Abstract
Rectal prolapse is a common and self-limiting condition in infancy and early childhood. Most cases respond to conservative management. Patients younger than 4 years with an associated condition have a better prognosis. Patients older than 4 years require surgery more often than younger children. Multiple operative and procedural approaches to rectal prolapse exist with variable recurrence rates and without a clearly superior operation. These include sclerotherapy, Thiersch's anal cerclage, Ekehorn's rectopexy, laparoscopic suture rectopexy, and posterior sagittal rectopexy.
Keywords: rectal prolapse, pediatric, sclerotherapy, surgery, rectopexy
Rectal prolapse (RP) refers to the extrusion of some or all of the rectal mucosa through the external anal sphincter. RP seldom occurs in children who do not have an underlying predisposing condition and usually occurs between infancy and 4 years of age, with the highest incidence in the first year of life. 1 During childhood, RP occurs with equal frequency in boys and girls. 2 3 In developing countries, most cases of RP have been attributed to acute diarrheal disease and intestinal parasite infestation, usually associated with malnutrition. 4 In the United states, most instances of RP are not related to cystic fibrosis (CF) but rather to stool abnormalities such as acute diarrhea and chronic constipation or neurologic or anatomic defects. 5
Types
There are two types of RP ( Fig. 1 ). Type I which is also called false procidentia, partial or mucosal prolapse, involves protrusion of the mucosa only and usually is less than 2 cm long. Partial RP produces radial folds at the junction with the anal skin; this is an important distinguishing characteristic. Type II which is also called true procidentia, or complete prolapse, involves full-thickness extrusion of the rectal wall characterized by concentric folds in the prolapsed mucosa. This is functionally intussusception of the rectum which can be further subdivided based on the level of intussusception. First-degree prolapse includes the mucocutaneous junction; the protrusion from the anal verge usually is greater than 5 cm. Second-degree prolapse is between 2 and 5 cm protrusion, and third-degree prolapse is internal, concealed, or occult and does not pass through the anal verge. 6
Fig. 1.
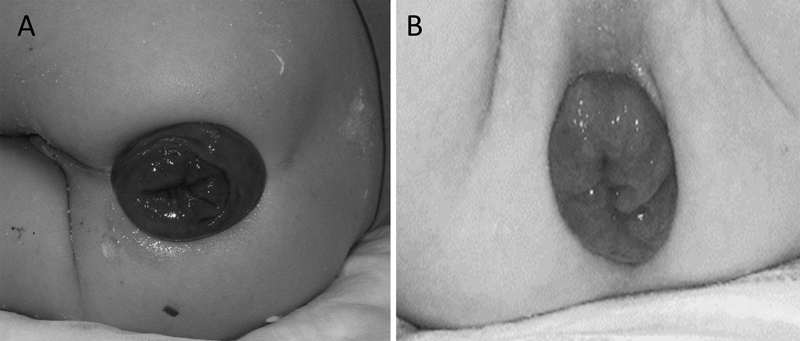
Rectal prolapse. ( A ) Complete rectal prolapse—the everted rectal wall appears as a tubular mass made up of concentric mucosal folds. ( B ) Mucosal rectal prolapse.
Pathogenesis
Children are predisposed to RP due to anatomic considerations which may account for the increased incidence in early childhood. They have a more vertical course of the rectum, flatter coccyx; relatively weak levator support and a relatively low position of the rectum in the pelvis; more sigmoid colon mobility; redundant rectal mucosa is attached only loosely to the underlying muscularis; and Houston's valves are absent in approximately 75% of infants younger than 1 year of age. 7 8 9 10 11 Based on a combination of information from adult anatomic, manometry, and dynamic radiologic studies in children, 12 RP is thought to develop as a result of circumferential intussusception of the upper rectum and rectosigmoid colon. These could all be reasons for the highest incidence in the first year of life. The gender incidence is almost equal in children compared with adults among whom females are six times more susceptible. 13
Predisposing conditions
The finding of RP should be considered as a symptom of an underlying condition that predisposes to RP. Conditions associated or predisposing to development of RP include increased bowel motility (organisms causing infectious diarrhea— Amebiasis , Giardiasis , Trichuriasis , Salmonella , Shigella , and Escherichia coli 0157:H7 and noninfectious ulcerative colitis and laxative abuse), increased abdominal pressure (chronic constipation, protracted coughing, excessive vomiting, straining at urination with phimosis), congenital conditions (CF, myelomeningocele, Hirschsprung's disease, spina bifida, congenital hypothyroidism), and miscellaneous conditions (mucosal polyps/tumors, imperforate anus postrepair, malnutrition). 14 RP was attributed to chronic constipation in 28%, diarrheal disease in 20%, CF in 11%, and to neurologic or anatomic conditions in 24%; no underlying cause was identified in the remaining 17%. 15
RP has been associated with many underlying diseases and incidence varies with geographic and socioeconomic factors. It is much more common in undeveloped countries, where parasitic disease, malnutrition, and diarrheal illness are high. Contributing factors in the industrialized world include constipation, pertussis, CF, and polyps. In the United States, constipation is the most common association. CF is associated with nearly a 20% incidence of prolapse in some reports, but most children with prolapse do not have undiagnosed CF, particularly in the absence of symptoms. 16 17 Interestingly, none of the patients with CF have a history of constipation and instead most frequently present with a history consistent with fat malabsorption. 5
Clinical Manifestations
RP often presents as a dark red mass (70%) with or without mucous and blood (25%) that protrudes from the rectum during straining. 18 The mass typically is detected by the parents, who bring the child to medical attention. As a general rule, prolapse is painless, but it can be associated with mild discomfort. A digital rectal exam may reveal decreased or absent anal tone initially that returns to normal within a few hours. In patients with a neurologic condition, decreased sphincter tone may persist. Adolescent patients usually complain of tenesmus, anorectal pain, and passage of blood and mucous. 6
Diagnosis
Evaluation of children with RP ranges from straightforward to difficult. Physical examination is often normal because the prolapse cannot be evoked in the clinic setting, and the diagnosis is based on history. Many children have often undergone gastrointestinal consultation, barium enemas, colonoscopies, etc. However, an extensive evaluation is unnecessary in most uncomplicated cases, particularly in the absence of a history of rectal bleeding, constipation/diarrhea, or associated abnormalities.
The diagnosis of RP can be made based on history alone and, when presently active, is obvious on physical exam. A painless, dark-red mass at the anal verge with or without mucous is present on physical examination if the prolapse has not spontaneous reduced. If spontaneous reduction has occurred, the parent's description of such a mass that appeared during straining may be the information to make the diagnosis. When a swelling or protruding anal lesion in a child is discovered by parents and visual inspection of the anal region and digital rectal examination is normal, parents should be encouraged to take photos or videos of the anal region before performing additional diagnostic testing such as air contrast enema and/or sigmoidoscopy. These photos and videos can provide a definitive diagnosis and prevent unnecessary diagnostic procedures. 19
Examining the child in the squatting position or asking him or her to strain may demonstrate the RP. Palpation of the prolapsed mucosa between the thumb and forefinger permits the examiner to distinguish between mucosal or complete RP. 6 The diagnosis of occult RP usually is made during sigmoidoscopy that reveals erythema and granularity of the distal rectum, or a polypoid white-toped mucosal lesion on the anterior rectal wall. 20 Occult RP may be an early finding in solitary ulcer of the rectum syndrome. 20 21
In the era of newborn screening for CF, 3.6% of patients with RP had CF, and conversely, 3.5% of patients with CF have RP. 22 Earlier studies quoted a higher incidence of RP in children with CF, often occurring in approximately 20% of cases usually between 6 months and 3 years of age and often preceding the diagnosis of CF. 17 Some authors recommend a sweat test for all cases of RP. 5 17 However, in children with CF, the prolapse is often recurrent and probably related to malnutrition, poor muscle tone, and passage of voluminous stools. 22 23 In a series of 16 cases with CF screening, a positive result was found only in one patient. 18 CF screening should be reserved for cases that have no underlying diagnosis and have recurrent RP refractory to conservative treatment. 24
Colonoscopy can be performed if solitary rectal ulcer or rectal polyps are suspected. Patients with constipation or defecation disorders underwent contrast radiography of the colon and anorectal manometry. 25 Dynamic defecography (DD) is one of the most valuable methods for evaluation of pelvic floor disorders. While its utility has reached a consensus in adult disorders, it is limited in children. It can evaluate both structural and functional disorders not seen under resting conditions. 26 Defecography has a false-negative rate of 37% and in some patients, despite negative DD, surgical treatment for prolapse was performed on clinical grounds. Defecography has the advantage of displaying pathological sequences in a simulated defecation in a conscious patient, thus enabling the clinician to pay attention to correct that pathology. It is recommended in patients with atypical symptoms, with an age greater than 10 years or if rectal ulcer is suspected. It can be used as an adjunct to the assessment of RP and related disorders. 27 A study performed DD for all patients who were likely to be cooperative during the investigation 13 of 27 (48%) of patients. 25 If symptoms were consistent with radiologic imaging, operative intervention was recommended even if RP was not clinically evident. 25
Differential Diagnosis
Several entities should be considered in the differential diagnosis of RP. These include ileocecal intussusception, prolapsing rectal polyp, prolapsing rectal duplication cyst, and rectal hemorrhoids. 28 29 30 Children presenting with intussusception typically appear ill and presents with intermittent severe abdominal pain. For protruding intussusception, the examiner's finger can be passed between the apex of the prolapsed bowel and the anal sphincter. 6 In contrast to RP, the protruding mucosa is continuous with the perianal skin. Examination of the prolapsed tissue can distinguish polyps and hemorrhoids from RP because prolapsed polyps and hemorrhoids do not involve the entire rectal mucosa and do not have a hole in the middle.
Management
The primary management goal for RP is directed toward diagnosing and treating the predisposing condition ( Fig. 2 ). Manual reduction should be undertaken as soon as possible if spontaneous reduction does not occur as RP will eventually become more difficult to reduce. 6 While manual reduction seems intuitive, some of the finer points include administration of light sedation, child placed in knee-chest position on exam table or parent's lap, and the examiner's finger to be placed in the rectum to guide reversal of the prolapse. The prolapse should reduce in 5 to 15 minutes. Sugar can be applied if it is difficult which acutely reduces swelling by osmotic fluid shift. 31 After reduction, immediate recurrence can be tempered by taping of the buttocks. 6 Parents and patients can be taught to manually reduce the prolapse if it recurs. Patients should be sent home with a bowel regimen consisting of either stool softeners for constipation or adjustment of pancreatic enzyme for children with CF to treat the presumed underlying cause of the prolapse. Avoidance of prolonged straining along with softeners is a sufficient treatment for most children. The bowel regimen should continue until the child has regular bowel movements for several months without RP. It is important to achieve this early because the more the episodes of RP, especially those cases that do not reduce spontaneously and have a difficult reduction, the less responsive they are to conservative management. 18
Fig. 2.
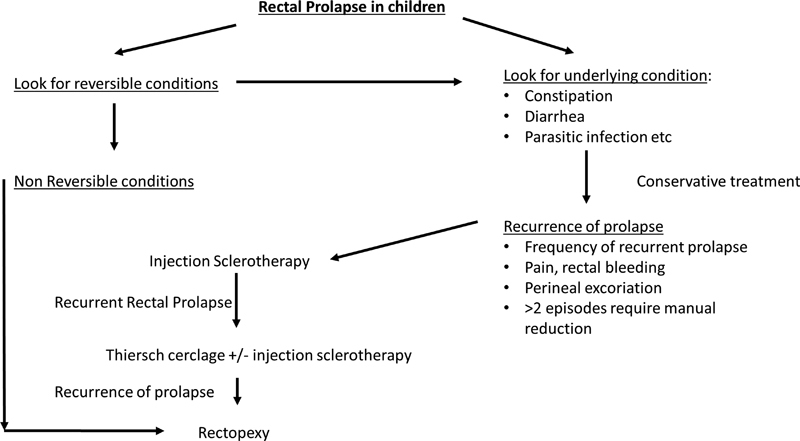
Algorithm for the management of rectal prolapse in children.
Medical and surgical treatment options are varied. The potential for spontaneous resolution of the RP complicates decision-making because reports of highly effective procedures in a relatively large number of children may be the result of patient selection rather than efficacy of the procedure. Additionally, no randomized clinical trials between the treatment options have been conducted. Surgical options include anal encirclement, excision and resection, injection sclerotherapy, packing, linear cauterization, and rectopexy. 14 Treatment for RP should be aimed at the anatomical defect rather than be chosen because of familiarity of the surgical procedure.
The decision about when to operate remains a difficult one. RP in children younger than 4 years is usually a self-limiting disorder, and most patients respond to conservative management within 1 year, 24 32 although some authors recommend only full 8 weeks. 33 While it is difficult to determine the length of conservative treatment, in a study of 341 patients, most responded within the first few weeks, and only 20 (5.8%) required treatment for longer than 3 months, with the average duration of presurgical treatment of 6 months. 34 Indications for operative intervention are not definite and include longstanding symptoms, rectal pain, bleeding, ulceration, and prolapse that requires frequent manual reductions or is difficult to reduce. 25 Predisposing conditions and older age (>4–5 years) may influence an earlier progression to operative repair. Several authors recommend a more aggressive approach in children older than 4 years, as they may have a higher failure rate when managed nonoperatively. 6 18 A 20-year retrospective review of RP in 45 children older than 3 years found that 53% had behavioral/psychiatric disorders and highlighted that this group of patients may benefit from a multidisciplinary approach with a combination of behavioral therapy, physical therapy, and surgical intervention to obtain the most optimal outcome. 35
When patients fail conservative therapy, a multitude of surgical options exist, and the difficulty becomes which of the 130 surgical procedures to choose from. 36 Much of the surgical literature on RP refers almost exclusively to adults. A Cochrane database review of the surgical management of RP in adults concluded that laparoscopic rectopexy was associated with less fever, postoperative complications, and shorter hospital stay than open rectopexy. 37 The disease is different in children and many adult techniques such as prosthetic slings are not often applicable. 38 The pediatric surgeon is faced with procedures that depend largely on individual surgeon experience and cohort case-controlled or case series data. For children with persistent RP, several surgical/procedural options exist. The four most commonly cited include sclerotherapy, Thiersch's anal cerclage, 39 40 41 Ekehorn's rectosacropexy, 34 transabdominal sacral rectopexy with or without sigmoid resection, 42 43 and sacral rectopexy through a posterior incision. 42 Importantly, operative and procedural therapies that are often successful in children younger than 4 years do not have the same success rate in older children, likely due to a difference in the comorbidities and etiology.
Sclerotherapy
Injection of a sclerosant is touted as the first-line therapy by several authors as quick, simple, painless procedure. It is performed as a submucosal injection above the dentate line and in general gives the advantages of simple technique, short hospitalization, fast healing without burdening patients, and minimal complications. 44 The sclerosing agents initiate an inflammatory reaction resulting in fibrosis outside the rectal wall and the perirectal tissue that leads to the wall of the rectum adhering to the perirectal tissue and this prevents recurrence of the prolapse. 45 46 The inflammation resulting from the sclerosant therapy carries the risk of being greater than what is desired. However, sclerotherapy requires general anesthesia and frequently may require more than one application. Many different sclerosing agents have been used in the treatment of RP. 1 2 24 31 33 44 46 47 48 49 50 51 The success rates and complications of the treatment reported in the literature differ depending on the sclerosing agent utilized. Some agents have good results but higher complication rates compared with other agents, while others have no complications but lower cure rates. Some agents were effective without complication, but the injection of the agent was very difficult. Often sclerotherapy can be used in combination with other methods of repair.
Several different agents have been used for sclerotherapy. For 98% ethyl alcohol, there was a reported recurrence rate of 11%, with two patients having mucosal sloughing and one patient developing rectovaginal fistula. 47 Seventy percent ethyl alcohol resulted in 96% cure rate after one injection with a complication rate of 2.3%. 33 50 Five percent phenol in almond oil achieved a 91% cure rate without any complication in one study, 48 but an 18% complication rate in another (mucosal sloughing, perianal fistula, and abscess formation). 47 A death due to phenol toxicity has been reported which should probably preclude its use given the benign nature of the condition. 14 Fifty percent dextrose was reported to be 64% successful with no complications. 1 The cure rate for 30% saline solution ranged from 78 to 83% after one injection and 94 to 97% after two injections, with a complication rate of 9.8 to 13.3% (perirectal space masses, perirectal abscess, and necrosis of the rectal mucosa). 2 45 Fifteen percent saline achieved a 93.7% cure rate after one injection without complications, 24 while another study demonstrated 83% success with the first injection for children younger than 5 years but no success with full-thickness prolapse in children older than 5 years. 51 Many authors have demonstrated that these injections often do not work on older children. 33 51 While submucosal injection is clearly a reasonable first option given the low risk and simplicity, we feel it is less likely to provide an enduring result with full-thickness prolapse that protrudes more than 2 to 3 cm. Minor full-thickness and partial-thickness prolapse responds well to injection, and we use Sotradecol.
Thiersch's Anal Cerclage
Thiersch's anal cerclage consists of placing a circumferential stich at the anal opening using electrocautery to create a small defect at the posterior border of the skin and anal mucosa and placing a circumferential absorbable suture such as a 1–0 PDS and tying it down over Hagar's dilators. Originally, this was described using a wire suture which had to be retrieved. 39 40 41 Modifications on this technique include the addition of sclerotherapy with D 25 W (dextrose 25% in water) and linear cauterization as well as tying the suture over a combination of two Hegar dilators. 39 Overall success rate is 90%, but of the successful percentage 67% of children required only one procedure and 23% requiring two or more Thiersch's procedures. The mean age at initial Thiersch's procedure was 5.3 years in the group without recurrence and 10.1 years in the group with recurrence following primary Thiersch. 39 Thiersch's cerclage was initially described using a wire suture 40 and this is still periodically performed resulting in complications such as erosion of the cerclage wire presenting as a perianal infection. 52 Of 24 cases, three cases (12%) had Thiersch's complication and two cases described the suture cutting through the mucosa and developed infection around the suture insertion site. 18 Although sclerotherapy and Thiersch's anal encirclement present as simple minimally invasive options, they are not without complications, as they can result in anal pain, stricture, ischiorectal abscesses, and fistula. They also have higher recurrence rates requiring the procedures to be repeated, subjecting the child to multiple general anesthetics. In addition, they are not suitable for patients with full-thickness RP.
Ekehorn's Rectosacropexy
In Ekehorn's rectosacropexy, a suture is placed in the rectal ampulla through the lowest part of the sacrum to induce inflammation and adhesions between the rectal wall and perirectal wall. The technique has been described as follows. The patient is placed in the left lateral decubitus position with retractors for exposure at the 12 and 6 o'clock positions (coccyx is at the 9 o'clock position). The sacroccygeal junction is palpated with the left thumb and the loose posterior rectal wall is pushed upward. A large curved needle with a braided 1-0 Silk suture is then passed from inside to outside at the level of the sacrococcygeal junction. A small piece of gauze is placed inside the “U” loop of the suture, and then both strands leaving the skin at the same horizontal level are tied over a large gauze. Suture is removed on the 10th postoperative day. 34 The average age of patients using this technique was 4.5 years with no recurrences demonstrated. 34 53
Lockhart Mummery
As for many of the procedures for RP there exist several versions of this procedure to induce inflammation in the presacral space. In the traditional Lockhart-Mummery operation, a mesh gauze packing is placed temporarily in the retrorectal space and then removed inciting inflammation in the pre-sacral space. This was studied in 25 patients with a 100% success rate, 54 and one recurrence in 8 children. 32 A variation including soaking the mesh in sterile talc that resulted in an infection rate of 2% was noted with a mean hospital stay of 6 days for children with a mean age of 5 years. 55
Laparoscopic Suture Rectopexy
Laparoscopic suture rectopexy (LSRP) consists of attaching the perirectal tissues to the presacral area to assure correct anatomical positioning and tissue adherence. Laparoscopy in the management of complete RP (using sutures, mesh, talc, resection, or levatorplasty) is safe, effective, and associated with improved functional outcome. 56 The main advantages of the laparoscopic approach include rapid return of intestinal function, early discharge from hospital, improved cosmetic result, low morbidity, and low recurrence rate. 38 We will perform the procedure on an outpatient basis. The fecal continence score and the functional outcome are also improved. Apart from these advantages, the results are similar to those with open procedures irrespective of the method used (suture, resection, or posterior mesh). Moreover, both diagnosis and treatment of RP can be done in the same setting. 57 58 59 60 61 62 The choice of LSRP technique is based on the pathophysiologic changes present. One group recommended applying a tailored technique depending on a combination of intraoperative and preoperative findings. 38 Among several series, operative time consistently ranged between 40 and 90 minutes for LSRP 38 63 64 with a hospital stay of 1 to 6 days.
Laparoscopic retrorectal dissection and suture rectopexy involve rectopexy to the sacral promontory and suture sigmoidopexy to the left lateral peritoneum without mesh. This operative approach was used for eight children without laxity of the pelvic floor. 38 The rectum is suture pexed to the fascia of the sacral promontory using three nonabsorbable sutures beginning distally on the right posterior wall of the rectum and progressing proximally. The rectum should extend straight into the pelvis with a mild degree of tension. According to some authors, the posterior rectal space and lateral rectal ligaments are not violated as the dissection stays above the level of the peritoneal reflection. 64 65 If rectosigmoid redundancy is an initiating factor for rectosigmoid intussusceptions, left lateral fixation of the sigmoid colon in a smooth course prevents postoperative kinking of the sigmoid over the edge of the mesh. 38 Simple suture rectopexy to secure the rectum to the presacral fascia was used in a 22-month-old child who presented with recurrent RP. 66 We completely dissect the retrorectal space to the pelvic floor prior to suturing to the sacral promontory to promote scarring between rectum and sacrum. This can be augmented with placement of glue ( Fig. 3 ).
Fig. 3.
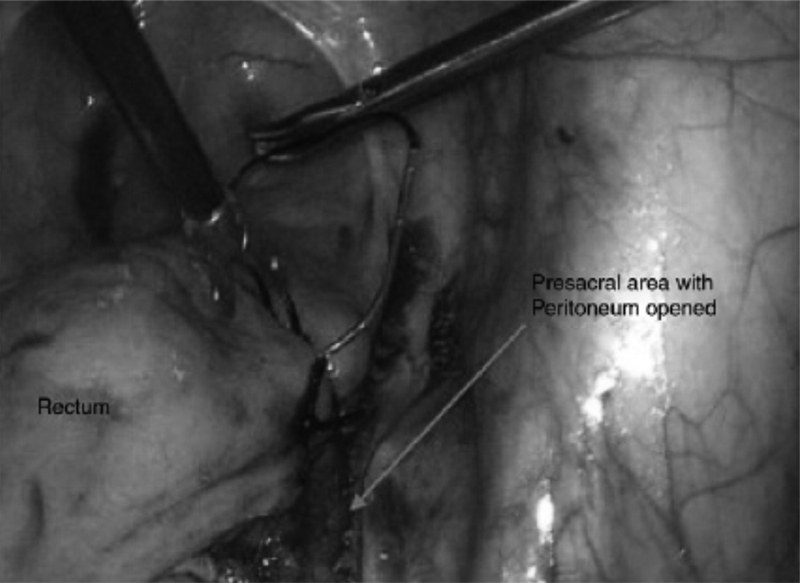
Laparoscopic view of the right pelvis during suturing of the seromuscular rectum to the presacral fascia.
Resection rectopexy in addition to LSRP is controversial. Ten children were repaired with resection rectopexy without mesh; these children had prolonged total colonic transit time and intractable constipation, and there was no recurrence of RP or constpiation. 38 Depending on the series of patients, laxative medications are continued following LSRP 65 for a total of 2 weeks postoperatively. 25
Levatorplasty has been demonstrated to improve RP accompanied by disabling incontinence. 67 Levatorplasty can also be performed by plication of the rectum and upward suspension through the entire thickness of the bony sacrum. 68 Levatorplasty was used for repair of the rectosacral hernia in five cases where the pelvic floor was lax. Laxity of the pelvic floor was defined as being able to move the pelvic floor 5 versus 2 cm in healthy children. The muscular hiatus posterior to the rectum was then treated as a hernia and was repaired by placing intracorporeal sutures to narrow the space posterior to the rectum and suspend it from the cut edge of the sacrum so that it cannot prolapse downward. 38
Patients with spinal dysraphism pose a unique RP patient population, as they have an associated lax rectosigmoid and poorly innervated pelvic floor due to inadequate innervation of the pelvic muscles as well as abnormalities of the sacrum. 6 69 These abnormalities can cause partial or total prolapse of the anterior wall, which is not fixed, producing invagination at the level of the rectosigmoid. 42 68 To avoid recurrent prolapse, rectopexy with fixation of the anterior border of the rectosigmoid to the lateral wall of the pelvic hollow has been proposed. With this fixation, there is no possibility of internal herniation because the sigmoid is in contact with the pelvic floor and adjacent to the lateral wall. 70 In a series of 22 patients with weakness of the pelvic floor from neuropathic conditions, the authors placed retrorectal Prolene mesh along with the addition of a levatorplasty in five of the patients. 38
Overall recurrence rates for LSRP are as high as 6.9% at 5 years and 10.8% at 10 years 38 with wide ranges across studies of 0 to 40%. One group reported complications in 17 of 27 patients when including anything outside a normal postoperative course including urinary retention; however, the recurrence rate was 8% with a median follow-up of 4 years. 25 It remains unclear if this was due to performing the procedure on a difference cohort of patients who were older (median age at operation was 14 years), different predisposing factors (mainly constipation and solitary rectal ulcer), or having had a longer median follow-up.
Constipation is the most significant problem postoperatively following LSRP 63 and has been demonstrated in each series. 25 38 71 Constipation may be due to the loss of compliance of rectum following rectopexy or creation of a more redundant sigmoid loop. 72 One group reported only one case of postoperative constipation and attributed this low number to routine correction of rectosigmoid redundancy by left lateral fixation and routine resection rectopexy for children with preoperative intractable constipation and prolonged total colonic transit times. 38 Others have reported 35% incidence of constipation following LSRP. 73 One group found that colonic transit times were reduced postoperatively in approximately 50% of those who had experienced constipation. 74 To avoid constipation postoperatively in patients, another author did not perform division of the lateral rectal ligaments, 63 as it appears that patients have less constipation when the ligaments can be preserved. 62 75 However, advantages of preservation of the rectal lateral ligaments are conflicting. 25 Further, as all retrospective series, the definition of constipation is variable, and the meaning of the clinical importance of this complication is not clarified. Laparoscopic colorectal resection with LSRP in children remains controversial. However, it is preferable in patients with history of intractable constipation and prolonged total colonic transit time due to the issues of chronic straining against a fixed rectum, 60 eliminates risk of volvulus, and has low morbidity and low recurrence rate. 38
Posterior Sagittal Rectopexy
Posterior sagittal rectopexy (PSRP) consists of a posterior sagittal midline incision, with division of the upper part of the sphincter mechanism. The anus itself is not divided and the rectal wall is identified and fixed with stitches to the cartilage of the coccyx and sacrum. The procedure is performed on an ambulatory basis. Four patients underwent this operation as an ambulatory procedure in one series at an average age 5.6 years (range, 2–8 years) with one recurrence. 76 No problems were reported in one series of 4, 77 and another of 35 patients. 78 Advantages include elimination of laparotomy, more direct approach, virtual elimination of postoperative anal prolapse, reconstruction of the attenuated and stretched sphincter muscles, and minimal morbidity and incapacitation. 79 The midline sacrococcygeal incision is carried down to but not through the external sphincter, and the patulous rectum is plicated back to normal size. Reapproximation of the levator sling and lower muscle complex was incorporated with the plicated rectum. 80 This repair is especially beneficial in children with a history of bladder extrophy who will have a 15 to 20% incidence of coexisting RP on the suggested basis of a pelvic floor weakness. Many pediatric surgeons feel that major rectal suspensions and pelvic reconstructions have no place in operative RP for children. Two anatomical areas are given special attention with this repair, namely, the retrorectal area fixed posteriorly to the levator ani and muscle complex 76 and plication of the dilated rectum. 81 Recurrence of posterior sagittal RP has been reported as high as 35 to 70%. 38 64
LSRP has been compared with PSRP. Sixteen children were evaluated with a median age of 6.5 years and no recurrences were found in the LSRP group compared with 25% (2 patients) in the PSRP group. Posterosagittal fixation may be inadequate in certain patients in whom RP is more likely the result of rectosigmoid intussusception with a high lead point or who are neurologically impaired. 42 63 This study also pointed out that mucosal RP occurs mainly in patients who have undergone surgery for anorectal malformations and requires different management than full-thickness RP. For mucosal RP, local excision and plasty without rectopexy are used. 63
Additional case reports and small series are numerous on approaches to treating RP. Many of these techniques are used in combination with other studied operations. These include linear cauterization studied on 73 patients with 1 failure and 5 patients requiring a second cauterization, 82 transsacral rectopexy with 1 partial recurrence as performed in 4 patients, 11 quadrant mucosal stripping in 21 patients with a 90 to 95% success rate, 81 transsacrococcygeal rectopexy in 46 patients with a 91% success rate, 42 and a transanal mucosal sleeve resection studied in 6 patients with a 100% success rate. 83 One case report demonstrated linear stapling—a modification of the Altemeier's procedure creating four stapler planes of resection and anastomosis to excise the prolapsed bowel. 84
Complications
While prolonged exposure of RP can lead to ulceration, venous obstruction, and thrombosis, it is usually self-limited and spontaneously reducing. 6 43 76 85 In contrast to hernias, strangulation is a rare occurrence. Solitary rectal ulcers and cloacogenic polyps can occur as a result of occult RP. 86 Solitary rectal ulcer syndrome is a benign condition usually diagnosed in adolescents. It is often apparent on endoscopy performed for complaints of rectal pain, bleeding, and passage of mucous. It may progress to the development of single or multiple localized areas of necrosis and ulceration of the mucosa, which are apparent on endoscopy. 6 86 Inflammatory cloacogenic polyps are polypoid formations, usually 0.4 to 1.2 cm in diameter. They are also diagnosed endoscopically following rectal bleeding. They arise from the transitional zone between the columnar rectal and squamous anal epithelium and represent a regeneration process. 6 Inflammatory cloacogenic polyps and the lesions of solitary rectal ulcer syndrome have been hypothesized to be caused by mucosal ischemia related to RP. 6 87
Additional complications have been noticed across series as mentioned earlier. These complications were collected using an inclusive complication index. Readmission is required in approximately 41% and reoperation, endoscopy, or other surgical procedure in 33%. Complications include severe fecal obstruction, constipation, fecal soiling, urinary retention, enuresis, infection, residual mucosal prolapse, discomfort at defecation, and recurrent RP. Mental retardation or behavioral disorders increased the risk of postoperative fecal obstruction and consptiation. 25
Prognosis
The prognosis of RP depends on the underlying etiology and is usually good. Approximately 90% of children who develop RP between the age of 9 months and 3 years respond to medical treatment and do not require surgery. Recurrences rarely occur after 6 years of age. The remaining 10% of children with RP have recurrences that persist into adulthood. 6 88 Children who present with RP after 4 years of age usually have neurologic or musculoskeletal defects of the pelvis and are less likely to respond to conservative measures. These children should be referred early for surgical intervention. 6 18 89
Conclusion
RP is an often self-limiting, self-reducing condition caused by constipation in children younger than 4 years. Attempts to find and treat the underlying condition should be sought. Older children are less likely to respond to conservative measures and often require operative intervention.
References
- 1.Chan W K, Kay S M, Laberge J M, Gallucci J G, Bensoussan A L, Yazbeck S. Injection sclerotherapy in the treatment of rectal prolapse in infants and children. J Pediatr Surg. 1998;33(02):255–258. doi: 10.1016/s0022-3468(98)90441-9. [DOI] [PubMed] [Google Scholar]
- 2.Dutta B N, Das A K. Treatment of prolapse rectum in children with injections of sclerosing agents. J Indian Med Assoc. 1977;69(12):275–276. [PubMed] [Google Scholar]
- 3.Narasanagi S S. Rectal prolapse in children. J Indian Med Assoc. 1974;62(11):378–380. [PubMed] [Google Scholar]
- 4.Eriksen C A, Hadley G P. Rectal prolapse in childhood--the role of infections and infestations. S Afr Med J. 1985;68(11):790–791. [PubMed] [Google Scholar]
- 5.Zempsky W T, Rosenstein B J. The cause of rectal prolapse in children. Am J Dis Child. 1988;142(03):338–339. doi: 10.1001/archpedi.1988.02150030112034. [DOI] [PubMed] [Google Scholar]
- 6.Siafakas C, Vottler T P, Andersen J M. Rectal prolapse in pediatrics. Clin Pediatr (Phila) 1999;38(02):63–72. doi: 10.1177/000992289903800201. [DOI] [PubMed] [Google Scholar]
- 7.Duhamel J, Pernin P. [Anal prolapse in the child] Ann Gastroenterol Hepatol (Paris) 1985;21(06):361–362. [PubMed] [Google Scholar]
- 8.Freeman N V. Rectal prolapse in children. J R Soc Med. 1984;77 03:9–12. [PMC free article] [PubMed] [Google Scholar]
- 9.Ripstein C B, Lanter B. Etiology and surgical therapy of massive prolapse of the rectum. Ann Surg. 1963;157:259–264. doi: 10.1097/00000658-196302000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Severijnen R, Festen C, van der Staak F, Rieu P. Rectal prolapse in children. Neth J Surg. 1989;41(06):149–151. [PubMed] [Google Scholar]
- 11.Chino E S, Thomas C G., Jr Transsacral approach to repair of rectal prolapse in children. Am Surg. 1984;50(02):70–75. [PubMed] [Google Scholar]
- 12.Suzuki H, Amano S, Matsumoto K, Tsukamoto Y. Anorectal motility in children with complete rectal prolapse. Prog Pediatr Surg. 1989;24:105–114. doi: 10.1007/978-3-642-74493-8_12. [DOI] [PubMed] [Google Scholar]
- 13.Corman M L. Rectal prolapse in children. Dis Colon Rectum. 1985;28(07):535–539. doi: 10.1007/BF02554107. [DOI] [PubMed] [Google Scholar]
- 14.Philip A T, Marraffa J M. Death following injection sclerotherapy due to phenol toxicity. J Forensic Sci. 2012;57(05):1372–1375. doi: 10.1111/j.1556-4029.2012.02224.x. [DOI] [PubMed] [Google Scholar]
- 15.Jacobs L K, Lin Y J, Orkin B A. The best operation for rectal prolapse. Surg Clin North Am. 1997;77(01):49–70. doi: 10.1016/s0039-6109(05)70532-6. [DOI] [PubMed] [Google Scholar]
- 16.Kopel F B. Gastrointestinal manifestations of cystic fibrosis. Gastroenterology. 1972;62(03):483–491. [PubMed] [Google Scholar]
- 17.Stern R C, Izant R J, Jr, Boat T F, Wood R E, Matthews L W, Doershuk C F. Treatment and prognosis of rectal prolapse in cystic fibrosis. Gastroenterology. 1982;82(04):707–710. [PubMed] [Google Scholar]
- 18.Antao B, Bradley V, Roberts J P, Shawis R. Management of rectal prolapse in children. Dis Colon Rectum. 2005;48(08):1620–1625. doi: 10.1007/s10350-005-0074-0. [DOI] [PubMed] [Google Scholar]
- 19.Akkoyun I, Akbiyik F, Soylu S G. The use of digital photos and video images taken by a parent in the diagnosis of anal swelling and anal protrusions in children with normal physical examination. J Pediatr Surg. 2011;46(11):2132–2134. doi: 10.1016/j.jpedsurg.2011.07.003. [DOI] [PubMed] [Google Scholar]
- 20.White C M, Findlay J M, Price J J. The occult rectal prolapse syndrome. Br J Surg. 1980;67(07):528–530. doi: 10.1002/bjs.1800670723. [DOI] [PubMed] [Google Scholar]
- 21.Alexander-Williams J.Solitary-ulcer syndrome of the rectum. Its association with occult rectal prolapse Lancet 19771(8004):170–171. [DOI] [PubMed] [Google Scholar]
- 22.El-Chammas K I, Rumman N, Goh V L, Quintero D, Goday P S. Rectal prolapse and cystic fibrosis. J Pediatr Gastroenterol Nutr. 2015;60(01):110–112. doi: 10.1097/MPG.0000000000000546. [DOI] [PubMed] [Google Scholar]
- 23.Robertson M B, Choe K A, Joseph P M. Review of the abdominal manifestations of cystic fibrosis in the adult patient. Radiographics. 2006;26(03):679–690. doi: 10.1148/rg.263055101. [DOI] [PubMed] [Google Scholar]
- 24.Abeş M, Sarihan H. Injection sclerotherapy of rectal prolapse in children with 15 percent saline solution. Eur J Pediatr Surg. 2004;14(02):100–102. doi: 10.1055/s-2004-815855. [DOI] [PubMed] [Google Scholar]
- 25.Koivusalo A I, Pakarinen M P, Rintala R J. Rectopexy for paediatric rectal prolapse: good outcomes but not without postoperative problems. Pediatr Surg Int. 2014;30(08):839–845. doi: 10.1007/s00383-014-3534-6. [DOI] [PubMed] [Google Scholar]
- 26.Zhang S C, Wang W L, Liu X. Defecography used as a screening entry for identifying evacuatory pelvic floor disorders in childhood constipation. Clin Imaging. 2014;38(02):115–121. doi: 10.1016/j.clinimag.2013.11.010. [DOI] [PubMed] [Google Scholar]
- 27.Koivusalo A I, Pakarinen M P, Rintala R I, Seuri R. Dynamic defecography in the diagnosis of paediatric rectal prolapse and related disorders. Pediatr Surg Int. 2012;28(08):815–820. doi: 10.1007/s00383-012-3125-3. [DOI] [PubMed] [Google Scholar]
- 28.Chen R, Zhao H, Sang X, Mao Y, Lu X, Yang Y. Severe adult ileosigmoid intussusception prolapsing from the rectum: a case report. Cases J. 2008;1(01):198. doi: 10.1186/1757-1626-1-198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Khushbakht S, ul Haq A. Rectal duplication cyst: a rare cause of rectal prolapse in a toddler. J Coll Physicians Surg Pak. 2015;25(12):909–910. [PubMed] [Google Scholar]
- 30.Mönig S P, Selzner M, Schmitz-Rixen T. Peutz-Jeghers syndrome in a child. Prolapse of a large colonic polyp through the anus. J Clin Gastroenterol. 1997;25(04):703–704. doi: 10.1097/00004836-199712000-00037. [DOI] [PubMed] [Google Scholar]
- 31.Coburn W M, III, Russell M A, Hofstetter W L. Sucrose as an aid to manual reduction of incarcerated rectal prolapse. Ann Emerg Med. 1997;30(03):347–349. doi: 10.1016/s0196-0644(97)70174-4. [DOI] [PubMed] [Google Scholar]
- 32.Qvist N, Rasmussen L, Klaaborg K E, Hansen L P, Pedersen S A. Rectal prolapse in infancy: conservative versus operative treatment. J Pediatr Surg. 1986;21(10):887–888. doi: 10.1016/s0022-3468(86)80015-x. [DOI] [PubMed] [Google Scholar]
- 33.Bahador A, Foroutan H R, Hosseini S M, Davani S Z. Effect of submucosal alcohol injection on prolonged rectal prolapse in infants and children. J Indian Assoc Pediatr Surg. 2008;13(01):11–13. doi: 10.4103/0971-9261.42566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sander S, Vural O, Unal M. Management of rectal prolapse in children: Ekehorn's rectosacropexy. Pediatr Surg Int. 1999;15(02):111–114. doi: 10.1007/s003830050528. [DOI] [PubMed] [Google Scholar]
- 35.Hill S R, Ehrlich P F, Felt B, Dore-Stites D, Erickson K, Teitelbaum D H. Rectal prolapse in older children associated with behavioral and psychiatric disorders. Pediatr Surg Int. 2015;31(08):719–724. doi: 10.1007/s00383-015-3733-9. [DOI] [PubMed] [Google Scholar]
- 36.Johansen O B, Wexner S D, Daniel N, Nogueras J J, Jagelman D G. Perineal rectosigmoidectomy in the elderly. Dis Colon Rectum. 1993;36(08):767–772. doi: 10.1007/BF02048369. [DOI] [PubMed] [Google Scholar]
- 37.Tou S, Brown S R, Malik A I, Nelson R L. Surgery for complete rectal prolapse in adults. Cochrane Database Syst Rev. 2008;(04):CD001758. doi: 10.1002/14651858.CD001758.pub2. [DOI] [PubMed] [Google Scholar]
- 38.Ismail M, Gabr K, Shalaby R. Laparoscopic management of persistent complete rectal prolapse in children. J Pediatr Surg. 2010;45(03):533–539. doi: 10.1016/j.jpedsurg.2009.09.013. [DOI] [PubMed] [Google Scholar]
- 39.Flum A S, Golladay E S, Teitelbaum D H. Recurrent rectal prolapse following primary surgical treatment. Pediatr Surg Int. 2010;26(04):427–431. doi: 10.1007/s00383-010-2565-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gabriel W B. Thiersch's operation for anal incontinence and minor degrees of rectal prolapse. Am J Surg. 1953;86(05):583–590. doi: 10.1016/0002-9610(53)90361-4. [DOI] [PubMed] [Google Scholar]
- 41.Oeconomopoulos C T, Swenson O. Thiersch's operation for rectal prolapse in infants and children. Am J Surg. 1960;100:457–461. doi: 10.1016/0002-9610(60)90388-3. [DOI] [PubMed] [Google Scholar]
- 42.Ashcraft K W, Garred J L, Holder T M, Amoury R A, Sharp R J, Murphy J P.Rectal prolapse: 17-year experience with the posterior repair and suspension J Pediatr Surg 19902509992–994., discussion 994–995 [DOI] [PubMed] [Google Scholar]
- 43.Henry L G, Cattey R P. Rectal prolapse. Surg Laparosc Endosc. 1994;4(05):357–360. [PubMed] [Google Scholar]
- 44.Green D. Mechanism of action of sclerotherapy. Semin Dermatol. 1993;12(02):88–97. [PubMed] [Google Scholar]
- 45.Beck D E. Rectal prolapse: an update. Curr Surg. 2000;57(03):185–189. doi: 10.1016/s0149-7944(00)00216-6. [DOI] [PubMed] [Google Scholar]
- 46.Wyllie G G. The injection treatment of rectal prolapse. J Pediatr Surg. 1979;14(01):62–64. doi: 10.1016/s0022-3468(79)80578-3. [DOI] [PubMed] [Google Scholar]
- 47.Fahmy M A, Ezzelarab S. Outcome of submucosal injection of different sclerosing materials for rectal prolapse in children. Pediatr Surg Int. 2004;20(05):353–356. doi: 10.1007/s00383-004-1197-4. [DOI] [PubMed] [Google Scholar]
- 48.Gysler R, Morger R. Sclerosing treatment with ethoxysclerol in anal prolapse in children [in German] Z Kinderchir. 1989;44(05):304–305. doi: 10.1055/s-2008-1043258. [DOI] [PubMed] [Google Scholar]
- 49.Kay N R, Zachary R B. The treatment of rectal prolapse in children with injections of 30 per cent saline solutions. J Pediatr Surg. 1970;5(03):334–337. doi: 10.1016/0022-3468(70)90190-9. [DOI] [PubMed] [Google Scholar]
- 50.Malyshev Y I, Gulin V A. Our experience with the treatment of rectal prolapse in infants and children. Am J Proctol. 1973;24(06):470–472. [PubMed] [Google Scholar]
- 51.Shah A, Parikh D, Jawaheer G, Gornall P. Persistent rectal prolapse in children: sclerotherapy and surgical management. Pediatr Surg Int. 2005;21(04):270–273. doi: 10.1007/s00383-005-1384-y. [DOI] [PubMed] [Google Scholar]
- 52.Saleem M M, Al-Momani H. Acute scrotum as a complication of Thiersch operation for rectal prolapse in a child. BMC Surg. 2006;6:19. doi: 10.1186/1471-2482-6-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schepens M A, Verhelst A A. Reappraisal of Ekehorn's rectopexy in the management of rectal prolapse in children. J Pediatr Surg. 1993;28(11):1494–1497. doi: 10.1016/0022-3468(93)90439-r. [DOI] [PubMed] [Google Scholar]
- 54.Balde I, Mbumbe-King A, Vinand P. [The Lockhart-Mummery technique in the treatment of the total rectal prolapse among children. Concerning 25 cases (author's transl)] Chir Pediatr. 1979;20(05):375–377. [PubMed] [Google Scholar]
- 55.Nazem M, Hosseinpour M, Farhadi M. Perineal mesh rectopexy with sterile talc in children with rectal prolapse. Eur J Pediatr Surg. 2010;20(05):321–324. doi: 10.1055/s-0030-1254121. [DOI] [PubMed] [Google Scholar]
- 56.Senagore A J. Management of rectal prolapse: the role of laparoscopic approaches. Semin Laparosc Surg. 2003;10(04):197–202. doi: 10.1177/107155170301000407. [DOI] [PubMed] [Google Scholar]
- 57.Boccasanta P, Rosati R, Venturi M et al. Comparison of laparoscopic rectopexy with open technique in the treatment of complete rectal prolapse: clinical and functional results. Surg Laparosc Endosc. 1998;8(06):460–465. [PubMed] [Google Scholar]
- 58.Boccasanta P, Venturi M, Reitano M C et al. Laparotomic vs. laparoscopic rectopexy in complete rectal prolapse. Dig Surg. 1999;16(05):415–419. doi: 10.1159/000018758. [DOI] [PubMed] [Google Scholar]
- 59.Heah S M, Hartley J E, Hurley J, Duthie G S, Monson J R. Laparoscopic suture rectopexy without resection is effective treatment for full-thickness rectal prolapse. Dis Colon Rectum. 2000;43(05):638–643. doi: 10.1007/BF02235579. [DOI] [PubMed] [Google Scholar]
- 60.Lechaux D, Trebuchet G, Siproudhis L, Campion J P. Laparoscopic rectopexy for full-thickness rectal prolapse: a single-institution retrospective study evaluating surgical outcome. Surg Endosc. 2005;19(04):514–518. doi: 10.1007/s00464-004-9088-2. [DOI] [PubMed] [Google Scholar]
- 61.Xynos E, Chrysos E, Tsiaoussis J, Epanomeritakis E, Vassilakis J S. Resection rectopexy for rectal prolapse. The laparoscopic approach. Surg Endosc. 1999;13(09):862–864. doi: 10.1007/s004649901120. [DOI] [PubMed] [Google Scholar]
- 62.Madiba T E, Baig M K, Wexner S D. Surgical management of rectal prolapse. Arch Surg. 2005;140(01):63–73. doi: 10.1001/archsurg.140.1.63. [DOI] [PubMed] [Google Scholar]
- 63.Koivusalo A, Pakarinen M, Rintala R. Laparoscopic suture rectopexy in the treatment of persisting rectal prolapse in children: a preliminary report. Surg Endosc. 2006;20(06):960–963. doi: 10.1007/s00464-005-0424-y. [DOI] [PubMed] [Google Scholar]
- 64.Laituri C A, Garey C L, Fraser J D et al. 15-Year experience in the treatment of rectal prolapse in children. J Pediatr Surg. 2010;45(08):1607–1609. doi: 10.1016/j.jpedsurg.2010.01.012. [DOI] [PubMed] [Google Scholar]
- 65.Potter D D, Bruny J L, Allshouse M J, Narkewicz M R, Soden J S, Partrick D A. Laparoscopic suture rectopexy for full-thickness anorectal prolapse in children: an effective outpatient procedure. J Pediatr Surg. 2010;45(10):2103–2107. doi: 10.1016/j.jpedsurg.2010.06.006. [DOI] [PubMed] [Google Scholar]
- 66.Saxena A K, Metzelder M L, Willital G H. Laparoscopic suture rectopexy for rectal prolapse in a 22-month-old child. Surg Laparosc Endosc Percutan Tech. 2004;14(01):33–34. doi: 10.1097/00129689-200402000-00009. [DOI] [PubMed] [Google Scholar]
- 67.Köhler A, Athanasiadis S. The value of posterior levator repair in the treatment of anorectal incontinence due to rectal prolapse--a clinical and manometric study. Langenbecks Arch Surg. 2001;386(03):188–192. doi: 10.1007/s004230100223. [DOI] [PubMed] [Google Scholar]
- 68.Tsugawa C, Matsumoto Y, Nishijima E, Muraji T, Higashimoto Y. Posterior plication of the rectum for rectal prolapse in children. J Pediatr Surg. 1995;30(05):692–693. doi: 10.1016/0022-3468(95)90692-4. [DOI] [PubMed] [Google Scholar]
- 69.Lasheen A E. Closed rectosacropexy for rectal prolapse in children. Surg Today. 2003;33(08):642–644. doi: 10.1007/s00595-003-2548-9. [DOI] [PubMed] [Google Scholar]
- 70.Montes-Tapia F, Cura-Esquivel I, Garza-Luna U, Martínez-Flores G, Muñoz-Maldonado G, Abrego-Moya V. Sigmoid fixation associated with rectopexy using a laparoscopic approach could prevent relapse of rectal prolapse in pediatric patients with spinal dysraphia. J Pediatr Surg. 2008;43(08):1551–1553. doi: 10.1016/j.jpedsurg.2008.02.082. [DOI] [PubMed] [Google Scholar]
- 71.Puri B. Rectal prolapse in children: laparoscopic suture rectopexy is a suitable alternative. J Indian Assoc Pediatr Surg. 2010;15(02):47–49. doi: 10.4103/0971-9261.70634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Brown A J, Anderson J H, McKee R F, Finlay I G. Strategy for selection of type of operation for rectal prolapse based on clinical criteria. Dis Colon Rectum. 2004;47(01):103–107. doi: 10.1007/s10350-003-0013-x. [DOI] [PubMed] [Google Scholar]
- 73.Kariv Y, Delaney C P, Casillas S et al. Long-term outcome after laparoscopic and open surgery for rectal prolapse: a case-control study. Surg Endosc. 2006;20(01):35–42. doi: 10.1007/s00464-005-3012-2. [DOI] [PubMed] [Google Scholar]
- 74.Demirbas S, Akin M L, Kalemoglu M, Ogün I, Celenk T. Comparison of laparoscopic and open surgery for total rectal prolapse. Surg Today. 2005;35(06):446–452. doi: 10.1007/s00595-004-2946-7. [DOI] [PubMed] [Google Scholar]
- 75.Fengler S A, Pearl R K, Prasad M L et al. Management of recurrent rectal prolapse. Dis Colon Rectum. 1997;40(07):832–834. doi: 10.1007/BF02055442. [DOI] [PubMed] [Google Scholar]
- 76.Peña A, Hong A. The posterior sagittal trans-sphincteric and trans-rectal approaches. Tech Coloproctol. 2003;7(01):35–44. doi: 10.1007/s101510300006. [DOI] [PubMed] [Google Scholar]
- 77.Ashcraft K W, Amoury R A, Holder T M. Levator repair and posterior suspension for rectal prolapse. J Pediatr Surg. 1977;12(02):241–245. doi: 10.1016/s0022-3468(77)80015-8. [DOI] [PubMed] [Google Scholar]
- 78.Davidian V A, Jr, Thomas C G., Jr Trans-sacral repair of rectal prolapse. Efficacy of treatment in thirty consecutive patients. Am J Surg. 1972;123(02):231–235. doi: 10.1016/0002-9610(72)90337-6. [DOI] [PubMed] [Google Scholar]
- 79.Sarin Y K, Sharma A K. Posterior sagittal rectopexy for rectal prolapse. Indian Pediatr. 1993;30(04):541–542. [PubMed] [Google Scholar]
- 80.Pearl R H, Ein S H, Churchill B. Posterior sagittal anorectoplasty for pediatric recurrent rectal prolapse. J Pediatr Surg. 1989;24(10):1100–1102. doi: 10.1016/s0022-3468(89)80228-3. [DOI] [PubMed] [Google Scholar]
- 81.Momoh J T. Quadrant mucosal stripping and muscle pleating in the management of childhood rectal prolapse. J Pediatr Surg. 1986;21(01):36–38. doi: 10.1016/s0022-3468(86)80648-0. [DOI] [PubMed] [Google Scholar]
- 82.Hight D W, Hertzler J H, Philippart A I, Benson C D. Linear cauterization for the treatment of rectal prolapse in infants and children. Surg Gynecol Obstet. 1982;154(03):400–402. [PubMed] [Google Scholar]
- 83.Chwals W J, Brennan L P, Weitzman J J, Woolley M M. Transanal mucosal sleeve resection for the treatment of rectal prolapse in children. J Pediatr Surg. 1990;25(07):715–718. doi: 10.1016/s0022-3468(05)80003-x. [DOI] [PubMed] [Google Scholar]
- 84.Lee J I, Vogel A M, Suchar A M, Glynn L, Statter M B, Liu D C. Sequential linear stapling technique for perineal resection of intractable pediatric rectal prolapse. Am Surg. 2006;72(12):1212–1215. [PubMed] [Google Scholar]
- 85.Chaloner E J, Duckett J, Lewin J. Paediatric rectal prolapse in Rwanda. J R Soc Med. 1996;89(12):688–689. doi: 10.1177/014107689608901208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Godbole P, Botterill I, Newell S J, Sagar P M, Stringer M D. Solitary rectal ulcer syndrome in children. J R Coll Surg Edinb. 2000;45(06):411–414. [PubMed] [Google Scholar]
- 87.Felt-Bersma R J, Tiersma E S, Cuesta M A.Rectal prolapse, rectal intussusception, rectocele, solitary rectal ulcer syndrome, and enterocele Gastroenterol Clin North Am 20083703645–668., ix [DOI] [PubMed] [Google Scholar]
- 88.Harris P R, Figueroa-Colon R. Rectal prolapse in children associated with Clostridium difficile infection. Pediatr Infect Dis J. 1995;14(01):78–80. doi: 10.1097/00006454-199501000-00022. [DOI] [PubMed] [Google Scholar]
- 89.Lopez E L, Devoto S, Fayad A, Canepa C, Morrow A L, Cleary T G.Association between severity of gastrointestinal prodrome and long-term prognosis in classic hemolytic-uremic syndrome J Pediatr 1992120(2, Pt 1):210–215. [DOI] [PubMed] [Google Scholar]


