Abstract
Purpose of Review
The purpose of this review was to establish the foundation of the major biologic adjuvants to rotator cuff repairs and review recent scientific findings.
Recent Findings
Platelet-rich plasma (PRP) overall has no significant impact on functional outcomes and repair integrity, but may be more advantageous in small to medium tears. Further studies should focus on leukocyte-rich versus poor preparations and the use of PRP in patients that are high risk for repair failure. Biologic and synthetic patches or augments provide mechanical stability for large and massive rotator cuff tears and decrease re-tear rates. Mesenchymal stem cells have demonstrated improved healing rates without an impact on outcomes. Cytokines and growth factors show promise in animal models, but require human trials to further evaluate.
Summary
In massive or revision repairs, allograft or synthetic patch augmentation should be considered. Platelet-rich plasma may have benefit in smaller tears. Further studies are needed to evaluate the value of mesenchymal stem cells and various cytologic chemical signals.
Keywords: Rotator cuff, Biologics, Platelet-rich plasma, Patches, Cytokines
Introduction
Rotator cuff tears are a growing problem within orthopedics as the incidence increases within an aging population [1], and repair of these tears has been estimated to save society approximately $ 3.4 Billion per year [2]. Despite the advances in open and, more recently, arthroscopic repairs of rotator cuff tears including suture material and constructs, re-tear rates have varied greatly from 11 to 90% depending on tear size and patient demographics [3–5]. A meta-analysis by Slabaugh et al. [6] found an average re-tear rate of 20.4% and those with intact cuff repairs trended towards improved functional scores. Later studies have contradicted this finding with no correlation between integrity of the repair and patient outcomes [7, 8]. Despite these findings, research has continued to investigate the use of adjuvants to improve patient outcomes and repair integrity as contemporary literature continues to reinforce the finding that an intact rotator cuff following repair is associated with improved strength and satisfaction. Many of these interventions are aimed towards patients who are at higher risk for failure based on age [9, 10], comorbidities (diabetes, hyperlipidemia, obesity), smoking, tendon retraction, and fatty degeneration [11]. Current literature reflects a wide array of adjuvants including platelet-rich plasma (PRP), patches (allo- and xeno-grafts), biologic scaffolds, and multiple cytokine and biologic signals to improve the healing of rotator cuff tears. The purpose of the paper is to review the foundational research in each area and provide an update on the most recent advances in each therapeutic option.
Platelet-Rich Plasma Products
Platelet-rich plasma (PRP) is one of the most studied adjuvants for rotator cuff tears and repairs. First described in the 1990s for use in plastic and maxillofacial procedures, the attraction of PRP is the consolidation or concentration of growth factors including transforming growth factor-β (TGF-β), fibroblast growth factor (FGF), platelet-derived growth factor (PDGF), and others that are involved in the healing process [12] (Fig. 1). Boswell et al. [13] outlined the numerous components, both cellular and chemical signals that are responsible for the benefits of PRP. The authors discuss that PRP is a milieu of factors that are both catabolic/anabolic and pro-/anti-inflammatory. The authors also highlighted that with this complexity comes difficulty in reproducing precise elements across each patient’s and company’s products [13]. One key difference is the cellular composition of PRP as increasing platelet concentrations are associated with anabolic signaling while increased leukocyte concentrations are correlated with catabolic signals [14]. This variability and tailoring of PRP products has resulted in numerous studies that look at the use of PRP and its formulations in rotator cuff pathology.
Fig. 1.
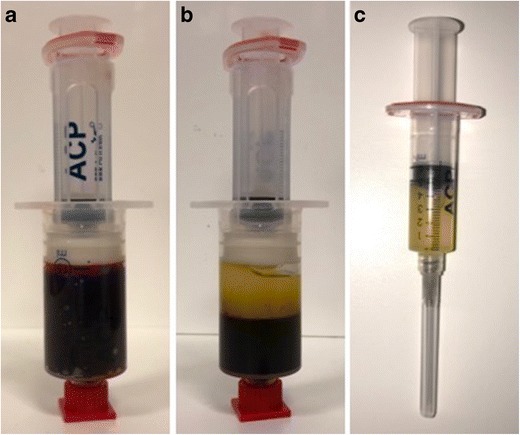
Platelet-rich plasma. a Venous blood prior to centrifugation collected in an Autologous Conditioned Plasma Double Syringe System (Arthrex, Naples, FL). b Double-syringe system after centrifugation showing separation of blood contents with platelet-rich plasma above and red blood cells below. c Platelet-rich plasma isolated from other blood contents and prepared for injection at the repair site
The role of PRP rotator cuff tears and repairs is to stimulate healing at the tendon-bone interface and decrease pain/inflammation. In non-operative cases, PRP has been used as an alternative to steroid in subacromial impingement and partial tears, especially in high-level athletes [12]. In higher-level studies, the impact of PRP has only been found equivalent to their control groups [15, 16]. Kesikburun et al. [15] performed a randomized control trial of 40 patients with rotator cuff tendinopathy or partial tears that received either PRP or a placebo injection followed by a rehabilitation program. There was no difference in pain or functional outcome scores at any time point up to 1 year. Filardo et al. [16•] performed a meta-analysis on various tendonopathies and included 32 studies regarding rotator cuff pathology (22 RCT, 18 surgical, and 3 conservative). The findings again demonstrated no difference in outcomes in the non-operative applications of PRP in rotator cuff tendinopathy and tears in single and multiple injection protocols [16•].
In the past 3 years, there has been a significant number of publications regarding the role of PRP in rotator cuff repairs. PRP injections for rotator cuff pathology are increasing and according to a recent database study are the third leading condition for use of PRP behind meniscal and unspecified shoulder pathology [17]. In terms of non-operative treatment, Shams et al. [18•] performed a randomized control trial comparing corticosteroid to PRP injections in symptomatic partial rotator cuff tears. Both groups saw significant improvement in all functional scores, but the PRP group demonstrated improved VAS, ASES, SST, and Constant-Murley Scores at 12 weeks, though there was no difference after 6 months. Neither treatment arm saw significant change in the degree of tendinopathy or tear grade at final follow-up [18•]. Additional studies have also shown that leukocyte reduced or LLO PRP has better anabolic characteristics stimulating normal collagen matrix and inhibiting cytokines responsible for inflammation and matrix degradation [19]. It is thought that these formulations may be preferred in future rotator cuff repairs and should be the focus of further studies. The heterogeneity among studies in terms of the formulation of the PRP and the various protocols and controls (one study was compared to dry needling) leaves the role of PRP in non-operative management of rotator cuff tendinopathy and tears to be inconclusive and requiring more randomized control studies with detailed information about the formulations used.
Evaluation of PRP in rotator cuff repairs has been previously analyzed through multiple randomized control studies [20–23] and systematic reviews/meta-analysis [24, 25•]. Chahal et al. [24] pooled five studies into their results and while risk ratio of re-tear was 0.77 [CI 0.48 to 1.23] in the PRP-treated groups, it did not prove significant. Patient-reported outcomes including Constant, Simple Shoulder Test (SST), Single Assessment Numeric Evaluation (SANE), and American Shoulder and Elbow Surgeons (ASES) scores were no different between the groups. A larger meta-analysis of 11 studies found similar results in that overall there was no difference in re-tear rates and patient outcomes between groups with and without PRP. Warth et al. [25•] did find on sub-group analysis that when tears were greater than 3 cm, PRP-treated groups showed significant decrease in re-tear rates (25.9 versus 57.1%; p = 0.046) in double row constructs. The authors found that a majority of these studies were powered only to detect large difference in outcome scores, which may have led to the common findings of no difference between the treatment groups. PRP can be injected directly, but is often delivered through a fibrin, thrombin, or collagen matrix. The fibrin matrix delivery system has had mixed results with two studies demonstrating lower re-tear rates [21] and mild improvement in outcomes [20] and two RCTs demonstrating increased risk of re-tears in the PRP-treated groups [23, 26]. A growing focus on leukocyte reduced PRP has shown promise with lower re-tears rates compared to regular PRP and other controls in multiple studies [27•, 28].
Evaluation of PRP adjuvants has been extensively studied in randomized control trials [27•, 28, 29, 30•, 31–35]. The findings of these studies are summarized in Table 1. One of the interesting modalities is the use of PRP in the immediate post-operative period. Two different randomized control trials analyzed the use of PRP 7 and 14 days post-operatively [34, 35]. Neither study was blinded nor showed a significant impact of PRP on functional outcomes or tendon integrity. Ebert et al. [35] did find a small increase in the strength of abduction in patients treated in PRP with improvements of 3.3 versus 0.9 when adjusted for baseline scores and sex. The 3.5-year follow-up also demonstrates that there does not appear to be any midterm benefit for function or tendon integrity with PRP-treated repairs. Jo et al. [27•] was the only recent trial to demonstrate a difference in either re-tear rates or functional outcomes. The key is that their study focused on larger tears, which previous studies have demonstrated the possibility of an increased impact with PRP [25•]. A common finding was that PRP injections improved the post-operative pain and use of pain medications through the first 30 days [30•, 31]. Both studies make the argument that this decreased pain could lead to better rehabilitation potential, but given that some physicians do not start full mobilization until 6 weeks post-operatively, this impact on therapy is limited. In light of the opiate epidemic, the role of PRP in pain reduction has merit, but cost analysis and further studies would have to be done to evaluate PRP’s impact on narcotic use.
Table 1.
Randomized control trials of PRP
| Study | Groups (# patients) | Type of PRP | Outcome | Conclusions |
|---|---|---|---|---|
| Jo et al. [27•] (2015) | Medium to large RC tears. Not blinded. Suture bridge repair PRP (37) versus repair alone (37) |
PRP gel at repair site. | -No difference between the groups for VAS pain, ROM, Strength, and Constant scores at all time points. -Re-tear rate: 3 versus 20% (p = 0.032) -Cross-sectional area of repair: − 36.76 ± 45.31 mm2 in PRP versus − 67.47 ± 47.26 mm2 (p = 0.014) |
Decrease in re-tear rates and increase surface area of tendon repair. No effect on outcomes or speed of healing. |
| Wang et al. [34] (2015) |
DR repair of SS tears, Not blinded -Post-operative injections at 7 and 14 days (30) versus -Repair alone (30) |
PRP at repair site with ultrasound visualization | -No difference in pain, ROM, or function at any time point up to 16 weeks. Repair integrity PRP: 77% intact, 23% partial, 0% full tear) Repair: 70% intact, 23% partial tear, 7% full tear (P = 0.35) |
Postoperative PRP injections on 2 occasion do not improve healing or functional recovery. |
| Barber et al. [29] (2016) |
< 3 cm tears -Triple loaded single row (20) versus -Suture-bridging double row (20) |
PRP fibrin matrix | Avg F/u 28 months SR: 3 of 20 repairs had tears on MRIs at 1 year DR: 3 of 20 repairs had tears Functional scores: all had improvements in ASES, Rowe, SST, Constant, SANE Scores. No difference between the groups. |
No difference in re-tear rates or reported outcomes between repair constructs with PRP fibrin matrix. |
| D’Ambrosi et al. [30•] (2016) |
Double blinded with full thickness SS tears -PRP (20) versus repair only (20). |
PRP injection into site of repair | -PRP lower VAS pain scores (1.5 ± 1.0 vs 3.2 ± 1.7; P < 0.05) in the first month. -No difference in DASH and Constant scores before and after 6 months between the groups -No difference in re-tears (ultrasound) at 6 months. |
PRP reduced pain in short term, but made no difference in re-tear rate or functional outcomes. |
| Holtby et al. [31] (2016) |
Double blinded, tears up to 3 cm -PRP (41) versus repair only (41) |
PRP injection into site of repair | -PRP with lower VAS scores (p = 0.012) -PRP took less pain pills (p = 0.026) -No difference in ROM post op at 6 months -NO difference in re-tear rates (14 vs 18%; p = 0.44) |
PRP improves pain in the short term, but no effect on re-tear or patient reported outcomes. |
| Flury et al. [32] (2016) |
Triple blinded, SS repairs with DR repairs PRP (60) versus ropivicaine (60) 2-year follow_up |
PRP at Footprint/Repair site, Ropivicaine in Subacromial space | -No difference in OSS, ASES, DASH, Constant scores at 3, 6, and 24 months. -Re-tear rates were 12.2% (PRP) vs 20.8% (control) p = 0.295 − 40.7 versus 30.5% (PRP v Control) had adverse local reactions p = 0.325 |
-No difference in re-tear rates or functional outcomes between groups up to 2 years. -Smoking appeared to impact the effects of PRP |
| Zumstein et al. [33] (2016) |
Blinded, double row-suture bridge L-PRP+ group (17) L-PRP− group (18) |
Leukocyte Rich PRP Fibrin Clots | -No complications in either group No difference between groups in outcome scores (SST, Contant, SSV) Watertight repair on 11 of 17 in L-PRP+ and 11 of 18 in L-PRP− groups (p = 0.73) on MRI -No difference in defect sizes or tendon quality at 12 months |
Leukocyte Rich PRP Fibrin does not improve functional outcomes, re-tear rates, or tendon quality at 12 months. |
| Ebert et al. [35] (2017) |
DR repair of SS tendon. PRP post-operatively at 7 and 14 days. (30) Versus Repair alone (30) |
PRP at repair site with ultrasound visualization | 3.5-year follow-up. No difference in PROMs -Higher strength subscore for Constant (3.3pts) for PRP group. (P = 0.006) No difference in MRI scores or re-tear rates. (Control had 2 symptomatic re-tears in first 16 weeks, while PRP had 2 re-tears between the 16-week and 3.5-year follow-up). |
PRP post-operative injections saw small improvement in Abduction strength, but otherwise provided no other functional improvement or benefit to tendon integrity. |
Two major meta-analysis and reviews have looked to pool the more recent data on PRP in rotator cuff repairs [36•, 37•]. Cai et al. [36•] included five studies of RCTs into their analysis, and while they also concluded that PRP had no impact on clinic outcomes, subgroup analysis showed PRP decrease re-tear rates in small to medium tears, which is contrary to the findings by Warth et al. [25•]. Saltzman et al. [37•] performed a systematic review of 7 different meta-analysis providing 3193 patients with 12- to 31-month follow-up. Overall, their findings support no clinical benefit in terms of functional outcomes (ASES, UCLA, Constant, and SST scores) or repair integrity at final follow-up. Interestingly, the findings of this broad analysis showed that there were improved outcomes with solid PRP versus liquid PRP preparations. They also support the findings of Cai et al. [36•] that PRP has a greater impact in re-tear rates in small to medium tears versus large tears [37•]. Finally, the group found that PRP placed directly at the bone-tendon interface had better outcomes than the PRP injected over the tendon and in double versus single row constructs.
The overall consensus of the current literature is that PRP does not provide a benefit for clinical outcomes and repair integrity in short and midterm follow-up. However, PRP may be more advantageous for small and medium tears to prevent re-tears. These findings are what influenced a recent cost analysis study that stated PRP is not cost effective for large tears and at current prices is also not cost effective for small and medium tears [38]. A Markov Model analysis demonstrated that PRP treatments would have to reduce re-tear rates by 9.1% at $750 and 12.1% at $1000 [39]. While numerous high-level RCTs and meta-analysis have been done on the role of PRP and show minimal benefit, it is important that this adjuvant still has role in rotator cuff repair. These studies are heterogeneous, and to date, there is limited data on the use of PRP in populations that are at high risk for failure. These would be the patients that may benefit most from these interventions and more focal studies on high-risk groups are required.
Patches and Augments
Beyond PRP, extracellular matrices (ECMs) or “patches” are a major adjuvant used in rotator cuff repairs. While PRP relies on cytokines to stimulate healing at rotator cuff tears, ECMs provide additional mechanical strength to increase load to failure in tissue that is stiffer and mechanically inferior [40]. Patches provide the framework for cellular in growth and eventually can demonstrate properties similar to autologous tendon [41]. Recently, these grafts have become the focus of other interventions in massive rotator cuff tears (superior capsule reconstruction). This section’s focus will be on the use of the ECMs or patches in augmenting or bridging repairs.
ECMs classically have been divided into two major groups: xenografts and allografts. Xenografts were some of the first studied and included porcine dermal and submucosal grafts. These grafts have had success in other portions of the body but proved detrimental for rotator cuff repair with Sclamberg et al. [42] finding 10 of 11 patients with large retracted tears at only 6 months following repair with no improvement in functional scores (five patients actually had worse scores). Two other studies of the porcine submucosal grafts had similar results with only 4 of 15 patients healing their tears in one study [43] and 6 of 10 in the other [44]. Iannotti et al. [43] reported increased pain in patients receiving the graft and Walton et al. [44] reported four patients with such severe reactions they required surgical treatment. The strong inflammatory response is presumed to be due to the DNA still present in these grafts [45]. Porcine dermal grafts have had more success than the submucosal grafts in part due to less DNA being present [45]. Badhe et al. [46] used the patch in massive rotator cuff tears, and at 3.5 years, 8 of 10 patients had intact repairs and Constant scores improved from 41 to 62. Similarly, a more recent mini-open technique by Cho et al. [47] used the graft in a suture bridge construct and saw significant improvements in functional scores at mean 20.6-month follow-up with 4 of 5 patients having intact repairs at 8 months after surgery. Gupta et al. [48] had probably the most successful cohort treated with dermal xenografts showing significant improvements in motion, strength, and ASES score (62.7 to 91.8; p = 0.0007). At 2-year follow-up, 16 of 21 patients had intact repair, 5 (22%) had partial tears, and only 1 patient (5%) had a complete re-tear, albeit due to a fall [48].
Allografts, specifically dermal allografts, have had early success in the augmentation of rotator cuff tears. Bond et al. [49] using GraftJacket (Wright Medical, Memphis, TN), saw significant improvement in UCLA, Constant, and SST scores, and 13 of 16 patients had full graft incorporation at mean 26 months. Similar findings in regard to functional improvement was published by Wong et al. [50], though they did not discuss the integrity of the repair in their study. Even in midterm follow-up for massive tears, case series have demonstrated improvements in functional outcome scores, strength, and range of motion with 76% with full intact repair [51]. Barber et al. [52] also provided convincing evidence with a randomized control trial of two-tendon repairs. Both ASES and Constant scores were statistically increased in the dermal allograft group, but the clinical difference in the scores was unclear. Furthermore, the patch group had intact repairs in 85% of patients versus 40% in the control group. This is one of the few level I studies on dermal allografts. Dermal allografts remain a significant tool in augmenting rotator cuff repairs.
In recent years, research has continued to examine the effectiveness of biological patches in augmenting repairs. In terms of basic science research, Smith et al. [53•] attempted to characterize the mechanical properties of commercially available scaffolds including xenografts, allografts, and synthetic scaffolds. None of the tested scaffolds matched the mechanical properties of the supraspinatus tendon, which the author’s highlight is in part because many of these products were not designed with the rotator cuff in mind. Smith et al. [53•] recommend that future grafts should aim to combine the macro mechanical properties of synthetic grafts with the micro mechanical properties of the biologic scaffolds. Another study used a second arthroscopic procedure to biopsy the repair site of rotator cuff repairs augmented with bovine collagen matrix [54]. By obtaining the seven specimens at different time points, the authors were able to outline a timeline of graft incorporation. Host cell infiltration with the presence of fibroblasts was seen in the earliest sample at 5 weeks, increasing organization was noted at 3 months, and finally full incorporation with solely host tissue seen at 6 months [54]. Beitzel et al. [55] tried to evaluate if the type of graft impacted the response of mesenchymal cells in a repair. While structurally stronger, the dermal allograft’s complex structure may impede infiltration of the tenocyte progenitor cells compared to the less organized porcine collagen scaffolds. A follow-up to this study looked at the impact of the biochemical composition of ECMs in rotator cuff repairs [56]. They found that scaffolds with increased type I and III collagen significantly increased the potential of bone marrow-derived mesenchymal stems cells to differentiate into bone and tendon. Future research should continue to discover or define the ideal graft in terms of the mechanical and biological inductive properties.
Smaller case series have demonstrated good results in augmentation of massive tears. In terms of primary repairs, Consigliere et al. [57•] found significant improvement in Constant (53 to 75) and Oxford scores (30 to 47) with a reduction of pain from 7 to 0.6 at a mean follow-up of 7 months after repair of large and massive rotator cuff tears augmented with dermal ECM. Two other case series looked at the role of patches in revision repairs [58•, 59]. Petri et al. [58•] had 13 shoulders in 12 patients augmented with acellular dermal allograft of which only one patient had re-tear at 2 months (patient had 4 previous repairs), and while the overall ASES score did not show statistically significant improvement, the functional score did significantly improve (p < 0.05) and median patient satisfaction was 9/10. The authors concluded that acellular dermal patches are safe and effective in massive rotator cuff repairs. In a separate review of 24 patients who received acellular dermal allografts in revision rotator cuff repair at a mean follow-up of 50 months [59], 10 of the 16 patients (63%) available for follow-up imaging had evidence of failed repair. Overall, excellent results were achieved in 24%, good in 13%, fair in 21%, and poor outcomes in 42% of patients based on ASES and SANE scores. Sears et al. [59] compared this to historical outcomes of revision repair without augmentation and found no significant improvements with dermal patches. A recent prospective blinded comparative study compared 20 patients who had their large to massive rotator cuff repairs augmented with dermal allograft to 15 patients with non-augmented repair [60]. At a mean follow-up of 24.9 months, the augmented group had only 10% of re-tears versus 26% in the controls (p = 0.0483). In addition, the patients treated with the dermal allograft saw significant improvements of their ASES, SF-12, and Western Ontario Rotator Cuff Index scores compared to the control group [60].
With primarily small cohorts and limited level I and II studies, systematic reviews and meta-analyses have tried to better define the role of patch augmentation in rotator cuff repairs. Three such reviews [61•, 62•, 63] re-enforced the advantages of allograft use in large to massive rotator cuff repairs. Ferguson et al. [62•] found that allograft augmented repairs had intact repairs 85 versus 40% at final follow-up. The authors also analyzed the use of polypropylene patches and found these to be superior to controls and xenograft results. Steinhaus et al. [61•] pooled the data on 24 studies with a minimum of 9 months of follow-up. They found that on average, augmented repairs saw forward elevation improve 58.6°, external rotation 16.6°, and internal rotation 16.1°. Statistically significant improvements in ASES, UCLA, Constant, Penn, and Oxford scores were found. The analysis confirms the inferiority of xenografts in terms of functional outcomes. The pooled data demonstrated an overall re-tear rate of 25% (34% for augments and 12% for interposition constructs) [61•]. Re-tear rates were highest for xenografts (44%) and lowest for synthetic grafts (15%) (Table 2).
Table 2.
Patches and scaffolds
| Source | Material | Summary of the literature |
|---|---|---|
| Porcine | Submucosal | High failure rate and little to no clinical improvement. Poor results likely secondary to severe inflammatory reaction from DNA content of the xenograft |
| Porcine | Dermal | Safe and effective for the augment for rotator cuff repairs. Patients showed significant symptomatic and functional improvement |
| Allograft | Dermal | Significantly improved outcomes scores and decreased re-tear rate in primary treatment. Also provides a safe and effective option for augmentation of revision and massive rotator cuff repairs. |
| Synthetic | Poly-L lactic acid | Successful outcomes in animal and human studies. Low re-tear rates and significant improvement in re-tear rates. |
| Synthetic | Polycarbonate polyurethane | Safe, low re-tear rate, and significant improvement in outcome scores at 12 months follow-up |
| Synthetic | Polypropylene | Low re-tear rates and significant clinical and outcome score improvement at 3-year follow-up |
Synthetic Scaffolds
Synthetic grafts or scaffolds, composed of various materials including polypropylene, Dacron, and even nylon [64], have had success in terms of re-tear rates [61•]. Synthetic material is often stronger biomechanically and does not have the theoretical risk of vector transmission [65, 66]. Variations of poly-L lactic acid have had success in both animal and human studies. Derwin et al. [67] augmented infraspinatus repairs in eight dogs and found at time 0 that the repair had 23% increase load to failure and at 12 weeks had less retraction with great surface area of repair increase stiffness, and ultimate load to failure. A case series by a single surgeon analyzed the outcomes and repair integrity in 18 consecutive patients with massive rotator cuff repairs at 12 and 42 months [68]. Proctor et al. [68] found 15 of 18 patients had intact repairs at 12 months, and 14 of 18 at 42 months. In addition, this cohort saw significant improvement in ASES scores from 25 to 71 at 12 months and to 70 at 42 months, with intact cuff repairs averaging 82 on the ASES scores. Another study utilizing polycarbonate polyurethane patch demonstrated 90% of intact repairs in ten patients at 12 months, with significant improvement in SST, ASES, and UCLA scores with no adverse reactions [69]. Even in the case of purely synthetic materials like polyester (Dacron), massive cuff tears re-enforced with synthetic scaffolds have led to decreased pain, increased range of motion, and significant improvement in patient-reported outcomes with minimal failures [70]. These studies lacked a control group but display the promise of synthetic grafts. One cohort study analyzed the outcomes of patients treated with repair only to repair with collagen patch and repair with polypropylene patch augmentation [71]. There were a total of 152 patients in the three groups; the authors found that all three groups saw significant improvements in VAS and UCLA scores at 2 and 36 months. The polypropylene group had significantly higher motion in forward elevation (140.68° versus 140.61° versus 174.71°) and strength (8.73 versus 9.03 versus 13.79 kg) at 36 months. The polypropylene group also had significantly lower re-tear rates (41% versus 51% versus 17%) at 36 months [71].
Some animal models have compared these synthetic scaffolds augmented with biologics like bone marrow-derived stem cells, and while there was an increase in collagen production, this did not always translate to a biomechanically stronger repair [72]. As highlighted by the biomechanical study by Smith et al. [53•], the focus of future research should continue to combine the biomechanical strength of synthetic grafts with integration of biological factors (Table 2).
Stem Cells, Cytokines, and Biologics
Tendon repair is complex system of chemical signaling that relies on inflammation and eventual re-organization of tissue. In order to improve healing rates, scientists have looked for ways to modify the milieu of factors to promote more reliable rotator cuff healing. One of the broadest approaches is the application of mesenchymal stem cells at the site of the repair. Snyder championed the approach of creating a “crimson duvet” to create a collection of stem cells and biological factors at rotator cuff repair sites [73]. Animal studies have supported that bone marrow-derived cells from the footprint can infiltrate the repair footprint and increase the strength of the repair [74]. Gulotta et al. [75] did not find the same improvement in the mechanical outcomes of their repairs, but still demonstrated that the bone marrow-derived cells were biologically active in the repair site. The use of mesenchymal cells to augment biologic scaffolds have proven very effective in strengthening repairs when compared to repairs with only the scaffold [76, 77]. In terms of human studies, Hernigou et al. [78•] performed a case control study comparing 45 patients who received mesechymal stem cells from bone marrow aspirate at single row rotator cuff repair sites to 45 control patients (Fig. 2). The stem cell group had 100% intact repairs at 6 months compared to 67% of controls, and at 10 years, the rate was 87 versus 44%. A recent matched pair case control group compared 35 patients with augmented repairs with adipose-derived mesenchymal stem cells to 35 control patients [79]. While there was no difference in functional outcomes between the two groups, the control group had a re-tear rate of 28.5 versus 14.3% for the stem cell group. While stem cells demonstrate promise in increasing healing rates and improved outcomes, higher-level studies are needed.
Fig. 2.
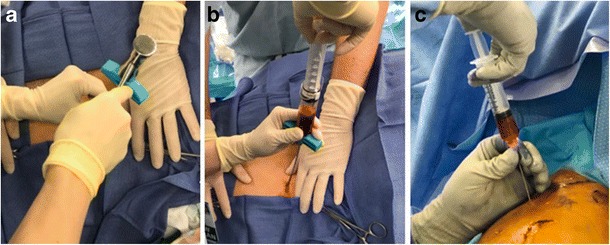
Bone marrow aspirate concentrate. a The trochar is placed at an inferolateral angle into the left iliac crest using a mallet. b A syringe is then attached and bone marrow is aspirated for processing. c Bone marrow aspirate concentrate is injected at the repair site
The effect of stem cells at healing sites is enacted through a complex expression of multiple chemical signals or cytokines. As highlighted by Sundman et al. [14], these factors can vary across various preparations of PRP, which is why researchers have looked to specific cytokines to increase healing rates with rotator cuff repairs. The enthesis of a tendon includes tendon, fibrocartilage, and bone, so early research investigated osteoinductive factors as possible adjuvants. Two sheep studies [80, 81] saw significant improvement in healing rates of rotator cuff tears, but as of yet, these results have not been reproduced in human trials. Transforming growth factor-beta (TGF-β) is a crucial signal in the inflammatory and healing phases of tissue repair; specifically, TGF-β3 has demonstrated some promise in accelerating the healing of rotator cuff tendons and had improved repairs in terms of histology and biomechanics [82, 83] but again is limited to animal models at this point. Matrix metalloprotease (MMP) enzymes have also been studied in soft tissue repairs. MMPs role in degrading collagen and other extra-cellular matrix proteins have been a focus of researchers to prevent re-tears. Gotoh et al. [84] found that MMP-3 was expressed at significantly higher levels in patients with cuff re-tears. Doxycycline, a common antibiotic, has been studied due to its role in MMP inhibition. In a rat study [85], doxycycline has showed promise of preventing re-tears. While MMP-3 has been established as involved with tissue degeneration, other MMPs are up-regulated in embryogenesis and genetically modified stem cells with over expression of membrane type 1-MMP (MT1-MMP) has improved production of fibrocartilage and lead to higher loads to failure and ultimate stress in rat models [86]. Specific growth factors, like platelet-derived growth factor (PDGF) and fibroblast growth factor (FGF), have also been studied. Recombinant PDGF has been shown to improve the biomechanics of repairs and increase bone-tendon interface when used with a collagen matrix [87], but only improve the histological characteristics of repairs when embedded in sutures alone [88]. Similarly, FGF-2 has shown in rat models to increase the histological integration of tendon repair and improve biomechanical properties when applied to rotator cuff repairs [89–91]. One explanation for FGF-2 role is that it stimulates tenogenic progenitor cells that lead to improved tendon to bone healing [92]. Even non-cytokines have been implicated in improving repairs. A recent rabbit study demonstrated that hyaluronic acid might play a role as an adjuvant to rotator cuff repairs [93].
Despite the numerous animal studies analyzing these cytokines and biologic compounds, none have been proven in a human model. Further studies are needed to evaluate the safety and efficacy of these adjuvants in rotator cuff repairs.
Conclusion
Rotator cuff tears are an ever-growing problem with an aging population and consistent healing of repairs remains a problem. While improvements in repair constructs have improved repair rates, researchers are looking for biological tools to augment repairs, especially in at risk populations. PRP has not been shown to improve healing rates or patient-reported outcomes in large level one studies and meta-analyses, but their role as an adjuvant could still provide a benefit in small to medium tears and higher-risk patients. The high-risk patient should be a focus of future studies. Biologic patches and augments are helpful in large and massive rotator cuff repairs. Xenografts, in general, are inferior to allografts due to the inflammatory response they elicit. Allografts provide mechanical reinforcement that leads to lower re-tear rates and better functional outcomes. Synthetic grafts have demonstrated to have superior biomechanical properties but are unable to provide the same biologic scaffold that allograft tissue provides. Combination of synthetic or allograft patches combined with cytokines or growth factors may provide the best environment for rotator cuff repairs. Mesenchymal stem cells have been shown to improve healing rates but not functional outcomes, though high-level human trials are lacking. Chemical messengers, despite their promise in animal models, lack human studies to support their use at this time, but should remain a focus of future research.
Compliance with Ethical Standards
Conflict of Interest
Brian J. Cole reports other from Aesculap/B.Braun, other from American Journal of Orthopedics, other from American Orthopedic Society for Sports Medicine, other from American Shoulder and Elbow Surgeons, personal fees and other from Arthrex, Inc., other from Arthroscopy, other from Arthroscopy Association of North America, other from Athletico, other from Cytori, other from Elsevier Publishing, other from international Cartilage Repair Society, other from Journal of Bone and Joint Surgery-American, other from Journal of Shoulder and Elbow Surgery, other from Journal of the American Academy of Orthopedic Surgeons, other from National Institutes of Health (NIAMS & NICHD), other from Ossur, personal fees and other from Regentis, other from Saunders/Mosby-Elsevier, other from Smith & Nephew, other from Tornier, outside the submitted work.
The other authors declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Footnotes
This article is part of the Topical Collection on Rotator Cuff Repair
References
Papers of particular interest, published recently, have been highlighted as: • Of importance
- 1.Yamaguchi K, Ditsios K, Middleton WD, Hildebolt CF, Galatz LM, Teefey SA. The demographic and morphological features of rotator cuff disease. A comparison of asymptomatic and symptomatic shoulders. J Bone Joint Surg Am. 2006;88(8):1699–1704. doi: 10.2106/JBJS.E.00835. [DOI] [PubMed] [Google Scholar]
- 2.Mather RC, 3rd, Koenig L, Acevedo D, Dall TM, Gallo P, Romeo A, et al. The societal and economic value of rotator cuff repair. J Bone Joint Surg Am. 2013;95(22):1993–2000. doi: 10.2106/JBJS.L.01495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lafosse L, Brozska R, Toussaint B, Gobezie R. The outcome and structural integrity of arthroscopic rotator cuff repair with use of the double-row suture anchor technique. J Bone Joint Surg Am. 2007;89(7):1533–1541. doi: 10.2106/JBJS.F.00305. [DOI] [PubMed] [Google Scholar]
- 4.Galatz LM, Ball CM, Teefey SA, Middleton WD, Yamaguchi K. The outcome and repair integrity of completely arthroscopically repaired large and massive rotator cuff tears. J Bone Joint Surg Am. 2004;86-A(2):219–224. doi: 10.2106/00004623-200402000-00002. [DOI] [PubMed] [Google Scholar]
- 5.Derwin KA, Badylak SF, Steinmann SP, Iannotti JP. Extracellular matrix scaffold devices for rotator cuff repair. J Shoulder Elb Surg. 2010;19(3):467–476. doi: 10.1016/j.jse.2009.10.020. [DOI] [PubMed] [Google Scholar]
- 6.Slabaugh MA, Nho SJ, Grumet RC, Wilson JB, Seroyer ST, Frank RM, Romeo AA, Provencher MT, Verma NN. Does the literature confirm superior clinical results in radiographically healed rotator cuffs after rotator cuff repair? Arthroscopy. 2010;26(3):393–403. doi: 10.1016/j.arthro.2009.07.023. [DOI] [PubMed] [Google Scholar]
- 7.Kim KC, Shin HD, Lee WY. Repair integrity and functional outcomes after arthroscopic suture-bridge rotator cuff repair. J Bone Joint Surg Am. 2012;94(8):e48. doi: 10.2106/JBJS.K.00158. [DOI] [PubMed] [Google Scholar]
- 8.Russell RD, Knight JR, Mulligan E, Khazzam MS. Structural integrity after rotator cuff repair does not correlate with patient function and pain: a meta-analysis. J Bone Joint Surg Am. 2014;96(4):265–271. doi: 10.2106/JBJS.M.00265. [DOI] [PubMed] [Google Scholar]
- 9.Verma NN, Bhatia S, Baker CL, 3rd, Cole BJ, Boniquit N, Nicholson GP, et al. Outcomes of arthroscopic rotator cuff repair in patients aged 70 years or older. Arthroscopy. 2010;26(10):1273–1280. doi: 10.1016/j.arthro.2010.01.031. [DOI] [PubMed] [Google Scholar]
- 10.Tashjian RZ, Hollins AM, Kim HM, Teefey SA, Middleton WD, Steger-May K, Galatz LM, Yamaguchi K. Factors affecting healing rates after arthroscopic double-row rotator cuff repair. Am J Sports Med. 2010;38(12):2435–2442. doi: 10.1177/0363546510382835. [DOI] [PubMed] [Google Scholar]
- 11.Angeline ME, Dines JS. Factors affecting the outcome of rotator cuff surgery. In: Nicholson GP, editor. Orthopaedic knowledge update 4: shoulder and elbow. Rosemont: American Academy of Orthopaedic Surgeons; 2013. pp. 217–228. [Google Scholar]
- 12.Garbis N, Romeo AA, Van Thiel G, Ghodadra N, Provencher MT, Cole BJ, Verma N. Clinical indications and techniques for the use of platelet-rich plasma in the shoulder. Oper Tech Sports Med. 2011;19(3):165–169. doi: 10.1053/j.otsm.2011.03.002. [DOI] [Google Scholar]
- 13.Boswell SG, Cole BJ, Sundman EA, Karas V, Fortier LA. Platelet-rich plasma: a milieu of bioactive factors. Arthroscopy. 2012;28(3):429–439. doi: 10.1016/j.arthro.2011.10.018. [DOI] [PubMed] [Google Scholar]
- 14.Sundman EA, Cole BJ, Fortier LA. Growth factor and catabolic cytokine concentrations are influenced by the cellular composition of platelet-rich plasma. Am J Sports Med. 2011;39(10):2135–2140. doi: 10.1177/0363546511417792. [DOI] [PubMed] [Google Scholar]
- 15.Kesikburun S, Tan AK, Yilmaz B, Yasar E, Yazicioglu K. Platelet-rich plasma injections in the treatment of chronic rotator cuff tendinopathy: a randomized controlled trial with 1-year follow-up. Am J Sports Med. 2013;41(11):2609–2616. doi: 10.1177/0363546513496542. [DOI] [PubMed] [Google Scholar]
- 16.• Filardo G, Di Matteo B, Kon E, Merli G, Marcacci M. Platelet-rich plasma in tendon-related disorders: results and indications. Knee Surg Sports Traumatol Arthrosc. 2016; Meta-analysis of the effect of PRP on tendinopathy including 32 studies investigating rotator cuff pathology. They found no significant difference in non-operative outcomes between single and multiple injection protocols.
- 17.Zhang JY, Fabricant PD, Ishmael CR, Wang JC, Petrigliano FA, Jones KJ. Utilization of platelet-rich plasma for musculoskeletal injuries: an analysis of current treatment trends in the United States. Orthop J Sports Med. 2016;4(12):2325967116676241. doi: 10.1177/2325967116676241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shams A, El-Sayed M, Gamal O, Ewes W. Subacromial injection of autologous platelet-rich plasma versus corticosteroid for the treatment of symptomatic partial rotator cuff tears. Eur J Orthop Surg Traumatol. 2016;26(8):837–842. doi: 10.1007/s00590-016-1826-3. [DOI] [PubMed] [Google Scholar]
- 19.Cross JA, Cole BJ, Spatny KP, Sundman E, Romeo AA, Nicholson GP, Wagner B, Fortier LA. Leukocyte-reduced platelet-rich plasma normalizes matrix metabolism in torn human rotator cuff tendons. Am J Sports Med. 2015;43(12):2898–2906. doi: 10.1177/0363546515608157. [DOI] [PubMed] [Google Scholar]
- 20.Randelli P, Arrigoni P, Ragone V, Aliprandi A, Cabitza P. Platelet rich plasma in arthroscopic rotator cuff repair: a prospective RCT study, 2-year follow-up. J Shoulder Elb Surg. 2011;20(4):518–528. doi: 10.1016/j.jse.2011.02.008. [DOI] [PubMed] [Google Scholar]
- 21.Castricini R, Longo UG, De Benedetto M, Panfoli N, Pirani P, Zini R, Maffulli N, Denaro V. Platelet-rich plasma augmentation for arthroscopic rotator cuff repair: a randomized controlled trial. Am J Sports Med. 2011;39(2):258–265. doi: 10.1177/0363546510390780. [DOI] [PubMed] [Google Scholar]
- 22.Weber SC, Kauffman JI, Parise C, Weber SJ, Katz SD. Platelet-rich fibrin matrix in the management of arthroscopic repair of the rotator cuff: a prospective, randomized, double-blinded study. Am J Sports Med. 2013;41(2):263–270. doi: 10.1177/0363546512467621. [DOI] [PubMed] [Google Scholar]
- 23.Rodeo SA, Delos D, Williams RJ, Adler RS, Pearle A, Warren RF. The effect of platelet-rich fibrin matrix on rotator cuff tendon healing: a prospective, randomized clinical study. Am J Sports Med. 2012;40(6):1234–1241. doi: 10.1177/0363546512442924. [DOI] [PubMed] [Google Scholar]
- 24.Chahal J, Van Thiel GS, Mall N, Heard W, Bach BR, Cole BJ, Nicholson GP, Verma NN, Whelan DB, Romeo AA. The role of platelet-rich plasma in arthroscopic rotator cuff repair: a systematic review with quantitative synthesis. Arthroscopy. 2012;28(11):1718–1727. doi: 10.1016/j.arthro.2012.03.007. [DOI] [PubMed] [Google Scholar]
- 25.Warth RJ, Dornan GJ, James EW, Horan MP, Millett PJ. Clinical and structural outcomes after arthroscopic repair of full-thickness rotator cuff tears with and without platelet-rich product supplementation: a meta-analysis and meta-regression. Arthroscopy. 2015;31(2):306–320. doi: 10.1016/j.arthro.2014.09.007. [DOI] [PubMed] [Google Scholar]
- 26.Bergeson AG, Tashjian RZ, Greis PE, Crim J, Stoddard GJ, Burks RT. Effects of platelet-rich fibrin matrix on repair integrity of at-risk rotator cuff tears. Am J Sports Med. 2012;40(2):286–293. doi: 10.1177/0363546511424402. [DOI] [PubMed] [Google Scholar]
- 27.Jo CH, Shin JS, Shin WH, Lee SY, Yoon KS, Shin S. Platelet-rich plasma for arthroscopic repair of medium to large rotator cuff tears: a randomized controlled trial. Am J Sports Med. 2015;43(9):2102–2110. doi: 10.1177/0363546515587081. [DOI] [PubMed] [Google Scholar]
- 28.Malavolta EA, Gracitelli ME, Ferreira Neto AA, Assuncao JH, Bordalo-Rodrigues M, de Camargo OP. Platelet-rich plasma in rotator cuff repair: a prospective randomized study. Am J Sports Med. 2014;42(10):2446–2454. doi: 10.1177/0363546514541777. [DOI] [PubMed] [Google Scholar]
- 29.Barber FA. Triple-loaded single-row versus suture-bridge double-row rotator cuff tendon repair with platelet-rich plasma fibrin membrane: a randomized controlled trial. Arthroscopy. 2016;32(5):753–761. doi: 10.1016/j.arthro.2015.11.020. [DOI] [PubMed] [Google Scholar]
- 30.D'Ambrosi R, Palumbo F, Paronzini A, Ragone V, Facchini RM. Platelet-rich plasma supplementation in arthroscopic repair of full-thickness rotator cuff tears: a randomized clinical trial. Musculoskelet Surg. 2016;100(Suppl 1):25–32. doi: 10.1007/s12306-016-0415-2. [DOI] [PubMed] [Google Scholar]
- 31.Holtby R, Christakis M, Maman E, MacDermid JC, Dwyer T, Athwal GS, Faber K, Theodoropoulos J, Woodhouse LJ, Razmjou H. Impact of platelet-rich plasma on arthroscopic repair of small- to medium-sized rotator cuff tears: a randomized controlled trial. Orthop J Sports Med. 2016;4(9):2325967116665595. doi: 10.1177/2325967116665595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Flury M, Rickenbacher D, Schwyzer HK, Jung C, Schneider MM, Stahnke K, Goldhahn J, Audigé L. Does pure platelet-rich plasma affect postoperative clinical outcomes after arthroscopic rotator cuff repair? A randomized controlled trial. Am J Sports Med. 2016;44(8):2136–2146. doi: 10.1177/0363546516645518. [DOI] [PubMed] [Google Scholar]
- 33.Zumstein MA, Rumian A, Thelu CE, Lesbats V, O'Shea K, Schaer M, et al. SECEC research grant 2008 II: use of platelet- and leucocyte-rich fibrin (L-PRF) does not affect late rotator cuff tendon healing: a prospective randomized controlled study. J Shoulder Elb Surg. 2016;25(1):2–11. doi: 10.1016/j.jse.2015.09.018. [DOI] [PubMed] [Google Scholar]
- 34.Wang A, McCann P, Colliver J, Koh E, Ackland T, Joss B, Zheng M, Breidahl B. Do postoperative platelet-rich plasma injections accelerate early tendon healing and functional recovery after arthroscopic supraspinatus repair? A randomized controlled trial. Am J Sports Med. 2015;43(6):1430–1437. doi: 10.1177/0363546515572602. [DOI] [PubMed] [Google Scholar]
- 35.Ebert JR, Wang A, Smith A, Nairn R, Breidahl W, Zheng MH, et al. A Midterm Evaluation of Postoperative Platelet-Rich Plasma Injections on Arthroscopic Supraspinatus Repair: A Randomized Contreolled Trial. Am J Sports Med 2017;45(13):2965–74. Randomized control trial comparing rotator cuff repair with post-operative PRP injections versus repair alone. PRP post-operative injections improved abduction strength, but otherwise provided no other functional improvement or benefit to tendon integrity. [DOI] [PubMed]
- 36.Cai YZ, Zhang C, Lin XJ. Efficacy of platelet-rich plasma in arthroscopic repair of full-thickness rotator cuff tears: a meta-analysis. J Shoulder Elb Surg. 2015;24(12):1852–1859. doi: 10.1016/j.jse.2015.07.035. [DOI] [PubMed] [Google Scholar]
- 37.Saltzman BM, Jain A, Campbell KA, Mascarenhas R, Romeo AA, Verma NN, Cole BJ. Does the use of platelet-rich plasma at the time of surgery improve clinical outcomes in arthroscopic rotator cuff repair when compared with control cohorts? A systematic review of meta-analyses. Arthroscopy. 2016;32(5):906–918. doi: 10.1016/j.arthro.2015.10.007. [DOI] [PubMed] [Google Scholar]
- 38.Vavken P, Sadoghi P, Palmer M, Rosso C, Mueller AM, Szoelloesy G, Valderrabano V. Platelet-rich plasma reduces Retear rates after arthroscopic repair of small- and medium-sized rotator cuff tears but is not cost-effective. Am J Sports Med. 2015;43(12):3071–3076. doi: 10.1177/0363546515572777. [DOI] [PubMed] [Google Scholar]
- 39.Samuelson EM, Odum SM, Fleischli JE. The cost-effectiveness of using platelet-rich plasma during rotator cuff repair: a Markov model analysis. Arthroscopy. 2016;32(7):1237–1244. doi: 10.1016/j.arthro.2015.12.018. [DOI] [PubMed] [Google Scholar]
- 40.Rodeo SA. Biologic augmentation of rotator cuff tendon repair. J Shoulder Elb Surg. 2007;16(5 Suppl):S191–S197. doi: 10.1016/j.jse.2007.03.012. [DOI] [PubMed] [Google Scholar]
- 41.Adams JE, Zobitz ME, Reach JS, Jr, An KN, Steinmann SP. Rotator cuff repair using an acellular dermal matrix graft: an in vivo study in a canine model. Arthroscopy. 2006;22(7):700–709. doi: 10.1016/j.arthro.2006.03.016. [DOI] [PubMed] [Google Scholar]
- 42.Sclamberg SG, Tibone JE, Itamura JM, Kasraeian S. Six-month magnetic resonance imaging follow-up of large and massive rotator cuff repairs reinforced with porcine small intestinal submucosa. J Shoulder Elb Surg. 2004;13(5):538–541. doi: 10.1016/j.jse.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 43.Iannotti JP, Codsi MJ, Kwon YW, Derwin K, Ciccone J, Brems JJ. Porcine small intestine submucosa augmentation of surgical repair of chronic two-tendon rotator cuff tears. A randomized, controlled trial. J Bone Joint Surg Am. 2006;88(6):1238–1244. doi: 10.2106/JBJS.E.00524. [DOI] [PubMed] [Google Scholar]
- 44.Walton JR, Bowman NK, Khatib Y, Linklater J, Murrell GA. Restore orthobiologic implant: not recommended for augmentation of rotator cuff repairs. J Bone Joint Surg Am. 2007;89(4):786–791. doi: 10.2106/JBJS.F.00315. [DOI] [PubMed] [Google Scholar]
- 45.Gilbert TW, Freund JM, Badylak SF. Quantification of DNA in biologic scaffold materials. J Surg Res. 2009;152(1):135–139. doi: 10.1016/j.jss.2008.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Badhe SP, Lawrence TM, Smith FD, Lunn PG. An assessment of porcine dermal xenograft as an augmentation graft in the treatment of extensive rotator cuff tears. J Shoulder Elb Surg. 2008;17(1 Suppl):35S–39S. doi: 10.1016/j.jse.2007.08.005. [DOI] [PubMed] [Google Scholar]
- 47.Cho CH, Lee SM, Lee YK, Shin HK. Mini-open suture bridge repair with porcine dermal patch augmentation for massive rotator cuff tear: surgical technique and preliminary results. Clin Orthop Surg. 2014;6(3):329–335. doi: 10.4055/cios.2014.6.3.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gupta AK, Hug K, Boggess B, Gavigan M, Toth AP. Massive or 2-tendon rotator cuff tears in active patients with minimal glenohumeral arthritis: clinical and radiographic outcomes of reconstruction using dermal tissue matrix xenograft. Am J Sports Med. 2013;41(4):872–879. doi: 10.1177/0363546512475204. [DOI] [PubMed] [Google Scholar]
- 49.Bond JL, Dopirak RM, Higgins J, Burns J, Snyder SJ. Arthroscopic replacement of massive, irreparable rotator cuff tears using a GraftJacket allograft: technique and preliminary results. Arthroscopy. 2008;24(4):403–409.e1. doi: 10.1016/j.arthro.2007.07.033. [DOI] [PubMed] [Google Scholar]
- 50.Wong I, Burns J, Snyder S. Arthroscopic GraftJacket repair of rotator cuff tears. J Shoulder Elb Surg. 2010;19(2 Suppl):104–109. doi: 10.1016/j.jse.2009.12.017. [DOI] [PubMed] [Google Scholar]
- 51.Gupta AK, Hug K, Berkoff DJ, Boggess BR, Gavigan M, Malley PC, Toth AP. Dermal tissue allograft for the repair of massive irreparable rotator cuff tears. Am J Sports Med. 2012;40(1):141–147. doi: 10.1177/0363546511422795. [DOI] [PubMed] [Google Scholar]
- 52.Barber FA, Burns JP, Deutsch A, Labbe MR, Litchfield RB. A prospective, randomized evaluation of acellular human dermal matrix augmentation for arthroscopic rotator cuff repair. Arthroscopy. 2012;28(1):8–15. doi: 10.1016/j.arthro.2011.06.038. [DOI] [PubMed] [Google Scholar]
- 53.• Smith RDJ, Zargar N, Brown CP, Nagra NS, Dakin SG, Snelling SJB, et al. Characterizing the macro and micro mechanical properties of scaffolds for rotator cuff repair. J Shoulder Elbow Surg 2017. Characterized the mechanical properties of available scaffolds and none matched the mechanics of the supraspinatus tendon. [DOI] [PubMed]
- 54.Arnoczky SP, Bishai SK, Schofield B, Sigman S, Bushnell BD, Hommen JP, van Kampen C. Histologic evaluation of biopsy specimens obtained after rotator cuff repair augmented with a highly porous collagen implant. Arthroscopy. 2017;33(2):278–283. doi: 10.1016/j.arthro.2016.06.047. [DOI] [PubMed] [Google Scholar]
- 55.Beitzel K, McCarthy MB, Cote MP, Russell RP, Apostolakos J, Ramos DM, Kumbar SG, Imhoff AB, Arciero RA, Mazzocca AD. Properties of biologic scaffolds and their response to mesenchymal stem cells. Arthroscopy. 2014;30(3):289–298. doi: 10.1016/j.arthro.2013.11.020. [DOI] [PubMed] [Google Scholar]
- 56.Voss A, McCarthy MB, Hoberman A, Cote MP, Imhoff AB, Mazzocca AD, Beitzel K. Extracellular matrix of current biological scaffolds promotes the differentiation potential of mesenchymal stem cells. Arthroscopy. 2016;32(11):2381–2392.e1. doi: 10.1016/j.arthro.2016.04.033. [DOI] [PubMed] [Google Scholar]
- 57.Consigliere P, Polyzois I, Sarkhel T, Gupta R, Levy O, Narvani AA. Preliminary results of a consecutive series of large & massive rotator cuff tears treated with arthroscopic rotator cuff repairs augmented with extracellular matrix. Arch Bone Jt Surg. 2017;5(1):14–21. [PMC free article] [PubMed] [Google Scholar]
- 58.Petri M, Warth RJ, Horan MP, Greenspoon JA, Millett PJ. Outcomes after open revision repair of massive rotator cuff tears with biologic patch augmentation. Arthroscopy. 2016;32(9):1752–1760. doi: 10.1016/j.arthro.2016.01.037. [DOI] [PubMed] [Google Scholar]
- 59.Sears BW, Choo A, Yu A, Greis A, Lazarus M. Clinical outcomes in patients undergoing revision rotator cuff repair with extracellular matrix augmentation. Orthopedics. 2015;38(4):e292–e296. doi: 10.3928/01477447-20150402-57. [DOI] [PubMed] [Google Scholar]
- 60.Gilot GJ, Alvarez-Pinzon AM, Barcksdale L, Westerdahl D, Krill M, Peck E. Outcome of large to massive rotator cuff tears repaired with and without extracellular matrix augmentation: a prospective comparative study. Arthroscopy. 2015;31(8):1459–1465. doi: 10.1016/j.arthro.2015.02.032. [DOI] [PubMed] [Google Scholar]
- 61.Steinhaus ME, Makhni EC, Cole BJ, Romeo AA, Verma NN. Outcomes after patch use in rotator cuff repair. Arthroscopy. 2016;32(8):1676–1690. doi: 10.1016/j.arthro.2016.02.009. [DOI] [PubMed] [Google Scholar]
- 62.Ferguson DP, Lewington MR, Smith TD, Wong IH. Graft utilization in the augmentation of large-to-massive rotator cuff repairs: a systematic review. Am J Sports Med. 2016;44(11):2984–2992. doi: 10.1177/0363546515624463. [DOI] [PubMed] [Google Scholar]
- 63.Ono Y, Davalos Herrera DA, Woodmass JM, Boorman RS, Thornton GM, Lo IK. Can grafts provide superior tendon healing and clinical outcomes after rotator cuff repairs?: a meta-analysis. Orthop J Sports Med. 2016;4(12):2325967116674191. doi: 10.1177/2325967116674191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gillespie RJ, Knapik DM, Akkus O. Biologic and synthetic grafts in the reconstruction of large to massive rotator cuff tears. J Am Acad Orthop Surg. 2016;24(12):823–828. doi: 10.5435/JAAOS-D-15-00229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.McCormack RA, Shreve M, Strauss EJ. Biologic augmentation in rotator cuff repair--should we do it, who should get it, and has it worked? Bull Hosp Jt Dis (2013) 2014;72(1):89–96. [PubMed] [Google Scholar]
- 66.Cole BJ, Gomoll AH, Yanke A, Pylawka T, Lewis P, Macgillivray JD, et al. Biocompatibility of a polymer patch for rotator cuff repair. Knee Surg Sports Traumatol Arthrosc. 2007;15(5):632–637. doi: 10.1007/s00167-006-0187-6. [DOI] [PubMed] [Google Scholar]
- 67.Derwin KA, Codsi MJ, Milks RA, Baker AR, McCarron JA, Iannotti JP. Rotator cuff repair augmentation in a canine model with use of a woven poly-L-lactide device. J Bone Joint Surg Am. 2009;91(5):1159–1171. doi: 10.2106/JBJS.H.00775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Proctor CS. Long-term successful arthroscopic repair of large and massive rotator cuff tears with a functional and degradable reinforcement device. J Shoulder Elb Surg. 2014;23(10):1508–1513. doi: 10.1016/j.jse.2014.01.010. [DOI] [PubMed] [Google Scholar]
- 69.Encalada-Diaz I, Cole BJ, Macgillivray JD, Ruiz-Suarez M, Kercher JS, Friel NA, et al. Rotator cuff repair augmentation using a novel polycarbonate polyurethane patch: preliminary results at 12 months' follow-up. J Shoulder Elb Surg. 2011;20(5):788–794. doi: 10.1016/j.jse.2010.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Nada AN, Debnath UK, Robinson DA, Jordan C. Treatment of massive rotator-cuff tears with a polyester ligament (Dacron) augmentation: clinical outcome. J Bone Joint Surg Br. 2010;92(10):1397–1402. doi: 10.1302/0301-620X.92B10.24299. [DOI] [PubMed] [Google Scholar]
- 71.Ciampi P, Scotti C, Nonis A, Vitali M, Di Serio C, Peretti GM, Fraschini G. The benefit of synthetic versus biological patch augmentation in the repair of posterosuperior massive rotator cuff tears: a 3-year follow-up study. Am J Sports Med. 2014;42(5):1169–1175. doi: 10.1177/0363546514525592. [DOI] [PubMed] [Google Scholar]
- 72.Depres-Tremblay G, Chevrier A, Snow M, Hurtig MB, Rodeo S, Buschmann MD. Rotator cuff repair: a review of surgical techniques, animal models, and new technologies under development. J Shoulder Elb Surg. 2016;25(12):2078–2085. doi: 10.1016/j.jse.2016.06.009. [DOI] [PubMed] [Google Scholar]
- 73.Snyder SJ, Burns J. Rotator cuff healing and the bone marrow “crimson duvet” from clinical observations to science. Tech Should Surg. 2009;10(4):130–137. doi: 10.1097/BTE.0b013e3181c2a940. [DOI] [Google Scholar]
- 74.Kida Y, Morihara T, Matsuda K, Kajikawa Y, Tachiiri H, Iwata Y, et al. Bone marrow-derived cells from the footprint infiltrate into the repaired rotator cuff. J Shoulder Elb Surg. 2013;22(2):197–205. doi: 10.1016/j.jse.2012.02.007. [DOI] [PubMed] [Google Scholar]
- 75.Gulotta LV, Kovacevic D, Ehteshami JR, Dagher E, Packer JD, Rodeo SA. Application of bone marrow-derived mesenchymal stem cells in a rotator cuff repair model. Am J Sports Med. 2009;37(11):2126–2133. doi: 10.1177/0363546509339582. [DOI] [PubMed] [Google Scholar]
- 76.Tornero-Esteban P, Hoyas JA, Villafuertes E, Rodriguez-Bobada C, Lopez-Gordillo Y, Rojo FJ, et al. Efficacy of supraspinatus tendon repair using mesenchymal stem cells along with a collagen I scaffold. J Orthop Surg Res 2015;10:124-015-0269-6. [DOI] [PMC free article] [PubMed]
- 77.Thangarajah T, Sanghani-Kerai A, Henshaw F, Lambert SM, Pendegrass CJ, Blunn GW. Application of a Demineralized Cortical Bone Matrix and Bone Marrow-Derived Mesenchymal Stem Cells in a Model of Chronic Rotator Cuff Degeneration. Am J Sports Med 2017;46(1):98–108. [DOI] [PubMed]
- 78.Hernigou P, Flouzat Lachaniette CH, Delambre J, Zilber S, Duffiet P, Chevallier N, Rouard H. Biologic augmentation of rotator cuff repair with mesenchymal stem cells during arthroscopy improves healing and prevents further tears: a case-controlled study. Int Orthop. 2014;38(9):1811–1818. doi: 10.1007/s00264-014-2391-1. [DOI] [PubMed] [Google Scholar]
- 79.Kim YS, Sung CH, Chung SH, Kwak SJ, Koh YG. Does an injection of adipose-derived mesenchymal stem cells loaded in fibrin glue influence rotator cuff repair outcomes? A clinical and magnetic resonance imaging study. Am J Sports Med. 2017;45(9):2010–2018. doi: 10.1177/0363546517702863. [DOI] [PubMed] [Google Scholar]
- 80.Seeherman HJ, Archambault JM, Rodeo SA, Turner AS, Zekas L, D'Augusta D, et al. rhBMP-12 accelerates healing of rotator cuff repairs in a sheep model. J Bone Joint Surg Am. 2008;90(10):2206–2219. doi: 10.2106/JBJS.G.00742. [DOI] [PubMed] [Google Scholar]
- 81.Rodeo SA, Potter HG, Kawamura S, Turner AS, Kim HJ, Atkinson BL. Biologic augmentation of rotator cuff tendon-healing with use of a mixture of osteoinductive growth factors. J Bone Joint Surg Am. 2007;89(11):2485–2497. doi: 10.2106/JBJS.C.01627. [DOI] [PubMed] [Google Scholar]
- 82.Kovacevic D, Fox AJ, Bedi A, Ying L, Deng XH, Warren RF, Rodeo SA. Calcium-phosphate matrix with or without TGF-beta3 improves tendon-bone healing after rotator cuff repair. Am J Sports Med. 2011;39(4):811–819. doi: 10.1177/0363546511399378. [DOI] [PubMed] [Google Scholar]
- 83.Manning CN, Kim HM, Sakiyama-Elbert S, Galatz LM, Havlioglu N, Thomopoulos S. Sustained delivery of transforming growth factor beta three enhances tendon-to-bone healing in a rat model. J Orthop Res. 2011;29(7):1099–1105. doi: 10.1002/jor.21301. [DOI] [PubMed] [Google Scholar]
- 84.Gotoh M, Mitsui Y, Shibata H, Yamada T, Shirachi I, Nakama K, Okawa T, Higuchi F, Nagata K. Increased matrix metalloprotease-3 gene expression in ruptured rotator cuff tendons is associated with postoperative tendon retear. Knee Surg Sports Traumatol Arthrosc. 2013;21(8):1807–1812. doi: 10.1007/s00167-012-2209-x. [DOI] [PubMed] [Google Scholar]
- 85.Bedi A, Fox AJ, Kovacevic D, Deng XH, Warren RF, Rodeo SA. Doxycycline-mediated inhibition of matrix metalloproteinases improves healing after rotator cuff repair. Am J Sports Med. 2010;38(2):308–317. doi: 10.1177/0363546509347366. [DOI] [PubMed] [Google Scholar]
- 86.Gulotta LV, Kovacevic D, Montgomery S, Ehteshami JR, Packer JD, Rodeo SA. Stem cells genetically modified with the developmental gene MT1-MMP improve regeneration of the supraspinatus tendon-to-bone insertion site. Am J Sports Med. 2010;38(7):1429–1437. doi: 10.1177/0363546510361235. [DOI] [PubMed] [Google Scholar]
- 87.Hee CK, Dines JS, Dines DM, Roden CM, Wisner-Lynch LA, Turner AS, Mcgilvray KC, Lyons AS, Puttlitz CM, Santoni BG. Augmentation of a rotator cuff suture repair using rhPDGF-BB and a type I bovine collagen matrix in an ovine model. Am J Sports Med. 2011;39(8):1630–1639. doi: 10.1177/0363546511404942. [DOI] [PubMed] [Google Scholar]
- 88.Uggen C, Dines J, McGarry M, Grande D, Lee T, Limpisvasti O. The effect of recombinant human platelet-derived growth factor BB-coated sutures on rotator cuff healing in a sheep model. Arthroscopy. 2010;26(11):1456–1462. doi: 10.1016/j.arthro.2010.02.025. [DOI] [PubMed] [Google Scholar]
- 89.Ide J, Kikukawa K, Hirose J, Iyama K, Sakamoto H, Fujimoto T, et al. The effect of a local application of fibroblast growth factor-2 on tendon-to-bone remodeling in rats with acute injury and repair of the supraspinatus tendon. J Shoulder Elb Surg. 2009;18(3):391–398. doi: 10.1016/j.jse.2009.01.013. [DOI] [PubMed] [Google Scholar]
- 90.Ide J, Kikukawa K, Hirose J, Iyama K, Sakamoto H, Mizuta H. The effects of fibroblast growth factor-2 on rotator cuff reconstruction with acellular dermal matrix grafts. Arthroscopy. 2009;25(6):608–616. doi: 10.1016/j.arthro.2008.11.011. [DOI] [PubMed] [Google Scholar]
- 91.Tokunaga T, Karasugi T, Arimura H, Yonemitsu R, Sakamoto H, Ide J, Mizuta H. Enhancement of rotator cuff tendon-bone healing with fibroblast growth factor 2 impregnated in gelatin hydrogel sheets in a rabbit model. J Shoulder Elb Surg. 2017;26(10):1708–1717. doi: 10.1016/j.jse.2017.03.020. [DOI] [PubMed] [Google Scholar]
- 92.Tokunaga T, Shukunami C, Okamoto N, Taniwaki T, Oka K, Sakamoto H, Ide J, Mizuta H, Hiraki Y. FGF-2 stimulates the growth of Tenogenic progenitor cells to facilitate the generation of Tenomodulin-positive tenocytes in a rat rotator cuff healing model. Am J Sports Med. 2015;43(10):2411–2422. doi: 10.1177/0363546515597488. [DOI] [PubMed] [Google Scholar]
- 93.Honda H, Gotoh M, Kanazawa T, Ohzono H, Nakamura H, Ohta K, et al. Hyaluronic Acid Accelerates Tendon-to-Bone Healing After Rotator Cuff Repair. Am J Sports Med 2017;45(14):3322–30. [DOI] [PubMed]


