Abstract
Purpose of Review
The purpose of this review is to discuss the indications for reverse shoulder arthroplasty (RSA) in the treatment of massive rotator cuff tear (MCT), review the reported outcomes in the literature, and outline our approach and surgical technique for treating these patients.
Recent Findings
While RSA remains a successful and well-accepted treatment for cuff tear arthropathy (CTA), management of MCT in the absence of arthritis is controversial. In this particular setting, patients best suited for RSA are elderly, lower-demand individuals with chronic, irreparable MCT, and pseudoparalysis. Age < 60, better pre-operative function and upper extremity neurologic dysfunction are potential risk factors for poor outcome with RSA in this population. Long-term follow-up studies of RSA for CTA and MCT show good functional outcomes and implant survival > 90% at 10 years.
Summary
Treatment of MCT must be individualized for each patient. When patient selection is optimized, RSA is a reliable means of relieving pain and improving function with excellent success. Further investigation is necessary to better define its indications and assess the role of alternative, joint-salvaging procedures.
Keywords: Acromiohumeral interval, Fatty atrophy, Massive rotator cuff tear, Reverse shoulder arthroplasty, Rotator cuff repair
Introduction
Rotator cuff tears are common injuries in the adult population, with a prevalence as high as 22% in patients older than 65 [1]. Appropriate management depends on multiple factors, including both the size and chronicity of the tear, along with patient-specific factors such as age, functional status, and associated medical comorbidities. Rotator cuff tears have previously been classified based on tear size to help better characterize the pathology and guide treatment options. Although there is no consensus on the precise definition of a massive cuff tear, criteria such as tears ≥ 5 cm in greatest dimension and those involving two or more tendons are commonly referenced [2, 3]. Massive cuff tears (MCT) pose a challenge to surgeons given their size and potential for irreparability. They are also of increasing importance and interest, as they are reported to represent up to 40% of all rotator cuff tears [4].
The treatment options for MCT are numerous. In the majority of cases, management should start with conservative measures with an emphasis on physical therapy. Shoulder rehabilitation exercises focusing on deltoid and periscapular muscle strengthening can help restore functional shoulder range of motion, even in the absence of a fully intact rotator cuff. In a traumatic setting, a period of rest, ice, and activity modification may be necessary to alleviate symptoms. NSAIDs and corticosteroid injections can also help to relieve pain. When conservative management is unsuccessful, surgical intervention is often warranted. Specific options include debridement with and without subacromial decompression, biceps tenotomy, partial or complete rotator cuff repair, tendon transfer, various grafting and tendon augmentation techniques, superior capsular reconstruction, and reverse total shoulder arthroplasty. This article will focus on the rationale and indications for reverse shoulder arthroplasty (RSA) in the setting of MCT. We will also review the outcomes reported in the literature as well as our personal experience, technique, and general considerations in treating these patients.
Pathomechanics
The rotator cuff muscle-tendon unit serves as a critical dynamic stabilizer of the shoulder joint. The force couples of the rotator cuff in both the transverse and coronal planes provide a “concavity compression” effect, which forces the humeral head into the center of the glenoid [5, 6]. This compensates for the lack of inherent stability of the glenohumeral articulation and creates a stable fulcrum, allowing the more powerful deltoid muscle to elevate the arm and position the hand in space. In the setting of a massive rotator cuff tear, these force couples are disrupted, and the compressive stabilizing effect is lost. Without a stable fulcrum to resist translation of the humeral head, shoulder instability and dysfunction occurs. Clinically, this can manifest as pseudoparalysis, in which a patient is unable to actively abduct or elevate the arm despite full passive motion. When the force couples cannot be re-established by other surgical means, the inverse ball and socket design of the RSA prosthesis provides an inherently stable fulcrum at the shoulder joint and allows the deltoid to regain its normal function (Fig. 1).
Fig. 1.
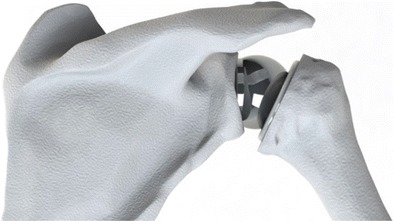
Reverse ball and socket total shoulder arthroplasty design
Indications for RSA
Popular use of the reverse total shoulder prosthesis began to flourish after it was re-engineered by Grammont in 1985 [7]. While initial reports were limited to the treatment of rotator cuff tear arthropathy, implant design and surgical technique have evolved, and the indications for RSA have rapidly expanded to include treatment of acute 4-part proximal humerus fractures [8–10], humeral fracture sequelae [11, 12], osteoarthritis with glenoid bone loss [13], revision arthroplasty [14–16], and oncologic reconstruction [17], as well as the treatment of MCT with and without glenohumeral arthritis [18–21]. This article focuses on those pathologies specifically relating to primary deficiency of the rotator cuff.
Massive Cuff Tear with Glenohumeral Arthritis
The primary indication for RSA has been and remains rotator cuff deficiency in the setting of painful glenohumeral arthritis. The term “cuff tear arthropathy” was first formally described by Neer in 1983, as a complex condition of shoulder dysfunction due to rotator cuff deficiency [22]. The proposed pathomechanics involve mechanical factors brought on by superior migration of the humeral head and gross instability as well as nutritional factors related to the reduced motion with resultant disuse osteopenia and the leakage of synovial fluid with loss of normal joint pressure. Together these factors lead to a characteristic pattern of joint destruction [22]. Radiographic changes are progressive and include narrowing of the acromiohumeral interval, acetabularization of the acromion, superior erosion of the glenoid, femoralization and ultimate collapse of the humeral head, and medialization of the joint [23]. Hamada et al. proposed a radiographic classification of massive cuff tears reflecting these changes (Table 1). Stages 4 and 5 of the classification demonstrate pathologic changes of the glenohumeral joint and, in this article, are more generally referred to as cuff tear arthropathy. Stages 1, 2, and 3 indicate MCT without arthritis.
Table 1.
Hamada classification of radiographic changes in MCT [23]
| Grade 1 | AHI ≥ 6 mm |
| Grade 2 | AHI ≤ 5 mm |
| Grade 3 | Grade 2 + “acetabulization” of the acromion |
| Grade 4 | Grade 3 + glenohumeral arthritis |
| Grade 5 | Grade 4 + humeral head collapse (cuff tear arthropathy) |
MCT massive cuff tear, AHI acromiohumeral interval
Historically, cuff tear arthropathy (CTA) was a very difficult problem to treat, as neither rotator cuff repair nor conventional shoulder prostheses were able to successfully address the pathology. Surgical options, including hemiarthroplasty and other more constrained implants, offered less than satisfactory outcomes and were often fraught with complications [22, 24–28]. The advent of RSA was revolutionary in the treatment of this condition. The reverse prosthesis was able to address both the glenohumeral joint pathology and the instability incurred from the deficient rotator cuff. As our knowledge has increased and technology has advanced, surgical technique and implant design have improved. With the modern reverse prosthesis, we are able to offer these patients a reliable means of relieving pain and improving function with excellent success [18, 29–31].
Massive Cuff Tear without Glenohumeral Arthritis
While CTA is a clear indication for RSA, the optimal treatment for patients with MCT in the absence of arthritis is less obvious and remains controversial. It is imperative that treatment will be individualized for every patient. The first question is that of the reparability of the tendon. The success of rotator cuff repair (RCR) depends on a number of patient-related factors, including age, health, and pre-operative function as well as certain characteristics specific to the tear, including size, chronicity, and quality of the remaining cuff [32–37]. There is also the variable of the surgeon’s technical skill and expertise. Advances in technology and improved repair techniques have expanded our ability to repair larger tears. However, in the case of a chronic, MCT, the tendon is often retracted and atrophic, and obtaining an appropriately tensioned anatomic repair may not be possible [38]. Furthermore, poor tendon quality and host biology, as in the case of an elderly patient, may limit the ability of the repaired tendon to heal [39]. All of these factors can have an impact on the feasibility of tendon repair and subsequent healing and should be used to guide treatment.
For the massive cuff tears deemed to be irreparable, surgical options are limited. There are newer techniques such as allograft tissue augmentation and superior capsular reconstruction, both of which have shown promise in early reports, but there is relatively little data to support their widespread use at this time [40–42]. In certain patients with MCT without osteoarthritis, reverse shoulder arthroplasty may be a reasonable solution. Even in the absence of articular cartilage pathology, RSA has proven to be a reliable option to relieve pain and restore function [20]. It has been a mission of ours to study these patients carefully and analyze our own surgical results so that we may better understand who might best benefit from RSA in this population and optimize patient selection.
A patient being considered for RSA should have a painful, irreparable rotator cuff tear and evidence of pseudoparalysis with active forward elevation less than 90° [20, 43]. One should look closely at the patient’s age, health status, and comorbid conditions. While multiple reports have shown RSA to be a reliable procedure with good outcomes in patients less than 65 years of age [44, 45, 46•, 47], Muh et al. reported that patient satisfaction was lower for younger patients [45]. We have recently found younger age to be a risk factor for poor functional improvement after RSA in the specific setting of MCT without arthritis [48•]. Accordingly, we rarely consider performing RSA as an index procedure for MCT without arthritis in patients < 65, and one should exercise caution in this population. However, the elderly patient, particularly if they have poor prognostic indicators for rotator cuff healing such as smoking or diabetes, may be ideally served with RSA [43, 49, 50].
Careful consideration should be given to a patient’s prior history of shoulder surgery, particularly a previously failed attempt at RCR. Denard et al. previously found that revision RCR was able to reverse pseudoparalysis in only 43% of patients with MCT [51]. Moreover, Shamshudin et al. reported that revision cuff repair was associated with declining functional outcomes after 6 months, more re-tears, more pain with activities of daily living, lower activity level, and decreased overall satisfaction at 2 years post-operatively compared with primary cuff repair [52•]. Importantly, Sadoghi et al. found that previously failed arthroscopic rotator cuff surgery did not have a negative impact on outcomes and survival rate after reverse shoulder arthroplasty [53]. Thus, RSA is an excellent salvage operation in these patients and may be more prudent than a repeat attempt at repair.
In general, advanced imaging should reveal atrophy and fatty infiltration of the rotator cuff, as this has been shown to be an indicator of poor outcomes with attempted repair [33, 34, 54–56] (Fig. 2). Dwyer et al. found medial-lateral tear dimension, as measured on a coronal MRI, to be the most predictive of tear irreparability [57]. Additionally, we carefully assess the patient’s shoulder stability. On physical exam, anterosuperior escape of the humeral head can be observed with attempted abduction of the arm, indicating dynamic instability [43]. The loss of humeral containment within the coracoacromial arch accounts for the observed pseudoparalysis. These patients may or may not have a narrowed acromiohumeral interval on radiographic imaging, which indicates more chronic instability and subluxation. Patients with a painful MCT, obvious instability, and moderate to severe anterosuperior escape are appropriate candidates for RSA (Fig. 3).
Fig. 2.
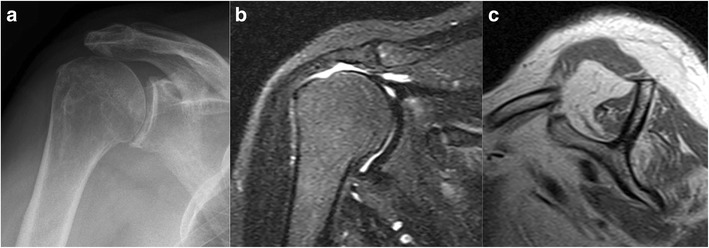
Pre-operative imaging of a 72-year-old male with a painful, massive rotator cuff tear and pseudoparalysis. a AP radiograph with a narrowed acromiohumeral interval and preserved glenohumeral joint. b Coronal MRI shows a complete disruption of the rotator cuff with medial retraction of the supraspinatus tendon. c Sagittal MRI shows extensive fatty infiltration within the rotator cuff musculature. The patient underwent successful reverse shoulder arthroplasty
Fig. 3.
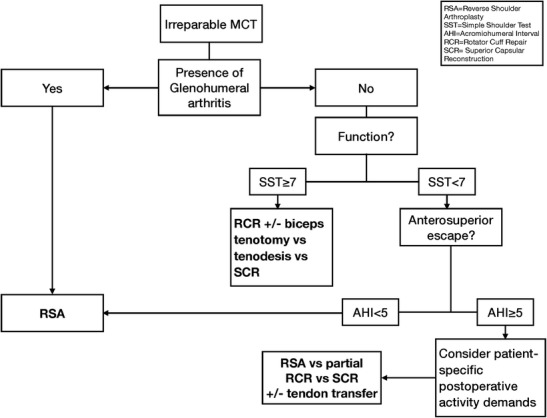
A proposed treatment algorithm for massive, irreparable rotator cuff tear
Contraindications
RSA is specifically contraindicated in patients with a nonfunctional deltoid. This may be a result of cervical radiculopathy, axillary nerve injury, or deltoid injury from prior open shoulder surgery. Careful physical examination is critical and can differentiate deltoid weakness from rotator cuff deficiency. Furthermore, a potentially reparable MCT in a younger patient with mild to no osteoarthritis of the glenohumeral joint is not an appropriate indication for RSA. Similarly, acute traumatic MCT in active individuals is best managed with an attempt at arthroscopic RCR.
In an attempt to better delineate which patients might be at greater risk for having a poor outcome with RSA, we looked closely at a group of 74 patients who underwent RSA for MCT without osteoarthritis [48•]. Out of the entire population, we selected out those who were deemed to have a poor outcome defined by an improvement in SST score of 1 point or less. When we compared this group with the rest of the study cohort, the risk factors for poor improvement following RSA were age < 60, pre-operative SST score ≥ 7, and upper extremity neurologic dysfunction [48•]. Similarly, Werner et al. found that high pre-operative function as measured by increased ASES scores was associated with poor functional improvement after RSA [58•]. Previously, the absence of pseudoparalysis has been reported to be associated with worse outcomes after RSA [20, 59]. Interestingly, we did not find pre-operative active forward elevation > 90 degrees to be an independent risk factor for poor functional improvement [48•]. While we would certainly caution against using RSA in patients with preserved forward elevation, there may be a role for RSA in a small subset of these patients. Specifically, patients with a painful, massive rotator cuff tear with loss of active external rotation and preserved forward elevation may do well with an RSA and a simultaneous latissimus dorsi transfer. For this reason, we prefer to focus more on one’s overall pre-operative function as opposed to any particular motion measurement, and we recommend exercising caution when considering RSA in patients with SST ≥7 [48•].
Outcomes
Frankle et al. evaluated 60 patients who underwent RSA for rotator cuff tear arthropathy and glenohumeral osteoarthritis [19]. At a mean follow-up of 33 months, there were significant improvements in forward elevation (55 to 105), abduction (41 to 102) and external rotation (12 to 41). VAS pain scores improved from 6.2 to 2.2, ASES scores improved from 34.3 to 68.2, and 95% of patients were satisfied with the procedure. An overall complication rate of 17% was observed with no cases of scapular notching and a 12% revision rate due to glenoid baseplate failure [19]. After a critical review of failure modes and a thorough biomechanical investigation, the baseplate was re-designed to incorporate 5.0-mm peripheral locking screws [60]. A subsequent report on 96 RSAs performed for various indications showed an improved complication rate of 9.4% with no cases of mechanical baseplate failure [18]. These functional improvements with RSA for CTA have been reproducible in a large number of patients by multiple other surgeons with comparable complication rates [29–31, 61].
In the setting of massive irreparable rotator cuff tear without glenohumeral arthritis, Mulieri et al. reported on 60 patients who had RSA specifically for this indication [20]. They observed significant improvements in range of motion, VAS pain scores, and all functional outcome measures investigated (ASES, SST, and SF-36). There was an overall complication rate of 20% [20]. In 2015, Hartzler et al. reported on another 74 patients with RSA for the same indication, and similar results were observed with significant improvements in range of motion and all outcome measures [48•]. The overall complication rate was 17% with a 1.4% revision rate at a minimum of 2-year follow-up. Recent analysis of a cohort of 92 patients over the age of 65 who underwent RSA for MCT without osteoarthritis by the senior author (M.A.F.) again demonstrated improvements in all clinical and functional outcome measures with a re-operation rate of 2.2% at a minimum of 2 years. This outcome data from our institution is summarized in Table 2.
Table 2.
Outcome data for 92 patients > 65 years of age who underwent RSA by the senior author (M.A.F.) for MCT without arthritis at a minimum 2-year follow-up
| Mean ± range | p value | ||
|---|---|---|---|
| Pre-operative | Post-operative | ||
| FE | 57 ± 34 | 136 ± 46 | < 0.0001 |
| AB | 53 ± 35 | 129 ± 44 | < 0.0001 |
| ER | 32 ± 28 | 57 ± 32 | < 0.0001 |
| IR | 2.6 ± 1.8 | 4.7 ± 2.4 | < 0.0001 |
| VSAS pain | 5.6 ± 2.5 | 1.6 ± 2.5 | < 0.0001 |
| ASES | 39 ± 16 | 77 ± 22 | < 0.0001 |
| SST | 1.9 ± 1.7 | 7.1 ± 3.4 | < 0.0001 |
FE forward elevation, AB abduction, ER external rotation, IR internal rotation, VAS visual analog score, ASES American Shoulder and Elbow Surgeons score, SST Simple Shoulder Test score
A recent systematic review of the literature by Petrillo et al. evaluated 408 RSAs in 396 patients, all performed for either CTA or massive, irreparable rotator cuff tear [62•]. Post-operatively, there was statistically significant improvement in all clinical outcome scores and improved range of motion in all planes. They found an overall complication rate of 17.4%, resulting in a revision rate of 7.3% [62•]. A more recent series by Groh and Groh, which included RSA for indications other than rotator cuff pathology, observed a more favorable overall complication rate of 7% in 114 shoulders and a re-operation rate of 5.3% [63]. Moreover, when revision arthroplasties were excluded, the complication rate in 93 primary RSAs was 4.3% with a re-operation rate of 2.2%. These improved complication and re-operation rates may reflect more recent mastery of the procedure.
Implant Survival and Long-term Follow-up
One concern regarding the routine use of RSA, particularly in younger patients, has been that of the longevity of the implant. This is of critical importance given the known high complication rate and technical difficulty of revision RSA [64•, 65]. Two early reports of the long-term survivorship of RSA both demonstrated a survival rate of 95% at 120 and 97 months, respectively, when performed specifically for MCT with arthropathy [66, 67]. More recently, Bacle et al. observed an implant survival rate of 93% at a minimum of 10 years after RSA performed for multiple indications [68•]. We recently reviewed our 10-year follow-up data and found implant survivorship to be 91% [69•].
While post-operative complications and long-term implant survival appear to be reasonable, there is the additional concern of potentially decreasing functional outcomes over time with RSA. Both Guery et al. and Sirveaux et al. noted deterioration in functional scores after 6 years [66, 67]. A multicenter study by Favard et al. reviewed a large number of RSAs performed by multiple surgeons using different approaches and implants and found a decrease in Constant-Murley scores after 8 years [70]. Most recently, Bacle et al. found a similar decrease in Constant-Murley scores at a long-term follow-up (average 150 months) when compared to the scores at mid-term follow-up (average 39 months) [68•]. It is worth noting that RSAs performed for the indications of CTA and MCT were associated with less functional decline. In a review of our own most recent follow-up data, we found that our patients maintained their improvements in ASES, SST, and pain scores at 10 years [69•]. We observed a small decrease in range of motion in all planes between the 5-year and 10-year studies, which we attribute to the advanced age of the cohort (average age of 78 years at last follow-up). It has been observed in most series that RSA performed for MCT and CTA has faired better in the short and long term when compared to RSA performed for other indications [21, 66, 68•, 70].
Surgical Technique
We prefer a standard deltopectoral approach to the shoulder. The incision begins 5 cm medial to the acromioclavicular joint at the anterior border of the clavicle and extends distally over the coracoid to the lateral aspect of the humerus at the deltoid insertion. Upon subcutaneous dissection to the deltopectoral interval, the cephalic vein is taken medially with the pectoralis, taking care to cauterize all lateral tributaries to the deltoid. The medial border of the deltoid is elevated. It is essential to release all subdeltoid adhesions and debride the bursa from the subdeltoid and subacromial spaces. The subscapularis muscle is released directly off of the bone at the lesser tuberosity. The subscapularis should be adequately mobilized to facilitate later repair by debriding capsular tissue and releasing adhesions deep to the muscle belly from the anterior wall of the scapula, as well as those from the subcoracoid space. The proximal humerus is dislocated anteriorly, and an anatomical humeral head cut is made. Loose edges of irreparable rotator cuff should be debrided to prevent impingement with the humerosocket and glenosphere. The glenoid is exposed and prepared with thorough debridement of the labral tissue circumferentially.
A central line for the baseplate center screw is utilized, taking care not to distalize the glenoid component (Fig. 4). Many patients with chronic rotator cuff deficiency will have pre-existing superior humeral head migration. In this setting, distalizing the components may produce excessive soft tissue tension and generate undue stress across the implant-bone interface and on the acromion. Furthermore, distalization of the humerus disrupts the normal glenohumeral joint mechanics. Particularly in the setting of MCT without osteoarthritis when the bony structures are relatively preserved, we feel that it is critical to restore the patient’s anatomy to as close to normal as possible. Most patients have some residual rotator cuff tissue attached to the humerus, and by restoring the native anatomy, one can appropriately tension the remaining cuff and maximize its function. We find this to be critical to preserve and/or restore active external rotation after RSA. Thus, we recommend the use of an anatomic humerosocket neck-shaft angle and a lateralized glenosphere with an anatomic center of rotation. After the glenosphere is inserted, the humerus is prepared for component implantation. The humeral cup should be at the level of the cortical rim such that it is inset into the humerus (Fig. 5). After trialing and final implantation of the components, the subscapularis is repaired whenever possible.
Fig. 4.
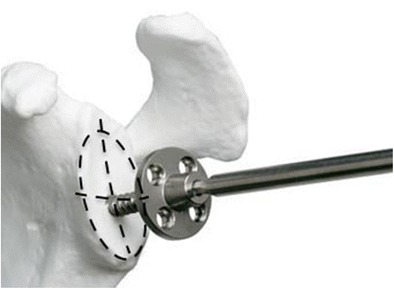
The central screw of the glenoid baseplate is placed at the centerline of the glenoid fossa
Fig. 5.
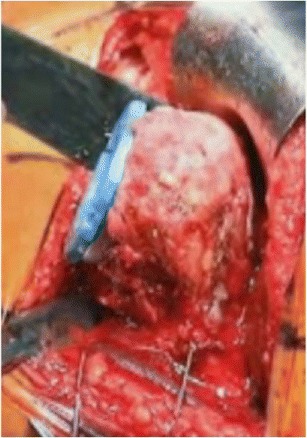
Intraoperative photo during humeral component trailing. The humeral cup should be at the level of the cortical rim so that it is inset into the humerus to avoid unnecessary distalization
General Considerations
While RSA has proven to be a reliable and lasting means of offering pain relief and improving function in patients with massive, irreparable rotator cuff tears, we must continue to exercise caution against its widespread use, particularly in patients without arthropathy. In addition to the concerns over potential surgical complications and long-term implant survivability, there is the separate issue of its increased cost. Makhni et al. analyzed the cost-effectiveness of RSA versus arthroscopic RCR in patients with large or massive rotator cuff tear without arthropathy and found repair to be a more cost-effective procedure based on the available data in the literature [71•]. This was largely due to the increased cost of implants and inpatient hospital stays with RSA, combined with the good functional results after RCR. Indeed, Henry et al. performed a meta-analysis of 954 patients with chronic, MCT who underwent either complete or partial arthroscopic RCR and found consistent improvement in range of motion and functional outcome scores [72•]. It is worth noting that the average pre-operative forward flexion of patients included in the meta-analysis was 125 degrees, which is much higher than that seen in the patients we would normally be considering for RSA. Nevertheless, the necessity of a complete repair and importance of tendon healing has recently been called into question, as reasonable short-term results have been observed even with partial repairs and in the absence of healing [33, 72•, 73, 74•]. Thus, an attempt at RCR may be a reasonable index procedure for MCT without arthritis, even in an elderly patient when the odds of obtaining an anatomic repair are minimal. Certainly, those patients unwilling to undergo a joint replacement procedure and/or have more labor-intensive lifestyles are best treated with complete or partial RCR. The observed re-tear rate of 79% in the meta-analysis by Henry et al. is worrisome, however, and should be discussed with patients prior to surgery [72•]. Although this did not seem to have an identifiable impact on patient outcomes at early follow-up in their study, other authors have reported recurrent tears to be associated with worse outcomes, and the long-term consequences of re-tear are unknown [75]. As mentioned by Makhni et al., RSA may still be appropriate and cost-effective as an index procedure for MCT without arthritis when the likelihood of a re-tear is high, as is the case for the patients for whom we consider this procedure [71•].
Future Directions
Future studies should closely examine the long-term functional results after both RSA and RCR for MCT. Despite the good outcomes after RSA at 10 years from recent reports, patients are living longer, and we still cannot predict how these implants will perform at longer term follow-up. We also need to better understand the role of superior capsular reconstruction, as this may prove to be a good option for those patients without arthritis who suffer from pseudoparalysis and isolated anterosuperior escape. As surgeons, careful and critical analysis of our results with these different procedures should help us continue to better define their indications and maximize their benefit while minimizing complications and the overall burden to the healthcare system.
Conclusions
RSA has shown excellent results for the treatment of massive cuff tear with and without glenohumeral arthritis. Cuff tear arthropathy remains the classic indication for RSA. Even in the absence of arthritis, RSA may be a reasonable option for certain patients with a massive, irreparable tear. Patient history and peri-operative activity demands should be investigated thoroughly so that treatment can be individualized for each patient. Ideal candidates for RSA in this population are elderly, lower-demand individuals with chronic rotator cuff deficiency, pseudoparalysis, and clinical and/or radiographic evidence of anterosuperior escape. RSA is also a reliable salvage procedure in the setting of prior failed RCR. Risk factors for poor outcome with RSA for MCT without arthritis include age < 60, better pre-operative function, and upper extremity neurologic dysfunction. With regard to operative technique, we recommend restoring the patient’s native anatomy to as close to normal as possible by using an implant with a lateralized center of rotation and avoiding distalization of the humerus. Future studies should further investigate the role of superior capsular reconstruction, which may be a reasonable alternative for some of these patients.
Compliance with Ethical Standards
Conflict of Interest
Mark Frankle reports personal fees from DJO Surgical and Cayenne Medical, outside of the submitted work. In addition, he reports patent 6,790,234, issued to DJO Surgical. The other authors declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Footnotes
This article is part of the Topical Collection on Rotator Cuff Repair
References
Papers of particular interest, published recently, have been highlighted as: • Of importance
- 1.Fehringer EV, Sun J, VanOeveren LS, Keller BK, Matsen FA., III Full-thickness rotator cuff tear prevalence and correlation with function and co-morbidities in patients sixty-five years and older. J Shoulder Elb Surg. 2008;17(6):881–885. doi: 10.1016/j.jse.2008.05.039. [DOI] [PubMed] [Google Scholar]
- 2.DeOrio JK, Cofield RH. Results of a second attempt at surgical repair of a failed initial rotator-cuff repair. J Bone Joint Surg Am. 1984;66(4):563–567. doi: 10.2106/00004623-198466040-00011. [DOI] [PubMed] [Google Scholar]
- 3.Gerber C, Fuchs B, Hodler J. The results of repair of massive tears of the rotator cuff. J Bone Joint Surg Am. 2000;82(4):505–515. doi: 10.2106/00004623-200004000-00006. [DOI] [PubMed] [Google Scholar]
- 4.Bedi A, Dines J, Warren RF, Dines DM. Massive tears of the rotator cuff. J Bone Joint Surg Am. 2010;92(9):1894–1908. doi: 10.2106/JBJS.I.01531. [DOI] [PubMed] [Google Scholar]
- 5.Lippitt S, Matsen F. Mechanisms of glenohumeral joint stability. Clin Orthop Relat Res. 1993;291:20–28. [PubMed] [Google Scholar]
- 6.Lipitt SB, Vanderhooft JE, Harris SL, Sidles JA, Harryman DT, II, Matsen FA., III Glenohumeral stability from concavity-compression: a quantitative analysis. J Shoulder Elb Surg. 1993;2(1):27–35. doi: 10.1016/S1058-2746(09)80134-1. [DOI] [PubMed] [Google Scholar]
- 7.Grammont PM, Baulot E. Delta shoulder prosthesis for rotator cuff rupture. Orthopedics. 1993;16(1):65–68. doi: 10.3928/0147-7447-19930101-11. [DOI] [PubMed] [Google Scholar]
- 8.Boyle MJ, Youn SM, Frampton CM, Ball CM. Functional outcomes of reverse shoulder arthroplasty compared with hemiarthroplasty for acute proximal humeral fractures. J Shoulder Elb Surg. 2013;22(1):32–37. doi: 10.1016/j.jse.2012.03.006. [DOI] [PubMed] [Google Scholar]
- 9.Cuff DJ, Pupello DR. Comparison of hemiarthroplasty and reverse shoulder arthroplasty for the treatment of proximal humeral fractures in elderly patients. J Bone Joint Surg Am. 2013;95(22):2050–2055. doi: 10.2106/JBJS.L.01637. [DOI] [PubMed] [Google Scholar]
- 10.Sebastia-Forcada E, Cebrian-Gomez R, Lisaur-Utrilla A, Gil-Guillen V. Reverse shoulder arthroplasty versus hemiarthroplasty for acute proximal humeral fractures. A blinded, randomized, controlled, prospective study. J Shoulder Elb Surg. 2014;23(10):1419–1426. doi: 10.1016/j.jse.2014.06.035. [DOI] [PubMed] [Google Scholar]
- 11.Raiss P, Edwards TB, da Silva MR, Bruckner T, Loew M, Walch G. Reverse shoulder arthroplasty for the treatment of nonunions of the surgical neck of the proximal part of the humerus (type-3 fracture sequelae) J Bone Joint Surg Am. 2014;96(24):2070–2076. doi: 10.2106/JBJS.N.00405. [DOI] [PubMed] [Google Scholar]
- 12.Willis M, Min W, Brooks JP, Mulieri P, Walker M, Pupello D, Frankle M. Proximal humeral malunion treated with reverse shoulder arthroplasty. J Shoulder Elb Surg. 2012;21(4):507–513. doi: 10.1016/j.jse.2011.01.042. [DOI] [PubMed] [Google Scholar]
- 13.Mizuno N, Denard PJ, Raiss P, Walch G. Reverse total shoulder arthroplasty for primary glenohumeral osteoarthritis in patients with a biconcave glenoid. J Bone Joint Surg Am. 2013;95(14):1297–1304. doi: 10.2106/JBJS.L.00820. [DOI] [PubMed] [Google Scholar]
- 14.Kelly JD, 2nd, Zhao JX, Hobgood ER, Norris TR. Clinical results of revision shoulder arthroplasty using the reverse prosthesis. J Shoulder Elb Surg. 2012;21(11):1516–1525. doi: 10.1016/j.jse.2011.11.021. [DOI] [PubMed] [Google Scholar]
- 15.Levy J, Frankle M, Mighell M, Pupello D. The use of the reverse shoulder prosthesis for the treatment of failed hemiarthroplasty for proximal humeral fracture. J Bone Joint Surg Am. 2007;89(2):292–300. doi: 10.2106/JBJS.E.01310. [DOI] [PubMed] [Google Scholar]
- 16.Walker M, Willis MP, Brooks JP, Pupello D, Mulieri PJ, Frankle MA. The use of the reverse shoulder arthroplasty for treatment of failed total shoulder arthroplasty. J Shoulder Elb Surg. 2012;21(4):514–522. doi: 10.1016/j.jse.2011.03.006. [DOI] [PubMed] [Google Scholar]
- 17.De Wilde L, Boileau P, Van der Bracht H. Does reverse shoulder arthroplasty for tumors of the proximal humerus reduce impairment? Clin Orthop Relat Res. 2011;469(9):2489–2495. doi: 10.1007/s11999-010-1758-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cuff D, Pupello D, Virani N, Levy J, Frankle M. Reverse shoulder arthroplasty for the treatment of rotator cuff deficiency. J Bone Joint Surg Am. 2008;90(6):1244–1251. doi: 10.2106/JBJS.G.00775. [DOI] [PubMed] [Google Scholar]
- 19.Frankle M, Siegal S, Pupello D, Saleem A, Mighell M, Vasey M. The reverse shoulder prosthesis for glenohumeral arthritis associated with severe rotator cuff deficiency. A minimum two-year follow-up study of sixty patients. J Bone Joint Surg Am. 2005;87(8):1697–1705. doi: 10.2106/JBJS.D.02813. [DOI] [PubMed] [Google Scholar]
- 20.Mulieri P, Dunning P, Klein S, Pupello D, Frankle M. Reverse shoulder arthroplasty for the treatment of irreparable rotator cuff tear without glenohumeral arthritis. J Bone Joint Surg Am. 2010;92(15):2544–2556. doi: 10.2106/JBJS.I.00912. [DOI] [PubMed] [Google Scholar]
- 21.Wall B, Nove-Josserand L, O'Connor DP, Edwards TB, Walch G. Reverse total shoulder arthroplasty: a review of results according to etiology. J Bone Joint Surg Am. 2007;89(7):1476–1485. doi: 10.2106/JBJS.F.00666. [DOI] [PubMed] [Google Scholar]
- 22.Neer CS, 2nd, Craig EV, Fukuda H. Cuff-tear arthropathy. J Bone Joint Surg Am. 1983;65(9):1232–1244. doi: 10.2106/00004623-198365090-00003. [DOI] [PubMed] [Google Scholar]
- 23.Hamada K, Fukuda H, Mikasa M, Kobayashi Y. Roentgenographic findings in massive rotator cuff tears. A long-term observation. Clin Orthop Relat Res. 1990:92–6. [PubMed]
- 24.Field LD, Dines DM, Zabinski SJ, Warren RF. Hemiarthroplasty of the shoulder for rotator cuff arthropathy. J Shoulder Elb Surg. 1997;6(1):18–23. doi: 10.1016/S1058-2746(97)90066-5. [DOI] [PubMed] [Google Scholar]
- 25.Nwakama AC, Cofield RH, Kavanagh BF, Loehr JF. Semiconstrained total shoulder arthroplasty for glenohumeral arthritis and massive rotator cuff tearing. J Shoulder Elb Surg. 2000;9(4):302–307. doi: 10.1067/mse.2000.106467. [DOI] [PubMed] [Google Scholar]
- 26.Post M, Jablon M. Constrained total shoulder arthroplasty: long-term follow-up observations. Clin Orthop Relat Res. 1983;173:109–116. [PubMed] [Google Scholar]
- 27.Sanchez-Sotelo J, Cofield RH, Rowland CM. Shoulder hemiarthroplasty for glenohumeral arthritis associated with severe rotator cuff deficiency. J Bone Joint Surg Am. 2001;83(12):1814–1822. doi: 10.2106/00004623-200112000-00008. [DOI] [PubMed] [Google Scholar]
- 28.Zuckerman JD, Scott AJ, Gallagher MA. Hemiarthroplasty for cuff tear arthropathy. J Shoulder Elb Surg. 2000;9(3):169–172. doi: 10.1067/mse.2000.105138. [DOI] [PubMed] [Google Scholar]
- 29.Al-Hadithy N, Domos P, Sewell MD, Pandit R. Reverse shoulder arthroplasty in 41 patients with cuff tear arthropathy with a mean follow-up period of 5 years. J Shoulder Elb Surg. 2014;23(11):1662–1668. doi: 10.1016/j.jse.2014.03.001. [DOI] [PubMed] [Google Scholar]
- 30.Boileau P, Watkinson D, Hatzidakis AM, Hovorka I. Neer Award 2005: the Grammont reverse shoulder prosthesis: results in cuff tear arthritis, fracture sequelae, and revision arthroplasty. J Shoulder Elb Surg. 2006;15(5):527–540. doi: 10.1016/j.jse.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 31.Nolan BM, Ankerson E, Wiater JM. Reverse total shoulder arthroplasty improves function in cuff tear arthropathy. Clin Orthop Relat Res. 2011;469(9):2476–2482. doi: 10.1007/s11999-010-1683-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Boileau P, Brassart N, Watkinson DJ, Carles M, Hatzidakis AM, Krishnan SG. Arthroscopic repair of full-thickness tears of the supraspinatus: does the tendon really heal? J Bone Joint Surg Am. 2005;87(6):1229–1240. doi: 10.2106/JBJS.D.02035. [DOI] [PubMed] [Google Scholar]
- 33.Chung SW, Kim JY, Kim MH, Kim SH, Oh JH. Arthroscopic repair of massive rotator cuff tears: outcome and analysis of factors associated with healing failure or poor postoperative function. Am J Sports Med. 2013;41(7):1674–1683. doi: 10.1177/0363546513485719. [DOI] [PubMed] [Google Scholar]
- 34.Goutallier D, Postel JM, Gleyze P, Leguilloux P, Van Driessche S. Influence of cuff muscle fatty degeneration on anatomic and functional outcomes after simple suture of full-thickness tears. J Shoulder Elb Surg. 2003;12(6):550–554. doi: 10.1016/S1058-2746(03)00211-8. [DOI] [PubMed] [Google Scholar]
- 35.Meyer DC, Wieser K, Farshad M, Gerber C. Retraction of supraspinatus muscle and tendon as predictors of success of rotator cuff repair. Am J Sports Med. 2012;40(10):2242–2247. doi: 10.1177/0363546512457587. [DOI] [PubMed] [Google Scholar]
- 36.Rhee YG, Cho NS, Yoo JH. Clinical outcome and repair integrity after rotator cuff repair in patients older than 70 years versus patients younger than 70 years. Arthroscopy. 2014;30(5):546–554. doi: 10.1016/j.arthro.2014.02.006. [DOI] [PubMed] [Google Scholar]
- 37.Wu XL, Briggs L, Murrell GA. Intraoperative determinants of rotator cuff repair integrity: an analysis of 500 consecutive repairs. Am J Sports Med. 2012;40(12):2771–2776. doi: 10.1177/0363546512462677. [DOI] [PubMed] [Google Scholar]
- 38.Meyer DC, Farshad M, Amacker NA, Gerber C, Wieser K. Quantitative analysis of muscle and tendon retraction in chronic rotator cuff tears. Am J Sports Med. 2012;40(3):606–610. doi: 10.1177/0363546511429778. [DOI] [PubMed] [Google Scholar]
- 39.Mall NA, Tanaka MJ, Choi LS, Paletta GA., Jr Factors affecting rotator cuff healing. J Bone Joint Surg Am. 2014;96(9):778–788. doi: 10.2106/JBJS.M.00583. [DOI] [PubMed] [Google Scholar]
- 40.Gupta AK, Hug K, Berkoff DJ, Boggess BR, Gavigan M, Malley PC, Toth AP. Dermal tissue allograft for the repair of massive irreparable rotator cuff tears. Am J Sports Med. 2012;40(1):141–147. doi: 10.1177/0363546511422795. [DOI] [PubMed] [Google Scholar]
- 41.Mihata T, Lee TQ, Watanabe C, Fukunishi K, Ohue M, Tsujimura T, Kinoshita M. Clinical results of arthroscopic superior capsule reconstruction for irreparable rotator cuff tears. Arthroscopy. 2013;29(3):459–470. doi: 10.1016/j.arthro.2012.10.022. [DOI] [PubMed] [Google Scholar]
- 42.Mihata T, McGarry MH, Pirolo JM, Kinoshita M, Lee TQ. Superior capsule reconstruction to restore superior stability in irreparable rotator cuff tears: a biomechanical study. Am J Sports Med. 2012;40(10):2248–2255. doi: 10.1177/0363546512456195. [DOI] [PubMed] [Google Scholar]
- 43.Harreld KL, Puskas BL, Frankle M. Massive rotator cuff tears without arthropathy: when to consider reverse shoulder arthroplasty. J Bone Joint Surg Am. 2011;93(10):973–984. [PubMed] [Google Scholar]
- 44.Ek ET, Neukom L, Catanzaro S, Gerber C. Reverse total shoulder arthroplasty for massive irreparable rotator cuff tears in patients younger than 65 years old: results after five to fifteen years. J Shoulder Elb Surg. 2013;22(9):1199–1208. doi: 10.1016/j.jse.2012.11.016. [DOI] [PubMed] [Google Scholar]
- 45.Muh SJ, Streit JJ, Wanner JP, Lenarz CJ, Shishani Y, Rowland DY, Riley C, Nowinski RJ, Edwards TB, Gobezie R. Early follow-up of reverse total shoulder arthroplasty in patients sixty years of age or younger. J Bone Joint Surg Am. 2013;95(20):1877–1883. doi: 10.2106/JBJS.L.10005. [DOI] [PubMed] [Google Scholar]
- 46.Samuelsen BT, Wagner ER, Houdek MT, Elhassan BT, Sanchez-Sotelo J, Cofield R, Sperling JW. Primary reverse shoulder arthroplasty in patients aged 65 years or younger. J Shoulder Elb Surg. 2017;26(1):e13–e17. doi: 10.1016/j.jse.2016.05.026. [DOI] [PubMed] [Google Scholar]
- 47.Sershon RA, Van Thiel GS, Lin EC, McGill KC, Cole BJ, Verma NN, Romeo AA, Nicholson GP. Clinical outcomes of reverse total shoulder arthroplasty in patients aged younger than 60 years. J Shoulder Elb Surg. 2014;23(3):395–400. doi: 10.1016/j.jse.2013.07.047. [DOI] [PubMed] [Google Scholar]
- 48.Hartzler RU, Steen BM, Hussey MM, Cusick MC, Cottrell BJ, Clark RE, Frankle MA. Reverse shoulder arthroplasty for massive rotator cuff tear: risk factors for poor functional improvement. J Shoulder Elb Surg. 2015;24(11):1698–1706. doi: 10.1016/j.jse.2015.04.015. [DOI] [PubMed] [Google Scholar]
- 49.Mallon WJ, Misamore G, Snead DS, Denton P. The impact of preoperative smoking habits on the results of rotator cuff repair. J Shoulder Elb Surg. 2004;13(2):129–132. doi: 10.1016/j.jse.2003.11.002. [DOI] [PubMed] [Google Scholar]
- 50.Tashjian RZ, Hollins AM, Kim HM, Teefey SA, Middleton WD, Steger-May K, Galatz LM, Yamaguchi K. Factors affecting healing rates after arthroscopic double-row rotator cuff repair. Am J Sports Med. 2010;38(12):2435–2442. doi: 10.1177/0363546510382835. [DOI] [PubMed] [Google Scholar]
- 51.Denard PJ, Ladermann A, Jiwani AZ, Burkhart SS. Functional outcome after arthroscopic repair of massive rotator cuff tears in individuals with pseudoparalysis. Arthroscopy. 2012;28(9):1214–1219. doi: 10.1016/j.arthro.2012.02.026. [DOI] [PubMed] [Google Scholar]
- 52.Shamsudin A, Lam PH, Peters K, Rubenis I, Hackett L, Murrell GA. Revision versus primary arthroscopic rotator cuff repair: a 2-year analysis of outcomes in 360 patients. Am J Sports Med. 2015;43(3):557–564. doi: 10.1177/0363546514560729. [DOI] [PubMed] [Google Scholar]
- 53.Sadoghi P, Vavken P, Leithner A, Hochreiter J, Weber G, Pietschmann MF, Muller PE. Impact of previous rotator cuff repair on the outcome of reverse shoulder arthroplasty. J Shoulder Elb Surg. 2011;20(7):1138–1146. doi: 10.1016/j.jse.2011.01.013. [DOI] [PubMed] [Google Scholar]
- 54.Gladstone JN, Bishop JY, Lo IK, Flatow EL. Fatty infiltration and atrophy of the rotator cuff do not improve after rotator cuff repair and correlate with poor functional outcome. Am J Sports Med. 2007;35(5):719–728. doi: 10.1177/0363546506297539. [DOI] [PubMed] [Google Scholar]
- 55.Shen PH, Lien SB, Shen HC, Lee CH, Wu SS, Lin LC. Long-term functional outcomes after repair of rotator cuff tears correlated with atrophy of the supraspinatus muscles on magnetic resonance images. J Shoulder Elb Surg. 2008;17(Suppl 1):1S–7S. doi: 10.1016/j.jse.2007.04.014. [DOI] [PubMed] [Google Scholar]
- 56.Yoo JC, Ahn JH, Koh KH, Lim KS. Rotator cuff integrity after arthroscopic repair for large tears with less-than-optimal footprint coverage. Arthroscopy. 2009;25(10):1093–1100. doi: 10.1016/j.arthro.2009.07.010. [DOI] [PubMed] [Google Scholar]
- 57.Dwyer T, Razmjou H, Henry P, Gosselin-Fournier S, Holtby R. Association between pre-operative magnetic resonance imaging and reparability of large and massive rotator cuff tears. Knee Surg Sports Traumatol Arthrosc. 2015;23(2):415–422. doi: 10.1007/s00167-013-2745-z. [DOI] [PubMed] [Google Scholar]
- 58.Werner BC, Wong AC, Mahony GT, Craig EV, Dines DM, Warren RF, Gulotta LV. Causes of poor postoperative improvement after reverse total shoulder arthroplasty. J Shoulder Elb Surg. 2016;25(8):e217–e222. doi: 10.1016/j.jse.2016.01.002. [DOI] [PubMed] [Google Scholar]
- 59.Boileau P, Gonzalez JF, Chuinard C, Bicknell R, Walch G. Reverse total shoulder arthroplasty after failed rotator cuff surgery. J Shoulder Elb Surg. 2009;18(4):600–606. doi: 10.1016/j.jse.2009.03.011. [DOI] [PubMed] [Google Scholar]
- 60.Harman M, Frankle M, Vasey M, Banks S. Initial glenoid component fixation in ‘reverse’ total shoulder arthroplasty: a biomechanical evaluation. J Shoulder Elb Surg. 2005;14(1):162S–167S. doi: 10.1016/j.jse.2004.09.030. [DOI] [PubMed] [Google Scholar]
- 61.Naveed MA, Kitson J, Bunker TD. The Delta III reverse shoulder replacement for cuff tear arthropathy: a single-centre study of 50 consecutive procedures. J Bone Joint Surg (Br) 2011;93(1):57–61. doi: 10.1302/0301-620X.93B1.24218. [DOI] [PubMed] [Google Scholar]
- 62.Petrillo S, Longo UG, Papalia R, Denaro V. Reverse total shoulder arthroplasty for massive irreparable rotator cuff tears and cuff tear arthropathy: a systematic review. Musculoskelet Surg. 2017;101(2):105–112. doi: 10.1007/s12306-017-0474-z. [DOI] [PubMed] [Google Scholar]
- 63.Groh GI, Groh GM. Complications rates, reoperation rates, and the learning curve in reverse shoulder arthroplasty. J Shoulder Elb Surg. 2014;23(3):388–394. doi: 10.1016/j.jse.2013.06.002. [DOI] [PubMed] [Google Scholar]
- 64.Black EM, Roberts SM, Siegel E, Yannopoulos P, Higgins LD, Warner JJ. Failure after reverse total shoulder arthroplasty: what is the success of component revision? J Shoulder Elb Surg. 2015;24(12):1908–1914. doi: 10.1016/j.jse.2015.05.029. [DOI] [PubMed] [Google Scholar]
- 65.Boileau P, Melis B, Duperron D, Moineau G, Rumian AP, Han Y. Revision surgery of reverse shoulder arthroplasty. J Shoulder Elb Surg. 2013;22(10):1359–1370. doi: 10.1016/j.jse.2013.02.004. [DOI] [PubMed] [Google Scholar]
- 66.Guery J, Favard L, Sirveaux F, Oudet D, Mole D, Walch G. Reverse total shoulder arthroplasty. Survivorship analysis of eighty replacements followed for five to ten years. J Bone Joint Surg Am. 2006;88(8):1742–1747. doi: 10.2106/JBJS.E.00851. [DOI] [PubMed] [Google Scholar]
- 67.Sirveaux F, Favard L, Oudet D, Huquet D, Walch G, Mole D. Grammont inverted total shoulder arthroplasty in the treatment of glenohumeral osteoarthritis with massive rupture of the cuff. Results of a multicentre study of 80 shoulders. J Bone Joint Surg (Br) 2004;86(3):388–395. doi: 10.1302/0301-620X.86B3.14024. [DOI] [PubMed] [Google Scholar]
- 68.Bacle G, Nove-Josserand L, Gaurad P, Walch G. Long-term outomes of reverse total shoulder arthroplasty: a follow-up of a previous study. J Bone Joint Surg Am. 2017;99(6):454–461. doi: 10.2106/JBJS.16.00223. [DOI] [PubMed] [Google Scholar]
- 69.• Cuff DJ, Pupello DR, Santoni BG, Clark RE, Frankle MA. Reverse shoulder arthroplasty for the treatment of rotator cuff deficiency: a concise follow-up, at a minimum of 10 years, of previous reports. J Bone Joint Surg Am. 2018. Study reports outcomes and implant survival of RSA after a minimum of 10 years.
- 70.Favard L, Levigne C, Nerot C, Gerber C, De Wilde L, Mole D. Reverse prostheses in arthropathies with cuff tear: are survivorship and function maintained over time? Clin Orthop Relat Res. 2011;469(9):2469–2475. doi: 10.1007/s11999-011-1833-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Makhni EC, Swart E, Steinhaus ME, Mather RC, 3rd, Levine WN, Bach BR, Jr, Romeo AA, Verma NN. Cost-effectiveness of reverse total shoulder arthroplasty versus arthroscopic rotator cuff repair for symptomatic large and massive rotator cuff tears. Arthroscopy. 2016;32(9):1771–1780. doi: 10.1016/j.arthro.2016.01.063. [DOI] [PubMed] [Google Scholar]
- 72.Henry P, Wasserstein D, Park S, Dwyer T, Chahal J, Slobogean G, Schemitsch E. Arthroscopic repair for chronic massive rotator cuff tears: a systematic review. Arthroscopy. 2015;31(12):2472–2480. doi: 10.1016/j.arthro.2015.06.038. [DOI] [PubMed] [Google Scholar]
- 73.Holtby R, Razmjou H. Relationship between clinical and surgical findings and reparability of large and massive rotator cuff tears: a longitudinal study. BMC Musculoskelet Disord. 2014;15(1):180. doi: 10.1186/1471-2474-15-180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Namdari S, Donegan RP, Chamberlain AM, Galatz LM, Yamaguchi K, Keener JD. Factors affecting outcome after structural failure of repaired rotator cuff tears. J Bone Joint Surg Am. 2014;96(2):99–105. doi: 10.2106/JBJS.M.00551. [DOI] [PubMed] [Google Scholar]
- 75.Kim HM, Caldwell JM, Buza JA, Fink LA, Ahmad CS, Bigliani LU, Levine WN. Factors affecting satisfaction and shoulder function in patients with a recurrent rotator cuff tear. J Bone Joint Surg Am. 2014;96(2):106–112. doi: 10.2106/JBJS.L.01649. [DOI] [PubMed] [Google Scholar]


