Abstract
Background and Purpose
Attempts to generate active site‐directed cathepsin K (CatK) inhibitors for the treatment of osteoporosis have failed because of side effects. We have previously shown that an ectosteric tanshinone CatK inhibitor isolated from Salvia miltiorrhiza blocked, selectively, the collagenase activity of CatK, without affecting the active site and demonstrated its bone‐preserving activity in vivo. Here, we have characterize the antiresorptive potential of other tanshinones, which may provide a scaffold for side effect‐free CatK inhibitors.
Experimental Approach
Thirty‐one tanshinones were tested for their activity against CatK in enzymic and cell‐based assays. The inhibitory potency against triple helical and fibrillar collagen degradation was determined in enzymic assays, by scanning electron microscopy and mechanical strength measurements. Human osteoclast assays were used to determine the effects of the inhibitors on bone resorption, its reversibility and osteoclastogenesis. Binding sites were characterized by molecular docking.
Key Results
Twelve compounds showed highly effective anti‐collagenase activity and protected collagen against destruction and mechanical instability without inhibiting the hydrolysis of non‐collagenous substrates. Six compounds were highly effective in osteoclast bone resorption assays with IC50 values of <500 nM. None of these tanshinones had effects on cell viability, reversibility of bone resorption inhibition and osteoclastogenesis. The core pharmacophore of the tanshinones appears to be the three‐ring system with either a para‐ or ortho‐quinone entity.
Conclusions and Implications
Our study identified several potent ectosteric antiresorptive CatK inhibitors from the medicinal plant, S. miltiorrhiza, which may avoid side effects seen with active site‐directed inhibitors in clinical trials.
Abbreviations
- CatK
cathepsin K
- CTx‐I
carboxy‐terminal cross‐linking telopeptide of type 1 collagen
- E64
(1S,2S)‐2‐(((S)‐1‐((4‐guanidinobutyl)amino)‐4‐methyl‐1‐oxopentan‐2‐yl)carbamoyl)cyclopropanecarboxylic acid
- GAGs
glycosaminoglycans
- M‐CSF
macrophage colony‐stimulating factor
- OVX
ovariectomized
- RANKL
receptor activator of NF‐κB ligand
- SEM
scanning electron microscopy
- TRAcP
tartrate‐resistant acid phosphatase
- Z‐FR‐MCA
carbobenzoxy‐phenylalanyl‐arginyl‐4‐methyl‐coumaryl‐7‐amide
Introduction
Currently, several mechanistically different approaches to treat osteoporosis are clinically utilized. Most of them are antiresorptive and include hormone replace therapy, selective oestrogen receptor modulators (SERMs), denosumab and bisphosphonates (Chen and Sambrook, 2011). Only parathyroid hormone‐related peptides are effective as anabolic compounds (Whitfield, 2006). The common feature of most antiresorptives is the suppression of osteoclast numbers. This can be achieved by replacing oestrogen in hormone‐deficient postmenopausal women where oestrogen plays a major role in osteoclast differentiation or mimicking an oestrogen effect with SERMs. Other antiresorptives directly reduce the number of osteoclasts by either interfering with osteoclastogenesis (denosumab) via neutralizing the receptor activator of NF‐κB ligand (RANKL) or by killing osteoclasts (bisphosphonates) via apoptosis (Baron et al., 2011). All these methods have various side effects. A common problem is that they unbalance the cooperation between bone‐forming osteoblasts and bone‐degrading osteoclasts, which ultimately leads to poor bone quality.
Major efforts have been undertaken to circumvent the disruption of the crosstalk between bone cells. One promising approach is the selective inhibition of the bone‐resorbing enzyme without interfering with the viability of osteoclasts. The perfect target for this approach was cathepsin K (CatK), which is the predominant protease in osteoclasts and exclusively responsible for the degradation of type I collagen in bone (Duong et al., 2016). Type I collagen constitutes 90% of the organic bone matrix. Many CatK inhibitors were synthesized (Yamashita and Dodds, 2000; Bromme and Lecaille, 2009), and at least five have entered clinical trials for osteoporosis and to a lesser degree for osteoarthritis (Bromme et al., 2016). With the exception of relacatib, all compounds went into phase II or even III trials and demonstrated high efficacy in terms of increasing bone mineral density and reducing the risk of bone fractures. However, none of the drug candidates were ever approved, and this was mostly due to various side effects. Relacatib failed because of undesirable drug interactions, balicatib because of increased risks for skin fibrosis (Runger et al., 2012), ONO‐5334 because of marketing reasons and likely skin fibrosis risk issues as well (Eastell et al., 2011), and finally, odanacatib after completing a multicentre phase III trial with about 17 000 patients was withdrawn after a careful adjudication of side effects (Mullard, 2016; Drake et al., 2017). The main reasons were increased risks of stroke and other cardiovascular events and, to a lesser degree, skin problems as well (Merck, 2014). Reasons for the failure of CatK inhibitors were pointed out as a lack of specificity leading to the inhibition of closely related cathepsins. However, this is rather unlikely as most of the clinical drug candidates had excellent specificity parameters, and despite differences in their potential to accumulate in lysosomes, all appear to have a fibrosis‐promoting activity. This may rather indicate an on‐target instead of an off‐target effect (Bromme and Lecaille, 2009; Bromme et al., 2016). We have previously shown that odanacatib increases TGF‐ß1 concentrations in skin fibroblast cultures by preventing a CatK‐mediated TGF‐ß1 degradation (Panwar et al., 2016). In contrast, a so‐called ectosteric inhibitor of CatK, dihydrotanshinone I (DHT1 or T12 in this report), did not block TGF‐ß1 degradation but maintained a highly potent antiresorptive activity by specifically blocking the collagenase activity of CatK (Panwar et al., 2016). The mechanism is based on the interference of the inhibitors with an ectosteric site needed for the formation of CatK dimers. We have previously demonstrated that the degradation of triple helical collagens and collagen fibres requires a CatK dimer (Aguda et al., 2014). These oligomers are not needed for any other known CatK proteolytic activity (Sharma et al., 2015). DHT1 is a diterpenoid isolated from Salvia miltiorrhiza, a Chinese herb widely used in bone and cardiovascular diseases (Guo et al., 2014; Wang et al., 2017). Initially, we screened several Chinese herbs that are traditionally used for the treatment of bone diseases for ectosteric CatK inhibitors. S. miltiorrhiza, also known as Danshen, revealed a strong inhibition of the collagenase activity of CatK. Considering that the herb produces more than 100 chemical constituents, of which about half are tanshinone‐like compounds (Wang, 2010; Su et al., 2015), this medicinal plant might represent a unique source of potent ectosteric inhibitors of CatK. Therefore, we decided to analyse additional tanshinones made by the herb for their potential antiresorptive activities.
In the present study, we evaluated 31 tanshinones for their anti‐collagenase and antiresorptive activities. We analysed the inhibition of CatK‐mediated degradation of soluble triple‐helical collagen, insoluble fibrillar collagen and non‐helical gelatine. Twelve compounds showed highly effective anti‐collagenase activity in soluble and fibrillar collagen assays. None of these compounds interfered with the active site of the protease. Six of these compounds showed efficacy in in vitro bone resorption assays and importantly did not show an anti‐osteoclastogenesis effect at even 10 times the effective IC50 concentration for bone resorption and did not interfere in the degradation of TGF‐ß1, a known profibrotic growth factor. Structure activity relationship analysis and molecular docking experiments identified structural elements needed for the ectosteric inhibitor activity of tanshinones.
Methods
Collagenase assay
Powdered herbal material from Danshen (Radix et Rhizoma Salviae miltiorrhizae), Di Huang (Radix Rehmannia), Bai Zhu (Rhizoma Atractylodis Macrocephalae) and Huang Qi (Radix Astragali) were methanol extracted over 1 week. Herbal extracts (100 μg·mL−1) were incubated with 0.6 mg·mL−1 soluble bovine type I collagen (USB, Cleveland, OH, USA) and 400 nM human recombinant CatK, in the presence or absence of 200 nM chondroitin 4‐sulfate (Sigma‐Aldrich, St. Louis, MO, USA) in 100 mM sodium acetate buffer, pH 5.5, containing 2.5 mM DTT and EDTA and incubated at 28°C. Recombinant human CatK was expressed in Pichia pastoris and purified as previously described (Linnevers et al., 1997). The same assay was used for the evaluation of 31 individual tanshinones (ChemFaces, Wuhan, China) for their potential anti‐collagenase activity. Compounds were dissolved in DMSO or ethanol, and solvent concentrations were kept below 0.2% where no inhibitory effect is observed. Assay inhibitor concentrations were between 0.5 and 100 μM. After 4 h of incubation of collagen with CatK in the absence or presence of herbal extracts or tanshinones, 1 μM (1S,2S)‐2‐(((S)‐1‐((4‐guanidinobutyl)amino)‐4‐methyl‐1‐oxopentan‐2‐yl)carbamoyl)cyclopropanecarboxylic acid (E64) was added to each reaction to inhibit the residual activity of CatK, and samples were analysed on 9% SDS‐PAGE gels. Coomassie‐stained bands representing the residual amount of α‐collagen chains were quantitatively assessed to determine IC50 values. CatK‐mediated collagen degradation assays with corresponding solvents were used as control. All assays were done in five independent experiments.
Degradation of insoluble collagen
Collagen fibres were isolated from mouse tails and incubated with 1 μM CatK with or without tanshinone inhibitors at 20 μM concentrations in 100 mM sodium acetate buffer, pH 5.5, containing 2.5 mM DTT and EDTA at 28°C for 10 h. Most potent inhibitors were tested at different concentrations to obtain IC50 values. The reaction was stopped by adding 10 μM E64, a pan‐cathepsin inhibitor. The degree of the collagenase inhibition was quantified on the basis of released soluble collagen fragments using the hydroxyproline assay as previously described (Panwar et al., 2015). For scanning electron microscopy (SEM) analysis, fibres were rinsed with nanopure water and fixed with 2.5% glutaraldehyde, followed by extensive washing with water. Subsequently, fibres were dried by passing through increasing concentrations of ethanol and transferred into a critical point dryer. Upon drying, fibres were mounted on metal stubs using double‐sided carbon tape and coated with iradium in Leica EM MED020 coating system (Leica Microsystems Inc., Ontario, Canada). Images from 20 fibres of each treatment were taken using a Helios NanoLabTM 650 (FEI, Hillsboro, OR, USA) scanning electron microscope at operating voltages between 2 and 10 kV (Panwar et al., 2013).
Gelatinase and Z‐FR‐MCA activity measurement assay
Gelatine (prepared by heating 0.6 mg·mL−1 soluble bovine type I collagen at 75°C for 30 min) was incubated with 5 nM human recombinant CatK in 100 mM sodium acetate buffer, pH 5.5, containing 2.5 mM DTT and EDTA in the presence of tanshinones (12.5 μM) at 28°C. After 1 h, 1 μM E64 was added to inhibit the remaining CatK activity. Gelatine alone and gelatine with CatK with corresponding solvents were used as control. Samples were analysed on 9% SDS‐PAGE gels. The density of the α1 band was quantified to obtain the percentage of inhibition as described above. All assays were done in five independent experiments. For fluorogenic peptide substrate hydrolysis, 5 nM CatK was added with or without 12.5 μM tanshinones in 1 mL sodium acetate buffer, pH 5.5, containing 2.5 mM DTT and EDTA, and 5 μM carbobenzoxy‐phenylalanyl‐arginyl‐4‐methyl‐coumaryl‐7‐amide (Z‐FR‐MCA, Bachem Inc, Torrence, CA). Z‐FR‐MCA is a fluorogenic peptide substrate for cathepsins. We also tested the potential inhibitory activity of tanshinones at the enzyme to inhibitor molar ratio equal to the IC50 values obtained in the soluble collagenase assays. Activities were measured using a spectro‐fluorometer PerkinElmer LB50 with wavelengths set at 380 and 460 nm respectively.
Micro‐tensile testing of collagen fibres
Collagen fibres were evaluated for their mechanical stability before and after CatK digestion in the presence or absence of tanshinones as previously described (Panwar et al., 2013; Panwar et al., 2015). Briefly, optical microscopy was used for diameter measurements. Tensile strength of control (n = 25), CatK digested (n = 25) or inhibitor‐treated fibres (n = 25) were measured using a KES‐G1 tensile testing machine (Kato Tech Co., Ltd., Kyoto, Japan). Tensile tests were performed with a 50 N capacity load cell, and a gauge length of 10 mm was selected. Both control and digested fibres were stretched in a uniaxial direction until failure at a cross‐head speed of 0.1 mm·min−1.
In vitro bone resorption assay
Osteoclasts were generated from mononuclear cells isolated from human bone marrow purchased from Lonza (Walkersville, MD, USA). The bone marrow cell suspension was layered over 10 mL Ficoll‐Paque media solution and centrifuged for 30 min at 500 × g. Following centrifugation, the white interface containing the monocytes was harvested and washed twice with α‐MEM. Cells were cultured in 96‐well cell culture plates (Corning Inc. Corning, NY, USA; product no. 351172) using α‐MEM containing 10% FBS and 25 ng·mL−1 macrophage colony‐stimulating factor (M‐CSF) for 24 h and then cultured with 25 ng·mL−1 RANKL and 25 ng·mL−1 M‐CSF for 7 days. Differentiated osteoclasts at a density of 100 000 cells were seeded on individual bovine bone slices (prepared from bovine cortical femur bone: 5.5 mm diameter and 0.4 mm thickness) in the presence or absence of tanshinones and incubated for 72 h at 5% CO2 and 37°C. The metabolic activity of osteoclasts (viability) was determined using CellTiter‐Blue Viability Assay (Promega BioSciences, Madison, WI, USA). The total number of osteoclasts was determined after fixing them in 4% formaldehyde and subsequently stained using the tartrate‐resistant acid phosphatase (TRAcP) kit (Sigma‐Aldrich.). Bone slices were incubated in distilled H2O to lyse the cells and wiped clean with a cotton stick. Subsequently, resorption cavities were stained with toluidine blue and analysed by light microscopy. The number of resorption events and eroded bone surface area were determined as previously described (Soe and Delaisse, 2010; Panwar et al., 2016). Cells with more than two nuclei were counted to determine the total number of osteoclasts per bone slice in each experimental condition. Images were acquired using a Nikon Eclipse LV100 microscope. Collagenolysis by osteoclasts was determined by measuring carboxy‐terminal cross‐linking telopeptide of type 1 collagen (CTx‐I) in cell culture media (MyBiosorce elisa kit, San Diego, CA, USA).
Osteoclastogenesis assay
Osteoclastogenesis experiments were performed to determine the effect of tanshinones on osteoclast differentiation. Isolated mononuclear cells (100 000 cells) were seeded on bone slices in 96‐well plate in the presence or absence of tanshinones (5 μM) and incubated for 10 days at 5% CO2 and 37°C. Medium was changed every second day containing the appropriate tanshinones. Cells were stained for TRAcP activity as described above to quantify the effect of inhibitors on multinucleated osteoclast formation. TRAcP‐positive cells containing at least two nuclei were counted as multinucleated osteoclasts.
Reversibility of tanshinone activity in osteoclasts
The reversibility of tanshinone‐mediated inhibition of human osteoclast‐mediated bone resorption was determined by removal of the inhibitors after 2 days of incubation. Briefly, osteoclasts were seeded at a density of 100 000 cells per bone slice in the presence or absence of tanshinones (1 μM) in a two‐phase experiment. Cells were treated with either vehicle or 1 μM tanshinones for 2 days. Subsequently, osteoclasts on bone slices were washed twice with media and transferred into a new culture plate, and the culture medium was either switched from vehicle to vehicle, vehicle to tanshinones, tanshinones to tanshinones or tanshinones to vehicle for an additional 2 days. Appropriate controls for different conditions were cultured for 2 and 4 days in parallel experiments.
Effect of tanshinones on fibroblast‐mediated TGF‐ß1 degradation
Human skin fibroblasts (PromoCell GmBH, Heidelberg, Germany) were seeded in a six‐well plate in the presence or absence of tanshinones or an E64‐containing protease inhibitor cocktail (PIC) (Sigma‐Aldrich, St. Louis, MO, USA) in DMEM containing 10% FBS at 37°C and 5% CO2 as previously described (Panwar et al., 2016). Western blot detection of TGF‐β1 and β‐actin in fibroblast cell lysates was performed with human anti‐TGF‐β1 primary antibody (G1221, Promega BioSciences, Madison, WI, USA) and anti‐β‐actin antibody (622102, BioLegend, San Diego, CA, USA) (1:1000 dilution) incubated overnight at 4°C. As secondary antibody, HRP‐conjugated anti‐IgG was used (W4018, Promega) at a 1:2500 dilution and incubated for 1 h at room temperature. Immuno‐positive bands were visualized using an enhanced chemiluminescence assay kit (GE Healthcare Life Sciences, Mississauga, ON, Canada).
Molecular docking of tanshinones to CatK
The ligand sets for the tanshinones were generated using LigPrep and OPLS3 force fields and ionization states generated at pH 5.5. The enzyme molecule used for docking was an inhibitor‐bound CatK (PDBID: 1ATK) with the inhibitor, and heteroatoms removed, as an inhibitor‐free CatK structure was unavailable at the time. Theoretical LogP were also calculated using QikProp in Maestro. Molecular modelling with Autodock Vina was performed using PyRx 0.8 with the previously prepared ligand and protein data sets. Ser95 was used as the centre of the docking grid in a cube with 8 Å sides. The ligands were docked to the area with an exhaustiveness of 10. The resulting poses were exported along with the theoretical binding affinities and visualized in PyMOL. The theoretical K i values were calculated from the theoretical binding affinities using Autodock Vina.
Data and statistical analysis
The data and statistical analysis comply with the recommendations on experimental design and analysis in pharmacology (Curtis et al., 2015). All assays were performed in five independent experiments. Cell assays were performed in five independent experiments where different bone marrow samples were used to generate osteoclasts. Five to six technical repeats were run in each experiment. No randomization or blinding was used for the enzymic and cell‐culture assays. However, some experiments were performed by two researchers in independent experiments, and data were then compiled for final analysis. The data are expressed as means ± SD. Statistical significance was determined by one‐way ANOVA followed by Bonferroni post hoc test. In all cases, P values of ≤0.05 were taken as significant, and the Bonferroni post hoc test was performed with F at P < 0.05 and no significant variance inhomogeneity. GraphPad Prism version 4.01 and Sigma plot 12.0 software packages were used for analysis and statistical tests.
Materials
Compounds used in these experiments were supplied as follows: E64 by Sigma‐Aldrich Canada Co, Oakville, ON; odanacatib by Selleckchem.com, Houston, TX; Z‐FR‐MCA by Bachem USA, Torrance, CA.
Nomenclature of targets and ligands
Key proteins targets and ligands in this article are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Harding et al., 2018), and are permanently archived in the Concise Guide to PHARMACOLOGY 2017/18 (Alexander et al., 2017).
Results
Inhibition of CatK‐mediated degradation of soluble collagen by herbal extracts and tanshinones
From four methanol extracts of Chinese herbs (Danshen, Di Huang, Bai Zhu and Huang Qi), only the Danshen extract (S. miltiorrhiza) showed a strong inhibition of the collagenase activity of CatK (Figure 1A). None of the extracts inhibited the degradation of non‐helical gelatine (data not shown). Subsequently, we analysed 31 commercially available tanshinones isolated from Danshen. Twelve of the 31 tested tanshinones (T02, T05, T06, T07, T08, T09, T11, T12, T17, T20, T23 and T27 at 10 μM) exhibited inhibition of collagen degradation, of more than 70%, at a 25‐fold molar excess of the inhibitor over the protease concentration. The IC50 values for these compounds was less than 10 μM when 400 nM CatK was used (Table 1 and Supporting Information Table S3). The most potent compounds had IC50 values of 2.7 μM (T06), 3.6 μM (T23) and between 5.9 and 6.3 μM (T05, T08, T17 and T20) reflecting molar CatK : tanshinone ratios of 1:5, 1:9 and about 1:15. The compounds T10, T15, T16, T18, T27, T28, T30 and T31 did not display significant inhibition at 50 μM (1:125 molar ratio). SDS‐PAGE analyses of all 31 tanshinones tested at 10 μM inhibitor concentration and their respective quantification are shown in Figure 1B–D and their quantification in Figure 1E.
Figure 1.
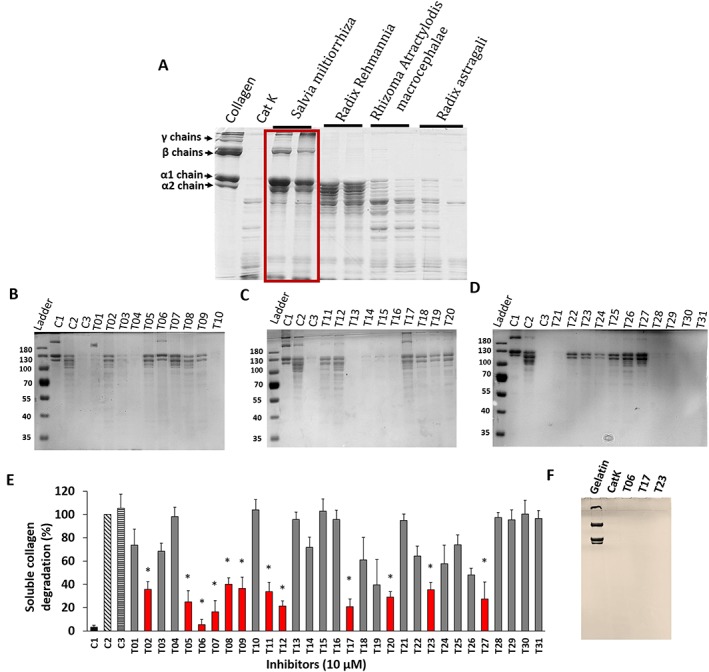
Inhibition of soluble collagen degradation by ectosteric inhibitors. (A) Screening of four herbal extracts traditionally used in Chinese medicine for skeletal diseases. (B–D) Representative SDS‐PAGE analysis of type I collagen degradation inhibition by tanshinone inhibitors (31 inhibitors) at 10 μM concentration. Assays were carried out with 400 nM CatK and 200 nM chondroitin 4‐sulfate. C1, control (untreated); C2, CatK digested; C3, CatK digested (0.2% solvent). (E) The degradation of collagen was quantified on the basis of α1 band density on the Coomassie blue‐stained gel (n = 5) at a constant inhibitor concentration of 10 μM. * Red bars show tanshinones exhibiting a reduction of collagen degradation of more than 50% in the presence of 10 μM inhibitor. (F) Gelatine degradation by CatK in presence or absence of selected tanshinones (12.5 μM), which showed no effect of tanshinones on non‐helical substrate degradation. All assays were done in five independent experiments and data shown are means±SD; n = 5.
Table 1.
Inhibition of soluble, insoluble collagen and gelatine by tanshinone inhibitors
| Tanshinone name and number | Structure | Soluble type I collagen degradation IC50 (μM) | Insoluble collagen degradation % inhibition IC50 (μM) | Gelatine degradation % inhibition 12.5 μM (1:2500) | Z‐FR‐MCA % inhibition Hydrolysis at IC50 molar ratio |
|---|---|---|---|---|---|
| Miltirone (T01) |
(1).
|
31.9 ± 3.4 | 75.6 ± 12.4 | 0 | 0 |
| 15,16‐Dihydrotanshindiol (T02) |
(2).
|
14 ± 1.9 | 12.3 ± 2.6 | 0 | 0.8 ± 0.6 |
| Danshenol A (T03) |
(3).
|
25.4 ± 2.4 | 21.4 ± 3.5 | 0 | 0 |
| epi‐Cryptoacetalide (T04) |
(4).
|
63.1 ± 7.3 | 17.2 ± 5.1 | 0 | 0 |
| 1,2,5,6‐Tetrahydro‐tanshinone I (T05) |
(5).
|
5.9 ± 0.5 | 22.3 ± 4.8 | 0 | 0 |
| Tanshinone IIA sulfonic sodium (T06) |
(6).
|
2.7 ± 0.2 | 3.6 ± 1.7 | 0 | 0 |
| Methylenedihydrotanshinquinone (T07) |
(7).
|
7.8 ± 2.4 | 18.5 ± 2.9 | 0 | 1.5 ± 0.7m |
| Salvianan (T08) |
(8).
|
6.0 ± 0.8 | 14.4 ± 2.6 | 0 | 0 |
| Trijuganone C (T09) |
(9).
|
11.3 ± 0.7 | 18.3 ± 4.2 | 0 | 0 |
| Epidanshenspiroketallactone (T10) |
(10).
|
>50 | 93.2 ± 10.8 | 0 | 0 |
| Dihydrotanshinone (T11) |
(11).
|
13.3 ± 2.3 | 20.6 ± 6.1 | 0 | 1.73 ± 1.2 |
| DHT1 (T12) |
(12).
|
8.9 ± 1.1 | 9.5 ± 3.8 | 0 | 0 |
| Cryptotanshinone (T13) |
(13).
|
26.3 ± 4.9 | 65.2 ± 9.4 | 0 | 0 |
| Tanshinone I (T14) |
(14).
|
16.6 ± 1.5 | 19.6 ± 5.2 | 0 | 0 |
| Tanshinlactone (T15) |
(15).
|
>50 | 88.1 ± 13.4 | 0 | 0 |
| Tanshinone IIA (T16) |
(16).
|
>50 | 72.8 ± 7.3 | 0 | 0 |
| Isotanshinone II (T17) |
(17).
|
5.9 ± 0.7 | 10.3 ± 4.1 | 0 | 0 |
| Danshenxinkun D (T18) |
(18).
|
>50 | >100 | 0 | 0 |
| Przewaquinone A (T19) |
(19).
|
11.2 ± 2.7 | 20 ± 5.8 | 0 | 1.8 ± 0.7 |
| Neocryptotanshinone II (T20) |
(20).
|
6.3 ± 1.7 | 11 ± 3.4 | 0 | 0 |
| Salviolone (T21) |
(21).
|
12.2 ± 2.3 | 18.5 ± 3.1 | 0 | 0 |
| Sageone (T22) |
(22).
|
17.2 ± 2.3 | 21 ± 5.5 | 0 | 0 |
| Methylenetanshinquinone (T23) |
(23).
|
3.6 ± 0.6 | 6.7 ± 1.8 | 0 | 1.3 ± 0.7 |
| Neocryptotanshinone (T24) |
(24).
|
16.8 ± 2.6 | 20.0 ± 3.4 | 0 | 0 |
| 1,2‐Didehydrocryptotanshinone (T25) |
(25).
|
21.3 ± 3.1 | 27 ± 3.2 | 0 | 3.1 ± 2.2 |
| 15‐epi‐Danshenol‐A (T26) |
(26).
|
>50 | >100 | 0 | 1.3 ± 0.6 |
| Tanshindiol A (T27) |
(27).
|
16.9 ± 3.7 | 18.8 ± 2.5 | 0 | 0 |
| 6,7‐Dehydroferruginol (T28) |
(28).
|
>50 | >100 | 0 | 0 |
| Salvinolone (T29) |
(29).
|
>50 | >100 | 0 | 0 |
| Sugiol (T30) |
(30).
|
>50 | >100 | 0 | 1.7 ± 1.2 |
| Hydroxymethylenetanshinquinone (T31) |
(31).
|
>50 | >100 | 0 | 0 |
Inhibition of CatK‐mediated collagen fibre degradation by tanshinones
Inhibition of insoluble collagen fibre degradation by tanshinones was analysed using the hydroxyproline assay. The CatK‐solubilized collagen fractions were analysed and quantified in the assay buffer at 20 μM tanshinone concentration (Figure 2A). Forty per cent of the compounds, including T02, T06, T11, T12, T17, T20, T23 and T27, showed more than 50% inhibition of fibrillar collagen degradation at a 20‐fold excess over CatK. The potencies of the tanshinones for soluble collagen and fibrous collagen were in most cases comparable. The IC50 values were about 2–3 times higher for the fibrous collagen when compared with the values obtained with soluble collagen, as the enzyme concentration was 2.5 times higher. IC50 values are shown in Table 1. Further, we analysed structural changes of the collagen fibres by SEM in the presence or absence of tanshinone inhibitors when treated with CatK. In the absence of inhibitors, collagen fibres were completely degraded. However, in the presence of tanshinone inhibitors, fibres remained mostly intact but showed an increase in fibre diameter. This is likely caused by the proteolytic removal of collagen‐associated proteoglycans. We observed a few nicks at the surface of fibres, which varied from one inhibitor specimen treatment to the other, reflecting different degrees of inhibition. The comparative structure analysis of degraded fibres and fibres whose degradation was inhibited is shown in Figure 2B.
Figure 2.
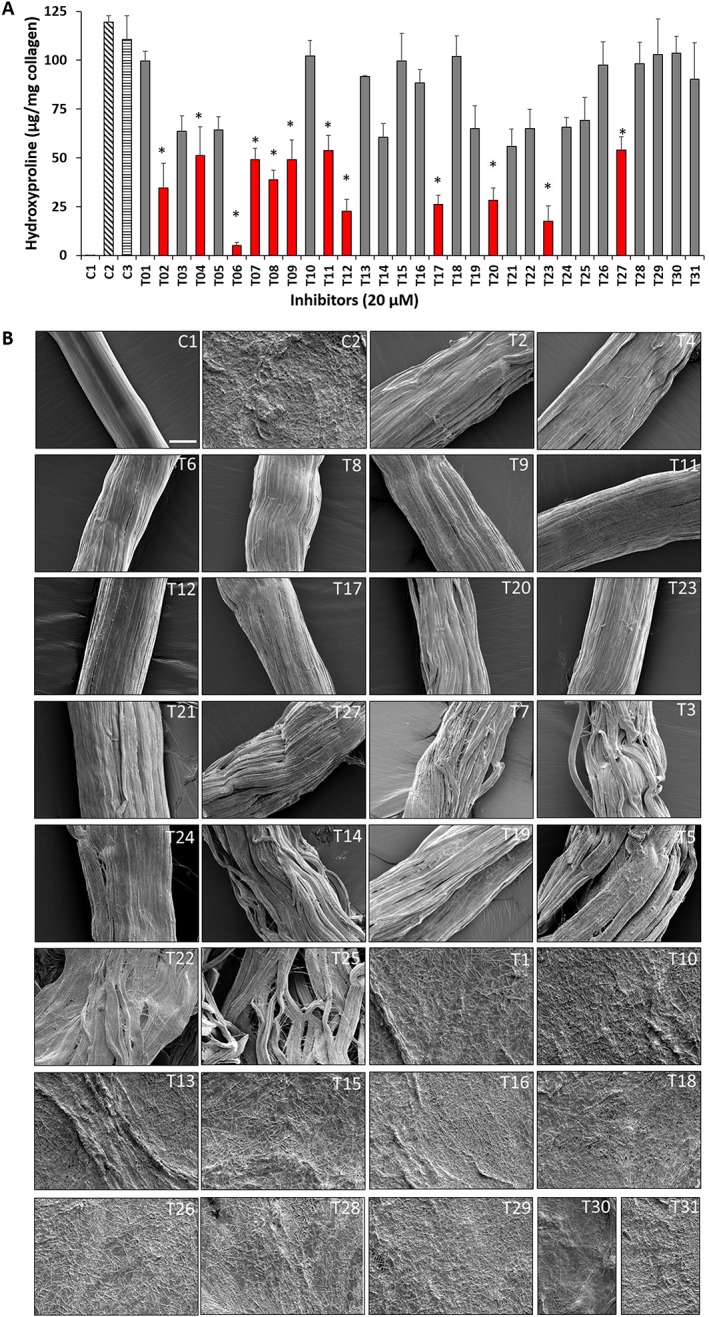
Inhibition of insoluble collagen fibre degradation by tanshinone compounds. (A) Hydroxyproline analysis (n = 5) of control (untreated), CatK digested (1 μM for 10 h at 28°C) and tanshinone inhibitor (20 μM)‐treated collagen fibre degradation. C1, control (untreated); C2, CatK digested; C3, CatK digested (0.2% solvent). * Red bars show tanshinones exhibiting a reduction of collagen degradation by more than 50% in the presence of 10 μM inhibitor. Data shown are means ± SD; n = 5. (B) Structural analysis of collagen fibre degradation inhibition by tanshinone inhibitors. SEM micrographs of control (untreated), CatK digested (1 μM for 10 h at 28°C) and tanshinone inhibitor‐treated collagen fibres. Scale bars = 25 μm. CatK‐mediated fibre degradation was inhibited in the presence of 20 μM of selected tanshinones (T02, T06, T11, T12, T17, T20, T23 and T27). In contrast, fibres were completely digested by CatK in the absence of inhibitors.
Lack of inhibition of the degradation of non‐collagen substrates
To determine whether tanshinones are active site‐directed inhibitors, we evaluated the inhibitory potency of these compounds on the hydrolysis of a synthetic fluorogenic CatK peptide substrate, Z‐FR‐MCA, and on gelatine. Twenty‐three out of 31 compounds showed no inhibition of Z‐FR‐MCA hydrolysis with the remaining eight compounds revealing some minor inhibition (0.8–3.4%) at a molar CatK : tanshinone ratio of the IC50 concentrations for the collagenase activity of CatK. Despite their potent anti‐collagenase activities, none of these compounds inhibited the degradation of non‐helical gelatine even at a 2500‐fold molar excess of these inhibitors (Figure 1E and Table 1). This clearly indicated that these tanshinones did not significantly block the active site of CatK.
Mechanical properties of collagen fibres
The most effective collagenase inhibitors, T02, T06, T11, T12, T17, T20, T23 and T27, were selected for the evaluation of their effects on the mechanical stability of collagen fibres exposed to CatK. We found a large variation in the mechanical characteristics of untreated and CatK‐treated collagen fibres. Collagen fibres whose degradation was inhibited by tanshinones remained mechanically stable. However, strain and stress at break of these fibres was significantly lower compared with the untreated fibres (Supporting Information Table S1 and Figure 3). This can be explained by the fact that tanshinone inhibitors prevented CatK‐mediated collagen fibre destruction but allowed the removal of glycosaminoglycan (GAG)‐containing proteoglycans. GAGs and proteoglycans function as cross‐linking threads in collagen fibres (Osborne et al., 1999), and their degradation directly interferes with the mechanical strength of the fibres.
Figure 3.
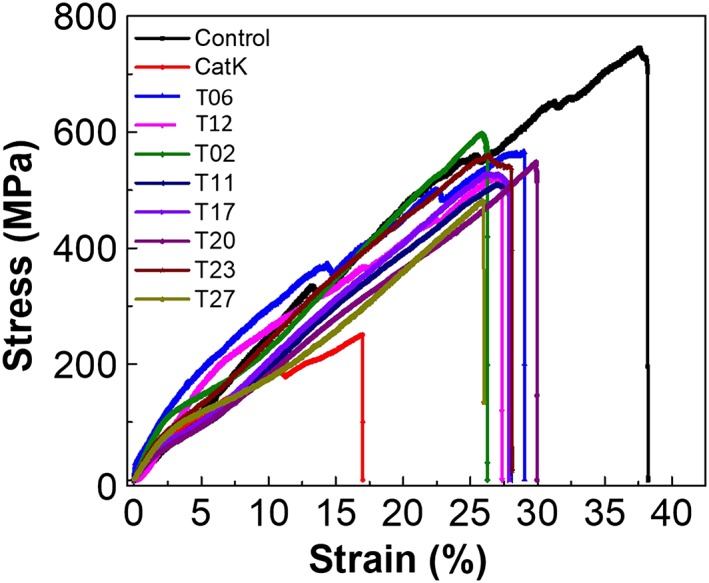
Mechanical properties of untreated, CatK and tanshinone‐treated collagen fibres. Stress–strain plots for control, CatK‐treated, T12, T06, T02, T11, T17, T20, T23 and T27 (20 μM)‐treated collagen fibres. Plots represent the comparison of stress–strain between undigested (n = 25), CatK‐digested fibres (n = 25) and each inhibitor‐treated collagen fibres (n = 25). Fibres were digested with CatK for 2 h, and stress–strain curves were obtained from the displacement of 0.1 mm·min−1.
Effect of tanshinones on osteoclast‐mediated bone resorption
As eight tanshinones prevented the collagenolytic activity of CatK in in vitro assays (T02, T06, T11, T12, T17, T20, T23 and T27), we analysed their effect on osteoclast viability and bone resorption activity of osteoclasts. The resorption cavities formed by osteoclasts in the presence and absence of selective tanshinones are shown in Figure 4A. First, we evaluated the overall metabolic activity and the total number of osteoclasts. None of the tanshinones tested affected osteoclast viability (Figure 4B) and the number of osteoclasts (Figure 4C) at the inhibitor concentration of 1 μM. Next, we determined the antiresorptive potential of the tanshinones towards mature osteoclasts. Measuring CTx‐I release from the bone slices provides an overall assessment of the inhibition of osteoclasts. CTx‐I is a CatK‐generated carboxy‐terminal cross‐linking telopeptide fragment of type 1 collagen and thus reflects the bone resorptive activity of CatK. Of the nine tanshinones tested, seven (T06, T08, T11, T12, T17, T20 and T23) showed a significant inhibition of bone resorption, whereas T02 and T27 were very weak inhibitors of the release of CTx‐I (Figure 4D).
Figure 4.
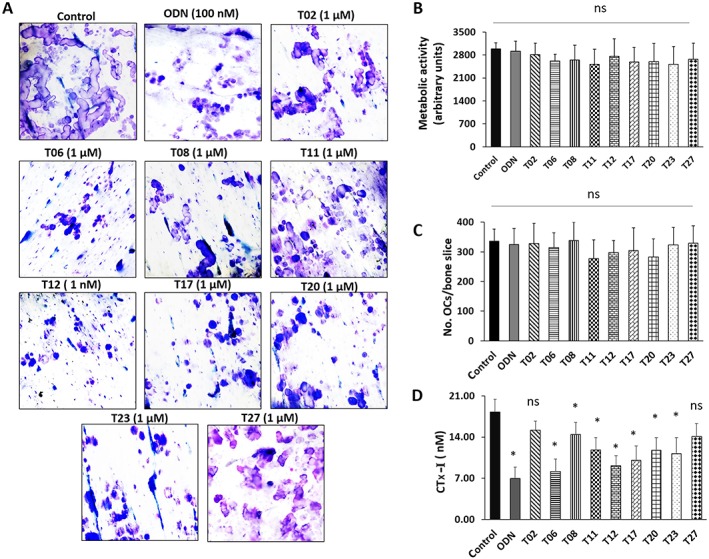
Effect of tanshinone inhibitors on bone resorption parameters. (A) The effect of tanshinones was observed on the basis of the inhibition of the formation of resorption cavities generated by human osteoclasts (OCs), cultured on bovine bone slices for 72 h. (B) Metabolic activity of OCs after treatment with tanshinone inhibitors when compared with untreated OCs. (C) TRAcP‐positive OCs with two nuclei or more were counted manually using light microscopy. The number of TRAcP‐positive multinucleated OCs was unaffected by the use of any of these inhibitors. (D) Effect of inhibitors on CTx‐I concentration in conditioned media after 72 h in untreated, odanacatib (ODN) and tanshinone inhibitor‐treated cultures. and data shown are means ± SD; n = 5. *P < 0.05 significantly different from untreated control; ns, not significant.
To analyse the antiresorptive activity of tanshinones in more detail, we evaluated the bone slices for individual resorption events. Figure 4A shows representative images of resorption events on bone surfaces in the absence or presence of inhibitors (1 μM). In untreated cultures, osteoclasts made long deep excavation trenches and smaller round excavation pits. Odanacatib, an active site inhibitor, was used as control, which strongly inhibited trench formation. These resorption pattern changes are a typical response of osteoclasts to CatK inhibition as previously reported (Panwar et al., 2016). Different tanshinones showed variable degrees of the inhibition of bone resorption. Evaluation of the resorption parameters revealed that T06, T11, T12, T17, T20 and T23 effectively reduced the total eroded surface area (Figure 5A). T12 showed effective reduction in the total bone eroded surface (~50%) at 1 μM concentration as described previously (Panwar et al., 2016). Similarly, T06, T11, T17, T20 and T23 reduced the total eroded surface area between 40 and 70% at 1 μM inhibitor concentration. As with the odanacatib control, we observed clear differences in the pattern of resorption cavities formed by osteoclasts in the presence and absence of tanshinones. In general, the number of trenches reduced in the presence of inhibitors, and more pits appeared as a consequence of the osteoclastic bone demineralization process. Therefore, the resorption events appearing as trenches and pits were quantified separately (Figure 5B, C). T12 and T06 almost completely abolished the formation of trenches. More pits were observed with few smaller trenches after treatment with T06, T12, T17, T20 and T23. Other inhibitors (T08, T11 and T27) showed higher number of pits and less trenches compared with untreated conditions. In addition, the size of trenches was comparatively smaller than in the absence of inhibitors. The IC50 values for the inhibition of osteoclastic bone resorption observed with these compounds are given in Figure 5D. The release of CTx‐I correlated well with the determined IC50 values for the inhibition of trench and pit formation.
Figure 5.
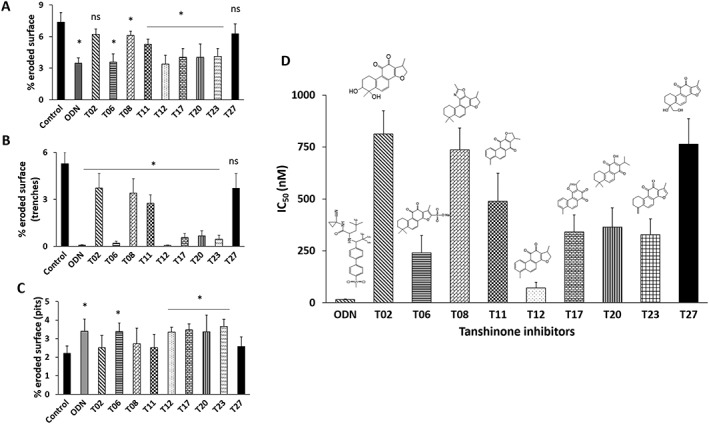
Effect of tanshinone inhibitors on pit and trench formation: Effect of tanshinones was observed on the basis of resorption cavities generated by human osteoclasts (A) total eroded surface (%), (B) % trench surface and (C) % pit surface. Six bone slices per condition were used and data shown are means ± SD from 5 independent experiments. *P < 0.05 significantly different from untreated control; ns, not significant. (D) IC50 values of % eroded surface/bone surface for odanacatib (ODN), T02, T06, T08, T11, T12, T17, T20 and T23 tanshinones. Data shown are means ± SD from 5 independent experiments.
Effect of tanshinones on osteoclastogenesis
Next, we determined whether any of the antiresorptive tanshinones (T02, T06, T08, T11, T12, T17, T20, T23 and T27) had an inhibitory effect on osteoclastogenesis. None of the compounds inhibited the fusion of monocytes into multinucleated osteoclast‐like cells up to concentrations of 5 μM (more than 10 times the concentration of IC50 values of bone resorption inhibition). The number of TRAcP‐positive osteoclasts was comparable with their untreated controls. However, at concentrations of >10 μM, some inhibition of osteoclastogenesis was observed (data not shown). Multinucleated osteoclasts formed in the presence or absence of tanshinones, and the quantification of TRAcP‐positive cells are shown in Figure 6A, B.
Figure 6.
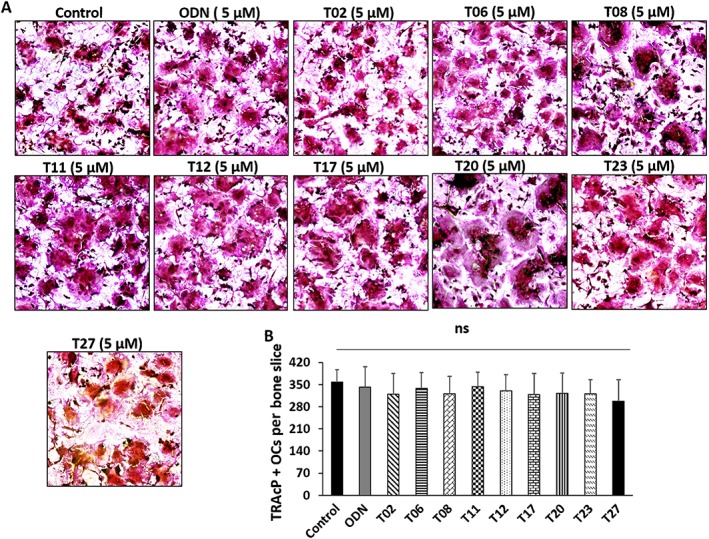
Inhibitory effects of tanshinones on osteoclastogenesis. (A) Effect of tanshinone inhibitors on osteoclastogenesis compared with the DMSO‐treated control group. Tanshinone inhibitors showed no effect on osteoclastogenesis up to 5 μM concentration. Mononuclear cells were seeded on bone slices in the presence of RANKL and M‐CSF and treated with individual tanshinones for 10 days and analysed by TRAcP staining. (B) TRAcP‐stained osteoclasts were counted by measuring the number of cells containing two or more nuclei in a single cell. The quantification data from five experiments (n = 5) are presented as means ± SD. ‘ns’, not significant.
Effect of tanshinones on reversibility of osteoclasts
We performed drug removal experiments to determine whether the antiresorptive activity of tanshinones on osteoclasts was reversible and quantified the trench surface and total eroded surface and also determined the effect on osteoclast numbers (Figure 7A–F). Representative images of eroded surface in the presence or absence of T12 are shown in Figure 7A. Trench formation was strongly suppressed when osteoclasts were cultured on bone slices for 4 days in the presence of T06, T12, T17, T20 and T23 (Figure 7B–F). When tanshinones were removed after 2 days, trench formation recurred and reached about 70% of the vehicle‐only treatment. Neither the addition nor the removal of T06 had any significant effect on osteoclast numbers (Figure 7B–F, right panel).
Figure 7.
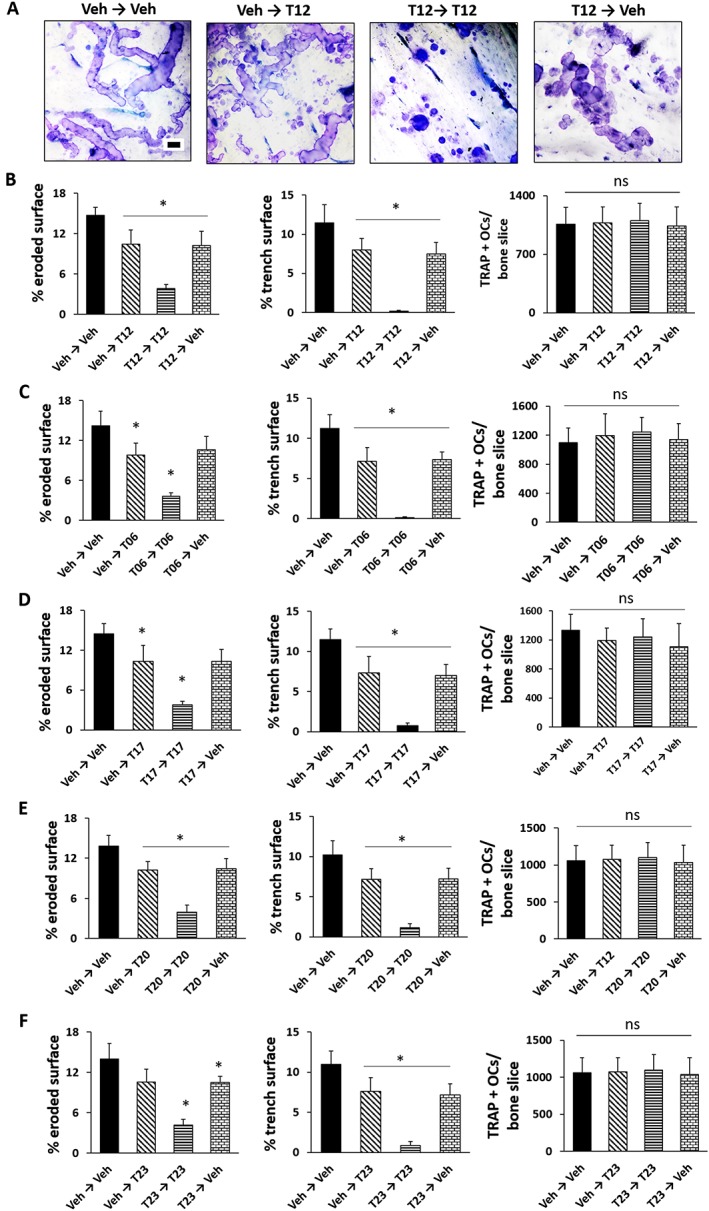
Effect of tanshinones on the reversibility of osteoclasts. (A) Representative images of resorption cavities generated by osteoclasts cultured on bone slices in the presence of T12 under different treatments received during phase I (2 day) and phase II (2 day) treatments. Effect of (B) T12, (C) T06, (D) T17, (E) T20 and (F) T23 on the % eroded surface per bone slice, % trench surface and number of osteoclasts (n = 5 independent experiments). Data represent mean ± SD. *P < 0.05 significantly different from untreated samples; ns, not significant Scale bar: 40 μm. Veh, vehicle.
Effect of tanshinones on fibroblast‐mediated TGF‐ß1 turnover
In our previous study, we have shown that the active site‐directed CatK inhibitor, odanacatib, blocked the degradation of TGF‐ß1 in fibroblasts, whereas the tanshinone DHT1 had no inhibitory effect. Here, we demonstrate that the tanshinones T17, T20 and T23 also did not inhibit the degradation of active TGF‐ß1 (Figure 8A, B). As expected, TGF‐ß1 degradation was blocked by an E64‐containing PIC. None of these tanshinones affected the metabolic activity of fibroblasts (data not shown).
Figure 8.
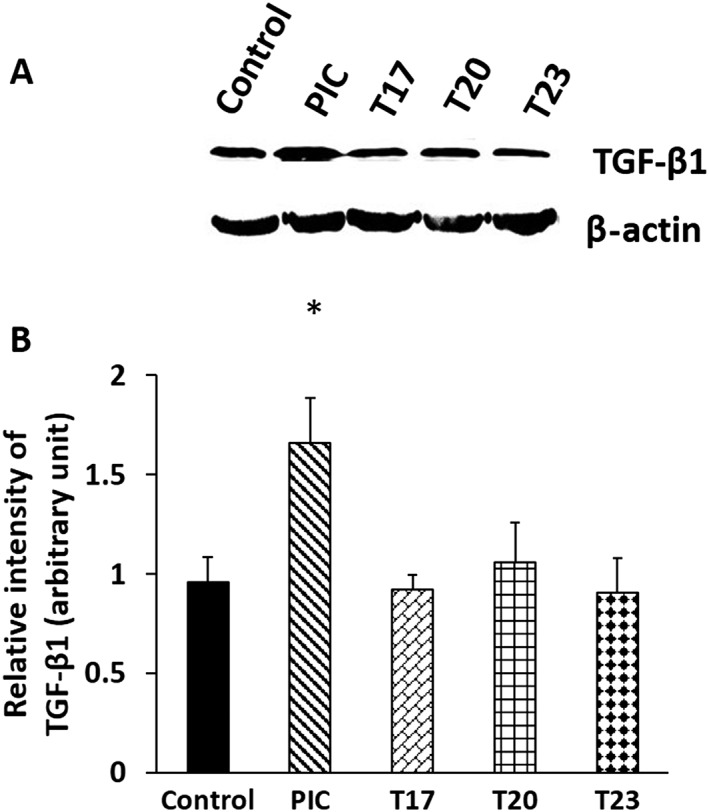
(A) Western blot and (B) quantification for TGF‐β1 protein from the fibroblast lysates of tanshinones T17, T20 and T23‐treated, E64‐containing PIC‐treated and untreated cells for 48 h. Data represent mean ± SD (n = 4 independent experiments). *P < 0.05 significantly different from untreated samples; ns, not significant.
Structural–activity relationship and molecular docking analysis
The activity results obtained with the 31 tanshinone analogues defined a picture of their structure–activity relationship in the following terms: first, the natural products occur either as para‐quinones (type 1) or ortho‐quinones, the latter being found either as structural type 2 or 3 (Scheme 1). Ortho‐quinones exhibited consistently better activity, one exception being the unusually potent para‐quinone T20. The latter, nonetheless, displays an OH group in place of one of the carbonyls of the ortho‐quinones, suggesting that the presence of oxygen at that position is important for activity. This conclusion is reinforced by the observation that the non‐quinoid compound T08 was also appreciably active.
Scheme 1.
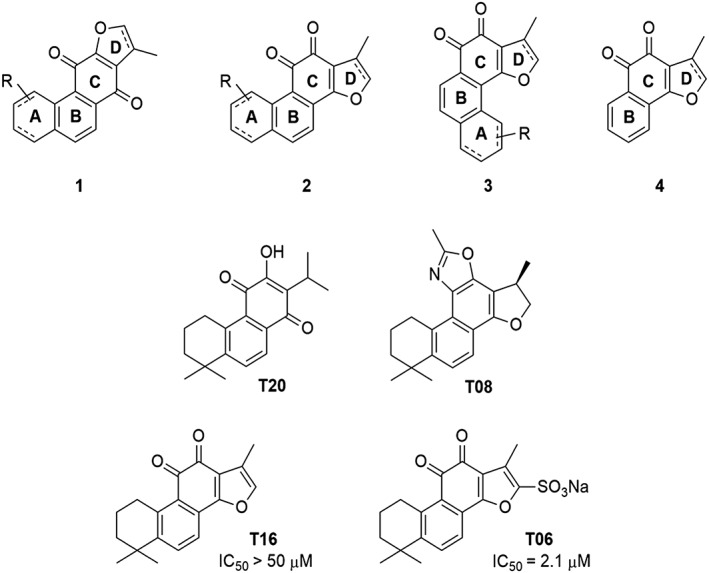
Tanshinone structures.
Rings A and D in structural types 1–3 (Scheme 1) may be aromatic or aliphatic (dashed bonds), suggesting that the degree of unsaturation of these cycles is not an important factor. The fact that a slightly better activity was found in ring D saturated tanshinones may only be a consequence of improved solubility. Moreover, the observation that structural types 2 and 3 showed comparable activity suggests that the orientation of ring A is immaterial. Therefore, the pharmacophore of tanshinones may be defined as structure 4 (Scheme 1), wherein the furan ring (D) may be saturated or unsaturated, and ring B may sustain additional substituents.
Tanshinones are generally rather lipophilic compounds, with calculated partition coefficients (LogP) ranging from 1.2 to 3.5 and polar surface areas between 28 and 120 Å2 (Supporting Information Table S2). Increasing aqueous solubility seems to have a favourable influence on activity and showed slight correlation with collagenase activities (Supporting Information Table S2). To illustrate, the best tanshinone analogue identified was compound T06, a sulfonylated derivative of T16. The IC50 of T06 is equal to 2.7 μM (Log P 1.6); however, the IC50 of T16 is greater than 50 μM (Log P 2.8).
In order to determine the putative binding of tanshinones to ectosteric site 1, we used a molecular modelling approach to provide insight into the interactions that the inhibitors may form with the enzyme. Figure 9A displays the docking of T23 into ectosteric site 1 using Autodock Vina. The zoomed‐in view shows the compound fits into the defined cleft of the binding site and reveals favourable interactions with the site, including hydrophobic stacking interactions with Tyr87 and hydrogen bonding interactions with Glu93 and Glu94.
Figure 9.
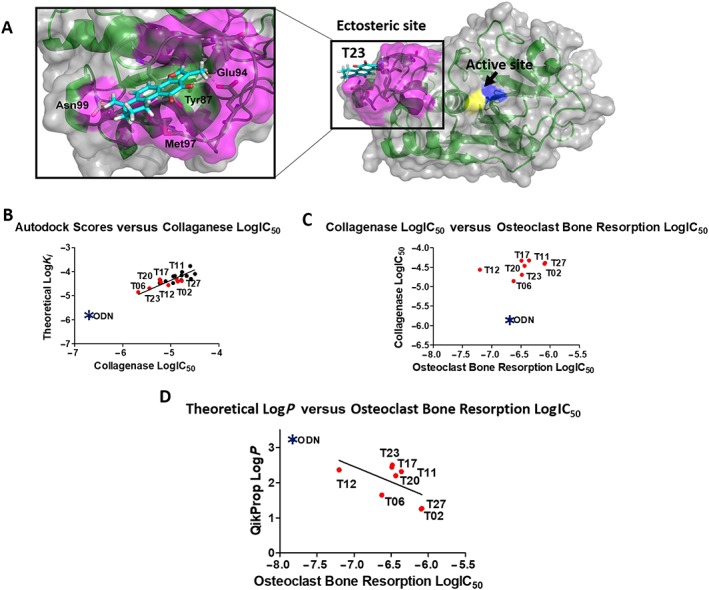
Structural similarity map of tanshinones: (A) the binding of T23 was modelled using Autodock Vina to ectosteric site 1 (coloured in pink), and the resulting pose with the highest affinity is shown with the entire CatK molecule (right panel). The active site residues Cys25 and His162 are coloured in yellow and blue respectively. The zoomed‐in view (left panel) shows the compound fits into the defined cleft of the binding site and shows favourable interactions with the site, including hydrophobic stacking interactions with Tyr87 and hydrogen bonding interactions with Glu93 and Glu94. (B) Plot of Autodock scores (LogK i) versus LogIC50 values for the inhibition of CatK collagenase activity. Tanshinones active in the osteoclast resorption assay are shown in red. Odanacatib (ODN) as positive reference is shown as a blue star. The theoretical K i values are calculated by Autodock from the best pose of each of the tanshinones. R 2 = 0.622. (C) Plot of LogIC50 for the collagenase inhibition versus the LogIC50 values for the osteoclast‐mediated bone resorption inhibition. Odanacatib served as positive control (blue star). R 2 = 0.182. (D) Plot of LogP versus the LogIC50 values for the osteoclast‐mediated bone resorption inhibition. Odanacatib served as positive control (blue star). R 2 = 0.337.
Furthermore, we calculated the binding affinities (theoretical K i) for the active 21 compounds, which ranged from 14 to 200 μM (Supporting Information Table S2). A linear regression analysis of the experimental IC50 values for the collagenase inhibition assays and the theoretical K i values indicated a correlation between both (Figure 9B). Odanacatib, a highly potent CatK active site‐directed inhibitor had a higher theoretical binding affinity than the tanshinones and was more potent in collagenase assays, falling in the expected range predicted by the linear regression.
However, since the IC50 values for the collagenase inhibition assays did not correlate with the appropriate IC50 values obtained in the osteoclast bone resorption assays (Figure 9C), we plotted the LogP values against later IC50 values. Here, a good correlation was observed, which also included the values for odanacatib (Figure 9D). Inhibitors that were less effective in the osteoclast assay (T02 and T27) had lower theoretical LogP values (1.2 and 1.3), whereas the more effective inhibitors (T11, T12, T17, T20 and T23) had LogP values ranging from 2.2 to 2.5. T06 was an exception and had a low LogP value of 1.6; however, its high potency in inhibiting the collagenase activity may have contributed to its effectiveness in the cell‐based assay as well. Odanacatib had a calculated LogP of 3.3 and was 20 times more potent (Panwar et al., 2016) than T06 in the cell‐based assays and also fell in the expected range predicted by the linear regression model.
Discussion
We have previously shown that the collagenase activity of CatK depends on CatK complexes formed in the presence of GAGs (Li et al., 2002; Li et al., 2008; Aguda et al., 2014). In the absence of GAGs, CatK exerts only a collagen telopeptide‐cleaving activity (Li et al., 2000; Li et al., 2002). All other non‐collagenolytic activities, including its gelatinase activity, do not require GAG‐dependent CatK complexes. Therefore, inhibitors that prevent CatK oligomerization without interfering with the active site could be an alternative approach to osteoporosis treatment with compounds targeting CatK, that avoids the side effects seen with active site‐directed inhibitors. This novel type of exosite inhibitors was coined ectosteric inhibitors to discriminate them from allosteric inhibitors. Allosteric inhibitors bind in an exosite, which leads to a modification of the active site. Thus, allosteric inhibitors would have similar problems as active site‐directed inhibitors as they are likely to affect all substrates of the target protease. We conducted a screening of several herbs used in traditional Chinese medicine for the treatment of skeletal and cardiovascular diseases and identified S. miltiorrhiza as a rich source of ectosteric inhibitors (Guo et al., 2014). The largest compound class synthesized by S. miltiorrhiza are diterpenoids, called tanshinones (Su et al., 2015). In our study, we evaluated 31 tanshinones, several of which showed remarkable inhibition of CatK‐mediated degradation of soluble and insoluble collagen. The most effective collagenase inhibitor was T06 (tanshinone IIA sulfonate) with IC50 values of 2.7 and 3.6 μM for soluble and fibre collagen. The IC50 values reflect molar CatK to inhibitor ratios of 1:6 and 1:3.6, respectively, and thus point to high potency and specificity. The theoretical limits for the IC50 value would be 0.2 and 0.5 μM, indicating 1:1 inhibitor protease complexes, which were obtained with the active site‐directed inhibitor, odanacatib (Panwar et al., 2016). IC50 values for several of the other tanshinones were in the range between 5 and 10 μM for soluble collagen (12‐fold to 25‐fold molar excess over CatK) and about twice higher towards collagen fibres. This indicates that most tanshinones bind at the same site, which is necessary for the hydrolysis of soluble and fibrillar collagens. However, several of tanshinones studied (T02, T03, T04, T12, T14 and T27) showed equal or even lower IC50 values for the inhibition of fibrillar collagen degradation, compared with the respective IC50 values for soluble collagen, which suggests different binding sites for the inhibition of the two collagen forms. We have unpublished results that suggest, in contrast to the dimeric CatK complex needed for fibrillar collagen degradation (Aguda et al., 2014), the degradation of soluble collagen is catalysed by an oligomeric complex (Li et al., 2008; Cherney et al., 2011). Here, different protein–protein and protein–GAG interactions sites are present.
The IC50 values of these tanshinones in osteoclast‐mediated bone resorption assays were between 60 and 800 nM and thus between 4 and 50 times less potent than odanacatib with an IC50 value of 14 nM (Panwar et al., 2016). However, the physical outcome of the inhibition of bone resorption was indistinguishable between the effects from tanshinones and odanacatib. Both reduced the formation of trenches, which reflects the collagen removal and cavity formation (Soe et al., 2013). The formation of demineralized pits is less affected by tanshinones and odanacatib, as the inhibition of CatK does not interfere with the demineralization step (Panwar et al., 2016). Tanshinones also protected the mechanical stability of type I collagen fibres. Fibres partly digested by CatK, lost their stability in the absence of inhibitors, whereas fibres in the presence of tanshinones mostly maintained their mechanical integrity. The partial loss is likely to be due to the proteolytic removal of collagen fibre‐associated proteoglycans by monomeric CatK. This is also reflected by the swelling of the otherwise intact fibres (Panwar et al., 2015). However, this is likely to be of little relevance in vivo, as collagen fibres in bone are embedded in the hydroxyl apatite matrix, which shields them against CatK‐mediated degradation when the overall bone resorption is blocked.
As observed with odanacatib, none of the antiresorptive tanshinones affected osteoclastogenesis and cell viability at concentrations of up to 5 μM. This is important as current osteoporosis treatment regimes reduce the number of osteoclasts either by apoptosis (bisphosphonates) or preventing osteoclast formation (denusomab) (Baron et al., 2011). Osteoclast deficiency disrupts osteoclast–osteoblast communication, which is required for the homeostasis of bone remodelling (Andersen et al., 2013). Inhibitors, which specifically inhibit osteoclastic bone resorption without affecting osteoclast viability, osteoclast formation and osteoclast–osteoblast crosstalk are thought to be more desirable, as they will interfere less with bone formation. However, at higher tanshinone concentrations, anti‐osteoclastogenesis effects were observed, as described previously for T05, T13 and T16 (Kim et al., 2004; Lee et al., 2005). Kim et al. (2004) reported the inhibition of osteoclast formation via the inhibition of the NF‐kB pathway at 30 μM in cellular assays. This is about 40–500 times higher than the CatK‐targeted antiresorptive activity of the most effective tanshinones evaluated in this report. T16 (tanshinone IIA), which is the most thoroughly investigated tanshinone derivative, had no CatK inhibitor activity at 50 μM.
Tanshinones active in the osteoclast bone resorption assay revealed full reversibility of their inhibitory activity. Osteoclast resorptive activity was fully restored 2 days after tanshinone removal. The same reversibility has been previously described for odanacatib (Zhuo et al., 2014). Drug reversibility is considered an advantage as this allows for drug removal in case of side effects. Adverse events such as jaw bone necrosis, which has been reported with bisphosphonates (Heng et al., 2012), cannot be easily stopped when the drug has a long‐lasting effect in bone. Additionally, no effect on the degradation of TGF‐ß1 in fibroblasts was observed after treatment with tanshinones T17, T20 and T23. We have previously demonstrated for T12 and T06, such a lack of inhibition of the TGF‐ß1 degradation mediated by CatK (Panwar et al., 2016; Panwar et al., 2017).
Extracts of salvia alone or in combination with other medicinal plant extracts have been evaluated in ovariectomized (OVX) rats and in several clinical trials and proved efficacious in reducing bone loss (Chae et al., 2004; Cui et al., 2011; Guo et al., 2014). Considering that at least 12 tanshinone compounds had a significant anti‐collagenase activity towards CatK (IC50 < 10 μM) and 6 showed inhibitory activities in osteoclast bone resorption assays below 500 nM, the in vivo efficacy of the herbal extract is likely to be due to the combined CatK inhibition by several tanshinones present in S. miltiorrhiza. We have recently corroborated the in vivo activity of tanshinones by demonstrating that orally administered T06 (40 mg·kg·−1·day−1) reduced bone loss in OVX mice, via inhibition of the collagenase activity of CatK (Panwar et al., 2017).
Structure‐activity studies of tanshinone derivatives provided information regarding similarity and dissimilarity among effective and non‐effective inhibitors. Tanshinones share several common structural features, the most prominent being a system of linked five‐membered or six‐membered rings, which are mostly conjugated. A common structural motif includes a scaffold of two conjugated aromatic rings and one cyclic hexanedione. However, changes in the substituent groups have a significant effect on the potency of these compounds as this appears to affect the binding of the compounds to the enzyme and compound solubility and membrane permeability. Based on the structural similarity map generated for the 31 tanshinones characterized, there are three major groups of structurally related compounds (Scheme 2). Compounds in group I have four rings with ring D as a dihydrofurane. Group II contains compounds with a methylfurane ring D. Finally, group III contains compounds with a slightly greater variety of structural elements but maintains the common tanshinone backbone. Compounds in groups I and II contain a higher proportion of active compounds. For compounds in groups I and II, we observe that changes in ring A have little effect on the potency of the compounds. Ring A can be aromatic, saturated or have different substituents. Whereas in group III, there is a higher proportion of inactive compounds, and structural similarities between compounds are not easily observed. The most potent compound observed, T06, tanshinone IIA sodium sulfonate, is unique among the tanshinones tested in that it contains a sulfonate group and is charged. The IC50 value for this compound is the lowest identified in all groups, possibly because of a better binding, which is due to the introduced charge. Based on molecular docking simulations, the sulfonate forms a hydrogen bond with Ser95 in ectosteric site 1 (Sharma et al., 2015). The in vitro cellular activity of the tested tanshinones correlated very well with the theoretic LogP values indicating solubility and membrane permeability (Supporting Information Table S2).
Scheme 2.
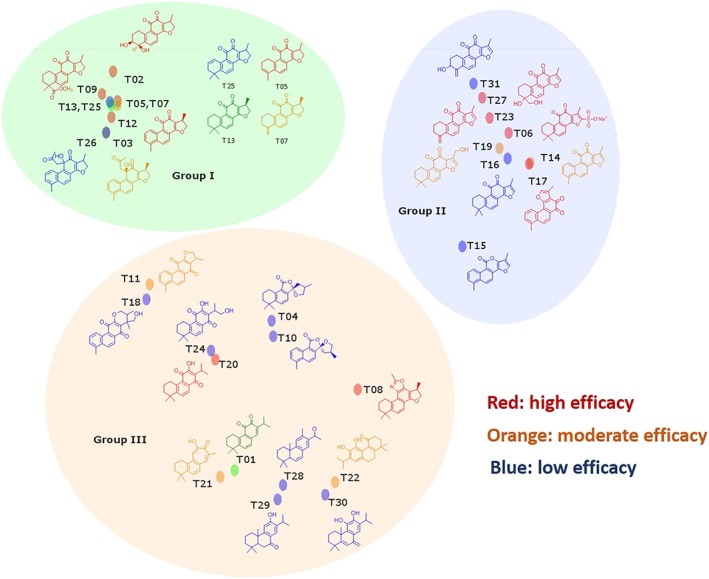
Grouping of tanshinones in three structurally related classes. Compounds are separated into three different groups with common structural motifs. Compounds showed inhibition for both soluble and insoluble collagen at low IC50 values are highlighted in red colour. Compounds with moderate effectiveness in inhibition of soluble and insoluble collagen are indicated in orange colour. Compounds with lowest inhibitory effect are shown in blue colour.
In conclusion, we characterized 31 tanshinone derivatives produced in S. miltiorrhiza. Twelve out of 31 compounds revealed a substantial anti‐collagenase activity, and six of the compounds were highly efficient in suppressing osteoclast‐mediated trench formation, an assay which best reflects the efficacy of antiresorptive drugs (Merrild et al., 2015). An SAR analysis revealed a minimal pharmacophore of three rings and provides a foundation for the design of more potent and bioavailable synthetic ectosteric inhibitors. Our results clearly suggest that the clinical efficacy of S. miltiorrhiza extracts in preventing bone resorption in osteoporosis patients (Guo et al., 2014) is based on the ectosteric activity of several tanshinones on the collagenase activity of CatK.
Author contributions
P.P. and D.B. designed the project; P.P. performed all the osteoclast assays and their evaluation, SEM and mechanical studies and enzymatic assays; S.L. performed the molecular docking experiments, enzymic assay and the evaluation of physical parameters of tanshinones; A.J. and P.A. contributed to the enzymic assays; D.Z. performed the initial screening of Chinese herbs; M.C. performed the SAR analysis; and P.P., S.L. and D.B. were responsible for the final draft.
Conflict of interest
The authors declare no conflicts of interest.
Declaration of transparency and scientific rigour
This Declaration acknowledges that this paper adheres to the principles for transparent reporting and scientific rigour of preclinical research recommended by funding agencies, publishers and other organisations engaged with supporting research.
Supporting information
Table S1 Mechanical properties of collagen fibres after CatK digestion in the presence and absence of tanshinones.
Table S2 Physicochemical data for selected tanshinones.
Table S3 Chemical information and commercial source of tanshinones.
Acknowledgements
This work was supported by grants from the Canadian Institutes of Health Research, MOP89974 and MOP201209 (D.B.) and the National Natural Science Foundation of China (NSFC81273995) (D.Z.). D.B. was supported by a Canada Research Chair award. We are grateful to the UBC Centre for High‐Throughput Phenogenomics facility for the use of the SEM.
Panwar, P. , Law, S. , Jamroz, A. , Azizi, P. , Zhang, D. , Ciufolini, M. , and Brömme, D. (2018) Tanshinones that selectively block the collagenase activity of cathepsin K provide a novel class of ectosteric antiresorptive agents for bone. British Journal of Pharmacology, 175: 902–923. doi: 10.1111/bph.14133.
References
- Aguda AH, Panwar P, Du X, Nguyen NT, Brayer GD, Bromme D (2014). Structural basis of collagen fiber degradation by cathepsin K. Proc Natl Acad Sci U S A 111: 17474–17479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Fabbro D, Kelly E, Marrion NV, Peters JA, Faccenda E et al (2017). The Concise Guide to PHARMACOLOGY 2017/18: Enzymes. Br J Pharmacol 174: S272–S359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen TL, Abdelgawad ME, Kristensen HB, Hauge EM, Rolighed L, Bollerslev J et al (2013). Understanding coupling between bone resorption and formation: are reversal cells the missing link? Am J Pathol 183: 235–246. [DOI] [PubMed] [Google Scholar]
- Baron R, Ferrari S, Russell RG (2011). Denosumab and bisphosphonates: different mechanisms of action and effects. Bone 48: 677–692. [DOI] [PubMed] [Google Scholar]
- Bromme D, Lecaille F (2009). Cathepsin K inhibitors for osteoporosis and potential off‐target effects. Expert Opin Investig Drugs 18: 585–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bromme D, Panwar P, Turan S (2016). Cathepsin K osteoporosis trials, pycnodysostosis and mouse deficiency models: commonalities and differences. Expert Opin Drug Discovery 11: 457–472. [DOI] [PubMed] [Google Scholar]
- Chae HJ, Chae SW, Yun DH, Keum KS, Yoo SK, Kim HR (2004). Prevention of bone loss in ovariectomized rats: the effect of Salvia miltiorrhiza extracts. Immunopharmacol Immunotoxicol 26: 135–144. [DOI] [PubMed] [Google Scholar]
- Chen JS, Sambrook PN (2011). Antiresorptive therapies for osteoporosis: a clinical overview. Nat Rev Endocrinol 8: 81–91. [DOI] [PubMed] [Google Scholar]
- Cherney MM, Lecaille F, Kienitz M, Nallaseth FS, Li Z, James MN et al (2011). Structure–activity analysis of cathepsin K/chondroitin 4‐sulfate interactions. J Biol Chem 286: 8988–8998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui Y, Bhandary B, Marahatta A, Lee GH, Li B, Kim DS et al (2011). Characterization of Salvia miltiorrhiza ethanol extract as an anti‐osteoporotic agent. BMC Complement Altern Med 11: 120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis MJ, Bond RA, Spina D, Ahluwalia A, Alexander SPA, Giembycz MA et al (2015). Experimental design and analysis and their reporting: new guidance for publication in BJP. Brit J Pharmacol 172: 3461–3471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drake MT, Clarke BL, Oursler MJ, Khosla S (2017). Cathepsin K inhibitors for osteoporosis: biology, potential clinical utility, and lessons learned. Endocr Rev 38: 325–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duong LT, Leung AT, Langdahl B (2016). Cathepsin K inhibition: a new mechanism for the treatment of osteoporosis. Calcif Tissue Int 98: 381–397. [DOI] [PubMed] [Google Scholar]
- Eastell R, Nagase S, Ohyama M, Small M, Sawyer J, Boonen S et al (2011). Safety and efficacy of the cathepsin K inhibitor ONO‐5334 in postmenopausal osteoporosis: the OCEAN study. J Bone Miner Res 26: 1303–1312. [DOI] [PubMed] [Google Scholar]
- Guo Y, Li Y, Xue L, Severino RP, Gao S, Niu J et al (2014). Salvia miltiorrhiza: an ancient Chinese herbal medicine as a source for anti‐osteoporotic drugs. J Ethnopharmacol 155: 1401–1416. [DOI] [PubMed] [Google Scholar]
- Harding SD, Sharman JL, Faccenda E, Southan C, Pawson AJ, Ireland S et al (2018). The IUPHAR/BPS Guide to PHARMACOLOGY in 2018: updates and expansion to encompass the new guide to IMMUNOPHARMACOLOGY. Nucl Acids Res 46: D1091–D1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heng C, Badner VM, Vakkas TG, Johnson R, Yeo Y (2012). Bisphosphonate‐related osteonecrosis of the jaw in patients with osteoporosis. Am Fam Physician 85: 1134–1141. [PubMed] [Google Scholar]
- Kim HH, Kim JH, Kwak HB, Huang H, Han SH, Ha H et al (2004). Inhibition of osteoclast differentiation and bone resorption by tanshinone IIA isolated from Salvia miltiorrhiza Bunge. Biochem Pharmacol 67: 1647–1656. [DOI] [PubMed] [Google Scholar]
- Lee SY, Choi DY, Woo ER (2005). Inhibition of osteoclast differentiation by tanshinones from the root of Salvia miltiorrhiza bunge. Arch Pharm Res 28: 909–913. [DOI] [PubMed] [Google Scholar]
- Li Z, Hou WS, Bromme D (2000). Collagenolytic activity of cathepsin K is specifically modulated by cartilage‐resident chondroitin sulfates. Biochemistry 39: 529–536. [DOI] [PubMed] [Google Scholar]
- Li Z, Hou WS, Escalante‐Torres CR, Gelb BD, Bromme D (2002). Collagenase activity of cathepsin K depends on complex formation with chondroitin sulfate. J Biol Chem 277: 28669–28676. [DOI] [PubMed] [Google Scholar]
- Li Z, Kienetz M, Cherney MM, James MN, Bromme D (2008). The crystal and molecular structures of a cathepsin K:chondroitin sulfate complex. J Mol Biol 383: 78–91. [DOI] [PubMed] [Google Scholar]
- Linnevers CJ, McGrath ME, Armstrong R, Mistry FR, Barnes M, Klaus JL et al (1997). Expression of human cathepsin K in Pichia pastoris and preliminary crystallographic studies of an inhibitor complex. Protein Sci 6: 919–921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merck (2014). Merck announces data from pivotal phase 3 fracture outcomes study for odanacatib, an investigational oral, once‐weekly treatment for osteoporosis [Online] Available from http://www.mercknewsroom.com/news‐release/research‐and‐development‐news/merck‐announces‐data‐pivotal‐phase‐3‐fracture‐outcomes‐st (last accessed 10 March 2016)
- Merrild DM, Pirapaharan DC, Andreasen CM, Kjaersgaard‐Andersen P, Moller AM, Ding M et al (2015). Pit‐ and trench‐forming osteoclasts: a distinction that matters. Bone Res 3: 15032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullard A (2016). Merck & Co. drops osteoporosis drug odanacatib. Nat Rev Drug Discov 15: 669. [DOI] [PubMed] [Google Scholar]
- Osborne CS, Reid WH, Grant MH (1999). Investigation into the biological stability of collagen/chondroitin‐6‐sulphate gels and their contraction by fibroblasts and keratinocytes: the effect of crosslinking agents and diamines. Biomaterials 20: 283–290. [DOI] [PubMed] [Google Scholar]
- Panwar P, Du X, Sharma V, Lamour G, Castro M, Li H et al (2013). Effects of cysteine proteases on the structural and mechanical properties of collagen fibers. J Biol Chem 288: 5940–5950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panwar P, Lamour G, Mackenzie NC, Yang H, Ko F, Li H et al (2015). Changes in structural–mechanical properties and degradability of collagen during aging‐associated modifications. J Biol Chem 290: 23291–23306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panwar P, Soe K, Guido RV, Bueno RV, Delaisse JM, Bromme D (2016). A novel approach to inhibit bone resorption: exosite inhibitors against cathepsin K. Br J Pharmacol 173: 396–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panwar P, Xue L, Soe K, Srivastava K, Law S, Delaisse JM et al (2017). An ectosteric inhibitor of cathepsin K inhibits bone resorption in ovariectomized mice. J Bone Miner Res 32: 2415–2430. [DOI] [PubMed] [Google Scholar]
- Runger TM, Adami S, Benhamou CL, Czerwinski E, Farrerons J, Kendler DL et al (2012). Morphea‐like skin reactions in patients treated with the cathepsin K inhibitor balicatib. J Am Acad Dermatol 66: e89–e96. [DOI] [PubMed] [Google Scholar]
- Sharma V, Panwar P, O'Donoghue AJ, Cui H, Guido RV, Craik CS et al (2015). Structural requirements for the collagenase and elastase activity of cathepsin K and its selective inhibition by an exosite inhibitor. Biochem J 465: 163–173. [DOI] [PubMed] [Google Scholar]
- Soe K, Delaisse JM (2010). Glucocorticoids maintain human osteoclasts in the active mode of their resorption cycle. J Bone Miner Res 25: 2184–2192. [DOI] [PubMed] [Google Scholar]
- Soe K, Merrild DM, Delaisse JM (2013). Steering the osteoclast through the demineralization‐collagenolysis balance. Bone 56: 191–198. [DOI] [PubMed] [Google Scholar]
- Su CY, Ming QL, Rahman K, Han T, Qin LP (2015). Salvia miltiorrhiza: traditional medicinal uses, chemistry, and pharmacology. Chin J Nat Med 13: 163–182. [DOI] [PubMed] [Google Scholar]
- Wang BQ (2010). Salvia miltiorrhiza: chemical and pharmacological review of a medicinal plant. J Med Plant Res 4: 2813–2820. [Google Scholar]
- Wang L, Ma R, Liu C, Liu H, Zhu R, Guo S et al (2017). Salvia miltiorrhiza: a potential red light to the development of cardiovascular diseases. Curr Pharm Des 23: 1077–1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitfield JF (2006). Osteoporosis‐treating parathyroid hormone peptides: what are they? What do they do? How might they do it? Curr Opin Investig Drugs 7: 349–359. [PubMed] [Google Scholar]
- Yamashita DS, Dodds RA (2000). Cathepsin K and the design of inhibitors of cathepsin K. Curr Pharm Des 6: 1–24. [DOI] [PubMed] [Google Scholar]
- Zhuo Y, Gauthier JY, Black WC, Percival MD, Duong LT (2014). Inhibition of bone resorption by the cathepsin K inhibitor odanacatib is fully reversible. Bone 67: 269–280. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 Mechanical properties of collagen fibres after CatK digestion in the presence and absence of tanshinones.
Table S2 Physicochemical data for selected tanshinones.
Table S3 Chemical information and commercial source of tanshinones.