Abstract
Blastocystis hominis (B. hominis) is a protozoan zoonosis which clinical signs of infection with this parasite has been reported to be more severe in patients with weakened immune systems than healthy controls. So, the aim of the study was to evaluate genomic analysis of B. hominis isolates obtained from patients with HIV-positive using locus SSU-rDNA. At first, 268 stool samples were randomly collected from patients with HIV-positive referred to health centers of Khuzestan province, southwest of Iran. Formol-ether and direct smear techniques were used for the detection of parasitic agents. After extracting DNA, the samples were analyzed by the PCR method. Finally, the subtypes were determined by the sequencing and PCR methods. New samples were used for the preparation of positive control sample; they were cultured in coagulant-serum biphasic cultivation media. Of 268 stool samples, 33 (12.3%) cases were detected positive for B. hominis using Formol-Ether technique but 51 (19%) cases were positive using molecular method. The most common isolates were related to the subtype III with 29 positive cases (56.8%), then, genotype I with 11 (21.6%) cases, 6 cases (11.8%) with genotype II, 3 (5.9%) combined cases with genotypes I and III as well as 2 cases (3.9%) with genotype VI. There was a significant difference between two groups of HIV-positive patients (infected with the parasite and/or without the parasite) in the term of the mean of TCD4-positive cells. The results indicated a relatively high prevalence of B. hominis in HIV-positive patients as well as our findings may represent that the number reduction of TCD4-positive cells has an effective role in the increased risk of the parasitic infection in HIV-positive patients.
Keywords: Blastocystis hominis, HIV-positive, PCR, Khuzestan province, Iran
Introduction
Blastocystis hominis (B. hominis) is a protozoan zoonosis which lives in the large intestine of humans and other vertebrates as anaerobic (Jones et al. 2009). B. hominis was detected as a fungal yeast in human stool sample, for the first time in 1911 (Badparva et al. 2015). Different morphological forms of the parasite are including vacuolar, granules and amoeboid (Zhang et al. 2012). B. hominis can cause blastocystosis with the diarrhea accompanied by dizziness, vomiting, abdominal pain, anorexia, nausea, weight loss and intestinal tympanites (Zhang et al. 2012). This infection happens worldwide with a prevalence rat of 1–30%; developing regions and areas with poor sanitation have a higher outbreak than developed countries with good sanitation (Cirioni et al. 1999; Graczyk et al. 2005; Guignard et al. 2000; Hellard et al. 2000). In addition, the parasite is transmitted by cysts via contaminated water and food (Badparva et al. 2015).
Previous studies showed that Blastocystis has the different subtypes; 13 subtypes of this parasite are identified using molecular methods and subtypes 1–9 have been isolated from human (Parkar et al. 2010; Stensvold et al. 2007, 2009b). Some investigations reported subtype 4 as the most common subtypes in humans (Domínguez-Márquez et al. 2009; Poirier et al. 2011) but other studies indicated that subtype 3 were as the most dominant genotype that causing of more gastrointestinal symptoms (Stensvold et al. 2009a; Tan et al. 2008). Simultaneous infection of HIV and parasites is common and the effects of simultaneous infection of parasitic diseases and HIV have recently been receiving much attention (John et al. 2006). The clinical symptoms of the infection with B. hominis has been reported to be more severe in patients with immune deficiency than healthy controls (Eroglu et al. 2009). Considering patients with acquired immune deficiency syndrome (AIDS) and the prevalence of Blastocystis parasite and the unknown strains responsible for the infections in these patients; no recorded data is available in Khuzestan province, southwest of Iran. Therefore, the aim of the study was to evaluate genomic analysis of B. hominis isolates obtained from patients with HIV-positive using locus SSU-rDNA.
Methods
Specimen collection
At first, the purpose and nature of this study were expressed to the HIV-positive patients. Then, 286 stool samples were randomly collected among HIV-positive patients referred to the health centers in Khuzestan province, southwest of Iran during 2013–2014 (Safi et al. 2016). In the next stage, the samples were transferred to Department of Parasitology, Ahvaz Jundishapur University of Medical Sciences. AIDS in all patients was previously proved and these patients had the documents in the health centers that their TCD4 count was also determined.
Stool examination
At first, the samples were condensed using Formol-ether technique and the prevalence rate of Blastocystis was determined by the examination of the samples sediment using microscopic method. The samples sediment was kept at − 20 °C. Then, the DNA samples were extracted by Stool DNA Extraction mini Kit Bioneer (K-3036 AccuPrep® Stool DNA Extraction Kit 100 reactions) and kept at − 20 °C in a sterilized condition. Forward and Reverse primers were used to do PCR on the extracted DNA (Yoshikawa et al. 2000). These primers are able to reproduce SSU-rDNA gene fragment. The length of these gene segments is about 500 bp. Organic base sequence of each primer is given below:
SR1F (GCTTATCTGGTTGATCCTGCCAGTAGT)
SR1R (TGATCCTTCCGCAGGTTCACCTA)
Then, the genotype of the parasite was examined by using of seven pairs of primers sequenc-es-tagged site (STS) (Table 1). The parasite genotype was determined based on the size of amplified fragment.
Table 1.
Seven pairs of STS primers for genotyping of Blastocystis hominis
| STS primer set product | Size (bp) | Sequences of forward (F) and reverse (R) primers (5′–3′) | GenBank accession no. |
|---|---|---|---|
| SB83sub I | 351 | F: GAAGGACTCTCTGACGATGA R: GTCCAAATGAAAGGCAGC |
AF166086 |
| SB340sub II | 704 | F: ATCAGCCTACAATCTCCTC R: ATCGCCACTTCTCCAAT |
AF166087 |
| SB227sub III | 526 | F: TAGGATTTGGTGTTTGGAGA R: TTAGAAGTGAAGGAGATGGAAG |
AF166088 |
| SB337sub IV | 487 | F: GCATCCAGACTACTATCAACATT R: CCATTTTCAGACAACCACTTA |
AF166091 |
| SB336sub V | 317 | F: TGTTCTTGTGTCTTCTCAGCTC R: TTCTTTCACACTCCCGTCAT |
AY048752 |
| SB332sub VI | 338 | F: GTGGGTAGAGGAAGGAAAACA R: AGAACAAGTCGATGAAGTGAGAT |
AY048751 |
| SB155sub VII | 650 | F: GTCTTTCCCTGTCTATTCTGCA R: AATTCGGTCTGCTTCTTCTG |
AY048750 |
Sequencing
For genotyping, the positive samples from the PCR assay were sequenced by the Bioneer Company (Daejeon, South Korea). Afterwards, the specified sequence was compared with the sequences of the registered isolates available in the GenBank library (NCBI), and the homology between sequences was examined using BLAST software.
Culture characteristics of B. hominis
In addition to work, fresh samples were used for the preparation of positive control samples and they were cultured in coagulant-serum-media based on Markell Medical Parasitology Book (John et al. 2006). Streptomycin (600 µg/ml) was used for preventing bacterial contamination and the sample was cultured in the liquid phase of the media. After 48–72 h keeping the samples at 35 °C, the smear was prepared from them and the samples were examined.
Statistical analysis
SPSS statistical software of version 17 was used for the data analysis and Chi square test for significance differences. The P value less than 0.05 were considered significant.
Results
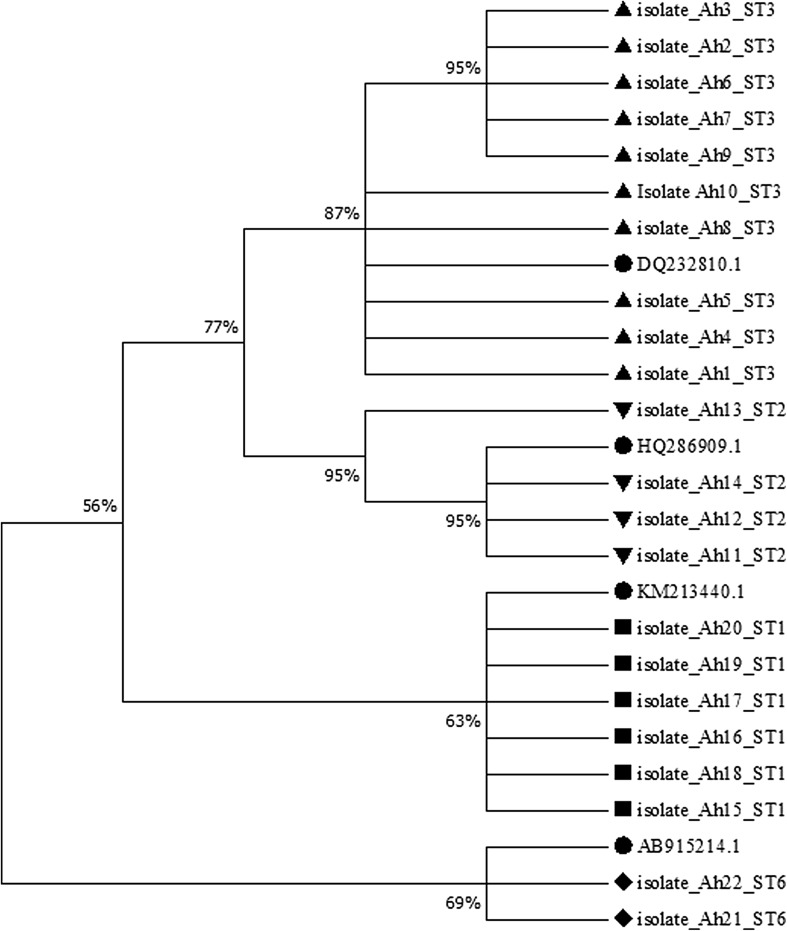
Table 2 indicates the comparison of positive cases of B. hominis using Formol-Ether and molecular methods. Based on Table 2, of 268 stool samples, 33 samples were detected positive for B. hominis using Formol-Ether technique (12.3%). Figure 1 shows the result of the electrophoresis of B. hominis samples with a single pair of primer of SSU-rDNA. Of 268 stool samples, 51 samples were positive using molecular method (19%). Also, Fig. 2 demonstrates the electrophoresis of the result of PCR of B. hominis isolations using STS primers as well as Table 3 shows genotyping of positive cases of B. hominis using PCR and sequencing methods. According to this table, the most common isolates were related to genotype III with 29 (56.8%) out of 51 cases, then, genotype I with 11 (21.6%) out of 51 cases, genotype II with 6 (11.8%) out of 51 and 3 (5.9%) combined cases of genotypes I and III as well as 2 (3.9%) cases with genotype VI was identified. The mean number of TCD4-positive cells in the group of HIV-positive patients with B. hominis was 232 cells per cubic meters. While, in patients without B. hominis was 258 cells per cubic meters. There was a significant difference between two groups of HIV-positive patients (infected with the parasite and/or without the parasite) in the term of the mean of TCD4-positive cells (P < 0.05. The data of nucleotide sequences reported in the paper are available in GenBank at accession numbers KP244334, KP244338-44, and KP244346-59. In addition, Fig. 3 shows phylogenetic analysis of ITS sequences of B. hominis isolates recovered from HIV-positive in Khuzestan province, southwest of Iran.
Table 2.
Comparison of positive cases of B. hominis using Formol-Ether and molecular methods
| Used method | Number | Positive samples (%) |
|---|---|---|
| Formol-Ether | 268 | 33 (12.3) |
| Molecular | 268 | 51 (19) |
Fig. 1.
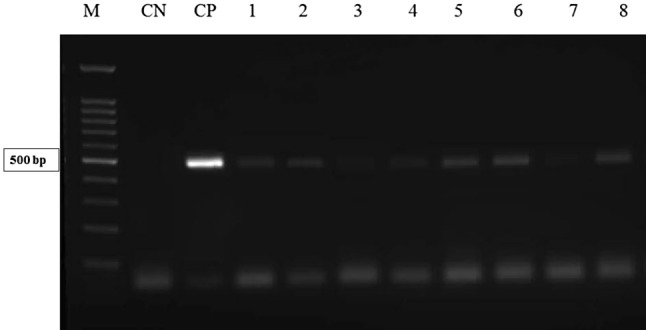
The result of the electrophoresis of Blastocystis hominis samples with a single pair of primer of SSU-rDNA. M, molecular marker 100 bp; CP, positive control; CN, negative control; 1–8, positive samples
Fig. 2.

The electrophoresis of the result of PCR of B. hominis isolations using STS primers, no. 1: DNA marker size 100 bp, no. 2: negative control, numbers 3, 4 and 5: subtype 3-band 526 bp, numbers 6 and 7: subtype 2-band 704 bp, numbers 8 and 12: combination of subtypes 1 and 3, no. 9: band 338 bp subtype 6, numbers 10 and 12: subtype 1 band 351 bp
Table 3.
Genotyping of positive cases of B. hominis using PCR and sequencing methods
| Subtype | Number | Percentage (%) |
|---|---|---|
| Subtype I | 11 | 21.6 |
| Subtype II | 6 | 11.8 |
| Subtype III | 29 | 56.8 |
| Subtype VI | 2 | 3.9 |
| Subtype I and subtype III | 3 | 5.9 |
| Total | 51 | 100 |
Fig. 3.
Phylogenetic analysis of ITS sequences of B. hominis isolates recovered from HIV-positive in Khuzestan province, southwest of Iran. The phylogenetic tree was drawn using the MEGA (version 7) software and Neighbor-Joining method
Discussion
Opportunistic infections are one of the major factors in mortality of immunodeficient patients and the clinical symptoms of the infection with B. hominis were more severe in patients with immune deficiency (Eroglu et al. 2009). Considering the prevalence of the parasite and the unknown strains responsible for the infections in patients with HIV-positive; therefore, the aim of the study was to evaluate genomic analysis of B. hominis isolates obtained from patients with HIV-positive using locus SSU-rDNA.
In the study, the prevalence of B. hominis was determined 51 cases (19%) among 268 samples of patients with HIV-positive by molecular method. The results indicated a relatively high prevalence of B. hominis in HIV-positive patients and the prevalence is higher than similar studies in Iran. For example, in Tehran (center of Iran) in 2004, a study was conducted on 206 stool samples of patients with HIV-positive that the prevalence rate of B.hominis has been reported 4.4% (Zali et al. 2004). Also in Mashhad (east of Iran), the prevalence of the organism has been reported 6.22% out of 31 samples of patients with HIV-positive (Fariba et al. 2010). In the present study, two different methods were used for the detection and isolation of B. hominis; in the first stage, the Formol-Ether technique and the microscopic observation were applied and the prevalence of the parasites was reported 12.3% in this method. In the second method, molecular methods were applied that the prevalence rate was determined 19%. These results showed that molecular methods have more sensitivity compared to other methods. Dispersion of the results can be due to using the various methods in the diagnosis. The prevalence rate obtained in above studies was via using the direct and the Formol-Ether methods and cultivation in suitable media. However, it should be noted that the dispersion of the results can also be related to several factors including health status of the area, studied target groups, sampling methods, survey methods and techniques applied for identification.
One obvious point in HIV-positive patients was decreasing TCD4+ cells in these patients. In this study, the mean number of TCD4-positive cells in the group of HIV-positive patients with B. hominis was 232 cells per cubic meters. While, in patients without B. hominis was 258 cells per cubic meters. There was a significant difference between two groups of HIV-positive patients (infected with the parasite and/or without the parasite) in the term of the mean of TCD4-positive cells (P < 0.05). This can express that the number reduction of TCD4-positive cells has an effective role in the increased risk of the parasitic infection in HIV-positive patients. That’s why there should be more efforts to prevent, control and monitor on these patients.
Our finding indicated that the most common isolates were related to genotype III with 29 (56.8%) out of 51 cases, then, genotype I with 11 (21.6%) out of 51 cases, genotype II with 6 (11.8%) out of 51 and 3 (5.9%) combined cases of genotypes I and III as well as 2 (3.9%) cases with genotype VI was identified. Consistent with these results, in a study by Yoshikawa et al. in 2000, the detection and the determination of Blastocystis subtypes in five countries of Bangladesh, Germany, Japan, Thailand and Pakistan were conducted that the most common subtype in all countries except Thailand was ST3 and then ST1 (Yoshikawa et al. 2000). In addition, two studies that were conducted in Iran by Motazedian using PCR–RFLP method and Mousavi using STS primers method, ST1 and ST3 had the highest prevalence among the determined subtypes, respectively (Moosavi et al. 2012; Motazedian et al. 2008). Also, the studies on patients with HIV-positive and cancer in Turkey showed ST3 as the dominant subtype (Tan et al. 2009). In most studies, other subtypes of the parasites are less frequently observed. The above results propose this hypothesis that ST3 is a subtype of human origin. Also our results showed that coagulant-serum-media was not ideal for cultivation of Blastocystis, because despite increasing the size of the parasite, not much parasite was observed in each field. It is recommended to use Modified RPMI and IMDM media.
This result was similar with other studies on patients with immune deficiency. The results of this study indicated the high prevalence rate of the parasite especially in susceptible individuals particularly in HIV-positive patients. Additionally, patients with HIV-positive and other patients with weak immune system are at the risk of this parasitic infection. The highest risk of this infection is at individuals with impaired immune systems that these organisms can be life-threatening in people with immune system dysfunction. It is essential that the high-risk people receive the information about the risk of direct and indirect contact with the infected individuals and animals.
Conclusion
The results indicated a relatively high prevalence of B. hominis in HIV-positive patients as well as our findings may represent that the number reduction of TCD4-positive cells has an effective role in the increased risk of the parasitic infection in HIV-positive patients. Coagulant-serum-media was not ideal for cultivation of Blastocystis in this study, because despite increasing the size of the parasites, not many parasites were observed in each field.
Acknowledgments
This article is the result of a research project with number OG-93112 approved by the Ahvaz Jundishapur University of Medical Sciences, Iran. The authors thank the esteemed group of Parasitology and Research Center of Tropical Infectious Diseases.
Author’s contributions
All authors contributed to the study design. MT and SK were leader of the research. ARP carried out experimental tests and prepared the Manuscript. All authors read and approved the final version of the manuscript.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no competing interests
Ethical standard
All experimental procedures were approved by the Ethics Committee of Ahvaz Jundishapur University of Medical Sciences, Iran.
Informed consent
Informed consent was obtained from subjects involved in this study.
References
- Badparva E, Sadraee J, Kheirandish F. Genetic diversity of blastocystis isolated from cattle in Khorramabad, Iran. Jundishapur J Microbiol. 2015;8(3):e14810. doi: 10.5812/jjm.14810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cirioni O, Giacometti A, Drenaggi D, Ancarani F, Scalise G. Prevalence and clinical relevance of Blastocystis hominis in diverse patient cohorts. Eur J Epidemiol. 1999;15(4):387–391. doi: 10.1023/A:1007551218671. [DOI] [PubMed] [Google Scholar]
- Domínguez-Márquez MV, Guna R, Muñoz C, Gómez-Muñoz MT, Borrás R. High prevalence of subtype 4 among isolates of Blastocystis hominis from symptomatic patients of a health district of Valencia (Spain) Parasitol Res. 2009;105(4):949–955. doi: 10.1007/s00436-009-1485-y. [DOI] [PubMed] [Google Scholar]
- Eroglu F, Genc A, Elgun G, Koltas IS. Identification of Blastocystis hominis isolates from asymptomatic and symptomatic patients by PCR. Parasitol Res. 2009;105(6):1589–1592. doi: 10.1007/s00436-009-1595-6. [DOI] [PubMed] [Google Scholar]
- Fariba B, et al. A study of the prevalence of intestinal parasitic infection in HIV positive individuals in Mashhad, Northeast Iran. Jundishapur J Microbiol. 2010;2010(2, Spring):61–65. [Google Scholar]
- Graczyk TK, Shiff CK, Tamang L, Munsaka F, Beitin AM, Moss WJ. The association of Blastocystis hominis and Endolimax nana with diarrheal stools in Zambian school-age children. Parasitol Res. 2005;98(1):38–43. doi: 10.1007/s00436-005-0003-0. [DOI] [PubMed] [Google Scholar]
- Guignard S, Arienti H, Freyre L, Lujan H, Rubinstein H. Prevalence of enteroparasites in a residence for children in the Cordoba Province, Argentina. Eur J Epidemiol. 2000;16(3):287–293. doi: 10.1023/A:1007651714790. [DOI] [PubMed] [Google Scholar]
- Hellard ME, Sinclair MI, Hogg GG, Fairley CK. Prevalence of enteric pathogens among community based asymptomatic individuals. J Gastroenterol Hepatol. 2000;15(3):290–293. doi: 10.1046/j.1440-1746.2000.02089.x. [DOI] [PubMed] [Google Scholar]
- John DT, Petri WA, Markell EK, Voge M. Markell and Voge’s medical parasitology. Amsterdam: Elsevier Health Sciences; 2006. [Google Scholar]
- Jones MS, Whipps CM, Ganac RD, Hudson NR, Boroom K. Association of Blastocystis subtype 3 and 1 with patients from an Oregon community presenting with chronic gastrointestinal illness. Parasitol Res. 2009;104(2):341–345. doi: 10.1007/s00436-008-1198-7. [DOI] [PubMed] [Google Scholar]
- Moosavi A, et al. Genetic variability of Blastocystis sp. isolated from symptomatic and asymptomatic individuals in Iran. Parasitol Res. 2012;111(6):2311–2315. doi: 10.1007/s00436-012-3085-5. [DOI] [PubMed] [Google Scholar]
- Motazedian H, Ghasemi H, Sadjjadi S. Genomic diversity of Blastocystis hominis from patients in southern Iran. Ann Trop Med Parasitol. 2008;102(1):85–88. doi: 10.1179/136485908X252197. [DOI] [PubMed] [Google Scholar]
- Parkar U, et al. Molecular characterization of Blastocystis isolates from zoo animals and their animal-keepers. Vet Parasitol. 2010;169(1):8–17. doi: 10.1016/j.vetpar.2009.12.032. [DOI] [PubMed] [Google Scholar]
- Poirier P, Wawrzyniak I, Albert A, El Alaoui H, Delbac F, Livrelli V. Development and evaluation of a real-time PCR assay for detection and quantification of Blastocystis parasites in human stool samples: prospective study of patients with hematological malignancies. J Clin Microbiol. 2011;49(3):975–983. doi: 10.1128/JCM.01392-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safi M, Tavalla M, Mardani M, Afrisham R. Prevalence of intestinal parasitic infections among applicants for health cards attending Ahvaz East Health Center during 2012–2013. Asian Pac J Trop Dis. 2016;6(2):151–154. doi: 10.1016/S2222-1808(15)61002-7. [DOI] [Google Scholar]
- Stensvold CR, et al. Terminology for Blastocystis subtypes—a consensus. Trends Parasitol. 2007;23(3):93–96. doi: 10.1016/j.pt.2007.01.004. [DOI] [PubMed] [Google Scholar]
- Stensvold CR, et al. Subtype distribution of Blastocystis isolates from synanthropic and zoo animals and identification of a new subtype. Int J Parasitol. 2009;39(4):473–479. doi: 10.1016/j.ijpara.2008.07.006. [DOI] [PubMed] [Google Scholar]
- Stensvold CR, Lewis H, Hammerum AM, Porsbo LJ, Nielsen SS, Olsen KE, Arendrup MC, Nielsen HV, Mølbak K. Blastocystis: unravelling potential risk factors and clinical significance of a common but neglected parasite. Epidemiol Infect. 2009;137:1655–1663. doi: 10.1017/S0950268809002672. [DOI] [PubMed] [Google Scholar]
- Tan T, Suresh K, Smith H. Phenotypic and genotypic characterisation of Blastocystis hominis isolates implicates subtype 3 as a subtype with pathogenic potential. Parasitol Res. 2008;104(1):85–93. doi: 10.1007/s00436-008-1163-5. [DOI] [PubMed] [Google Scholar]
- Tan T, Ong S, Suresh K. Genetic variability of Blastocystis sp. isolates obtained from cancer and HIV/AIDS patients. Parasitol Res. 2009;105(5):1283–1286. doi: 10.1007/s00436-009-1551-5. [DOI] [PubMed] [Google Scholar]
- Yoshikawa H, et al. Genomic analysis of Blastocystis hominis Strains isolated from two long-term health care facilities. J Clin Microbiol. 2000;38(4):1324–1330. doi: 10.1128/jcm.38.4.1324-1330.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zali MR, Mehr AJ, Rezaian M, Meamar AR, Vaziri S, Mohraz M. Prevalence of intestinal parasitic pathogens among HIV-positive individuals in Iran. Jpn J Infect Dis. 2004;57(6):268–270. [PubMed] [Google Scholar]
- Zhang X, Qiao J, Wu X, Da R, Zhao L, Wei Z. In vitro culture of Blastocystis hominis in three liquid media and its usefulness in the diagnosis of blastocystosis. Int J Infect Dis. 2012;16(1):e23–e28. doi: 10.1016/j.ijid.2011.09.012. [DOI] [PubMed] [Google Scholar]