Abstract
Purpose of Review
The purposes of this review were to provide an overview of the current practice of evaluating the postoperative rotator cuff on imaging and to review the salient imaging findings of the normal and abnormal postoperative rotator cuff, as well as of postoperative complications.
Recent Findings
The repaired rotator cuff frequently appears abnormal on magnetic resonance imaging (MRI) and ultrasound (US). Recent studies have shown that while the tendons typically normalize, they can demonstrate clinically insignificant abnormal imaging appearances for longer than 6 months. Features of capsular thickening or subacromial-subdeltoid bursal thickening and fluid distension were found to decrease substantially in the first 6-month postoperative period. MRI and US were found to be highly comparable in the postoperative assessment of the rotator cuff, although they had a lower sensitivity for partial thickness tears. Imaging evaluation of newer techniques such as patch augmentation and superior capsular reconstruction needs to be further investigated.
Summary
MRI and US are useful in the postoperative assessment of the rotator cuff, not only for evaluation of the integrity of the rotator cuff, but also for detecting hardware complications and other etiologies of shoulder pain.
Keywords: Rotator cuff repair, Magnetic resonance imaging, Ultrasound, Rotator cuff re-tear
Introduction
The rotator cuff plays an important role in shoulder abduction and rotation, with the supraspinatus aiding in abduction [1] and the infraspinatus and subscapularis functioning as the main external and internal rotators, respectively [2]. After repair of rotator cuff tendon tears, approximately 25% of patients may experience persistent or new pain and disability [3]. In the symptomatic patient after rotator cuff repair, the primary etiologies to consider include, but are not limited to, rotator cuff re-tear, impingement, and postoperative synovitis. Hardware dislodgement can also be an unexpected finding on imaging, which may cause significant pain and impairment [4, 5].
The rate of re-tears reported in the literature varies tremendously, ranging from 9 to 94% [6–8], with re-tears typically occurring within the first three postoperative months [9]. The risk of re-tear increases with patient age [6] and size of the preoperative rotator cuff tear [7]. Additional risk factors associated with re-tears include preoperative fatty infiltration of the rotator cuff musculature [4] and double-row repairs [8]. Magnetic resonance imaging (MRI) and ultrasound (US) are both used in evaluating pathology after rotator cuff repair. Our goal is to highlight both expected postoperative and abnormal findings on MRI, while underscoring the use of static and dynamic ultrasound as a complementary exam, particularly when a high likelihood of re-tear is suspected clinically.
MRI Evaluation of the Postoperative Rotator Cuff
After clinical and physical examination, radiographs are typically obtained for first-line evaluation [4, 10•]. If radiographs do not answer the clinical question, MRI can be performed to evaluate the rotator cuff integrity and assess for complications such as re-tear of the tendon or displacement of suture anchors. A recent study by Collin et al. found inter-observer agreement regarding MRI interpretation of the postoperative rotator cuff to range from 0.76 to 0.90 [11•]. At our institution, MRI of the repaired rotator cuff is acquired on a 1.5-T (Tesla) system (GE Healthcare, Waukesha, Wis) with our imaging parameters described in Table 1. To overcome artifacts from metallic shavings, the bandwidth can be increased to 80 kHz. In our experience, 3-T magnets have not provided additional diagnostic benefit over 1.5-T units in assessing the postoperative shoulder, while accentuating susceptibility artifact from metal [12].
Table 1.
MRI protocol for imaging of the shoulder
| PPlane/sequence | FOV (cm) | Matrix (frequency × phase) | Slice thickness/gap (mm) | TE (msec) | TR (msec) | TI (msec) | Bandwidth (kHz) | NEX |
|---|---|---|---|---|---|---|---|---|
| 3 plane localizer SS FSE | 20 | 256 × 128 | 7/0 | Min | Min | – | 31 | 1 |
| Oblique coronal IR FSE | 17 | 288 × 288 | 3.5/0 | 17 | 4000 | 150 | 31 | 2 |
| Oblique coronal PD FSE | 16 | 512 × 384 | 3/0 | 24 | 4000 | – | 31 | 2 |
| Axial PD FSE | 15 | 512 × 384 | 3/0 | 24 | 4000 | – | 31 | 2 |
| Oblique sagittal PD FSE | 15 | 512 × 320 | 4/0.5 | 24 | 4000 | – | 31 | 2 |
FOV field of view, TE time to echo, TR time to repetition, TI inversion time, NEX number of excitations, ETL echo train length, SS FSE single shot fast spin echo, IR inversion recovery, FSE fast spin echo, PD proton density
MRI Appearance of the Repaired Rotator Cuff Tendon
Normal, native tendons on MRI should demonstrate homogenous low signal intensity without tendon thickening or attenuation, interposed fluid-intensity high signal, or discontinuity [13]. Repaired rotator cuff tendons, on the other hand, commonly demonstrate increased signal intensity on MRI; such appearance can be attributable to postoperative granulation tissue and inflammation or preexisting tendinosis, rather than a re-tear [14]. A small study by Spielmann et al. determined that only 10% of asymptomatic patients who had undergone rotator cuff repair had a normal MRI appearance of the rotator cuff tendon [15]. Patients who are asymptomatic with good function of their rotator cuff may still demonstrate tendon thinning on imaging [10•]. The repaired tendon may continue to appear thin or hyperintense up to a year after surgery (Fig. 1a–d) [14]. At the site of debridement, the tendon may have a smooth defect that is classically wider than it is deep, which should not be mistaken for a re-tear [16, 17]. Intermediate to low signal intensity foci may be identified within the repaired tendon, typically from either suture material, granulation tissue, fibrosis, or metal artifact from instrumentation [10•, 15, 16]. After the first postoperative year, the repaired tendon will begin to have a more normal appearance, but may demonstrate abnormal characteristics even years after surgery [14]. Additionally, it is important to be aware that portions of the rotator cuff may not have been repaired secondary to poor tissue quality, therefore continuing to demonstrate features of partial or full-thickness tear similar to the prior study; these should not be mistaken for re-tears [10•].
Fig. 1.
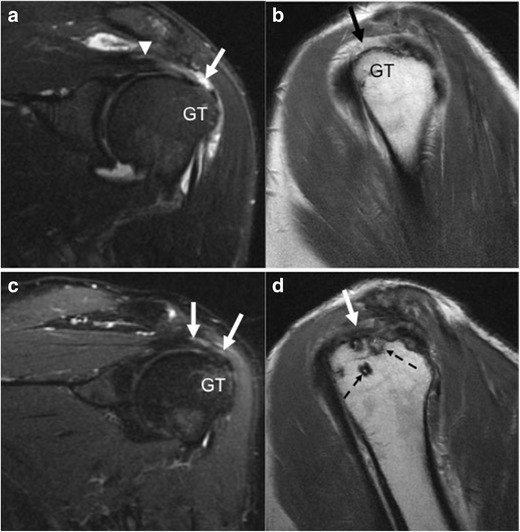
a–d Fifty-six-year-old man initially presenting with rotator cuff tear: preoperative and postoperative imaging. Preoperative oblique coronal IR (a) and oblique sagittal PD (b) images demonstrate the supraspinatus tendon (arrowhead) with a fluid-filled gap (arrow) and no normal fibers inserting onto the greater tuberosity (GT), consistent with a full thickness tear. Ten months after rotator cuff repair, oblique coronal IR (c) and oblique sagittal PD (d) images demonstrate somewhat irregular and slightly hyperintense supraspinatus tendon fibers (arrows) inserting onto the greater tuberosity, without a fluid filled gap to suggest a re-tear. Suture anchors (dashed arrows) are noted within the greater tuberosity
In irreparable or chronic, massive rotator cuff tears, surgical augmentation with graft may be performed to bridge the rotator cuff defect, or a superior capsular reconstruction to stabilize the humeral head [18•, 19, 20]. Dermal allograft, fascia lata autograft, or synthetic material may be used [18•, 21]. On MRI, the grafts typically demonstrate low signal intensity on proton density images; however, the literature on the MRI evaluation of rotator cuff and superior capsular reconstruction grafts is sparse [18•, 21, 22]. In patch augmentation, the two ends of the torn rotator cuff may not be in complete apposition, but the graft should bridge the rent in the rotator cuff; in superior capsular reconstruction, the graft should be continuous from the superior glenoid to the greater tuberosity for stabilization of the glenohumeral joint (Fig. 2a–f) [18•, 22, 23].
Fig. 2.
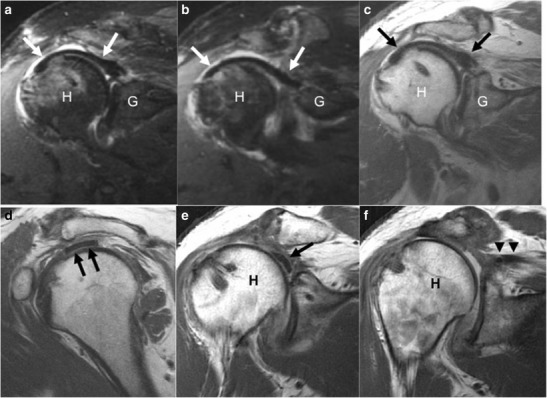
a–f Sixty-year-old man, 4 months after superior capsular reconstruction. Oblique coronal IR (a, b), oblique coronal PD (c), and oblique sagittal PD (d) images demonstrate low signal intensity of the dermal allograft (arrows). The graft is closely apposed to both the greater tuberosity at the humeral head (H), as well as the glenoid (G). In a different patient (e, f), there is discontinuity of the low signal intensity graft (arrow) seen far anteriorly, with detachment from both the humeral head (H) and the medially retracted supraspinatus tendon (arrowheads). Previously, the graft had been surgically placed from the humeral head to the torn supraspinatus tendon
Increased signal within the greater tuberosity may also be a normal finding at the site of suture anchor placement, as this is seen even in asymptomatic patients [15]. Subacromial bursitis may be a finding after rotator cuff repair and can be seen in both symptomatic and asymptomatic patients (Fig. 3a, b) [17]. Causative factors of subacromial bursal fluid include leakage of joint fluid through a repaired tendon, synovitis inciting a bursitis, or postoperative scarring [17].
Fig. 3.
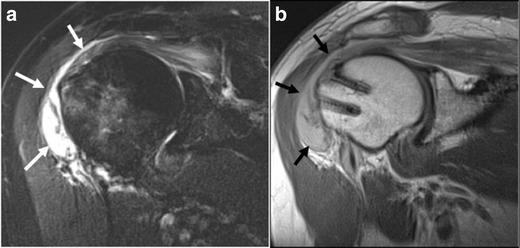
a, b Fifty-year-old man with shoulder pain, 9 months after rotator cuff repair. Oblique coronal IR (a) and oblique coronal PD (b) demonstrate marked subacromial-subdeltoid bursitis (arrows) with fluid distension and debris
At our institution, intra-articular gadolinium is not used in the setting of prior rotator cuff repair. In our experience, optimized high-resolution noncontrast MRI on 1.5T units is accurate for identifying clinically significant re-tears of the rotator cuff. A study by Duc et al. came to a similar conclusion, as did a recent meta-analysis by Roy et al., finding that MR arthrography does not provide improved diagnostic performance when compared to standard unenhanced MRI [24, 25•].
Complications of Rotator Cuff Repair on MRI
Familiarity with the typical appearance of the postoperative tendon is important when approaching the repaired rotator cuff, as most repaired tendons demonstrate irregularity on MRI and US in the 1-year postoperative period, which should not be mistaken for a failed tendon repair or re-tear [14]. Re-tears are typically thought to be a result of failed tendon healing due to inadequate tissue quality or failure of fixation, either at the bone-tendon interface or from suture breakage [10•, 26, 27]. A re-tear typically manifests as a full-thickness discontinuity with gap of tendon fibers where there is intersecting fluid signal, reflecting a large rotator cuff defect usually occurring at the supraspinatus tendon (Fig. 4a–c) [14, 15, 17, 28]. Medial retraction of the proximal tendon and muscle edge can be seen as a secondary sign of re-tear [28]. However, as stated already, a full-thickness or partial-thickness defect of the cuff may be a normal postoperative appearance if that portion of the tear could not be successfully repaired intraoperatively. This can pose a challenge for interpretation and knowledge of the exact surgical technique as well as comparison to prior MRI becomes critical. Additionally, similar findings of discontinuity with hyperintense, fluid intensity signal within the gap indicate a failure of surgical grafts [18•].
Fig. 4.
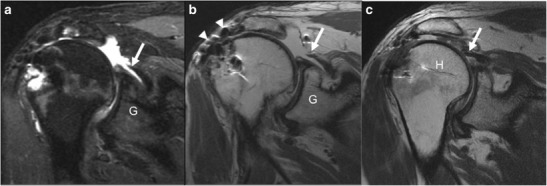
a–c Forty-seven-year-old man, 5 months after most recent rotator cuff repair. Oblique coronal IR (a) and oblique coronal PD (b) images demonstrate a fluid filled defect with retraction of the supraspinatus tendon (arrow) to the level of the glenoid (G), consistent with complete rotator cuff re-tear. There is susceptibility artifact (arrowheads) from prior repair. In a different patient, oblique coronal PD (c) image demonstrates a complete tear of the repaired supraspinatus tendon (arrow) with retraction of the tendon fibers to the level of the humeral head (H)
While one of the limitations of MRI includes artifact from metallic material, bioabsorbable suture anchors are well demonstrated on MRI and result in minimal artifact in contradistinction to older metallic screws [11•, 29]. Partial or complete dislodgement of the rotator cuff anchors from the humeral head is yet another complication of the repaired cuff (Fig. 5a–b). In a small study by Magee et al., 13 of 30 patients who presented with pain after rotator cuff repair demonstrated dislodgement of the rotator cuff anchors, all presenting in the first 6-month postoperative period [30]. As bioabsorbable anchors are not visible on radiographs, MRI is important for assessing their integrity [30, 31]. Anchors displaced into the glenohumeral joint can incite a synovitis, causing new pain after arthroscopy or result in chondral injury and decreased range of motion (Fig. 6a–d) [5, 30]. If displaced into the glenohumeral joint, suture anchors can also cause a locking sensation of the shoulder [4, 5]. One case study found a suture anchor displaced into the acromioclavicular joint [29]. MRI can determine the number of anchors that are displaced and their location and can aid in surgical planning. Anchor dislodgement may or may not be accompanied by a cuff tear, and the rotator cuff tendons should be closely examined when there is identification of suture anchor failure [30].
Fig. 5.
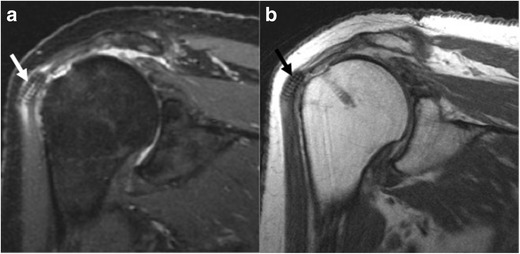
a, b Seventy-nine-year-old woman, 6 months after rotator cuff repair, presents with clicking and soreness of the shoulder. Oblique coronal IR (a) and oblique coronal PD (b) images demonstrate a screw (arrow) displaced into the substance of the proximal deltoid muscle, inciting surrounding soft tissue edema, and subacromial-subdeltoid bursitis
Fig. 6.

a–d Fifty-six-year-old woman, 9 months after rotator cuff repair, presents with shoulder pain. Oblique coronal IR (a) and oblique coronal PD (b) images demonstrate a screw (arrow) displaced into the glenohumeral joint, generating a reactive synovitis. Axial PD (c) image best shows the entirety of the screw (arrow) within the joint, superior to the level of the humeral head. Oblique coronal CT image (d) again shows the faintly radiopaque screw (bracket) displaced superior to the humeral head
Even without dislodgement, certain implants including suture anchors can incite an inflammatory reaction or result in foreign body granulomas in the susceptible host [32–34]. On MRI, prominent and persistent bone marrow edema around the anchors associated with bony resorption is suspicious for an inflammatory reaction (Fig. 7a–c) [33]. The imaging features can overlap with infection particularly if these are accompanied by soft tissue edema, subacromial bursitis, and synovitis. While subacromial-subdeltoid bursitis can be seen in asymptomatic patients after rotator cuff repair (Spielman et al. found 67% of asympatomatic patients to have subacromial-subdeltoid bursitis), in the absence of focal tendon abnormality, a large amount of fluid in the bursa should raise suspicion for other etiologies such as infection or bursitis related to bioabsorbable surgical anchors [15, 28].
Fig. 7.
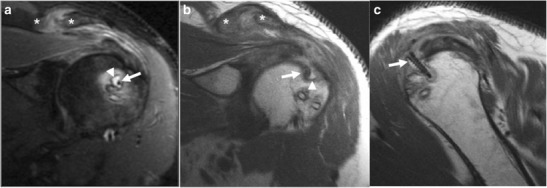
a–c Forty-nine-year-old man, 4 months after rotator cuff repair, presents with shoulder pain. Oblique coronal IR (a) and PD (b) images demonstrate hyperintense signal (arrowhead) surrounding a screw (arrow), reflecting bony resorption with reactive bone marrow edema. The supraspinatus muscle belly and adjacent acromioclavicular joint (asterisks) demonstrate hyperintense signal, reflecting inflammatory changes. Sagittal PD (c) image demonstrates the same screw (arrow) proud to the humeral cortex
Other postoperative complications and etiologies of pain include acromial fracture, suprascapular or axillary nerve injury, deltoid dehiscence, capsular scarring, and biceps tendon subluxation or rupture [4, 10•, 28]. The glenohumeral joint should be examined for articular damage, either from dislodged suture anchors or post-arthroscopic glenohumeral chondrolysis [35–37]. Of course, other causes of pain such as labral tears and pathology unrelated to rotator cuff repair can also be identified on MRI [10•].
US Evaluation of the Postoperative Rotator Cuff
Indications for US
As US possesses high spatial resolution and allows the performance of dynamic maneuvers in real time, it can be very useful in the postoperative evaluation of the rotator cuff, particularly in the setting of metal artifact making MRI non-diagnostic [10•, 38]. Recent meta-analysis by Roy et al. found similar diagnostic accuracies between US and MRI in the evaluation for re-tears, with sensitivities and specificities over 0.90 for both imaging modalities in their detection of full-thickness tears, and over 0.90 specificities but somewhat lower sensitivities of 0.67–0.83 for partial-thickness tears [25•]. A more recent study by Okoroha et al. showed that MRI demonstrated greater inter-observer reliability in the evaluation of tear size, retraction, and muscle atrophy as compared to US [39]. In patients with contraindications for MRI, such as cardiac pacemakers, US is a valuable tool in assessing the repaired rotator cuff tendons [40].
A systematic approach should be taken when performing real-time diagnostic US of the postoperative shoulder. While US is highly operator dependent, a standardized examination can be useful in guiding treatment. At our institution, US is performed with the patient in a seated position using a high-frequency (6–15 MHz) linear broad-array transducer. The anterior structures (long head of the biceps tendon and subscapularis tendon) are examined first, followed by superior structures (acromioclavicular joint, supraspinatus tendon, and subacromial-subdeltoid bursa), and the posterior structures (infraspinatus and teres minor tendons and posterior glenohumeral joint). While sonographic evaluation of the rotator cuff tendons is dependent on patient cooperation, such as the ability to maneuver into the Crass and modified Crass positions for evaluation of the supraspinatus tendon footprint [41], complete supraspinatus tears at the footprint can still be identified with the patient imaged in the neutral position. Unlike an MRI, which is a static exam, the ability to perform dynamic maneuvers is an advantage of US. Abduction of the shoulder during real-time scanning of the supraspinatus tendon can assess for subacromial impingement [42].
US Appearance of the Repaired Rotator Cuff
A normal tendon such as the rotator cuff should have a homogeneously hyperechoic (bright) and fibrillar appearance on US [43]. However, as on MRI, an intact postoperative rotator cuff may exhibit a varied and abnormal appearance on US [40]. Serial ultrasound evaluation of postoperative rotator cuff tendons has shown that the tendons may appear abnormal for years after surgery [10•, 44]. Gulotta et al. found defects within 20–50% of the repaired tendons using sonography [44]. The repaired tendons demonstrate abnormal echogenicity and thickness: the tendons may appear hyper or hypoechoic from disorganization of the tendon fibers and healing mechanisms and may demonstrate varying degrees of thickening or attenuation (Fig. 8a, b) [10•, 38, 40, 44, 45•]. A study by Yoo et al. following tendons up to 6 months after surgery determined that postoperative tendon echotexture and fibrillar architecture normalized over time, as did irregularities along the tendon surface [45•]. However, the normal fibrillar architecture of the tendon may remain abnormal for up to 5 years after surgery [44]. Increased peritendinous vascularity of the repaired tendon should also decrease by approximately 6 months after surgery [46].
Fig. 8.
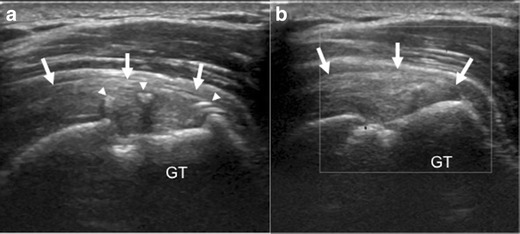
a, b A 51-year-old man, 1 year after rotator cuff repair, demonstrates a normal appearance on ultrasound after rotator cuff repair. The supraspinatus tendon (arrows) appears thickened and slightly hypoechoic, but there is no fluid filled defect disrupting its insertion onto the greater tuberosity (GT). Hyperechoic sutures (arrowheads) with posterior acoustic shadowing are identified within the substance of the supraspinatus tendon (arrows), and there is no increased vascularity seen on doppler images (b)
Complications of Rotator Cuff Repair and Features on US
Yoo et al. found that recurrent tears occurred within the first 3 months of surgical repair, confirming results from Miller et al. [45•, 47]. Tears were defined as an absence of continuous tendon fibers visualized over the humeral head and attaching to the greater tuberosity (Fig. 9a, b) [45•]. Dynamic sonographic imaging with shoulder abduction may also be performed to confirm tendon continuity; identification of fibers moving in tandem with the greater tuberosity upon abduction typically confirms preserved tendon integrity [48]. The greater tuberosity should also be examined for dislodged screws (Fig. 8e, f). Additionally, sutures, appearing as thin curvilinear, hyperechoic structures sometimes with posterior acoustic shadowing or reverberation artifact (Fig. 8a, b) [48], should also be evaluated for displacement. As on MRI, full-thickness or partial-thickness defects in the cuff on US may represent a re-tear or may represent a normal postoperative appearance if that portion of the tear was irreparable. Differentiation of the two may not be possible without knowing how the repair was performed. However, if a full-thickness defect of the cuff is associated with a bare suture anchor in the greater tuberosity, this is indicative of a new tear (Fig. 9c, d).
Fig. 9.
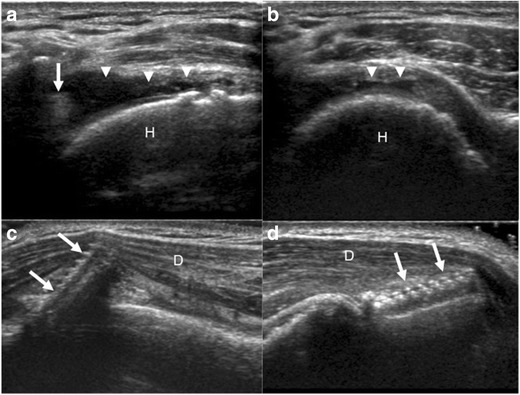
a–d Sonographic images of a 76-year-old man (a, b), 10 years after rotator cuff repair with shoulder pain after lifting boxes. Longitudinal (a) and transverse (b) to the supraspinatus tendon demonstrate a full thickness tear with retraction of the supraspinatus tendon (arrow) to the level of the humeral head (H) and fluid filled gap (arrowheads). Sonographic images (c, d) of a 38-year-old woman, 1 month after rotator cuff repair with shoulder pain, demonstrate a screw (arrows), backing out of the greater tuberosity and into the deltoid musculature (D)
Subacromial-subdeltoid bursal abnormalities can also be identified on ultrasound. Fluid collection superficial to the supraspinatus tendon is indicative of subacromial-subdeltoid fluid distension [43]. Yoo et al. found that 58 and 34% of patients had bursal thickening and fluid distension, respectively, in the 3-month postoperative period, although the study did not determine pain status [45•]. The posterior glenohumeral capsule may also appear thickened [49]. However, over time, there is typically a significant decrease in bursal and capsular thickness, as well as subacromial-subdeltoid fluid distension, shoulder stiffness, and pain [45•, 49].
Role of Contrast in Imaging the Rotator Cuff After Repair
In patients with contraindications to MRI in whom there are further clinical concerns for rotator cuff abnormality after sonographic evaluation, computed tomography (CT) arthrogram may be obtained [50, 51]. Conventional arthrogram, however, has been considered unreliable because contrast material may leak through a repaired, incompletely healed tendon, and arthrography has been shown to have a lower sensitivity, specificity, and accuracy compared to ultrasound [40]. While CT arthrogram may be able to detect full thickness rotator cuff tears, sensitivity and specificity for partial thickness tears are significantly lower [51]. Even in the setting of a full thickness tear, postoperative scar formation and anatomic distortion may prevent contrast from dispersing into the subacromial bursa [26, 40]. MRI in this instance would better characterize the location and size of a defect, reveal postoperative scar tissue, and allow for preoperative planning if a tear is identified.
Conclusion
Familiarity with the typical imaging appearance of the postoperative tendon is important when evaluating the repaired rotator cuff, as tendons commonly demonstrate an abnormal appearance on both MRI and US in the 1-year postoperative period that should not be mistaken for a failed tendon repair or re-tear. Imaging appearance of the postoperative rotator cuff is variable and should be correlated with clinical symptoms and the operative history, inclusive of graft utilization. Not only can imaging be used to diagnose re-tear and integrity of the rotator cuff, but it can also identify other etiologies for pain, such as hardware dislodgement, infection, or capsular scarring/synovitis. Identification of suture anchor dislodgement or displacement of suture wires themselves can be helpful in aiding surgical planning.
Compliance with ethical standards
Conflict of Interest
All authors declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Footnotes
This article is part of the Topical Collection on Rotator Cuff Repair
References
Papers of particular interest, published recently, have been highlighted as: • Of importance
- 1.Thompson WO, Debski RE, Boardman ND, 3rd, Taskiran E, Warner JJ, Fu FH, et al. A biomechanical analysis of rotator cuff deficiency in a cadaveric model. Am J Sports Med. 1996;24(3):286–292. doi: 10.1177/036354659602400307. [DOI] [PubMed] [Google Scholar]
- 2.Kronberg M, Nemeth G, Brostrom LA. Muscle activity and coordination in the normal shoulder. An electromyographic study. Clin Orthop Relat Res. 1990;257:76–85. [PubMed] [Google Scholar]
- 3.Neviaser RJ. Evaluation and management of failed rotator cuff repairs. Orthop Clin North Am. 1997;28(2):215–224. doi: 10.1016/S0030-5898(05)70281-9. [DOI] [PubMed] [Google Scholar]
- 4.Thakkar RS, Thakkar SC, Srikumaran U, McFarland EG, Fayad LM. Complications of rotator cuff surgery-the role of post-operative imaging in patient care. Br J Radiol. 2014;87(1039):20130630. doi: 10.1259/bjr.20130630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Abrams JS. Management of the failed rotator cuff surgery: causation and management. Sports Med Arthrosc. 2010;18(3):188–197. doi: 10.1097/JSA.0b013e3181eb6cc1. [DOI] [PubMed] [Google Scholar]
- 6.Diebold G, Lam P, Walton J, Murrell GAC. Relationship between age and rotator cuff retear: a study of 1600 consecutive rotator cuff repairs. J Bone Joint Surg Am. 2017;99(14):1198–1205. doi: 10.2106/JBJS.16.00770. [DOI] [PubMed] [Google Scholar]
- 7.Le BT, XL W, Lam PH, Murrell GA. Factors predicting rotator cuff retears: an analysis of 1000 consecutive rotator cuff repairs. Am J Sports Med. 2014;42(5):1134–1142. doi: 10.1177/0363546514525336. [DOI] [PubMed] [Google Scholar]
- 8.McElvany MD, McGoldrick E, Gee AO, Neradilek MB, Matsen FA., 3rd Rotator cuff repair: published evidence on factors associated with repair integrity and clinical outcome. Am J Sports Med. 2015;43(2):491–500. doi: 10.1177/0363546514529644. [DOI] [PubMed] [Google Scholar]
- 9.Kim JH, Hong IT, Ryu KJ, Bong ST, Lee YS, Kim JH. Retear rate in the late postoperative period after arthroscopic rotator cuff repair. Am J Sports Med. 2014;42(11):2606–2613. doi: 10.1177/0363546514547177. [DOI] [PubMed] [Google Scholar]
- 10.Pierce JL, Nacey NC, Jones S, Rierson D, Etier B, Brockmeier S, Anderson MW. Postoperative shoulder imaging: rotator cuff, labrum, and biceps tendon. Radiographics. 2016;36(6):1648–1671. doi: 10.1148/rg.2016160023. [DOI] [PubMed] [Google Scholar]
- 11.Collin P, Yoshida M, Delarue A, Lucas C, Jossaume T, Ladermann A, et al. Evaluating postoperative rotator cuff healing: prospective comparison of MRI and ultrasound. Orthop Traumatol Surg Res. 2015;101(6 Suppl):S265–S268. doi: 10.1016/j.otsr.2015.06.006. [DOI] [PubMed] [Google Scholar]
- 12.Bernstein MA, Huston J, 3rd, Ward HA. Imaging artifacts at 3.0T. J Magn Reson Imaging. 2006;24(4):735–746. doi: 10.1002/jmri.20698. [DOI] [PubMed] [Google Scholar]
- 13.Chang A, Miller TT. Imaging of tendons. Sports Health. 2009;1(4):293–300. doi: 10.1177/1941738109338361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Crim J, Burks R, Manaster BJ, Hanrahan C, Hung M, Greis P. Temporal evolution of MRI findings after arthroscopic rotator cuff repair. AJR Am J Roentgenol. 2010;195(6):1361–1366. doi: 10.2214/AJR.10.4436. [DOI] [PubMed] [Google Scholar]
- 15.Spielmann AL, Forster BB, Kokan P, Hawkins RH, Janzen DL. Shoulder after rotator cuff repair: MR imaging findings in asymptomatic individuals--initial experience. Radiology. 1999;213(3):705–708. doi: 10.1148/radiology.213.3.r99dc09705. [DOI] [PubMed] [Google Scholar]
- 16.Mohana-Borges AV, Chung CB, Resnick D. MR imaging and MR arthrography of the postoperative shoulder: spectrum of normal and abnormal findings. Radiographics. 2004;24(1):69–85. doi: 10.1148/rg.241035081. [DOI] [PubMed] [Google Scholar]
- 17.Zanetti M, Jost B, Hodler J, Gerber CMR. Imaging after rotator cuff repair: full-thickness defects and bursitis-like subacromial abnormalities in asymptomatic subjects. Skelet Radiol. 2000;29(6):314–319. doi: 10.1007/s002560000203. [DOI] [PubMed] [Google Scholar]
- 18.Zerr J, McDermott JD, Beckmann NM, Fullick RK, Chhabra A. Case study: failure of superior capsular reconstruction using dermal allograft. Skelet Radiol. 2017;46(11):1585–1589. doi: 10.1007/s00256-017-2716-4. [DOI] [PubMed] [Google Scholar]
- 19.Petri M, Greenspoon JA, Moulton SG, Millett PJ. Patch-augmented rotator cuff repair and superior capsule reconstruction. Open Orthop J. 2016;10(Suppl 1: M7):315–323. doi: 10.2174/1874325001610010315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thangarajah T, Pendegrass CJ, Shahbazi S, Lambert S, Alexander S, Blunn GW. Augmentation of rotator cuff repair with soft tissue scaffolds. Orthop J Sports Med. 2015;3(6):2325967115587495. doi: 10.1177/2325967115587495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thorsness R, Romeo A. Massive rotator cuff tears: trends in surgical management. Orthopedics. 2016;39(3):145–151. doi: 10.3928/01477447-20160503-07. [DOI] [PubMed] [Google Scholar]
- 22.Nada AN, Debnath UK, Robinson DA, Jordan C. Treatment of massive rotator-cuff tears with a polyester ligament (Dacron) augmentation: clinical outcome. J Bone Joint Surg Br. 2010;92(10):1397–1402. doi: 10.1302/0301-620X.92B10.24299. [DOI] [PubMed] [Google Scholar]
- 23.Varvitsiotis D, Papaspiliopoulos A, Antipa E, Papacharalampous X, Flevarakis G, Feroussis J. Results of reconstruction of massive irreparable rotator cuff tears using a fascia lata allograft. Indian J Orthop. 2015;49(3):304–311. doi: 10.4103/0019-5413.156202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Duc SR, Mengiardi B, Pfirrmann CW, Jost B, Hodler J, Zanetti M. Diagnostic performance of MR arthrography after rotator cuff repair. AJR Am J Roentgenol. 2006;186(1):237–241. doi: 10.2214/AJR.04.1818. [DOI] [PubMed] [Google Scholar]
- 25.Roy JS, Braen C, Leblond J, Desmeules F, Dionne CE, MacDermid JC, et al. Diagnostic accuracy of ultrasonography, MRI and MR arthrography in the characterisation of rotator cuff disorders: a systematic review and meta-analysis. Br J Sports Med. 2015;49(20):1316–1328. doi: 10.1136/bjsports-2014-094148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zlatkin MB. MRI of the postoperative shoulder. Skelet Radiol. 2002;31(2):63–80. doi: 10.1007/s00256-001-0460-1. [DOI] [PubMed] [Google Scholar]
- 27.Ruzek KA, Bancroft LW, Peterson JJ. Postoperative imaging of the shoulder. Radiol Clin N Am. 2006;44(3):331–341. doi: 10.1016/j.rcl.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 28.Gusmer PB, Potter HG, Donovan WD, O'Brien SJ. MR imaging of the shoulder after rotator cuff repair. AJR Am J Roentgenol. 1997;168(2):559–563. doi: 10.2214/ajr.168.2.9016248. [DOI] [PubMed] [Google Scholar]
- 29.Medina G, Garofo G, D'Elia CO, Bitar AC, Castropil W, Schor B. Bioabsorbable suture anchor migration to the acromioclavicular joint: how far can these implants go? Case Rep Orthop. 2014;2014:834896–834894. doi: 10.1155/2014/834896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Magee T, Shapiro M, Hewell G, Williams D. Complications of rotator cuff surgery in which bioabsorbable anchors are used. AJR Am J Roentgenol. 2003;181(5):1227–1231. doi: 10.2214/ajr.181.5.1811227. [DOI] [PubMed] [Google Scholar]
- 31.Major NM, Banks MC. MR imaging of complications of loose surgical tacks in the shoulder. AJR Am J Roentgenol. 2003;180(2):377–380. doi: 10.2214/ajr.180.2.1800377. [DOI] [PubMed] [Google Scholar]
- 32.Nusselt T, Freche S, Klinger HM, Baums MH. Intraosseous foreign body granuloma in rotator cuff repair with bioabsorbable suture anchor. Arch Orthop Trauma Surg. 2010;130(8):1037–1040. doi: 10.1007/s00402-010-1125-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pawaskar AC, Kekatpure A, Cho NS, Rhee YG, Jeon IH. Magnetic resonance appearance of bioabsorbable anchor screws for double row arthroscopic rotator cuff repairs. Indian J Orthop. 2015;49(2):164–170. doi: 10.4103/0019-5413.152452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Freehill MQ, Harms DJ, Huber SM, Atlihan D, Buss DD. Poly-L-lactic acid tack synovitis after arthroscopic stabilization of the shoulder. Am J Sports Med. 2003;31(5):643–647. doi: 10.1177/03635465030310050201. [DOI] [PubMed] [Google Scholar]
- 35.Marecek GS, Saltzman MD. Complications in shoulder arthroscopy. Orthopedics. 2010;33(7):492–497. doi: 10.3928/01477447-20100526-15. [DOI] [PubMed] [Google Scholar]
- 36.Hansen BP, Beck CL, Beck EP, Townsley RW. Postarthroscopic glenohumeral chondrolysis. Am J Sports Med. 2007;35(10):1628–1634. doi: 10.1177/0363546507304136. [DOI] [PubMed] [Google Scholar]
- 37.Bailie DS, Ellenbecker TS. Severe chondrolysis after shoulder arthroscopy: a case series. J Shoulder Elb Surg. 2009;18(5):742–747. doi: 10.1016/j.jse.2008.10.017. [DOI] [PubMed] [Google Scholar]
- 38.Mack LA, Nyberg DA, Matsen FR, 3rd, Kilcoyne RF, Harvey D. Sonography of the postoperative shoulder. AJR Am J Roentgenol. 1988;150(5):1089–1093. doi: 10.2214/ajr.150.5.1089. [DOI] [PubMed] [Google Scholar]
- 39.Okoroha KR, Mehran N, Duncan J, Washington T, Spiering T, Bey MJ, van Holsbeeck M, Moutzouros V. Characterization of rotator cuff tears: ultrasound versus magnetic resonance imaging. Orthopedics. 2017;40(1):e124–ee30. doi: 10.3928/01477447-20161013-04. [DOI] [PubMed] [Google Scholar]
- 40.Crass JR, Craig EV, Feinberg SB. Sonography of the postoperative rotator cuff. AJR Am J Roentgenol. 1986;146(3):561–564. doi: 10.2214/ajr.146.3.561. [DOI] [PubMed] [Google Scholar]
- 41.Lee MH, Sheehan SE, Orwin JF, Lee KS. Comprehensive shoulder US examination: a standardized approach with multimodality correlation for common shoulder disease. Radiographics. 2016;36(6):1606–1627. doi: 10.1148/rg.2016160030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jacobson JA. Shoulder US: anatomy, technique, and scanning pitfalls. Radiology. 2011;260(1):6–16. doi: 10.1148/radiol.11101082. [DOI] [PubMed] [Google Scholar]
- 43.Moosikasuwan JB, Miller TT, Burke BJ. Rotator cuff tears: clinical, radiographic, and US findings. Radiographics. 2005;25(6):1591–1607. doi: 10.1148/rg.256045203. [DOI] [PubMed] [Google Scholar]
- 44.Gulotta LV, Nho SJ, Dodson CC, Adler RS, Altchek DW, MacGillivray JD, HSS Arthroscopic Rotator Cuff Registry Prospective evaluation of arthroscopic rotator cuff repairs at 5 years: part II--prognostic factors for clinical and radiographic outcomes. J Shoulder Elb Surg. 2011;20(6):941–946. doi: 10.1016/j.jse.2011.03.028. [DOI] [PubMed] [Google Scholar]
- 45.Yoo HJ, Choi JY, Hong SH, Kang Y, Park J, Kim SH, Kang HS. Assessment of the postoperative appearance of the rotator cuff tendon using serial sonography after arthroscopic repair of a rotator cuff tear. J Ultrasound Med. 2015;34(7):1183–1190. doi: 10.7863/ultra.34.7.1183. [DOI] [PubMed] [Google Scholar]
- 46.Fealy S, Adler RS, Drakos MC, Kelly AM, Allen AA, Cordasco FA, Warren RF, O'Brien SJ. Patterns of vascular and anatomical response after rotator cuff repair. Am J Sports Med. 2006;34(1):120–127. doi: 10.1177/0363546505280212. [DOI] [PubMed] [Google Scholar]
- 47.Miller BS, Downie BK, Kohen RB, Kijek T, Lesniak B, Jacobson JA, Hughes RE, Carpenter JE. When do rotator cuff repairs fail? Serial ultrasound examination after arthroscopic repair of large and massive rotator cuff tears. Am J Sports Med. 2011;39(10):2064–2070. doi: 10.1177/0363546511413372. [DOI] [PubMed] [Google Scholar]
- 48.Chun KA, Cho KH. Postoperative ultrasonography of the musculoskeletal system. Ultrasonography. 2015;34(3):195–205. doi: 10.14366/usg.15006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tham ER, Briggs L, Murrell GA. Ultrasound changes after rotator cuff repair: is supraspinatus tendon thickness related to pain? J Shoulder Elb Surg. 2013;22(8):e8–15. doi: 10.1016/j.jse.2012.10.047. [DOI] [PubMed] [Google Scholar]
- 50.De Filippo M, Bertellini A, Sverzellati N, Pogliacomi F, Costantino C, Vitale M, Zappia M, Corradi D, Garlaschi G, Zompatori M. Multidetector computed tomography arthrography of the shoulder: diagnostic accuracy and indications. Acta Radiol. 2008;49(5):540–549. doi: 10.1080/02841850801935559. [DOI] [PubMed] [Google Scholar]
- 51.JH O, Kim JY, Choi JA, Kim WS. Effectiveness of multidetector computed tomography arthrography for the diagnosis of shoulder pathology: comparison with magnetic resonance imaging with arthroscopic correlation. J Shoulder Elb Surg. 2010;19(1):14–20. doi: 10.1016/j.jse.2009.04.012. [DOI] [PubMed] [Google Scholar]


