Abstract
Breast cancer is the most common cancer among women worldwide, and survival rates are increasing. Chemotherapy-associated peripheral neuropathy (PN) is clinically important because of effects on quality of life (QOL) and potential effects on dose limitations. This adverse drug reaction is associated with certain classes of chemotherapy and commonly presents as peripheral sensory neuropathy whose natural course is largely unknown. The literature was reviewed to determine the frequency and characteristics of PN associated with adjuvant chemotherapy in early-stage breast cancer (ESBC) to explore the potential impact on long-term (one or more years after diagnosis) health outcomes and QOL. MEDLINE, PubMed, Embase, and the Cochrane Library were searched for relevant English-language randomized controlled trials, systematic reviews, meta-analyses, and case-control and cohort studies published between January 1990 and July 1996. Included studies were limited to current adjuvant regimens (eg, anthracyclines, taxanes, cyclophosphamide, platinum compounds). Two investigators independently reviewed abstracts, full-text articles, and extracted data from fair- and good-quality studies. Discrepancies in quality assessment and data extraction were resolved by consensus. We identified 364 articles; 60 were eligible for full-text review. Only five reports of four studies provided data beyond one year post–treatment initiation. Studies used different measures to assess PN. Neuropathic symptoms persisted in 11.0% to more than 80% of participants at one to three years following treatment. There is a paucity of data describing persistent PN in ESBC patients. Consistent use of validated measures and well-conducted randomized clinical trials or observational studies are needed to evaluate the incidence, persistence, and QOL associated with the long-term effects of PN.
Increased survival for patients with early-stage breast cancer (ESBC) has steadily transformed the field of breast oncology to require a survivor-centered focus (1). Adjuvant chemotherapy use in the treatment of ESBC is well established (2–6). Adjuvant therapy regimen choice may need to consider toxicity, especially in ESBC patients, whose excellent prognosis requires careful consideration of long-term adverse effects. Current therapies provide meaningful benefits to patients; however, they carry the risk of long-term adverse outcomes including cardiac, neurologic, and hematologic toxicity, as well as secondary malignancies.
Chemotherapy-associated peripheral neuropathy (PN) is a clinically important treatment toxicity, which may be dose-limiting and carry the risk of long-term effects on quality of life (QOL) (7,8). Chemotherapy-associated PN results from specific chemotherapy agents and can cause disruption and dysfunction of the somatic (sensory or motor) and/or autonomic nervous systems, leading to discomfort and decreased QOL. Taxanes, platinum compounds, and vinca alkaloids are the major classes of oncology drugs where exposure produces the highest PN risk (9). Over the past two decades, randomized clinical trials (RCTs) have demonstrated the benefits of adding taxanes to adjuvant chemotherapy (2). Dose-dependent exposure relationships are established for these therapies and may result in both acute toxicity and persistent symptoms. PN is related to drug-specific factors, including mechanism of action, dose, schedule (intensity and cumulative dosing), and regimen selection (multi-agent synergy) (7–13).
The duration and prevalence of persistent PN are not well studied, and the underlying mechanisms are only partially understood. Estimates of chemotherapy-associated PN prevalence vary widely. Evidence-based strategies for PN clinical management are very limited, with no effective treatments, and the impact of preexisting comorbid conditions is unknown (7,14). Better information about predisposing factors and long-term treatment risks is important for shared decision-making between ESBC patients and physicians considering adjuvant chemotherapy (15). We conducted a systematic literature review to summarize current evidence on the 1) incidence and prevalence of PN present at one or more years from diagnosis of ESBC among women receiving adjuvant chemotherapy (doxorubicin, epirubicin, paclitaxel, docetaxel, and carboplatin) and 2) effects of PN on patient-reported outcomes (PROs) and QOL.
We conducted a comprehensive search of MEDLINE, PubMed, Embase, and the Cochrane Library for relevant English-language studies published between January 1990 and July 2016 to identify studies of PN following chemotherapy for ESBC. A full description of the key word search can be found in the Supplementary Materials (available online). Searches were supplemented with reference lists from other relevant systematic reviews and pertinent articles.
We included studies of women with ESBC (stages I–IIIA) receiving adjuvant chemotherapy regimens including anthracyclines, cyclophosphamide, taxanes, or platinum compounds with at least 12 months of follow-up. We excluded studies limited to neoadjuvant chemotherapy or older regimens. RCTs, case-control studies, cohort studies, systematic reviews, and meta-analyses were eligible for inclusion. We excluded studies conducted in countries not ranked as “very high human development” (16) or including women with advanced stage (IIIB–IV) or distant metastases.
Studies were rated as “fair” or “good” quality using the Newcastle-Ottawa Scale for nonrandomized studies and the Cochrane criteria for RCTs (17,18). Two investigators independently extracted data and reviewed for accuracy, resolving any discrepancies by consensus. Data extracted included demographics, treatment regimens, and any PN-related outcomes broadly defined to include incidence, prevalence, severity, symptoms, duration, and resolution. Pooled estimates were not possible because of the scant number of studies and variation in PN outcome measures.
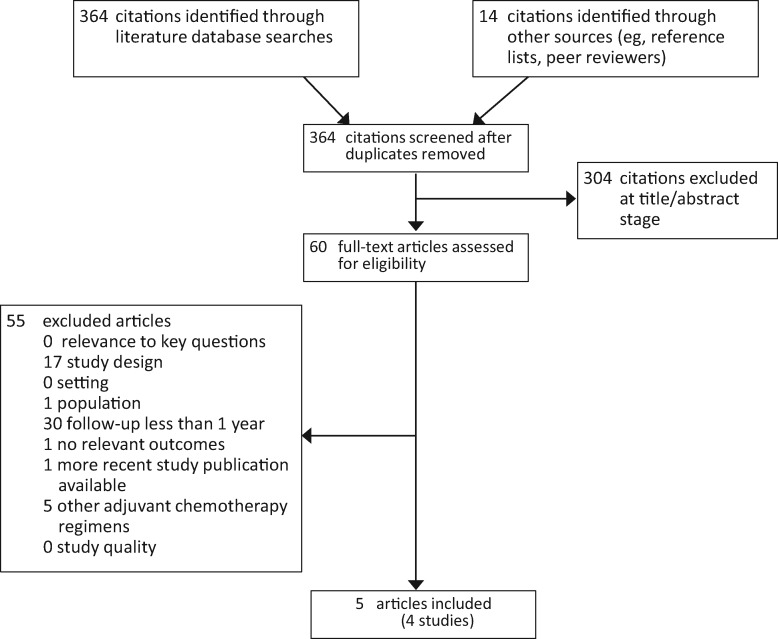
We identified 364 articles, from which 60 potentially relevant full-text articles were considered for inclusion. Only five publications describing four studies provided data on relevant outcomes one or more years postdiagnosis (Figure 1). The most common reason for exclusion was follow-up of less than one year (29 studies). Key characteristics of the five included publications are shown in Table 1, and study outcomes are shown in Table 2. Three publications on two good-quality studies (19–21) and two fair-quality studies (22,23) measured PN for one to three years post-treatment.
Figure 1.
Systematic review exclusion chart.
Table 1.
Systematic review of peripheral neuropathy after breast cancer chemotherapy: Study characteristics*
| Study, y | Design | Country | Aim | Population | Quality rating |
|---|---|---|---|---|---|
| Hershman et al., 2011 (22) | Prospective cohort study | United States | To evaluate the prevalence and severity of long-term symptoms of neuropathy following adjuvant taxane-based chemotherapy in women with early-stage breast cancer |
|
Fair |
| Cross-sectional study | United States | To evaluate the prevalence and severity of long-term symptoms of neuropathy following adjuvant taxane-based chemotherapy in women with early-stage breast cancer |
|
||
| Nitz et al., 2014 (23) | Open-label randomized prospective phase III trial (WSG-AGO EC-DOC vs FEC/CMF)* | Germany | To compare outcomes and toxicities of 4 cycles of epirubicin 90 mg/m2 and cyclophosphamide 500 mg/m2 followed by 4 cycles of docetaxel 100 mg/m2 with nontaxane therapy (CMF or FEC)* |
|
Fair |
| Eckhoff et al., 2015 (19) | Open-label randomized, prospective phase III trial (DBCG 07 READ Trial) | Denmark | To assess duration and severity of PN after cessation of docetaxel by analyzing patient-reported outcomes in a longitudinal study and to evaluate the impact of persistence of PN on health-related quality of life |
|
Good |
| Fontes et al., 2016 (20) | Prospective cohort study | Portugal | To estimate the incidence of neurologic complication after breast cancer treatment, including peripheral neuropathy and neuropathic pain |
|
Good |
| Pereira et al., 2015 (21) (Supplementary to Fontes et al. [20]) | Prospective cohort study | Portugal | To estimate the incidence of chemotherapy-induced peripheral neuropathy and to identify its main determinants and impact in patient-reported outcomes |
|
BMI = Body Mass Index; CMF = cyclophosphamide, methotrexate, and 5-fluorouracil; EC-DOC = epirubicin, cyclophosphamide, and docetaxel; FEC = 5-fluorouracil, epirubicin, and cyclophosphamide; WSG-AGO = West German Study Group–German Gynecological Oncology Group.
Table 2.
Systematic review of peripheral neuropathy after breast cancer chemotherapy: Study outcomes and results*
| Study, y | Primary outcome(s) | Treatment | Results | Grading | Persistence | Follow-up period |
|---|---|---|---|---|---|---|
| Hershman et al., 2011 (22) | Adjuvant paclitaxel treatment: Cohort study: followed to 12 mo from treatment |
|
|
|
1 y | |
| Cross-sectional study: 6–24 mo from treatment |
|
|
Not reported | |||
| Nitz et al., 2014 (23) |
|
|
Peripheral neuropathy measures not reported |
|
2–3 y | |
| Eckhoff et al., 2015 (19) |
|
|
|
|
1–3.2 y | |
| Fontes et al., 2016 (20) |
|
|
|
|
1–3 y | |
| Pereira et al., 2015 (21) (Supplementary to Fontes et al. [20]) |
|
Same as above |
|
|
|
1 y |
CMF = cyclophosphamide, methotrexate and 5-fluorouracil; CTCAE = Common Terminology Criteria of Adverse Events; EORTC CIPN20 = European Organisation for Research and Treatment of Cancer Chemotherapy Induced Peripheral Neuropathy 20; EORTC QLQ C-30 = European Organisation for Research and Treatment of Cancer Quality of Life; FACT = Functional Assessment of Cancer Therapy; FACT/GOG-Ntx = Functional Assessment of Cancer Therapy/Gynecologic Oncology Group-Neurotoxicity; FEC = 5-fluorouracil, epirubicin, and cyclophosphamide; HADS = Hospital Anxiety and Depression Scale; OR = odds ratio; PQSI = Pittsburgh Sleep Quality Index; TNSc = Total Neuropathy Score, clinical version.
In 2011, Hershman et al. examined the prevalence and severity of persistent PN in ESBC patients receiving taxane therapy in both a prospective cohort and a cross-sectional study (22). Adjuvant paclitaxel regimens at varying doses were evaluated (most common: 175 mg/m2 every two weeks for four cycles). The cross-sectional study included 50 women treated six to 24 months prior to evaluation (median = 12 months). More than 80% reported ongoing numbness or discomfort in hands or feet; severe symptoms were reported in hands (27.0%) and feet (25.0%). Severe PN resulted in treatment alterations (10.0%) and discontinuation (4.0%). Outcomes were assessed using the FACT/GOG-Ntx, CTCAE, v. 3.0, and QST (defined in Table 3). FACT physical well-being scores were more likely to be lower for those reporting severe symptoms.
Table 3.
Reported measures of chemotherapy-induced peripheral neuropathy across studies
| Measure name | Use | Scoring |
|---|---|---|
| Functional Assessment of Cancer Therapy/Gynecologic Oncology Group–Neurotoxicity (FACT/GOG-Ntx) (30) | This measure incorporates the FACT G (29) subscales OG-Ntx, a standardized self-report questionnaire for neurotoxicity to score functioning as well as QOL. The FACT G assesses the areas of physical well-being, social/family well-being, emotional well-being, and functional well-being, as well as 5 additional questions related to arthralgia, myalgia, and skin discoloration. The first 4 questions of the 11-question OG-Ntx ask about numbness/tingling and discomfort in the hands and feet. | Responses are given on a 5-point scale, with 4 meaning ‘‘not at all’’ and 0 meaning ‘‘very much,” in which higher scores reflect better quality of life. The maximum score is 44. |
| Quantitative Neurosensory Testing (QST) (31) | A group of sensory diagnostic tests for peripheral nerve function, this particular version includes measures for tactile threshold (TT) and vibration threshold (VT), measured by technological evaluation. | Higher QST scores reflect decreased sensation or worsening neuropathy. |
| Common Terminology Criteria of Adverse Events (CTCAE versions 3.0 and 4.0) (32,33) | CTCAE defines peripheral neuropathy as a disorder characterized by functional disturbances of sensory neurons resulting in abnormal cutaneous sensations of tingling, numbness, pressure, cold, and warmth that are experienced in the absence of a stimulus. | The adverse event is graded on a scale of 1 to 5, with 5 being the worst. |
| Total Neuropathy Score, clinical version (TNSc) (34) | This instrument tests strength, deep tendon reflexes, vibration sensibility (128 Hz tuning fork), and pain sensation to assess neuropathy severity. Pain classified by the International Association for the Study of Pain System. | Each item is rated on a 0 to 4 scale, with a higher score reflecting poorer functioning. |
| Self-reported questionnaire based on the National Cancer Institute Common Toxicity Criteria (NCI-CTC version 2.0) (35) | This physician-guided measure was transformed to a patient-reported assessment of peripheral neuropathy severity by assigning a grade. The questionnaire was not validated at the time of assessment. | The grading scale ranges from 0 to 4, with 4 being the worst. |
| European Organisation for Research and Treatment of Cancer Chemotherapy Induced Peripheral Neuropathy 20 (EORTC QLQ CIPN 20) (36) | This 20-question instrument measures docetaxel-induced peripheral neuropathy along three subscales assessing sensory, motor, and autonomic symptoms. The items are scored from 1, meaning “not at all,” to 4, meaning “very much.” | For this study, scores were transformed to a 100-point scale. A higher score indicates more experiences, symptoms, or complaints. |
The FACT/GOG-Ntx was administered before and after treatment and every three months for one year post-treatment in the prospective study of 50 ESBC patients (22). Differences between baseline and 12-month post-treatment measures were statistically significant (Table 2). After 12 months, the social well-being and the neurotoxicity subscale scores decreased, indicating a reduced QOL (Table 3). The PRO and sensory evaluations showed statistically significant correlations one year post-treatment. At one year, 67.0% of patients reported persistent numbness in hands or feet; 27.0% reported severe PN symptoms.
In 2014, Nitz et al. reported on neurotoxicity with follow-up of up to three years in a phase III trial for intermediate-risk ESBC (23). In the docetaxel arm (EC-Doc) showing superior survival outcomes, 19.1% of patients reported grade 3 or 4 neurotoxicity at the end of treatment compared with 6.5% of those receiving CMF or FEC (control group). Persisting neurotoxicity of any grade was reported by EC-Doc patients: 14.2% at six months, 11.0% at one year, and 7.4% after two to three years. PN of any grade persisting after two years of follow-up was reported in 3.2% and 0.6% of the EC-Doc and FEC groups, respectively.
Studies by Eckhoff et al. (19) and Fontes et al. (20) explored risk factors and dose relationships related to PN onset and persistence in similarly aged cohorts of ESBC patients; Pereira et al. (21) provided supplementary data to the Fontes study (20). Eckhoff et al. administered an adapted NCI-CTC v. 2.0 (Table 3) to a cohort of 1174 patients in Denmark (1031 analyzed) to assess PROs for PN (20). Docetaxel-associated PN was evaluated by the EORTC QLQ-CIPN20 (Table 3), and health-related QOL was assessed by the European Organization for Research and Treatment of Cancer quality of life questionnaire (EORTC QLQ C-30 v. 3.0) (24). All treatment regimens included use of docetaxel administered for either three 100 mg/m2 cycles or six 75 mg/m2 cycles. At the initial assessment (immediately post-treatment), 241 patients (23.4%) reported grade 2 to 4 PN, which persisted for one to three years in 81 patients (33.6%). Among patients not reporting PN at the initial assessment, 9.6% developed PN within one to three years. Patients with PN at initial assessment were more likely to report persistent PN (P < .0001). In the entire cohort, 43.0% of women reported grades 1 to 4 PN at one to three years after treatment with docetaxel.
Among all treated patients completing the EORTC QLQ-CIPN20, chief sensory complaints were tingling of the fingers/hands and toes/feet (Table 3). The most prominently reported motor symptoms were difficulty opening a jar/bottle and foot cramps. Statistically significant differences on all EORTC QLQ-C30 functioning and symptom subscales were observed between patient groups with grade 0 to 1, 2, or 3 to 4 PN. Clinically important differences between no PN and grade 3 or 4 PN were detected in the areas of role functioning, social functioning, global health status/QOL, fatigue, and pain (19).
Independent risk factors for PN persistence included age 55 years or older (odds ratio [OR] = 1.99, 95% confidence interval [CI] = 1.35 to 2.95) and maximum grade of neuropathy during treatment vs no PN: grade 1 (OR = 2.21, 95% CI = 1.12 to 4.39), grade 2 (OR = 7.49, 95% CI = 3.86 to 14.55), and grade 3 or 4 (OR = 9.94, 95% CI = 4.77 to 20.70). Grade severity of other symptoms (persistent muscle and joint pain, fatigue, and stomatitis) were also associated with persistent PN (P < .05).
Fontes et al. focused on determinants of the course of PN (20). In this prospective cohort study, 475 women (93.9%) completed the three-year evaluation (31 lost to follow-up). In the first year after diagnosis, 288 received either neoadjuvant (11.1%) or adjuvant (88.9%) chemotherapy. FEC-D (three cycles of concomitant 5-FU 500 mg/m,2 epirubicin 100 mg/m,2 and cyclophosphamide 500 mg/m,2 followed by three cycles of docetaxel 100 mg/m2) was the most commonly received regimen (59.4%).
In the overall cohort, PN symptoms were present in 14.1% at one year and 12.6% at three years. Of 288 patients who received chemotherapy, 23.3% were diagnosed with PN within one year after diagnosis and 20.5% had persisting symptoms at three years. Pereira reported an increased PN cumulative incidence of 38% at one year among patients in the same cohort receiving docetaxel-based regimens (21). Nearly all patients with PN at one and three years had a sensory neuropathy (98.1% with grade 1–2), whereas a smaller percentage had a motor neuropathy (15.1% with grade 1–3) using CTCAE v. 4.0 (Table 3). The severity of disease in patients first presenting with PN at three-year follow-up was reflected in the high median TNSc score (Table 3) and percentage reporting sensory (100%) and motor (57.1%) PN. The adjusted odds ratio for PN among patients receiving taxane-based regimens was 14.76 (95% CI = 3.31 to 65.79). Among other factors evaluated, only cancer stage at baseline showed a statistically significant association with PN at three years (OR = 3.73, 95% CI = 1.70 to 8.14). After one year of follow-up, the use of docetaxel-based regimens with a cumulative dose of less than 300 mg/m2 and greater than 300 mg/m2 produced dose-dependent PN risk (relative risk [RR] = 6.96, 95% CI = 2.46 to 19.71; RR = 13.32, 95% CI = 4.11 to 43.14, respectively) compared with those without exposure (21).
We identified only five publications of chemotherapy-associated PN with at least 12 months of follow-up among ESBC patients in this systematic review. The paucity of reports is unexpected given the high frequency of neurotoxic adjuvant chemotherapy use and the expected long-term survival of the patients receiving these treatments. These studies had limited sample size and were conducted in four different countries. There was wide variation in study design, chemotherapy regimens, drug dosing, variables collected, follow-up time, outcome measures, and PN prevalence. Only one study evaluated the PN incidence between comparable women exposed and unexposed to adjuvant chemotherapy (20). More research is needed on this long-term toxicity of contemporary adjuvant chemotherapy in ESBC patients.
Within the included studies, frequency estimates of persistent PN one or more years post-treatment ranged from 11.0% to greater than 80%. Data synthesis and interpretation were limited by disparate measures of PN and lack of comparison control groups. There is no standard measurement approach for evaluating chemotherapy-associated PN, limiting interpretability. Various multimodal options include the use of clinician-rated instruments (eg, NCI-CTCAE), PRO and QOL instruments, and physical symptom assessments (eg, QST, TNSc) (Table 3). Clinician-rated scales may introduce bias due to the subjective mapping of symptoms to a grade (25), underestimating the severity of the patient experience, as seen in a comparative study of this question (26). Although not without limitations, PROs have face validity because PN manifestations are primarily subjective symptoms.
No biomarkers are available to predict the relationship between a baseline score and length or severity of PN symptoms (higher-grade PN during treatment was shown to increase the risk of persistent PN) (24). Pharmacogenetic variants potentially associated with PN development are under evaluation (27,28). Integration of newer PROs and investigation of circulating biomarkers may identify risk stratification opportunities (14). Further research employing standardized measurements of PN is needed to understand risk factors, persistence, and severity.
Improved understanding of PN incidence, prevalence, and severity would enable breast cancer patients and physicians to consider this potential adverse effect of adjuvant chemotherapy. Clinical trials play a role in capturing adverse events; however, longitudinal observational research is complementary, allowing for evaluation of late effects unable to be detected by RCTs of shorter duration. More precise treatment decision-making requires the availability of well-conducted, high-quality comparative longitudinal studies measuring long-term PN as an outcome (29). The development, collection, and synthesis of this evidence would ultimately enable tools for more informed treatment choices for ESBC patients.
We have shown that data describing long-term PN in ESBC patients are very sparse. Validated consensus measurement scales in well-conducted RCTs and observational studies are needed to evaluate the incidence, persistence, and QOL associated with persistent PN. Evidence on the risk of persistent PN is needed to facilitate more comprehensive decision-making about selection of adjuvant therapy regimens.
Funding
This review was supported by National Cancer Institute contract #HHSN261201400163P and by National Institute of Health NCI HHS PHS U10CA-180868, -180822, and UG1-CA-189867; and the Breast Cancer Research Foundation (PAG).
Notes
DRR contributed to the literature search, study design, figures, writing, methods, and data interpretation. PAG contributed to study design, figures, writing, methods, and data interpretation. HB contributed to writing and data interpretation. MSW contributed to the literature search, study design, figures, writing, and methods. JM contributed to the literature search, study design, figures, writing, methods, and data interpretation.
PAG discloses that she is a member of the Scientific Advisory Board of the Breast Cancer Research Foundation.All other authors declare no other potential conflict(s) of interest.
The authors thank Bruce Abbott, Medical Librarian, University of California, Davis, for literature search support.
Supplementary Material
References
- 1. Howlader N, Noone AM, Krapcho M, et al. , eds. SEER Cancer Statistics Review, 1975–2013. Bethesda, MD: National Cancer Institute; 2016. http://seer.cancer.gov/csr/1975_2013/. Accessed January 9, 2017. [Google Scholar]
- 2. Denduluri N, Somerfield MR, Eisen A, et al. Selection of optimal adjuvant chemotherapy regimens for human epidermal growth factor receptor 2 (HER2) – negative and adjuvant targeted therapy for HER2-positive breast cancers: An American Society of Clinical Oncology guideline adaptation of the Cancer Care Ontario clinical practice guideline. J Clin Oncol. 2016;34(20):2416–2429. [DOI] [PubMed] [Google Scholar]
- 3. Lee KS, Ro J, Nam BH, et al. A randomized phase-III trial of docetaxel/capecitabine vs doxorubicin/cyclophosphamide as primary chemotherapy for patients with stage II/III breast cancer. Breast Cancer Res Treat. 2008;109(3):481–489. [DOI] [PubMed] [Google Scholar]
- 4. Toledano A, Azria D, Garaud P, et al. Phase III trial of concurrent or sequential adjuvant chemoradiotherapy after conservative surgery for early-stage breast cancer: Final results of the ARCOSEIN trial. J Clin Oncol. 2007;25(4):405–410. Erratum in: J Clin Oncol. 2007;25(16):2334. [DOI] [PubMed] [Google Scholar]
- 5. Loprinzi CL, Thomé SD.. Understanding the utility of adjuvant systemic therapy for primary breast cancer. J Clin Oncol. 2001;19(4):972–979. [DOI] [PubMed] [Google Scholar]
- 6. Joensuu H, Kellokumpu-Lehtinen PL, Bono P, et al. Adjuvant docetaxel or vinorelbine with or without trastuzumab for breast cancer. N Engl J Med. 2006;354(8):809–820. [DOI] [PubMed] [Google Scholar]
- 7. Hershman DL, Lacchetti C, Dworkin RH, et al. Prevention and management of chemotherapy-induced peripheral neuropathy in survivors of adult cancers: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol. 2014;32(18):1941–1967. [DOI] [PubMed] [Google Scholar]
- 8. Sykes N, Bennet M, Yuan C.. Clinical Pain Management Cancer Pain. 2nd ed. Boca Raton, FL: CRC Press; 2008. https://www.crcpress.com/Clinical-Pain-Management-Second-Edition-Cancer-Pain/Sykes-Bennet-Yuan/p/book/9780340940075. Accessed January 9, 2017. [Google Scholar]
- 9. Hausheer FH, Schilsky RL, Bain S, Berghorn EJ, Lieberman F.. Diagnosis, management, and evaluation of chemotherapy-induced peripheral neuropathy. Semin Oncol. 2006;33(1):15–49. [DOI] [PubMed] [Google Scholar]
- 10. Park SB, Goldstein D, Krishnan AV, et al. Chemotherapy-induced peripheral neurotoxicity: A critical analysis. CA Cancer J Clin. 2013;63(6):419–437. [DOI] [PubMed] [Google Scholar]
- 11. Eckhoff L, Knoop AS, Jensen MB, Ejlertsen B, Ewertz M.. Risk of docetaxel-induced peripheral neuropathy among 1,725 Danish patients with early stage breast cancer. Breast Cancer Res Treat. 2013;142(1):109–118. [DOI] [PubMed] [Google Scholar]
- 12. van Gerven JM, Moll JW, van den Bent MJ, et al. Paclitaxel (Taxol) induces cumulative mild neurotoxicity. Eur J Cancer. 1994;30A(8):1074–1077. [DOI] [PubMed] [Google Scholar]
- 13. Chaudhry V, Chaudhry M, Crawford TO, et al. Toxic neuropathy in patients with pre-existing neuropathy. Neurology. 2003;60(2):337–340. [DOI] [PubMed] [Google Scholar]
- 14. Schneider BP, Hershman DL, Loprinzi C.. Symptoms: Chemotherapy-induced peripheral neuropathy. Adv Exp Med Biol. 2015;862:77–87. [DOI] [PubMed] [Google Scholar]
- 15. Wolf S, Barton D, Kottschade L, Grothey A, Loprinzi C.. Chemotherapy-induced peripheral neuropathy: Prevention and treatment strategies. Eur J Cancer. 2008;44(11):1507–1515. [DOI] [PubMed] [Google Scholar]
- 16. Jahan S. 2015 Human Development Report, United Nations Development Programme. http://hdr.undp.org/sites/default/files/2015_human_development_report.pdf. Accessed January 16, 2017.
- 17. Wells GA, Shea B, O’Connell D, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality if nonrandomized studies in meta-analyses. Department of Epidemiology and Community Medicine, University of Ottawa, Canada. http://www.ohri.ca/programs/clinical_epidemiology/oxford.htm. Accessed January 9, 2017.
- 18. Higgins JP, Altman DG, Gøtzsche PC; Cochrane Bias Methods Group; Cochrane Statistical Methods Group. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Eckhoff L, Knoop A, Jensen MB, Ewertz M.. Persistence of docetaxel-induced neuropathy and impact on quality of life among breast cancer survivors. Eur J Cancer. 2015;51(3):292–300. [DOI] [PubMed] [Google Scholar]
- 20. Fontes F, Pereira S, Castro-Lopes JM, Lunet N.. A prospective study on the neurological complications of breast cancer and its treatment: Updated analysis three years after cancer diagnosis. Breast. 2016;29:31–38. [DOI] [PubMed] [Google Scholar]
- 21. Pereira S, Fontes F, Sonin T, et al. Chemotherapy-induced peripheral neuropathy after neoadjuvant or adjuvant treatment of breast cancer: A prospective cohort study. Support Care Cancer. 2016;24(4):1571–1581. [DOI] [PubMed] [Google Scholar]
- 22. Hershman DL, Weimer LH, Wang A, et al. Association between patient reported outcomes and quantitative sensory tests for measuring long-term neurotoxicity in breast cancer survivors treated with adjuvant paclitaxel chemotherapy. Breast Cancer Res Treat. 2011;125(3):767–774. [DOI] [PubMed] [Google Scholar]
- 23. Nitz U, Gluz O, Huober J, et al. Final analysis of the prospective WSG-AGO EC-Doc versus FEC phase III trial in intermediate-risk (pN1) early breast cancer: Efficacy and predictive value of Ki67 expression. Ann Oncol. 2014;25(8):1551–1557. [DOI] [PubMed] [Google Scholar]
- 24. Aaronson NK, Ahmedzai S, Bergman B, et al. The European Organization for Research and Treatment of Cancer QLQ-C30: A quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. 1993;85(5):365–376. [DOI] [PubMed] [Google Scholar]
- 25. Atkinson TM, Li Y, Coffey CW, et al. Reliability of adverse symptom event reporting by clinicians. Qual Life Res. 2012;21(7):1159–1164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Shimozuma K, Ohashi Y, Takeuchi A, et al. Feasibility and validity of the Patient Neurotoxicity Questionnaire during taxane chemotherapy in a phase III randomized trial in patients with breast cancer: N-SAS BC 02. Support Care Cancer. 2009;17(12):1483–1491 [DOI] [PubMed] [Google Scholar]
- 27. Eckhoff L, Feddersen S, Knoop AS, Ewertz M, Bergmann TK.. Docetaxel-induced neuropathy: A pharmacogenetic case-control study of 150 women with early-stage breast cancer. Acta Oncol. 2015;54(4):530–537. [DOI] [PubMed] [Google Scholar]
- 28. Schneider BP, Li L, Radovich M, et al. Genome-wide association studies for taxane-induced peripheral neuropathy in ECOG-5103 and ECOG-1199. Clin Cancer Res. 2015;21(22):5082–5091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bandos H, Melnikow J, Rivera DR, et al. Long-term peripheral neuropathy in breast cancer patients treated with adjuvant chemotherapy: NRG Oncology/NSABP B-30. J Natl Cancer Inst. 2018;110(2):149–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Calhoun EA, Welshman EE, Chang CH, et al. Psychometric evaluation of the Functional Assessment of Cancer Therapy/Gynecologic Oncology Group-Neurotoxicity (Fact/GOG-Ntx) questionnaire for patients receiving systemic chemotherapy. Int J Gynecol Cancer. 2003;13(6):741–748. [DOI] [PubMed] [Google Scholar]
- 31. Goldberg JM, Lindblom U.. Standardised method of determining vibratory perception thresholds for diagnosis and screening in neurological investigation. J Neurol Neurosurg Psychiatr. 1979;42(9):793–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Cancer Therapy Evaluation Program. Common Terminology Criteria for Adverse Events, version 3.0. DCTD, NCI, NIH, DHHS. 2006. https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/ctcaev3.pdf. Accessed January 16, 2017.
- 33. Cancer Therapy Evaluation Program. Common Terminology Criteria for Adverse Events, version 4.0. DCTD, NCI, NIH, DHHS. 2010. Available at: http://ctep.cancer.gov/. Accessed: January 16, 2017.
- 34. Cornblath DR, Chaudhry V, Carter K, et al. Total neuropathy score: Validation and reliability study. Neurology. 1999;53(8):1660–1664. [DOI] [PubMed] [Google Scholar]
- 35. Cancer Therapy Evaluation Program in coordination with the Common Toxicity Criteria Review Committee. NCI Common Toxicity Criteria (CTC), version 2.0. DCTD, NCI, NIH, DHHS. 1999. https://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/ctcmanual_v4_10-4-99.pdf. Accessed January 16, 2017.
- 36. Postma TJ, Aaronson NK, Heimans JJ, et al. The development of an EORTC quality of life questionnaire to assess chemotherapy-induced peripheral neuropathy: The QLQ-CIPN20. Eur J Cancer. 2005;41(8):1135–1139. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.