ABSTRACT
Hematopoietic stem cells (HSCs) develop in discrete anatomical niches, migrating during embryogenesis from the aorta-gonad-mesonephros (AGM) region to the fetal liver, and finally to the bone marrow, where most HSCs reside throughout adult life. These niches provide supportive microenvironments that specify, expand and maintain HSCs. Understanding the constituents and molecular regulation of HSC niches is of considerable importance as it could shed new light on the mechanistic principles of HSC emergence and maintenance, and provide novel strategies for regenerative medicine. However, controversy exists concerning the cellular complexity of the bone marrow niche, and our understanding of the different HSC niches during development remains limited. In this Review, we summarize and discuss what is known about the heterogeneity of the HSC niches at distinct stages of their ontogeny, from the embryo to the adult bone marrow, drawing predominantly on data from mouse studies.
KEY WORDS: Bone marrow, Hematopoietic stem cell, Hemogenic endothelium, Microenvironment, Niche
Summary: This Review gives an overview of what is known regarding niche heterogeneity at distinct stages of HSC ontogeny, from embryonic life to the adult bone marrow.
Introduction
Hematopoietic stem cells (HSCs) are a rare cell population that sit at the top of the hematopoietic hierarchy; they have the capacity to self-renew and to differentiate to give rise to all blood cell lineages on demand (Abkowitz et al., 2002; Orkin and Zon, 2008). Definitive HSCs (Glossary, Box 1) emerge from the hemogenic endothelium (Glossary, Box 1) within the aorta-gonad-mesonephros (AGM; a region of embryonic mesoderm, described in more detail below). HSCs then migrate to the fetal liver through the circulation before colonizing the adult bone marrow (Bertrand et al., 2010; Boisset et al., 2010; Dzierzak and Speck, 2008; Kissa and Herbomel, 2010). HSCs can also migrate to extramedullary sites (sites outside of the bone marrow) to bring about hematopoiesis in response to stress. As mature blood cells are predominantly short-lived, HSCs are required throughout the lifetime of an individual to replenish the blood system (Orkin and Zon, 2008). Functionally, they are defined by their capacity to reconstitute the entire blood system in an irradiated recipient by stem cell transplantation, a method now widely used clinically to treat patients with hematological diseases, including leukemia, lymphoma, and sickle cell disease (Appelbaum, 2007). However, given the limited number of matching donors and of cord blood-derived HSCs, obtaining sufficient numbers of compatible HSCs remains a limiting factor for bone marrow transplantation therapy. Thus, there is a major need to develop new strategies to expand HSCs ex vivo efficiently for transplantation therapies. Improving our understanding of the endogenous regulatory mechanisms that are involved in HSC specification, maintenance and expansion is of paramount importance for accomplishing this goal.
Box 1. Glossary.
BCR-ABL1-induced CML. A mouse model of chronic myeloid leukemia (CML) induced by retrovirus engineered to express the oncogene BCR-ABL1.
Catecholamine. Catecholamines, including dopamine, adrenaline (also called epinephrine) and noradrenaline (or norepinephrine), are important neurotransmitters produced by sympathetic nerve. In the bone marrow, they can be delivered to the tissue by secretion from nerve endings or by the blood circulation.
CFU-F, colony forming unit-fibroblast and mesensphere. Clonogenic mesenchymal stem/progenitor cell activity measured by the formation in vitro of multicellular fibroblast colonies or spheres.
Definitive HSCs. Defined as cells capable of reconstituting the entire hematopoietic system of an irradiated adult recipient. They have the capacity to self-renew and differentiate to give rise to all lineages of the adult hematopoietic system.
Endothelial-to-hematopoietic transition (EHT). A key developmental event forming hematopoietic cells from hemogenic endothelial cells within the dorsal aorta.
Erythromyeloid progenitors (EMPs) and lymphoid progenitors. EMPs are a specific type of hematopoietic progenitor that can differentiate into erythroid and myeloid lineages. EMPs first emerge in the yolk sac at E8.25 and serve as a major source of hematopoiesis in the developing embryos before the generation of definitive HSCs. Lymphoid progenitors are cells are unipotent progenitors restricted to giving rise to B and/or T lymphocytes.
Hematopoietic stem and progenitor cells (HSPCs). Rare populations of cells residing predominantly in the bone marrow that can support blood cell development by self-renewal and differentiation.
Hemogenic endothelium. A special subset of endothelial cells that can give rise to multilineage HSPCs.
HSC repopulating capacity/activity. The capacity/activity of HSC to repopulate the hematopoietic system of an irradiated recipient. HSC repopulating activity is commonly evaluated by transplantation assay, in which cells from tested tissue are transplanted into irradiated recipients.
MLL-AF9-driven murine AML. A mouse model of acute myeloid leukemia (AML) induced by the MLL-AF9 fusion gene.
Sinusoidal endothelial cells (SECs). The endothelial cells that line the sinusoidal blood vessels of the bone marrow.
The stem cell ‘niche’, as proposed by Schofield (1978), provides a specialized microenvironment that preserves their repopulating capacity (Glossary, Box 1). For the past few decades, considerable efforts have been devoted to elucidating the key components of this niche, with recent evidence showing that the HSC niche is composed of diverse cell types that have specific regulatory roles, working in concert to support HSC induction, differentiation and maintenance (Birbrair and Frenette, 2016). However, many questions remain to be answered about the HSC niche, such as how the different HSC niches differ anatomically and developmentally, and what the exact roles of the distinct cellular components are that constitute the HSC niche. We also do not know whether and how the many cell types within the bone marrow niche contribute to HSC heterogeneity. As we discuss in this Review, these important issues are beginning to be addressed, advancing our understanding of the heterogeneity of the HSC niche, from embryonic development to adult life and into ageing.
Aorta-gonad-mesonephros: the origin of definitive HSCs
The hematopoietic system is of mesodermal origin, and in mammals, hematopoiesis occurs in three distinct waves in a variety of sites during embryonic development (Bertrand et al., 2005; McGrath et al., 2015; Palis, 2014; Tober et al., 2007). In the mouse, the first wave of hematopoiesis occurs in the yolk sac at embryonic day (E) 7, a developmental event that is known as primitive hematopoiesis (Bertrand et al., 2005; Chen et al., 2011; McGrath et al., 2015; Nakano et al., 2013; Palis, 2014; Palis et al., 2001, 1999; Tober et al., 2007). The primary function of primitive hematopoiesis is to produce transient hematopoietic cells to meet the immediate needs of the embryo, including the generation of primitive erythroid progenitors, required for oxygenation; embryonic macrophages, required for tissue remodeling and defense; and primitive megakaryocytes, which function in vascular maintenance (Bertrand et al., 2005; Kingsley et al., 2004; Palis, 2014). However, the first wave of hematopoiesis does not produce lymphoid cells or HSCs.
Primitive hematopoiesis is very transient and rapidly replaced by adult-type definitive hematopoiesis. This so-called second hematopoietic wave originates in the yolk sac at E8.25 and is marked by the emergence of erythromyeloid progenitors (Glossary, Box 1) and lymphoid progenitors (Glossary, Box 1) (Böiers et al., 2013; Chen et al., 2011; McGrath et al., 2015; Nakano et al., 2013; Palis et al., 2001, 1999). This second wave of hematopoiesis is considered to be ‘definitive hematopoiesis’ because erythromyeloid progenitors differentiate into blood cells with adult characteristics and functions (Palis, 2014). Although this wave generates erythromyeloid progenitors, there is no evidence that it produces HSCs; before E10.5, no transplantable HSCs are detectable in the yolk sac. The third wave, which commences at E10.5, is marked by the generation of HSCs in the AGM region of the embryo (Ivanovs et al., 2011; Medvinsky and Dzierzak, 1996; Müller et al., 1994). The AGM is an embryonic tissue derived from the mesodermal germ layer that contains the dorsal aorta, the genital ridge (which gives rise to the gonads), and the mesonephros (which gives rise to the kidneys), and that extends from the anterior limbs to the posterior limbs of the E9.5 to E12.5 mouse embryo (Fig. 1A) (de Bruijn et al., 2000; Medvinsky et al., 2011). HSCs are generated from hemogenic endothelium within the AGM as a result of endothelial-to-hematopoietic transitions (Glossary, Box 1) (Bertrand et al., 2010; Boisset et al., 2010; de Bruijn et al., 2002; Jaffredo et al., 1998; Kissa and Herbomel, 2010). AGM-derived HSCs migrate to the fetal liver where they can give rise to all the cell types of the hematopoietic hierarchy (Crisan and Dzierzak, 2016). Interestingly, HSCs are also detectable in two other main blood vessels in the mouse embryo, the vitelline and umbilical arteries, coincident with HSC generation in the AGM (de Bruijn et al., 2000).
Fig. 1.
HSC niche during mouse embryonic development. (A,B) Schematics of mouse embryos at E10.5 (A) and E12.5 (B). Rostral is top, dorsal to the right. (A) At E10.5, HSCs are generated from hemogenic endothelium within the dorsal aorta (A), which is located in the AGM (shown in magnified view), between the mesenchymal (M) tissue that gives rise to the gonads (G) and mesonephros (Me). The magnified image (right) shows a cross-section of the dorsal aorta, in which stromal cells and catecholamines, released from sympathetic nervous system, contribute to the embryonic HSC niche and regulate HSC specification within the AGM. (B) At E12.5, HSCs migrate through the circulation to the fetal liver (FL) via the portal sinus. In the fetal liver, HSCs undergo a dramatic expansion before colonizing the bone marrow. The magnified image shows a cross-section showing NG2+ nestin+ pericytes, which associate with portal vessels, contributing to a niche that promotes HSC expansion.
AGM-derived stromal cells: HSC maintenance and repopulating potential
The observation that HSCs emerge from the hemogenic endothelium within the AGM suggests that this endothelium provides a unique microenvironment that can specify HSCs. High-resolution live imaging in a transgenic zebrafish line [using the mouse Runx1+23 enhancer, which marks definitive hematopoietic stem and progenitor cells (HSPCs; Glossary, Box 1) in the zebrafish] has revealed that single HSPCs directly interact with the surrounding perivascular niche; endothelial cells remodel to surround a single HSPC that is anchored to mesenchymal stromal cells (Tamplin et al., 2015). Distinct AGM-derived stromal cell lines have been shown to have the capacity to support both mouse and human HSC repopulating activity after co-culture of stromal and hematopoietic cells ex vivo (Durand et al., 2006; Matsuoka et al., 2001; Ohneda et al., 1998; Xu et al., 1998).
The adult bone marrow niche has predominantly been studied in vivo in genetic mouse models; by contrast, studies of HSC niches during embryonic development predominantly rely on ex vivo co-cultures of stromal and hematopoietic cells (Matsuoka et al., 2001; Ohneda et al., 1998; Xu et al., 1998). The first well-characterized stromal clone, derived from the AGM region of the E10.5 mouse embryo, was AGM-S3 (Xu et al., 1998). Co-culture studies have revealed that AGM-S3 can maintain HSPCs from mouse bone marrow and human cord blood in vitro (Matsuoka et al., 2001; Xu et al., 1998). Around the same time, two AGM-derived endothelial cell lines, DAS-104-8 and DAS-104-4, were found to support HSC differentiation and maintenance in the fetal liver, respectively (Ohneda et al., 1998). Distinct AGM sub-regions exhibit different HSC repopulating activity. Specifically, HSC activity is first detected in the dorsal aorta mesenchyme (AM) at E11 and later in the urogenital region (UR) at E12 (de Bruijn et al., 2000; Oostendorp et al., 2002a). Further studies of their localization have revealed that HSCs distribute almost exclusively to the ventral wall of the dorsal aorta and are enriched in the middle region of this vessel at E11 (Mascarenhas et al., 2009; Taoudi and Medvinsky, 2007), suggesting that specialized sub-microenvironments exist within the AGM region. Explant co-cultures of AGM with surrounding ventral and dorsal tissue have also revealed that ventral tissue from E10 mouse embryo increases AGM HSC repopulating activity, whereas dorsal tissue decreases it (Peeters et al., 2009).
To compare the microenvironment provided by different regions, the Dzierzak group generated hundreds of stromal cell lines from sub-dissected tissues of the E11 mouse AGM, AM and UR, and also from fetal liver and gastrointestinal tissue (Oostendorp et al., 2002a). This comparison revealed that these different anatomical sub-regions have a distinctive capacity to support HSPCs (Oostendorp et al., 2002a). Subsequently, the transcriptional profiles of two highly HSC-supportive stromal cell lines (EL08-1D2 and UG26-1B6) were compared with those of four less or non-supportive stromal lines (UG15-1B7, AM20-1B4, EL28-1B3 and AM30-3F4). This comparison revealed that the HSC-supportive clones more frequently expressed a range of growth factors at a higher level, including the putative secreted factors fibroblast growth factor 7 and insulin-like growth factor-binding proteins 3 and 4 (Abkowitz et al., 2002; Oostendorp et al., 2002b, 2005). However, no specific factor has been identified that can distinguish between supportive and non-supportive stromal cell lines. More recently, co-culture studies have identified that stem cell factor (SCF; also known as KITL), but not interleukin 3 (IL3), is a major HSC maturation factor during early embryonic development from E9 to E10 (Rybtsov et al., 2014). This finding is consistent with the HSC deficiency phenotype reported for the Scf knockout mouse (Barker, 1994; Ding et al., 2012).
Thus far, co-culture assays have been the main approach used to assess the supportive role of stromal cells for HSC emergence (Ohneda et al., 1998; Oostendorp et al., 2002a). However, considering that in vitro co-cultures are by their very nature imperfect substitutes for examining in vivo processes, it is important to develop alternative in vivo approaches (such as genetically engineered mice) to determine whether a candidate cell and/or factor functions in inducing the generation of HSCs in the AGM microenvironment.
Sympathetic nervous system: a key AGM niche component
Nerves from the sympathetic nervous system (SNS) have been functionally implicated in the earliest stage of HSC generation during development (Fitch et al., 2012) (Fig. 1A). Fitch et al. discovered that the deletion of Gata3 significantly impaired HSC generation in the mouse AGM. Surprisingly, this effect on HSC emergence was independent of a cell-intrinsic role for Gata3, but secondary to its role in regulating the expression of tyrosine hydroxylase (Th), an enzyme required for the biosynthesis of catecholamines (Glossary, Box 1) (Fitch et al., 2012). The external supply of α- and β-adrenergic agonists can rescue the HSC generation defect in the Gata3-deficient AGM (Fitch et al., 2012). However, only the β2-adrenergic receptor was highly expressed in nascent HSCs in the AGM, suggesting that catecholamines might act directly on HSCs through this receptor (Fitch et al., 2012). In addition, this HSC defect was recapitulated in Th knockout mice, in which catecholamine production was deficient (Fitch et al., 2012). This study revealed that the SNS serves as a key component of the AGM HSC niche, and highlights that HSC generation does not occur in isolation in the AGM, but is exposed to signals from other organs that are developing at the same time.
Placenta: an extra-embryonic niche for HSC development
In 2005, two research groups independently identified the placenta as an extra-embryonic niche for HSC development and expansion (Gekas et al., 2005; Li, 2005; Ottersbach and Dzierzak, 2005). These studies showed that the emergence of HSCs with repopulating activity in the placenta occurs in parallel with that of the AGM, starting at E10.5 to E11, and that this HSC pool expands until E12.5-E13.5. Analyses of Ncx1 (sodium-calcium exchanger; Slc8a1) knockout mouse embryos, which lack a heartbeat, demonstrated the presence of phenotypic HSCs that express HSC surface markers in the placenta even in the absence of blood circulation (Gekas et al., 2010; Rhodes et al., 2008), indicating that the placenta can generate HSCs de novo. However, transplantation of cultured E10 mouse placenta, at a time point at which it contains no detectable HSCs, did not repopulate the irradiated recipient mice. By contrast, E10 AGM explant culture autonomously generated HSCs with repopulating activity (Robin et al., 2006). Whether the placenta has the capacity to generate de novo HSCs or only supports HSC expansion remains debated and will need further investigation.
The human placenta is also a potent niche that contains hematopoietic progenitors (Robin et al., 2009; Zovein and Iruela-Arispe, 2009), highlighting its potential use for HSC transplantation therapy (in which healthy stem cells are infused into the body of the patient to replace the diseased bone marrow) because the progenitor content of the placenta is large relative to that of the umbilical cord (a more traditional source of stem cells for transplantation) and is easily accessible. Furthermore, stromal cell lines generated from human placenta at different developmental time points have been shown to have pericyte characteristics and the capacity to support human hematopoiesis (Robin et al., 2009). Together, these studies suggest that the placenta is an important extra-embryonic niche for HSCs during development. These HSCs generated at distinct anatomical sites will convene in the fetal liver for rapid expansion before colonizing the bone marrow in adult.
Fetal liver: an important niche for HSC expansion
Following the generation of HSCs in the AGM, the fetal liver becomes the predominant embryonic niche for HSC expansion. HSCs are released into circulation and migrate into the mouse fetal liver at E11, and then undergo a dramatic expansion until E16 (Fig. 1B). Unlike adult bone marrow HSCs, which are mostly quiescent, fetal liver HSCs are highly proliferative (Bowie et al., 2007, 2006; Morrison et al., 1995), leading to a ∼38-fold expansion from E12 to E16 (Ema and Nakauchi, 2000). A comparison of the quiescent adult bone marrow HSC niche (Arai and Suda, 2007; Wilson et al., 2008) with the highly proliferative fetal liver HSC niche might provide mechanistic insights into how to achieve HSC expansion in vitro, and reveal the key factors involved.
Stromal cells constitute the primary component of the fetal liver niche for hematopoietic cells based on findings obtained from their co-culture with HSCs in vitro. To dissect the microenvironment of the fetal liver HSC niche, >200 immortalized stromal cell lines derived from mouse fetal liver were generated and found to exhibit extensive heterogeneity in their ability to support hematopoiesis in vitro (Moore et al., 1997b; Wineman et al., 1996). A particular single stromal clone, AFT024, was capable of maintaining human and mouse hematopoietic repopulating activity after co-culturing in vitro (Moore et al., 1997a; Nolta et al., 2002). In addition, stromal cells generated from either primary cultures or stromal lines showed features characteristic of an epithelial-to-mesenchymal transition (EMT), and their hematopoietic supportive capacity was abrogated upon hepatocytic maturation (Chagraoui et al., 2003). A recent study has revealed an important role for activating transcription factor 4 (ATF4, a basic region-leucine zipper transcription factor) in the fetal liver microenvironment. Stroma cells from Atf4−/− fetal liver exhibited abrogated capacity to support HSC repopulating activity ex vivo. ATF4 contributes to HSC maintenance by controlling the transcription of cytokines, such as angiopoietin-like 3 (Angptl3, a known cytokine that supports HSC activity in the fetal liver) (Zhao et al., 2015).
Although immortalized stromal cell lines can transiently maintain HSCs in vitro, they do not have the capacity to markedly expand HSCs in culture. SCF+ DLK+ fetal liver hepatic progenitors have been identified as an important stromal cell population that supports HSC expansion in the fetal liver (Chou et al., 2013; Chou and Lodish, 2010; Zhang and Lodish, 2004). These cells were reported to express growth factors important for supporting HSC activity, including Angptl3, insulin-like growth factor 2 (IGF2), SCF and thrombopoietin (TPO; THPO). Mouse HSPCs expand more than 20-fold upon being co-cultured with SCF+ DLK+ cells in vitro for 2-3 weeks. Together, these findings suggest that stromal cells play an important, supportive role in the fetal liver niche, though their contributions in vivo require further investigation.
To characterize the endogenous fetal liver niche, our group has evaluated the presence of perivascular nestin-GFP+ cells in the mouse fetal liver. Rare nestin-GFP+ cells, marked by NG2 (CSPG4) expression, exist in the developing liver in association with portal vessels (Khan et al., 2016) (Fig. 1B). The depletion of NG2+ cells using the NG2-Cre/iDTA genetic mouse model, in which diphtheria toxin A (DTA) is selectively expressed in NG2+ cells, significantly reduced HSCs in the E14.5 fetal liver. A comparison of the transcriptome of bone marrow nestin-GFP+ cells with fetal liver nestin-GFP+ cells revealed they share similar levels of expression of all the major factors known to influence niche activity. However, fetal liver nestin-GFP+ cells, as expected, differentially expressed cell cycle genes and were highly proliferative relative to nestin-GFP+ cells from adult bone marrow. In addition, between E12 and E14.4, the number of niche cells expanded at the same scale as HSCs and the vasculature according to fractal geometries, highlighting the notion that during the fetal liver stage, expansion of the vascular tree, rather than a change in the expression profile of niche cells, might drive HSC expansion. Together, these studies revealed an important role of fetal liver niche to support rapid HSC expansion before transiting into adult bone marrow.
Perinatal transition: fetal spleen and bone marrow
Following significant expansion in the fetal liver, HSCs migrate to the spleen and later to the bone marrow, thus making fetal spleen and bone marrow the main hematopoietic niche sites during the early postnatal period. Active seeding of HSCs begins in the mouse spleen at E15.5; HSC activity increases daily in the spleen from E15.5 to E17.5, whereas it decreases in the fetal liver after E15.5 (Christensen et al., 2004; Morita et al., 2011). HSC-repopulating activity can be first detected in the bone marrow at E17.5 (Christensen et al., 2004). Once HSCs seed in the bone marrow, their frequency and number are higher in the bone marrow than in the fetal spleen, although the repopulating activity of spleen HSCs, on average, is similar to that of bone marrow HSCs (Ikuta and Weissman, 1992). HSC activity is still detectable in the spleen until a few weeks after birth, but the commitment is considered to be limited to erythropoiesis in adult mice under steady-state conditions (Wolber et al., 2002).
To test the capacity of the fetal spleen environment to support HSC activity, an organ culture system that used fetal liver or fetal spleen was developed (Bertrand et al., 2006). After 4 days of in vitro culture, cells from fetal liver, but not from fetal spleen, could reconstitute the recipient mice, suggesting that although the fetal spleen is colonized by HSCs, it has a more limited capacity for HSC maintenance. Stromal cell lines derived from spleen are capable of driving myeloid differentiation, resulting in large numbers of macrophages, but are incapable of supporting HSC repopulating activity (Bertrand et al., 2006). In addition, removal of the spleen from neonatal mouse pups did not reduce the number of HSCs in the bone marrow, indicating that bone marrow-destined HSCs do not have to migrate through the fetal spleen.
The bone marrow is colonized by HSCs, beginning late in fetal development (Christensen et al., 2004) and also during the first week of life after the profound remodeling of portal vessels in the fetal liver (Khan et al., 2016). HSCs proliferate markedly during the first 3 weeks in the neonatal bone marrow and then become quiescent (Bowie et al., 2006). This rapid expansion may correspond to the development of bone marrow vasculature and definitive niches. HSC emergence in the bone marrow occurs coincidently with marrow vascularization and ossification, around E16.5 (Coşkun et al., 2014) Fetal-derived bone marrow cells from osterix (Sp7) null (Osx−/−) mice, which can form normal vasculature but lack ossification, failed to home to and to engraft transplanted recipients (Coşkun et al., 2014), highlighting the important role of the fetal bone marrow niche in regulating HSC activity during the perinatal stage.
Bone marrow: the primary niche for adult HSC maintenance
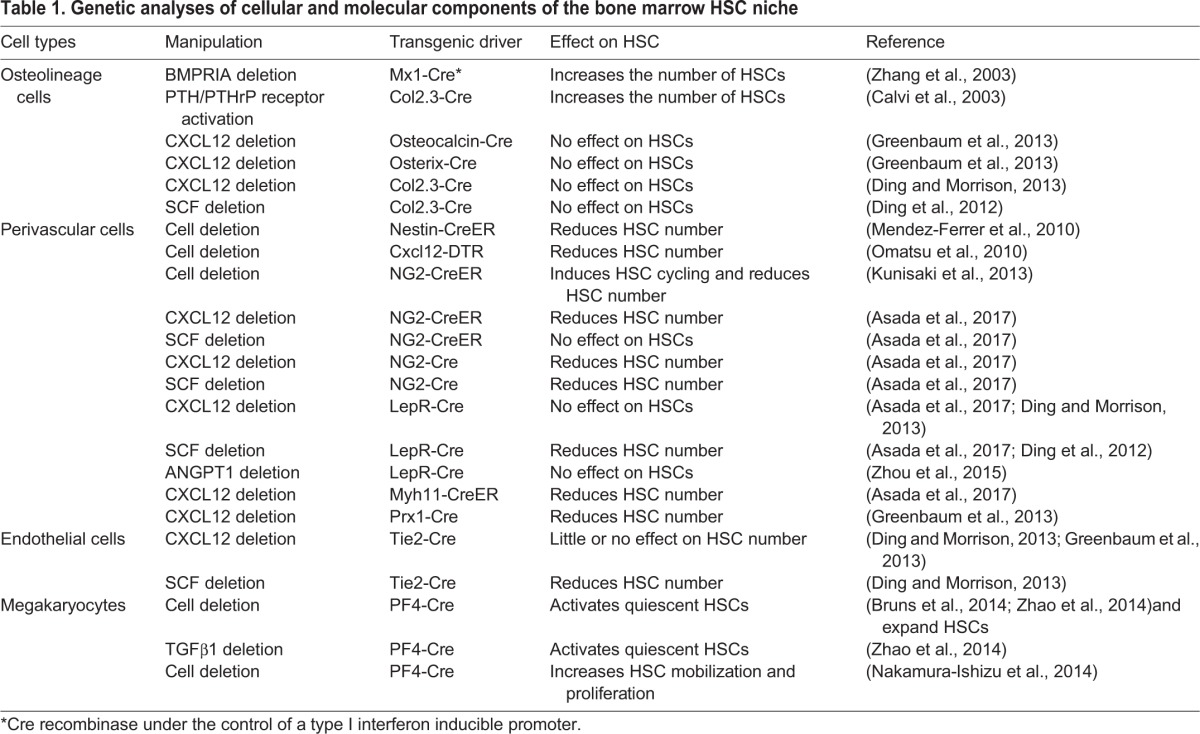
Considerable efforts have been devoted to uncovering the molecular and cellular components of this niche and it has become clear that the bone marrow niche is composed of distinct cell types with different regulatory functions (Birbrair and Frenette, 2016) (Fig. 2, Table 1). In this section, we review our current knowledge of its components.
Fig. 2.
HSC niche players in the adult bone marrow. Schematic of the adult bone marrow, showing various cell types that contribute to the HSC bone marrow niche. Sinusoid endothelial cells regulate HSC maintenance by producing SCF and Jag1. Peri-arteriolar NG2- and Nes-GFP-expressing stromal cells (dark red) secrete CXCL12, which is essential for HSC maintenance. CXCL12-abundant reticular (CAR) cells that co-express LepR and Nes-GFP (green), mainly locate around sinusoids. CXCL12 secreted from these cells controls the mobilizing pool of HSCs. CXCL12 secreted from arteriole-associated stromal cells controls HSC quiescence. SCF secreted from peri-sinusoidal stromal cells (CAR, LepR+, Nes-GFP+ cell) contributes to maintaining HSCs. Non- myelinating Schwann cells activate latent TGFβ into its active form, which is important for HSC maintenance. Mature hematopoietic cells also participate in the orchestration of HSC function; CD169+ bone marrow macrophages promote HSC retention by increasing CXCL12 production by Nes-GFP+ stromal cells and megakaryocytes maintain HSC quiescence via CXCL4, TGFβ and thrombopoietin (TPO).
Table 1.
Genetic analyses of cellular and molecular components of the bone marrow HSC niche
Role of the osteolineage
Given that the predominant site for HSC maintenance in adulthood is the bone marrow, it has been assumed that HSC maintenance requires input from osteolineage cells. In vitro co-culture assays with osteoblast cells and hematopoietic progenitors have revealed that osteoblasts can support hematopoietic progenitor cells by producing a wide array of supportive growth factors, including G-CSF (granulocyte-colony stimulating factor; CSF3) (Taichman et al., 2000). Subsequently, two research groups simultaneously discovered that mutant mice that exhibit an increased number of osteoblasts also have increased HSC numbers in the bone marrow. In one mutant, the BMP receptor type IA was conditionally inactivated and in the other, the PTH/PTHrP receptors (PTH1R) were constitutively activated, with both genetic modifications targeted to osteoblastic cells (Calvi et al., 2003; Zhang et al., 2003). This finding provided the first in vivo evidence that osteoblasts might function within the bone marrow to regulate the number and function of HSCs (Calvi et al., 2003; Zhang et al., 2003).
However, subsequent studies have raised questions as to whether osteoblasts play a direct role in regulating HSC function. This is because the depletion of osteoblasts using genetic or chemical approaches has been reported to not significantly affect HSC numbers (Kiel et al., 2007; Visnjic et al., 2004; Zhu et al., 2007). These findings were made in biglycan-deficient mice, which have multiple metabolic defects in their bone marrow stromal cells (Kiel et al., 2007), and in transgenic mice that express herpesvirus thymidine kinase under the Col1a1 promoter treated with ganciclovir (GCV), in which osteoblast lineage cells are abrogated (Visnjic et al., 2004; Zhu et al., 2007). The conditional deletion of key cytokines, CXCL12 or SCF, from osteoblasts also did not result in significant alterations of HSC numbers in bone marrow (Ding and Morrison, 2013; Ding et al., 2012). These discrepancies regarding the osteoblastic niche could be due to the lack of specificity of the genetic promoters that mark subsets of mesenchymal lineage cells. One study suggested that CXCL12 derived from osteoblasts contributed to the development of common lymphoid progenitors (CLPs) (Ding and Morrison, 2013), whereas a recent analysis found that CXCL12 derived from osteoblasts plays no role in the support of CLPs (Greenbaum et al., 2013). Both CLPs and multipotent progenitors (MPPs) are maintained by IL7-producing stromal cells, which appear to overlap with stromal cells expressing high levels of CXCL12 (also called CXCL12-abundant reticular cells or CAR cells) suggesting that some putative HSC niches might be shared with lineage-committed progenitors (Cordeiro Gomes et al., 2016). Whether HSC niches are shared with committed progenitors will require a direct evaluation of their localization by real-time imaging.
Role of perivascular cells
Local neurotransmitters, delivered by SNS perivascular nerves tightly associated with the vasculature, regulate HSC egress from the bone marrow (Katayama et al., 2006; Méndez-Ferrer et al., 2008). This discovery led to a search for the stromal cells that are targeted by SNS nerves, leading to the identification of nestin-GFP+ cells that express very high levels of key factors known to be involved in HSC maintenance, such as SCF and CXCL12 (Méndez-Ferrer et al., 2010). Nestin-GFP+ putative niche cells physically associate with HSCs, and are enriched in mesenchymal stem cell (MSC) activity as determined by CFU-F and mesensphere formation (Glossary, Box 1), raising the possibility that the two stem cell populations of the bone marrow physically associate with each other to form a niche. Further studies have shown, using the surface markers αV integrin (CD51; ITGAV) and PDGFRα – which mark most nestin-GFP+ cells in the bone marrow – that MSC activity segregates with the expression of HSC niche factors (Pinho et al., 2013). This supports the idea that the niche activity (which supports HSC maintenance and expansion) and MSC activity might come from the same stromal cell subset but more specific markers are needed to demonstrate that this is indeed the case. In human bone marrow, stromal progenitors, marked by high expression of MCAM (melanoma-associated cell adhesion molecule; also known as CD146), were identified as cells that can organize and transfer hematopoietic microenvironment in vivo upon transplantation (Sacchetti et al., 2007).
Further studies clearly show that various perivascular cells, including CAR cells (Omatsu et al., 2010; Pinho et al., 2013), LepR-cre-marked stromal cells (Kunisaki et al., 2013) and nestin-GFP+ cells (Asada et al., 2017), highly overlap in the bone marrow (90% of LepR-Cre marked stromal cells are nestin-GFP+; likewise, the vast majority of nestin-GFP+ cells would be CAR cells). The role of perivascular cells in HSC maintenance is also supported by their cell-specific depletion, mediated by diphtheria toxin, as shown in Nes-CreERT2; iDTR (cre-inducible diphtheria toxin receptor, DTR) mice, in which nestin+ cells are depleted upon diphtheria toxin treatment (Méndez-Ferrer et al., 2010), and in CXCL12-DTR-GFP mice, in which CAR cells are depleted (Omatsu et al., 2010). In vivo depletion of nestin+ cells or CAR cells significantly reduced HSC content in the bone marrow (Méndez-Ferrer et al., 2010; Omatsu et al., 2010). By conditionally inactivating genes in LepR-Cre cells, the cell-specific deletion of Scf, but not of Cxcl12, has been found to severely reduce HSC numbers (Asada et al., 2017; Ding and Morrison, 2013; Ding et al., 2012).
Whole-mount three-dimensional imaging of the bone marrow has also revealed that two major subsets of nestin-GFP+ cells exist, one that is abundant and broadly distributed and that closely matches CAR cells or LepR+ cells, and one that is less abundant (∼10%) and that expresses the pericyte marker NG2 (CSPG4) associated with arterioles (Kunisaki et al., 2013). Imaging studies have shown that quiescent HSCs are enriched close to NG2+ arteriolar perivascular stromal cells, and that depletion of NG2+ cells activates HSC cycling and re-distributes this HSC subset away from arterioles (Kunisaki et al., 2013). The selective deletion of Cxcl12 from arteriolar NG2+ cells, but not from sinusoidal LepR+ cells, reduced HSC numbers in the bone marrow (Asada et al., 2017). By contrast, the deletion of Scf in LepR+ cells, but not in NG2+ cells, reduced HSC numbers in the bone marrow (Asada et al., 2017). Together, these studies suggest that cytokines produced by the different perivascular lineages of the HSC niche make distinct contributions to HSC maintenance.
Role of endothelial cells
Endothelial cells form the inner lining of all blood vessels and help supply nutrients and oxygen to different organs throughout the body, including the bone marrow. HSCs, like most hematopoietic cells, reside close to bone marrow sinusoidal endothelial cells (Glossary, Box 1) (Kiel et al., 2005; Kunisaki et al., 2013). The role of endothelial cells in the bone marrow was first studied in the hematopoietic recovery model after myeloablation (Hooper et al., 2009). The specific deletion of mouse Vegfr2 (Kdr) in endothelial cells prevented the recovery of damaged sinusoidal endothelial cells [VE-cadherin (CDH5)+ VEGFR2+ VEGFR3 (FLT4)+ Sca-1 (Ly6a)−] and also the ensuing hematopoietic recovery in mice after non-lethal irradiation. A blockade of VEGFR2 signaling with antibodies also significantly impaired the engraftment and reconstitution of transplanted bone marrow cells in the lethally irradiated mice (Hooper et al., 2009). Further studies showed that repopulating HSPCs (CD34− FLT3− Kit+ Lineage− Sca-1+) were expanded by Akt-activated endothelial cells in vitro via cell-cell contact (Butler et al., 2010). Interestingly, the selective activation of Akt in the endothelial cells of adult mice increased the number of functional HSCs in both spleen and bone marrow (Kobayashi et al., 2010).
The expression of Notch ligands by endothelial cells also promotes HSC proliferation and prevents HSC exhaustion in vitro, whereas shRNA-mediated downregulation of the angiocrine factors of endothelial cells reduced the repopulation of HSCs in vivo (Kobayashi et al., 2010). The specific deletion of the Notch ligand Jag1 from endothelial cells using VE-cadherin-Cre mice resulted in lower HSC numbers in steady state and reduced hematopoietic recovery after sublethal irradiation (Poulos et al., 2013). Deletion of Scf from endothelial cells using Tie2-Cre mice also led to a dramatic reduction in HSC numbers in the bone marrow and spleen (Ding et al., 2012). Notably, the role of endothelial cell-derived SCF postnatally is unclear because HSC numbers in the livers of neonatal (P0) mice were significantly reduced in mice lacking SCF in their endothelial cells (Ding et al., 2012). A specialized endothelial cell subset, called type H cells, has been identified near the bone growth plate and endosteum of young mice where they regulate osteogenesis in a Notch-dependent manner (Kusumbe et al., 2014; Ramasamy et al., 2014). Interestingly, Notch signaling in endothelial cells also expanded HSCs in vivo, and the reactivation of Notch signaling in endothelial cells rescued the reduced niche-forming vasculature in aged mice, but did not rescue the phenotype of aging HSCs (Kusumbe et al., 2016). The permeability of different endothelial cells has been suggested to regulate the quiescence and proliferation of HSCs, wherein less permeable arterial endothelial cells maintain HSCs in a low reactive oxygen species (ROS) quiescent state whereas the more permeable, sinusoidal endothelial cells, might activate HSCs as a result of plasma leakage (Itkin et al., 2016). The role of individual endothelial cell types in maintaining HSCs in the bone marrow niche remains poorly understood, and novel genetic mouse models are needed to provide new insights into the role of endothelial cell subtypes in this niche.
Other specific niches
The progeny of HSCs can also regulate HSCs in a feedback loop. Three-dimensional imaging of HSC localization in the mouse bone marrow has revealed an unexpected relationship between a subset of HSCs and megakaryocytes (Mks). Mks appear to contribute significantly to the maintenance of HSC quiescence because their depletion leads to the proliferation and expansion of HSCs (Bruns et al., 2014; Nakamura-Ishizu et al., 2015; Zhao et al., 2014). Mk-mediated HSC quiescence could occur via several mechanisms, including the secretion of CXCL4 (PF4) (Bruns et al., 2014), TGFβ1 (Zhao et al., 2014) or thrombopoietin (Nakamura-Ishizu et al., 2014). Other studies show that non-myelinating GFAP+ Schwann cells can also regulate HSC quiescence by converting the latent form of TGFβ into an active form (Yamazaki et al., 2011). These findings highlight that two distinct areas in the bone marrow niche (near Mks or GPAP+ nerves, which are associated with arterioles) promote HSC quiescence. Defining whether these areas harbor specific HSC subsets will be of importance in the future.
Niche-regulating cells
SNS nerves regulate G-CSF-induced HSC mobilization from the bone marrow. When adrenergic neurotransmission is ablated, pharmacologically or genetically, the HSC migratory response to G-CSF stimulation is prevented, owing to the loss of CXCL12 downregulation (Katayama et al., 2006). The cyclical circadian release of HSCs into the bloodstream under hemostasis is also orchestrated through the rhythmic release of adrenergic signals from sympathetic nerves in the bone marrow, which tightly controls CXCL12 expression from stromal cells (Méndez-Ferrer et al., 2008). Further studies have shown that chemotherapy-induced bone marrow nerve injury in mice impairs hematopoietic regeneration (Lucas et al., 2013), highlighting the potential use of neuroprotective drugs to protect hematopoietic niches from chemotherapeutic injury. An additional link between brain and bone marrow is also underscored by the role of the type-1 muscarinic receptor (Chrm1) in the hypothalamus, which regulates G-CSF-mediated HSC mobilization from bone marrow via a glucocorticoid signaling relay (Pierce et al., 2017). Overall, these studies reveal that the nervous system is a key regulator of the HSC bone marrow niche.
Myeloid and lymphoid HSC progeny have also been shown to play an important role in the HSC niche. Among these, macrophages are involved in regulating G-CSF-induced HSC mobilization. In vivo depletion of macrophages, determined either in macrophage Fas-induced apoptosis transgenic mice or by administration of clodronate-loaded liposomes to wild-type mice, led to the mobilization of HSCs into the blood (Winkler et al., 2010). This was confirmed independently by studies revealing that CD169 (SIGLEC1)+ bone marrow macrophages promote the retention of HSCs in the bone marrow by secreting a factor that increases CXCL12 production specifically by nestin+ MSCs (Chow et al., 2011). Recent studies have suggested that this factor might be oncostatin M (Albiero et al., 2015). Other studies have shown that G-CSF-receptor signaling in macrophages plays an important role in eliciting HSC mobilization (Christopher et al., 2011). Thus, these studies indicate that macrophages and the SNS exert parallel and antagonistic functions in regulating the HSC niche.
Neutrophils are the most abundant myeloid leukocytes and have a short lifespan (12 h) in the circulation. Neutrophil homeostasis is orchestrated through a balance of neutrophil production and release from bone marrow into blood, and migration back to bone marrow for elimination (Christopher and Link, 2007). Neutrophil clearance in the bone marrow has been shown to trigger signals that modulate the HSC bone marrow niche, leading to the rhythmic egress of hematopoietic progenitors into the circulation (Casanova-Acebes et al., 2013). Specifically, elevated levels of circulating neutrophils promote the release of hematopoietic progenitors into the blood; by contrast, neutrophil depletion promotes their retention in the bone marrow. Thus, this study indicates that neutrophils might act as a HSC niche-regulating cell.
In vivo imaging of mouse bone marrow demonstrated the colocalization of transplanted HSPCs with regulatory T cells (Treg) in the endosteal area of the bone marrow (Fujisaki et al., 2011). Moreover, transplanted donor-derived HSCs can persist in non-irradiated recipient mice for 30 days without immunosuppression. However, depletion of Treg using FoxP3-GFP; iDTR mice abrogated this colocalization, leading to a marked reduction in the number of donor HSCs surviving in the bone marrow after allo-transplantation. Treg might thus confer an immune privilege site in the bone marrow.
Overall, these studies suggest the contribution of the SNS, macrophages, neutrophils and Treg in regulating the HSC niche. HSCs traffic between niches from developmental stages to adulthood and enormous progress has been made in clarifying the complexity behind the HSC niche by evaluating the contribution of niche cells and niche-regulating cells to HSC development and maintenance. These common principles could guide the development of new approaches to treat hematological diseases and should foster studies on altered niche in blood disorders.
The HSC niche during aging and hematologic malignancies
Clinically, the aging of the hematopoietic system is associated with impaired immune functions, increased risk of infection, myeloproliferative diseases and anemia (Geiger et al., 2013; Rossi et al., 2005). The aging of HSCs partially contributes to the impairments of an aged hematopoietic system (Fig. 3). Aged HSCs are more proliferative and their numbers increase in the aged bone marrow (de Haan et al., 1997; Dykstra et al., 2011; Morrison et al., 1996; Rossi et al., 2005). However, their capacity to migrate to the bone marrow and their regenerative potential are both reduced compared with young HSCs, as measured by transplantation assays (Dykstra et al., 2011; Kamminga et al., 2005; Morrison et al., 1996). Additionally, the differentiation of aged HSCs is biased toward the myeloid lineage; aged HSCs produce more myeloid progenitor cells and fewer lymphoid progenitor cells relative to younger HSCs (Dykstra et al., 2011; Sudo et al., 2000).
Fig. 3.
The aging HSC niche. Schematic of the aging HSC niche and its associated alterations. The number of phenotypic HSCs (e.g. identified by fluorescence-activated cell sorting analysis) in the bone marrow increases with aging. Aged HSCs exhibit increased proliferation, reduced homing and regenerative capacity, and myeloid-biased differentiation. The potential effects of HSC aging on the bone marrow include various abnormalities, such as altered vasculature, increased adipogenesis, reduced osteogenesis, altered secretion of extrinsic factors from niche cells (increased secretion of CCL5 and decreased OPN levels), and reduced adhesion between HSCs and stroma cells.
Although HSC aging is primarily attributed to cell-intrinsic mechanisms, including differential transcriptional and epigenetic profiles, altered ROS levels, and DNA damage (Beerman and Rossi, 2014; Flach et al., 2014), recent data have highlighted an important role for extrinsic regulation by the surrounding HSC niche (Nakamura-Ishizu and Suda, 2014). Aged MSCs are reported to show skewed differentiation toward adipogenesis at the expense of osteogenesis (Fig. 3) (Bellantuono et al., 2009; Singh et al., 2016). Adipocytes are increasingly present in the bone marrow upon aging, and their presence might negatively regulate hematopoiesis and B lymphopoiesis (Naveiras et al., 2009). Extrinsic factors that are secreted by niche cells, such as the inflammatory cytokine CC-chemokine ligand 5 (CCL5), might contribute to the age-associated myeloid bias of HSCs (Ergen et al., 2012). The aged bone marrow also features alterations to the vasculature, including decreased type H endothelial cells, which connect to arterioles (Kusumbe et al., 2016). These endothelial cells are regulated by Notch-mediated signals; although Notch signaling activation improved the vascular defects of old mice, it did not boost the functionality of aged HSCs (Kusumbe et al., 2016). Additionally, the expression of osteopontin (OPN; SPP1), a known regulator of HSCs, is reportedly reduced in the aged stroma (Fig. 3) (Guidi et al., 2017). When old HSCs were treated with protease-cleaved OPN, their aging was attenuated in transplantation settings (Guidi et al., 2017). As the aging of the bone marrow microenvironment is under intensive investigation, more knowledge should soon emerge on the interplay between the intrinsic and extrinsic mechanisms involved in HSC aging.
Alterations to the microenvironment upon aging might also contribute to age-associated diseases. Among these are hematologic malignancies, in which an altered microenvironment has been suggested to initiate or to be co-opted by the malignancy. Loss of the retinoblastoma protein (RB; RB1) or the retinoic acid receptor gamma (RARγ) in mice alters the bone marrow microenvironment and is sufficient to induce myeloproliferative syndrome (Walkley et al., 2007a,b). In vivo deletion of the microRNA-processing gene Dicer1 specifically in murine mesenchymal stem and progenitor cells (MSPCs) (using the constitutive osterix promoter) led to acute myeloid leukemia (AML), but not when the gene was deleted in mature osteoblasts (via the osteocalcin promoter) (Raaijmakers et al., 2010). In addition, the expression of a stabilized β-catenin in mouse osteolineage cells, driven by the constitutive Col1a promoter, resulted in AML as a result of increased Notch signaling (Kode et al., 2014). Interestingly, the specific activation of the PTH (parathyroid hormone) receptor in mouse osteoblasts inhibited BCR-ABL1-induced chronic myelogenous leukemia (CML)-like myeloproliferation but enhanced MLL-AF9-driven AML (Glossary, Box 1) (Krause et al., 2013), suggesting that distinct signals from the microenvironment might differentially regulate malignant transformation.
By contrast, recent studies in murine AML models have suggested that signals from malignant cells can further remodel the bone marrow niche to create a cancer-supportive environment that advances disease progression. MLL-AF9-driven murine AML cells disrupt SNS nerves, causing the proliferation of nestin+ MSPCs that are primed for osteoblastic differentiation and a reduction in HSC-maintaining NG2+ periarteriolar niche cells (Hanoun et al., 2014). Blockade of the β2-, but not of the β3-adrenergic receptor enhanced AML development, and treatment with a β2-adrenergic receptor agonist delayed AML progression (Hanoun et al., 2014). By contrast, in a murine myeloproliferative neoplasm (MPN) model generated by expression of a mutated Janus kinase 2 (Jak2V617F), neuropathy was reported to reduce MSC numbers (Arranz et al., 2014). When MPN mice were treated with a β3-adrenergic agonist, this restored the sympathetic regulation of MSCs and reduced the number of leukemia stem cells (LSCs), thereby reducing MPN progression (Arranz et al., 2014). Interestingly, in a chronic model of BCR-ABL CML, MPN expanded the osteoblastic lineage cells in the bone marrow, which progressively remodeled the endosteal bone marrow niche into a self-reinforcing leukemic niche that supports LSCs, but impairs normal hematopoiesis (Schepers et al., 2013), a finding that is consistent with independent studies of MLL-AF9-driven murine AML (Hanoun et al., 2014). In a model of BCR-ABL CML crisis (which resembles AML), the formation of mature osteoblasts was reduced (Frisch et al., 2012). These studies point to the existence of a differentiation block from osteoprogenitors to mature osteoblasts. Considering the important role of the bone marrow microenvironment in regulating hematological diseases, targeting the HSC niche within the bone marrow could provide an important future therapeutic strategy for hematologic malignancies. For example, therapies that preserve innervation and protect healthy niche cells might prevent the formation of a cancer-promoting niche and maintain healthy HSC numbers.
Future perspectives
Although major advances have been made in identifying the components of the adult bone marrow HSC niche, it appears clear that Schofield's original concept of the niche (Schofield, 1978) – that it is both necessary and sufficient for HSC specification and maintenance – remains to be fully validated experimentally. For example, Schofield suggested that a stem cell niche could reprogram a CFU-S progenitor into a stem cell, a notion that has been recently validated in the hair follicle (Rompolas et al., 2013) and intestine (Takeda et al., 2011; Tian et al., 2011), but not for the hematopoietic system. Although results so far have clearly suggested that mesenchymal cells are required for HSC maintenance, no single niche cell type appears to be sufficient for HSC maintenance. The HSC niche appears to be highly complex and regulated by both local and long-range acting factors that coordinate the intricate tasks of forming blood and titrating its lineages on a daily basis. Recent data suggest that cell-autonomous epigenetic memory might underlie the heterogeneous behavior of HSCs (Yu et al., 2016). This raises the possibility that HSCs could in fact model or specify the type of niche that they need. Future studies using specific genetic tracers will undoubtedly uncover different subtypes of HSCs that might each have their own niche specificities.
Acknowledgements
We apologize to the investigators whose work could not be cited owing to space limitation.
Footnotes
Competing interests
The authors declare no competing or financial interests.
References
- Abkowitz J. L., Catlin S. N., McCallie M. T. and Guttorp P. (2002). Evidence that the number of hematopoietic stem cells per animal is conserved in mammals. Blood 100, 2665-2667. 10.1182/blood-2002-03-0822 [DOI] [PubMed] [Google Scholar]
- Albiero M., Poncina N., Ciciliot S., Cappellari R., Menegazzo L., Ferraro F., Bolego C., Cignarella A., Avogaro A. and Fadini G. P. (2015). Bone marrow macrophages contribute to diabetic stem cell mobilopathy by producing Oncostatin M. Diabetes 64, 2957-2968. 10.2337/db14-1473 [DOI] [PubMed] [Google Scholar]
- Appelbaum F. R. (2007). Hematopoietic-cell transplantation at 50. N. Engl. J. Med. 357, 1472-1475. 10.1056/NEJMp078166 [DOI] [PubMed] [Google Scholar]
- Arai F. and Suda T. (2007). Maintenance of quiescent hematopoietic stem cells in the osteoblastic niche. Ann. N. Y. Acad. Sci. 1106, 41-53. 10.1196/annals.1392.005 [DOI] [PubMed] [Google Scholar]
- Arranz L., Sánchez-Aguilera A., Martín-Pérez D., Isern J., Langa X., Tzankov A., Lundberg P., Muntión S., Tzeng Y.-S., Lai D.-M. et al. (2014). Neuropathy of haematopoietic stem cell niche is essential for myeloproliferative neoplasms. Nature 512, 78-81. 10.1038/nature13383 [DOI] [PubMed] [Google Scholar]
- Asada N., Kunisaki Y., Pierce H., Wang Z., Fernandez N. F., Birbrair A., Ma'ayan A. and Frenette P. S. (2017). Differential cytokine contributions of perivascular haematopoietic stem cell niches. Nat. Cell Biol. 19, 214-223. 10.1038/ncb3475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker J. E. (1994). Sl/Sld hematopoietic progenitors are deficient in situ. Exp. Hematol. 22, 174-177. [PubMed] [Google Scholar]
- Beerman I. and Rossi D. J. (2014). Epigenetic regulation of hematopoietic stem cell aging. Exp. Cell Res. 329, 192-199. 10.1016/j.yexcr.2014.09.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellantuono I., Aldahmash A. and Kassem M. (2009). Aging of marrow stromal (skeletal) stem cells and their contribution to age-related bone loss. Biochim. Biophys. Acta 1792, 364-370. 10.1016/j.bbadis.2009.01.008 [DOI] [PubMed] [Google Scholar]
- Bertrand J. Y., Jalil A., Klaine M., Jung S., Cumano A. and Godin I. (2005). Three pathways to mature macrophages in the early mouse yolk sac. Blood 106, 3004-3011. 10.1182/blood-2005-02-0461 [DOI] [PubMed] [Google Scholar]
- Bertrand J. Y., Desanti G. E., Lo-Man R., Leclerc C., Cumano A. and Golub R. (2006). Fetal spleen stroma drives macrophage commitment. Development 133, 3619-3628. 10.1242/dev.02510 [DOI] [PubMed] [Google Scholar]
- Bertrand J. Y., Chi N. C., Santoso B., Teng S., Stainier D. Y. R. and Traver D. (2010). Haematopoietic stem cells derive directly from aortic endothelium during development. Nature 464, 108-111. 10.1038/nature08738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birbrair A. and Frenette P. S. (2016). Niche heterogeneity in the bone marrow. Ann. N. Y. Acad. Sci. 1370, 82-96. 10.1111/nyas.13016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Böiers C., Carrelha J., Lutteropp M., Luc S., Green J. C. A., Azzoni E., Woll P. S., Mead A. J., Hultquist A., Swiers G. et al. (2013). Lymphomyeloid contribution of an immune-restricted progenitor emerging prior to definitive hematopoietic stem cells. Cell Stem Cell 13, 535-548. 10.1016/j.stem.2013.08.012 [DOI] [PubMed] [Google Scholar]
- Boisset J.-C., van Cappellen W., Andrieu-Soler C., Galjart N., Dzierzak E. and Robin C. (2010). In vivo imaging of haematopoietic cells emerging from the mouse aortic endothelium. Nature 464, 116-120. 10.1038/nature08764 [DOI] [PubMed] [Google Scholar]
- Bowie M. B., McKnight K. D., Kent D. G., McCaffrey L., Hoodless P. A. and Eaves C. J. (2006). Hematopoietic stem cells proliferate until after birth and show a reversible phase-specific engraftment defect. J. Clin. Invest. 116, 2808-2816. 10.1172/JCI28310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowie M. B., Kent D. G., Dykstra B., McKnight K. D., McCaffrey L., Hoodless P. A. and Eaves C. J. (2007). Identification of a new intrinsically timed developmental checkpoint that reprograms key hematopoietic stem cell properties. Proc. Natl. Acad. Sci. USA 104, 5878-5882. 10.1073/pnas.0700460104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruns I., Lucas D., Pinho S., Ahmed J., Lambert M. P., Kunisaki Y., Scheiermann C., Schiff L., Poncz M., Bergman A. et al. (2014). Megakaryocytes regulate hematopoietic stem cell quiescence through CXCL4 secretion. Nat. Med. 20, 1315-1320. 10.1038/nm.3707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler J. M., Nolan D. J., Vertes E. L., Varnum-Finney B., Kobayashi H., Hooper A. T., Seandel M., Shido K., White I. A., Kobayashi M. et al. (2010). Endothelial cells are essential for the self-renewal and repopulation of Notch-dependent hematopoietic stem cells. Cell Stem Cell 6, 251-264. 10.1016/j.stem.2010.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvi L. M., Adams G. B., Weibrecht K. W., Weber J. M., Olson D. P., Knight M. C., Martin R. P., Schipani E., Divieti P., Bringhurst F. R. et al. (2003). Osteoblastic cells regulate the haematopoietic stem cell niche. Nature 425, 841-846. 10.1038/nature02040 [DOI] [PubMed] [Google Scholar]
- Casanova-Acebes M., Pitaval C., Weiss L. A., Nombela-Arrieta C., Chèvre R., A-González N., Kunisaki Y., Zhang D., van Rooijen N., Silberstein L. E. et al. (2013). Rhythmic modulation of the hematopoietic niche through neutrophil clearance. Cell 153, 1025-1035. 10.1016/j.cell.2013.04.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chagraoui J., Lepage-Noll A., Anjo A., Uzan G. and Charbord P. (2003). Fetal liver stroma consists of cells in epithelial-to-mesenchymal transition. Blood 101, 2973-2982. 10.1182/blood-2002-05-1341 [DOI] [PubMed] [Google Scholar]
- Chen M. J., Li Y., De Obaldia M. E., Yang Q., Yzaguirre A. D., Yamada-Inagawa T., Vink C. S., Bhandoola A., Dzierzak E. and Speck N. A. (2011). Erythroid/myeloid progenitors and hematopoietic stem cells originate from distinct populations of endothelial cells. Cell Stem Cell 9, 541-552. 10.1016/j.stem.2011.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou S. and Lodish H. F. (2010). Fetal liver hepatic progenitors are supportive stromal cells for hematopoietic stem cells. Proc. Natl. Acad. Sci. USA 107, 7799-7804. 10.1073/pnas.1003586107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou S., Flygare J. and Lodish H. F. (2013). Fetal hepatic progenitors support long-term expansion of hematopoietic stem cells. Exp. Hematol. 41, 479-490.e474. 10.1016/j.exphem.2013.02.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow A., Lucas D., Hidalgo A., Méndez-Ferrer S., Hashimoto D., Scheiermann C., Battista M., Leboeuf M., Prophete C., van Rooijen N. et al. (2011). Bone marrow CD169+ macrophages promote the retention of hematopoietic stem and progenitor cells in the mesenchymal stem cell niche. J. Exp. Med. 208, 261-271. 10.1084/jem.20101688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen J. L., Wright D. E., Wagers A. J. and Weissman I. L. (2004). Circulation and chemotaxis of fetal hematopoietic stem cells. PLoS Biol. 2, e75 10.1371/journal.pbio.0020075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christopher M. J. and Link D. C. (2007). Regulation of neutrophil homeostasis. Curr. Opin Hematol. 14, 3-8. 10.1097/00062752-200701000-00003 [DOI] [PubMed] [Google Scholar]
- Christopher M. J., Rao M., Liu F., Woloszynek J. R. and Link D. C. (2011). Expression of the G-CSF receptor in monocytic cells is sufficient to mediate hematopoietic progenitor mobilization by G-CSF in mice. J. Exp. Med. 208, 251-260. 10.1084/jem.20101700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordeiro Gomes A., Hara T., Lim V. Y., Herndler-Brandstetter D., Nevius E., Sugiyama T., Tani-Ichi S., Schlenner S., Richie E., Rodewald H.-R. et al. (2016). Hematopoietic stem cell niches produce lineage-instructive signals to control multipotent progenitor differentiation. Immunity 45, 1219-1231. 10.1016/j.immuni.2016.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coşkun S., Chao H., Vasavada H., Heydari K., Gonzales N., Zhou X., de Crombrugghe B. and Hirschi K. K. (2014). Development of the fetal bone marrow niche and regulation of HSC quiescence and homing ability by emerging osteolineage cells. Cell Rep. 9, 581-590. 10.1016/j.celrep.2014.09.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crisan M. and Dzierzak E. (2016). The many faces of hematopoietic stem cell heterogeneity. Development 143, 4571-4581. 10.1242/dev.114231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Bruijn M. F. T. R., Speck N. A., Peeters M. C. and Dzierzak E. (2000). Definitive hematopoietic stem cells first develop within the major arterial regions of the mouse embryo. EMBO J. 19, 2465-2474. 10.1093/emboj/19.11.2465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Bruijn M. F. T. R., Ma X., Robin C., Ottersbach K., Sanchez M.-J. and Dzierzak E. (2002). Hematopoietic stem cells localize to the endothelial cell layer in the midgestation mouse aorta. Immunity 16, 673-683. 10.1016/S1074-7613(02)00313-8 [DOI] [PubMed] [Google Scholar]
- de Haan G., Nijhof W. and Van Zant G. (1997). Mouse strain-dependent changes in frequency and proliferation of hematopoietic stem cells during aging: correlation between lifespan and cycling activity. Blood 89, 1543-1550. [PubMed] [Google Scholar]
- Ding L. and Morrison S. J. (2013). Haematopoietic stem cells and early lymphoid progenitors occupy distinct bone marrow niches. Nature 495, 231-235. 10.1038/nature11885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding L., Saunders T. L., Enikolopov G. and Morrison S. J. (2012). Endothelial and perivascular cells maintain haematopoietic stem cells. Nature 481, 457-462. 10.1038/nature10783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durand C., Robin C. and Dzierzak E. (2006). Mesenchymal lineage potentials of aorta-gonad-mesonephros stromal clones. Haematologica 91, 1172-1179. [PubMed] [Google Scholar]
- Dykstra B., Olthof S., Schreuder J., Ritsema M. and de Haan G. (2011). Clonal analysis reveals multiple functional defects of aged murine hematopoietic stem cells. J. Exp. Med. 208, 2691-2703. 10.1084/jem.20111490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dzierzak E. and Speck N. A. (2008). Of lineage and legacy: the development of mammalian hematopoietic stem cells. Nat. Immunol. 9, 129-136. 10.1038/ni1560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ema H. and Nakauchi H. (2000). Expansion of hematopoietic stem cells in the developing liver of a mouse embryo. Blood 95, 2284-2288. [PubMed] [Google Scholar]
- Ergen A. V., Boles N. C. and Goodell M. A. (2012). Rantes/Ccl5 influences hematopoietic stem cell subtypes and causes myeloid skewing. Blood 119, 2500-2509. 10.1182/blood-2011-11-391730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitch S. R., Kimber G. M., Wilson N. K., Parker A., Mirshekar-Syahkal B., Göttgens B., Medvinsky A., Dzierzak E. and Ottersbach K. (2012). Signaling from the sympathetic nervous system regulates hematopoietic stem cell emergence during embryogenesis. Cell Stem Cell 11, 554-566. 10.1016/j.stem.2012.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flach J., Bakker S. T., Mohrin M., Conroy P. C., Pietras E. M., Reynaud D., Alvarez S., Diolaiti M. E., Ugarte F., Forsberg E. C. et al. (2014). Replication stress is a potent driver of functional decline in ageing haematopoietic stem cells. Nature 512, 198-202. 10.1038/nature13619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frisch B. J., Ashton J. M., Xing L., Becker M. W., Jordan C. T. and Calvi L. M. (2012). Functional inhibition of osteoblastic cells in an in vivo mouse model of myeloid leukemia. Blood 119, 540-550. 10.1182/blood-2011-04-348151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujisaki J., Wu J., Carlson A. L., Silberstein L., Putheti P., Larocca R., Gao W., Saito T. I., Lo Celso C., Tsuyuzaki H. et al. (2011). In vivo imaging of Treg cells providing immune privilege to the haematopoietic stem-cell niche. Nature 474, 216-219. 10.1038/nature10160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiger H., de Haan G. and Florian M. C. (2013). The ageing haematopoietic stem cell compartment. Nat. Rev. Immunol. 13, 376-389. 10.1038/nri3433 [DOI] [PubMed] [Google Scholar]
- Gekas C., Dieterlen-Lièvre F., Orkin S. H. and Mikkola H. K. A. (2005). The placenta is a niche for hematopoietic stem cells. Dev. Cell 8, 365-375. 10.1016/j.devcel.2004.12.016 [DOI] [PubMed] [Google Scholar]
- Gekas C., Rhodes K. E., Van Handel B., Chhabra A., Ueno M. and Mikkola H. K. A. (2010). Hematopoietic stem cell development in the placenta. Int. J. Dev. Biol. 54, 1089-1098. 10.1387/ijdb.103070cg [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenbaum A., Hsu Y.-M. S., Day R. B., Schuettpelz L. G., Christopher M. J., Borgerding J. N., Nagasawa T. and Link D. C. (2013). CXCL12 in early mesenchymal progenitors is required for haematopoietic stem-cell maintenance. Nature 495, 227-230. 10.1038/nature11926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guidi N., Sacma M., Ständker L., Soller K., Marka G., Eiwen K., Weiss J. M., Kirchhoff F., Weil T., Cancelas J. A. et al. (2017). Osteopontin attenuates aging-associated phenotypes of hematopoietic stem cells. EMBO J. 36, 840-853. 10.15252/embj.201694969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanoun M., Zhang D., Mizoguchi T., Pinho S., Pierce H., Kunisaki Y., Lacombe J., Armstrong S. A., Dührsen U. and Frenette P. S. (2014). Acute myelogenous leukemia-induced sympathetic neuropathy promotes malignancy in an altered hematopoietic stem cell niche. Cell Stem Cell 15, 365-375. 10.1016/j.stem.2014.06.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooper A. T., Butler J. M., Nolan D. J., Kranz A., Iida K., Kobayashi M., Kopp H.-G., Shido K., Petit I., Yanger K. et al. (2009). Engraftment and reconstitution of hematopoiesis is dependent on VEGFR2-mediated regeneration of sinusoidal endothelial cells. Cell Stem Cell 4, 263-274. 10.1016/j.stem.2009.01.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikuta K. and Weissman I. L. (1992). Evidence that hematopoietic stem cells express mouse c-kit but do not depend on steel factor for their generation. Proc. Natl. Acad. Sci. USA 89, 1502-1506. 10.1073/pnas.89.4.1502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itkin T., Gur-Cohen S., Spencer J. A., Schajnovitz A., Ramasamy S. K., Kusumbe A. P., Ledergor G., Jung Y., Milo I., Poulos M. G. et al. (2016). Distinct bone marrow blood vessels differentially regulate haematopoiesis. Nature 532, 323-328. 10.1038/nature17624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanovs A., Rybtsov S., Welch L., Anderson R. A., Turner M. L. and Medvinsky A. (2011). Highly potent human hematopoietic stem cells first emerge in the intraembryonic aorta-gonad-mesonephros region. J. Exp. Med. 208, 2417-2427. 10.1084/jem.20111688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffredo T., Gautier R., Eichmann A. and Dieterlen-Lievre F. (1998). Intraaortic hemopoietic cells are derived from endothelial cells during ontogeny. Development 125, 4575-4583. [DOI] [PubMed] [Google Scholar]
- Kamminga L. M., van Os R., Ausema A., Noach E. J. K., Weersing E., Dontje B., Vellenga E. and de Haan G. (2005). Impaired hematopoietic stem cell functioning after serial transplantation and during normal aging. Stem Cells 23, 82-92. 10.1634/stemcells.2004-0066 [DOI] [PubMed] [Google Scholar]
- Katayama Y., Battista M., Kao W.-M., Hidalgo A., Peired A. J., Thomas S. A. and Frenette P. S. (2006). Signals from the sympathetic nervous system regulate hematopoietic stem cell egress from bone marrow. Cell 124, 407-421. 10.1016/j.cell.2005.10.041 [DOI] [PubMed] [Google Scholar]
- Khan J. A., Mendelson A., Kunisaki Y., Birbrair A., Kou Y., Arnal-Estape A., Pinho S., Ciero P., Nakahara F., Ma'ayan A. et al. (2016). Fetal liver hematopoietic stem cell niches associate with portal vessels. Science 351, 176-180. 10.1126/science.aad0084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiel M. J., Yilmaz O. H., Iwashita T., Yilmaz O. H., Terhorst C. and Morrison S. J. (2005). SLAM family receptors distinguish hematopoietic stem and progenitor cells and reveal endothelial niches for stem cells. Cell 121, 1109-1121. 10.1016/j.cell.2005.05.026 [DOI] [PubMed] [Google Scholar]
- Kiel M. J., Radice G. L. and Morrison S. J. (2007). Lack of evidence that hematopoietic stem cells depend on N-cadherin-mediated adhesion to osteoblasts for their maintenance. Cell Stem Cell 1, 204-217. 10.1016/j.stem.2007.06.001 [DOI] [PubMed] [Google Scholar]
- Kingsley P. D., Malik J., Fantauzzo K. A. and Palis J. (2004). Yolk sac-derived primitive erythroblasts enucleate during mammalian embryogenesis. Blood 104, 19-25. 10.1182/blood-2003-12-4162 [DOI] [PubMed] [Google Scholar]
- Kissa K. and Herbomel P. (2010). Blood stem cells emerge from aortic endothelium by a novel type of cell transition. Nature 464, 112-115. 10.1038/nature08761 [DOI] [PubMed] [Google Scholar]
- Kobayashi H., Butler J. M., O'Donnell R., Kobayashi M., Ding B.-S., Bonner B., Chiu V. K., Nolan D. J., Shido K., Benjamin L. et al. (2010). Angiocrine factors from Akt-activated endothelial cells balance self-renewal and differentiation of haematopoietic stem cells. Nat. Cell Biol. 12, 1046-1056. 10.1038/ncb2108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kode A., Manavalan J. S., Mosialou I., Bhagat G., Rathinam C. V., Luo N., Khiabanian H., Lee A., Murty V. V., Friedman R. et al. (2014). Leukaemogenesis induced by an activating beta-catenin mutation in osteoblasts. Nature 506, 240-244. 10.1038/nature12883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause D. S., Fulzele K., Catic A., Sun C. C., Dombkowski D., Hurley M. P., Lezeau S., Attar E., Wu J. Y., Lin H. Y. et al. (2013). Differential regulation of myeloid leukemias by the bone marrow microenvironment. Nat. Med. 19, 1513-1517. 10.1038/nm.3364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunisaki Y., Bruns I., Scheiermann C., Ahmed J., Pinho S., Zhang D., Mizoguchi T., Wei Q., Lucas D., Ito K. et al. (2013). Arteriolar niches maintain haematopoietic stem cell quiescence. Nature 502, 637-643. 10.1038/nature12612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusumbe A. P., Ramasamy S. K. and Adams R. H. (2014). Coupling of angiogenesis and osteogenesis by a specific vessel subtype in bone. Nature 507, 323-328. 10.1038/nature13145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusumbe A. P., Ramasamy S. K., Itkin T., Mäe M. A., Langen U. H., Betsholtz C., Lapidot T. and Adams R. H. (2016). Age-dependent modulation of vascular niches for haematopoietic stem cells. Nature 532, 380-384. 10.1038/nature17638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L. (2005). Finding the hematopoietic stem cell niche in the placenta. Dev. Cell 8, 297-298. 10.1016/j.devcel.2005.02.005 [DOI] [PubMed] [Google Scholar]
- Lucas D., Scheiermann C., Chow A., Kunisaki Y., Bruns I., Barrick C., Tessarollo L. and Frenette P. S. (2013). Chemotherapy-induced bone marrow nerve injury impairs hematopoietic regeneration. Nat. Med. 19, 695-703. 10.1038/nm.3155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mascarenhas M. I., Parker A., Dzierzak E. and Ottersbach K. (2009). Identification of novel regulators of hematopoietic stem cell development through refinement of stem cell localization and expression profiling. Blood 114, 4645-4653. 10.1182/blood-2009-06-230037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuoka S., Tsuji K., Hisakawa H., Xu M., Ebihara Y., Ishii T., Sugiyama D., Manabe A., Tanaka R., Ikeda Y. et al. (2001). Generation of definitive hematopoietic stem cells from murine early yolk sac and paraaortic splanchnopleures by aorta-gonad-mesonephros region-derived stromal cells. Blood 98, 6-12. 10.1182/blood.V98.1.6 [DOI] [PubMed] [Google Scholar]
- McGrath K. E., Frame J. M., Fegan K. H., Bowen J. R., Conway S. J., Catherman S. C., Kingsley P. D., Koniski A. D. and Palis J. (2015). Distinct sources of hematopoietic progenitors emerge before HSCs and provide functional blood cells in the mammalian embryo. Cell Rep. 11, 1892-1904. 10.1016/j.celrep.2015.05.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medvinsky A. and Dzierzak E. (1996). Definitive hematopoiesis is autonomously initiated by the AGM region. Cell 86, 897-906. 10.1016/S0092-8674(00)80165-8 [DOI] [PubMed] [Google Scholar]
- Medvinsky A., Rybtsov S. and Taoudi S. (2011). Embryonic origin of the adult hematopoietic system: advances and questions. Development 138, 1017-1031. 10.1242/dev.040998 [DOI] [PubMed] [Google Scholar]
- Méndez-Ferrer S., Lucas D., Battista M. and Frenette P. S. (2008). Haematopoietic stem cell release is regulated by circadian oscillations. Nature 452, 442-447. 10.1038/nature06685 [DOI] [PubMed] [Google Scholar]
- Méndez-Ferrer S., Michurina T. V., Ferraro F., Mazloom A. R., Macarthur B. D., Lira S. A., Scadden D. T., Ma'ayan A., Enikolopov G. N. and Frenette P. S. (2010). Mesenchymal and haematopoietic stem cells form a unique bone marrow niche. Nature 466, 829-834. 10.1038/nature09262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore K. A., Ema H. and Lemischka I. R. (1997a). In vitro maintenance of highly purified, transplantable hematopoietic stem cells. Blood 89, 4337-4347. [PubMed] [Google Scholar]
- Moore K. A., Pytowski B., Witte L., Hicklin D. and Lemischka I. R. (1997b). Hematopoietic activity of a stromal cell transmembrane protein containing epidermal growth factor-like repeat motifs. Proc. Natl. Acad. Sci. USA 94, 4011-4016. 10.1073/pnas.94.8.4011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morita Y., Iseki A., Okamura S., Suzuki S., Nakauchi H. and Ema H. (2011). Functional characterization of hematopoietic stem cells in the spleen. Exp. Hematol. 39, 351-359.e353. 10.1016/j.exphem.2010.12.008 [DOI] [PubMed] [Google Scholar]
- Morrison S. J., Hemmati H. D., Wandycz A. M. and Weissman I. L. (1995). The purification and characterization of fetal liver hematopoietic stem cells. Proc. Natl. Acad. Sci. USA 92, 10302-10306. 10.1073/pnas.92.22.10302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison S. J., Wandycz A. M., Akashi K., Globerson A. and Weissman I. L. (1996). The aging of hematopoietic stem cells. Nat. Med. 2, 1011-1016. 10.1038/nm0996-1011 [DOI] [PubMed] [Google Scholar]
- Müller A. M., Medvinsky A., Strouboulis J., Grosveld F. and Dzierzakt E. (1994). Development of hematopoietic stem cell activity in the mouse embryo. Immunity 1, 291-301. 10.1016/1074-7613(94)90081-7 [DOI] [PubMed] [Google Scholar]
- Nakamura-Ishizu A. and Suda T. (2014). Aging of the hematopoietic stem cells niche. Int. J. Hematol. 100, 317-325. 10.1007/s12185-014-1641-8 [DOI] [PubMed] [Google Scholar]
- Nakamura-Ishizu A., Takubo K., Fujioka M. and Suda T. (2014). Megakaryocytes are essential for HSC quiescence through the production of thrombopoietin. Biochem. Biophys. Res. Commun. 454, 353-357. 10.1016/j.bbrc.2014.10.095 [DOI] [PubMed] [Google Scholar]
- Nakamura-Ishizu A., Takubo K., Kobayashi H., Suzuki-Inoue K. and Suda T. (2015). CLEC-2 in megakaryocytes is critical for maintenance of hematopoietic stem cells in the bone marrow. J. Exp. Med. 212, 2133-2146. 10.1084/jem.20150057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakano H., Liu X., Arshi A., Nakashima Y., van Handel B., Sasidharan R., Harmon A. W., Shin J.-H., Schwartz R. J., Conway S. J. et al. (2013). Haemogenic endocardium contributes to transient definitive haematopoiesis. Nat. Commun. 4, 1564 10.1038/ncomms2569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naveiras O., Nardi V., Wenzel P. L., Hauschka P. V., Fahey F. and Daley G. Q. (2009). Bone-marrow adipocytes as negative regulators of the haematopoietic microenvironment. Nature 460, 259-263. 10.1038/nature08099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolta J. A., Thiemann F. T., Arakawa-Hoyt J., Dao M. A., Barsky L. W., Moore K. A., Lemischka I. R. and Crooks G. M. (2002). The AFT024 stromal cell line supports long-term ex vivo maintenance of engrafting multipotent human hematopoietic progenitors. Leukemia 16, 352-361. 10.1038/sj.leu.2402371 [DOI] [PubMed] [Google Scholar]
- Ohneda O., Fennie C., Zheng Z., Donahue C., La H., Villacorta R., Cairns B. and Lasky L. A. (1998). Hematopoietic stem cell maintenance and differentiation are supported by embryonic aorta-gonad-mesonephros region-derived endothelium. Blood 92, 908-919. [PubMed] [Google Scholar]
- Omatsu Y., Sugiyama T., Kohara H., Kondoh G., Fujii N., Kohno K. and Nagasawa T. (2010). The essential functions of adipo-osteogenic progenitors as the hematopoietic stem and progenitor cell niche. Immunity 33, 387-399. 10.1016/j.immuni.2010.08.017 [DOI] [PubMed] [Google Scholar]
- Oostendorp R. A. J., Harvey K. N., Kusadasi N., de Bruijn M. F., Saris C., Ploemacher R. E., Medvinsky A. L. and Dzierzak E. A. (2002a). Stromal cell lines from mouse aorta-gonads-mesonephros subregions are potent supporters of hematopoietic stem cell activity. Blood 99, 1183-1189. 10.1182/blood.V99.4.1183 [DOI] [PubMed] [Google Scholar]
- Oostendorp R. A., Medvinsky A. J., Kusadasi N., Nakayama N., Harvey K., Orelio C., Ottersbach K., Covey T., Ploemacher R. E., Saris C. et al. (2002b). Embryonal subregion-derived stromal cell lines from novel temperature-sensitive SV40 T antigen transgenic mice support hematopoiesis. J. Cell Sci. 115, 2099-2108. [DOI] [PubMed] [Google Scholar]
- Oostendorp R. A. J., Robin C., Steinhoff C., Marz S., Bräuer R., Nuber U. A., Dzierzak E. A. and Peschel C. (2005). Long-term maintenance of hematopoietic stem cells does not require contact with embryo-derived stromal cells in cocultures. Stem Cells 23, 842-851. 10.1634/stemcells.2004-0120 [DOI] [PubMed] [Google Scholar]
- Orkin S. H. and Zon L. I. (2008). Hematopoiesis: an evolving paradigm for stem cell biology. Cell 132, 631-644. 10.1016/j.cell.2008.01.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ottersbach K. and Dzierzak E. (2005). The murine placenta contains hematopoietic stem cells within the vascular labyrinth region. Dev. Cell 8, 377-387. 10.1016/j.devcel.2005.02.001 [DOI] [PubMed] [Google Scholar]
- Palis J. (2014). Primitive and definitive erythropoiesis in mammals. Front. Physiol. 5, 3 10.3389/fphys.2014.00003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palis J., Robertson S., Kennedy M., Wall C. and Keller G. (1999). Development of erythroid and myeloid progenitors in the yolk sac and embryo proper of the mouse. Development 126, 5073-5084. [DOI] [PubMed] [Google Scholar]
- Palis J., Chan R. J., Koniski A., Patel R., Starr M. and Yoder M. C. (2001). Spatial and temporal emergence of high proliferative potential hematopoietic precursors during murine embryogenesis. Proc. Natl. Acad. Sci. USA 98, 4528-4533. 10.1073/pnas.071002398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peeters M., Ottersbach K., Bollerot K., Orelio C., de Bruijn M., Wijgerde M. and Dzierzak E. (2009). Ventral embryonic tissues and Hedgehog proteins induce early AGM hematopoietic stem cell development. Development 136, 2613-2621. 10.1242/dev.034728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce H., Zhang D., Magnon C., Lucas D., Christin J. R., Huggins M., Schwartz G. J. and Frenette P. S. (2017). Cholinergic signals from the CNS regulate G-CSF-mediated HSC mobilization from bone marrow via a glucocorticoid signaling relay. Cell Stem Cell 20, 648-658.e4. 10.1016/j.stem.2017.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinho S., Lacombe J., Hanoun M., Mizoguchi T., Bruns I., Kunisaki Y. and Frenette P. S. (2013). PDGFRalpha and CD51 mark human nestin+ sphere-forming mesenchymal stem cells capable of hematopoietic progenitor cell expansion. J. Exp. Med. 210, 1351-1367. 10.1084/jem.20122252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulos M. G., Guo P., Kofler N. M., Pinho S., Gutkin M. C., Tikhonova A., Aifantis I., Frenette P. S., Kitajewski J., Rafii S. et al. (2013). Endothelial Jagged-1 is necessary for homeostatic and regenerative hematopoiesis. Cell Rep. 4, 1022-1034. 10.1016/j.celrep.2013.07.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raaijmakers M. H. G. P., Mukherjee S., Guo S., Zhang S., Kobayashi T., Schoonmaker J. A., Ebert B. L., Al-Shahrour F., Hasserjian R. P., Scadden E. O. et al. (2010). Bone progenitor dysfunction induces myelodysplasia and secondary leukaemia. Nature 464, 852-857. 10.1038/nature08851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramasamy S. K., Kusumbe A. P., Wang L. and Adams R. H. (2014). Endothelial Notch activity promotes angiogenesis and osteogenesis in bone. Nature 507, 376-380. 10.1038/nature13146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes K. E., Gekas C., Wang Y., Lux C. T., Francis C. S., Chan D. N., Conway S., Orkin S. H., Yoder M. C. and Mikkola H. K. A. (2008). The emergence of hematopoietic stem cells is initiated in the placental vasculature in the absence of circulation. Cell Stem Cell 2, 252-263. 10.1016/j.stem.2008.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robin C., Ottersbach K., Durand C., Peeters M., Vanes L., Tybulewicz V. and Dzierzak E. (2006). An unexpected role for IL-3 in the embryonic development of hematopoietic stem cells. Dev. Cell 11, 171-180. 10.1016/j.devcel.2006.07.002 [DOI] [PubMed] [Google Scholar]
- Robin C., Bollerot K., Mendes S., Haak E., Crisan M., Cerisoli F., Lauw I., Kaimakis P., Jorna R., Vermeulen M. et al. (2009). Human placenta is a potent hematopoietic niche containing hematopoietic stem and progenitor cells throughout development. Cell Stem Cell 5, 385-395. 10.1016/j.stem.2009.08.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rompolas P., Mesa K. R. and Greco V. (2013). Spatial organization within a niche as a determinant of stem-cell fate. Nature 502, 513-518. 10.1038/nature12602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi D. J., Bryder D., Zahn J. M., Ahlenius H., Sonu R., Wagers A. J. and Weissman I. L. (2005). Cell intrinsic alterations underlie hematopoietic stem cell aging. Proc. Natl. Acad. Sci. USA 102, 9194-9199. 10.1073/pnas.0503280102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rybtsov S., Batsivari A., Bilotkach K., Paruzina D., Senserrich J., Nerushev O. and Medvinsky A. (2014). Tracing the origin of the HSC hierarchy reveals an SCF-dependent, IL-3-independent CD43(-) embryonic precursor. Stem Cell Rep. 3, 489-501. 10.1016/j.stemcr.2014.07.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacchetti B., Funari A., Michienzi S., Di Cesare S., Piersanti S., Saggio I., Tagliafico E., Ferrari S., Robey P. G., Riminucci M. et al. (2007). Self-renewing osteoprogenitors in bone marrow sinusoids can organize a hematopoietic microenvironment. Cell 131, 324-336. 10.1016/j.cell.2007.08.025 [DOI] [PubMed] [Google Scholar]
- Schepers K., Pietras E. M., Reynaud D., Flach J., Binnewies M., Garg T., Wagers A. J., Hsiao E. C. and Passegué E. (2013). Myeloproliferative neoplasia remodels the endosteal bone marrow niche into a self-reinforcing leukemic niche. Cell Stem Cell 13, 285-299. 10.1016/j.stem.2013.06.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schofield R. (1978). The relationship between the spleen colony-forming cell and the haemopoietic stem cell. Blood Cells 4, 7-25. [PubMed] [Google Scholar]
- Singh L., Brennan T. A., Russell E., Kim J.-H., Chen Q., Brad Johnson F. and Pignolo R. J. (2016). Aging alters bone-fat reciprocity by shifting in vivo mesenchymal precursor cell fate towards an adipogenic lineage. Bone 85, 29-36. 10.1016/j.bone.2016.01.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudo K., Ema H., Morita Y. and Nakauchi H. (2000). Age-associated characteristics of murine hematopoietic stem cells. J. Exp. Med. 192, 1273-1280. 10.1084/jem.192.9.1273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taichman R. S., Reilly M. J. and Emerson S. G. (2000). The hematopoietic microenvironment: osteoblasts and the hematopoietic microenvironment. Hematology 4, 421-426. 10.1080/10245332.1999.11746468 [DOI] [PubMed] [Google Scholar]
- Takeda N., Jain R., LeBoeuf M. R., Wang Q., Lu M. M. and Epstein J. A. (2011). Interconversion between intestinal stem cell populations in distinct niches. Science 334, 1420-1424. 10.1126/science.1213214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamplin O. J., Durand E. M., Carr L. A., Childs S. J., Hagedorn E. J., Li P., Yzaguirre A. D., Speck N. A. and Zon L. I. (2015). Hematopoietic stem cell arrival triggers dynamic remodeling of the perivascular niche. Cell 160, 241-252. 10.1016/j.cell.2014.12.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taoudi S. and Medvinsky A. (2007). Functional identification of the hematopoietic stem cell niche in the ventral domain of the embryonic dorsal aorta. Proc. Natl. Acad. Sci. USA 104, 9399-9403. 10.1073/pnas.0700984104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian H., Biehs B., Warming S., Leong K. G., Rangell L., Klein O. D. and de Sauvage F. J. (2011). A reserve stem cell population in small intestine renders Lgr5-positive cells dispensable. Nature 478, 255-259. 10.1038/nature10408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tober J., Koniski A., McGrath K. E., Vemishetti R., Emerson R., de Mesy-Bentley K. K. L., Waugh R. and Palis J. (2007). The megakaryocyte lineage originates from hemangioblast precursors and is an integral component both of primitive and of definitive hematopoiesis. Blood 109, 1433-1441. 10.1182/blood-2006-06-031898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visnjic D., Kalajzic Z., Rowe D. W., Katavic V., Lorenzo J. and Aguila H. L. (2004). Hematopoiesis is severely altered in mice with an induced osteoblast deficiency. Blood 103, 3258-3264. 10.1182/blood-2003-11-4011 [DOI] [PubMed] [Google Scholar]
- Walkley C. R., Olsen G. H., Dworkin S., Fabb S. A., Swann J., McArthur G. A., Westmoreland S. V., Chambon P., Scadden D. T. and Purton L. E. (2007a). A microenvironment-induced myeloproliferative syndrome caused by retinoic acid receptor gamma deficiency. Cell 129, 1097-1110. 10.1016/j.cell.2007.05.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walkley C. R., Shea J. M., Sims N. A., Purton L. E. and Orkin S. H. (2007b). Rb regulates interactions between hematopoietic stem cells and their bone marrow microenvironment. Cell 129, 1081-1095. 10.1016/j.cell.2007.03.055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson A., Laurenti E., Oser G., van der Wath R. C., Blanco-Bose W., Jaworski M., Offner S., Dunant C. F., Eshkind L., Bockamp E. et al. (2008). Hematopoietic stem cells reversibly switch from dormancy to self-renewal during homeostasis and repair. Cell 135, 1118-1129. 10.1016/j.cell.2008.10.048 [DOI] [PubMed] [Google Scholar]
- Wineman J., Moore K., Lemischka I. and Muller-Sieburg C. (1996). Functional heterogeneity of the hematopoietic microenvironment: rare stromal elements maintain long-term repopulating stem cells. Blood 87, 4082-4090. [PubMed] [Google Scholar]
- Winkler I. G., Sims N. A., Pettit A. R., Barbier V., Nowlan B., Helwani F., Poulton I. J., van Rooijen N., Alexander K. A., Raggatt L. J. et al. (2010). Bone marrow macrophages maintain hematopoietic stem cell (HSC) niches and their depletion mobilizes HSCs. Blood 116, 4815-4828. 10.1182/blood-2009-11-253534 [DOI] [PubMed] [Google Scholar]
- Wolber F. M., Leonard E., Michael S., Orschell-Traycoff C. M., Yoder M. C. and Srour E. F. (2002). Roles of spleen and liver in development of the murine hematopoietic system. Exp. Hematol. 30, 1010-1019. 10.1016/S0301-472X(02)00881-0 [DOI] [PubMed] [Google Scholar]
- Xu M. J., Tsuji K., Ueda T., Mukouyama Y. S., Hara T., Yang F. C., Ebihara Y., Matsuoka S., Manabe A., Kikuchi A. et al. (1998). Stimulation of mouse and human primitive hematopoiesis by murine embryonic aorta-gonad-mesonephros-derived stromal cell lines. Blood 92, 2032-2040. [PubMed] [Google Scholar]
- Yamazaki S., Ema H., Karlsson G., Yamaguchi T., Miyoshi H., Shioda S., Taketo M. M., Karlsson S., Iwama A. and Nakauchi H. (2011). Nonmyelinating Schwann cells maintain hematopoietic stem cell hibernation in the bone marrow niche. Cell 147, 1146-1158. 10.1016/j.cell.2011.09.053 [DOI] [PubMed] [Google Scholar]
- Yu V. W. C., Yusuf R. Z., Oki T., Wu J., Saez B., Wang X., Cook C., Baryawno N., Ziller M. J., Lee E. et al. (2016). Epigenetic memory underlies cell-autonomous heterogeneous behavior of hematopoietic stem cells. Cell 167, 1310-1322.e1317. 10.1016/j.cell.2016.10.045 [DOI] [PubMed] [Google Scholar]
- Zhang C. C. and Lodish H. F. (2004). Insulin-like growth factor 2 expressed in a novel fetal liver cell population is a growth factor for hematopoietic stem cells. Blood 103, 2513-2521. 10.1182/blood-2003-08-2955 [DOI] [PubMed] [Google Scholar]
- Zhang J., Niu C., Ye L., Huang H., He X., Tong W.-G., Ross J., Haug J., Johnson T., Feng J. Q. et al. (2003). Identification of the haematopoietic stem cell niche and control of the niche size. Nature 425, 836-841. 10.1038/nature02041 [DOI] [PubMed] [Google Scholar]
- Zhao M., Perry J. M., Marshall H., Venkatraman A., Qian P., He X. C., Ahamed J. and Li L. (2014). Megakaryocytes maintain homeostatic quiescence and promote post-injury regeneration of hematopoietic stem cells. Nat. Med. 20, 1321-1326. 10.1038/nm.3706 [DOI] [PubMed] [Google Scholar]
- Zhao Y., Zhou J., Liu D., Dong F., Cheng H., Wang W., Pang Y., Wang Y., Mu X., Ni Y. et al. (2015). ATF4 plays a pivotal role in the development of functional hematopoietic stem cells in mouse fetal liver. Blood 126, 2383-2391. 10.1182/blood-2015-03-633354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou B. O., Ding L. and Morrison S. J. (2015). Hematopoietic stem and progenitor cells regulate the regeneration of their niche by secreting Angiopoietin-1. Elife 4, e05521 10.7554/eLife.05521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J., Garrett R., Jung Y., Zhang Y., Kim N., Wang J., Joe G. J., Hexner E., Choi Y., Taichman R. S. et al. (2007). Osteoblasts support B-lymphocyte commitment and differentiation from hematopoietic stem cells. Blood 109, 3706-3712. 10.1182/blood-2006-08-041384 [DOI] [PubMed] [Google Scholar]
- Zovein A. C. and Iruela-Arispe M. L. (2009). Time to cut the cord: placental HSCs grow up. Cell Stem Cell 5, 351-352. 10.1016/j.stem.2009.09.003 [DOI] [PubMed] [Google Scholar]





