Abstract
Purpose of Review
With an increasing rate of adolescent elbow injuries, especially in throwing athletes, the purpose of this review is to investigate the current literature regarding the diagnosis, treatment, and non-operative and operative outcomes of medial epicondyle fractures, ulnar collateral ligament repair, osteochondritis dissecans of the elbow, and olecranon stress fractures.
Recent Findings
Acceptable outcomes with both non-operative and operative treatments of medial epicondyle fractures have been reported, with surgical indications continuing to evolve. Unstable osteochondritis dissecans lesions, especially in patients with closed growth plates, require operative fixation, and emerging open and arthroscopic techniques including lesion debridement, marrow stimulation, autograft transfer, and allograft transplantation are described with good outcomes. Ulnar collateral repair has emerged as an exciting treatment option for an avulsion of either end of the ligament in young throwing athletes, with faster rehabilitation times than traditional ulnar collateral ligament reconstruction. Olecranon stress fractures are increasing in prevalence, and when a non-operative treatment course is unsuccessful, athletes have a high return-to-play rate after percutaneous cannulated screw placement.
Summary
With proper indications, non-operative and operative treatment modalities are reported with a high return-to-play and acceptable clinical outcomes for common elbow injuries, including medial epicondyle fractures, ulnar collateral ligament repair, osteochondritis dissecans of the elbow, and olecranon stress fractures, in adolescent throwing athletes. Further research is needed to better define treatment algorithms, surgical indications, and outcomes.
Keywords: Medial epicondyle fracture, Ulnar collateral ligament repair, Elbow osteochondritis dissecans, Olecranon stress fractures, Persistent olecranon physis, Adolescent elbow injuries
Medial Epicondyle Fractures
Introduction
Medial epicondyle fractures account for 10–20% of all pediatric elbow fractures [1]. They most commonly occur in boys 9 to 14 years of age. The medial epicondyle ossifies at age 3 years in girls and 5 years in boys and then fuses to the humerus at age 13 years in girls and 15 years in boys [2]. The ulnar collateral ligament and flexor-pronator mass attach to the medial epicondyle, acting to resist valgus forces on the elbow. Multiple mechanisms of injury have been described including elbow dislocation, valgus stress avulsion, and direct blow.
Assessing displacement of medial epicondyle fractures requires an understanding of normal anatomy (Fig. 1). Due to the variable age at fusion in upper extremity athletes, contralateral elbow radiographs and physical exam are important to distinguish anatomic variation from an acute injury. Typically, fragment displacement is distal and anterior, so an isolated AP radiograph may underestimate true displacement. Maximum displacement of the fracture should be determined using a combination of AP, lateral, and 45° internal rotation oblique radiographs [3]. Computed tomography (CT) and magnetic resonance imaging is typically not helpful in assessing these injuries.
Fig. 1.
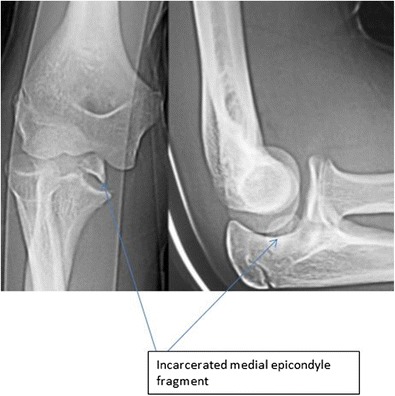
Medial epicondyle fracture that is incarcerated following closed reduction of an elbow dislocation
Treatment Indications
Non-operative treatment is recommended for non-displaced or minimally displaced medial epicondyle fractures. Absolute indications for operative intervention include an incarcerated fracture fragment, open fracture, and ulnar nerve entrapment [4]. Relative indications include elbow instability following a dislocation, significant displacement, and patients that participate in upper extremity sports, including gymnastics, baseball, softball, and volleyball.
There is no consensus in the literature to define significant displacement, and recommendations have ranged from 2 to 10 mm [1]. Obtaining a complete injury history is important to determine whether the injury involved a subluxation or dislocation of the elbow with spontaneous reduction. In these cases, the displacement of the epicondyle may be minimal, but significant laxity of the elbow may exist, making operative intervention prudent.
The theoretical concern with non-operative treatment of medial epicondyle fractures is symptomatic non-union. Non-union has been reported to occur in up to 90% of displaced medial epicondyle fractures, a rate which is reduced to 7% in operatively treated patients [5]. Additionally, in elbow dislocations with associated medial epicondyle fractures, non-operative management requires longer immobilization, which risks arthrofibrosis.
Surgical Technique
Open reduction and internal fixation (ORIF) with a screw is the standard operative treatment of displaced fractures. The patient is placed supine, an arm table is used, and a medial approach is made to the elbow. Branches of the medial antebrachial cutaneous and ulnar nerves are identified and protected. Neurolysis and transposition is rarely indicated. In acute settings, the zone of injury is readily identified by the presence of a large hematoma as well as capsular disruption if a dislocation occurred. The fracture is debrided and reduced under direct visualization. Reduction is assisted by placing the elbow in 30°–60° of flexion to relax the flexor-pronator muscles. A temporary traction stitch may be placed just distal to the fragment in the flexor-pronator tendon to aid reduction and improve access to the small fragment for placement of the guide pin. In adolescents, debridement of the physeal remnant, primarily on the medial epicondyle fragment, encourages physeal closure. For fixation, the author prefers to use a fully threaded 4.0-mm cannulated screw (Fig. 2). The medial epicondyle fragment is slightly over drilled to allow for compression at the fracture site. A fully threaded screw allows excellent purchase along the anterior aspect of the distal humerus and also allows for easy removal without concern for bony overgrowth of the non-threaded portion of a partially threaded screw. For small or fragmented fractures, the use of a washer may be considered, though evidence suggests this may increase screw prominence and pain [6].
Fig. 2.
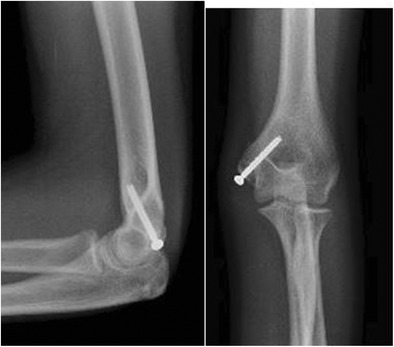
Fixation of a medial epicondyle fracture with a fully threaded screw
Following fracture fixation, the ulnar nerve should be examined to ensure that it is not subluxating with elbow motion. Valgus elbow stability should also be assessed, although concomitant ulnar collateral ligament injury is rare in these apophyseal fractures. The elbow is immobilized with a splint for 5–7 days, and range of motion is started immediately following splint removal. Valgus moments and resisted finger and wrist flexion and pronation should be avoided to protect the repair.
Outcomes
Outcomes in the treatment of medial epicondyle fractures in children are generally good with both operative and non-operative treatments but are limited to small retrospective series [7, 8•, 9–11]. Historically, non-operative management of medial epicondyle fractures with 35-year follow-up resulted in non-union in more than half of patients, yet all reported good long-term outcomes [5]. However, contemporary patient reported outcome measures were not utilized, which may create a ceiling effect that would fail to detect loss of function in athletes who place high demands on their elbows.
Increasing concern for symptomatic valgus instability and arthrofibrosis has led surgeons to consider operative intervention for displaced fractures. Computer simulations of medial epicondyle fractures suggest that for each 1 mm of anterior fragment displacement, a 2% reduction in wrist flexion strength occurs; yet, the clinical significance of this is unknown [12].
Two recent retrospective studies have compared outcomes of non-operative and operative treatment of medial epicondyle fractures. The first series assessed 2-year outcomes in a select cohort of 20 upper extremity athletes with mean initial fracture displacement of 5.3 mm in the non-operative group and 7.6 mm in the operative group [13]. The authors reported union in all patients. The most common complication was loss of motion, occurring in approximately 30% of each group. Ulnar nerve symptoms were noted in 43% of operatively treated patients and 16% of non-operatively treated patients. All patients achieved excellent DASH scores and returned to sport at the same or higher level. The second series included 31 medial epicondyle fractures with 2-year follow-up and found no difference in elbow outcome scores based on treatment. Radiographic valgus instability was noted in two patients with non-operatively treated displaced fractures.
Complications
Symptomatic non-union is the ultimate complication that has influenced the trend toward operative fixation (Fig. 3). Symptoms include medial elbow pain and prominence, valgus instability, and ulnar nerve paresthesias. In the setting of symptomatic non-union, ORIF has been reported to give excellent clinical and functional outcomes in the majority of patients [14•, 15–17]. Acute ulnar nerve palsies may be seen in the setting of an elbow fracture dislocation or as a result of entrapment of the nerve with a displaced fracture. Additionally, ulnar nerve subluxation may occur postoperatively, reinforcing the importance of assessing this after fixation and prior to closure. Additional risks associated with operative treatment include infection and painful hardware. Hardware removal is reported to be highly variable, occurring in 0–100% of patients based on surgeon preference [14•, 11, 17].
Fig. 3.
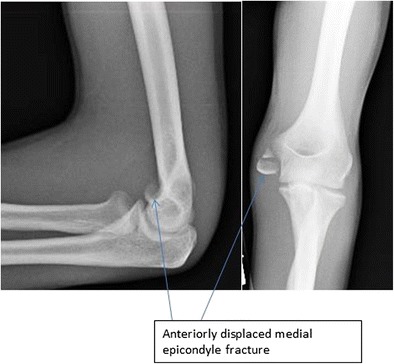
Anterior displacement of the medial epicondyle fracture fragment resulting from the pull of the flexor-pronator mass
Conclusion
Medial epicondyle fractures are a common elbow fracture in children and adolescents. Unlike many areas in orthopedics, there is not a clear treatment algorithm for these fractures, and this represents an area of opportunity for prospective, randomized studies. The surgeon must consider each patient’s injury mechanism, fracture displacement, associated injuries, and sports activities prior to electing a treatment course. Good outcomes can be expected with non-operative and operative treatment of these fractures, with decreased rates of non-union with operative treatment.
Osteochondritis Dissecans of the Elbow
Introduction
Osteochondritis dissecans (OCD) is a pathologic condition affecting subchondral bone and articular cartilage. The true etiology of OCD lesions in the capitellum is unknown but is likely multifactorial secondary to repetitive microtrauma, altered biomechanics, and ischemia from the limited vascularity. Additionally, ossification abnormalities and endocrine and genetic predisposition have also been suggested [18].
Humeral capitellar OCD occurs in the second decade of life primarily between 10 and 15 years old. It has been reported to occur in roughly 4 out of every 1000 males [19]. A large percentage of cases involve Little League pitchers, and at the time of presentation, many adolescents have already undergone physeal closure [20]. Kida et al. evaluated 2433 baseball players with a mean age of 14 years old involved in junior high and high school baseball teams. They found the prevalence of capitellar OCD lesions to be 3.4% among adolescent baseball players. Interestingly, unlike other throwing injuries, the player’s baseball position was not strongly related to the development of an OCD lesion [21•].
Classification
Various classifications exist to help standardize and define treatment algorithms. Early classification systems were primarily based upon the AP elbow X-ray. However, Kijowski and De Smet demonstrated that routine elbow X-rays are often normal or show only subtle changes in early stages of OCD of the capitellum [22]. Kajiyama et al. [23] compared OCD lesions in baseball players and gymnasts and determined that due to the differential pattern of applied stress, capitellar OCD lesions in baseball players were located more anteriorly and AP radiographs with the elbow in 45° of flexion are better for detecting OCD lesions in that group (Fig. 4) [23].
Fig. 4.
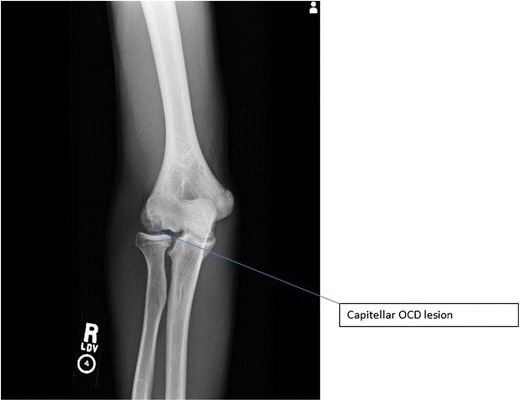
AP radiograph of capitellar OCD lesion
The International Cartilage Repair Society (ICRS) general classification system for OCD lesions is based on arthroscopic findings [24]. In this system, grade 1 lesions demonstrate a continuous softened area with intact cartilage, grade 2 lesions have a partial discontinuity which is stable when probed, grade 3 lesions have complete discontinuity that is not yet dislocated, and grade 4 lesions have a defect with a dislocated or loose fragment (Table 1).
Table 1.
ICRS classification of OCD lesions
| Grade 1 | Continuous softened area with intact cartilage |
| Grade 2 | Partial discontinuity which is stable when probed |
| Grade 3 | Complete discontinuity that is not yet dislocated |
| Grade 4 | Defect with a dislocated or loose fragment |
Brittberg and Winalski [24]
Itsubo et al. [25] introduced a capitellar magnetic resonance imaging (MRI) staging system (Fig. 5) in an attempt to estimate stability. The authors found that preoperative MRI grading correctly matched ICRS classification in 94% of patients and felt their MRI staging system provided reliable evidence for estimating ICRS classification and bony fragment instability [25] (Table 2).
Fig. 5.
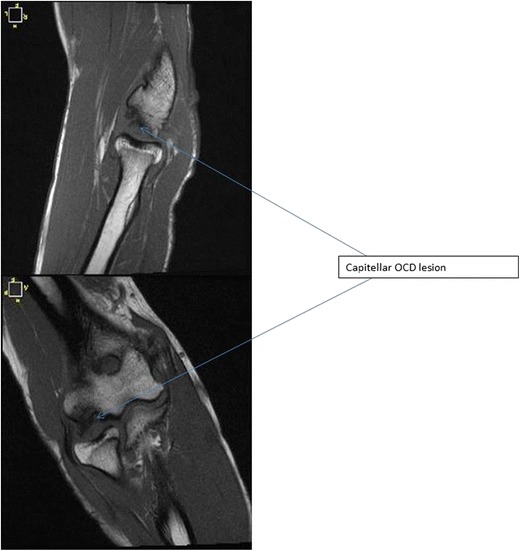
T1-weighted coronal and sagittal MRI images of capitellar OCD lesion
Table 2.
Capitellar MRI staging to estimate stability
| Stage | Characteristics | Stability |
|---|---|---|
| 1 | Capitellum is normally shaped with several spotted areas of high signal intensity lower than that of cartilage | Stable |
| 2 | Several spotted areas of higher intensity than that of cartilage | Stable |
| 3 | Discontinuity and non-circularity of the chondral surface signal of the capitellum and no high signal interface apparent between the lesion and the floor | Unstable |
| 4 | Lesion separated by a high intensity line in comparison with cartilage | Unstable |
| 5 | Capitellar lesion displaced from the floor or defect of the capitellar lesion noted | Unstable |
Itsubo et al. [25]
Prompt recognition of OCD lesions in the earlier stages may prevent the loss of the native articular surface. Since surgical intervention may not be protective against degenerative changes [20], quality imaging studies are needed to differentiate between stable and unstable OCD lesions. Stable lesions can be managed successfully with non-operative measures including rest and activity modification. Factors associated with a good prognosis include early lesions, patients with open capitellar growth plates [26], and localized subchondral bone flattening without fragmentation [27, 28]. However, healing with non-operative management becomes very unlikely in those with closed growth plates and lesion instability even without displacement [26, 28, 27, 29].
Surgical Management
Operative management is most commonly arthroscopic. For unstable fragmented lesions, this typically includes debridement of the lesion, removal of loose bodies, and concomitant marrow stimulation with abrasion chondroplasty or microfracture [30•, 31–35]. Alternatively, several open and arthroscopic techniques exist for lesion repair with fragment fixation as well as restorative procedures such as osteochondral autografting and allografting and cellular-induced cartilage scaffolds [36–42].
Arthroscopic Management
Arthroscopic management of OCD lesions offers the advantages of direct visualization, smaller incisions, decreased adhesions, and earlier rehabilitation [43]. A standard 30° offset scope allows for adequate viewing. O’Driscoll and Morrey described the lateral radiocapitellar portal [44] for the management of capitellar OCD lesions, although others have proposed the use of a distal ulnar portal for improved exposure to the posterolateral capitellum providing access for drilling, burring, and local debridement [43]. Utilizing a shaver or curettes, all unstable cartilage and necrotic bone is removed. Bleeding channels can be created in the subchondral bone by drilling or microfracture awl.
When the OCD lesion has an intact cartilage cap, drilling can stimulate a healing response. Although drilling from both inside the joint and outside has been described, it is important not to disrupt the cartilage cap. Drilling can be accomplished with the use of a 0.062 Kirschner wire, making multiple perforations in the subchondral plate while minimizing trauma to the articular surface by redirecting the drill through the same perforation. When using the outside in technique, fluoroscopy is used to identify the lesion and an ACL guide can be useful for targeting the lesion.
When the OCD lesion is intact but unstable, internal fixation has been found to be successful for larger osteochondral fragments [45]. After identification of the lesion arthroscopically, the fragment is gently elevated and the bed is prepared by removing sclerotic bone and fibrous tissue which can be enhanced with cancellous bone graft. Many fixation techniques have been described, including metal and bioscrews, either headed or headless variable-pitched compression screws [46–49].
When the OCD fragment is not salvageable, autograft osteochondral plugs can be taken from the knee [50–52] or harvested from either the fifth or sixth costal-osteochondral junction and implanted [41, 53, 54]. Most surgeons who perform capitellar OATS more commonly utilize the knee as a source for donor graft due to the familiarity of the anatomy and avoidance of complications associated with iatrogenic injury to the costal pleura. Several commercially available osteochondral autograft transfer systems can be used for donor site preparation and graft harvest. Donor plugs from the knee can be harvested from the lateral trochlear ridge, although the inferior medial trochlear ridge can be utilized and may offer the added benefit of improved congruity [55].
Surgical Outcomes
Several studies have reported surgical outcomes for capitellar OCD lesions. Most data would support good to excellent outcomes with debridement and marrow stimulation procedures with 80–90% returning to preinjury level of play [35, 32, 56]. However, results appear to deteriorate in larger lesions with advanced stage. Ueda et al. reported on mid- to long-term outcomes following arthroscopic fragment excision in adolescent athletes and found that with larger lesions, overall outcomes were acceptable, although motion and patient satisfaction were inferior to those with small lesions [57]. Similarly, Bexkens et al. demonstrated a much lower return-to-play rate of 62% in those with advanced stage [58]. Westerman et al. performed a systematic review and meta-analysis on return to sport after operative management of OCD lesions [59]. They evaluated 24 studies reporting outcomes in 492 patients. They found that the overall return-to-sport rate was 86% at a mean 5.6 months. Furthermore, osteochondral autografting allowed for a much higher return-to-play rate at 94%, compared with debridement and marrow stimulation (71%) or OCD fixation (64%). Although much research has surrounded cell-based therapies and biologic scaffolds for cartilage regeneration [60], routine use in the elbow has yet to be supported in the literature.
Ulnar Collateral Ligament Repair
Introduction
Although the medial ulnar collateral ligament (MUCL) does not play an important role in functions of activities of daily living, its stabilizing effect against valgus instability is critical in many sports especially those involving throwing [61]. Rupture of this ligament historically represented a devastating, career-ending injury for the throwing athletes until Dr. Jobe described the MUCL reconstruction surgery in 1986 utilizing palmaris autograft which he passed through bone tunnels on the medial aspect of the elbow joint [62]. Previous attempts at MUCL repair had been unreliable without a high rate of return to sport [63]. Variations of this technique continue to represent the gold standard for the management of MUCL tears in the throwing athlete today [64]. An epidemic of MUCL tears has occurred over the past couple of decades due to increased volume of play by youth baseball players, and MUCL reconstruction has been utilized to return college, high school, and even junior high school players back to sport [65]. Despite its excellent track record for return to sport, MUCL reconstruction has some significant drawbacks including morbidity associated with graft harvesting, prolonged postoperative rehabilitation program often lasting more than a year, and most recently, a rising failure rate requiring revision [66]. Recently, several investigators have revisited the issue of attempted MUCL repair in younger athletes with the premise that these players often have less damage to their ligament than older athletes and may be good candidates for repair rather than reconstruction [67]. This section will explore the current state of MUCL repair including indications, technique, early results, and future direction of this promising technique.
Indications for Surgery
This procedure is currently indicated in young athletes with symptomatic instability of the elbow who have failed a course of non-operative management and have physical exam findings and radiographic evidence (stress X-rays and MR arthrogram) of valgus instability of the elbow due to MUCL deficiency [67]. Results have not been reported on professional athletes in recent series. Currently, this technique is recommended in players who demonstrate rupture of the ligament from the proximal or distal attachment or both but is contraindicated in players with broad ligament damage. Proponents of this technique report that they still perform over twice as many reconstructions as they do repairs for MUCL deficiency reflective of the population that fulfills the above-stated criteria [67].
Repair Techniques
The patient is positioned supine with the affected arm on a hand table. In cases in which arthroscopy is indicated prior to ligament repair, the patient is placed prone and the arm is then placed in an internally rotated position parallel to the trunk to access the medial elbow [67]. A 5-mm curvilinear incision beginning at the medial epicondyle and passing distally is then made. The ligament is approached via a flexor-pronator muscle splitting approach or a muscle elevating approach through the bed of the ulnar nerve depending on surgeon preference. The ligament is evaluated for injury proximally and distally. If no injury is apparent, the ligament is split in the direction of its fibers for evaluation of the deep surface. In cases of direct repair without augmentation, the bone in the area of ligament damage proximally or distally is lightly debrided and rasped. The anchor is placed and sutures are placed in the intact portion of the ligament (Fig. 6). For proximal avulsions, the anchor is placed at the apex of the “V” formed by the junction of the trochlea and the medial epicondyle. For distal avulsions, the anchor is placed directly into the sublime tubercle. Protection of the ulnar nerve is critical during repair.
Fig. 6.
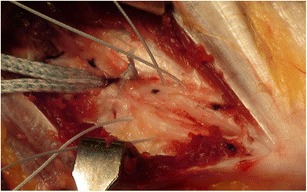
The split ulnar collateral ligament is seen with sutures in place for repair and the fiber tape visible distally for later bracing. Courtesy of Dr. Christopher Ahmad
Recently, a technique utilizing a collagen-coated fiber tape augmentation to serve as an internal brace for the healing ligament has been described. Although clinical results of internal bracing are not currently available, it is a promising technique. A biomechanical study comparing this augmented repair technique to a typical modified Jobe technique of reconstruction revealed greater resistance to gap formation in the repair group and comparable results in all other parameters including maximum torque at failure [68•]. Two suture anchors with fiber tape as well as a high-tensile suture are used to repair the native ligament to bone and brace the ligament while it is healing. The suture anchor is placed at the location of injury first. The high-tensile suture is used to repair the ligament in the area of damage. The isometric point on the opposite bone is identified, and the second suture anchor is placed, tightening the fiber tape appropriately (Fig. 7).
Fig. 7.
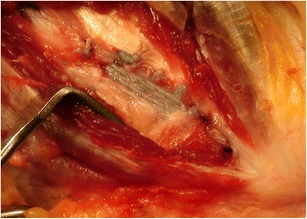
Final repair with sutures tied and brace tensioned. Courtesy of Dr. Christopher Ahmad
Postoperative Care
The elbow is immobilized for 1 week only, and range of motion is then begun in a double-hinged brace from 30° to full flexion. Strengthening of the core, shoulder, wrist, and forearm is introduced at 2–4 weeks. Full range of motion is allowed in the brace at 6 weeks along with progressive activity-specific exercises. Throwing and hitting can be introduced within 8–12 weeks depending on strength. The brace is discontinued at 12 weeks with return to sport between 16 and 24 weeks.
Outcomes
Earlier comparison series favored MUCL reconstruction to repair [63]. In the largest and most recent individual series, Savoie and co-investigators reported that 56/60 (93%) of players returned to the same or higher level of sport mostly within 6 months with 93% of patients achieving an excellent or good result according to the Andrews-Carson score [67]. There were six complications, two of which required return to the operating room all occurring in athletes who were able to return to sport. A recent systematic review identified three clinical series with a total of 92 patients undergoing MUCL repair including this and older series and reported similar results [64, 69, 63, 67].
Future Direction
The universally accepted priority in MUCL injuries in young athletes is prevention. Within this realm of repair, the ideal patient population must be identified, the benefits of bracing over primary repair clarified, and the challenges of revision surgery realized.
Conclusions
Medial ulnar collateral ligament repair is a viable option in the treatment of young players who have an otherwise healthy elbow with ligament damage limited to either end. It offers the advantages of less initial morbidity, faster return to sport, and potentially easier revision surgery in the case of subsequent failure. The role of this procedure in the treatment of professional athletes will require further investigation.
Olecranon Stress Fractures
Introduction
Olecranon stress fractures and symptomatic, persistent olecranon physeal injuries of the elbow, though relatively rare, have become increasingly common in adolescent throwing athletes [70]. These repetitive throwing injuries represent the combined sequelae of olecranon impingement with valgus extension overload and excessive pull of the triceps muscle during the acceleration phase of throwing [71]. Throwing athletes with pain related to an olecranon stress fracture, or symptomatic persistent olecranon physis, have difficulty performing at their highest level due to pain, a prolonged period of healing, and a high likelihood of recurrence. The diagnosis is confirmed with clinical examination of the throwing athlete for olecranon tenderness, painful resisted elbow extension, and loss of range of motion; combined with plain radiographs and advanced imaging, either with the use of MRI or CT scans [72•]. A persistent olecranon physis is diagnosed radiographically as that which occurs after the typical fusion age of 12 to 15 years or as a throwing arm olecranon physis that persists in the presence of a confirmed closed contralateral olecranon physis [73] (Fig. 8). MRI scans are useful in identifying bone marrow edema patterns in olecranon stress fractures, while both MRI and CT scans provide further fracture characterization and localization.
Fig. 8.
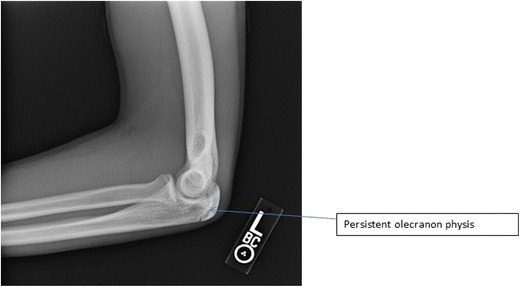
Lateral X-ray image demonstrating a stage 2 persistent olecranon physis
Radiographic Staging
Radiographic staging has been demonstrated as valuable for treatment decision-making for the persistent symptomatic olecranon physis. Matsuura et al. described staging of these lesions. Stage 1 involves simple widening of the olecranon physis relative to the contralateral side. Stage 2 lesions are characterized as having sclerotic edges of the physis, indicating chronicity and a lack of radiographic healing. The authors’ staging correlated with successful conservative treatment, as 91.7% of patients with a stage 1 lesion healed with conservative modalities and those with stage 2 lesions all required eventual surgical treatment [74]. Frank et al., in 2017, described two unique radiographic patterns of physeal non-union, distal and proximal, in patients requiring operative treatment. Distal non-unions feature sclerotic lucency at the olecranon physis, while a proximal persistent olecranon physis was identified by radiolucency proximal to the triceps insertion where an accessory ossification center did not close. No difference in union rates after surgery was noted when comparing the two unique types [75•]. Advanced imaging is often attained in addition to plain radiographs for detailed fracture characterization (Fig. 9). An MR arthrogram may be performed if concurrent ulnar collateral ligament pathology or additional intra-articular pathology is suspected on clinical examination.
Fig. 9.
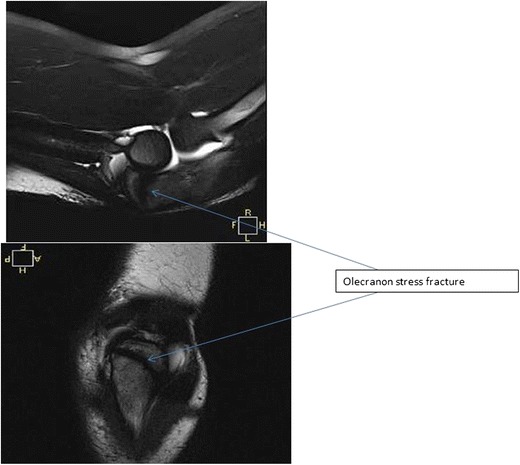
Axial and sagittal T2 MRI images of an olecranon stress fracture
Surgical Indications
Both surgical and non-surgical treatment modalities have been described for the treatment of olecranon stress fractures and persistent symptomatic olecranon physeal injuries of the elbow in the literature. With early recognition after the onset of symptoms, non-operative treatment with rest, rehabilitation, bracing, and correction of throwing technique are favored and often successful [76]. Schickendantz et al. reported successful non-operative treatment of olecranon stress fractures in a series of seven throwers with a symptom duration of 3 months or less. The authors reported a 12 to 14-week rehabilitation program that involved avoidance of throwing, two initial weeks of bracing 20° short of extension, progressive resistance exercises at 2 to 6 weeks, and a sport-specific rehabilitation program at 6 weeks, with a 4 to 6-week progressive throwing program initiated at an average of 8 weeks. All players returned to throwing, and six of seven returned to their prior level of play [77]. With a failure of a comprehensive non-operative treatment program for 3 months, including failure of an attempted progressive throwing program, surgical treatment is warranted [72•].
Techniques
Multiple surgical techniques have been described for treatment of olecranon stress fractures. These include open reduction and internal fixation with tension band wiring, bone grafting, cannulated screw placement, and cannulated screw placement with adjunctive tension band wiring. Though cannulated screw fixation and tension band wiring have demonstrated equivalent radiographic and clinical outcomes in skeletally immature patients with displaced traumatic olecranon fractures, there has been no direct comparison of techniques in those patients with olecranon stress fractures and persistent symptomatic olecranon physeal injuries [78]. Recent literature has focused on treatment with percutaneous cannulated screws placed under radiographic guidance. Cannulated screws are placed perpendicular to the fracture orientation in both the coronal and sagittal planes, paying careful attention to preoperative imaging, with a goal of providing balanced compression across the fracture site (Fig. 10). Postoperative rehabilitation includes posterior splint placement at 90° of flexion for 7 days followed by immediate extension motion and avoidance of flexion past 90° for 6 weeks. Full range of motion with initiation of strengthening occurs at 8 weeks. An interval throwing program is initiated at 14 weeks [71, 72•].
Fig. 10.
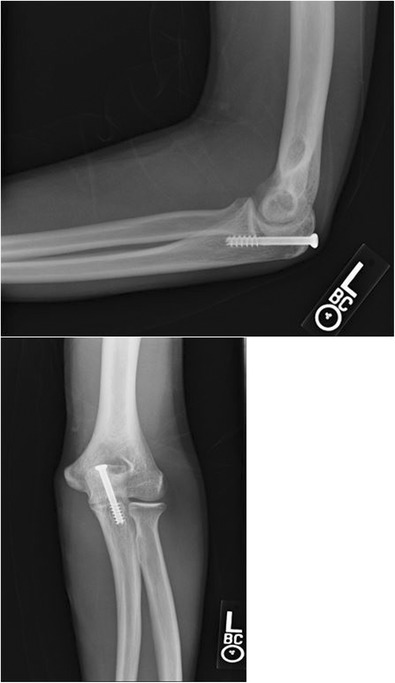
Persistent olecranon physis from Fig. 1 successfully treated with cannulated screw placement
Outcomes
Paci et al., in the largest series of throwing athletes with olecranon stress fractures requiring operative treatment in the literature, reported on 18 male baseball players. A single percutaneous cannulated titanium screw was placed under fluoroscopic guidance perpendicular to the orientation of the fracture, followed by a 14-week rehabilitation program and then progressive throwing program. All 18 stress fractures healed, and 94% of patients returned to baseball at or above their prior level [72•]. Fujioka et al. described results in six throwing athletes with olecranon stress fractures with similar treatment with a percutaneously placed headless screw. All athletes returned to their previous level of play and healed clinically and radiographically by 6 months after surgery [71]. Complications described after surgery include painful hardware, infection, and recurrence of olecranon fracture. These complications did not appear to affect ultimate return-to-play outcomes [79, 72•].
Author’s Preferred Approach
When evaluating the adolescent throwing athlete, an initial history and examination is performed and correlated with radiographic and advanced imaging, with MRI as our preference. A comprehensive conservative treatment regimen as described by Schickendantz et al. is initiated including a progressive throwing program [77]. If the athlete fails this protocol, the preferred surgical treatment is with a percutaneous cannulated screw placement and rehabilitation protocol as according to Paci et al. [72•].
Conclusions
Olecranon stress fractures, when managed acutely, typically respond well to conservative treatment with activity modification, splinting, physical therapy, and a progressive throwing program. With failure of a comprehensive non-operative treatment regimen, surgical treatment is warranted and reported surgical outcomes with percutaneous cannulated screw placement are excellent.
Summary
With an increasing rate of adolescent elbow injuries, especially in throwing athletes, comprehensive understanding of the diagnosis, treatment, and non-operative and operative outcomes of medial epicondyle fractures, ulnar collateral ligament repair, osteochondritis dissecans of the elbow, and olecranon stress fractures is of paramount importance for the treating orthopedic elbow surgeon. Acceptable outcomes with both non-operative and operative treatment of medial epicondyle fractures have been reported, with surgical indications continuing to evolve. When surgery is indicated, treatment with open reduction and internal fixation with a fully threaded cannulated screw yields satisfactory results. Unstable osteochondritis dissecans lesions, especially in patients with closed growth plates, require operative fixation, and emerging open and arthroscopic techniques including lesion debridement, marrow stimulation, autograft transfer, and allograft transplantation are described with good outcomes. Ulnar collateral repair has emerged as an exciting treatment option for an avulsion of either end of the ligament in young throwing athletes, with faster rehabilitation times than traditional ulnar collateral ligament reconstruction. Biocomposite anchors and braided non-absorbable sutures are utilized to create an internal brace to support the healing ulnar collateral ligament and provide structural support with early throwing. Olecranon stress fractures and persistent olecranon physes in throwers are increasing in prevalence, and when a non-operative treatment course is unsuccessful, athletes have a high return-to-play rate after percutaneous cannulated screw placement. Painful hardware and recurrence are described complications. With proper indications, non-operative and operative treatment modalities are reported with a high return-to-play and acceptable clinical outcomes for common elbow injuries, including medial epicondyle fractures, ulnar collateral ligament repair, osteochondritis dissecans of the elbow, and olecranon stress fractures, in adolescent throwing athletes. Further research is needed to better define treatment algorithms, surgical indications and outcomes, and the comparison of described techniques.
Compliance with Ethical Standards
Conflict of Interest
The authors declare that they have no conflicts of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Footnotes
This article is part of the Topical Collection on Injuries in Overhead Athletes
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
- 1.Gottschalk HP, Eisner E, Hosalkar HS. Medial epicondyle fractures in the pediatric population. J Am Acad Orthop Surg. 2012;20(4):223–232. doi: 10.5435/JAAOS-20-04-223. [DOI] [PubMed] [Google Scholar]
- 2.Patel B, Reed M, Patel S. Gender-specific pattern differences of the ossification centers in the pediatric elbow. Pediatr Radiol. 2009;39(3):226–231. doi: 10.1007/s00247-008-1078-4. [DOI] [PubMed] [Google Scholar]
- 3.Gottschalk HP, Bastrom TP, Edmonds EW. Reliability of internal oblique elbow radiographs for measuring displacement of medial epicondyle humerus fractures: a cadaveric study. J Pediatr Orthop. 2013;33(1):26–31. doi: 10.1097/BPO.0b013e318279c673. [DOI] [PubMed] [Google Scholar]
- 4.Fowles JV, Slimane N, Kassab MT. Elbow dislocation with avulsion of the medial humeral epicondyle. J Bone Joint Surg Br. 1990;72(1):102–104. doi: 10.1302/0301-620X.72B1.2298765. [DOI] [PubMed] [Google Scholar]
- 5.Josefsson PO, Danielsson LG. Epicondylar elbow fracture in children. 35-year follow-up of 56 unreduced cases. Acta Orthop Scand. 1986;57(4):313–315. doi: 10.3109/17453678608994399. [DOI] [PubMed] [Google Scholar]
- 6.Louahem DM, Bourelle S, Buscayret F, Mazeau P, Kelly P, Dimeglio A, Cottalorda J. Displaced medial epicondyle fractures of the humerus: surgical treatment and results. A report of 139 cases. Arch Orthop Trauma Surg. 2010;130(5):649–655. doi: 10.1007/s00402-009-1009-3. [DOI] [PubMed] [Google Scholar]
- 7.Farsetti P, Potenza V, Caterini R, Ippolito E. Long-term results of treatment of fractures of the medial humeral epicondyle in children. J Bone Joint Surg Am. 2001;83-A(9):1299–1305. doi: 10.2106/00004623-200109000-00001. [DOI] [PubMed] [Google Scholar]
- 8.Knapik DM, Fausett CL, Gilmore A, Liu RW. Outcomes of nonoperative pediatric medial humeral epicondyle fractures with and without associated elbow dislocation. J Pediatr Orthop. 2017;37(4):e224–e2e8. doi: 10.1097/BPO.0000000000000890. [DOI] [PubMed] [Google Scholar]
- 9.Lee HH, Shen HC, Chang JH, Lee CH, Wu SS. Operative treatment of displaced medial epicondyle fractures in children and adolescents. J Shoulder Elb Surg. 2005;14(2):178–185. doi: 10.1016/j.jse.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 10.Pace GI, Hennrikus WL. Fixation of displaced medial epicondyle fractures in adolescents. J Pediatr Orthop. 2017;37(2):e80–ee2. doi: 10.1097/BPO.0000000000000743. [DOI] [PubMed] [Google Scholar]
- 11.Stepanovich M, Bastrom TP, Munch J, 3rd, Roocroft JH, Edmonds EW, Pennock AT. Does operative fixation affect outcomes of displaced medial epicondyle fractures? J Child Orthop. 2016;10(5):413–419. doi: 10.1007/s11832-016-0757-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Edmonds EW, Santago AC, 2nd, Saul KR. Functional loss with displacement of medial epicondyle humerus fractures: a computer simulation study. J Pediatr Orthop. 2015;35(7):666–671. doi: 10.1097/BPO.0000000000000371. [DOI] [PubMed] [Google Scholar]
- 13.Lawrence JT, Patel NM, Macknin J, Flynn JM, Cameron D, Wolfgruber HC, et al. Return to competitive sports after medial epicondyle fractures in adolescent athletes: results of operative and nonoperative treatment. Am J Sports Med. 2013;41(5):1152–1157. doi: 10.1177/0363546513480797. [DOI] [PubMed] [Google Scholar]
- 14.Biggers MD, Bert TM, Moisan A, Spence DD, Warner WC, Jr, Beaty JH, et al. Fracture of the medial humeral epicondyle in children: a comparison of operative and nonoperative management. J Surg Orthop Adv. 2015;24(3):188–192. doi: 10.3113/JSOA.2015.0188. [DOI] [PubMed] [Google Scholar]
- 15.Kulkarni VS, Arora N, Gehlot H, Saxena S, Kulkarni SG, Bajwa S. Symptomatic medial humeral epicondylar fracture non-union- rare presentation of a relatively common injury. Injury. 2017;48(Suppl 2):S50–SS3. doi: 10.1016/S0020-1383(17)30494-1. [DOI] [PubMed] [Google Scholar]
- 16.Smith JT, McFeely ED, Bae DS, Waters PM, Micheli LJ, Kocher MS. Operative fixation of medial humeral epicondyle fracture nonunion in children. J Pediatr Orthop. 2010;30(7):644–648. doi: 10.1097/BPO.0b013e3181ed4381. [DOI] [PubMed] [Google Scholar]
- 17.Vuillermin C, Donohue KS, Miller P, Bauer AS, Kramer DE, Yen YM. Incarcerated medial epicondyle fractures with elbow dislocation: risk factors associated with morbidity. J Pediatr Orthop. 2017:1. 10.1097/BPO.0000000000000991. [DOI] [PubMed]
- 18.Reinker KA. CORR insights(R): osteochondritis dissecans lesions in family members: does a positive family history impact phenotypic potency? Clin Orthop Relat Res. 2017;475(6):1581–1582. doi: 10.1007/s11999-016-5102-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nissen CW. Osteochondritis dissecans of the elbow. Clin Sports Med. 2014;33(2):251–265. doi: 10.1016/j.csm.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 20.Takahara M, Mura N, Sasaki J, Harada M, Ogino T. Classification, treatment, and outcome of osteochondritis dissecans of the humeral capitellum. J Bone Joint Surg Am. 2007;89(6):1205–1214. doi: 10.2106/00004623-200706000-00007. [DOI] [PubMed] [Google Scholar]
- 21.Kida Y, Morihara T, Kotoura Y, Hojo T, Tachiiri H, Sukenari T, Iwata Y, Furukawa R, Oda R, Arai Y, Fujiwara H, Kubo T, Matsui T, Azuma Y, Seo K, Hiramoto M. Prevalence and clinical characteristics of osteochondritis dissecans of the humeral capitellum among adolescent baseball players. Am J Sports Med. 2014;42(8):1963–1971. doi: 10.1177/0363546514536843. [DOI] [PubMed] [Google Scholar]
- 22.Kijowski R, De Smet AA. Radiography of the elbow for evaluation of patients with osteochondritis dissecans of the capitellum. Skelet Radiol. 2005;34(5):266–271. doi: 10.1007/s00256-005-0899-6. [DOI] [PubMed] [Google Scholar]
- 23.Kajiyama S, Muroi S, Sugaya H, Takahashi N, Matsuki K, Kawai N, Osaki M. Osteochondritis dissecans of the humeral capitellum in young athletes: comparison between baseball players and gymnasts. Orthop J Sports Med. 2017;5(3):2325967117692513. doi: 10.1177/2325967117692513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brittberg M, Winalski CS. Evaluation of cartilage injuries and repair. J Bone Joint Surg Am. 2003;85-A(Suppl 2):58–69. doi: 10.2106/00004623-200300002-00008. [DOI] [PubMed] [Google Scholar]
- 25.Itsubo T, Murakami N, Uemura K, Nakamura K, Hayashi M, Uchiyama S, Kato H. Magnetic resonance imaging staging to evaluate the stability of capitellar osteochondritis dissecans lesions. Am J Sports Med. 2014;42(8):1972–1977. doi: 10.1177/0363546514532604. [DOI] [PubMed] [Google Scholar]
- 26.Mihara K, Tsutsui H, Nishinaka N, Yamaguchi K. Nonoperative treatment for osteochondritis dissecans of the capitellum. Am J Sports Med. 2009;37(2):298–304. doi: 10.1177/0363546508324970. [DOI] [PubMed] [Google Scholar]
- 27.Takahara M, Ogino T, Takagi M, Tsuchida H, Orui H, Nambu T. Natural progression of osteochondritis dissecans of the humeral capitellum: initial observations. Radiology. 2000;216(1):207–212. doi: 10.1148/radiology.216.1.r00jl29207. [DOI] [PubMed] [Google Scholar]
- 28.Bradley JP, Petrie RS. Osteochondritis dissecans of the humeral capitellum. Diagnosis and treatment. Clin Sports Med. 2001;20(3):565–590. doi: 10.1016/S0278-5919(05)70270-2. [DOI] [PubMed] [Google Scholar]
- 29.Takahara M, Ogino T, Sasaki I, Kato H, Minami A, Kaneda K. Long term outcome of osteochondritis dissecans of the humeral capitellum. Clin Orthop Relat Res. 1999;363:108–115. doi: 10.1097/00003086-199906000-00014. [DOI] [PubMed] [Google Scholar]
- 30.Camp CL, Dines JS, Degen RM, Sinatro AL, Altchek DW. Arthroscopic microfracture for osteochondritis dissecans lesions of the capitellum. Arthrosc Tech. 2016;5(3):e477–e481. doi: 10.1016/j.eats.2016.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bexkens R, van den Ende KIM, Ogink PT, van Bergen CJA, van den Bekerom MPJ, Eygendaal D. Clinical outcome after arthroscopic debridement and microfracture for osteochondritis dissecans of the capitellum. Am J Sports Med. 2017;363546517704842(10):2312–2318. doi: 10.1177/0363546517704842. [DOI] [PubMed] [Google Scholar]
- 32.Koehler SM, Walsh A, Lovy AJ, Pruzansky JS, Shukla DR, Hausman MR. Outcomes of arthroscopic treatment of osteochondritis dissecans of the capitellum and description of the technique. J Shoulder Elb Surg. 2015;24(10):1607–1612. doi: 10.1016/j.jse.2015.06.013. [DOI] [PubMed] [Google Scholar]
- 33.Smith MV, Bedi A, Chen NC. Surgical treatment for osteochondritis dissecans of the capitellum. Sports Health. 2012;4(5):425–432. doi: 10.1177/1941738112444707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Miyake J, Masatomi T. Arthroscopic debridement of the humeral capitellum for osteochondritis dissecans: radiographic and clinical outcomes. J Hand Surg Am. 2011;36(8):1333–1338. doi: 10.1016/j.jhsa.2011.05.024. [DOI] [PubMed] [Google Scholar]
- 35.Jones KJ, Wiesel BB, Sankar WN, Ganley TJ. Arthroscopic management of osteochondritis dissecans of the capitellum: mid-term results in adolescent athletes. J Pediatr Orthop. 2010;30(1):8–13. doi: 10.1097/BPO.0b013e3181c3be83. [DOI] [PubMed] [Google Scholar]
- 36.Caldwell PE, 3rd, Auerbach B, Pearson SE. Arthroscopic treatment of capitellum osteochondritis dissecans with micronized allogeneic cartilage scaffold. Arthrosc Tech. 2017;6(3):e815–ee20. doi: 10.1016/j.eats.2017.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bexkens R, Ogink PT, Doornberg JN, Kerkhoffs G, Eygendaal D, Oh LS, et al. Donor-site morbidity after osteochondral autologous transplantation for osteochondritis dissecans of the capitellum: a systematic review and meta-analysis. Knee Surg Sports Traumatol Arthrosc. 2017;25(7):2237–2246. doi: 10.1007/s00167-017-4516-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mirzayan R, Lim MJ. Fresh osteochondral allograft transplantation for osteochondritis dissecans of the capitellum in baseball players. J Shoulder Elb Surg. 2016;25(11):1839–1847. doi: 10.1016/j.jse.2016.08.006. [DOI] [PubMed] [Google Scholar]
- 39.Maruyama M, Harada M, Satake H, Tomohiro U, Takagi M, Takahara M. Bone-peg grafting for osteochondritis dissecans of the humeral capitellum. J Orthop Surg (Hong Kong) 2016;24(1):51–56. doi: 10.1177/230949901602400113. [DOI] [PubMed] [Google Scholar]
- 40.Lyons ML, Werner BC, Gluck JS, Freilich AM, Dacus AR, Diduch DR, Chhabra AB. Osteochondral autograft plug transfer for treatment of osteochondritis dissecans of the capitellum in adolescent athletes. J Shoulder Elb Surg. 2015;24(7):1098–1105. doi: 10.1016/j.jse.2015.03.014. [DOI] [PubMed] [Google Scholar]
- 41.Nishinaka N, Tsutsui H, Yamaguchi K, Uehara T, Nagai S, Atsumi T. Costal osteochondral autograft for reconstruction of advanced-stage osteochondritis dissecans of the capitellum. J Shoulder Elb Surg. 2014;23(12):1888–1897. doi: 10.1016/j.jse.2014.06.047. [DOI] [PubMed] [Google Scholar]
- 42.Takeba J, Takahashi T, Hino K, Watanabe S, Imai H, Yamamoto H. Arthroscopic technique for fragment fixation using absorbable pins for osteochondritis dissecans of the humeral capitellum: a report of 4 cases. Knee Surg Sports Traumatol Arthrosc. 2010;18(6):831–835. doi: 10.1007/s00167-009-0945-3. [DOI] [PubMed] [Google Scholar]
- 43.van den Ende KI, McIntosh AL, Adams JE, Steinmann SP. Osteochondritis dissecans of the capitellum: a review of the literature and a distal ulnar portal. Arthroscopy. 2011;27(1):122–128. doi: 10.1016/j.arthro.2010.08.008. [DOI] [PubMed] [Google Scholar]
- 44.O’Driscoll SW, Morrey BF. Arthroscopy of the elbow. Diagnostic and therapeutic benefits and hazards. J Bone Joint Surg Am. 1992;74(1):84–94. doi: 10.2106/00004623-199274010-00010. [DOI] [PubMed] [Google Scholar]
- 45.Hennrikus WP, Miller PE, Micheli LJ, Waters PM, Bae DS. Internal fixation of unstable in situ osteochondritis dissecans lesions of the capitellum. J Pediatr Orthop. 2015;35(5):467–473. doi: 10.1097/BPO.0000000000000308. [DOI] [PubMed] [Google Scholar]
- 46.Inoue G. Bilateral osteochondritis dissecans of the elbow treated by Herbert screw fixation. Br J Sports Med. 1991;25(3):142–144. doi: 10.1136/bjsm.25.3.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nobuta S, Ogawa K, Sato K, Nakagawa T, Hatori M, Itoi E. Clinical outcome of fragment fixation for osteochondritis dissecans of the elbow. Ups J Med Sci. 2008;113(2):201–208. doi: 10.3109/2000-1967-232. [DOI] [PubMed] [Google Scholar]
- 48.Takeba J, Takahashi T, Watanabe S, Imai H, Kikuchi S, Umakoshi K, Matsumoto H, Ohshita M, Miura H, Aibiki M. Short-term clinical results of arthroscopic osteochondral fixation for elbow osteochondritis dissecans in teenaged baseball players. J Shoulder Elb Surg. 2015;24(11):1749–1756. doi: 10.1016/j.jse.2015.07.009. [DOI] [PubMed] [Google Scholar]
- 49.Uchida S, Utsunomiya H, Taketa T, Sakoda S, Hatakeyama A, Nakamura T, Sakai A. Arthroscopic fragment fixation using hydroxyapatite/poly-L-lactate acid thread pins for treating elbow osteochondritis dissecans. Am J Sports Med. 2015;43(5):1057–1065. doi: 10.1177/0363546515570871. [DOI] [PubMed] [Google Scholar]
- 50.Stone KR, Pelsis JR, Crues JV, 3rd, Walgenbach AW, Turek TJ. Osteochondral grafting for failed knee osteochondritis dissecans repairs. Knee. 2014;21(6):1145–1150. doi: 10.1016/j.knee.2014.09.003. [DOI] [PubMed] [Google Scholar]
- 51.Maruyama M, Takahara M, Harada M, Satake H, Takagi M. Outcomes of an open autologous osteochondral plug graft for capitellar osteochondritis dissecans: time to return to sports. Am J Sports Med. 2014;42(9):2122–2127. doi: 10.1177/0363546514538759. [DOI] [PubMed] [Google Scholar]
- 52.Iwasaki N, Kato H, Ishikawa J, Masuko T, Funakoshi T, Minami A. Autologous osteochondral mosaicplasty for osteochondritis dissecans of the elbow in teenage athletes: surgical technique. J Bone Joint Surg Am. 2010;92(Suppl 1 Pt 2):208–216. doi: 10.2106/JBJS.J.00214. [DOI] [PubMed] [Google Scholar]
- 53.Oka Y, Ikeda M. Treatment of severe osteochondritis dissecans of the elbow using osteochondral grafts from a rib. J Bone Joint Surg Br. 2001;83(5):738–739. doi: 10.1302/0301-620X.83B5.11767. [DOI] [PubMed] [Google Scholar]
- 54.Sato K, Nakamura T, Toyama Y, Ikegami H. Costal osteochondral grafts for osteochondritis dissecans of the capitulum humeri. Tech Hand Up Extrem Surg. 2008;12(2):85–91. doi: 10.1097/BTH.0b013e31815b2e05. [DOI] [PubMed] [Google Scholar]
- 55.Vezeridis AM, Bae DS. Evaluation of knee donor and elbow recipient sites for osteochondral autologous transplantation surgery in capitellar osteochondritis dissecans. Am J Sports Med. 2016;44(2):511–520. doi: 10.1177/0363546515620184. [DOI] [PubMed] [Google Scholar]
- 56.Rahusen FT, Brinkman JM, Eygendaal D. Results of arthroscopic debridement for osteochondritis dissecans of the elbow. Br J Sports Med. 2006;40(12):966–969. doi: 10.1136/bjsm.2006.030056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ueda Y, Sugaya H, Takahashi N, Matsuki K, Tokai M. Mid to long term outcome after arthroscopic fragment resection for capitellar osteochondritis dissecans in adolescent athlete. Orthopaedic Journal of Sports Medicine. 2017;5(7 suppl6):2325967117S00286. doi: 10.1177/2325967117744537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bexkens R, van den Ende KIM, Ogink PT, van Bergen CJA, van den Bekerom MPJ, Eygendaal D. Clinical outcome after arthroscopic debridement and microfracture for osteochondritis dissecans of the capitellum. Am J Sports Med. 2017;45(10):2312–2318. doi: 10.1177/0363546517704842. [DOI] [PubMed] [Google Scholar]
- 59.Westermann RW, Hancock KJ, Buckwalter JA, Kopp B, Glass N, Wolf BR. Return to Sport After Operative Management of Osteochondritis Dissecans of the Capitellum: A Systematic Review and Meta-analysis. Orthop J Sports Med. 2016;4(6):2325967116654651. 10.1177/2325967116654651 [DOI] [PMC free article] [PubMed]
- 60.Iwasaki N, Yamane S, Nishida K, Masuko T, Funakoshi T, Kamishima T, Minami A. Transplantation of tissue-engineered cartilage for the treatment of osteochondritis dissecans in the elbow: outcomes over a four-year follow-up in two patients. J Shoulder Elb Surg. 2010;19(8):e1–e6. doi: 10.1016/j.jse.2010.05.016. [DOI] [PubMed] [Google Scholar]
- 61.Morrey BF, An KN. Articular and ligamentous contributions to the stability of the elbow joint. Am J Sports Med. 1983;11(5):315–319. doi: 10.1177/036354658301100506. [DOI] [PubMed] [Google Scholar]
- 62.Jobe FW, Stark H, Lombardo SJ. Reconstruction of the ulnar collateral ligament in athletes. J Bone Joint Surg Am. 1986;68(8):1158–1163. doi: 10.2106/00004623-198668080-00004. [DOI] [PubMed] [Google Scholar]
- 63.Conway JE, Jobe FW, Glousman RE, Pink M. Medial instability of the elbow in throwing athletes. Treatment by repair or reconstruction of the ulnar collateral ligament. J Bone Joint Surg Am. 1992;74(1):67–83. doi: 10.2106/00004623-199274010-00009. [DOI] [PubMed] [Google Scholar]
- 64.Erickson BJ, Chalmers PN, Bush-Joseph CA, Verma NN, Romeo AA. Ulnar collateral ligament reconstruction of the elbow: a systematic review of the literature. Orthop J Sports Med. 2015;3(12):2325967115618914. doi: 10.1177/2325967115618914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cain EL, Jr, Andrews JR, Dugas JR, Wilk KE, McMichael CS, Walter JC, 2nd, et al. Outcome of ulnar collateral ligament reconstruction of the elbow in 1281 athletes: results in 743 athletes with minimum 2-year follow-up. Am J Sports Med. 2010;38(12):2426–2434. doi: 10.1177/0363546510378100. [DOI] [PubMed] [Google Scholar]
- 66.Dugas JR. Ulnar collateral ligament repair: an old idea with a new wrinkle. Am J Orthop (Belle Mead NJ) 2016;45(3):124–127. [PubMed] [Google Scholar]
- 67.Savoie FH, 3rd, Trenhaile SW, Roberts J, Field LD, Ramsey JR. Primary repair of ulnar collateral ligament injuries of the elbow in young athletes: a case series of injuries to the proximal and distal ends of the ligament. Am J Sports Med. 2008;36(6):1066–1072. doi: 10.1177/0363546508315201. [DOI] [PubMed] [Google Scholar]
- 68.Dugas JR, Walters BL, Beason DP, Fleisig GS, Chronister JE. Biomechanical comparison of ulnar collateral ligament repair with internal bracing versus modified Jobe reconstruction. Am J Sports Med. 2016;44(3):735–741. doi: 10.1177/0363546515620390. [DOI] [PubMed] [Google Scholar]
- 69.Argo D, Trenhaile SW, Savoie FH, 3rd, Field LD. Operative treatment of ulnar collateral ligament insufficiency of the elbow in female athletes. Am J Sports Med. 2006;34(3):431–437. doi: 10.1177/0363546505281240. [DOI] [PubMed] [Google Scholar]
- 70.Brucker J, Sahu N, Sandella B. Olecranon stress injury in an adolescent overhand pitcher: a case report and analysis of the literature. Sports Health. 2015;7(4):308–311. doi: 10.1177/1941738114567868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Fujioka H, Tsunemi K, Takagi Y, Tanaka J. Treatment of stress fracture of the olecranon in throwing athletes with internal fixation through a small incision. Sports Med Arthrosc Rehabil Ther Technol. 2012;4(1):49. doi: 10.1186/1758-2555-4-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Paci JM, Dugas JR, Guy JA, Cain EL, Jr, Fleisig GS, Hurst C, Wilk KE, Andrews JR. Cannulated screw fixation of refractory olecranon stress fractures with and without associated injuries allows a return to baseball. Am J Sports Med. 2013;41(2):306–312. doi: 10.1177/0363546512469089. [DOI] [PubMed] [Google Scholar]
- 73.Rettig AC, Wurth TR, Mieling P. Nonunion of olecranon stress fractures in adolescent baseball pitchers: a case series of 5 athletes. Am J Sports Med. 2006;34(4):653–656. doi: 10.1177/03635465281802. [DOI] [PubMed] [Google Scholar]
- 74.Matsuura T, Kashiwaguchi S, Iwase T, Enishi T, Yasui N. The value of using radiographic criteria for the treatment of persistent symptomatic olecranon physis in adolescent throwing athletes. Am J Sports Med. 2010;38(1):141–145. doi: 10.1177/0363546509342677. [DOI] [PubMed] [Google Scholar]
- 75.Frank RM, Lenart BA, Cohen MS. Olecranon physeal nonunion in the adolescent athlete: identification of two patterns. J Shoulder Elb Surg. 2017;26(6):1044–1051. doi: 10.1016/j.jse.2016.11.036. [DOI] [PubMed] [Google Scholar]
- 76.Lowery WD, Jr., Kurzweil PR, Forman SK, Morrison DS. Persistence of the olecranon physis: a cause of “little league elbow”. J Shoulder Elb Surg 1995;4(2):143-147, DOI: 10.1016/S1058-2746(05)80070-9. [DOI] [PubMed]
- 77.Schickendantz MS, Ho CP, Koh J. Stress injury of the proximal ulna in professional baseball players. Am J Sports Med. 2002;30(5):737–741. doi: 10.1177/03635465020300051801. [DOI] [PubMed] [Google Scholar]
- 78.Corradin M, Marengo L, Andreacchio A, Paonessa M, Giacometti V, Samba A, Rousset M, Canavese F. Outcome of isolated olecranon fractures in skeletally immature patients: comparison of open reduction and tension band wiring fixation versus closed reduction and percutaneous screw fixation. Eur J Orthop Surg Traumatol. 2016;26(5):469–476. doi: 10.1007/s00590-016-1774-y. [DOI] [PubMed] [Google Scholar]
- 79.Stephenson DR, Love S, Garcia GG, Mair SD. Recurrence of an olecranon stress fracture in an elite pitcher after percutaneous internal fixation: a case report. Am J Sports Med. 2012;40(1):218–221. doi: 10.1177/0363546511422796. [DOI] [PubMed] [Google Scholar]


