Abstract
Constipation is a common childhood problem, but an anatomic or physiologic cause is identified in fewer than 5% of children. By definition, idiopathic constipation is a diagnosis of exclusion. Careful clinical evaluation and thoughtful use of imaging and other testing can help exclude specific causes of constipation and guide therapy. Medical management with laxatives is effective for the majority of constipated children. For those patients unresponsive to medications, however, several surgical options can be employed, including anal procedures, antegrade colonic enemas, colorectal resection, and intestinal diversion. Judicious use of these procedures in properly selected patients and based on appropriate preoperative testing can lead to excellent outcomes. This review summarizes the surgical options available for managing refractory constipation in children and provides guidance on how to choose the best procedure for a given patient.
Keywords: constipation, functional constipation, idiopathic constipation, antegrade colonic enemas, colorectal resection
Constipation accounts for an estimated 3 to 5% of visits to a pediatrician and $3.9 billion in annual health care costs in the United States alone. Chronic idiopathic constipation, also known as functional constipation, is defined as difficult and infrequent defecation without an identifiable organic (i.e., anatomic, metabolic, or neurologic) cause. 1 Despite the prevalence of pediatric constipation, an underlying cause is identified in fewer than 5% of cases. 2 3 In children, constipation often develops during the introduction of solid foods, around the time of toilet training, or at the start of school. 2 4 Regardless of the cause, as constipation progresses, it can lead to a cycle of fecal retention and rectal dilatation, which impairs normal colorectal motility and leads to worsening retention of stool and increasing rectal dilatation. Defecation may become painful or unpleasant for the child, further exacerbating the problem. This cycle emphasizes the importance of treating constipation early in its course.
Clinical Presentation
The normal frequency of bowel movements in children varies with age and diet. Most newborns (98%) pass meconium within the first 24 hours of life. 5 Infants have several stools daily in the first week of life, although breast-fed infants may stool only once every several days. Stool frequency decreases with age, averaging once daily by the age of 4 years. 2
The signs and symptoms of functional constipation are described by the Rome III criteria 6 7 8 ( Table 1 ). In children, this includes a history of infrequent defecation (less than two times per week), stool retention, painful or hard bowel movements, large caliber stools, and the presence of a fecal mass in the rectum. 6 7 Fecal incontinence can occur due to overflow soiling. 4 Chronic abdominal pain is also a common complaint. 2 Withholding behavior is also common, as children who experience painful defecation learn to withhold stool by contracting their gluteal muscles and external anal sphincter (EAS).
Table 1. Rome III criteria for diagnosis of functional constipation in children.
| Infants/Toddlers (< 4 y old) 7 | Child/Adolescent (≥ 4 y old) 6 |
|---|---|
| • ≥ 2 of the following for ≥ 1 mo: – ≤ 2 defecations/wk – ≥ 1 episode/wk of incontinence after acquisition of toileting skills – History of excessive stool retention – History of painful or hard bowel movements – Presence of a large fecal mass in the rectum – History of large diameter stools which may obstruct the toilet |
• ≥ 2 of the following for ≥ 2 mo: – ≤ 2 defecations/wk – ≥ 1 episode/wk of fecal incontinence – History of retentive posturing or excessive volitional stool retention – History of painful or hard bowel movements – Presence of a large fecal mass in the rectum – History of large diameter stools which may obstruct the toilet • Insufficient criteria for diagnosis of IBS |
Abbreviation: IBS, irritable bowel syndrome.
Diagnostic Evaluation
Physical Examination
Idiopathic constipation is a clinical diagnosis, but anatomic or physiologic causes can be excluded by careful history, physical exam, and appropriate diagnostic testing. History and physical exam should include a thorough assessment of “alarm” signs and symptoms (i.e., delayed growth, delayed passage of meconium, decreased tone, neurologic defects, congenital anomalies), which are associated with specific causes of constipation. 1 9 Prior to making the diagnosis of idiopathic constipation, these potential etiologies should be excluded ( Table 2 ).
Table 2. Differential diagnosis of constipation in children.
| Pelvic |
|---|
| • Anorectal malformations • Pelvic or rectal mass • Rectal prolapse • Rectocele • Dysfunctional voiding • Pelvic floor dyssynergia • Internal anal sphincter hypertonicity or achalasia |
| Colorectal |
| • Hirschsprung's disease • Intestinal neuronal dysplasia • Visceral myopathies or neuropathies |
| Systemic |
| • Spinal cord anomalies • Cystic fibrosis • Connective tissue disorders • Hypothyroidism • Diabetes mellitus • Hypercalcemia • Hypokalemia • Celiac disease • Cow's milk protein allergy • Drugs or toxins • Psychosocial issues |
On abdominal examination, the clinician may appreciate distension or a palpable scybala (i.e., fecal mass) in the lower abdomen. Rectal exam should be performed to identify the presence of impacted stool or a presacral or intrarectal mass. Visual and digital anal inspection is also important to ensure normal size and positioning of the anal opening and to assess for rectal prolapse with bear-down. Explosive expulsion of stool or gas with digital rectal exam (referred to as the “blast sign”) may suggest Hirschsprung's disease.
Many of the diagnostic tests commonly used in adults are not validated in children. Current guidelines from the North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition (NASPGHAN) and the European Society for Pediatric Gastroenterology, Hepatology, and Nutrition (ESPGHAN) do not recommend the routine use of any specific imaging or laboratory tests in the diagnosis of pediatric functional constipation. 9 However, when symptoms fail to improve with the usual medical management as described below, further diagnostic evaluation can elucidate potential causes and help guide targeted therapy. In our practice, we favor a “bottom-up” approach starting distally with assessment of the anus and pelvic floor and progressing proximally to consider rectal and colonic. We review these diagnostic tests briefly because of the role they can play in surgical decision making.
Anorectal Manometry
Anorectal manometry (ARM) is a test in which motor and sensory anorectal function is measured to identify potential causes of constipation or fecal incontinence. ARM is useful in the diagnosis of Hirschsprung's disease and dyssynergic defecation. In Hirschsprung's disease, the normal relaxation of the internal anal sphincter (IAS) in response to rectal distention (rectoanal inhibitory reflex [RAIR]) is absent. 10 Children without a RAIR should be referred for rectal suction biopsy, the gold standard for diagnosing Hirschsprung's disease. 9 Patients with a hypertonic or nonrelaxing IAS may benefit from sphincter myectomy/myotomy or injection of Clostridium botulinum toxin (Botox) injection. 11 In dyssynergic defecation, ARM may show a paradoxical contraction of pelvic floor muscles during defecation or insufficient rectal pressures during bear-down. 12 New advances with solid-state catheters can now provide three-dimensional (3D), high-definition visualization of defecation dynamics, which enhances the diagnostic evaluation of constipated children. 13
Colonic Manometry
Colonic manometry involves colonoscopic insertion of a catheter along the length of the colon to measure segmental pressure changes over time. This test can identify neurogenic and myogenic causes of constipation, and some have suggested that it can help guide surgical therapy. 14 15 16 No specific motility pattern is diagnostic of idiopathic constipation, although regional deficits in colonic motility or pan-colonic dysfunction can be identified. Comparing baseline manometry to results obtained after surgery (e.g., intestinal diversion or antegrade colonic enema [ACE]) can help determine whether colonic function is improving. Colonic manometry after ACE shows improvement over time in nearly all patients, with normal motility achieved in up to 83% of cases, 17 18 thereby identifying those who can discontinue antegrade enemas. Similarly, normalization of colonic manometry after intestinal diversion can guide timing of stoma reversal. 15
Colonic Transit Studies
Colonic transit studies typically employ radiopaque markers, radioisotope scintigraphy, or a wireless motility capsule to measure the speed of intestinal transit. Transit studies may help identify anatomic sites of fecal retention, distinguish retentive (constipation-associated) fecal incontinence from nonretentive fecal incontinence, and document slow transit. Some studies in children suggest that functional constipation can be divided into normal transit, slow transit, and outlet obstruction based on the results of colonic transit studies. 19 However, it is unclear if children with slow transit constipation represent a distinct disease or if slow transit simply represents the result of severe constipation. 20
Imaging Studies
Plain abdominal radiographs do not generally add much to the history and physical exam. They may, however, help assess stool burden when physical exam is unreliable, such as in obese patients, or when rectal exam is either refused or contraindicated for psychological reasons. Plan films of the abdomen can also be useful in following stool burden over time, but is neither a sensitive nor specific method of diagnosing constipation. 9 21 A contrast enema may show a dilated rectum in functional constipation, whereas in short-segment Hirschsprung's disease, an abnormally narrow, funnel-shaped rectum is seen. Spinal cord abnormalities have been found in up to 9% of children with constipation, with tethered cord being the most common. 22 In these cases, spinal magnetic resonance imaging (MRI) is the diagnostic test of choice.
Medical Management
Laxatives—including osmotics, stimulants, lubricants, and stool softeners—represent the mainstay of medical therapy for childhood constipation, and the majority of children will have symptomatic improvement with an appropriate regimen. 23 In some cases, enemas and/or suppositories may be helpful, but many clinicians try to avoid the rectal route in children as it may add psychological trauma to patients already sensitive to and anxious about painful defecation.
Consensus guidelines from NASPGHAN/ESPGHAN recommend daily polyethylene glycol (PEG) at a dose of 1 to 1.5 g/kg/day for 3 to 6 days for initial fecal disimpaction, followed by a daily maintenance dose of 0.4 g/kg/day for a minimum of 2 months. 9 A Cochrane review comparing the efficacy of various laxatives found that PEG preparations were more effective than lactulose or magnesium hydroxide and were well tolerated. 24 Lactulose, magnesium hydroxide, lubricants (e.g., mineral oil), and stimulants (e.g., bisacodyl, senna) are recommended as second-line or alternative treatments. While not formally recommended, stool softeners (e.g., docusate) can be considered. A normal intake of fluids and fiber is recommended. 9
Few alternative medications have been found to be helpful in childhood constipation. Due to lack of strong evidence in children, current guidelines from NASPGHAN/ESPGHAN recommend against routine use of prebiotics, probiotics, lubiprostone, linaclotide, or prucalopride. 9
Biofeedback and Physical Therapy
A randomized controlled trial in 203 constipated children found that while biofeedback improved defecation dynamics, it had no effect on constipation. 25 Similarly, a recent Cochrane review in adults with chronic idiopathic constipation found insufficient evidence to support the use of biofeedback. 26 While consensus guidelines from the American Neurogastroenterology and Motility Society (ANMS) and the European Society of Neurogastroenterology and Motility (ESNM) support the use of biofeedback therapy for adults with dyssynergic defecation, the available evidence does not support the use of biofeedback in constipated children. 27
Pelvic floor physical therapy has been used to treat children with dysfunctional voiding, but has not been rigorously evaluated in constipated children. A large multicenter randomized controlled trial is currently underway to evaluate the effectiveness of pelvic physical therapy for functional constipation in children. 28 In our experience, physical therapy has been helpful for children with dyssynergic defecation and outlet obstruction due to a variety of etiologies, but evidence-based data to support this practice are needed.
Nerve Stimulation
Sacral nerve stimulation (SNS) has been used to treat refractory constipation in both adults and children. 29 It is believed that stimulation of the sacral nerves aids extrinsic neural control of the large bowel and modulates inhibitory reflexes. 30 However, a recent Cochrane review found no benefit to SNS for constipation in adults. 31 Retrospective data suggest that SNS may improve bowel movement frequency in children with functional constipation, but prospective randomized studies have not been done. 32 In a few small studies, transcutaneous electrical stimulation (TES) has been shown to improve quality of life and increase bowel movement frequency in children with slow transit constipation. 33 34 35 However, beneficial effects of TES last less than 6 months in a third of patients and long-term data are lacking. 36
Surgical Management
Surgical management for idiopathic constipation is reserved for those patients who are refractory to medical management. It is estimated that approximately 10% of constipated children who are referred to a pediatric surgeon will need an operation. 37 Surgery can be very effective, but needs to be tailored based on the results of preoperative testing and individual patient factors. Ideally, an operation is aimed at treating the underlying pathophysiology when it is known, but in idiopathic constipation, it is most often directed toward symptom alleviation. A recent systematic review of 45 pediatric studies including 1,157 children with idiopathic constipation found no single operation to represent “best practice.” 38 Small study sizes, lack of prospective randomized studies, heterogeneous patient populations, as well as differences in indications for surgery, preoperative testing, operative techniques, postoperative care, and outcome measures account for much of the variability in the literature. 38 39 40
Surgical options for treating refractory constipation in children include anal procedures, antegrade enemas, colorectal resection, and intestinal diversion ( Table 3 ). In a recent review, Siminas and Losty proposed an algorithm that starts with less invasive procedures, such as Botox injection for suspected anal sphincter dysfunction, and progresses stepwise toward more invasive procedures, including antegrade enemas, temporary diversion, and bowel resection. 38 Permanent diversion is reserved as an option of last resort. 41 We review these surgical options, considering the clinical indications, technical aspects, and outcomes associated with each.
Table 3. Surgical options for refractory constipation.
| Anal procedures |
|---|
| • Sphincter myectomy or myotomy |
| • Botulinum toxin (Botox) injection |
| Antegrade colonic enemas |
| • Appendicostomy |
| • Cecostomy |
| Colorectal resection |
| • Segmental resection (e.g., rectosigmoidectomy) |
| • Subtotal colectomy |
| • Total proctocolectomy |
| Intestinal diversion |
| • Ileostomy |
| • Colostomy |
Anal Procedures
IAS dysfunction can lead to obstructive defecation. If identified by ARM, IAS hypertonicity and/or achalasia should be treated. Children with idiopathic constipation have a normal IAS resting pressure and RAIR, but have increased sphincter thickness, frequency, and amplitude of IAS contraction—possibly due to the constant stimulus of stool in the rectum—which may perpetuate the vicious cycle of fecal retention and difficult defecation. 42 43 44 45 A variety of anal procedures have been utilized to address these issues, including anal dilatation, IAS myectomy or myotomy, and Botox injection.
An early prospective study performed anal dilatation on a cohort of children with chronic idiopathic constipation and found symptomatic improvement, 45 but a more recent double-blinded randomized controlled trial found no benefit to anal dilatation over intensive medical treatment. 46 Anal dilatation is no longer recommended for treating constipation.
Sphincter myectomy or myotomy has long been used to treat constipation associated with IAS achalasia 47 and hypertonicity, 48 but has also been applied to treat idiopathic constipation. 43 49 Recently, Botox injection has been favored over myotomy/myectomy. Botox is safe, easy to use, and causes transient inhibition of acetylcholine release at the neuromuscular junction, eliminating the long-term risk of fecal incontinence associated with sphincter myotomy/myectomy. Botox injection of the IAS has been shown to be an effective treatment for children with Hirschsprung's disease who develop obstructive symptoms following pull-through surgery. 50 51 Botox has also been used in children with idiopathic constipation, 52 achieving a reduction in symptom severity equivalent to that achieved with sphincter myectomy. 11 53 Though the effect of Botox is transient, usually lasting 6 months, the symptomatic improvement can last indefinitely. 11 54 This may be due to Botox leading to improved evacuation and diminished rectal distention, thereby allowing recovery of normal rectal sensation and motor function. 55 Botox injection of the EAS has also been described and is suggested for children with constipation due to voluntary withholding or paradoxical contraction of the EAS, 56 but more studies are needed to determine its long-term efficacy.
When an anal procedure is indicated, we recommend starting with Botox because of its low risk. With the child under conscious sedation or general anesthesia, a solution of Botox type A is diluted in sterile saline to a concentration of 20 to 100 unit/mL (for a dose of 6 unit/kg, up to 100 units total) and injected in four quadrants of the IAS, with or without ultrasound guidance. 11 54 When ARM is available, an immediate reduction in IAS pressure can be documented. When Botox is effective, but has required many repeated injections due to recurrent symptoms, sphincter myectomy/myotomy may be an appropriate next step.
Antegrade Colonic Enema
In 1990, Malone et al first described a continent catheterizable appendicostomy for the delivery of ACEs in children with fecal incontinence. 57 ACE is now widely used to treat idiopathic childhood constipation as well. NASPGHAN/ESPGHAN currently recommends ACE for children with intractable constipation that is refractory to medical management, 9 though some studies suggest it may be more appropriate in older children (>5 years of age) due to the need for patient compliance. 38 In constipated patients, the goal of the ACE procedure is to allow direct access to the colon so that the patient can deliver antegrade enemas in cases where they are refractory to medications or dependent on rectal enemas.
Since 1990, several modifications to the Malone appendicostomy have been described. 58 The original procedure reversed the appendix and reimplanted it through a submucosal cecal tunnel to prevent reflux. 57 An orthotopic appendicocecostomy with plication of the cecum to prevent reflux is now more commonly used. 59 The stoma can be hidden in the umbilical fold to improve aesthetic appearance. 59 60 An open or laparoscopic approach can be employed. 61 If the appendix is absent or cannot be used, a neoappendix can be fashioned from cecum, ileum, or defunctionalized ureter. 58 62 A percutaneous cecostomy tube or button device can also be placed directly into the cecum using an open, laparoscopic, or percutaneous technique. 63 64 65 Distal colonic access via a catheter inserted into the sigmoid colon to deliver “left-sided” ACE has also been described. Comparison of left-sided and traditional ACE suggests that left-sided ACE may be associated with fewer complications, lower volume enema fluid requirement, and faster enema transit time. 66 However, left-sided ACE may not be as effective in resolving constipation and achieving continence. 67
Our preference is to perform a laparoscopic-assisted percutaneous endoscopic cecostomy (LAPEC). 68 We have modified our original procedure and now place a balloon-type button at the initial operation. Briefly, a laparoscope is placed through the umbilicus and the cecum is accessed colonoscopically. After insufflating the cecum, two sutures or T-fasteners are passed through the abdominal wall and the anterior cecal wall to hold up the cecum. A needle is passed through the right lower quadrant into the cecum and a wire is advanced. The tract is sequentially dilated over the wire and a 14F low-profile balloon-type gastrostomy button advanced into the cecum under both laparoscopic and endoscopic visualization. Additional ports are sometimes needed in the left lower quadrant and/or suprapubic area to facilitate the procedure. The button can be exchanged for a Chait trapdoor cecostomy tube, which is 10F and has a very low profile, 6 to 8 weeks after the procedure. Patients are discharged either the same day or the following day, and full-volume daily irrigations are started 5 to 7 days after the procedure.
Antegrade enema regimens vary and must be individually tailored based on patient response. Many patients (70%) start with saline enemas without additive. 39 However, most (61%) will have an inadequate response to saline alone and convert to a PEG-containing solution. 69 In our practice, we start irrigations with 10 to 15 mL/kg of PEG solution. 68 Addition of a stimulant laxative, such as bisacodyl, to enemas may improve efficacy. 70 Occasionally, patients benefit from addition of mineral oil or magnesium-containing solutions to the enema. Enema volume reported in the pediatric literature varies widely, averaging 23 mL/kg, and average administration time is 12 minutes (5–60 minutes). 58 69 Most patients will sit on the toilet for up to 60 minutes to complete evacuation. 69 71
Common complications of appendicostomy include pain at catheterization (27%), skin excoriation/granulation tissue (27%), stoma leakage (24%), stoma stenosis (22%), and superficial surgical site infection (17%). Rare complications include postoperative ileus (3%), stomal prolapse (3%), bowel perforation (2%), and parastomal hernia (1%). Revisions were required in 8% of patients. Common complications of cecostomy include tube leakage (18%) and skin excoriation/granulation tissue (14%). Revisions were required in 3% of patients. 38 A comparison of Malone appendicostomy to cecostomy button for children with idiopathic constipation and fecal soiling found that, while both procedures were effective in reducing soiling, the Malone appendicostomy was associated with a higher incidence of operative complications, the most common being stomal stenosis requiring reoperation (11%), followed by iatrogenic perforation of the appendix (5%). In contrast, cecostomy was associated with a higher incidence of minor nonoperative complications, including leakage (42%) and granulation tissue (33%). 72
Overall, 63 to 97% of patients experience symptomatic improvement in their constipation with ACE. 18 38 69 70 71 72 73 74 75 A systematic review of 25 pediatric studies including 505 operations for ACE reported good outcomes in an average of 82% of patients. 38 Interestingly, in contrast to the experience in children, ACE may not be as effective for adults. A recent meta-analysis reported a success rate of only 67.7% in adults with constipation, 76 although larger prospective studies are needed. Children with a massively dilated colon may not respond well to ACE and may do better with diversion, as described below. Similarly, children with severe behavioral or psychiatric issues who are unlikely to allow the enema to be performed should not be considered for this procedure. Some studies suggest that ACE may be less effective for idiopathic constipation than other etiologies, 77 78 but other studies have found no association between idiopathic constipation and worse outcome 70 74 and others have conversely found that idiopathic constipation is in fact associated with a better outcome. 79 One of the benefits of ACE over the other surgical options described below is that it does not burn any bridges. No bowel is removed and the procedure is easily reversed. Furthermore, it is easy to test for return of defecatory function simply by decreasing the frequency of antegrade enemas, or discontinuing them entirely. Failure of an ACE procedure can always be followed by colorectal resection or intestinal diversion when needed.
Colorectal Resection
Colorectal resection with primary reanastomosis can be useful in treating refractory constipation, but should be reserved for patients who do not respond to more conservative medical and surgical therapy. 38 Segmental resection, subtotal colectomy, and total proctocolectomy by various techniques have all been described with different outcomes. Segmental resection is especially indicated for children with a discrete focal abnormality limited to a segment of the colon, such as a very dilated rectum or rectosigmoid 41 ( Fig. 1 ). Several studies have reported good short-term outcomes following resection of a megarectosigmoid, with significant symptom improvement in up to 80%. 80 81 82 One study also reported good outcomes following segmental resection of a portion of colon based on abnormal colonic manometry, 75 while others have not found this approach to targeted segmental resection to be as effective. 18
Fig. 1.
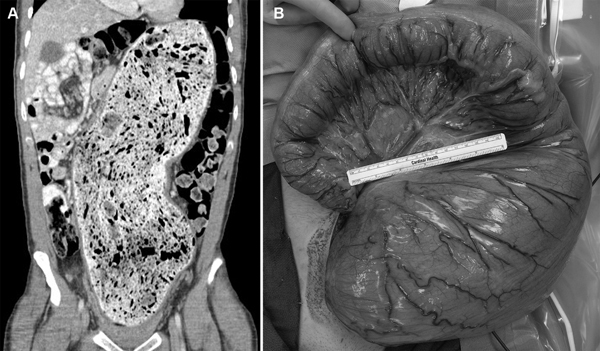
A 36-year-old man with severe, idiopathic constipation since childhood was found to have massive dilatation of a stool-filled rectosigmoid on computerized tomography (CT) scan ( A ). He underwent segmental resection of the megarectosigmoid, which was >18 cm in widest diameter ( B ), and has subsequently done well.
Segmental resection can be performed using an open, laparoscopic, or transanal approach. Transanal proctosigmoidectomy with coloanal anastomosis, similar to the Swenson procedure for Hirschsprung's disease, has been reported to have good outcomes for idiopathic constipation, 80 but is associated with a high rate of soiling or fecal incontinence, possibly due to loss of the rectal reservoir. 18 83 84 In contrast, children who had a Duhamel-type procedure with preservation of the rectum were reported to have good functional outcomes with no incontinence. 82 However, in an older cohort of patients with constipation since childhood, the Duhamel procedure resulted in improved bowel frequency in only half of the patients with abdominal pain and bloating common postoperatively. 85 Segmental resection in adults with megarectum has generally produced poorer outcomes compared with total or subtotal colectomy. 86 87 88 89
In adults with idiopathic constipation, subtotal colectomy with ileorectal anastomosis is the most commonly performed operation. 86 In children, this procedure is far less common and less effective. The largest case series of 10 constipated children found that while 70% had improved bowel movement frequency (3–6 per day), 30% suffered incontinence. 83 Other studies have reported varying success with frequent incontinence in 0 to 30% and persistent constipation in 0 to 50%. 15 37 75 90 Careful patient selection for this operation is essential, since it leaves the rectum in place, which lessens the likelihood of success in patients with anorectal or pelvic floor disorders, which are common in constipated children.
Restorative total proctocolectomy with ileal pouch reconstruction is infrequently performed for constipated children, but has been reported to be effective. The largest series in children reported improved bowel movement frequency (4–8 per day) in all five patients at 3-year follow-up, but three patients had nighttime soiling and one required antidiarrheal medications. 91 Similar results have been reported in adults, with improvement in constipation in all patients, but frequent problems with nighttime soiling in 7 to 46% and poor function leading to conversion to permanent ileostomy in 0 to 29%. 92 93 94
Studies of colorectal resection in children are limited by small sample sizes, differences in surgical techniques, and poor long-term follow-up. In a systematic review of 10 pediatric studies with 83 procedures for colorectal resection, a good outcome was reported in 84%. 38 Colorectal resection combined with ACE or temporary diversion has also been reported to lead to good results in 80 to 100%. 15 81 Complications occur in 17 to 24% of patients after colorectal resection and reoperations are needed in 12%. 38 When the rectum is resected, there is a high risk of fecal incontinence. However, preservation of an abnormal or dilated rectum may be associated with persistent symptoms of constipation. Given the permanence of resection and the higher risk of significant morbidity compared with other surgical options, colorectal resection should be reserved for patients with clear segmental abnormalities.
Intestinal Diversion
Intestinal diversion, either via ileostomy or colostomy, can be very effective in relieving symptoms of constipation. Though permanent intestinal diversion is considered by many to be a last resort, temporary diversion may be beneficial in select patients. This may be especially true for patients with marked pan-colonic dilatation, in whom ACE is unlikely to be effective. Some studies also suggest that temporary diversion is superior to ACE in younger children (<5 years of age) since ACE requires a cooperative and compliant patient. 38
Like ACE, temporary diversion can decrease colonic dilatation and improve colonic motility, with many children successfully reestablishing intestinal continuity ( Fig. 2 ). In the largest case series of intestinal diversion in constipated children, 19 patients had either an ileostomy or colostomy. A satisfactory outcome was reported in 95%, and 74% reestablished continuity, but 64% of those patients also underwent bowel resection. 18 In another study, good outcomes were reported in 12 children undergoing ileostomy or colostomy: 83% reestablished continuity and 50% of these also had bowel resections. 15 In one of the largest studies of colostomy alone, 10 children with functional constipation underwent a Hartmann procedure with a sigmoid end colostomy. Satisfaction was rated as 100% in short-term follow-up, but no patients in this series reestablished continuity. 95 There are no studies directly comparing ileostomy to colostomy in children, but ileostomy is the safer choice when the site of colorectal dysfunction is unknown.
Fig. 2.

Representative contrast enemas before ( A ) and 17 months after ( B ) diverting ileostomy in a 4-year-old boy with chronic idiopathic constipation. His ileostomy was reversed 18 months after diversion and he continues to do well with normal bowel movements every 1–2 days.
Complications of stoma formation are common. In a 20-year study of 1,616 stomas for various indications in children and adults, ileostomies had the highest rate of complication (49%) compared with colostomies (22–35%) and loop enteric stomas had more complications (37%) than end ostomies (30%). Increasing age was correlated with a higher risk of early complications, but complication rates were still high in children (29%). 96 Stoma formation specifically in children with constipation is associated with complications in 10 to 25% and needs reoperation in up to 30%. The most common complications include stomal prolapse (19%), diversion colitis (6%), skin excoriation (6%), and small bowel obstruction (6%). 38
In a systematic review of five pediatric studies with a total of 41 children who had intestinal diversion, success was reported in 93%. 38 In contrast, intestinal diversion in adult patients with severe constipation was only effective in 65% of cases and was generally not effective in improving abdominal pain and bloating. 86 97 Given the high morbidity of a stoma, temporary diversion can be considered as an alternative to ACE in select patients, but permanent diversion is recommended only when other management options have failed.
Long-Term Outcomes
There are few reliable long-term studies of idiopathic constipation in children. This is in part due to heterogeneity in the definition of the disease and a lack of validated outcome measures. 98 99 Most children managed with medical therapy will improve, and as many as half can discontinue laxatives after 1 year of treatment. 100 101 However, 25 to 30% of children will continue to have symptoms into adulthood. 100 102 Factors associated with a worse prognosis include female sex, older age at onset, longer time between onset of symptoms and treatment, longer colonic transit time, and greater severity of constipation. 18 100 102 103 104
ACE has become the most accepted procedure in children with refractory idiopathic constipation and is the only surgical procedure formally recommended in consensus guidelines from NASPGHAN/ESPGHAN. 9 Some children (13–42%) who are treated with ACE can discontinue enemas 6 to 24 months after beginning treatment and eventually have ACE reversal, at an average of 8.8 years after ACE. 17 70 104 105 106 However, in approximately 25% of patients, ACE alone will not be effective and other surgical management will be needed. 104 Intestinal diversion can be an effective alternative to ACE. The majority of patients who undergo intestinal diversion for idiopathic constipation will have improved colonic motility after diversion and can reestablish continuity, at an average of 2 years after diversion, although many of these patients will also require some type of bowel resection. 15 18 The long-term outcome of a diverting ostomy in constipated children is not well characterized, but short-term studies report frequent complications. Bowel resection alone or in combination with ACE or temporary diversion is reported to have good outcomes, but the long-term outcomes of these procedures in children remain unknown.
In comparison to children, adults with chronic idiopathic constipation have poorer outcomes in response to medical or surgical therapy. 107 It is uncertain if these adult patients represent the cohort of children with refractory disease, or if adult idiopathic constipation is a different disease entity. 108 A systematic review of 27 studies in adults with chronic idiopathic constipation found that surgical intervention was variably effective and invariably associated with significant morbidity and mortality. 86
In summary, constipation in children is a familiar and frustrating problem. A thorough history, examination, and diagnostic workup are essential to identify any potential underlying etiologies. In the majority of cases, however, no specific cause will be found. In these patients, when medical management fails, surgical options should be considered and can lead to significant improvement in symptoms and quality of life.
References
- 1.Walia R, Mulhearn N, Khan R, Cuffari C. Chronic constipation in children: an overview. Pract Gastroenterol. 2013;37(07):19–34. [Google Scholar]
- 2.Di Lorenzo C.Pediatric anorectal disorders Gastroenterol Clin North Am 20013001269–287., ix [DOI] [PubMed] [Google Scholar]
- 3.Liem O, Harman J, Benninga M, Kelleher K, Mousa H, Di Lorenzo C. Health utilization and cost impact of childhood constipation in the United States. J Pediatr. 2009;154(02):258–262. doi: 10.1016/j.jpeds.2008.07.060. [DOI] [PubMed] [Google Scholar]
- 4.Di Lorenzo C, Benninga M A. Pathophysiology of pediatric fecal incontinence. Gastroenterology. 2004;126(01) 01:S33–S40. doi: 10.1053/j.gastro.2003.10.012. [DOI] [PubMed] [Google Scholar]
- 5.Clark D A. Times of first void and first stool in 500 newborns. Pediatrics. 1977;60(04):457–459. [PubMed] [Google Scholar]
- 6.Rasquin A, Di Lorenzo C, Forbes D et al. Childhood functional gastrointestinal disorders: child/adolescent. Gastroenterology. 2006;130(05):1527–1537. doi: 10.1053/j.gastro.2005.08.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hyman P E, Milla P J, Benninga M A, Davidson G P, Fleisher D F, Taminiau J. Childhood functional gastrointestinal disorders: neonate/toddler. Gastroenterology. 2006;130(05):1519–1526. doi: 10.1053/j.gastro.2005.11.065. [DOI] [PubMed] [Google Scholar]
- 8.Longstreth G F, Thompson W G, Chey W D, Houghton L A, Mearin F, Spiller R C. Functional bowel disorders. Gastroenterology. 2006;130(05):1480–1491. doi: 10.1053/j.gastro.2005.11.061. [DOI] [PubMed] [Google Scholar]
- 9.Tabbers M M, DiLorenzo C, Berger M Y et al. Evaluation and treatment of functional constipation in infants and children: evidence-based recommendations from ESPGHAN and NASPGHAN. J Pediatr Gastroenterol Nutr. 2014;58(02):258–274. doi: 10.1097/MPG.0000000000000266. [DOI] [PubMed] [Google Scholar]
- 10.Noviello C, Cobellis G, Papparella A, Amici G, Martino A. Role of anorectal manometry in children with severe constipation. Colorectal Dis. 2009;11(05):480–484. doi: 10.1111/j.1463-1318.2008.01654.x. [DOI] [PubMed] [Google Scholar]
- 11.Irani K, Rodriguez L, Doody D P, Goldstein A M. Botulinum toxin for the treatment of chronic constipation in children with internal anal sphincter dysfunction. Pediatr Surg Int. 2008;24(07):779–783. doi: 10.1007/s00383-008-2171-3. [DOI] [PubMed] [Google Scholar]
- 12.Belkind-Gerson J, Surjanhata B, Kuo B, Goldstein A M. Bear-down maneuver is a useful adjunct in the evaluation of children with chronic constipation. J Pediatr Gastroenterol Nutr. 2013;57(06):775–779. doi: 10.1097/MPG.0b013e3182a698df. [DOI] [PubMed] [Google Scholar]
- 13.Lee Y Y, Erdogan A, Rao S S. High resolution and high definition anorectal manometry and pressure topography: diagnostic advance or a new kid on the block? Curr Gastroenterol Rep. 2013;15(12):360. doi: 10.1007/s11894-013-0360-2. [DOI] [PubMed] [Google Scholar]
- 14.Pensabene L, Youssef N N, Griffiths J M, Di Lorenzo C. Colonic manometry in children with defecatory disorders. role in diagnosis and management. Am J Gastroenterol. 2003;98(05):1052–1057. doi: 10.1111/j.1572-0241.2003.07412.x. [DOI] [PubMed] [Google Scholar]
- 15.Villarreal J, Sood M, Zangen T et al. Colonic diversion for intractable constipation in children: colonic manometry helps guide clinical decisions. J Pediatr Gastroenterol Nutr. 2001;33(05):588–591. doi: 10.1097/00005176-200111000-00014. [DOI] [PubMed] [Google Scholar]
- 16.Martin M J, Steele S R, Mullenix P S, Noel J M, Weichmann D, Azarow K S.A pilot study using total colonic manometry in the surgical evaluation of pediatric functional colonic obstruction J Pediatr Surg 20043903352–359., discussion 352–359 [DOI] [PubMed] [Google Scholar]
- 17.Aspirot A, Fernandez S, Di Lorenzo C, Skaggs B, Mousa H. Antegrade enemas for defecation disorders: do they improve the colonic motility? J Pediatr Surg. 2009;44(08):1575–1580. doi: 10.1016/j.jpedsurg.2008.11.061. [DOI] [PubMed] [Google Scholar]
- 18.Christison-Lagay E R, Rodriguez L, Kurtz M, St Pierre K, Doody D P, Goldstein A M.Antegrade colonic enemas and intestinal diversion are highly effective in the management of children with intractable constipation J Pediatr Surg 20104501213–219., discussion 219 [DOI] [PubMed] [Google Scholar]
- 19.Sutcliffe J R, King S K, Hutson J M, Cook D J, Southwell B R. Gastrointestinal transit in children with chronic idiopathic constipation. Pediatr Surg Int. 2009;25(06):465–472. doi: 10.1007/s00383-009-2374-2. [DOI] [PubMed] [Google Scholar]
- 20.Benninga M A, Büller H A, Tytgat G N, Akkermans L M, Bossuyt P M, Taminiau J A. Colonic transit time in constipated children: does pediatric slow-transit constipation exist? J Pediatr Gastroenterol Nutr. 1996;23(03):241–251. doi: 10.1097/00005176-199610000-00007. [DOI] [PubMed] [Google Scholar]
- 21.Reuchlin-Vroklage L M, Bierma-Zeinstra S, Benninga M A, Berger M Y. Diagnostic value of abdominal radiography in constipated children: a systematic review. Arch Pediatr Adolesc Med. 2005;159(07):671–678. doi: 10.1001/archpedi.159.7.671. [DOI] [PubMed] [Google Scholar]
- 22.Rosen R, Buonomo C, Andrade R, Nurko S. Incidence of spinal cord lesions in patients with intractable constipation. J Pediatr. 2004;145(03):409–411. doi: 10.1016/j.jpeds.2004.06.026. [DOI] [PubMed] [Google Scholar]
- 23.Russell K W, Barnhart D C, Zobell S, Scaife E R, Rollins M D. Effectiveness of an organized bowel management program in the management of severe chronic constipation in children. J Pediatr Surg. 2015;50(03):444–447. doi: 10.1016/j.jpedsurg.2014.08.006. [DOI] [PubMed] [Google Scholar]
- 24.Gordon M, Naidoo K, Akobeng A K, Thomas A G. Cochrane review: osmotic and stimulant laxatives for the management of childhood constipation. (Review) Evid Based Child Health. 2013;8(01):57–109. doi: 10.1002/ebch.1893. [DOI] [PubMed] [Google Scholar]
- 25.van der Plas R N, Benninga M A, Büller H Aet al. Biofeedback training in treatment of childhood constipation: a randomised controlled study Lancet 1996348(9030):776–780. [DOI] [PubMed] [Google Scholar]
- 26.Woodward S, Norton C, Chiarelli P. Biofeedback for treatment of chronic idiopathic constipation in adults. Cochrane Database Syst Rev. 2014;3(03):CD008486. doi: 10.1002/14651858.CD008486.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rao S S, Benninga M A, Bharucha A E, Chiarioni G, Di Lorenzo C, Whitehead W E. ANMS-ESNM position paper and consensus guidelines on biofeedback therapy for anorectal disorders. Neurogastroenterol Motil. 2015;27(05):594–609. doi: 10.1111/nmo.12520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.van Engelenburg-van Lonkhuyzen M L, Bols E M, Benninga M A, Verwijs W A, Bluijssen N M, de Bie R A. The effect of pelvic physiotherapy on reduction of functional constipation in children: design of a multicentre randomised controlled trial. BMC Pediatr. 2013;13:112. doi: 10.1186/1471-2431-13-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thomas G P, Dudding T C, Rahbour G, Nicholls R J, Vaizey C J. Sacral nerve stimulation for constipation. Br J Surg. 2013;100(02):174–181. doi: 10.1002/bjs.8944. [DOI] [PubMed] [Google Scholar]
- 30.van Wunnik B P, Baeten C G, Southwell B R. Neuromodulation for constipation: sacral and transcutaneous stimulation. Best Pract Res Clin Gastroenterol. 2011;25(01):181–191. doi: 10.1016/j.bpg.2010.12.008. [DOI] [PubMed] [Google Scholar]
- 31.Thaha M A, Abukar A A, Thin N N, Ramsanahie A, Knowles C H. Sacral nerve stimulation for faecal incontinence and constipation in adults. Cochrane Database Syst Rev. 2015;8(08):CD004464. doi: 10.1002/14651858.CD004464.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.van Wunnik B P, Peeters B, Govaert B, Nieman F H, Benninga M A, Baeten C G. Sacral neuromodulation therapy: a promising treatment for adolescents with refractory functional constipation. Dis Colon Rectum. 2012;55(03):278–285. doi: 10.1097/DCR.0b013e3182405c61. [DOI] [PubMed] [Google Scholar]
- 33.Ismail K A, Chase J, Gibb S et al. Daily transabdominal electrical stimulation at home increased defecation in children with slow-transit constipation: a pilot study. J Pediatr Surg. 2009;44(12):2388–2392. doi: 10.1016/j.jpedsurg.2009.07.063. [DOI] [PubMed] [Google Scholar]
- 34.Clarke M C, Chase J W, Gibb S, Hutson J M, Southwell B R.Improvement of quality of life in children with slow transit constipation after treatment with transcutaneous electrical stimulation J Pediatr Surg 200944061268–1272., discussion 1272 [DOI] [PubMed] [Google Scholar]
- 35.Clarke M C, Chase J W, Gibb S et al. Decreased colonic transit time after transcutaneous interferential electrical stimulation in children with slow transit constipation. J Pediatr Surg. 2009;44(02):408–412. doi: 10.1016/j.jpedsurg.2008.10.100. [DOI] [PubMed] [Google Scholar]
- 36.Leong L C, Yik Y I, Catto-Smith A G, Robertson V J, Hutson J M, Southwell B R. Long-term effects of transabdominal electrical stimulation in treating children with slow-transit constipation. J Pediatr Surg. 2011;46(12):2309–2312. doi: 10.1016/j.jpedsurg.2011.09.022. [DOI] [PubMed] [Google Scholar]
- 37.Sugarman I. Treatment of severe childhood constipation with restorative proctocolectomy: the surgeon's view. Arch Dis Child. 2010;95(11):861–862. doi: 10.1136/adc.2009.180810. [DOI] [PubMed] [Google Scholar]
- 38.Siminas S, Losty P D. Current surgical management of pediatric idiopathic constipation: a systematic review of published studies. Ann Surg. 2015;262(06):925–933. doi: 10.1097/SLA.0000000000001191. [DOI] [PubMed] [Google Scholar]
- 39.Kuizenga-Wessel S, Mousa H M, Benninga M A, Di Lorenzo C. Lack of agreement on how to use antegrade enemas in children. J Pediatr Gastroenterol Nutr. 2016;62(01):71–79. doi: 10.1097/MPG.0000000000000899. [DOI] [PubMed] [Google Scholar]
- 40.Wood R J, Yacob D, Levitt M A. Surgical options for the management of severe functional constipation in children. Curr Opin Pediatr. 2016;28(03):370–379. doi: 10.1097/MOP.0000000000000345. [DOI] [PubMed] [Google Scholar]
- 41.Levitt M A, Mathis K L, Pemberton J H. Surgical treatment for constipation in children and adults. Best Pract Res Clin Gastroenterol. 2011;25(01):167–179. doi: 10.1016/j.bpg.2010.12.007. [DOI] [PubMed] [Google Scholar]
- 42.Keshtgar A S, Ward H C, Clayden G S. Pathophysiology of chronic childhood constipation: functional and morphological evaluation by anorectal manometry and endosonography and colonic transit study. J Pediatr Surg. 2013;48(04):806–812. doi: 10.1016/j.jpedsurg.2012.08.037. [DOI] [PubMed] [Google Scholar]
- 43.Keshtgar A S, Ward H C, Clayden G S, Sanei A.Thickening of the internal anal sphincter in idiopathic constipation in children Pediatr Surg Int 200420(11-12):817–823. [DOI] [PubMed] [Google Scholar]
- 44.Hosie G P, Spitz L.Idiopathic constipation in childhood is associated with thickening of the internal anal sphincter J Pediatr Surg 199732071041–1043., discussion 1043–1044 [DOI] [PubMed] [Google Scholar]
- 45.Clayden G S, Lawson J O. Investigation and management of long-standing chronic constipation in childhood. Arch Dis Child. 1976;51(12):918–923. doi: 10.1136/adc.51.12.918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Keshtgar A S, Ward H C, Clayden G S, Sanei A. Role of anal dilatation in treatment of idiopathic constipation in children: long-term follow-up of a double-blind randomized controlled study. Pediatr Surg Int. 2005;21(02):100–105. doi: 10.1007/s00383-004-1336-y. [DOI] [PubMed] [Google Scholar]
- 47.De Caluwé D, Yoneda A, Akl U, Puri P. Internal anal sphincter achalasia: outcome after internal sphincter myectomy. J Pediatr Surg. 2001;36(05):736–738. doi: 10.1053/jpsu.2001.22949. [DOI] [PubMed] [Google Scholar]
- 48.Krebs C, Acuña R. Transanal internal sphincter myomectomy: indications, operative procedure and results. Eur J Pediatr Surg. 1994;4(03):151–157. doi: 10.1055/s-2008-1066090. [DOI] [PubMed] [Google Scholar]
- 49.Mishalany H. Seven years' experience with idiopathic unremitting chronic constipation. J Pediatr Surg. 1989;24(04):360–362. doi: 10.1016/s0022-3468(89)80269-6. [DOI] [PubMed] [Google Scholar]
- 50.Langer J C, Birnbaum E.Preliminary experience with intrasphincteric botulinum toxin for persistent constipation after pull-through for Hirschsprung's disease J Pediatr Surg 199732071059–1061., discussion 1061–1062 [DOI] [PubMed] [Google Scholar]
- 51.Minkes R K, Langer J C. A prospective study of botulinum toxin for internal anal sphincter hypertonicity in children with Hirschsprung's disease. J Pediatr Surg. 2000;35(12):1733–1736. doi: 10.1053/jpsu.2000.19234. [DOI] [PubMed] [Google Scholar]
- 52.Ahmadi J, Azary S, Ashjaei B, Paragomi P, Khalifeh-Soltani A. Intrasphincteric botulinum toxin injection in treatment of chronic idiopathic constipation in children. Iran J Pediatr. 2013;23(05):574–578. [PMC free article] [PubMed] [Google Scholar]
- 53.Keshtgar A S, Ward H C, Sanei A, Clayden G S. Botulinum toxin, a new treatment modality for chronic idiopathic constipation in children: long-term follow-up of a double-blind randomized trial. J Pediatr Surg. 2007;42(04):672–680. doi: 10.1016/j.jpedsurg.2006.12.045. [DOI] [PubMed] [Google Scholar]
- 54.Chumpitazi B P, Fishman S J, Nurko S. Long-term clinical outcome after botulinum toxin injection in children with nonrelaxing internal anal sphincter. Am J Gastroenterol. 2009;104(04):976–983. doi: 10.1038/ajg.2008.110. [DOI] [PubMed] [Google Scholar]
- 55.Keshtgar A S, Ward H C, Clayden G S. Diagnosis and management of children with intractable constipation. Semin Pediatr Surg. 2004;13(04):300–309. doi: 10.1053/j.sempedsurg.2004.10.018. [DOI] [PubMed] [Google Scholar]
- 56.Keshtgar A S, Ward H C, Clayden G S. Transcutaneous needle-free injection of botulinum toxin: a novel treatment of childhood constipation and anal fissure. J Pediatr Surg. 2009;44(09):1791–1798. doi: 10.1016/j.jpedsurg.2009.02.056. [DOI] [PubMed] [Google Scholar]
- 57.Malone P S, Ransley P G, Kiely E M.Preliminary report: the antegrade continence enema Lancet 1990336(8725):1217–1218. [DOI] [PubMed] [Google Scholar]
- 58.Graf J L, Strear C, Bratton B et al. The antegrade continence enema procedure: a review of the literature. J Pediatr Surg. 1998;33(08):1294–1296. doi: 10.1016/s0022-3468(98)90172-5. [DOI] [PubMed] [Google Scholar]
- 59.Levitt M A, Soffer S Z, Peña A. Continent appendicostomy in the bowel management of fecally incontinent children. J Pediatr Surg. 1997;32(11):1630–1633. doi: 10.1016/s0022-3468(97)90470-x. [DOI] [PubMed] [Google Scholar]
- 60.Wilcox D T, Kiely E M. The Malone (antegrade colonic enema) procedure: early experience. J Pediatr Surg. 1998;33(02):204–206. doi: 10.1016/s0022-3468(98)90432-8. [DOI] [PubMed] [Google Scholar]
- 61.Lynch A C, Beasley S W, Robertson R W, Morreau P N.Comparison of results of laparoscopic and open antegrade continence enema procedures Pediatr Surg Int 199915(5-6):343–346. [DOI] [PubMed] [Google Scholar]
- 62.Kiely E M, Ade-Ajayi N, Wheeler R A. Caecal flap conduit for antegrade continence enemas. Br J Surg. 1994;81(08):1215. doi: 10.1002/bjs.1800810847. [DOI] [PubMed] [Google Scholar]
- 63.Wong A L, Kravarusic D, Wong S L. Impact of cecostomy and antegrade colonic enemas on management of fecal incontinence and constipation: ten years of experience in pediatric population. J Pediatr Surg. 2008;43(08):1445–1451. doi: 10.1016/j.jpedsurg.2007.12.051. [DOI] [PubMed] [Google Scholar]
- 64.Rivera M T, Kugathasan S, Berger W, Werlin S L. Percutaneous colonoscopic cecostomy for management of chronic constipation in children. Gastrointest Endosc. 2001;53(02):225–228. doi: 10.1067/mge.2001.112182. [DOI] [PubMed] [Google Scholar]
- 65.Shandling B, Chait P G, Richards H F. Percutaneous cecostomy: a new technique in the management of fecal incontinence. J Pediatr Surg. 1996;31(04):534–537. doi: 10.1016/s0022-3468(96)90490-x. [DOI] [PubMed] [Google Scholar]
- 66.Sinha C K, Butler C, Haddad M. Left antegrade continent enema (LACE): review of the literature. Eur J Pediatr Surg. 2008;18(04):215–218. doi: 10.1055/s-2008-1038499. [DOI] [PubMed] [Google Scholar]
- 67.Chang H K, Chang E Y, Han S J, Choi S H, Oh J T. Long-term outcome of left- vs right-sided antegrade continence enema. J Pediatr Surg. 2012;47(10):1880–1885. doi: 10.1016/j.jpedsurg.2012.05.031. [DOI] [PubMed] [Google Scholar]
- 68.Rodriguez L, Flores A, Gilchrist B F, Goldstein A M. Laparoscopic-assisted percutaneous endoscopic cecostomy in children with defecation disorders (with video) Gastrointest Endosc. 2011;73(01):98–102. doi: 10.1016/j.gie.2010.09.011. [DOI] [PubMed] [Google Scholar]
- 69.Siddiqui A A, Fishman S J, Bauer S B, Nurko S. Long-term follow-up of patients after antegrade continence enema procedure. J Pediatr Gastroenterol Nutr. 2011;52(05):574–580. doi: 10.1097/MPG.0b013e3181ff6042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mugie S M, Machado R S, Mousa H M et al. Ten-year experience using antegrade enemas in children. J Pediatr. 2012;161(04):700–704. doi: 10.1016/j.jpeds.2012.04.042. [DOI] [PubMed] [Google Scholar]
- 71.Mousa H M, van den Berg M M, Caniano D A, Hogan M, Di Lorenzo C, Hayes J. Cecostomy in children with defecation disorders. Dig Dis Sci. 2006;51(01):154–160. doi: 10.1007/s10620-006-3101-7. [DOI] [PubMed] [Google Scholar]
- 72.Cascio S, Flett M E, De la Hunt M, Barrett A M, Jaffray B. MACE or caecostomy button for idiopathic constipation in children: a comparison of complications and outcomes. Pediatr Surg Int. 2004;20(07):484–487. doi: 10.1007/s00383-004-1220-9. [DOI] [PubMed] [Google Scholar]
- 73.Hoekstra L T, Kuijper C F, Bakx R, Heij H A, Aronson D C, Benninga M A. The Malone antegrade continence enema procedure: the Amsterdam experience. J Pediatr Surg. 2011;46(08):1603–1608. doi: 10.1016/j.jpedsurg.2011.04.050. [DOI] [PubMed] [Google Scholar]
- 74.King S K, Sutcliffe J R, Southwell B R, Chait P G, Hutson J M. The antegrade continence enema successfully treats idiopathic slow-transit constipation. J Pediatr Surg. 2005;40(12):1935–1940. doi: 10.1016/j.jpedsurg.2005.08.011. [DOI] [PubMed] [Google Scholar]
- 75.Bonilla S F, Flores A, Jackson C C, Chwals W J, Orkin B A. Management of pediatric patients with refractory constipation who fail cecostomy. J Pediatr Surg. 2013;48(09):1931–1935. doi: 10.1016/j.jpedsurg.2012.12.034. [DOI] [PubMed] [Google Scholar]
- 76.Chan D S, Delicata R J. Meta-analysis of antegrade continence enema in adults with faecal incontinence and constipation. Br J Surg. 2016;103(04):322–327. doi: 10.1002/bjs.10051. [DOI] [PubMed] [Google Scholar]
- 77.Peeraully M R, Lopes J, Wright A et al. Experience of the MACE procedure at a regional pediatric surgical unit: a 15-year retrospective review. Eur J Pediatr Surg. 2014;24(01):113–116. doi: 10.1055/s-0033-1357502. [DOI] [PubMed] [Google Scholar]
- 78.Curry J I, Osborne A, Malone P S. The MACE procedure: experience in the United Kingdom. J Pediatr Surg. 1999;34(02):338–340. doi: 10.1016/s0022-3468(99)90204-x. [DOI] [PubMed] [Google Scholar]
- 79.Randall J, Coyne P, Jaffray B. Follow up of children undergoing antegrade continent enema: experience of over two hundred cases. J Pediatr Surg. 2014;49(09):1405–1408. doi: 10.1016/j.jpedsurg.2014.02.090. [DOI] [PubMed] [Google Scholar]
- 80.Levitt M A, Martin C A, Falcone R A, Jr, Peña A.Transanal rectosigmoid resection for severe intractable idiopathic constipation J Pediatr Surg 200944061285–1290., discussion 1290–1291 [DOI] [PubMed] [Google Scholar]
- 81.Lee S L, DuBois J J, Montes-Garces R G, Inglis K, Biediger W. Surgical management of chronic unremitting constipation and fecal incontinence associated with megarectum: a preliminary report. J Pediatr Surg. 2002;37(01):76–79. doi: 10.1053/jpsu.2002.29431. [DOI] [PubMed] [Google Scholar]
- 82.Godbole P P, Pinfield A, Stringer M D. Idiopathic megarectum in children. Eur J Pediatr Surg. 2001;11(01):48–51. doi: 10.1055/s-2001-12203. [DOI] [PubMed] [Google Scholar]
- 83.Kerur B, Kantekure K, Bonilla S, Orkin B, Flores A F. Management of chronic intractable constipation in children. J Pediatr Gastroenterol Nutr. 2014;59(06):754–757. doi: 10.1097/MPG.0000000000000535. [DOI] [PubMed] [Google Scholar]
- 84.Levitt M A, Peña A. Surgery and constipation: when, how, yes, or no? J Pediatr Gastroenterol Nutr. 2005;41 01:S58–S60. doi: 10.1097/01.scs.0000180308.02052.b2. [DOI] [PubMed] [Google Scholar]
- 85.Stabile G, Kamm M A, Hawley P R, Lennard-Jones J E. Results of the Duhamel operation in the treatment of idiopathic megarectum and megacolon. Br J Surg. 1991;78(06):661–663. doi: 10.1002/bjs.1800780609. [DOI] [PubMed] [Google Scholar]
- 86.Gladman M A, Scott S M, Lunniss P J, Williams N S. Systematic review of surgical options for idiopathic megarectum and megacolon. Ann Surg. 2005;241(04):562–574. doi: 10.1097/01.sla.0000157140.69695.d3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kamm M A, Stabile G. Management of idiopathic megarectum and megacolon. Br J Surg. 1991;78(08):899–900. doi: 10.1002/bjs.1800780803. [DOI] [PubMed] [Google Scholar]
- 88.Stabile G, Kamm M A, Hawley P R, Lennard-Jones J E. Colectomy for idiopathic megarectum and megacolon. Gut. 1991;32(12):1538–1540. doi: 10.1136/gut.32.12.1538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Stabile G, Kamm M A. Surgery for idiopathic megarectum and megacolon. Int J Colorectal Dis. 1991;6(03):171–174. doi: 10.1007/BF00341241. [DOI] [PubMed] [Google Scholar]
- 90.Youssef N N, Pensabene L, Barksdale E, Jr, Di Lorenzo C. Is there a role for surgery beyond colonic aganglionosis and anorectal malformations in children with intractable constipation? J Pediatr Surg. 2004;39(01):73–77. doi: 10.1016/j.jpedsurg.2003.09.007. [DOI] [PubMed] [Google Scholar]
- 91.Asipu D, Jaffray B. Treatment of severe childhood constipation with restorative proctocolectomy. Arch Dis Child. 2010;95(11):867–870. doi: 10.1136/adc.2009.172973. [DOI] [PubMed] [Google Scholar]
- 92.Hosie K B, Kmiot W A, Keighley M R. Constipation: another indication for restorative proctocolectomy. Br J Surg. 1990;77(07):801–802. doi: 10.1002/bjs.1800770726. [DOI] [PubMed] [Google Scholar]
- 93.Stewart J, Kumar D, Keighley M R. Results of anal or low rectal anastomosis and pouch construction for megarectum and megacolon. Br J Surg. 1994;81(07):1051–1053. doi: 10.1002/bjs.1800810742. [DOI] [PubMed] [Google Scholar]
- 94.Thakur A, Fonkalsrud E W, Buchmiller T, French S. Surgical treatment of severe colonic inertia with restorative proctocolectomy. Am Surg. 2001;67(01):36–40. [PubMed] [Google Scholar]
- 95.Woodward M N, Foley P, Cusick E L. Colostomy for treatment of functional constipation in children: a preliminary report. J Pediatr Gastroenterol Nutr. 2004;38(01):75–78. doi: 10.1097/00005176-200401000-00017. [DOI] [PubMed] [Google Scholar]
- 96.Park J J, Del Pino A, Orsay C P et al. Stoma complications: the Cook County Hospital experience. Dis Colon Rectum. 1999;42(12):1575–1580. doi: 10.1007/BF02236210. [DOI] [PubMed] [Google Scholar]
- 97.Stabile G, Kamm M A, Hawley P R, Lennard-Jones J E. Results of stoma formation for idiopathic megarectum and megacolon. Int J Colorectal Dis. 1992;7(02):82–84. doi: 10.1007/BF00341291. [DOI] [PubMed] [Google Scholar]
- 98.Kuizenga-Wessel S, Benninga M A, Tabbers M M. Reporting outcome measures of functional constipation in children from 0 to 4 years of age. J Pediatr Gastroenterol Nutr. 2015;60(04):446–456. doi: 10.1097/MPG.0000000000000631. [DOI] [PubMed] [Google Scholar]
- 99.Kuizenga-Wessel S, Heckert S L, Tros W, van Etten-Jamaludin F S, Benninga M A, Tabbers M M. Reporting on outcome measures of functional constipation in children—a systematic review. J Pediatr Gastroenterol Nutr. 2016;62(06):840–846. doi: 10.1097/MPG.0000000000001110. [DOI] [PubMed] [Google Scholar]
- 100.van Ginkel R, Reitsma J B, Büller H A, van Wijk M P, Taminiau J A, Benninga M A. Childhood constipation: longitudinal follow-up beyond puberty. Gastroenterology. 2003;125(02):357–363. doi: 10.1016/s0016-5085(03)00888-6. [DOI] [PubMed] [Google Scholar]
- 101.Pijpers M A, Bongers M E, Benninga M A, Berger M Y. Functional constipation in children: a systematic review on prognosis and predictive factors. J Pediatr Gastroenterol Nutr. 2010;50(03):256–268. doi: 10.1097/MPG.0b013e3181afcdc3. [DOI] [PubMed] [Google Scholar]
- 102.Bongers M E, van Wijk M P, Reitsma J B, Benninga M A. Long-term prognosis for childhood constipation: clinical outcomes in adulthood. Pediatrics. 2010;126(01):e156–e162. doi: 10.1542/peds.2009-1009. [DOI] [PubMed] [Google Scholar]
- 103.Tran K, Staller K, Macklin E, Goldstein A, Belkind-Gerson J, Kuo B. Need for rectal biopsy for childhood constipation predicts severity of illness and need for laxatives. J Pediatr Gastroenterol Nutr. 2016;62(06):834–839. doi: 10.1097/MPG.0000000000001033. [DOI] [PubMed] [Google Scholar]
- 104.Jaffray B. What happens to children with idiopathic constipation who receive an antegrade continent enema? An actuarial analysis of 80 consecutive cases. J Pediatr Surg. 2009;44(02):404–407. doi: 10.1016/j.jpedsurg.2008.10.097. [DOI] [PubMed] [Google Scholar]
- 105.Pensabene L, Youssef N N, Di Lorenzo C. [Success of antegrade enemas in children with functional constipation] Pediatr Med Chir. 2003;25(02):126–130. [PubMed] [Google Scholar]
- 106.Youssef N N, Barksdale E, Jr, Griffiths J M, Flores A F, Di Lorenzo C. Management of intractable constipation with antegrade enemas in neurologically intact children. J Pediatr Gastroenterol Nutr. 2002;34(04):402–405. doi: 10.1097/00005176-200204000-00016. [DOI] [PubMed] [Google Scholar]
- 107.Platell C, Scache D, Mumme G, Stitz R. A long-term follow-up of patients undergoing colectomy for chronic idiopathic constipation. Aust N Z J Surg. 1996;66(08):525–529. doi: 10.1111/j.1445-2197.1996.tb00802.x. [DOI] [PubMed] [Google Scholar]
- 108.Hutson J M, Wheatley J, Uemura S, Hurley M.A long-term follow-up of patients undergoing colectomy for chronic idiopathic constipation Aust N Z J Surg 199767(2-3):136, author reply 137 [DOI] [PubMed] [Google Scholar]


