Abstract
Two adult pigeons were presented to the Teaching Veterinary Hospital (TVH), GADVASU, Ludhiana, Punjab with the history of weakness, anorexia, ruffled feathers and intermittent diarrhoea. Coproscopic examination revealed the presence of coccidian oocysts alongside eggs of Capillaria spp. Based on the morphological characters the eggs were designated as C. obsignata. Sporulation studies on the coccidian oocysts revealed mixed infection of Eimeria columbae and E. columbarum.
Keywords: Capillaria, Coccidia, Eimeria, Pigeons, Punjab
Introduction
Since long rearing of pigeons is a common practice in various regions of world, including India and are kept for meat, gaming and showy purposes. The pigeons suffer from many infectious diseases and some of those are considered to be pathogenic to humans (Zwart 1986). Amongst the various endoparasites reported in pigeons, the coccidia are considered to be the most common. About nine species belonging to genus Eimeria and one to genus Isospora have been described worldwide in pigeons, however, three species Eimeria columbae, E. columbarum and E. labbeana are of more significance, owing to their varying degrees of virulence (Zebisch 2005; Balicka-Ramisz and Pilarczyk 2014). These species have been reported from domestic (Columba livia domestica) as well as rock pigeons (Columba livia livia) from various regions of the world (Krautwald-Junghanns et al. 2009). Clinically the coccidian infections are often mixed, mostly sporting and carrier pigeons at greater risk, owing to a large number of flights especially in the racing season (Balicka-Ramisz and Pilarczyk 2014).
Furthermore, the coccidian infections increase the susceptibility of pigeons towards various bacterial, viral and other protozoal diseases, like salmonellosis, paramyxoviruses, circoviruses, trichomoniosis (Romaniuk 2000). In general, coccidian infections are mostly sub-clinical in nature and usually run without any clear-cut clinical manifestations. However, in young pigeons (1–4 months), disease is acute with mortality ranging from 5 to 70% and most deaths occurring in 3rd and 4th month (Mennemeier 1985). The affected pigeons may look healthy, but are less vigorous; feathers are brittle, there is anorexia, dehydration, cachexia, loss of body weight and foetid–watery diarrhoea (McDougald 2003).
Apart from coccidia, a nematode, Capillaria, is also frequently encountered in pigeons. Capillaria obsignata and C. caudinflata are two species found in the small intestine. The worms burrow deep into the intestinal mucosa thereby causing epithelial necrosis, inflammation and haemorrhages (Soulsby 1982). Clinically, the disease is of chronic gastro-enteritic type and is presented as emaciation, weight loss, diarrhoea, huddling and death if not treated (Pees 2008; Scullion and Scullion 2010).
There are sporadic published reports of endo- and ecto-parasitic infections in pigeons from various parts of India (Parsani et al. 2002; Senthivel and Pillai 2005; Shinde et al. 2008). However, there appears to be no published report on this aspect from Punjab state. The present communication describes two cases of mixed coccidia and Capillaria infection in pigeons presented to the Teaching Veterinary Hospital (TVH), GADVASU, Ludhiana, Punjab.
Materials and methods
Two adult pigeons were presented to the TVH, GADVASU, Ludhiana, Punjab. The clinical signs were recorded and faecal samples provided by the owner from the respective cages were examined microscopically for the presence of eggs/occysts, if any, by employing routinely used conventional qualitative faecal sample examination (sedimentation and floatation) methods (Soulsby 1982). The eggs/oocysts were identified as per the characters outlined by Soulsby (1982) and the samples, positive for coccidian oocysts, were further subjected to standard sporulation protocol in order to determine the genus and/or species. In brief, the faecal droppings positive for coccidian oocysts were mixed with 2.5% potassium dichromate solution and the suspension was transferred into a petri dish which was further kept at room temperature for sporulation. The species of coccidia was determined on the basis of morphological characteristics of oocysts and their sporulation time (Soulsby 1982). Micrometry of the eggs/oocysts was done by ocular micrometry on calibrated bright field microscope as well as with software DPZ-BSW (Olympus, Japan).
Results
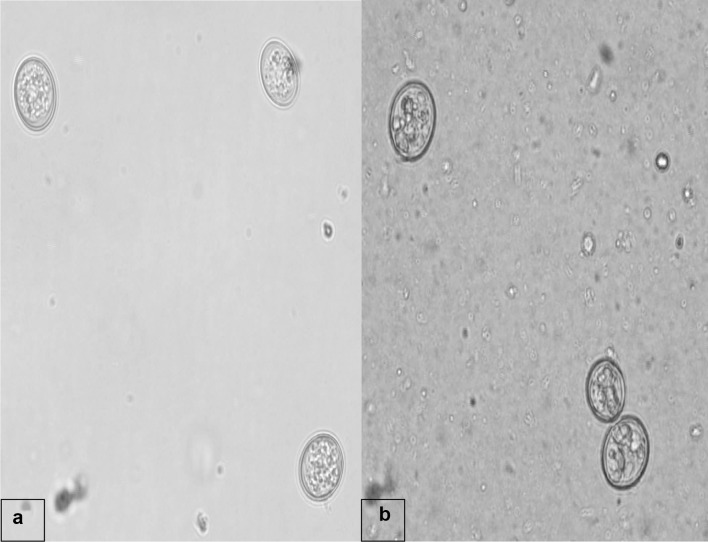
The clinical examination of the affected pigeons revealed emaciation, ruffled feathers and diarrhoeic faecal droppings. Microscopic examination revealed the presence of eggs belonging to genus Capillaria as well as coccidian oocysts (Fig. 1). The eggs were identified as Capillaria as they were characteristically medium sized, barrel shaped and with bipolar plugs. The egg shell had a reticulate pattern and the size was 50.25–53.90 µm × 24.50–26.95 µm based on which the eggs were designated as those of C. obsignata (Fig. 2). The sporulation studies revealed presence of oocysts of genus Eimeria, characterized by the presence of four sporocysts each containing two sporozoites which comprised of E. columbae and E. columbarum (Fig. 3).
Fig. 1.
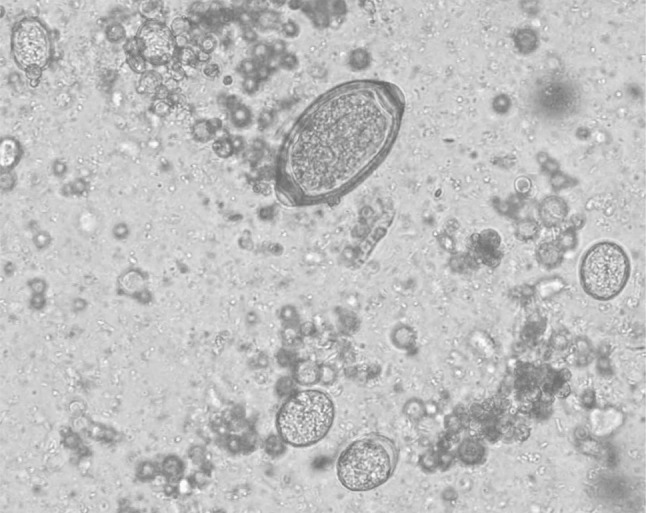
Mixed infection of Capillaria eggs and coccidian oocysts in faeces of pigeons (× 40)
Fig. 2.
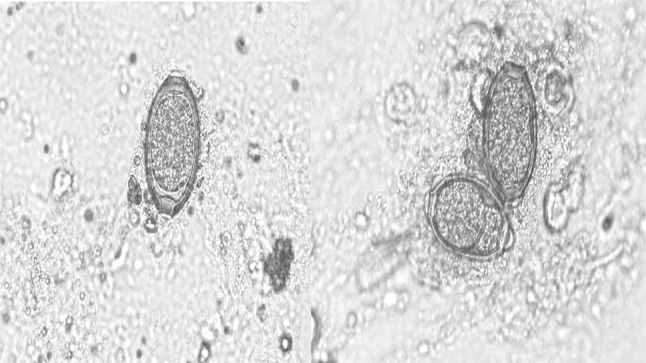
Eggs of Capillaria obsignata in faeces of pigeons (× 40)
Fig. 3.
Unsporulated (a) and sporulated (b) oocysts of coccidia in faeces of pigeons (× 40)
The oocysts of E. columbae were 16.4 × 14.3 µm in size, with a thin wall and those of E. columbarum were spherical in shape with no micropyle and 20.0 × 18.7 µm in size. The mature oocysts also contained an oocyst residuum composed of a number of inclusion bodies and the sporulation time recorded in the present investigation ranged from 2 to 4 days at room temperature. The wall of the oocysts was bi-layered, smooth and colorless.
Discussion
Many studies have been conducted globally regarding the endoparasites infections in pigeons, including India. Coccidian infections, species composition as well as mixed infections of intestinal nematodes and coccidia have also been on the records. Though it has been observed that young and growing pigeons are more commonly infected with Capillaria infection as compared to the adults (Soulsby 1982; Biu and Umoru 2010), yet, the infection can also occur in older pigeons which usually act as carriers, and a source of infection for other avian hosts (Parsani et al. 2006). Regarding Indian scenario, reports on prevalence of C. obsignata and coccidian parasites viz. E. columbae, E. labbanea and E. columbarum are available (Parsani et al. 2002; Senthivel and Pillai 2005; Shinde et al. 2008). The birds were successfully treated with fenbendazole (8 mg/kg for 6 days) for Capillaria infection while for coccidian oocysts amprolium (0.02% in water for 5 days) was prescribed. Furthermore, the owner was advised to practice the principles of general hygiene for rearing the pigeons along with provision of clean feed and water to reduce the infections. Therefore, it is concluded that while adopting deworming strategies the faecal samples must be examined to rule out the possibility of mixed infections as were observed in the present report.
Acknowledgements
The authors are thankful to the Director of Research, GADVASU, Ludhiana for providing facilities to carry out the research work. The authors are also thankful to the Head, Teaching Veterinary Clinical Services Complex, GADVASU, Ludhiana for his support.
Author’s contribution
NKS: pathological examination of faecal samples; collection of relevant literature and preparation of manuscript. HS: examination of faecal samples by conventional parasitological techniques, identification of parasitic stages encountered in faecal samples; collection of relevant literature, and manuscript preparation. SK: performed the sporulation of coccidian oocysts and micrometry of parasitic stages encountered during the study. AK and RSS: clinical examination; treatment and management of the cases.
Compliance with ethical standards
Conflict of interest
The authors declare they have no conflict of interest.
References
- Balicka-Ramisz A, Pilarczyk B. Occurrence of coccidia infection in pigeons in amateur husbandry: diagnosis and prevention. Ann Parasitol. 2014;60:93–97. [PubMed] [Google Scholar]
- Biu AA, Umoru A. Prevalence of pigeon capillariasis in Maiduguri, Nigeria. Anim Res Int. 2010;7:1226–1228. [Google Scholar]
- Krautwald-Junghanns ME, Zebisch R, Schmidt V. Relevance and treatment of coccidiosis in domestic pigeons (Columba livia forma domestica) with particular emphasis on toltrazuril. J Avian Med Surg. 2009;23:1–5. doi: 10.1647/2007-049R.1. [DOI] [PubMed] [Google Scholar]
- McDougald LR. Diseases of poultry. 11. Ames: Iowa State Press; 2003. Coccidiosis; pp. 974–991. [Google Scholar]
- Mennemeier G. Experimentelle Untersuchungen uer die Pathogenitat der Kokzidien der Taube [Dissertation] Hannover. Germany: Tierarztliche Hochschule Hannover; 1985. [Google Scholar]
- Parsani HR, Momin RR, Singh V. Incidence of parasitic infections in pigeons in Gujarat. J Vet Parasitol. 2002;16:43–46. [Google Scholar]
- Parsani HR, Momin RR, Shahu RK. Chemotherapy of nematodiasis in zoo pigeons. Zoos Print J. 2006;22:40–45. [Google Scholar]
- Pees M (2008) Pigeons: gastrointestinal tract disease. In: Manual of raptors, pigeons and passerine birds. BSAVA: Gloucester, pp 328–333
- Romaniuk K. Robaczyce gołębi. Magazyn Weterynaryjny. 2000;9:48. [Google Scholar]
- Scullion FT, Scullion MG (2010) Racing pigeons. In: BSAVA manual of exotic pets. 5th Edn. BSAVA: Gloucester, pp 188–199
- Senthivel K, Pillai KM. Coccidiosis of domestic pigeons (Columba livia domestica) J Vet Anim Sci. 2005;26:73–74. [Google Scholar]
- Shinde NG, Gatne ML, Singh A. Prevalence of parasites in pigeons (Columba livia domestica) of Mumbai. J Vet Parasitol. 2008;22:235–238. [Google Scholar]
- Soulsby EJL. Helminths, arthropods and protozoa of domesticated animals. 7. London: Bailliere Tindall; 1982. [Google Scholar]
- Zebisch R (2005) Untersuchungen zur Wirksamkeit und Vertraglichkeit von Baycox 5% Suspension bei der Brieftaube (Columba livia forma domestica) [Dissertation]. Leipzig, Germany: University Leipzig
- Zwart P. Pigeons and doves. In: Fowler ME, editor. Zoo & wild animal medicine. 2. Philadelphia: WB Saunders; 1986. pp. 440–445. [Google Scholar]