Abstract
The broad objective of this paper is to present an overview and synthesis of selected studies on reproduction and aging in two model tephritid fruit fly species including the Mediterranean fruit fly, Ceratitis capitata, and the Mexican fruit fly, Anastrepha ludens. We summarize the research findings from empirical studies and modeling investigations involving reproduction in the two tephritid species. At the end we identify and discuss four general principles regarding reproductive aging in tephritids including reciprocity of reproductive and aging costs, qualitative tradeoffs, plasticity of lifespan and reproduction, and life history constraints and determinacy.
Keywords: life tables, lifespan, fecundity, cost of reproduction, senescence, calorie restriction
Introduction
Aging and reproduction in insects are inextricably linked and mutually affecting, the former reducing egg-laying rate at older ages independent of previous egg laying, and the latter increasing the risk of death at each age with increasing reproductive effort. Although the tradeoffs between early reproduction and the risk of death are well known, the reciprocal relationship—i.e., the influence of aging on reproductive output—is less well understood. While age-patterns of reproduction play a role in the evolution of senescence, the reproductive system itself can also decrease in performance with age—reproduction may become less intense, of lower quality or cease (postreproductive lifespan/menopause) as an organism ages. Even though insects are important model systems in aging research, reproductive aging has rarely been addressed specifically in this group or is attributed to the depletion of resources.1 Thus, the main objective of this paper is to present an overview of the relationship between reproduction and aging based on the results of previous research on tephritid fruit flies by Carey et al.10
Reproductive aging in tephritids
Both the Mediterranean fruit fly, Ceratitis capitata, and the Mexican fruit fly, Anastrepha ludens, belong to the dipteran family Tephritidae—a group of about 4000 species referred to as the “true” fruit flies that is distributed throughout most of the world.2 Members of this group, most of which are roughly the size of a housefly, lay eggs in intact fruit using their sharp ovipositor rather than on decaying fruit as does their tiny, gnat-sized distant relative Drosophila melanogaster that is commonly known as the vinegar fly. Although less useful than D. melanogaster for research on genetics and development, the medfly and Mexfly are ideal models for demographic research for at least three reasons: (1) because members of these two species are relatively large and robust, they are much less prone to handling injury and can also be easily observed without the aid of a hand lens and microscope; (2) the willingness of females to lay eggs only when presented with an oviposition host enables researchers to manipulate their egg laying independent of dietary manipulations. In contrast, D. melanogaster females tend to lay their eggs on their food; and (3) because both of these tephritid species are reared at industrial scales, large numbers of individuals are always available for large-scale demographic studies.
Baseline patterns of reproductive aging
Model tephritids: Medfly and mexfly
The broad goal of the individual-level studies of reproduction in both the medfly3–5 and the Mexican fruit fly6 was to gather baseline information on their maturation rates, daily egg-laying patterns, age-specific survival, and lifespan. Survival and reproduction were monitored daily in a total of 1000 individual females of each species given access to ad libitum food, the results of which are summarized in Figure 1. Several aspects of this figure merit comment. First, the broad patterns of survival are similar in both cohorts including: (i) gentle decreases in survival for the first 10% of deaths; (ii) steeper declines in survival for the next 80% of deaths; and (iii) long tails for the remaining 10% of deaths. These patterns are manifestations of an underlying mortality schedule in female flies that accelerates at young and middle ages and decelerates at older ages.7–9 Second, both species of tephritids are relatively long lived with life expectancies at eclosion of the medfly and Mexfly of approximately 36 and 49 days, respectively. Thus, the average female Mexfly lives about a third longer than the average medfly. Third, both tephritid species are capable of laying 30–50 eggs per day during peak reproductive ages and 10–20 eggs per day at many of the older ages. Thus, the observed lifetime egg production in these species is extraordinarily high with fecundity in the medfly ranging from 640 to 1150 eggs/female10 and in the Mexfly 1400 eggs/female,6 a fecundity higher than any lifetime rate observed in earlier studies on the medfly.3,11 Fourth, two broad patterns were evident in egg laying of both species: (i) a weak correlation between longevity and ages of first reproduction and (ii) the retention of egg-laying capabilities at advanced ages.
Figure 1.
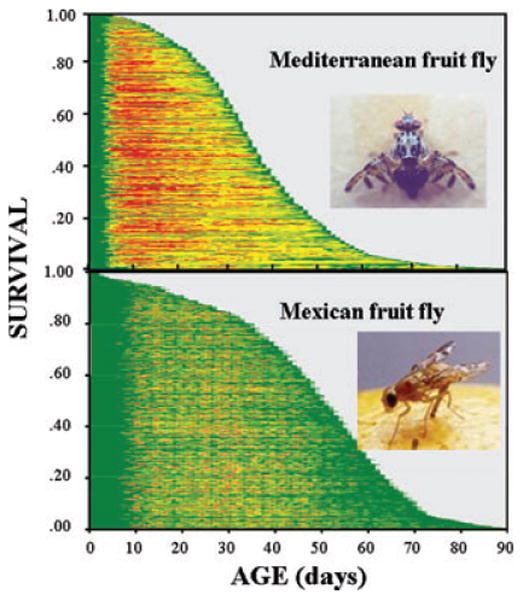
Event history charts of reproduction for female Mediterranean (top) and Mexican (bottom) fruit flies. Green = zero eggs/day; yellow = 1–40 and 1–50 eggs/day for medfly and Mexflies, respectively; red = >40 and >50 eggs/day for medfly and Mexflies, respectively.6,23
Main conclusions
The results of these studies of reproduction in the two tephritid fruit fly species: (i) provided important baseline information (i.e., maturation; egg-laying patterns; and postreproduction) for both species; and (ii) revealed that there was no correlation between either the intensity of early and late egg laying or the intensity of early egg laying and longevity.
Medfly biotypes
Because tephritid fruit flies such as the medfly have colonized a number of different temperate and tropical areas throughout the world, between-biotype comparisons of survival and reproduction shed important light on how different environments favor the evolution of different life history traits in these species. Thus, the purpose of the study by Greek entomologist Alexandros Diamantidis and his colleagues12 was to compare the life history traits of medfly populations obtained from different global regions. In a common garden environment, medfly populations were studied from six global regions including Africa (Kenya), the Pacific (Hawaii), Central America (Guatemala), South America (Brazil), extra-Mediterranean (Portugal), and Mediterranean (Greece). Substantial between-population differences were observed with female life expectancies ranging from 4 weeks in the shortest lived population (Guatemala) to 8 weeks in the longest lived population (Greece) (Table 1). Although Hawaiian and Kenyan females were relatively short-lived, the lifespans of males from these two regions were similar as were those for males of the long-lived populations (Brazil, Portugal, and Greece). Therefore, female cohorts could be classified into either short-lived (Guatemala, Hawaii, and Kenya) or long-lived (Brazil, Portugal, and Greece) biotypes. Although average fecundity rates were similar among populations, substantial differences existed in the age-specific reproduction schedule (Table 1). Short-lived populations (e.g., Guatemala) compressed the period of high egg-laying days into a limited “reproductive window” between days 10 and 40. In contrast, long-lived populations (e.g., Greece) spread out high egg-laying activity during their entire oviposition course (i.e., 4+ months). Furthermore, maturation rates as determined by the age at which the first egg of an individual is laid, were highly variable across populations.
Table 1. Longevity (both sexes) and reproduction (females only) in medfly populations originating from six different geographic areas12.
| Expectation of life (days) | Reproduction (eggs/female) | |||
|---|---|---|---|---|
|
|
|
|||
| Males | Females | Gross | Net | |
| Guatemala | 68.0 | 48.1 | 683.5 | 525.2 |
| Hawaii | 106.5 | 52.1 | 727.8 | 569.5 |
| Kenya | 115.9 | 58.3 | 701.1 | 655.0 |
| Brazil | 122.3 | 75.7 | 746.5 | 545.1 |
| Portugal | 107.1 | 75.6 | 700.5 | 549.1 |
| Greece | 112.1 | 72.3 | 1117.2 | 631.1 |
Main conclusions
The most important findings with respect to reproduction and aging included: (i) differences among populations in longevity were substantial (e.g., 48 versus 76 days for females); (ii) the gender gap favored males in all population by a wide margin (e.g., nearly 50 days in Kenyan populations); (iii) differences in net reproduction were substantially less relative to differences in longevity; and (iv) all populations experienced clear postreproductive periods in females.
Host effects
The ecology and demography of tephritid fruit flies can not be understood without understanding their relationship with their host fruit, which enters into their life histories in two respects: (i) as their sole source of larval nutrition; and (ii) as the site in which they lay their eggs as adult females. We summarize the results of two studies in this section including one on larval host effects on adult reproduction and survival in the medfly13 and the other on the effects of host deprivation on the life history traits of medfly females.14
Larval host
Inasmuch as the host upon which an adult fly develops as a larvae can have a profound effect on its survival and reproduction, the purpose of a study conducted in Hawaii was to compare the life history traits of medflies reared on a number of different larval hosts.13 A total of 30 different host species (e.g., plum, peach, mango, and papaya) were seeded with large numbers of medfly eggs, 24 of which produced viable adults in numbers (i.e., 15–60 pairs) that could be used for life table and egg-laying assays. The emerging adults from each host were placed in group cages and monitored for daily egg laying and survival. Large differences were observed in both sex-specific life expectancies and in female age-specific and lifetime reproduction across the 24 host species. Life expectancy was 22 days or greater for cohorts reared from 70% of the hosts. However, the average female fly reared from plum lived nearly twice as long as the average female reared from banana or mammee apple, two hosts that produced the shortest-lived flies. The highest gross and net reproductive rates from plum-reared females were twice the lowest rates that occurred in banana-reared flies as shown in Figure 2. These results reveal that the differences in lifetime reproduction in medfly females reared across these two different hosts were due to a combination of difference in the age-specific levels as well as to the differences in reproductive duration (i.e., lifespans).
Figure 2.
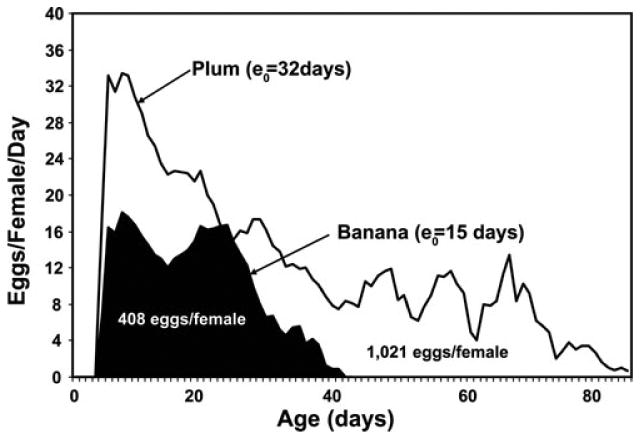
Age-specific reproduction in the medfly females reared from two different larval hosts.13 Inset contains lifetime reproduction for medflies reared from each host.
Main conclusion
The hosts uponwhich adult fruit flies develop as larvae can have a profound effect on both longevity (twofold) and lifetime reproduction (2.5-fold). Research is needed on the effects of larval nutrition on adult demographic traits since it would shed important light on the effects of early life conditions on mortality at advanced ages.
Adult host deprivation
Unlike D. melanogaster females, which feed on the same rotting fruit as they lay their eggs, adult tephritids lay their eggs in intact host fruit by boring a hole through the host skin using their ovipositor. Therefore the purpose of the study14 was to quantify the effect of different levels and patterns of host deprivation on survival and reproduction in medfly females that are maintained on a full diet. Female medflies in Hawaii were subjected to four different patterns of host deprivation at each of three levels for a 24-day period. Survival was recorded daily and egg production was recorded on the days in which hosts were present. Results revealed that host deprivation disrupts the reproductive cycle of females and reduces their overall reproductive effort. Reduced reproductive effort at young ages due to host deprivation increases survival (up to twofold) and daily reproduction (up to eightfold) at older ages. Therefore, host deprivation postpones senescence, but nevertheless some reproductive potential is lost. After host deprivation, flies laid large numbers of eggs, and the second day the number was lower than subsequent days (Fig. 3). Medfly egg production is not only affected by a female's chronological age but also by her previous reproductive effort. Any level or pattern of host deprivation increases survival by reducing reproductive effort.
Figure 3.
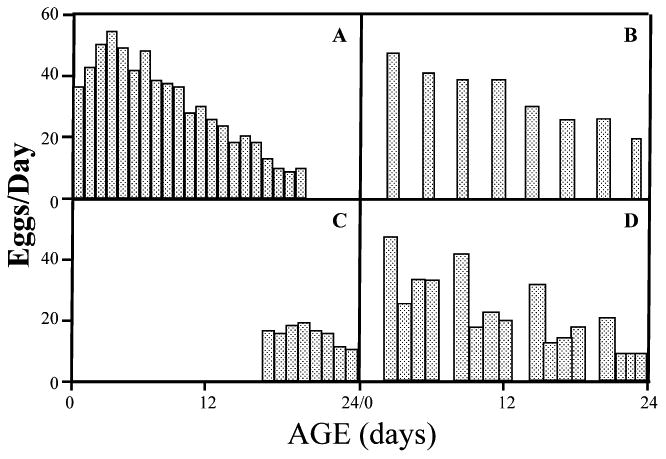
Reproduction in medflies given access to different patterns of oviposition hosts over a 24-day period; (A) control (host available all 24 days); (B) host available every third day; (C) host available days 16–24; (D) host available 4 of 5 days.14 Note the large differences in egg production levels between the control and treatment cohorts for days 21–24.
Main conclusion
The results of this study revealed that arresting reproduction in female medflies that are capable of reproducing extends their longevity. Although the same general longevity-extending, nonreproducing outcome is observed in medfly dietary restriction studies, the underlying mechanisms may be different.
Food restriction effects on reproductive aging
As noted in a seminal essay in ecology over two decades ago15 as well as by many gerontologists concerned with calorie restriction,16,17 food is the burning question in animal society—i.e., without adequate food individual development is arrested, reproductive rate slows, and mortality risk increases. It follows that any discussion of reproductive aging is incomplete without an understanding of and information on the effects of food on both survival and reproduction.
Dual modes of aging
The goal of the paper describing the phenomenon that was termed “dual modes of aging”18 was to test the hypothesis that medflies are capable of living to and reproducing at extreme ages if their reproduction is suspended at young ages due to lack of dietary protein. Experiments were conducted using the medfly at the rearing facility in Tapachula, Mexico. An initial pool of 2500 adults of each sex was maintained in single pair cages with sugar and water. At 30, 60, and 90 days, subgroups of 100 pairs were provided with a full diet (i.e., protein hydrolysate + sugar) ad libitum and their reproduction and survival were monitored until the last female died. The experiment revealed three important life expectancy patterns: (i) in all three treatments the remaining life expectancy of flies increased on the days when they were given a full diet after having been maintained on a sugar-only diet; (ii) life expectancy of the full-diet control flies at eclosion (age zero) was similar to remaining life expectancy of the treatment flies at the ages when they were first given a full diet; (iii) remaining life expectancy declines rapidly after medflies are switched to a full diet. One of the most remarkable discoveries was that 4- to 5-month-old medflies were capable of producing moderate numbers of eggs if they had been maintained on a sugar diet for the first 3 months (Fig. 4). A switch to full diet appears to have set the mortality clock back 90 days and to the slowly rising trajectory of the sugar-only cohort rather than the more-rapidly rising trajectories of the other cohorts switched to full diets.
Figure 4.
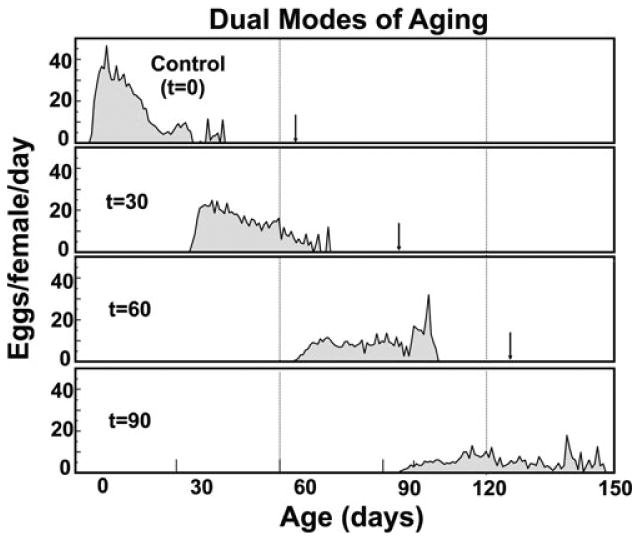
Event history graphs for medflies given access to different calorie restriction treatments.19 The time t (day) at which flies given access to the full (sugar + protein) diet indicated at left within each panel. Arrows indicate age of death of oldest flies within each treatment.
Main conclusions
The finding that female medflies are capable of switching mortality schedules suggests that the reproductive experience of females may include two distinct modes: (i) a waiting mode with low mortality, in which few or even no eggs are produced and (ii) a reproductive mode with prolific egg laying and low initial mortality followed by an acceleration in mortality and a reduction in egg laying. The results of this study also provided evidence for a reproductive cost (lost eggs) of aging; i.e., that some of the eggs that were not laid at young ages due to restricted access to protein source were not recovered at older ages when females were given access to a protein source.
Constant calorie restriction
The purpose of the study summarized here was to investigate medfly longevity and reproduction across a broad spectrum of diet restriction using a protocol similar to those applied to most rodent studies.19 Age-specific reproduction and age of death were monitored in 1200 individuals of both sexes that were individually maintained on 1-of-12 diets from ad libitum to 30% ad libitum. Diet was provided in a fixed volume of solution that was fully consumed each day, ensuring control of total nutrient consumption for each fly. Contrary to what would be expected under the “longevity extension through dietary restriction” paradigm,20 flies that were fed less experienced shorter life expectancies. Egg production continuously varied with diet, with average egg production declining more rapidly when diet was restricted to below 70%, and cohorts no longer containing individuals that laid more than 1800 eggs when diet was restricted by more than 50%. Even at the lowest food availability (30%) flies still laid some eggs. Although under food limitation reproduction is partly postponed, a fraction of a females' reproductive potential appears to be completely lost due to aging alone.
Main conclusions
The two main findings from this medfly calorie restriction study in which only calories but not dietary composition were manipulated included: (i) increased longevity was not observed in any of the food restricted cohorts and (ii) reproductive patterns revealed that when medfly females are given the opportunity to reproduce they do so at the expense of increased mortality even under the most harsh dietary conditions.
Nutritional gradients
The goal of this study on the Mexfly21 was to design an experiment in which both dietary composition and caloric content were altered in the adult food to address the question: Under what nutritional conditions do the longevity-enhancing effects resulting from food restriction either counteract, complement, or reinforce the mortality costs of reproduction? To answer this question a fine-grained dietary restriction study was designed involving 4800 individuals of A. ludens in which sex-specific survival and daily reproduction were measured in females in each of 20 different treatments (sugar:yeast ratios) plus four starvation controls. The contour graphs that were constructed from the response surface data reveal two nutritional thresholds: (i) a calorie restriction threshold (≈10%) below, which both female reproduction and survival decline precipitously and (ii) a sugar–yeast (compositional) threshold (≈5%) below which reproduction declines precipitously but where survival decreases gradually. The square contours in the reproductive surface showed that the yeast level sets a threshold in terms of the range of increasing reproduction due to increasing calories, and calories set a threshold in terms of the corresponding domain where yeast increase leads to reproduction increase. The intensity of egg laying is mainly regulated via yeast and not calories. Flies need to have access to high quality foods once they have a minimum of calories needed to sustain minimum longevity without which reproduction would be low.
Main conclusion
The finding that lifespan and reproductive maxima occur at much different nutritional coordinates underscored the well-known tradeoff between reproduction and survival. More importantly the magnitude of the nutritional differences between these maxima suggests that there is no dietary solution to maximize both of these life history parameters; that aging and reproduction are nutritionally inextricably coupled.
Models of reproductive aging
Exponential decline
The goal of the modeling study by statistician Hans Müller and his colleagues22 was to examine the relationship between reproduction and lifespan in the medfly based on age-specific reproductive data generated in the earlier studies.3,23 The trajectories of fecundity for individual flies were fitted to data from the peak to day 25 by nonlinear least squares. Egg-laying trajectories at the individual level followed simple exhaustion or decay dynamics and, therefore, the best predictor for subsequent mortality is the rate of decline in egg laying, i.e., the rate at which the egg supply is exhausted, rather than intensity of reproduction. This relationship is presented in Figure 5. Individual egg-laying trajectories rose sharply after egg laying began 5-17 days after emergence, reached a peak and then slowly declined. The rate of decline varied between individuals but one of the key findings was that this rate was approximately constant for each individual. The age trajectory of reproductive decline for each fly was accordingly modeled by the exponential function
Figure 5.
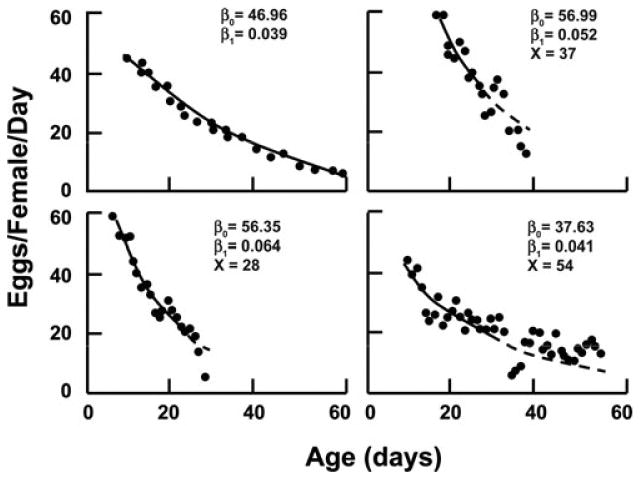
Trajectory of fecundity and mortality in the medfly.22 The trajectories of fecundity for individual flies are fitted to data from the peak to day 25 (solid line) by nonlinear least squares and predicted thereafter (dashed line). Each panel depicts the reproduction of individual females in which reproductive peak, the rate of decrease (i.e., slope) and age of death are denoted by β0, β1, and X, respectively. Note that the greater the slope (β1), the shorter the individual lifespan for each of the three example (i.e., upper right and two lower panels).
where f (x) is the fecundity (eggs/day) of the fly at age x days and θ is the age at peak egg laying. The two parameters β0 (mean 57.25 ± 16.70), the peak height of the trajectory and β1, the rate of decline (mean 0.090 ± 0.093), varied considerably from fly to fly. A modest but significant negative correlation between β0 and β1 indicated that fecundity tends to decline more slowly for flies with higher peak fecundity. The protracted decline in egg laying after the initial sharp rise was reasonably well predicted by the exponential model.
Main conclusions
The findings point to a fundamental link between reproductive dynamics and survival. This link is carried by the dynamics of reproduction and not by the absolute magnitude of reproduction as measured by the number of eggs produced.
A three-stage adult life model
The goal of the paper by Novoseltsev et al.24 was to use individual-level reproductive data derived from four different medfly studies to confirm that (i) individual fecundity in female flies is endowed as a flat pattern with a steady-state period of constant rate of egg laying; (ii) an individual female has three stages in her adult life: maturation, maturity, and senescence; (iii) an individual fecundity pattern has no max0imum; and (iv) two natural causes of death exist in flies including premature and senescence-caused. A model developed in a previous study was brought to bear on the four medfly populations that consisted of five parameters including Xonset denoting the age of first reproduction, T denoting the duration of the egg-laying plateau, RC denoting the plateau level, τtail denoting the exponential tail, and LS denoting lifespan. The model, depicted in Figure 6, sheds light on medfly aging and senescence in several respects, the most notable of which is use in comparing differences in the five parameter values across four different medfly populations (Table 2). One of the most remarkable results contained in this table are the large differences between the Greek flies derived from wild populations and the flies from the other populations—the egg-laying plateau (RC) was much lower and the maturation time (Xonset), duration of egg laying (T), and lifespan (LS) all much longer. However, the value of the exponential tail (senescence parameter) for the Greek flies did not stand apart from the value of this parameter in the other populations.
Figure 6.
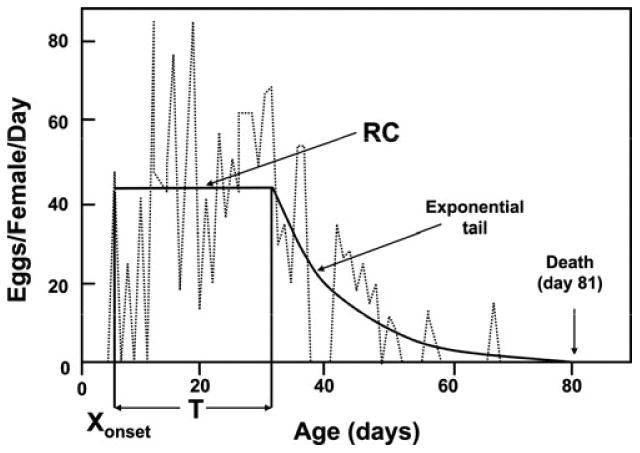
A typical individual fecundity pattern in medfly female.24 The pattern (solid line) has the following parameters: onset of reproduction Xonset = 4.0, duration of plateau period, T = 27.0, a plateau level, RC = 42.3, and a tail time constant (exponential tail) τtail = 10.6 in the exponent, t = x − T − Xonset. Death occurs at day 81.
Table 2. Parameter values of representative fecundity patterns24 where rc, t, x, τtail, and ls denotes height of egg-laying plateau (eggs/female), duration of plateau, age of maturity (first egg), exponential tail, and lifespan, respectively.
| Population (n) | RC | T | X | τtail | LS |
|---|---|---|---|---|---|
| Mexico (936) | 40.5 | 14.3 | 6.5 | 8.1 | 37.2 |
| Mexico (97) | 42.2 | 11 | 5.4 | 4.9 | 32.8 |
| Greek (50) | 14.6 | 22.3 | 19.1 | 6 | 65.6 |
| Israel (20) | 40 | 10.4 | 3.2 | 9.6 | 32.6 |
Main conclusions
(i) A practical implication is that the model makes it possible to store individual fecundity data in parametric form and (ii) the model parameters can be used for both inter-and intrapopulation comparisons as revealed in comparisons between Greek medflies and medflies from the other populations.
General principles derived from tephritid studies
The results of the studies of reproduction and aging using the two model tephritid species reveal several important relationships between reproduction and aging that are summarized as follows:
Reciprocity. Although a lifespan cost of reproduction has received much attention (i.e., the mortality cost of early reproduction), the results of many of the studies summarized in this paper reveal the importance of a reciprocal cost—the reproductive cost due to aging. A gradual decrease in egg production always occurred. Host deprivation as well as sugar– protein manipulations affected the level of egg output in that the high output days of young ages (in the full diet without host-deprivation trials) are never fully recovered. Therefore, there is an important cost to postponing reproduction, which we call a “reproduction cost of longevity,” which represents the reciprocal of the more traditional “mortality cost of reproduction.”
Qualitative tradeoffs. Tradeoffs in life history evolution are often viewed in terms of resource allocation (i.e., the “Y-model” in which resources are allocated to either maintenance or reproduction) with ecological tradeoffs as a separate class. Because there are many cases where such resource allocation tradeoffs do not emerge clearly from data, this resource allocation model has been criticized by endocrinologists who view the actions of signaling pathways as the underlying mechanism of tradeoffs.25 However, this may turn out to be a mere refinement of the “Y” model with the specific regulatory mechanisms added on and different “Y”s for different resources. To better understand tradeoffs we propose to take a more longitudinal view of the life history trajectories of individuals and recognize that there are qualitative tradeoffs that can not be understood simply as a resource allocation issue or an ecological tradeoff: e.g., a unit of reproduction can not simply be exchanged for a unit of lifespan. There are numerous examples of large qualitative differences in the tradeoffs between longevity and reproduction in the tephritid studies that can not be explained as differences in resource allocation.10
Plasticity. Both the lifespan and pattern of reproduction of fruit flies are highly plastic with enormous intercohort and between-biotype differences in both longevity and reproduction. With manipulations of diet and host deprivation, average longevity can differ fivefold. While it may be obvious that reproductive output can be reduced by providing a poor diet or host deprivation, the temporal pattern of reproduction also can be changed dramatically; e.g., dual modes shifting ages of last reproduction from around 30 to over 150 days. This within-species plasticity enables individuals to complete their life cycle under a wide range of environmental conditions. It also suggests that constraints on life history evolution are weak qualitatively. For example individuals may trade late reproduction for some reduction in total reproductive output, but still be able to reproduce successfully.
Constraints and determinacy. Previous work has advocated against a determined maximum lifespan. Is there a limit on total reproduction? The reproductive potential of fruit flies appears to be predestined by the early life experience in the host environment. Thus, adults eclose with a reproductive endowment that varies considerably by host species, has unknown limits and can be considered indeterminate. Whether they are able to then reach their potential depends on the availability of nutrients in the adult diet (to yolk eggs), host availability and the total number of life days remaining. Because yeast rich diets are best for yolking eggs, but not for lifespan, this tradeoff may frequently prevent flies from reaching their reproductive potential. Some particular flies, however, have postreproductive lifespans that are greater than their usual interoviposition intervals and this can be interpreted as having reached their potential.
Acknowledgments
Supported by NIA/NIH Grants P01 AG022500–01 and P01 AG08761–10.
Footnotes
Conflicts of interest: The authors declare no conflicts of interest.
References
- 1.Boggs CL. Understanding insect life histories and senescence through a resource allocation lens. Funct Ecol. 2009;23:27–37. [Google Scholar]
- 2.Christenson LD, Foote RH. Biology of fruit flies. Annu Rev Entomol. 1960;5:171–192. [Google Scholar]
- 3.Carey JR, et al. Relationship of age patterns of fecundity to mortality, longevity, and lifetime reproduction in a large cohort of Mediterranean fruit fly females. J Gerontol A Biol Sci Med Sci. 1998;53A:B245–B251. doi: 10.1093/gerona/53a.4.b245. [DOI] [PubMed] [Google Scholar]
- 4.Carey JR, et al. A simple graphical technique for displaying individual fertility data and cohort survival: case study of 1000 Mediterranean fruit fly females. Funct Ecol. 1998;12:359–363. [Google Scholar]
- 5.Carey JR. Insect biodemography. Annu Rev Entomol. 2001;46:79–110. doi: 10.1146/annurev.ento.46.1.79. [DOI] [PubMed] [Google Scholar]
- 6.Carey JR, et al. Biodemography of a long-lived tephritid: reproduction and longevity in a large cohort of Mexican fruit flies, Anastrepha ludens. Exp Gerontol. 2005;40:793–800. doi: 10.1016/j.exger.2005.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carey JR, Liedo P, Orozco D, Vaupel JW. Slowing of mortality rates at older ages in large medfly cohorts. Science. 1992;258:457–461. doi: 10.1126/science.1411540. [DOI] [PubMed] [Google Scholar]
- 8.Carey JR, et al. A male-female longevity paradox in medfly cohorts. J Anim Ecol. 1995;64:107–116. [Google Scholar]
- 9.Müller HG, et al. Early mortality surge in protein-deprived females causes reversal of sex differential of life expectancy in Mediterranean fruit flies. Proc Natl Acad Sci USA. 1997;94:2762–2765. doi: 10.1073/pnas.94.6.2762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carey JR. Longevity: the Biology and Demography of Life Span. Princeton University Press; Princeton: 2003. [Google Scholar]
- 11.Carey JR, et al. Food pulses increase longevity and induce cyclical egg production in Mediterranean fruit flies. Funct Ecol. 2002;16:313–325. [Google Scholar]
- 12.Diamantidis AD, et al. Life history evolution in a globally-invading tephritid: patterns of survival and reproduction in medflies from six world regions. Biol J Linn Soc. 2009;97:106–117. [Google Scholar]
- 13.Krainacker DA, Carey JR, Vargas R. Effect of larval host on the life history parameters of the Mediterranean fruit fly, Ceratitis capitata. Oecologia. 1987;73:583–590. doi: 10.1007/BF00379420. [DOI] [PubMed] [Google Scholar]
- 14.Carey JR, Krainacker D, Vargas R. Life history response of Mediterranean fruit fly females to periods of host deprivation. Entomol Exp Appl. 1986;42:159–167. [Google Scholar]
- 15.White TCR. The importance of a relative shortage of food in animal ecology. Oecologia. 1978;33:71–86. doi: 10.1007/BF00376997. [DOI] [PubMed] [Google Scholar]
- 16.Holliday R. Food, reproduction and longevity: is the extended lifespan of calorie-restricted animals an evolutionary adaptation? Bioessays. 1989;10:125–127. doi: 10.1002/bies.950100408. [DOI] [PubMed] [Google Scholar]
- 17.Masoro EJ. Subfield history: caloric restriction, slowing aging, and extending life. Sci Aging Knowledge Environ. 2003;re2:1–7. doi: 10.1126/sageke.2003.8.re2. [DOI] [PubMed] [Google Scholar]
- 18.Carey JR, et al. Dual modes of aging in Mediterranean fruit fly females. Science. 1998;281:996–998. doi: 10.1126/science.281.5379.996. [DOI] [PubMed] [Google Scholar]
- 19.Carey JR, et al. Life history response of Mediterranean fruit flies to dietary restriction. Aging Cell. 2002;1:140–148. doi: 10.1046/j.1474-9728.2002.00019.x. [DOI] [PubMed] [Google Scholar]
- 20.Masoro EJ. Caloric restriction and aging: controversial issues. J Gerontol A Biol Sci Med Sci. 2006;61A:14–19. doi: 10.1093/gerona/61.1.14. [DOI] [PubMed] [Google Scholar]
- 21.Carey JR, et al. Longevity-fertility trade-offs in the tephritid fruit fly, Anastrepha ludens, across dietary-restriction gradients. Aging Cell. 2008;7:470–477. doi: 10.1111/j.1474-9726.2008.00389.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Müller HG, Carey JR, Wu D, Vaupel JW. Reproductive potential determines longevity of female Mediterranean fruit flies. Proc R Soc Lond B Biol Sci. 2001;268:445–450. doi: 10.1098/rspb.2000.1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Carey JR, Liedo P. Mortality dynamics of insects: general principles derived from aging research on the Mediterranean fruit fly (Diptera: Tephritidae) Am Entomol. 1999;45:49–55. [Google Scholar]
- 24.Novoseltsev VN, et al. Systemic mechanisms of individual reproductive life history in female medflies. Mech Ageing Dev. 2004;125:77–87. doi: 10.1016/j.mad.2003.10.007. [DOI] [PubMed] [Google Scholar]
- 25.Zera AJ, Harshman LG. The physiology of life history trade-offs in animals. Annu Rev Ecol Syst. 2002;32:95–126. [Google Scholar]


