Abstract
Chronic intestinal pseudo-obstruction (CIP) is defined by either continuous or intermittent symptoms of bowel obstruction in the absence of fixed lumen excluding lesion. CIP includes a heterogeneous group of disorders which result either from diseases affecting the enteric neurons and smooth muscle lining or those involving the autonomic innervation of the bowel. Symptoms associated with CIP are nonspecific, which can sometimes contribute to the delay in recognizing the condition and making the correct diagnosis. The diagnostic workup should include imaging and manometry studies and, occasionally, full-thickness bowel biopsies for histopathological examination may be required. Multidisciplinary team approach for the management of these patients is recommended, and the team members should include a gastroenterologist, surgeon, chronic pain specialist, clinical nutritionist, and a psychologist. The treatment goals should include optimizing the nutritional status and preventing or delaying the development of intestinal failure. The majority of the patients require enteral or parenteral nutrition support, and chronic pain is a common and distressing symptom. Small bowel transplantation may be required if patients develop liver complications due to parenteral nutrition, have difficult central line access, or have poor quality of life and worsening pain despite aggressive medical management.
Keywords: chronic intestinal pseudo-obstruction, bowel motility, antroduodenal manometry
Intestinal pseudo-obstruction refers to a heterogeneous group of disorders with a similar phenotypic presentation characterized by obstructive intestinal symptoms in the absence of a true anatomical obstruction. 1 In Ogilvie's syndrome, patient develops acute intestinal pseudo-obstruction; on the other hand, if the obstructive symptoms are present for longer than 6 months, it is categorized as chronic intestinal pseudo-obstruction (CIP). This review will mainly focus on CIP and management of children with this disease. CIP was first reported in 1958 by Dudley and his colleagues when they failed to identify a cause for obstructive symptoms during exploratory laparotomy in a group of patients. It is usually diagnosed after the exclusion of a mechanical obstruction and documentation of disordered peristalsis resulting from abnormalities of the enteric neuromusculature and/or its autonomic innervation. Because of the nonspecific nature of symptoms and rarity of this disorder, it is not unusual that some patients have repeated and invasive workup before the correct diagnosis is made. There are only a few experienced pediatric motility centers that offer specialized diagnostic testing such as manometry studies which can be important in establishing the diagnosis.
Epidemiology
The exact prevalence of CIP is not known; it has been estimated that less than 100 infants are born with this condition in the United States every year. This study did not account for children who may have developed CIP later in life. A Japanese nationwide survey reported a prevalence of 3.7 in 1 million individuals. 2 More than half of the patients (56.5%) in this study developed symptoms in the neonatal period. The criteria used for the diagnosis of CIP were very stringent and so this study may have underestimated the prevalence.
Etiology
Several different classification schemes have been used to categorize pediatric patients with CIP ( Table 1 ). The authors prefer to categorize CIP patients into two broad categories: congenital and acquired. Each of these groups can be further subdivided into three histological categories: neuropathies, myopathies, and mesenchymopathies, although some patients may have coexisting pathological abnormalities. A large proportion of pediatric patients are classified under the idiopathic category because no histological abnormality can be identified despite extensive testing in these patients.
Table 1. Classification of chronic intestinal pseudo-obstruction.
| Onset: |
|---|
| Congenital |
| Acquired |
| Causes: |
| Familial |
| Sporadic |
| Degenerative |
| Viral |
| Inflammatory |
| Autoimmune |
| Toxic |
| Ischemia |
| Areas of the gastrointestinal tract involvement: |
| Entire gastrointestinal tract |
| Small bowel |
| Colon |
| Segments of the gastrointestinal tract |
| Pathology: |
| Myopathy |
| Neuropathy |
| Combined myopathy and neuropathy |
| Aganglionosis |
| Hypoganglionosis |
| Abnormalities of interstitial cells of Cajal |
| Connective tissue disorders |
Most congenital forms of CIP are sporadic with no family history of the condition. However, autosomal-dominant 3 4 and recessive 5 6 7 8 inheritance of enteric neuropathies as well as 9 10 11 12 myopathies have been reported. An X-linked recessive form of neuropathic CIP has been mapped to gene locus Xq28. 13 CIP is also a common manifestation of a variety of mitochondrial myopathies resulting from mutations in nuclear or mitochondrial genes involved in mitochondrial replication and/or oxidative respiration. Mitochondrial neurogastrointestinal encephalopathy (MNGIE) is an autosomal recessive condition caused by a mutation in the thymidine phosphorylase gene, which encodes enzymes for normal mitochondrial DNA synthesis. Similarly, CIP has also been reported in association with Alpers' disease (POLG mutations) or mitochondrial encephalopathy, lactic acidosis, and stroke-like episodes syndrome (MELAS).
Megacystis–microcolon–intestinal hypoperistalsis syndrome (MMIHS) is characterized by prenatal onset of urinary bladder distension with dilated loops of bowel and is associated with a mutation of ACTG2 gene. 14 This gene encodes c2 enteric actin which is an important contractile protein of enteric smooth muscle. 15 Interestingly, autosomal dominant rather than autosomal recessive inheritance was reported in the majority of the patients. Phenotypic variability has been recognized and distinct complications such as prune belly syndrome and hollow visceral myopathy have been reported.
Chronic atrial and intestinal dysrhythmia (CAID) syndrome is caused by mutations in SGOL1 gene, a component of the cohesin complex. 16 In addition to cardiac rhythm abnormalities, symptoms of CIP were reported in this adult cohort. Although this disorder has not been reported in children, the authors have identified this mutation in one of their patients who was diagnosed with CIP in early childhood and then died of respiratory complications not directly related to CIP.
Acquired causes of CIP include toxin exposure during critical developmental periods in utero; substances that affect neuronal migration or maturation may affect the development of the enteric plexus, leading to CIP ( Table 2 ). Fetal alcohol syndrome 17 as well as in utero exposure to narcotics can damage the enteric neurons, resulting in CIP. Other acquired conditions causing CIP can result from myenteric plexus neuritis secondary to persistent viral infections or autoimmune inflammatory disorders. CIP may be a rare sequela of infections such as cytomegalovirus, 18 Epstein–Barr, 19 and JC virus. 20
Table 2. Causes of secondary chronic intestinal pseudo-obstruction.
| Metabolic |
|---|
| Mitochondrial cytopathies |
| Autoimmune |
| SLE |
| Scleroderma |
| Dermatomyositis |
| Polymyositis |
| Autoimmune myositis |
| Autoimmune ganglionitis |
| Infectious/Postinfectious |
| Chagas' disease |
| Cytomegalovirus |
| Herpes zoster |
| Epstein–Barr virus |
| Kawasaki's disease |
| Post-viral neuropathy |
| Endocrine |
| Diabetes mellitus |
| Hypoparathyroidism |
| Hypothyroidism |
| Oncology/Hematology |
| Chemotherapy and/or bone marrow/stem cell transplant |
| Pheochromocytoma |
| Ganglioneuroblastoma (paraneoplastic syndrome) |
| Sickle cell disease |
| Muscle disorders |
| Myotonic dystrophy |
| Duchenne muscular dystrophy |
| Toxins |
| Fetal alcohol syndrome |
| Drugs |
| Diltiazem and nifedipine |
| Cyclopentolate/phenylephrine eye drops (neonates) |
| Developmental |
| Delayed maturation of interstitial cells of Cajal |
| Miscellaneous |
| Ehlers–Danlos syndrome |
| Eosinophilic gastroenteritis |
| Crohn's disease |
| Radiation injury |
Abbreviation: SLE, systemic lupus erythematosus.
Source: Adapted from Connor and Di Lorenzo.
Pathology
The London Classification of Gastrointestinal Neuromuscular Pathology was established by an international working group as an organizational framework for histopathologic diagnoses associated with intestinal dysmotility. 22 The normal development and changes in enteric neurons and muscular lining with age are poorly understood, and this makes the interpretation of the histopathology in pediatric patients with CIP challenging. 22 It is therefore not uncommon to find no recognizable abnormalities despite a thorough microscopic evaluation of full-thickness bowel biopsies in children with CIP. A standardized approach to the evaluation of enteric neuromusculature proposed by a group of experts is expected to help improve the understanding of histopathological abnormalities associated with this disorder. Because of the low diagnostic yield of full-thickness bowel biopsies in children, some pediatric motility centers in the United States do not routinely recommend full-thickness bowel biopsies in their patients.
The schematic representation of the enteric nervous system and how it regulates the bowel motor function is shown in Fig. 1 and the histopathological abnormalities of the bowel neurons and smooth muscle lining which are associated with CIP in children are summarized below ( Table 3 ).
Fig. 1.
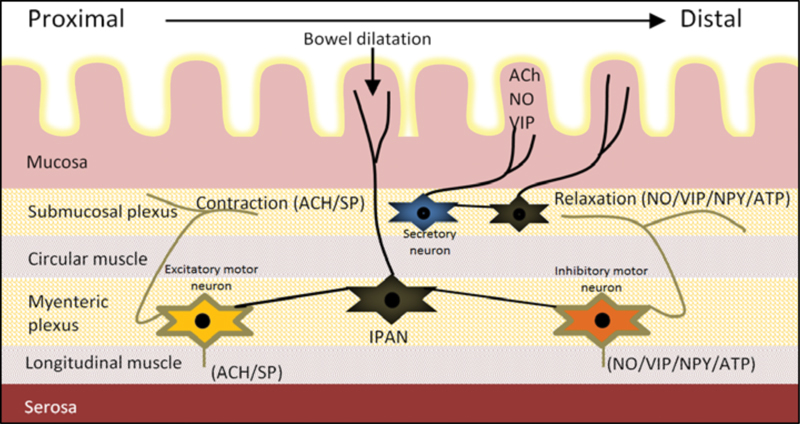
Schematic representation of the enteric nervous system and connections between the neurons in the submucosal and myenteric plexus. The submucosal plexus is mainly responsible for regulating bowel secretions and blood supply. The myenteric plexus which is located between the circular and longitudinal smooth muscle layers regulates the bowel motor function. The intrinsic primary afferent neurons (IPAN) receive input from the bowel mucosa and project in both oral and anal directions. Abnormalities of the enteric neurons and smooth muscle lining are associated with chronic intestinal pseudo-obstruction. The gastrointestinal tract also receives innervation from the autonomic nervous system (not shown).
Table 3. Newly described causes of chronic intestinal pseudo-obstruction.
| CAID syndrome (SGOL 1 mutation) |
| ACTG2 gene–associated megacystis–microcolon–intestinal hypoperistalsis syndrome |
| Mitochondrial diseases (MNGIE) |
| Deficient interstitial cells of Cajal |
| Viral infections (CMV, EBV, HSV, rotavirus) |
| Eosinophilic enterocolitis and neuropathy |
| Autoimmune (ANNA-1) |
| “Idiopathic” myositis and neuropathies |
| Systemic lupus erythematosus/Crohn's disease |
| New drug effects—perinatal zidovudine |
Abbreviations: CAID, chronic atrial and intestinal dysrhythmia; CMV, cytomegalovirus; EBV, Epstein–Barr virus; HSV, herpes simplex virus.
Intestinal Myopathies
The muscularis propria of the intestinal tract is normally composed of two layers, externa (longitudinal) and interna (circular), which are oriented perpendicular to one another. There are two forms of primary muscular malformation associated with CIP, the segmental additional muscle coat and diffuse abnormality of the muscle layering. 23 24 The segmental additional muscle coat has been associated with profound segmental dilatation of the colon, but small intestine involvement has not been described. The prominent separate band of smooth muscle is located internal to the muscularis interna and external to the muscularis mucosae of an otherwise normal muscularis propria. In diffuse disease of the smooth muscle lining, the abnormal muscle layering predominantly involves the small intestine. Portions of the muscularis propria retain normal bilayered architecture, but malformed regions harbor broad fascicles of smooth muscle that course obliquely or perpendicularly.
In degenerative leiomyopathy, there is a progressive loss of enteric smooth muscle and replacement with fibrous tissue. CIP symptoms are usually present, but urinary bladder involvement, age of presentation, and the inheritance pattern are very variable. 12 25 Many patients may not develop symptoms until adolescence. The two consistent histopathologic alterations reported are the myocyte degeneration and intramuscular fibrosis.
Intestinal leiomyositis is a rare disease which predominantly affects children, and less than 12 patients have been reported in world literature. 26 27 It is characterized by dense and diffuse lymphocytic infiltration of the muscularis propria, with limited extension into the myenteric plexus, submucosa, or lamina propria.
In addition to the aforementioned diseases, a variety of other conditions, such as bowel ischemia, drug toxicity, and autoimmune disorders, can cause secondary myopathies. 21
Intestinal Neuropathies
Neuronal intranuclear inclusion disease is a rare and heterogeneous neurodegenerative disorder that affects the central and peripheral nervous systems. There is progressive ganglion cell loss and in terminal phase, hypoganglionosis is seen. The ganglion cells show degenerative features including vacuolization, nuclear pyknosis, and irregular cytoplasmic contours. Large eosinophilic inclusions in nuclei of enteric ganglion cells are the pathognomonic histopathologic finding. In addition to CIP, other clinical features include ataxia, dysautonomia, dementia, and extrapyramidal symptoms. Infantile, juvenile, and adult forms of this disease have been described. 26
Aganglionosis in Hirschsprung's disease can rarely extend into varying parts of the small intestine. Rarely, segments of the bowel show normal ganglions and intervening parts are aganglionic; this is also referred to as skip segment Hirschsprung's disease. 28 It is thought that neural crest cells, during craniocaudal migration, cross the mesenteric border to a more distal part of the intestine and as a result end up well ahead of the wave front, and end up colonizing an area within the aganglionic segment.
Diffuse intestinal ganglioneuromatosis is characterized by diffusely distributed transmural hamartomatous lesions which are composed of benign ganglion cells. 26 It has been associated with multiple endocrine neoplasia type IIB, type I neurofibromatosis, and Cowden's disease. The hamartomatous lesions consist of mature ganglioneuromas with large ganglion cells with eccentric nuclei. Chronic constipation with megacolon is common and the pathogenesis of dysmotility is poorly understood.
Patients with intestinal hypoganglionosis present with CIP in early infancy, and the small intestine and colon show reduced number of ganglion cells per longitudinal centimeter bowel. Additionally, the plexus area and the number of ganglion cells per plexus are reduced and the ganglion distances are doubled compared with a healthy bowel. There is very low or absent activity of acetylcholinesterase in the mucosa along with significant reduction of nerve cells in the myenteric plexus and the submucous plexus ( Table 3 ). 29
In inflammatory enteric ganglionitis, there is inflammation within the enteric ganglia and along the enteric nerves. Eosinophilic inflammation has been reported with eosinophilic enterocolitis, connective tissue disease, and Hirschsprung's disease. The lymphoplasmacytic inflammation can be idiopathic or associated with infections, inflammatory bowel disease, cystic fibrosis, and paraneoplastic phenomenon (e.g., neuroblastoma).
Neuronal intestinal dysplasia is a controversial histopathological abnormality, first reported by Nezelof et al 30 to describe hyperplasia of the myenteric plexus and subsequently renamed by Meier-Ruge as IND (or IND type B). 31 It is now considered as a morphologic phenotype affecting the submucosal plexus of the intestine, either in an isolated form or with known neuropathies such as Hirschsprung's disease or neurofibromatosis. The histological diagnostic criteria have frequently been changed in the past four decades, causing confusion and leading to some experts questioning the validity of these findings. 22 The latest morphometric criteria require more than 8 neurons/ganglion (the so-called giant ganglia) in more than 20% of a minimum of 25 submucosal ganglia in patients older than 1 year. 22 These criteria were developed with 15-mm-thick frozen sections and enzyme histochemistry. The clinical significance remains unclear because of the lack of correlation between clinical symptoms and histological findings. In some patients, the histological abnormalities improve with age.
Mesenchymopathies
Interstitial cells of Cajal (ICC) are defined by the expression of the CD117 (c-kit) protein which is a membrane receptor with tyrosine kinase activity. They are present within the submucosal, intramuscular, and intermuscular layers of the gastrointestinal (GI) tract. The myenteric ICC serve as the pacemaker and generate the bioelectric slow wave potential that leads to the contraction of the smooth muscles. Decreased number of ICCs, along with structural abnormalities, such as loss of processes and damaged intracellular cytoskeleton and organelles, has been reported in adults and children with CIP. 32
The tendinous collagenous tissue of the muscularis propria constitutes an integral part of the peristaltic apparatus, but the details of the collagenous tissue complex of the bowel wall are not yet fully understood. Developmental and acquired abnormalities of the bowel connective tissue have been implicated in GI motility disorders. 33 Systemic sclerosis, an autoimmune disease of the connective tissue, can cause small bowel peristaltic dysfunction and CIP. 34
Clinical Features
The symptoms of CIP vary from patient to patient depending on the location and extent of the GI tract involved. The most common symptoms of CIP include abdominal pain, followed by nausea and vomiting, constipation, and loose stools. Abdominal distension can worsen during exacerbation of pseudo-obstruction which can be triggered by viral or bacterial infections, central line sepsis, general anesthesia, psychological stress, and malnutrition. 35 36 Almost half of the children with CIP present in infancy. Antenatal ultrasound may show polyhydramnios, megacystis, and distended bowel loops. Patients with severe phenotype are usually symptomatic within the first few hours of life with remarkable bowel obstruction, while less severe cases may present months later. Forty percent of patients presenting in infancy with CIP have associated intestinal malrotation. 1 35 If feeding intolerance and emesis persist after Ladd's procedure, CIP should be considered and repeated surgical interventions avoided. Adhesions are common in patients with CIP after laparotomy and can further compromise bowel propulsive function and precipitate intestinal failure.
Small intestinal bacterial overgrowth (SIBO) is a common complication and associated with worsening abdominal distention and pain. Nutritional complications due to malabsorption and micronutrition deficiencies can develop as a result of SIBO. 35 36 37 Instead of infrequent bowel movements, patients with SIBO can develop steatorrhea.
Dilated urinary bladder and ureters are associated with CIP. Timely diagnosis can help avoid complications such as urinary tract infections and renal damage.
Because of the nonspecific nature of the symptoms associated with CIP, the condition may go unrecognized for a long time and can be confused with aerophagia, gastroparesis, functional constipation, cyclic vomiting syndrome, drug toxicity, and hypothyroidism. Sometimes, patients may be wrongly diagnosed with Munchausen-by-proxy syndrome (also known as pediatric illness falsification).
Diagnosis
To accurately diagnose CIP, imaging studies and manometry evaluation are generally required ( Figs. 2 and 3 ). Nonspecific radiographic signs include dilated stomach, small intestine, and colon with air–fluid levels. Contrast studies help exclude an anatomical obstruction; it is crucial to plan the evacuation of barium or the use of water-soluble contrast as prolonged contrast stasis and bezoar formation are known complications of such studies in patients with CIP. Scintigraphy demonstrates delayed gastric emptying of solids or liquids. Breath hydrogen test may reveal elevation in fasting breath hydrogen and rapid increase with a carbohydrate meal in the presence of SIBO.
Fig. 2.
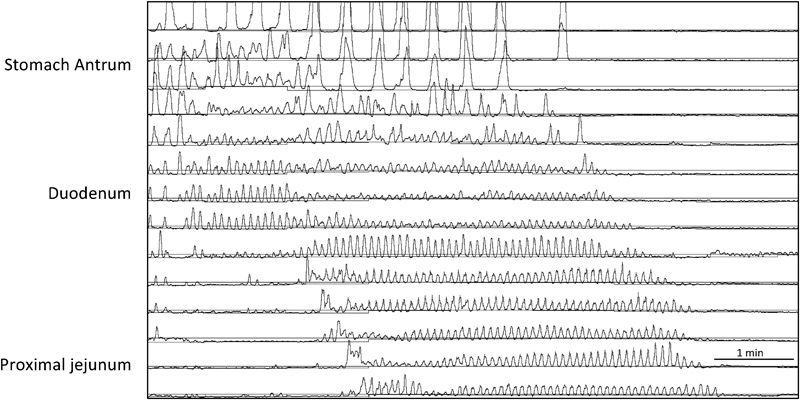
Fasting antroduodenal manometry showing three per minute gastric antrum contractions and migrating motor complex in the duodenum and proximal jejunum following intravenous erythromycin.
Fig. 3.
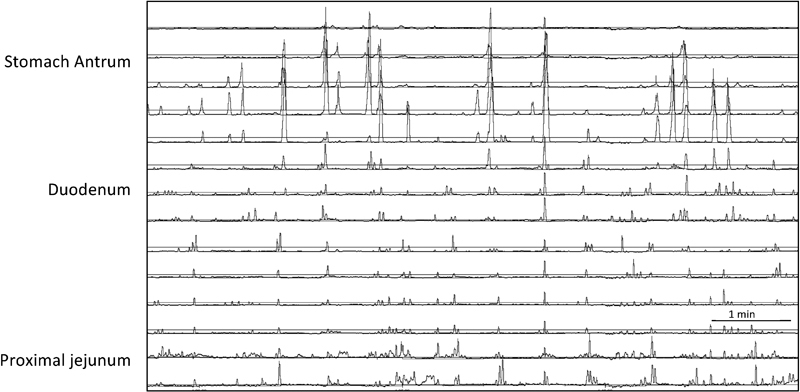
Abnormal fasting antroduodenal manometry in a child with chronic intestinal pseudo-obstruction. Following intravenous erythromycin, migrating motor complex was absent, instead there was random phasic contraction and some clusters were seen in the duodenum and proximal jejunum.
Manometry studies are more sensitive than radiographic studies and evaluate the strength and coordination of contractions in the esophagus, gastric antrum, small intestine, colon, and anorectum. 38 39 Severe abnormalities during manometry studies usually correlate with clinical symptoms. Esophageal manometry is abnormal in half of patients with CIP. Antroduodenal manometry is helpful to evaluate the stomach antrum and proximal small bowel motility. The stomach antrum normally contracts at a frequency of three contractions per minute and fasting normal small bowel contraction pattern is divided into three phases: phase I is a period of quiescence; during phase II, there are low-amplitude, random phasic contractions; and phase III/migrating motor complex (MMC) is associated with coordinated contractions lasting approximately 10 to 15 seconds ( Fig. 2 ). Smooth muscle disorders are characterized by low-amplitude, coordinated contractions, whereas neuropathic processes are characterized by uncoordinated contractions and the absence of MMC. 36 40 41 Differentiation of CIP into neuropathic and myopathic based on manometry findings can be difficult. A normal antroduodenal manometry and absence of dilated bowel essentially exclude the diagnosis of CIP and should prompt one to consider a psychological illness. Antroduodenal manometry is abnormal in partial and complete bowel obstruction as well.
Colon manometry can show lack of gastrocolic reflex and absence of high-amplitude propagating contractions either in the entire colon or a segment of the colon. In myopathic CIP, the colon contractions are lower in amplitude and this can be difficult to differentiate from low-amplitude colon contractions associated with dilated colon. There are several pitfalls with intestinal and colon manometry studies, and movement artifact in an uncooperative and crying child can make interpretation difficult. Ideally, manometry studies should be performed when the child has no acute illness and drugs known to affect motility should be discontinued.
Full-thickness gastric antrum, small intestine, and colon biopsies are not always necessary but essential if histological evaluation of the enteric neuromusculature is needed. Most pediatric surgeons in large academic centers are able to obtain adequate tissue laparoscopically. 42 It is anticipated that in the coming years, this will help improve our understanding of the histopathological abnormalities associated with childhood CIP. However, currently a vast majority of children with CIP have no identifiable abnormality of the enteric neurons or smooth muscle lining and are labeled as having idiopathic CIP. The authors' algorithm which is useful in the diagnostic workup of children with CIP is shown in Fig. 4 .
Fig. 4.
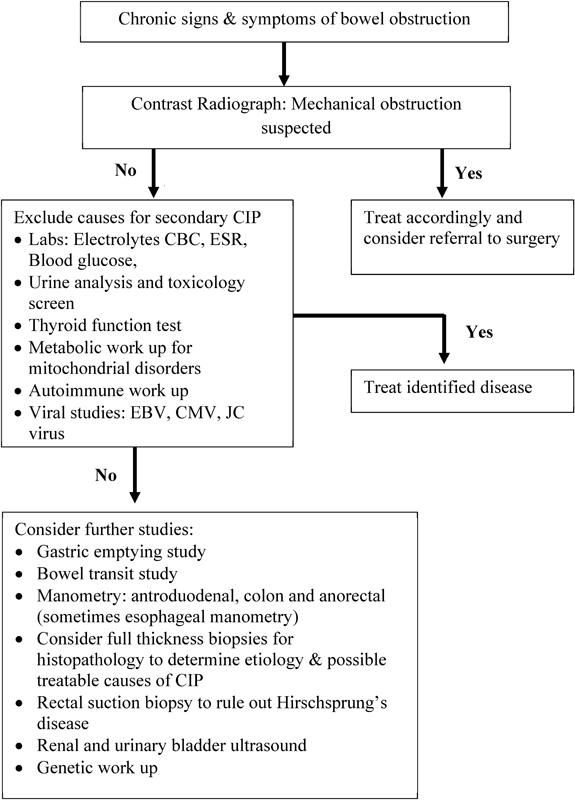
Algorithm useful in the diagnostic workup of children with CIP.
Treatment
Once the diagnosis of CIP is established, the treatment goal should be to minimize surgical intervention, preserve residual bowel motility and motor function, and optimize nutritional status. Almost one-third of patients with CIP require total or partial parenteral nutritional support and a third require total or partial enteral feeding support. 36 37 Ninety percent of the deaths due to CIP are related to complications of parenteral nutrition and include central line sepsis and parenteral nutrition induced liver cirrhosis and decompensation. 35 38 43 Therefore, optimal use of enteral nutrition and discontinuation of parenteral nutrition at the earliest opportunity is important. Continuous nasogastric tube or gastrostomy feeding may be used when patients are unable to take feed orally or do not tolerate bolus feeds. Children who have gastroparesis can be fed directly into the proximal small bowel through a gastrojejunal tube or a jejunostomy can be performed. The gastrostomy can also be used to vent the stomach and decompress the bowel during exacerbation of pseudo-obstruction.
Medications that stimulate intestinal motility help a minority of patients with CIP. Erythromycin (1–3 mg/kg intravenously, 3–5 mg/kg orally) 44 is a motilin receptor agonist that stimulates antral contractions (high amplitude: 3 per minute) and facilitates gastric emptying. Octreotide is a somatostatin analog that works on the small intestine and suppresses phase 2 contractions while inducing phase 3-like clustered contractions. However, these clusters may be nonpropagative or may propagate in both directions. 45 Amoxicillin-clavulanic acid can increase small bowel contractions and at the same time help treat SIBO. 46 Cisapride is a 5HT3 antagonist and 5HT4 agonist; it binds to the serotonin receptors in the myenteric plexus resulting in acetylcholine release and smooth muscle contraction. Cisapride has been shown to help patients with MMCs and without dilated bowels 47 by increasing the number and strength of duodenal contractions. Cisapride is no longer readily available due to concerns of rare fatal cardiac arrhythmias, and its use requires an Investigational New Drug application from the Food and Drug Administration. 48 Tegaserod, a 5HT4 agonist, is another drug that seemed to have potential therapeutic benefit for patients with CIP but was also withdrawn for cardiovascular concerns.
SIBO is a common complication of chronically dilated bowel and causes mucosal damage, malabsorption, fluid secretion, and gas production. 43 It has been associated with steatorrhea, fat-soluble vitamin, and vitamin B12 deficiency. Judicious use of oral antibiotics is recommended and can help improve abdominal distension and pain. Commonly used antibiotics include amoxicillin-clavulanic acid, trimethoprim-sulfamethoxazole, and aminoglycosides. Repeated and prolonged use of oral antibiotics is associated with risk of emergence of antibiotic-resistant organisms as well as yeast overgrowth.
Pain is often the most distressing symptom of CIP. A gastrostomy to vent the stomach and decompress small bowel can help alleviate pain and vomiting. Chronic pain can be managed with low-dose tricyclic antidepressants and gabapentin. 49 Opioids disrupt intestinal motility and should be avoided. Collaboration with a chronic pain team is usually recommended and should include mental health specialists. Other modalities for pain relief and control include cognitive behavioral therapy, hypnosis, relaxation, yoga, and massage therapy.
Constipation is initially treated with polyethylene glycol, suppositories, or enemas. Cecostomy for antegrade enemas, 50 and ileostomy with/without colonic resection in carefully selected patients with colon dysmotility, may help preserve small bowel motility and delay onset of intestinal failure. If the colon manometry shows no colon contractions, ileostomy is usually the best course. 35
Management of acute obstructive episodes is often challenging; the loss of fluid and electrolytes through vomiting and gastric suction can precipitate fluid and electrolyte imbalance. Careful monitoring and replacement of fluids and electrolytes is required. Unnecessary surgeries should be avoided as postoperative ileus is prolonged and development of adhesions can further compromise bowel function. Children with CIP, especially those with mega colon, are at risk of developing colon volvulus. 51 Prompt diagnosis and surgical intervention can be lifesaving in this situation. Differentiating colon volvulus from pseudo-obstruction crisis can be difficult, as clinical symptoms and signs are similar. Usually patients with a volvulus will report severe abdominal pain and obstipation.
Allogeneic hematopoietic stem cell transplantation can restore thymidine phosphorylase enzyme function in patients with MNGIE and can improve clinical manifestations in the long term. Patients who have advanced liver disease associated with intestinal failure tend to have poor outcomes despite hematopoietic stem cell transplantation. 52 Recently, it has been recognized that inflammatory enteric neuronal damage can be reversed by using immunosuppressive agents if diagnosed early. 53
Children with poor quality of life despite appropriate medical management or those who have developed complications of parenteral nutrition or venous access problems are candidates for intestinal transplantation. 36 54 The United Network for Organ Sharing data in children reported 1- and 5-year survival rates for transplantation for motility-related indications of 75 and 57%, respectively. 55 Although survival rates for intestinal transplantation have improved, they are not comparable to excellent outcome of other organ transplantation (e.g., liver and kidney). In our experience, patients who are able to attend school and have good quality of life despite needing parenteral nutrition support prefer to delay intestinal transplantation. Pediatric CIP is associated with significant morbidity and mortality usually due to the treatment these patients receive. Although quality of life of children and their families is significantly impacted due to CIP, the long-term survival and outcome for most patients have improved over the years. 56
References
- 1.Rudolph C D, Hyman P E, Altschuler S M et al. Diagnosis and treatment of chronic intestinal pseudo-obstruction in children: report of consensus workshop. J Pediatr Gastroenterol Nutr. 1997;24(01):102–112. doi: 10.1097/00005176-199701000-00021. [DOI] [PubMed] [Google Scholar]
- 2.Muto M, Matsufuji H, Tomomasa T et al. Pediatric chronic intestinal pseudo-obstruction is a rare, serious, and intractable disease: a report of a nationwide survey in Japan. J Pediatr Surg. 2014;49(12):1799–1803. doi: 10.1016/j.jpedsurg.2014.09.025. [DOI] [PubMed] [Google Scholar]
- 3.Mayer E A, Schuffler M D, Rotter J I, Hanna P, Mogard M. Familial visceral neuropathy with autosomal dominant transmission. Gastroenterology. 1986;91(06):1528–1535. doi: 10.1016/0016-5085(86)90211-8. [DOI] [PubMed] [Google Scholar]
- 4.Roy A D, Bharucha H, Nevin N C, Odling-Smee G W. Idiopathic intestinal pseudo-obstruction: a familial visceral neuropathy. Clin Genet. 1980;18(04):291–297. doi: 10.1111/j.1399-0004.1980.tb00888.x. [DOI] [PubMed] [Google Scholar]
- 5.Faulk D L, Anuras S, Gardner G D, Mitros F A, Summers R W, Christensen J.A familial visceral myopathy Ann Intern Med 197889(5, Pt 1):600–606. [DOI] [PubMed] [Google Scholar]
- 6.Haltia M, Somer H, Palo J, Johnson W G. Neuronal intranuclear inclusion disease in identical twins. Ann Neurol. 1984;15(04):316–321. doi: 10.1002/ana.410150403. [DOI] [PubMed] [Google Scholar]
- 7.Patel H, Norman M G, Perry T L, Berry K E. Multiple system atrophy with neuronal intranuclear hyaline inclusions. Report of a case and review of the literature. J Neurol Sci. 1985;67(01):57–65. doi: 10.1016/0022-510x(85)90022-x. [DOI] [PubMed] [Google Scholar]
- 8.Schuffler M D, Bird T D, Sumi S M, Cook A. A familial neuronal disease presenting as intestinal pseudoobstruction. Gastroenterology. 1978;75(05):889–898. [PubMed] [Google Scholar]
- 9.Anuras S, Mitros F A, Nowak T V et al. A familial visceral myopathy with external ophthalmoplegia and autosomal recessive transmission. Gastroenterology. 1983;84(02):346–353. [PubMed] [Google Scholar]
- 10.Ionasescu V, Thompson S H, Ionasescu R et al. Inherited ophthalmoplegia with intestinal pseudo-obstruction. J Neurol Sci. 1983;59(02):215–228. doi: 10.1016/0022-510x(83)90039-4. [DOI] [PubMed] [Google Scholar]
- 11.Schuffler M D, Lowe M C, Bill A H. Studies of idiopathic intestinal pseudoobstruction. I. Hereditary hollow visceral myopathy: clinical and pathological studies. Gastroenterology. 1977;73(02):327–338. [PubMed] [Google Scholar]
- 12.Schuffler M D, Pope C E., II Studies of idiopathic intestinal pseudoobstruction. II. Hereditary hollow visceral myopathy: family studies. Gastroenterology. 1977;73(02):339–344. [PubMed] [Google Scholar]
- 13.Auricchio A, Brancolini V, Casari G et al. The locus for a novel syndromic form of neuronal intestinal pseudoobstruction maps to Xq28. Am J Hum Genet. 1996;58(04):743–748. [PMC free article] [PubMed] [Google Scholar]
- 14.Thorson W, Diaz-Horta O, Foster J, II et al. De novo ACTG2 mutations cause congenital distended bladder, microcolon, and intestinal hypoperistalsis. Hum Genet. 2014;133(06):737–742. doi: 10.1007/s00439-013-1406-0. [DOI] [PubMed] [Google Scholar]
- 15.Wangler M F, Gonzaga-Jauregui C, Gambin T et al. Heterozygous de novo and inherited mutations in the smooth muscle actin (ACTG2) gene underlie megacystis-microcolon-intestinal hypoperistalsis syndrome. PLoS Genet. 2014;10(03):e1004258. doi: 10.1371/journal.pgen.1004258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chetaille P, Preuss C, Burkhard S et al. Mutations in SGOL1 cause a novel cohesinopathy affecting heart and gut rhythm. Nat Genet. 2014;46(11):1245–1249. doi: 10.1038/ng.3113. [DOI] [PubMed] [Google Scholar]
- 17.Van Goethem G, Schwartz M, Löfgren A, Dermaut B, Van Broeckhoven C, Vissing J. Novel POLG mutations in progressive external ophthalmoplegia mimicking mitochondrial neurogastrointestinal encephalomyopathy. Eur J Hum Genet. 2003;11(07):547–549. doi: 10.1038/sj.ejhg.5201002. [DOI] [PubMed] [Google Scholar]
- 18.Sonsino E, Mouy R, Foucaud P et al. Intestinal pseudoobstruction related to cytomegalovirus infection of myenteric plexus. N Engl J Med. 1984;311(03):196–197. doi: 10.1056/NEJM198407193110319. [DOI] [PubMed] [Google Scholar]
- 19.Besnard M, Faure C, Fromont-Hankard G et al. Intestinal pseudo-obstruction and acute pandysautonomia associated with Epstein-Barr virus infection. Am J Gastroenterol. 2000;95(01):280–284. doi: 10.1111/j.1572-0241.2000.01709.x. [DOI] [PubMed] [Google Scholar]
- 20.Selgrad M, De Giorgio R, Fini L et al. JC virus infects the enteric glia of patients with chronic idiopathic intestinal pseudo-obstruction. Gut. 2009;58(01):25–32. doi: 10.1136/gut.2008.152512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Connor F L, Di Lorenzo C. Chronic intestinal pseudo-obstruction: assessment and management. Gastroenterology. 2006;130(02) 01:S29–S36. doi: 10.1053/j.gastro.2005.06.081. [DOI] [PubMed] [Google Scholar]
- 22.Knowles C H, De Giorgio R, Kapur R P et al. The London Classification of gastrointestinal neuromuscular pathology: report on behalf of the Gastro 2009 International Working Group. Gut. 2010;59(07):882–887. doi: 10.1136/gut.2009.200444. [DOI] [PubMed] [Google Scholar]
- 23.Kapur R P, Correa H. Architectural malformation of the muscularis propria as a cause for intestinal pseudo-obstruction: two cases and a review of the literature. Pediatr Dev Pathol. 2009;12(02):156–164. doi: 10.2350/08-07-0495.1. [DOI] [PubMed] [Google Scholar]
- 24.Smith V V, Milla P J. Histological phenotypes of enteric smooth muscle disease causing functional intestinal obstruction in childhood. Histopathology. 1997;31(02):112–122. doi: 10.1046/j.1365-2559.1997.2250839.x. [DOI] [PubMed] [Google Scholar]
- 25.Smith J A, Hauser S C, Madara J L. Hollow visceral myopathy: a light- and electron-microscopic study. Am J Surg Pathol. 1982;6(03):269–275. [PubMed] [Google Scholar]
- 26.Kapur R P. Pathology of intestinal motor disorders in children. Surg Pathol Clin. 2010;3(03):711–741. doi: 10.1016/j.path.2010.06.005. [DOI] [PubMed] [Google Scholar]
- 27.Oton E, Moreira V, Redondo C et al. Chronic intestinal pseudo-obstruction due to lymphocytic leiomyositis: is there a place for immunomodulatory therapy? Gut. 2005;54(09):1343–1344. doi: 10.1136/gut.2005.071811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.O'Donnell A M, Puri P. Skip segment Hirschsprung's disease: a systematic review. Pediatr Surg Int. 2010;26(11):1065–1069. doi: 10.1007/s00383-010-2692-4. [DOI] [PubMed] [Google Scholar]
- 29.Dingemann J, Puri P. Isolated hypoganglionosis: systematic review of a rare intestinal innervation defect. Pediatr Surg Int. 2010;26(11):1111–1115. doi: 10.1007/s00383-010-2693-3. [DOI] [PubMed] [Google Scholar]
- 30.Nezelof C, Guy-Grand D, Thomine E. Megacolon with hyperplasia of the myenteric plexua. An anatomo-clinical entity, apropos of 3 cases [in French] Presse Med. 1970;78(34):1501–1506. [PubMed] [Google Scholar]
- 31.Meier-Ruge W. Casuistic of colon disorder with symptoms of Hirschsprung's disease [author's transl] Verh Dtsch Ges Pathol. 1971;55:506–510. [PubMed] [Google Scholar]
- 32.Negreanu L M, Assor P, Mateescu B, Cirstoiu C. Interstitial cells of Cajal in the gut--a gastroenterologist's point of view. World J Gastroenterol. 2008;14(41):6285–6288. doi: 10.3748/wjg.14.6285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bruhin-Feichter S, Meier-Ruge W, Martucciello G, Bruder E. Connective tissue in gut development: a key player in motility and in intestinal desmosis. Eur J Pediatr Surg. 2012;22(06):445–459. doi: 10.1055/s-0032-1322544. [DOI] [PubMed] [Google Scholar]
- 34.Gyger G, Baron M. Systemic sclerosis: gastrointestinal disease and its management. Rheum Dis Clin North Am. 2015;41(03):459–473. doi: 10.1016/j.rdc.2015.04.007. [DOI] [PubMed] [Google Scholar]
- 35.Heneyke S, Smith V V, Spitz L, Milla P J. Chronic intestinal pseudo-obstruction: treatment and long term follow up of 44 patients. Arch Dis Child. 1999;81(01):21–27. doi: 10.1136/adc.81.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mousa H, Hyman P E, Cocjin J, Flores A F, Di Lorenzo C. Long-term outcome of congenital intestinal pseudoobstruction. Dig Dis Sci. 2002;47(10):2298–2305. doi: 10.1023/a:1020199614102. [DOI] [PubMed] [Google Scholar]
- 37.Cucchiara S, Borrelli O. Nutritional challenge in pseudo-obstruction: the bridge between motility and nutrition. J Pediatr Gastroenterol Nutr. 2009;48 02:S83–S85. doi: 10.1097/MPG.0b013e3181a15bfe. [DOI] [PubMed] [Google Scholar]
- 38.Fell J M, Smith V V, Milla P J. Infantile chronic idiopathic intestinal pseudo-obstruction: the role of small intestinal manometry as a diagnostic tool and prognostic indicator. Gut. 1996;39(02):306–311. doi: 10.1136/gut.39.2.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Goulet O, Jobert-Giraud A, Michel J L et al. Chronic intestinal pseudo-obstruction syndrome in pediatric patients. Eur J Pediatr Surg. 1999;9(02):83–89. doi: 10.1055/s-2008-1072218. [DOI] [PubMed] [Google Scholar]
- 40.Di Lorenzo C, Lucanto C, Flores A F, Idries S, Hyman P E. Effect of sequential erythromycin and octreotide on antroduodenal manometry. J Pediatr Gastroenterol Nutr. 1999;29(03):293–296. doi: 10.1097/00005176-199909000-00010. [DOI] [PubMed] [Google Scholar]
- 41.Uc A, Hoon A, Di Lorenzo C, Hyman P E. Antroduodenal manometry in children with no upper gastrointestinal symptoms. Scand J Gastroenterol. 1997;32(07):681–685. doi: 10.3109/00365529708996518. [DOI] [PubMed] [Google Scholar]
- 42.Mazziotti M V, Langer J C. Laparoscopic full-thickness intestinal biopsies in children. J Pediatr Gastroenterol Nutr. 2001;33(01):54–57. doi: 10.1097/00005176-200107000-00009. [DOI] [PubMed] [Google Scholar]
- 43.Goulet O, Jobert-Giraud A, Michel J L et al. Chronic intestinal pseudo-obstruction syndrome in pediatric patients. Eur J Pediatr Surg. 1999;9(02):83–89. doi: 10.1055/s-2008-1072218. [DOI] [PubMed] [Google Scholar]
- 44.Di Lorenzo C, Flores A F, Tomomasa T, Hyman P E. Effect of erythromycin on antroduodenal motility in children with chronic functional gastrointestinal symptoms. Dig Dis Sci. 1994;39(07):1399–1404. doi: 10.1007/BF02088040. [DOI] [PubMed] [Google Scholar]
- 45.Di Lorenzo C, Lucanto C, Flores A F, Idries S, Hyman P E. Effect of octreotide on gastrointestinal motility in children with functional gastrointestinal symptoms. J Pediatr Gastroenterol Nutr. 1998;27(05):508–512. doi: 10.1097/00005176-199811000-00002. [DOI] [PubMed] [Google Scholar]
- 46.Gomez R, Fernandez S, Aspirot A et al. Effect of amoxicillin/clavulanate on gastrointestinal motility in children. J Pediatr Gastroenterol Nutr. 2012;54(06):780–784. doi: 10.1097/MPG.0b013e31824204e4. [DOI] [PubMed] [Google Scholar]
- 47.Di Lorenzo C, Reddy S N, Villanueva-Meyer J, Mena I, Martin S, Hyman P E. Cisapride in children with chronic intestinal pseudoobstruction. An acute, double-blind, crossover, placebo-controlled trial. Gastroenterology. 1991;101(06):1564–1570. doi: 10.1016/0016-5085(91)90393-y. [DOI] [PubMed] [Google Scholar]
- 48.Raphael B P, Nurko S, Jiang H et al. Cisapride improves enteral tolerance in pediatric short-bowel syndrome with dysmotility. J Pediatr Gastroenterol Nutr. 2011;52(05):590–594. doi: 10.1097/MPG.0b013e3181fe2d7a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rosner H, Rubin L, Kestenbaum A. Gabapentin adjunctive therapy in neuropathic pain states. Clin J Pain. 1996;12(01):56–58. doi: 10.1097/00002508-199603000-00010. [DOI] [PubMed] [Google Scholar]
- 50.Youssef N N, Barksdale E, Jr, Griffiths J M, Flores A F, Di Lorenzo C. Management of intractable constipation with antegrade enemas in neurologically intact children. J Pediatr Gastroenterol Nutr. 2002;34(04):402–405. doi: 10.1097/00005176-200204000-00016. [DOI] [PubMed] [Google Scholar]
- 51.Altaf M A, Werlin S L, Sato T T, Rudolph C D, Sood M R. Colonic volvulus in children with intestinal motility disorders. J Pediatr Gastroenterol Nutr. 2009;49(01):59–62. doi: 10.1097/MPG.0b013e3181879eb5. [DOI] [PubMed] [Google Scholar]
- 52.Halter J P, Michael W, Schüpbach Met al. Allogeneic haematopoietic stem cell transplantation for mitochondrial neurogastrointestinal encephalomyopathy Brain 2015138(Pt 10):2847–2858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cucchiara S, Borrelli O, Salvia G et al. A normal gastrointestinal motility excludes chronic intestinal pseudoobstruction in children. Dig Dis Sci. 2000;45(02):258–264. doi: 10.1023/a:1005491921972. [DOI] [PubMed] [Google Scholar]
- 54.Bond G J, Reyes J D. Intestinal transplantation for total/near-total aganglionosis and intestinal pseudo-obstruction. Semin Pediatr Surg. 2004;13(04):286–292. doi: 10.1053/j.sempedsurg.2004.10.016. [DOI] [PubMed] [Google Scholar]
- 55.Lao O B, Healey P J, Perkins J D, Horslen S, Reyes J D, Goldin A B. Outcomes in children after intestinal transplant. Pediatrics. 2010;125(03):e550–e558. doi: 10.1542/peds.2009-1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Schwankovsky L, Mousa H, Rowhani A, DI Lorenzo C, Hyman P E. Quality of life outcomes in congenital chronic intestinal pseudo-obstruction. Dig Dis Sci. 2002;47(09):1965–1968. doi: 10.1023/a:1019644022606. [DOI] [PubMed] [Google Scholar]


