Abstract
Hirschsprung disease (HD) is a common cause of neonatal intestinal obstruction in which a variable segment of the distal intestinal tract lacks the normal enteric nervous system elements. Affected individuals present with varying degrees of obstructive symptoms, but today most patients are diagnosed within the first several months of life owing to the well-recognized symptoms and the ease of making the diagnosis by way of the bedside suction rectal biopsy. Thus, for the adult general or colorectal surgeon, the vast majority of patients who present for evaluation will have already undergone surgical treatment within the first year of life by a pediatric surgeon. Despite several safe operative interventions to treat patients with HD, the long-term results are far from perfect. These patients may reach adult life with ongoing defecation disorders that require a systematic evaluation by a multidisciplinary group that should be led by a surgeon with a thorough knowledge of HD operations and the potential problems. The evaluation of these patients will form the basis for the majority of this review—however, some patients manage to escape diagnosis beyond the infant and childhood period—and a section herein will briefly address the case of an older patient who is suspected of having HD.
Keywords: Hirschsprung, complications, reoperation, incontinence, constipation
In 1886, a young Danish pediatrician named Harald Hirschsprung presented a treatise at the Society of Pediatrics in Berlin. His description of two patients entitled Constipation in newborns due to dilation and hypertrophy of the colon was the first credited description of the disease that now bears his name. 1 Hirschsprung disease (HD) is a common cause of neonatal intestinal obstruction in which a variable segment of the distal intestinal tract lacks the normal enteric nervous system elements. More specifically, the absence of ganglion cells of the myenteric and submucosal plexi is associated with the Hirschsprung phenotype that manifests clinically as functional obstruction of the affected distal intestine. For decades, surgeons believed that the dilated megacolon formed the basis of the problem. It was not until 50 years later when Swenson et al described the physiological findings of the affected colon in that the proximal contractions in the dilated colon did not enter the more distal segment. 2 These findings provided the basis for several subsequent surgical interventions that form the modern basis of surgical treatment. Nevertheless, the exact pathophysiology of HD remains unclear as there is incomplete understanding of how and if aganglionosis alone causes the observed functional obstruction. HD occurs in approximately 1 in 5,000 live births, and aganglionosis always begins at the anal verge. However, the length of the aganglionic segment varies: it is limited to the rectum and sigmoid colon in 75% of patients, involves an extended portion of the colon in 10%, involves the entire colon in another 10%, and, finally, involves the colon and varying lengths of the small bowel in 5%. Affected individuals present with varying degrees of obstructive symptoms, but today most patients are diagnosed within the first several months of life owing to the well-recognized symptoms and the ease of making the diagnosis by way of the suction rectal biopsy, which can be performed with ease in neonates at the bedside without anesthesia. Thus, for the adult general or colorectal surgeon, the vast majority of patients who present for evaluation will have already undergone surgical treatment. The evaluation of these patients will form the basis of the majority of this article—however, some patients manage to escape diagnosis beyond the infant and childhood period—and a section herein will briefly address the case of an older patient who is suspected of having HD.
Patients with a Previous Diagnosis of Hirschsprung Disease
Long-Term Outcomes of Hirschsprung Disease Treated in Childhood
There are essentially two categories of defecation dysfunction in children who have had previous surgery for HD: obstructive symptoms and problems with soiling. Obstructive symptoms can take the form of abdominal distension, bloating, vomiting, recurrent enterocolitis, or severe constipation requiring ongoing laxative therapy or enemas. The five major reasons for persistent obstructive symptoms following a pull-through are mechanical obstruction, persistent or acquired aganglionosis, disordered intestinal motility, internal anal sphincter dysfunction, and functional megacolon caused by stool-holding behavior. Soiling following a pull-through can be due to impaired continence as a result of internal anal sphincter dysfunction or poor sensation within the anal canal, or “pseudoincontinence” caused by either fecal loading with encopresis or hyperperistalsis in the pulled-through bowel. The evaluation of an older patient with a defecation disorder after an operative intervention for HD earlier in life requires an understanding of the extent of the original disease, the operation originally performed, the complications or problems encountered in infancy or childhood, and the current defecation issues for which the individual is being evaluated. A thorough and organized approach must be taken to correctly define the cause of the problem and to design an appropriate therapeutic plan. 3 4 A multidisciplinary team consisting of a general surgeon, an adult gastroenterologist, a pathologist, and a pediatric surgeon, where available, forms the ideal physician group to care for such a patient into adulthood.
It is clear that while HD is now rarely fatal, short- and long-term morbidities remain a considerable possibility into adulthood. Symptoms of constipation are generally believed to become milder or subside with time after operative correction of HD in infancy. 5 Reports of long-term outcomes among adolescents or young adults demonstrate a low incidence of constipation compared with a higher percentage of patients reporting constipation during childhood. 6 7 8 9 However, Rintala and Pakarinen noted that the overall incidence of constipation may increase again with advancing adult age, especially in those patients who had a Duhamel procedure, 10 and some have noted this finding independent of the initial operative reconstruction. 11
When fecal incontinence has been independently studied with specific scrutiny, the incidence exceeds 50% during childhood in nonsyndromic patients. 9 12 13 14 Nearly similar figures have been reported for adolescents and adults. In patients with Down's syndrome (trisomy 21), Catto-Smith et al found an 87% incidence of fecal incontinence. 15 Most of the literature examining long-term incontinence rates report patients with all degrees of incontinence, from frank incontinence with large volume fecal accidents to patients with milder symptoms such as occasional soiling or staining. Although the majority of patients suffer from minor soiling rather than frank incontinence, we believe that the social implications of this problem are a major problem for the affected individual.
The Antecedent History and Operative Intervention
Examination of previous operative record forms an essential element of establishing care for an older patient who presents with defecation issues in the setting of a previous diagnosis of HD. Understanding the extent of the initial disease and the operation performed are key first steps in the evaluation. Initially, the age at diagnosis and the original extent of disease will dictate the subsequent operative approach. Although many patients historically underwent a diverting colostomy or ileostomy at the level of bowel that contained ganglion cells (a “leveling” colostomy or ileostomy) when the diagnosis was established, many of the definitive operations are now performed in one stage without the need for antecedent intestinal diversion during the neonatal or infant period. 16 The common goal of the reconstructive operation is to remove the aganglionic bowel (or bypass it with a mixed conduit of ganglionic and aganglionic bowel) and provide normally innervated bowel as distally as possible while preserving the function of the anal sphincter mechanism to provide for normal defecation.
Rectosigmoid Hirschsprung Disease
The three most commonly performed reconstructive operations in patients with rectosigmoid or extended colonic disease are the Duhamel, Soave, and Swenson procedures, often termed pull-through operations ( Fig. 1 ). The pull-through segment of intestine contains a normal complement of ganglion cells by pathological analysis. Soave's operation involves pulling a segment of normally innervated intestine through a retained muscular cuff (of varying lengths depending on the preference of the surgeon) after extramucosal dissection of the distal rectum so as to theoretically avoid damaging the pelvic splanchnic nerves along the anterior rectum. Originally, Soave allowed the pull-through segment to extend from the anus without an anastomosis. 17 The distal pull-through segment was removed after it “adhered” to the anal tissues—hardly a scenario parents would find acceptable today. Boley is credited with performing a primary anastomosis at the time of the pull-through operation and immediately resecting the aganglionic colon, which is how the operation is currently performed. 18 However, many pediatric surgeons still refer to the modern operation as the Soave procedure. The muscular cuff is typically either left very short posteriorly, split, or segmentally excised in an attempt to allow for adequate defecation. Swenson's original operation has changed little since his first description, and although a component of the operation involves pulling the ganglionic bowel through the aganglionic segment that has been everted from the anus, the ultimate result is a very low hand-sewn coloanal (or ileoanal in a straight for total colonic disease) anastomosis. 2 Some authors use elements of both the Soave and Swenson operations, in what is colloquially termed a Soaveson procedure. In this modification, the posterior muscular cuff is removed as close to the dentate line as possible, while preserving the anterior muscular cuff consistent with the Soave operation. 19 This modification theoretically eliminates the potential of a constrictive retained muscular cuff, although this has not been well studied with long-term follow-up to date. In the Duhamel operation, a composite graft is created from a short segment of distal aganglionic colon retained anteriorly and a segment of normally innervated colon pulled through posteriorly. 20 The anterior aspect of the sphincter complex is left undisturbed, and the pull-through segment is sewn to the posterior half of the sphincter complex just above the dentate line. The composite Duhamel pouch is typically created by firing a GIA stapler inserted from the anus between the pull-through and the aganglionic rectum. 21 Other authors have described alternative approaches in an attempt to improve upon the potential anatomic pitfalls when constructing the composite pouch, 22 and some have described modifications to the original Duhamel operation. 23 24 The Soave, 25 Duhamel, 26 and Swenson 27 operations can all now be performed with the use of laparoscopy, and the Soave pull-through is performed completely transanally in some centers. 28 The Rehbein procedure is an anterior resection with a low coloanal anastomosis with an EEA stapler (Medtronic) that is not commonly performed in the United States due to concerns regarding a higher risk of postoperative constipation necessitating sphincter dilation or reoperative intervention. 29 This is also the case for the operation originally described by State, 30 which is not a reasonable modern operative correction strategy. There is no definitive evidence that any one of the three commonly performed operations is better than the other, but each lends itself to specific potential long-term pitfalls. The initial operation performed is often the one that the surgeon has been trained to perform and does frequently with his/her own optimal results. 31 32
Fig. 1.
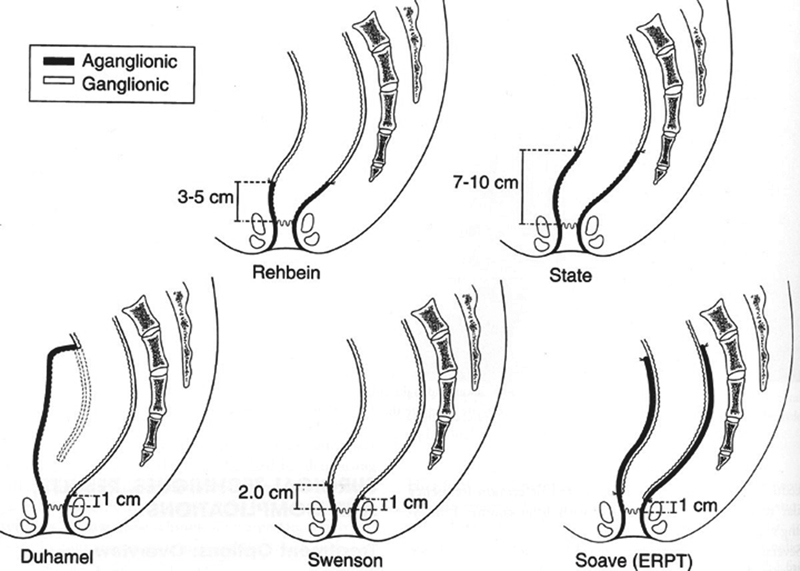
The different types of reconstruction “pullthrough” operations employed for the correction of Hirschsprung Disease.
Total Colonic Hirschsprung Disease
In total colonic HD (TCHD), the options for definitive reconstruction can be divided into three main categories: the straight pull-through, colonic composite procedures, and the J-pouch ileoanal anastomosis. We prefer to delay the definitive reconstructive procedure until the child is 1 year of age after a leveling ileostomy during the neonatal period. Some prefer to delay the reconstruction until later in life, 33 whereas others perform a pull-through without a diversion procedure in the neonatal period. 34 35 36 Another option is to perform the definitive reconstruction at diagnosis but leave a protective ileostomy until the child is toilet-trained for urine and is willing to tolerate rectal irrigations. 37 Straight pull-through procedures can be undertaken by using any of the standard techniques (Duhamel, Soave, or Swenson) and can be performed through a laparotomy or using laparoscopy. The postoperative issues with a straight pull-through are related to the liquid nature of the stool that may cause perineal excoriation, and aggressive skin care is tantamount to prevent this common postoperative problem. The concept of a colon composite is to perform a side-to-side anastomosis between normally innervated small bowel and aganglionic colon akin to the Duhamel reconstruction described previously. The retained aganglionic colon allows for water and electrolyte absorption, 38 whereas the normally innervated pull-through segment of small bowel allows for normal motility to the anal canal. Martin is credited with describing the first such operation that consisted of a long composite involving the entire left colon, 23 the Duhamel/Martin procedure. Nishijima et al prefer a technique using the right colon to take advantage of its theoretical higher capacity to absorb water and electrolytes. 39 However, this procedure requires a staged approach to bring the right colon/small bowel composite into the pelvis. Several authors have published modifications of the operation of Martin and Nishijima procedures. 40 41 Although the colon composite procedures theoretically permit decreased stool output from better water absorption, the aganglionic colon may dilate and some of these patients develop either enterocolitis, constipation, or pseudoincontinence, which requires removal of the patch, a permanent stoma, or conversion to another type of pull-through. A total colectomy with J-pouch reconstruction has also been described for reestablishing intestinal continuity in patients with TCHD, but there are little data available concerning long-term results. 42
Compared with patients with rectosigmoid HD, the long-term outcomes of initial procedures to resect the long aganglionic segment and restore intestinal continuity have not been as well elucidated. Ein et al described seven infants who underwent a Martin's modified Duhamel procedure for TCHD, all of whom are continent at follow-up between 1 to 13 years. These children had up to 10 formed stools per day, yet two out of seven had soiling at night. 43 Escobar et al reported the long-term outcome of 36 patients who underwent a pull-through for TCHD with a mean follow-up of 11 years. They noted that postoperative complications were more common after the Duhamel/Martin and Soave procedures. Of 29 patients, 21 (91%) had four to six bowel movements per day, and 17 (81%) of 21 were continent. The Kimura patch provided functional benefit in five patients with proximal disease. 44 Wildhaber et al reported the long-term follow-up of 20 patients with TCHD, 5 of whom had more extensive aganglionosis of the small bowel. One of these five patients underwent an endorectal pull-through, one underwent intestinal transplantation, and three died. Mean follow-up was 17.5 years. Postoperative complications included enterocolitis (55%), anal stricture (25%), and perineal excoriation (20%). Eighty-nine percent were free of recurrent enterocolitis. Eighty-two percent of patients reported one to five bowel movements a day, yet 18% experienced six or more bowel movements per day and occasional soiling was noted in 40%. 45 Barrena et al described 15 patients who underwent a modified Duhamel/Martin reconstruction and 18 who underwent a straight ileoanal pull-through for the treatment of TCHD. Out of 31 survivors, half the patients had more than three bowel movements per day, and the median continence score (normal = 14) was 11 (range: 6–14). Both operations resulted in comparable defecation and continence patterns, and quality of life was rated as good in 97%. 46 However, Blackburn et al painted a less optimistic picture in their report of seven patients with TCHD who underwent staged pull-through employing Soave, Duhamel, or Duhamel/Martin reconstructions. All patients had at least one readmission with enterocolitis, diarrhea, or high stoma output. Further procedures were required in four of the seven patients. In their small series, they observed high morbidity and poor functional outcome and concluded that patients with TCHD have a high probability of requiring a long-term stoma. 47 Shen et al reviewed 29 patients with TCHD who were treated with either a Duhamel/Martin procedure (14 patients) or a Soave procedure (15 patients). During the follow-up period, seven patients in the Duhamel/Martin group had enterocolitis, with four having severe enterocolitis leading to multiple hospitalizations. In contrast, only two patients had enterocolitis in the Soave group. At 6-month follow-up, 79% of patients in the Martin group and 62% patients in the Soave group had normal defecation. They found that patients managed with the Soave procedure had fewer operative complications compared with those who underwent the Duhamel/Martin procedure. However, the patients managed with the Soave procedure took longer to establish normal defecation. 48 Similar to patients with rectosigmoid disease, there is no clear superior operative method for the treatment of TCHD with respect to perioperative morbidity, mortality, enterocolitis, and functional outcomes. The operative technique performed should be based on surgeon familiarity and expertise, but long-term defecation disorders are common, and long-term follow-up is essential. 49
Internal Anal Sphincter Achalasia
Virtually, all children with HD lack the rectoanal inhibitory reflex (RAIR), whereby the internal anal sphincter normally relaxes in response to balloon dilation of the rectum. In patients who have refractory constipation despite a normal rectal biopsy and maximal medical therapy, anorectal manometry (ARM) adds value to the evaluation. Children with ganglion cells present on rectal biopsy who lack the RAIR are considered to have internal anal sphincter achalasia (IASA), 50 although some may misclassify these findings as ultrashort-segment HD, which should be used to describe a patient with a documented aganglionic segment of less than 1 to 2 cm. These children should be managed initially with a bowel management regimen, but many have already failed several medical interventions once they seek specialized evaluation. We advocate treating these patients with botulinum toxin A (botox A) delivered into the intersphincteric space using ultrasound guidance (100 units diluted in 1–2 mL of saline and injected at four quadrants), which often needs to be repeated for long-term efficacy. 51 Some advocate anal sphincter myectomy as first-line therapy or when botox A therapy no longer is effective after an initial response. 52 Botox A therapy is appealing due to the likelihood that the symptoms related to IASA will improve significantly over time in most of these children and that the effect of botox A is reversible.
The Common Initial Evaluation for a Patient with Stooling Problems after a Definitive Reconstructive Procedure
A detailed history and physical examination is performed with particular attention paid to the presence of symptoms consistent with either obstruction of stool evacuation (constipation) or incontinence (soiling). The use and outcome of stimulant laxatives, osmotic laxatives, antimotility agents, and other interventions that are used to evacuate the colon such as dilations or irrigations should also be noted. It is vital to note the specifics of the operative history including the type of pull-through and any postoperative complications. Each of the primary HD operations has potential pitfalls. In the Soave operation, the ganglionated bowel is pulled through a muscular cuff that may act as a constricting fibrotic ring that results in functional obstruction. The cuff is typically split or removed to prevent this potential problem. However, in some cases, the surgeon may have not split the cuff posteriorly, or if the cuff was split, it may still serve to act as an obstructive entity (i.e., the cuff anneals together in the healing process, or a subclinical abscess in the space between the cuff and pull-through segment causes a noncompliant scar). In the Duhamel operation, the composite pouch may become distended over time leading to stasis, constipation, and enterocolitis. In addition, a spur of aganglionic colon may also serve to cause similar problems. In patients who have undergone a Swenson procedure, the aganglionic colon left behind may lead to obstructive issues if the length of this segment is generous.
A water-soluble contrast enema is undertaken for all patients to evaluate the proximal pull-through anatomy. Thereafter, patients undergo an examination under anesthesia to examine the integrity of the anal canal, examine the location and status of the pull-through anastomosis, and evaluate the muscular cuff if a Soave pull-through was used as the initial operative intervention. At our center, we routinely perform concomitant ARM as a baseline to aid in selection of appropriate therapy, although it is not a required part of the evaluation. Finally, a full-thickness rectal biopsy just above the anastomosis is performed to determine the presence or absence of ganglion cells in the distal pull-through segment. Although it is tempting to reserve a biopsy for those patients with purely obstructive symptoms, patients with soiling due to pseudoincontinence secondary to obstipation should also undergo histological assessment of the pull-through segment.
Patients with Obstructive Symptoms
These children have symptoms that are seemingly identical to the symptoms with which they initially presented. Fig. 2 depicts the algorithm we employ after the results of the common initial evaluation. For patients with a restrictive Soave cuff, the biopsy and length of the restrictive cuff serve to allow for a decision about how best to proceed. In our experience, we prefer to perform a redo Soave pull-through in most cases regardless of the biopsy results; however, in the setting of a normal biopsy and a short restrictive muscular cuff, a posterior myectomy is also reasonable option if the surgeon has expertise with this technique. We perform the redo operation in the “Soaveson” manner as described previously, although a pure Swenson procedure may be necessary if a plane between the muscular cuff and the previous pull-through segment cannot be created. We believe that patients with an anastomotic stricture are best served with a redo pull-through. 53 Although serial dilations may serve to achieve an adequate anal outlet in some cases, the success of repetitive dilations may be impaired due to patient and/or parent compliance and the pain associated with dilating a noncompliant scar.
Fig. 2.
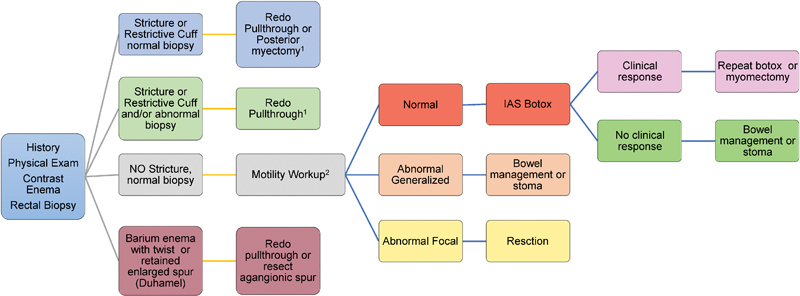
The algorithm for work-up and management of a patient with obstructive symptoms after a pull-through procedure for Hirschsprung disease. 1 Consideration for proximal intestinal diversion should be based on proximal intestinal dilation and dysfunction. 2 Motility work-up includes assessment of the contrast enema, anorectal manometry, and intestinal motility by manometric evaluation.
When the preoperative biopsy is normal , the surgeon can be assured that a redo with a relatively short pull-through segment is likely to be successful so long as the upstream colon is not significantly dysmotile. However, the proximal anastomotic margin should be analyzed for ganglion cells prior to leaving the operating room to confirm the adequacy of the pull-through. Long-standing outlet obstruction may lead to intestinal dilation and hypomotility, and this should not be taken lightly prior to a redo pull-through ( Fig. 3 ). Temporary intestinal diversion is an option to allow for adequate intestinal decompression; however, a primary redo pull-through may also be performed. In this setting, we prefer to divert the fecal stream with a laparoscopic-assisted loop ileostomy to allow the distal colon to decompress prior to the redo operation. Alternatively, the dilated bowel may be tapered along the antimesenteric border to ameliorate the size discrepancy between the pull-through intestine and the anal canal suture line with or without a temporary proximal intestinal diversion procedure.
Fig. 3.
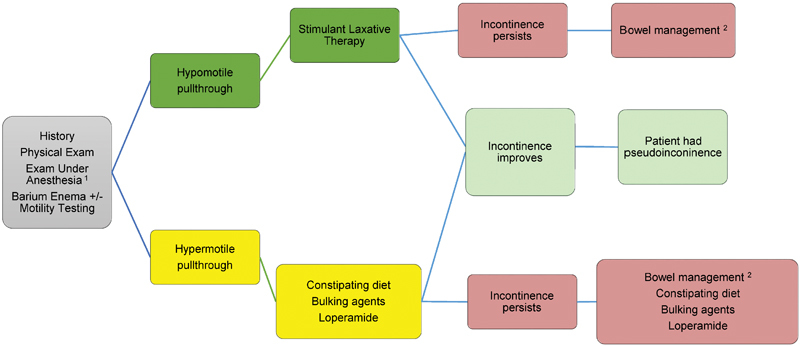
The algorithm for work-up and management of a patient with soiling symptoms after a pull-through procedure for Hirschsprung disease. 1 The examination under anesthesia serves to document the location of the previous anastomosis and the integrity of the dentate line and sphincter mechanism. A low anastomosis below the dentate line or a damaged sphincteric mechanism impairs anal sensation and normal defecation. 2 Bowel management is accomplished with either retrograde enemas or an antegrade washout program deployed with an appendicocecostomy or cecostomy.
Although rare, some children may have persistent aganglionosis due to pathologist error, a pull-through performed in the transition zone due to ganglion cell loss after a pull-through. When the preoperative biopsy is abnormal , the surgeon is prepared to perform a pull-through with specific attention to achieving a pull-through segment with normal histology (normal ganglion cells and no hypertrophic nerves), by taking biopsies of the aganglionic segment by laparotomy, laparoscopy, or sequentially with a total transanal approach. We recommend that these biopsies should be performed in full thickness to ensure accurate delineation of normal ganglionated bowel as the new pull-through. The same considerations for the proximal bowel dilation and hypomotility apply to this patient subgroup as addressed previously.
In a patient with obstructive symptoms, a normal biopsy, and a seemingly normal anal outlet (no stricture or restrictive cuff), one must then address the motility of the distal intestinal segment upstream from the anal anastomosis. Although much can be gleaned from the contrast enema (i.e., a dilated colon tends to hypomotility, whereas a normal caliber colon tends to normal motility), we prefer to perform a dedicated intestinal motility evaluation and ARM for this cohort. In the setting of normal motility, ARM nearly always demonstrates the absence of the RAIR due to the fact that the sphincter mechanism should be preserved with all of the pull-through operations. 54 Those patients with are treated with instillation of botox A into the intersphincteric space. We use 100 units deployed into the intersphincteric space by ultrasound guidance, although patients younger than 1 year receive half of that dose as initial therapy with escalation as needed. 55 The effect is reversible and the majority of patients need repeat injections, but the majority benefit and we find that it does improve the effectiveness of stimulant laxative therapy. 56 57 Patients with generalized abnormal motility are often treated with botox A without significant improvement. These patients typically require an aggressive bowel management program with stimulant laxatives or “washouts,” either by way of enemas or an antegrade washout program provided through a cecostomy (using the Malone antegrade continence procedure[MACE], a percutaneous cecostomy, or an appendicocecostomy tube). 58 59 In patients who have refractory outlet obstruction, intestinal diversion provides an option to improve quality of life when everything else has failed or when other issues preclude the ability to successfully engage in a bowel management program (i.e., patients with trisomy 21 or other behavioral disorders). Patients with segmental intestinal dysmotility are treated by segmental surgical resection, either with or without temporary intestinal diversion. We tend to perform an aggressive preoperative inpatient bowel preparation to avoid temporary intestinal diversion, but discuss the potential for doing so preoperatively with families depending on the findings at surgery.
A twist in the pull-through segment usually presents in the immediate perioperative period and should be corrected with redo pull-through once recognized. In some cases, the child will have a good initial response to surgery and then develop obstructive symptoms later. Mechanical obstruction may be the result of a stricture or a retained aganglionic spur from a Duhamel procedure that may fill with stool and obstruct the pulled-through bowel. The Duhamel procedure may also be complicated by a kink at the top of the anastomosis, which leads to obstruction. These complications are typically identified by a contrast enema and should be addressed with either resection of the aganglionic spur or by redoing the pull-through altogether.
Patients with Incontinence or Soiling
As with patients with obstructive symptoms, patients who soil after a pull-through should be approached in a systematic manner; our general approach is illustrated in Fig. 4 . There are several reasons for a child to be incontinent after a pull-through: isolated anal sphincter dysfunction, isolated anorectal sensory dysfunction, severe combined anorectal motor and sensory dysfunction, or “pseudoincontinence” due to either pull-through dysfunction due to fecal impaction or loss of the rectal reservoir and high amplitude colonic contractions, which leave little time to evacuate stool in a commode. The later problem is usually an issue with younger children who are toilet training and usually improves with time as the frequency of high-amplitude peristaltic contractions (HAPCs) decreases with age and patients learn to sense the urge associated with filling of the pull-through segment. The direct consequence of resecting the rectosigmoid colon during a standard pull-through operation is a tendency to pass stool frequently throughout the day because the natural fecal reservoir (the rectum) has been removed. These patients generally have normal anorectal anatomy with adequate sphincter and sensory function and exhibit excellent potential for long-term bowel control. These children benefit from behavioral modification (timed sits on the commode), patience and reassurance, and loperamide when necessary to decrease the frequency and amplitude of HAPCs. Nevertheless, soiling can persist even when a technically adequate pull-through is performed and can also occur in patients who do not have factors known be associated with higher rates of fecal incontinence, such as Down's syndrome 15 and TCHD. 60 61 This illustrates our incomplete understanding of the pathogenesis, treatment, and management of HD despite operations that should seemingly overcome the problem of segmental aganglionosis. Abnormal sphincter function may occur as the result of sphincter injury during the pull-through or a previous myectomy or sphincterotomy. This kind of injury is suspected based on an examination under anesthesia and can be objectively assessed using either ARM 62 or anal sonography. 63 Abnormal sensation may take the form of either lack of sensation for a full rectum (identified using awake ARM) or injury to the transitional epithelium, which normally permits differentiation between gas, liquid, and solid stool. This injury may occur during a pull-through, especially if the anastomosis is performed too low. However, the majority children with incontinence after a pull-through have overflow of stool because of ongoing constipation. Once sphincter injury and a problem with sensation have been excluded, the child should be evaluated and treated for obstructive symptoms as described in the previous section, and the majority of these patients achieve good bowel control with an appropriate bowel regimen. 64
Fig. 4.
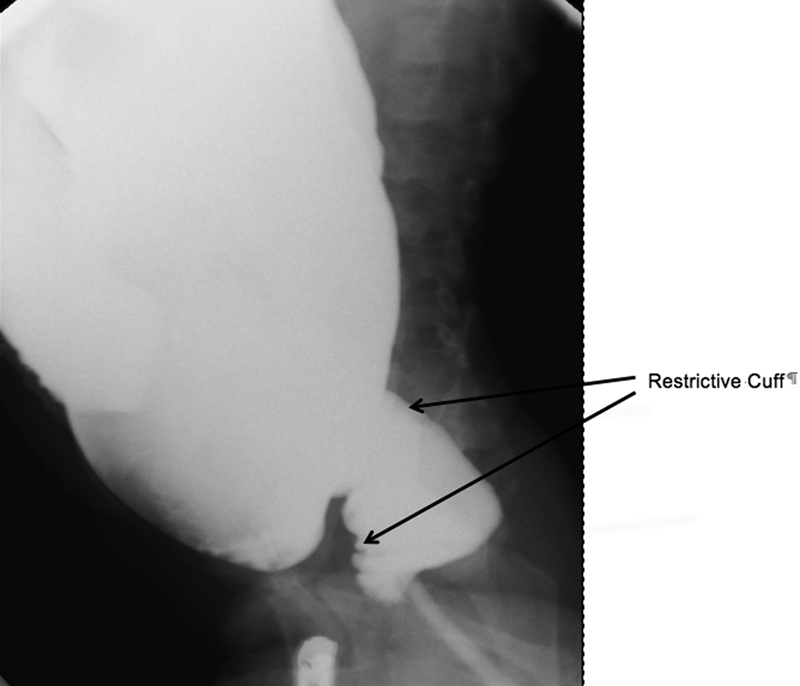
Long-standing outlet obstruction from a restrictive cuff after a Soave pull-through. Note the dilated proximal colon above the cuff.
Enterocolitis
Enterocolitis may be a presenting feature of HD or occur after surgical correction of the disease. Hirschsprung-associated enterocolitis (HAEC) is a condition with classic manifestations that include abdominal distention, fever, and foul-smelling stools. Although the clinical features of enterocolitis are generally agreed upon, a precise universal definition has not been developed accounting for the wide variation in the reported incidence of this problem postoperatively, with estimates ranging from 17 to 50%. 65 Reported rates are typically increased in patients with Down's syndrome or TCHD. 66 67 Scoring systems have emerged to quantify HAEC severity but are not widely implemented at the bedside. 68 The absence of enterocolitis preoperatively does not ensure that the child will not develop enterocolitis in the postoperative period. 69 The treatment of postoperative enterocolitis is largely symptomatic and involves nasogastric drainage, intravenous fluids, broad-spectrum antibiotics, and decompression of the rectum and colon by using rectal stimulation or irrigations. Investigation along the obstructive algorithm ( Fig. 1 ) should be considered for patients with recurrent HAEC. Decreasing the risk of HAEC in patients with recurrent disease can be accomplished by using preventive measures such as routine irrigations, chronic administration of metronidazole, particularly in those who are thought to be at higher risk for this complication based on clinical or histological grounds, or use of probiotics. 70 Intestinal diversion remains an option for patients with refractory and recurrent HAEC. Since HAEC is the most common cause of death in children with HD, it is extremely important that the surgeon educate the family about the risk of this complication and urge prompt medical attention should the child develop any concerning symptoms.
The Older Child or Adult with HD: “Late Diagnosis HD”
Although most patients in developed countries are diagnosed within the first year of life, patients occasionally escape diagnosis during infancy. The literature is riddled with case reports of “adult” HD commonly defined as age of diagnosis of greater than 10 years, 71 but an 11-year-old patient can hardly be considered an adult. Therefore, we prefer the term late diagnosis HD , as our general approach to these patients is similar regardless of age. These patients generally have long-standing constipation refractory to a bowel management regimen and most often do not experience HAEC. 72 Breast-fed infants with HD will often defecate normally during infancy, only to develop severe constipation around the time of weaning from breast milk. In other cases, children will present as toddlers or older children or adults with long-standing severe constipation that has never been investigated. As constipation is pervasive in childhood, it is unreasonable to expect a primary care physician to refer every patient with constipation to a surgeon for definitive evaluation for HD. In most cases, childhood constipation is successfully managed with diet, laxatives, and behavior modification. However, historical and examination features that should prompt suspicion for HD include failure to pass meconium in the first 48 hour of life, failure to thrive, gross abdominal distention, and dependence on enemas without significant encopresis. A child or adult with any of these features, or who does not respond adequately to intensive therapy, should be referred for rectal biopsy. A full-thickness biopsy under general anesthesia is required due to the fact that the suction rectal biopsy device cannot adequately sample the submucosa to render an adequate pathological assessment. Another option for older children is the use of ARM to evaluate for the absence of the RAIR in a patient with HD as discussed previously. However, suggestion of HD based on the absence of RAIR should be followed by a rectal biopsy, and we generally are prepared to perform the biopsy immediately after ARM under the same anesthetic.
Once the diagnosis of HD has been established in an older patient, it is prudent to consult with a pediatric surgeon who often has considerable expertise with the pull-through procedure. As stated previously, the type of procedure performed ultimately comes down to the preference of the operating surgeon, as the three main types of pull-through operations have been performed successfully in older patients. However, the difference between the infant and older patient is the often dilated and dysfunctional upstream bowel that may not function normally after a primary pull-through and pose a complicated coloanal anastomosis due to significant size discrepancy between the proximal anal incision and pull-through bowel. For that reason, we prefer a conservative approach to older patients by performing a laparoscopic-assisted leveling ostomy to determine the level of ganglionated colon and a concomitant resection of the dilated distal segment down to the peritoneal reflection. However, others have successfully performed one-stage procedures 73 or other delayed anastomotic approaches with reasonable outcomes. 74 We have had occasion to perform a one-stage procedure if the colon dilation is minimal and the patient can be adequately prepped. 75 If a one-stage operation is undertaken, we recommend an aggressive bowel preparation, tapering the pull-through segment, and heightened postoperative awareness for the possibility for anastomotic complications that should prompt immediate fecal diversion. In our practice, we generally wait 8 to 12 weeks after intestinal diversion and then return and perform another laparoscopic procedure to mobilize the rectum, take down the stoma, and then perform a Soave pull-through as is customary in our practice. Leaving the rectum at the principal operation allows for a Duhamel or Swenson reconstruction if desired by the operating surgeon. In the limited number of reports of late diagnosis HD, functional outcome is generally favorable, and complications are similar to those reported in patients diagnosed at an early age.
Conclusions
Operative correction for HD involves the general principals of removing or bypassing the aganglionic bowel, and establishing intestinal continuity by “pulling through” normally innervated bowel to the anus while preserving sphincter function. The vast majority of patients are diagnosed within the first year of life, yet long-term defection problems are common despite satisfactory operative interventions. The approach to the problem patient after a pull-through operation should be systematic and leave no stone unturned. Involving a pediatric surgeon in this process is highly recommended regardless of patient age, as some patients may require a redo operation and the pediatric surgeon often has considerable experience with many of the potential surgical options. No one surgical reconstruction has been shown to be definitively better than another. Prompt identification and aggressive treatment of HAEC is an important aspect of caring for a patient with HD even after a seemingly successful pull-through operation. Although late diagnosis HD is uncommon, a heightened awareness of this potential diagnosis should be entertained in the patient with long-standing and refractory constipation. Consultation with a pediatric surgeon familiar with the operative strategies for reconstruction is likely to be of help in formulating an appropriate treatment strategy.
References
- 1.Sergi C. Hirschsprung's disease: historical notes and pathological diagnosis on the occasion of the 100(th) anniversary of Dr. Harald Hirschsprung's death. World J Clin Pediatr. 2015;4(04):120–125. doi: 10.5409/wjcp.v4.i4.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Swenson O, Rheinlander H F, Diamond I. Hirschsprung's disease; a new concept of the etiology; operative results in 34 patients. N Engl J Med. 1949;241(15):551–556. doi: 10.1056/NEJM194910132411501. [DOI] [PubMed] [Google Scholar]
- 3.De La Torre L, Langer J C. Transanal endorectal pull-through for Hirschsprung disease: technique, controversies, pearls, pitfalls, and an organized approach to the management of postoperative obstructive symptoms. Semin Pediatr Surg. 2010;19(02):96–106. doi: 10.1053/j.sempedsurg.2009.11.016. [DOI] [PubMed] [Google Scholar]
- 4.Levitt M A, Dickie B, Peña A. The Hirschsprungs patient who is soiling after what was considered a “successful” pull-through. Semin Pediatr Surg. 2012;21(04):344–353. doi: 10.1053/j.sempedsurg.2012.07.009. [DOI] [PubMed] [Google Scholar]
- 5.Granström A L, Husberg B, Nordenskjöld A, Svensson P J, Wester T. Laparoscopic-assisted pull-through for Hirschsprung's disease, a prospective repeated evaluation of functional outcome. J Pediatr Surg. 2013;48(12):2536–2539. doi: 10.1016/j.jpedsurg.2013.07.017. [DOI] [PubMed] [Google Scholar]
- 6.Jarvi K, Laitakari E M, Koivusalo A, Rintala R J, Pakarinen M P. Bowel function and gastrointestinal quality of life among adults operated for Hirschsprung disease during childhood: a population-based study. Ann Surg. 2010;252(06):977–981. doi: 10.1097/SLA.0b013e3182018542. [DOI] [PubMed] [Google Scholar]
- 7.Heikkinen M, Rintala R, Luukkonen P. Long-term anal sphincter performance after surgery for Hirschsprung's disease. J Pediatr Surg. 1997;32(10):1443–1446. doi: 10.1016/s0022-3468(97)90557-1. [DOI] [PubMed] [Google Scholar]
- 8.Diseth T H, Bjørnland K, Nøvik T S, Emblem R. Bowel function, mental health, and psychosocial function in adolescents with Hirschsprung's disease. Arch Dis Child. 1997;76(02):100–106. doi: 10.1136/adc.76.2.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baillie C T, Kenny S E, Rintala R J, Booth J M, Lloyd D A. Long-term outcome and colonic motility after the Duhamel procedure for Hirschsprung's disease. J Pediatr Surg. 1999;34(02):325–329. doi: 10.1016/s0022-3468(99)90201-4. [DOI] [PubMed] [Google Scholar]
- 10.Rintala R J, Pakarinen M P. Long-term outcomes of Hirschsprung's disease. Semin Pediatr Surg. 2012;21(04):336–343. doi: 10.1053/j.sempedsurg.2012.07.008. [DOI] [PubMed] [Google Scholar]
- 11.Aworanti O M, McDowell D T, Martin I M, Quinn F. Does functional outcome improve with time postsurgery for Hirschsprung disease? Eur J Pediatr Surg. 2016;26(02):192–199. doi: 10.1055/s-0034-1544053. [DOI] [PubMed] [Google Scholar]
- 12.Reding R, de Ville de Goyet J, Gosseye S et al. Hirschsprung's disease: a 20-year experience. J Pediatr Surg. 1997;32(08):1221–1225. doi: 10.1016/s0022-3468(97)90686-2. [DOI] [PubMed] [Google Scholar]
- 13.Bai Y, Chen H, Hao J, Huang Y, Wang W. Long-term outcome and quality of life after the Swenson procedure for Hirschsprung's disease. J Pediatr Surg. 2002;37(04):639–642. doi: 10.1053/jpsu.2002.31625. [DOI] [PubMed] [Google Scholar]
- 14.Yanchar N L, Soucy P. Long-term outcome after Hirschsprung's disease: patients' perspectives. J Pediatr Surg. 1999;34(07):1152–1160. doi: 10.1016/s0022-3468(99)90588-2. [DOI] [PubMed] [Google Scholar]
- 15.Catto-Smith A G, Trajanovska M, Taylor R G. Long-term continence in patients with Hirschsprung's disease and Down syndrome. J Gastroenterol Hepatol. 2006;21(04):748–753. doi: 10.1111/j.1440-1746.2005.03996.x. [DOI] [PubMed] [Google Scholar]
- 16.Keckler S J, Yang J C, Fraser J Det al. Contemporary practice patterns in the surgical management of Hirschsprung's disease J Pediatr Surg 200944061257–1260., discussion 1260 [DOI] [PubMed] [Google Scholar]
- 17.Soave F. Hirschsprung's disease: a new surgical technique. Arch Dis Child. 1964;39:116–124. doi: 10.1136/adc.39.204.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Boley S J. New modification of the surgical treatment of Hirschsprung's disease. Surgery. 1964;56:1015–1017. [PubMed] [Google Scholar]
- 19.Yokoi A, Satoh S, Takamizawa S, Muraji T, Tsugawa C, Nishijima E. The preliminary study of modified Swenson procedure in Hirschsprung disease. J Pediatr Surg. 2009;44(08):1560–1563. doi: 10.1016/j.jpedsurg.2008.11.056. [DOI] [PubMed] [Google Scholar]
- 20.Duhamel B. A new operation for the treatment of Hirschsprung's disease. Arch Dis Child. 1960;35:38–39. doi: 10.1136/adc.35.179.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Canty T G. Modified Duhamel procedure for treatment of Hirschsprung's disease in infancy and childhood: review of 41 consecutive cases. J Pediatr Surg. 1982;17(06):773–778. doi: 10.1016/s0022-3468(82)80445-4. [DOI] [PubMed] [Google Scholar]
- 22.Suita S, Taguchi T, Yanai K, Kamimura T, Nakao M, Ikeda K. Longterm outcomes and quality of life after Z-shaped anastomosis for Hirschsprung's disease. J Am Coll Surg. 1998;187(06):577–583. doi: 10.1016/s1072-7515(98)00254-3. [DOI] [PubMed] [Google Scholar]
- 23.Martin L W. Total colonic aganglionosis preservation and utilization of entire colon. J Pediatr Surg. 1982;17(05):635–637. doi: 10.1016/s0022-3468(82)80125-5. [DOI] [PubMed] [Google Scholar]
- 24.Steichen F M, Talbert J L, Ravitch M M. Primary side-to-side colorectal anastomosis in the Duhamel operation for Hirschsprung's disease. Surgery. 1968;64(02):475–483. [PubMed] [Google Scholar]
- 25.Georgeson K E, Robertson D J. Laparoscopic-assisted approaches for the definitive surgery for Hirschsprung's disease. Semin Pediatr Surg. 2004;13(04):256–262. doi: 10.1053/j.sempedsurg.2004.10.013. [DOI] [PubMed] [Google Scholar]
- 26.de Lagausie P, Bruneau B, Besnard M, Jaby O, Aigrain Y. Definitive treatment of Hirschsprung's disease with a laparoscopic Duhamel pull-through procedure in childhood. Surg Laparosc Endosc. 1998;8(01):55–57. [PubMed] [Google Scholar]
- 27.Curran T J, Raffensperger J G. The feasibility of laparoscopic swenson pull-through. J Pediatr Surg. 1994;29(09):1273–1275. doi: 10.1016/0022-3468(94)90823-0. [DOI] [PubMed] [Google Scholar]
- 28.Langer J C, Minkes R K, Mazziotti M V, Skinner M A, Winthrop A L.Transanal one-stage Soave procedure for infants with Hirschsprung's disease J Pediatr Surg 19993401148–151., discussion 152 [DOI] [PubMed] [Google Scholar]
- 29.Rassouli R, Holschneider A M, Bolkenius M et al. Long-term results of Rehbein's procedure: a retrospective study in German-speaking countries. Eur J Pediatr Surg. 2003;13(03):187–194. doi: 10.1055/s-2003-41258. [DOI] [PubMed] [Google Scholar]
- 30.State D. Physiological operation for idiopathic congenital megacolon (Hirschsprung's disease) J Am Med Assoc. 1952;149(04):350–355. doi: 10.1001/jama.1952.02930210034010. [DOI] [PubMed] [Google Scholar]
- 31.Aworanti O M, Mcdowell D T, Martin I M, Hung J, Quinn F. Comparative review of functional outcomes post surgery for Hirschsprung's disease utilizing the paediatric incontinence and constipation scoring system. Pediatr Surg Int. 2012;28(11):1071–1078. doi: 10.1007/s00383-012-3170-y. [DOI] [PubMed] [Google Scholar]
- 32.Bradnock T J, Walker G M. Evolution in the management of Hirschsprung's disease in the UK and Ireland: a national survey of practice revisited. Ann R Coll Surg Engl. 2011;93(01):34–38. doi: 10.1308/003588410X12771863936846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dodero P, Magillo P, Scarsi P L. Total colectomy and straight ileo-anal soave endorectal pull-through: personal experience with 42 cases. Eur J Pediatr Surg. 2001;11(05):319–323. doi: 10.1055/s-2001-18549. [DOI] [PubMed] [Google Scholar]
- 34.Cheung S T, Tam Y H, Chong H M et al. An 18-year experience in total colonic aganglionosis: from staged operations to primary laparoscopic endorectal pull-through. J Pediatr Surg. 2009;44(12):2352–2354. doi: 10.1016/j.jpedsurg.2009.07.057. [DOI] [PubMed] [Google Scholar]
- 35.Mir E, Karaca I, Günsar C, Sencan A, Fesçekoglu O. Primary Duhamel-Martin operations in neonates and infants. Pediatr Int. 2001;43(04):405–408. doi: 10.1046/j.1442-200x.2001.01409.x. [DOI] [PubMed] [Google Scholar]
- 36.van der Zee D C, Bax N M. Duhamel-Martin procedure for Hirschsprung's disease in neonates and infants: one-stage operation. J Pediatr Surg. 1996;31(07):901–902. doi: 10.1016/s0022-3468(96)90406-6. [DOI] [PubMed] [Google Scholar]
- 37.Bischoff A, Levitt M A, Peña A. Total colonic aganglionosis: a surgical challenge. How to avoid complications? Pediatr Surg Int. 2011;27(10):1047–1052. doi: 10.1007/s00383-011-2960-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Heath A L, Spitz L, Milla P J. The absorptive function of colonic aganglionic intestine: are the Duhamel and Martin procedures rational? J Pediatr Surg. 1985;20(01):34–36. doi: 10.1016/s0022-3468(85)80388-2. [DOI] [PubMed] [Google Scholar]
- 39.Nishijima E, Kimura K, Tsugawa C, Muraji T. The colon patch graft procedure for extensive aganglionosis: long-term follow-up. J Pediatr Surg. 1998;33(02):215–219. doi: 10.1016/s0022-3468(98)90434-1. [DOI] [PubMed] [Google Scholar]
- 40.Sauer H, Fasching G. Preservation of the ileocecal valve and right colon in total colonic aganglionosis. J Pediatr Surg. 1993;28(12):1640–1643. doi: 10.1016/0022-3468(93)90128-8. [DOI] [PubMed] [Google Scholar]
- 41.Shandling B. Total colon aganglionosis--a new operation. J Pediatr Surg. 1984;19(05):503–505. doi: 10.1016/s0022-3468(84)80090-1. [DOI] [PubMed] [Google Scholar]
- 42.Hukkinen M, Koivusalo A, Rintala R J, Pakarinen M P. Restorative proctocolectomy with J-pouch ileoanal anastomosis for total colonic aganglionosis among neonates and infants. J Pediatr Surg. 2014;49(04):570–574. doi: 10.1016/j.jpedsurg.2013.07.021. [DOI] [PubMed] [Google Scholar]
- 43.Ein S H, Shandling B, So H. A new look at an old operation for aganglionosis. J Pediatr Surg. 1994;29(09):1228–1230. doi: 10.1016/0022-3468(94)90808-7. [DOI] [PubMed] [Google Scholar]
- 44.Escobar M A, Grosfeld J L, West K W et al. Long-term outcomes in total colonic aganglionosis: a 32-year experience. J Pediatr Surg. 2005;40(06):955–961. doi: 10.1016/j.jpedsurg.2005.03.043. [DOI] [PubMed] [Google Scholar]
- 45.Wildhaber B E, Teitelbaum D H, Coran A G.Total colonic Hirschsprung's disease: a 28-year experience J Pediatr Surg 20054001203–206., discussion 206–207 [DOI] [PubMed] [Google Scholar]
- 46.Barrena S, Andres A M, Burgos L et al. Long-term results of the treatment of total colonic aganglionosis with two different techniques. Eur J Pediatr Surg. 2008;18(06):375–379. doi: 10.1055/s-2008-1038895. [DOI] [PubMed] [Google Scholar]
- 47.Blackburn S, Corbett P, Griffiths D M, Burge D, Beattie R M, Stanton M. Total colonic aganglionosis: a 15-year single center experience. Eur J Pediatr Surg. 2014;24(06):488–491. doi: 10.1055/s-0033-1363159. [DOI] [PubMed] [Google Scholar]
- 48.Shen C, Song Z, Zheng S, Xiao X. A comparison of the effectiveness of the Soave and Martin procedures for the treatment of total colonic aganglionosis. J Pediatr Surg. 2009;44(12):2355–2358. doi: 10.1016/j.jpedsurg.2009.07.056. [DOI] [PubMed] [Google Scholar]
- 49.Marquez T T, Acton R D, Hess D J, Duval S, Saltzman D A.Comprehensive review of procedures for total colonic aganglionosis J Pediatr Surg 20094401257–265., discussion 265 [DOI] [PubMed] [Google Scholar]
- 50.Neilson I R, Yazbeck S. Ultrashort Hirschsprung's disease: myth or reality. J Pediatr Surg. 1990;25(11):1135–1138. doi: 10.1016/0022-3468(90)90748-x. [DOI] [PubMed] [Google Scholar]
- 51.Foroutan H R, Hosseini S M, Banani S A et al. Comparison of botulinium toxin injection and posterior anorectal myectomy in treatment of internal anal sphincter achalasia. Indian J Gastroenterol. 2008;27(02):62–65. [PubMed] [Google Scholar]
- 52.Friedmacher F, Puri P. Comparison of posterior internal anal sphincter myectomy and intrasphincteric botulinum toxin injection for treatment of internal anal sphincter achalasia: a meta-analysis. Pediatr Surg Int. 2012;28(08):765–771. doi: 10.1007/s00383-012-3123-5. [DOI] [PubMed] [Google Scholar]
- 53.Peña A, Elicevik M, Levitt M A.Reoperations in Hirschsprung disease J Pediatr Surg 200742061008–1013., discussion 1013–1014 [DOI] [PubMed] [Google Scholar]
- 54.Harrison M W, Deitz D M, Campbell J R, Campbell T J. Diagnosis and management of Hirschsprung's disease. A 25. year perspective. Am J Surg. 1986;152(01):49–56. doi: 10.1016/0002-9610(86)90138-8. [DOI] [PubMed] [Google Scholar]
- 55.Langer J C, Birnbaum E.Preliminary experience with intrasphincteric botulinum toxin for persistent constipation after pull-through for Hirschsprung's disease J Pediatr Surg 199732071059–1061., discussion 1061–1062 [DOI] [PubMed] [Google Scholar]
- 56.Han-Geurts I J, Hendrix V C, de Blaauw I, Wijnen M H, van Heurn E L. Outcome after anal intrasphincteric Botox injection in children with surgically treated Hirschsprung disease. J Pediatr Gastroenterol Nutr. 2014;59(05):604–607. doi: 10.1097/MPG.0000000000000483. [DOI] [PubMed] [Google Scholar]
- 57.Wester T, Granström A L. Botulinum toxin is efficient to treat obstructive symptoms in children with Hirschsprung disease. Pediatr Surg Int. 2015;31(03):255–259. doi: 10.1007/s00383-015-3665-4. [DOI] [PubMed] [Google Scholar]
- 58.Peeraully M R, Lopes J, Wright A et al. Experience of the MACE procedure at a regional pediatric surgical unit: a 15-year retrospective review. Eur J Pediatr Surg. 2014;24(01):113–116. doi: 10.1055/s-0033-1357502. [DOI] [PubMed] [Google Scholar]
- 59.DeFreest L, Smith J, Whyte C. Laparoscopic-assisted percutaneous cecostomy for antegrade continence enema. J Laparoendosc Adv Surg Tech A. 2014;24(04):261–264. doi: 10.1089/lap.2013.0292. [DOI] [PubMed] [Google Scholar]
- 60.Tsuji H, Spitz L, Kiely E M, Drake D P, Pierro A.Management and long-term follow-up of infants with total colonic aganglionosis J Pediatr Surg 19993401158–161., discussion 162 [DOI] [PubMed] [Google Scholar]
- 61.Anupama B, Zheng S, Xiao X. Ten-year experience in the management of total colonic aganglionosis. J Pediatr Surg. 2007;42(10):1671–1676. doi: 10.1016/j.jpedsurg.2007.05.021. [DOI] [PubMed] [Google Scholar]
- 62.Zaslavsky C, Loening-Baucke V. Anorectal manometric evaluation of children and adolescents postsurgery for Hirschsprung's disease. J Pediatr Surg. 2003;38(02):191–195. doi: 10.1053/jpsu.2003.50041. [DOI] [PubMed] [Google Scholar]
- 63.Kuwahara M, Iwai N, Yanagihara J, Tokiwa K, Fukata R. Endosonographic study of anal sphincters in patients after surgery for Hirschsprung's disease. J Pediatr Surg. 1999;34(03):450–453. doi: 10.1016/s0022-3468(99)90497-9. [DOI] [PubMed] [Google Scholar]
- 64.Levitt M A, Martin C A, Olesevich M, Bauer C L, Jackson L E, Peña A.Hirschsprung disease and fecal incontinence: diagnostic and management strategies J Pediatr Surg 20094401271–277., discussion 277 [DOI] [PubMed] [Google Scholar]
- 65.Teitelbaum D H, Coran A G. Enterocolitis. Semin Pediatr Surg. 1998;7(03):162–169. doi: 10.1016/s1055-8586(98)70012-5. [DOI] [PubMed] [Google Scholar]
- 66.Menezes M, Puri P. Long-term outcome of patients with enterocolitis complicating Hirschsprung's disease. Pediatr Surg Int. 2006;22(04):316–318. doi: 10.1007/s00383-006-1639-2. [DOI] [PubMed] [Google Scholar]
- 67.Murphy F, Puri P. New insights into the pathogenesis of Hirschsprung's associated enterocolitis. Pediatr Surg Int. 2005;21(10):773–779. doi: 10.1007/s00383-005-1551-1. [DOI] [PubMed] [Google Scholar]
- 68.Pontarelli E M, Ford H R, Gayer C P. Recent developments in Hirschsprung's-associated enterocolitis. Curr Gastroenterol Rep. 2013;15(08):340. doi: 10.1007/s11894-013-0340-6. [DOI] [PubMed] [Google Scholar]
- 69.Hackam D J, Filler R M, Pearl R H. Enterocolitis after the surgical treatment of Hirschsprung's disease: risk factors and financial impact. J Pediatr Surg. 1998;33(06):830–833. doi: 10.1016/s0022-3468(98)90652-2. [DOI] [PubMed] [Google Scholar]
- 70.Wang X, Li Z, Xu Z, Wang Z, Feng J. Probiotics prevent Hirschsprung's disease-associated enterocolitis: a prospective multicenter randomized controlled trial. Int J Colorectal Dis. 2015;30(01):105–110. doi: 10.1007/s00384-014-2054-0. [DOI] [PubMed] [Google Scholar]
- 71.Starling J R, Croom R D, III, Thomas C G., Jr Hirschsprung's disease in young adults. Am J Surg. 1986;151(01):104–109. doi: 10.1016/0002-9610(86)90019-x. [DOI] [PubMed] [Google Scholar]
- 72.Haricharan R N, Seo J M, Kelly D R et al. Older age at diagnosis of Hirschsprung disease decreases risk of postoperative enterocolitis, but resection of additional ganglionated bowel does not. J Pediatr Surg. 2008;43(06):1115–1123. doi: 10.1016/j.jpedsurg.2008.02.039. [DOI] [PubMed] [Google Scholar]
- 73.Ammar S A, Ibrahim I A. One-stage transanal endorectal pull-through for treatment of Hirschsprung's disease in adolescents and adults. J Gastrointest Surg. 2011;15(12):2246–2250. doi: 10.1007/s11605-011-1662-z. [DOI] [PubMed] [Google Scholar]
- 74.Jarry J, Faucheron J L. Laparoscopic rectosigmoid resection with transanal colonic pull-through and delayed coloanal anastomosis: a new approach to adult Hirschsprung disease. Dis Colon Rectum. 2011;54(10):1313–1319. doi: 10.1097/DCR.0b013e3182270c41. [DOI] [PubMed] [Google Scholar]
- 75.Vorobyov G I, Achkasov S I, Biryukov O M. Clinical features' diagnostics and treatment of Hirschsprung's disease in adults. Colorectal Dis. 2010;12(12):1242–1248. doi: 10.1111/j.1463-1318.2009.02031.x. [DOI] [PubMed] [Google Scholar]


