Smith CR, Mitchell J, Balmer BC, Barry KP, McDonald T, Mori CS, Rosel PE, Rowles TK, Speakman TR, Townsend FI, Tumlin MC, Wells RS, Zolman ES, Schwacke LH. Reproductive outcome and survival of common bottlenose dolphins sampled in Barataria Bay, Louisiana, USA, following the Deepwater Horizon oil spill. Proc Biol Sci. 2015;282 doi: 10.1098/rspb.2015.1944. 1818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemos LS, de Moura JF, Hauser-Davis RA, de Campos RC, Siciliano S. Small cetaceans found stranded or accidentally captured in southeastern Brazil: bioindicators of essential and non-essential trace elements in the environment. Ecotoxicol Environ Saf. 2013;97:166–175. doi: 10.1016/j.ecoenv.2013.07.025. [DOI] [PubMed] [Google Scholar]
- Li Chen T, LaCerte C, Wise SS, Holmes A, Martino J, Wise JP, Jr, Thompson WD, Wise JP., Sr Comparative cytotoxicity and genotoxicity of particulate and soluble hexavalent chromium in human and sperm whale (Physeter macrocephalus) skin cells. Comp Biochem Physiol, Part C: Toxicol Pharmacol. 2012;155(1):143–150. doi: 10.1016/j.cbpc.2011.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Liu J, Zhu Q, Wu W. The weathering of oil after the Deepwater Horizon oil spill: insights from the chemical composition of the oil from the sea surface, salt marshes and sediments. Environ Res Lett. 2012;7(3):035302. [Google Scholar]
- MacDonald IR, Guinasso NL, Jr, Sassen R, Brooks JM, Lee L, Scott KT. Gas hydrate that breaches the sea floor on the continental slope of the Gulf of Mexico. Geology. 1994;22:699–702. [Google Scholar]
- MacDonald IR, Reilly JF, Jr, Best SE, Venkataramaiah R, Sassen R, Guinasso NL, Jr, Amos J. Remote sensing inventory of active oil seeps and chemosynthetic communities in the Northern Gulf of Mexico. In: Schumacher D, Abrams MA, editors. Hydrocarbon Migration and Its Near-Surface Expression. Vol. 66. AAPG Memoir; 1996. pp. 27–37. [Google Scholar]
- MacDonald IR, Leifer I, Sassen R, Stine R, Mitchell R, Guinasso NL., Jr Transfer of hydrocarbons from natural seeps to the water column and atmosphere. Geofluids. 2002;2(2):95–107. [Google Scholar]
- McCoy MA, Salerno JA. Assessing the Effects of the Gulf of Mexico Oil Spill on Human Health: A Summary of the June 2010 Workshop. 2010 [PubMed] [Google Scholar]
- McNutt MK, Camilli R, Crone TJ, Guthrie GD, Hsieh PA, Ryerson TB, Savas O, Shaffer F. Review of flow rate estimates of the Deepwater Horizon oil spill. Proc Natl Acad Sci U S A. 2012;109(50):20260–20267. doi: 10.1073/pnas.1112139108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millemann DR, Portier RJ, Olson G, Bentivegna CS, Cooper KR. Particulate accumulations in the vital organs of wild Brevoortia patronus from the northern Gulf of Mexico after the Deepwater Horizon oil spill. Ecotoxicology. 2015;24(9):1831–1847. doi: 10.1007/s10646-015-1520-y. [DOI] [PubMed] [Google Scholar]
- O’Hern JE, Biggs DC. Sperm whale (Physeter macrocephatus) habitat in the Gulf of Mexico: satellite observed ocean color and altimetry applied to small-scale variability in distribution. Aquat Mamm. 2009;35(3):358–366. [Google Scholar]
- Ortega-Ortiz JG, Engelhaupt D, Winsor M, Mate BR, Hoelzel AR. Kinship of long-term associates in the highly social sperm whale. Mol Ecol. 2012;21(3):732–744. doi: 10.1111/j.1365-294X.2011.05274.x. [DOI] [PubMed] [Google Scholar]
- Osuji LC, Onojake CM. Trace heavy metals associated with crude oil: a case study of Ebocha-8 oil-spill-polluted site in Niger Delta, Nigeria. Chem Biodivers. 2004;1(11):1708–1715. doi: 10.1002/cbdv.200490129. [DOI] [PubMed] [Google Scholar]
- Pabuwal V, Boswell M, Pasquali A, Wise SS, Kumar S, Shen Y, Garcia T, Lacerte C, Wise JP, Jr, Wise JP, Sr, Warren W, Walter RB. Transcriptomic analysis of cultured whale skin cells exposed to hexavalent chromium [Cr(VI)] Aquat Toxicol. 2013:134–135. 74–81. doi: 10.1016/j.aquatox.2013.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson CH, Rice SD, Short JW, Esler D, Bodkin JL, Ballachey BE, Irons DB. Long-term ecosystem response to the Exxon Valdez oil spill. Science. 2003;302(5653):2082–2086. doi: 10.1126/science.1084282. [DOI] [PubMed] [Google Scholar]
- Pozebon D, Lima EC, Maia SM, Fachel JMG. Heavy metals contribution of non-aqueous fluids used in off short oil drilling. Fuel. 2005;84(1):53–61. [Google Scholar]
- Ramseur JL. Deepwater Horizon: The Fate of the Oil. Congressional Research Service; 2010. www.crs.gov. [Google Scholar]
- Rosel PE, Wilcox LA. Genetic evidence reveals a unique lineage of Bryde’s whales in the Northern Gulf of Mexico. Endanger Species Res. 2014;25:19–34. [Google Scholar]
- Ryerson TB, Aikin KC, Angevine WM, Atlas EL, Blake DR, Brock CA, Fehsenfeld FC, Gao RS, de Gouw JA, Fahey DW, Holloway JS, Lack DA, Lueb RA, Meinardi S, Middlebrook AM, Murphy DM, Neuman JA, Nowak JB, Parrish DD, Peischl J, Perring AE, Pollack IB, Ravishankara AR, Roberts JM, Schwarz JP, Spackman JR, Stark H, Warneke C, Watts LA. Atmospheric emissions from the Deepwater Horizon spill constrain air-water partitioning hydrocarbon fate, and leak rate. Geophys Res Lett. 2011;38(7) [Google Scholar]
- Sammarco PW, Kolian SR, Warby RA, Bouldin JL, Subra WA, Porter SA. Concentrations in human blood of petroleum hydrocarbons associated with the BP/Deepwater Horizon oil spill, Gulf of Mexico. Arch Toxicol. 2015;90(4):829–837. doi: 10.1007/s00204-015-1526-5. [DOI] [PubMed] [Google Scholar]
- Schaum J, Cohen M, Perry S, Artz R, Draxler R, Frithsen JB, Heist D, Lorber M, Phillips L. Screening level assessment of risks due to dioxin emissions from burning oil from the BP Deepwater Horizon Gulf of Mexico spill. Environ Sci Technol. 2010;44(24):9383–9389. doi: 10.1021/es103559w. [DOI] [PubMed] [Google Scholar]
- Schwacke LH, Smith CR, Townsend FI, Wells RS, Hart LB, Balmer BC, Collier TK, De Guise S, Fry MM, Guillette LJ, Jr, Lamb SV, Lane SM, McFee WE, Place NJ, Tumlin MC, Ylitalo GM, Zolman ES, Rowles TK. Health of common bottlenose dolphins (Tursiops truncatus) in Barataria Bay, Louisiana, following the Deepwater Horizon oil spill. Environ Sci Technol. 2014;48(1):93–103. doi: 10.1021/es403610f. [DOI] [PubMed] [Google Scholar]
- Singleton B, Turner J, Walter L, Lathan N, Thorpe D, Ogbevoen P, Daye J, Alcorn D, Wilson S, Semien J, Richard T, Johnson T, McCabe K, Estrada JJ, Galvez F, Velasco C, Reiss K. Environmental stress in the Gulf of Mexico and its potential impact on public health. Environ Res. 2016;146:108–115. doi: 10.1016/j.envres.2015.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steffy DA, Nichols AC, Morgan LJ, Gibbs R. Evidence that the Deepwater Horizon oil spill caused a change in the nickel, chromium, and lead average seasonal concentrations occurring in sea bottom sediment collected from the eastern Gulf of Mexico continental shelf between the years 2009 and 2011. Water Air Soil Pollut. 2013;224:1756. [Google Scholar]
- Stowers D. Deepwater Gulf of Mexico rebounding from Macondo: GOM drilling and production on the upswing. Oil Gas Finan. 2013 J(Tulsa, OK, Pennwell Corporation) http://www.ogfj.com/articles/print/volume-10/issue-4/on-the-cover/deepwater-gulf-of-mexico-rebounding-from-macondo.html accessed 8 May 2017.
- Tershy B. Body size, diet, habitat use, and social behavior of Balaenoptera whales in the Gulf of California. J Mammal. 1992;73(3):477–486. [Google Scholar]
- Tershy BR, Acevedo GA, Breese D, Strong CS. Diet and feeding behavior of fin and Bryde’s whales in the Central Gulf of California, Mexico. Rev Inv Cient. 1993;1(1):31–38. [Google Scholar]
- Waring GT, Josephson E, Maze-Foley K, Rosel PE. Bryde’s Whale (Balaenoptera edeni): Northern Gulf of Mexico Stock. In: Fisheries N, editor. US Atlantic and Gulf of Mexico Marine Mammal Stock Assessments – 2012. Vol. 1. National Oceanic and Atmospheric Administration; Woods Hole, MA: 2013a. pp. 120–124. [Google Scholar]
- Waring GT, Josephson E, Maze-Foley K, Rosel PE. Mammal Stock Assessments – 2012. Vol. 1. N. O. a. A. Administration; Woods Hole, MA: 2013b. Short-Finned Pilot Whale (GLobicephala macrorhynchus): Northern Gulf of Mexico Stock. U.S. Atlantic and Gulf of Mexico Marine; pp. 298–302. [Google Scholar]
- Waring GT, Josephson E, Maze-Foley K, Rosel PE. Sperm Whale (Physeter macrocephalus): Northern Gulf of Mexico Stock. In: Fisheries N, editor. US Atlantic and Gulf of Mexico Marine Mammal Stock Assessments – 2012. Vol. 1. Woods Hole; MA: 2013c. pp. 112–119. [Google Scholar]
- Wise JP, Jr, Wise JT, Wise CF, Wise SS, Gianios C, Jr, Xie H, Thompson WD, Perkins C, Falank C, Wise JP., Sr Concentrations of the genotoxic metals, chromium and nickel, in whales, tar balls, oil slicks, and released oil from the gulf of Mexico in the immediate aftermath of the Deepwater Horizon oil crisis: is genotoxic metal exposure part of the Deepwater Horizon legacy? Environ Sci Technol. 2014;48(5):2997–3006. doi: 10.1021/es405079b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise JP, Sr, Payne R, Wise SS, LaCerte C, Wise J, Gianios C, Jr, Thompson WD, Perkins C, Zheng T, Zhu C, Benedict L, Kerr I. A global assessment of chromium pollution using sperm whales (Physeter macrocephalus) as an indicator species. Chemosphere. 2009;75(11):1461–1467. doi: 10.1016/j.chemosphere.2009.02.044. [DOI] [PubMed] [Google Scholar]
- Wise JP, Sr, Wise SS, LaCerte C, Wise JP, Jr, Aboueissa AM. The genotoxicity of particulate and soluble chromate in sperm whale (Physeter macrocephalus) skin fibroblasts. Environ Mol Mutagen. 2011;52(1):43–49. doi: 10.1002/em.20579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wursig B. Marine mammals of the Gulf of Mexico. In: Ward CH, editor. Habitats and Biota of the Gulf of Mexico: Before the Deepwater Horizon Oil Spill. Springer; New York, NY: 2017. [Google Scholar]
- Yang J, Miyazaki N. Moisture content in Dall’s porpoise (Phocoenoides Dalli) tissues: a reference base for conversion factors between dry and wet weight trace element concentrations in cetaceans. Environ Pollut. 2003;121(3):345–347. doi: 10.1016/s0269-7491(02)00239-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


