Abstract
Neuronal remodeling is crucial for formation of the mature nervous system and disruption of this process can lead to neuropsychiatric diseases. Global gene expression changes in neurons during remodeling as well as the factors that regulate these changes remain poorly defined. To elucidate this process, we performed RNA-seq on isolated Drosophila larval and pupal neurons and found upregulated synaptic signaling and downregulated gene expression regulators as a result of normal neuronal metamorphosis. We further tested the role of alan shepard (shep), which encodes an evolutionarily conserved RNA-binding protein required for proper neuronal remodeling. Depletion of shep in neurons prevents the execution of metamorphic gene expression patterns, and shep-regulated genes correspond to Shep chromatin and/or RNA-binding targets. Reduced expression of a Shep-inhibited target gene that we identified, brat, is sufficient to rescue neuronal remodeling defects of shep knockdown flies. Our results reveal direct regulation of transcriptional programs by Shep to regulate neuronal remodeling during metamorphosis.
KEY WORDS: shep, Neuronal transcriptome, Metamorphosis, Neuronal remodeling, BMP signaling, Chromatin insulator
Summary: We provide the first report of the transition of the neuronal transcriptome during metamorphic remodeling and further elucidate how Shep regulates transcription of its chromatin targets to regulate neuronal remodeling.
INTRODUCTION
The nervous system is highly dynamic during development and undergoes dramatic remodeling to achieve mature innervation and function. Neuronal remodeling often involves pruning of existing neurites and subsequent outgrowth for proper function and behavior at the adult stage. This conserved process has been characterized and studied in a wide range of organisms including worms, flies, mammals and humans (Gao et al., 1999; Bagri et al., 2003; Zhao et al., 2008; Garin-Aguilar et al., 2012; Yaniv and Schuldiner, 2016). Dysregulated neuronal remodeling leads to abnormal neuronal connections and can result in neuropsychiatric diseases such as schizophrenia and autism (Cocchi et al., 2016; Sekar et al., 2016; Thomas et al., 2016).
Drosophila melanogaster undergoes dramatic reorganization of the nervous system during metamorphosis and therefore offers an excellent opportunity to study neuronal remodeling. Multiple populations of Drosophila neurons have been well-characterized with respect to their remodeling during metamorphosis (Schubiger et al., 1998; Zheng et al., 2003; Boulanger et al., 2012; Yaniv et al., 2012; Yaniv and Schuldiner, 2016). For instance, all bursicon neurons undergo extensive changes including enlargement of cell bodies, pruning of linear larval projections and regrowth of highly branched adult projections as a result of neuronal remodeling (Peabody et al., 2008; Zhao et al., 2008). After eclosion, bursicon neurons in the abdominal ganglion (BAG cells) are activated by commander bursicon neurons in the subesophageal ganglion (BSEG cells) to secrete peptidergic hormones that promote wing expansion behaviors (Luan et al., 2006). Disrupted remodeling of bursicon neurons leads to lethality and defects in adult wing expansion (Luan et al., 2006; Zhao et al., 2008). These readily scored phenotypes, together with easily detectable dispersed somata and projections, make bursicon neurons an ideal model to investigate neuronal remodeling and neuropeptidergic signaling during development. Previous studies have identified a variety of pathways that act as key regulators of neuronal remodeling including, but not limited to, TGFβ signaling, ecdysone signaling, TOR/insulin signaling and the ubiquitin-proteasome system (Schubiger et al., 1998; Zheng et al., 2003; Gu et al., 2014). To understand the global transcriptional programs during normal neuronal remodeling, a study of brain transcriptome changes was performed 18 h after the onset of metamorphosis (Li and White, 2003). However, how gene expression is shaped specifically in neurons during late metamorphosis and how key regulators control gene expression during neuronal remodeling remains unknown.
The shep gene encodes an evolutionarily conserved RNA-binding protein that regulates neuronal remodeling during Drosophila metamorphosis. Although neurite organization defects are observed during the embryonic stage at low penetrance and in the larval peripheral nervous system (Schachtner et al., 2015), shep-depleted larvae display largely normal behaviors and development (Chen et al., 2014; Schachtner et al., 2015). In contrast, loss of shep at late pupal and adult stages leads to lethality, reduced neuropil sizes and severe behavioral defects, suggesting an essential function of shep during metamorphic development (Chen et al., 2014). Specifically, shep mutants display wing expansion defects as well as smaller soma and reduced axonal projections of both BAG and BSEG neurons. Genetic evidence indicates that shep promotes neuronal remodeling of bursicon neurons by antagonizing Myc and Dad, which encodes a Smad protein that inhibits bone morphogenetic protein (BMP) signaling (Chen et al., 2017). Loss of Myc rescues the wing expansion defects and neurite morphology of the BSEG neurons in shep-depleted animals, and either loss of Dad or activation of BMP signaling is sufficient to rescue the wing expansion defects and remodeling defects of both BAG and BSEG neurons.
Although the molecular mechanisms by which shep functions are not yet understood, the ability of Shep to associate with RNA and chromatin may provide clues to its activity. Shep binds to RNA both in vivo and in vitro (Matzat et al., 2012; Ray et al., 2013; Dale et al., 2014), and Shep interacts directly with the chromatin insulator proteins Su(Hw) and Mod(mdg4)67.2 (Matzat et al., 2012). These two core gypsy insulator proteins together associate with DNA to regulate chromatin conformation and control gene expression (Ghosh et al., 2001). Shep colocalizes with either Su(Hw) or Mod(mdg4)67.2 throughout the genome and negatively regulates gypsy insulator activities specifically in the central nervous system (CNS) (Matzat et al., 2012). However, how shep regulates gene expression during neuronal remodeling and whether its ability to antagonize chromatin insulators is involved in this process remain unknown.
In order to identify mechanisms of neuronal remodeling, we performed RNA-seq on neurons isolated before metamorphosis and 82 h after its onset, at a late pupal stage. Analyses of shep-depleted neurons detected strong effects on gene expression specifically at the pupal stage, and we found that the vast majority of shep-regulated genes contribute to the normal transition of the neuronal transcriptome into late metamorphosis. Consistent with direct shep regulation, many of these genes are bound by Shep and are controlled at the level of transcription. We further demonstrated that reduced expression of one of its targets, brat, is sufficient to rescue loss-of-shep phenotypes in certain neurons. Taken together, our findings elucidate the transition of the neuronal transcriptome through normal metamorphosis and further define how Shep regulates transcriptional programs during this process to promote neuronal remodeling.
RESULTS
Loss of shep strongly affects pupal but not larval neuronal transcriptome
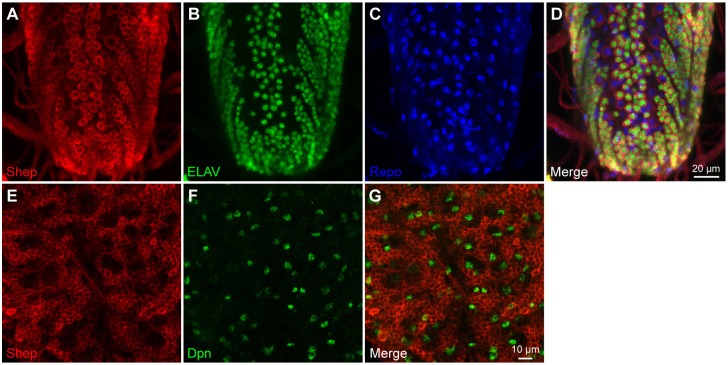
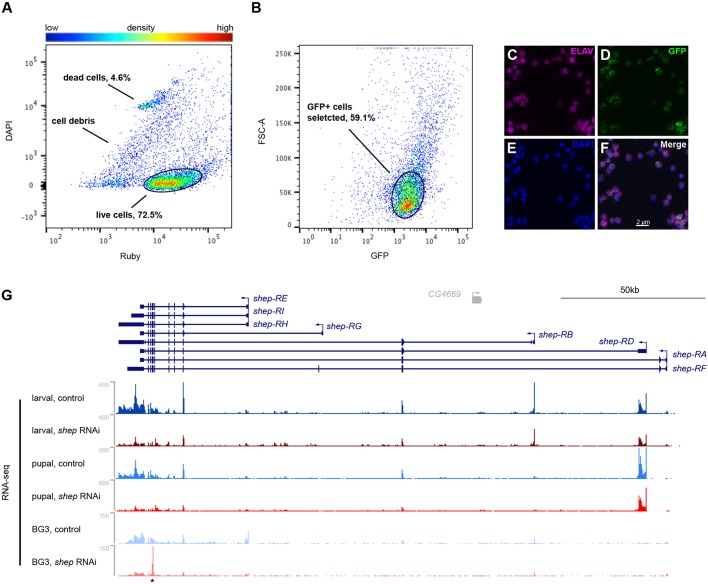
Shep is required for normal neuronal remodeling; however, its cell type expression pattern in the CNS has not been determined. Immunofluorescence detected high Shep levels in ELAV-positive neurons, low levels in Repo-positive glial cells, and no expression in Dpn-positive neuroblasts of third instar larval CNS (Fig. 1), suggesting that Shep functions in a wide range of differentiated neurons and glia. Higher magnification detected Shep in the cytoplasm but also overlapping the nuclear lamina (Fig. S1). To determine the specific role of shep in controlling neuronal gene expression, we used fluorescence-activated cell sorting (FACS) to isolate shep-depleted neurons from dissected third instar larval CNS. We employed the pan-neuronal driver elav-Gal4 (Fig. S2A-E) to drive expression of transgenes UAS-mCD8::GFP, UAS-shep-RNAi and UAS-Dcr-2 (elav>shep-RNAi, Dcr-2, mCD8::GFP) in order to achieve stronger shep depletion than that in the available shep allele mutants (Chen et al., 2014). After dissociation, signal from the dead cell stain DAPI and the non-selective cell stain Ruby was used to select live cells from dead cells and debris (Fig. 2A, Fig. S3), and live cells were further selected for GFP expression (Fig. 2B). These isolated GFP-positive cells were positive for ELAV immunostaining (Fig. 2C-F), confirming that they are differentiated neurons. We also applied a similar FACS strategy to isolate neurons from shep-depleted brains at pupal stage 14 (P14) (Fig. S2), a late stage of metamorphosis at which shep-dependent cellular defects are detected (Chen et al., 2014). Neurons from control elav>Dcr-2, mCD8::GFP larvae and pupae were also collected, and we constructed stranded total RNA-seq libraries. Downregulation of shep was confirmed at both stages (Fig. 2G).
Fig. 1.
Shep is expressed in neurons and glial cells. (A-D) Immunostaining detects high levels of Shep (A, red) in neurons marked by ELAV (B, green) and low levels in glial cells marked by Repo (C, blue) in third instar larva. Merge is shown in D. (E-G) Shep (E, red) is absent in neuroblasts marked by Dpn (F, green). Merge is shown in G.
Fig. 2.
FACS isolation of live shep-depleted neurons. (A) Based on DAPI and Ruby signals, FACS sorts dissociated cells into dead cells, cell debris and live cells, which account for 72.5% of total sorting counts and are selected for next gating. (B) GFP-positive cells selected for downstream analysis account for 59.1% of total sorting counts. (C-F) FACS-isolated cells are positive for both GFP (D) and ELAV (C) by immunostaining. DAPI staining (E) and merge (F) are also shown. (G) RNA-seq data at the shep locus. These data include RNA-seq of isolated neurons from third instar larvae and P14 pupae and RNA-seq of control and loss-of-shep BG3 cells. Asterisk indicates target site of shep dsRNA, which persists after transfection.
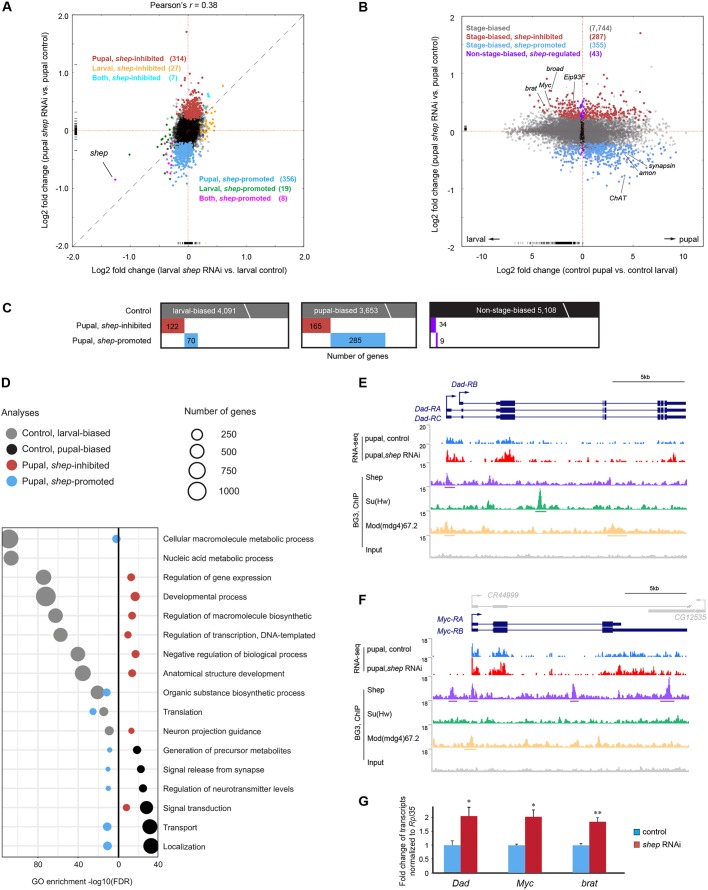
Consistent with Shep functioning primarily during metamorphosis, Shep depletion had a substantially stronger effect on gene expression in pupae than in larvae. In larval neurons, we detected only 34 significantly upregulated and 27 downregulated genes in shep-depleted neurons, indicating a modest effect at this stage of development (Fig. 3A, Table S1). Despite equal efficiency of knockdown at both stages, we found 321 upregulated (hereafter termed shep-inhibited) and 364 downregulated (shep-promoted) genes in pupal neurons depleted of shep (Fig. 3A, Table S1). Strong pupal-biased effects on gene expression are consistent with previous findings that loss of shep results in behavioral and cellular phenotypes specifically in pupal and later stages (Chen et al., 2014).
Fig. 3.
Loss of shep strongly affects transcriptome of P14 pupal neurons. (A) Genome-wide fold changes of gene expression in response to loss of shep at third instar larval and P14 pupal stages. Dashed line indicates where gene expression is equally affected between the two stages. Note that the expression of dawdle is upregulated to more than 10-fold at both stages, which is out of plot range in both A and B. Genes inhibited by shep in pupae (red), larvae (yellow) and both (aqua) or promoted by shep in pupae (sky blue), larvae (green) or both (pink) are indicated. (B) The shep gene specifically regulates stage-biased genes during metamorphosis. Genes with (gray) or without (black) stage-biased expression during normal metamorphosis are indicated. Stage-biased genes that are shep-inhibited (red) or shep-promoted (blue) in pupae are indicated. Non-stage biased shep-regulated genes are indicated in purple. (C) The binary heat maps apply the same color scheme and summarize overlap between shep-regulated genes and genes with expression changes during normal metamorphosis. (D) Top GO terms of gene expression changes as a result of normal metamorphosis that are either larval-biased (gray) or pupal-biased (black) overlaid with changes due to loss of shep in pupal neurons that are shep-inhibited (red) or shep-promoted (blue). (E,F) Screenshots of RNA-seq signals in loss-of-shep P14 pupal neurons, and Shep, Su(Hw) and Mod(mdg4)67.2 ChIP-seq signals in BG3 cells at the Dad (E) and Myc (F) loci. (G) Validation of RNA-seq results by RT-qPCR in sorted pupal neurons. Three biological replicates for each genotype were analyzed with Student's t-test (*P<0.05, **P<0.01).
Characterization of gene expression changes in neurons as a result of normal metamorphosis
We first compared the transcriptomes of larval versus pupal neurons in control animals and detected transcripts of 12,936 genes expressed at either stage. Among these genes, 4091 are larval-biased and 3653 are pupal-biased by the P14 stage (Fig. 3B,C, Table S1). Larval-biased genes were most enriched for gene expression regulators such as transcription factors and RNA-binding proteins (Fig. 3D, ‘Regulation of gene expression’, ‘Nucleic acid metabolic process’). Among these larval-biased genes, 35 components involved in Notch-mediated neuronal differentiation and ten factors involved in ecdysone-mediated cell death (Fig. 3D, ‘Developmental process’) were downregulated in pupal neurons, consistent with completion of these processes by the end of metamorphosis. In contrast, pupal-biased neuronal genes include membrane transporters and factors involved in synaptic signaling as well as factors that control the generation of metabolites (Fig. 3D, ‘Transport’, ‘Signal release from synapse’, ‘Generation of precursor metabolites’). The increased expression of these effector proteins likely reflects preparation for more complex behaviors and functions of the adult nervous system.
Shep specifically regulates the expression of metamorphic genes
Next, we found that shep was required to achieve accurate transition of the neuronal transcriptome during metamorphosis. In shep-depleted pupal neurons, 94% (642/685) of dysregulated genes are stage-biased genes, suggesting that shep specifically regulates the transition of the transcriptional program during neuronal metamorphosis (Fig. 3B,C). Loss of shep leads to decreased expression of presynaptic signaling factors involved in biosynthesis, transport and release of transmitters that would otherwise be upregulated as a result of normal metamorphosis (Fig. 3D). For example, ChAT and Synapsin, which encode presynaptic choline acetyltransferase and vesicle-associated proteins, respectively, are both pupal-biased in expression by approximately 20-fold in control neurons (Table S1). However, depletion of Shep reduces their pupal expression by 1.8-fold and 1.3-fold, respectively (Fig. 3B, Table S1). Consistent with these results, both ChAT and Synapsin are known to be downregulated at the protein level in neurons of shep knockdown pupae (Chen et al., 2014). In spite of overall mild gene expression changes that probably result from incomplete shep depletion (Fig. 3A), detection of these known shep-regulated genes validates the sensitivity and design of our approach. Also downregulated by loss of shep are 11 peptidergic factors, including amon, which encodes a key biosynthetic enzyme that cleaves and activates a wide range of neuropeptides (Wegener et al., 2011). The normal expression of amon is 18-fold pupal-biased, but loss of shep reduces amon expression by 1.3-fold in pupal neurons (Table S1). These results reveal shep regulation of factors involved in synaptic signaling, particularly peptidergic signaling, consistent with specific shep expression in neuropeptidergic neurons (Chen et al., 2014).
In contrast to the effector proteins discussed above, loss of shep leads to persistent expression of gene expression regulators normally downregulated during neuronal metamorphosis. Among these genes, we detected strong enrichment of factors that regulate transcription, biosynthesis of macromolecules, neuron projection guidance and development (Fig. 3B-D), as well as multiple factors that are known to regulate neuronal remodeling. For example, Shep depletion leads to upregulation of Dad (Fig. 3E), Myc (Fig. 3F) and broad (Fig. S4A) specifically in pupal neurons. In control neurons, expression of these genes declines by 3.2-fold, 9-fold and 8-fold by the end of metamorphosis, respectively (Table S1). However, loss of shep results in elevation of their expression to 1.4-fold, 1.3-fold and 1.6-fold compared with their normal pupal expression levels, and we validated these results by RT-qPCR in FACS-sorted pupal neurons (Fig. 3G) (see Table S2 for primer sequences). Loss of either Dad or Myc suppresses defects in wing expansion and neuronal remodeling of bursicon neurons caused by shep knockdown (Chen et al., 2017). The broad gene encodes an ecdysone-responding transcription factor that regulates neuronal outgrowth during metamorphosis (Scott et al., 2011). The persistent expression of these transcription factors indicates failure to restrict activities of particular regulatory programs that function in earlier development, which might lead to defective neuronal development during metamorphosis.
Shep binds to and regulates transcription of shep-inhibited genes
Given that Shep antagonizes gypsy chromatin insulator activity in the CNS, we next tested whether Shep-dependent genes are regulated specifically at the transcription level by monitoring nascent RNA synthesis in experimentally tractable BG3 cells derived from larval CNS tissue. As a first step, we examined steady-state transcript levels in cultured BG3 cells treated with dsRNA (see Table S2 for primer sequences) directed against shep or GFP. RNA-seq analysis detected 121 downregulated and 231 upregulated genes after shep depletion (Table S1). To monitor the newly synthesized pool of transcripts specifically, we treated cells with 5-ethynyl uridine (EU), which penetrates cells and incorporates into nascent transcripts after a short pulse, allowing biotinylation and capture of these RNAs (Jao and Salic, 2008). RT-qPCR of 11 selected shep-inhibited genes (see Table S2 for primer sequences) confirmed significantly higher nascent transcript levels in shep knockdown cells for four of these genes (Fig. 4A). These transcriptionally inhibited genes in BG3 cells include NFAT, corto and brat, which are also inhibited by shep specifically in pupal neurons (Fig. S5). These results suggest that shep can indeed regulate gene expression by inhibiting the transcription of at least a subset of affected genes.
Fig. 4.
Shep regulates expression of the genes to which it binds. (A) EU-qPCR of shep-inhibited genes reveals shep inhibition of nascent transcript levels. Cells transfected with GFP dsRNA were used as control. Student's t-test (*P<0.05). Only genes differentially expressed in BG3 cells were selected for quantification. (B) Binary heat maps of ChIP and RIP binding targets of Shep and insulator proteins in BG3 cells, ordered by overlap with shep-inhibited or shep-promoted genes in BG3 cells. Numbers in parentheses indicate total number of genes within each category. Fisher's exact test with Bonferroni correction was applied to two groups of genes represented by different colors, and P-values and odds ratios are indicated.
We hypothesized that global shep-dependent gene expression changes might be due to the insulator-related function of Shep on chromatin. Therefore, we compared our BG3 genome-wide RNA-seq results with previously determined chromatin immunoprecipitation (ChIP)-seq profiles of Shep and insulator proteins in the same cell type (Matzat et al., 2012). This analysis revealed statistically significant enrichment of binding of Shep or insulator proteins specifically at shep-inhibited genes. Of the 4710 genes harboring 4882 Shep ChIP-seq peaks in their gene bodies or within 1 kb upstream (Matzat et al., 2012), we identified 81% (186/231; Fig. 4B) of upregulated and 60% (72/121) of downregulated genes in shep-depleted BG3 cells. These results suggest that Shep can either inhibit or promote gene expression through action on chromatin. Among the 72 shep-promoted Shep ChIP target genes, Su(Hw) and Mod(mdg4)67.2 binding were detected for only ten and 18 genes, respectively. However, for the 186 Shep ChIP target genes that are also shep-inhibited, Mod(mdg4)67.2 and Su(Hw) binding were found for 91 and 75 genes, respectively, including the shep-inhibited NFAT, corto, brat, Tet, Dad and Myc genes. The binding of insulator proteins specifically at shep-inhibited ChIP targets suggests that Shep interacts with insulator proteins on chromatin in order to inhibit gene expression.
Shep is an RNA-binding protein and, interestingly, transcripts of shep-inhibited genes are also bound by Shep protein. All Shep isoforms have two RNA recognition motifs and bind nuclear RNA; thus, we intersected our RNA-seq data with 498 transcripts bound by Shep that were identified by nuclear native RNA immunoprecipitation (RIP) (Dale et al., 2014). Among the 121 shep-promoted genes in BG3 cells, only three of them express transcripts bound by Shep. In contrast, we identified 45 of 231 shep-inhibited genes producing transcript binding targets of Shep (Fig. 4B) in BG3 cells. Remarkably, 92% (42/45) of these upregulated RIP targets of Shep derive from loci that also harbor Shep ChIP binding, suggesting co-transcriptional recruitment of Shep specifically at these shep-inhibited genes. Taken together, we observed colocalization of Shep and insulator proteins on chromatin of shep-inhibited genes, which also produce transcripts bound by Shep.
Knockdown of brat rescues loss-of-shep phenotypes
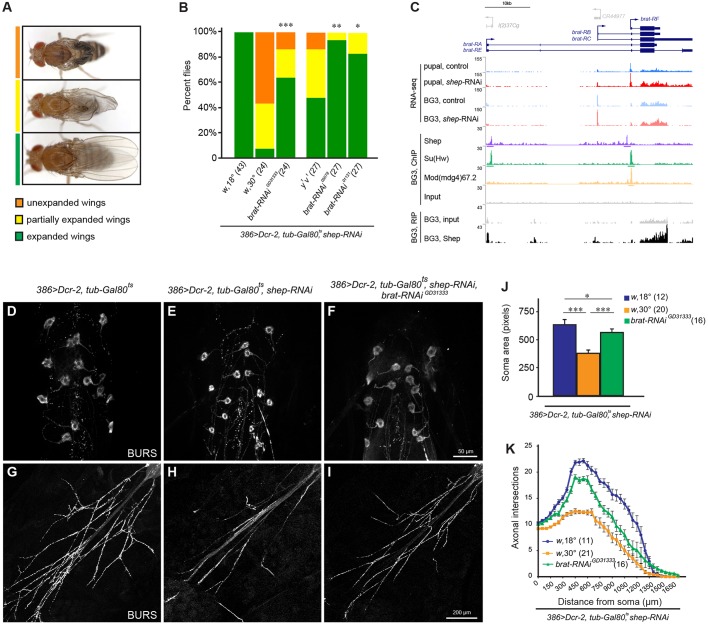
To examine the biological relevance of Shep regulation of its ChIP and RIP targets, we tested whether genetic manipulation of Shep targets that we identified could alter previously reported shep-dependent phenotypes. To generate neuronal phenotypes, we knocked shep down in peptidergic neurons in a temperature-dependent manner. At the permissive temperature of 18°C, 386>shep-RNAi, Dcr-2, mCD8::GFP, tub-Gal80ts flies grew normally but at the restrictive temperature of 30°C, flies displayed wing expansion defects (WEDs) (Fig. 5A,B). Consistent with WEDs, we observed smaller soma sizes (Fig. 5D,E) as well as fewer neurites and boutons of BAG neurons (Fig. 5G,H) and BSEG neurons (data not shown), due to remodeling defects when shep is knocked down.
Fig. 5.
Restoration of brat suppresses loss-of-shep phenotypes. (A) Representative images show fully expanded, partially expanded and unexpanded wings. (B) RNAi-mediated loss of brat suppresses WEDs caused by loss of shep. Transgenes with different genetic backgrounds were tested against appropriate controls. Fisher's exact test with Bonferroni correction (*P<0.05, **P<0.01, ***P<0.001). Sample sizes are shown in parentheses. All experiments were performed at 30°C except the 18°C control. (C) Screenshot of brat locus. Shown are control and shep RNAi RNA-seq data of P14 pupal neurons and BG3 cells, Shep RIP-seq and ChIP-seq data, and ChIP-seq data of insulator proteins. (D-I) Representative images showing rescued soma size (D-F) and projections (G-I) of bursicon neurons of abdominal ganglion by loss of brat. (J) Quantification of soma size of bursicon neurons of abdominal ganglion. One-way ANOVA with Tukey's post hoc test (*P<0.05, ***P<0.001). Sample sizes are shown in parentheses. (K) Sholl analysis of axonal projections of bursicon neurons of abdominal ganglion. Sample sizes are shown in parentheses.
Among the shep-regulated genes, we were particularly interested in testing the functional effects of brat depletion. Our results indicate that Shep binds to and transcriptionally inhibits brat (Fig. 3G, Fig. 4A, Fig. 5C). Moreover, brat inhibits neuronal growth (Olesnicky et al., 2012) and negatively regulates BMP signaling (Shi et al., 2013), which is a key pathway promoted by shep to regulate neuronal remodeling (Chen et al., 2017). Therefore, ectopic overexpression of brat as a result of shep depletion in neurons might contribute to neuronal remodeling defects. In order to control for potential off-target effects, we employed three independently derived UAS-brat-RNAi strains to knock brat down in neurons (Fig. S6A,B), and each of these knockdown constructs significantly rescued WEDs in 386>shep-RNAi, Dcr-2, mCD8::GFP animals (Fig. 5B). Consistent with rescued wing expansion, brat knockdown resulted in larger soma size (Fig. 5F,J) and increased neurites of BAG neurons of shep-depleted pupae (Fig. 5I,K), indicating that decreased expression of brat is sufficient to partially restore neuronal remodeling of BAG neurons during metamorphosis and post-eclosion wing expansion in shep knockdown flies. Decreased expression of brat did not rescue morphological defects of BSEG neurons, implying population-specific regulatory mechanisms during neuronal remodeling.
DISCUSSION
In this study, we have elucidated how shep controls gene expression in neurons during the larval-to-pupal transition, integrating multiple factors known to regulate neuronal remodeling. These shep-dependent genes are bound by Shep and insulator proteins, consistent with a direct effect on their transcription levels. Decreased expression of brat, a key Shep target gene we identified, is sufficient to rescue cellular and behavioral defects caused by loss of shep. Our findings characterize for the first time the transition of the neuronal transcriptome that is achieved during metamorphosis. Moreover, our results highlight the central role of Shep as a direct regulator of BMP signaling, within the larger network of factors controlling neuronal metamorphosis.
Transition of neuronal transcriptome during metamorphosis
Our work provides much needed insight into the global transcription program of the nervous system during development. A previous microarray study examined the transcriptome of whole brains of third instar larvae compared with 18 h pupae and detected 61 neural genes differentially expressed by early metamorphosis (Li and White, 2003). In contrast, our study compared neurons isolated from third instar larvae and 82 h pupae, extending our understanding of gene expression changes at this previously uninvestigated late metamorphic stage. Remarkably, we detected two orders of magnitude more differential expression than identified in the previous study, yet none of this differential expression is detected by both studies. These results suggest highly dynamic or stage-specific gene expression in neurons throughout metamorphosis.
Updated technical approaches allowed us to learn key features of the neuronal remodeling process. We applied cell sorting to provide higher specificity and sensitivity to the detection of differential expression by eliminating interference from a large variety of cell types. Furthermore, we employed RNA-seq, which allows discernment of a much higher dynamic range of expression differences than microarray studies. Our results enabled us to detect that synaptic signaling factors are boosted whereas certain gene expression regulators are restricted during this critical window of neuronal development. Having sampled a time point in late metamorphosis, we detected downregulation of factors that promote neuronal pruning during early metamorphosis, such as Sox14 (Kirilly et al., 2009), hdc (Loncle and Williams, 2012) and Uba1 (Watts et al., 2003). These results imply that transcriptional programs of neuronal remodeling are temporally distinct and strictly regulated throughout metamorphosis.
Shep is required for expression of synaptic and peptidergic signaling factors during metamorphosis
In shep-depleted pupal neurons, expression of synaptic and peptidergic signaling factors is reduced, consistent with behavioral and physiological phenotypes of shep mutants at the adult stage. Depletion of shep leads to reduction of transmitter biosynthesis factors, transmitter transporters, synaptic structural proteins, vesicle-associated transport factors and membrane-associated secretion factors, all of which are crucial for function and homeostasis of synaptic signaling. Decreased levels of synaptic signaling might lead to insufficient transmitter release and defective repletion during neuronal activities, resulting in the uncoordinated locomotion, weakness during eclosion, and behavioral defects observed in shep mutants and loss-of-function alleles (Armstrong et al., 2006; Tunstall et al., 2012; Chen et al., 2014; Arya et al., 2015).
In particular, depletion of shep reduces expression of 11 neuropeptidergic factors, indicating shep regulation of peptidergic signaling. Interestingly, the shep gene is bound by a peptidergic cell fate determinant transcription factor, Dimm (Hamanaka et al., 2010; Hadžić et al., 2015), which also binds and activates the amon gene (Park et al., 2004). Our data indicate that Shep associates with amon and positively regulates its expression (Fig. S6C), and overexpression of amon is sufficient to rescue wing expansion defects without affecting cellular morphology of shep-depleted bursicon neurons (data not shown). These results raise the possibility that shep could act as a relay between dimm and amon to regulate peptidergic signaling. Importantly, expression of other neuropeptide-processing enzymes was not found to be affected by loss of shep, suggesting that shep regulation of peptidergic signaling is achieved through specific regulation of amon.
Shep represses inhibitors of BMP signaling in order to regulate neuronal remodeling
In contrast to synaptic signaling factors, loss of shep allows persistent expression of a wide range of regulatory factors that normally decline during metamorphosis. These regulatory factors include key regulators of gene expression, which negatively regulate neuronal remodeling and peptidergic signaling. We find that Shep antagonizes Dad by binding and repressing its expression, consistent with the finding that loss of Dad suppresses remodeling defects of shep-depleted bursicon neurons (Chen et al., 2017). In addition to Dad, we also identified shep-inhibited sbb (Table S1) and brat as Shep chromatin-binding targets, both of which inhibit expression of key components of BMP signaling. BMP signaling is required for peptidergic capacity in peptidergic neurons and neuronal remodeling during metamorphosis (Veverytsa and Allan, 2011; Boulanger et al., 2012; Chen et al., 2017). Whereas sbb represses expression of the BMP receptor tkv (Funakoshi et al., 2001), brat reduces translation and transcript stability of Mad, a Smad factor that transduces BMP signaling (Shi et al., 2013; Newton et al., 2015). It is also known that brat negatively regulates larval dendrite growth (Olesnicky et al., 2012). Therefore, our results suggest that Shep represses multiple inhibitory factors of BMP signaling to promote neuronal remodeling and neuropeptidergic signaling. Consistent with our results, zebrafish Shep has been shown to promote TGFβ/BMP signaling during neural crest development (Jayasena and Bronner, 2012), suggesting conserved regulation of TGFβ/BMP signaling by Shep.
Notably, we observed population-specific rescue of shep-depleted bursicon neurons by shep-interacting genes. Genetic manipulation of BMP signaling components modifies morphology of both shep-depleted BAG and BSEG neurons (Chen et al., 2017) whereas brat RNAi affects only BAG neurons (Fig. 5D-K). Although restoration of remodeling in different neurons could be due to different required thresholds of regulatory factors, it is possible that distinct regulatory mechanisms are required during normal remodeling of different neuronal populations, as observed for other shep-interacting genes (Chen et al., 2017). As none of the known shep-interacting genes (Chen et al., 2017) (Fig. 5) is able to fully rescue all loss-of-shep phenotypes (Chen et al., 2014), this panel of shep-regulated genes that we identified might be required to work in concert to ensure normal remodeling of the nervous system.
Direct Shep regulation of Myc to regulate neuronal remodeling
We find that Shep associates and represses pupal expression of Myc in neurons. These results provide evidence of direct Shep regulation of Myc, consistent with the previous finding that loss of Myc rescues shep-dependent neuronal remodeling defects. Notably, human Shep also binds to Myc (Iguchi-Ariga et al., 1988) and antagonizes its ability to activate transcription (Niki et al., 2000). In conjunction with these vertebrate data, our findings suggest evolutionarily conserved shep antagonism of Myc. Although Brat post-transcriptionally inhibits Myc in stem cells of the Drosophila larval nervous system (Betschinger et al., 2006), we did not observe increased Myc protein levels after pan-neuronal knockdown of brat by western blotting brains at the P14 stage (data not shown), suggesting that Brat post-transcriptional inhibition of Myc is specific to differentiating cells or to an earlier stage of development. Furthermore, pan-neuronal knockdown of Myc or brat did not affect Shep protein levels (data not shown), indicating that these factors are downstream targets of Shep.
Shep represses ecdysone-transducing factors that regulate neuronal remodeling
We find that Shep associates with broad and Eip93F, and depletion of Shep leads to their persistent expression specifically in pupal neurons. Both broad and Eip93F are transcription factors that transduce ecdysone signals into transcriptional cascades during metamorphosis (Karim et al., 1993; Mou et al., 2012), which play key roles to regulate neuronal remodeling (Schubiger et al., 1998; Zheng et al., 2003). Overexpression of broad or Eip93F results in dendrite remodeling defects during neuronal remodeling (Scott et al., 2011) and cell death (Lee et al., 2000), respectively. Therefore, dysregulation of broad and Eip93F might lead to ectopic ecdysone signaling, thus contributing to the remodeling defects of shep-depleted neurons. Importantly, the expression of all key regulatory factors discussed above are regulated by shep exclusively in the pupal stage, implying developmental regulatory programs mediated by shep that are distinct from initial neuronal growth.
Shep regulates expression of the genes to which it binds
The majority of shep-dependent genes are bound by Shep in chromatin, suggesting that Shep directly regulates their gene expression. This is supported by our findings that Shep inhibits transcription levels of its ChIP target genes. Enrichment of insulator protein binding specifically at shep-inhibited genes raises the possibility that Shep regulates chromatin conformation and gene expression through interactions with chromatin insulators during neuronal metamorphosis. This hypothesis is supported by the observation that loss of su(Hw) is sufficient to rescue WEDs in shep-depleted flies (Chen et al., 2017). Consistent with previous findings of mutually exclusive three-way genome-wide localization (Matzat et al., 2012), we found that Shep, Mod(mdg4)67.2 and Su(Hw) rarely bind identical genomic locations within shep-inhibited genes. This result supports antagonistic interaction between Shep and insulator proteins on chromatin, perhaps at the level of chromatin looping. Given the localization of Shep at the nuclear lamina, it is also possible that Shep and insulator proteins regulate gene expression by mediating chromatin interactions with a nuclear scaffold. More detailed analysis will be required to examine the precise functional relationship between Shep and gypsy insulator factors in gene regulation of the nervous system.
As an RNA-binding protein, Shep could be co-transcriptionally recruited to inhibit gene transcription as a negative-feedback loop. Previous studies have noted that 58% of nuclear transcripts bound by Shep also harbor chromatin-binding sites for Shep (Dale et al., 2014). We found an even stronger enrichment in that 93% (42/45) of upregulated Shep nuclear-bound transcripts are transcribed from genomic loci harboring Shep ChIP peaks in BG3 cells. This enrichment is almost exclusive for shep-inhibited genes, suggesting that Shep-associated transcripts play a role in Shep inhibition of gene expression on chromatin. A recent study showed that depletion of shep in S2 cells results in alternative-splicing defects (Brooks et al., 2015). Accordingly, we found that 63% (209/330, P=2.3e−10, odds ratio=4.7) of genes with altered splicing after shep depletion in S2 cells harbor Shep ChIP association in BG3 cells; thus, it is also possible that Shep is co-transcriptionally recruited to chromatin to regulate alternative splicing. In spite of its thus far described functions in the nucleus (Matzat et al., 2012; Dale et al., 2014), Shep localizes predominantly to the cytoplasm in all tissues examined; therefore, it is possible that Shep is also involved in later steps of gene regulation.
In conclusion, our findings reveal previously unknown changes in the neuronal transcriptome as a result of metamorphosis, and we discovered mechanisms by which Shep contributes to this developmental transition. These results collectively establish a molecular genetic network in which shep promotes neuronal remodeling using bursicon neurons as a model system (Fig. 6). Mechanisms of shep regulation of neuronal remodeling include repression of Myc, which regulates neuronal remodeling of BSEG neurons, and multiple inhibitory factors of BMP signaling that are involved in remodeling of BAG neurons (brat), or both BAG and BSEG neurons (BMP signaling components).
Fig. 6.
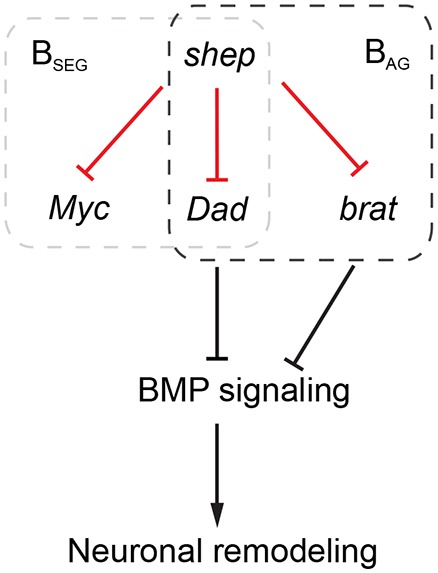
Diagram of shep-dependent genetic network of neuronal remodeling. Novel negative genetic interactions identified in our study (red) in the context of known remodeling factors (black). Myc and brat rescue morphological defects in loss-of-shep BSEG and BAG neurons, respectively, whereas components of BMP signaling pathway affect remodeling of both bursicon neuron populations.
MATERIALS AND METHODS
Fly stocks
Drosophila stocks and crosses were grown on standard cornmeal-yeast-agarose medium at 25°C unless otherwise noted. Only female animals were used for data collection and analysis. Fly strains are included in the supplementary Materials and Methods.
Cell dissociation and fluorescence-activated cell sorting (FACS)
The FACS procedure was modified and developed based on a protocol for fly neuroblasts (Harzer et al., 2013). All steps were conducted at room temperature (RT) unless otherwise noted. Cells were sorted on a FACSAria II machine of the Flow Cytometry Core at the National Heart, Lung and Blood Institute. See supplementary Materials and Methods for details.
Immunostaining, imaging and quantification
The sorted cell solution was dropped onto a slide coated with 0.01% poly-L-lysine for 10 min at RT. Attached cells or dissected tissues underwent immunostaining according to a previously published procedure (Hewes et al., 2003). Primary antibodies used for immunostaining include rat anti-ELAV [Developmental Studies Hybridoma Bank (DSHB), 7E8A10, 1:50], mouse anti-LaminB (DSHB, ADL67.10, 1:50), guinea pig anti-Shep (Matzat et al., 2012) (1:200), rat anti-Dpn (Abcam, ab195172, 1:100), rat anti-Miranda (Abcam, ab197788, 1:400), mouse anti-Repo (DSHB, 8D12, 1:100) and rabbit anti-BURS (Luan et al., 2006) (1:5000). Secondary antibodies (Thermo Fisher Scientific) were used at 1:1000. Images were taken as maximum-intensity z-series projections with a Leica780 confocal microscope. Soma areas of the six most anterior bursicon neurons in the abdominal ganglia at the P14 pupal stage were quantified using Adobe Photoshop and averaged for each preparation as one replicate. Projections of bursicon neurons in the abdominal ganglia were counted in Adobe Illustrator by Sholl analysis (Milošević and Ristanović, 2007) as previously described (Chen et al., 2014) with concentric circles spaced 50 μm apart.
RNA-seq libraries, sequencing and analysis
RNA was extracted from sorted neurons with RNeasy Plus Micro Kit (Qiagen) according to the manufacturer's protocol and was quantified with Quant-iT RiboGreen RNA Assay Kit (Thermo Fisher Scientific). RNA integrity was estimated with Agilent RNA 6000 Pico Kit with Agilent Bioanalyzer, and 45-100 ng total RNA was used to generate RNA-seq libraries with Ovation RNA-Seq Systems 1-16 for Model Organisms (Nugen), along with Covaris cDNA fragmentation. All samples were sequenced with HiSeq2500 (Illumina) at the NIDDK Genomics Core Facility by 50 bp single-end sequencing. See supplementary Materials and Methods for details of gene oncology and computational analyses. Primer sequences of neuronal RT-qPCR are included in Table S2.
Cell culture and electroporation of dsRNA
BG3-c2 cells (Drosophila Genomics Resource Center) were cultured at 25°C in Schneider's medium supplemented with 10% fetal bovine serum and 10 μg/ml insulin. The MEGAscript T7 Kit (Thermo Fisher Scientific) was used to generate dsRNA, and electroporation program T30 was performed with Amaxa Cell Line Nucleofector Kit V (Lonza) according to the manufacturer's protocol. See supplementary Materials and Methods for details. Sequences of primers used to generate dsRNA are included in Table S2.
RNA labeling with 5-ethynyl uridine (EU) and RNA isolation
EU-RNA labeling and capture were performed with Click-iT Nascent RNA Capture Kit (Thermo Fisher Scientific) according to the manufacturer's protocol with the following minor modifications. Four days after transfection with dsRNA, BG3 cells were incubated with 0.6 mM EU for 1 h, and RNA was extracted with Trizol (Thermo Fisher Scientific). The Click-iT reaction was performed with 0.25 mM biotin azide, and biotinylated RNA was captured with 12 μl T1 beads. After washing, RNA captured on beads was re-suspended with 20 μl reaction system containing SuperScript III Reverse Transcriptase (Thermo Fisher Scientific), oligo (dT)20 and random primers for cDNA synthesis. Quantification of RNA and cDNA was performed with Quant-iT RiboGreen RNA Assay Kit (Thermo Fisher Scientific) and Qubit ssDNA Kit (Thermo Fisher Scientific), respectively. Primer sequences of EU RT-qPCR are included in Table S2.
Wing expansion scoring
Wings were scored as unexpanded when the distal tip of the wing opened less than 90° relative to the long (proximal to distal) axis of the wing. All intermediate degrees of wing expansion were scored as partially expanded wings (Zhao et al., 2008), and only straight and flat wings were scored as expanded.
Statistics
All experiments were performed with three biological replicates unless noted. The P-values of Fisher's exact tests are reported as two-tailed values, and statistical significance of all tests is reported as *P<0.05, **P<0.01, ***P<0.001. Average values in all bar plots and the Sholl analysis are reported as mean±s.e.m.
Supplementary Material
Acknowledgements
We thank B. White for comments on the manuscript, fly strains, and reagents; J. Kassis for comments on the manuscript; T. Gu and R. Hewes for fly strains; J. Yin and Q. Yuan for sharing FACS protocols; P. Boyle and N. Moshkovich for design of dsRNA primers; and members of the Lei laboratory for discussions and comments on the manuscript.
Footnotes
Competing interests
The authors declare no competing or financial interests.
Author contributions
Conceptualization: D.C., R.K.D., E.P.L.; Methodology: D.C., R.K.D.; Software: R.K.D.; Validation: D.C.; Formal analysis: D.C., R.K.D.; Investigation: D.C.; Data curation: D.C., R.K.D.; Writing - original draft: D.C.; Writing - review & editing: D.C., R.K.D., E.P.L.; Visualization: D.C., R.K.D.; Supervision: E.P.L.; Project administration: E.P.L.; Funding acquisition: E.P.L.
Funding
This work was funded by the Intramural Program of the National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health (DK015602 to E.P.L.). Deposited in PMC for release after 12 months.
Data availability
The RNA-seq data have been deposited in the Gene Expression Omnibus database under accession number GSE93737.
Supplementary information
Supplementary information available online at http://dev.biologists.org/lookup/doi/10.1242/dev.154047.supplemental
References
- Armstrong J. D., Texada M. J., Munjaal R., Baker D. A. and Beckingham K. M. (2006). Gravitaxis in Drosophila melanogaster: a forward genetic screen. Genes Brain Behav. 5, 222-239. 10.1111/j.1601-183X.2005.00154.x [DOI] [PubMed] [Google Scholar]
- Arya G. H., Magwire M. M., Huang W., Serrano-Negron Y. L., Mackay T. F. C. and Anholt R. R. H. (2015). The genetic basis for variation in olfactory behavior in Drosophila melanogaster. Chem. Senses 40, 233-243. 10.1093/chemse/bjv001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagri A., Cheng H.-J., Yaron A., Pleasure S. J. and Tessier-Lavigne M. (2003). Stereotyped pruning of long hippocampal axon branches triggered by retraction inducers of the semaphorin family. Cell 113, 285-299. 10.1016/S0092-8674(03)00267-8 [DOI] [PubMed] [Google Scholar]
- Betschinger J., Mechtler K. and Knoblich J. A. (2006). Asymmetric segregation of the tumor suppressor brat regulates self-renewal in Drosophila neural stem cells. Cell 124, 1241-1253. 10.1016/j.cell.2006.01.038 [DOI] [PubMed] [Google Scholar]
- Boulanger A., Farge M., Ramanoudjame C., Wharton K. and Dura J.-M. (2012). Drosophila motor neuron retraction during metamorphosis is mediated by inputs from TGF-beta/BMP signaling and orphan nuclear receptors. PLoS ONE 7, e40255 10.1371/journal.pone.0040255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks A. N., Duff M. O., May G., Yang L., Bolisetty M., Landolin J., Wan K., Sandler J., Booth B. W., Celniker S. E. et al. (2015). Regulation of alternative splicing in Drosophila by 56 RNA binding proteins. Genome Res. 25, 1771-1780. 10.1101/gr.192518.115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen D., Qu C., Bjorum S. M., Beckingham K. M. and Hewes R. S. (2014). Neuronal remodeling during metamorphosis is regulated by the alan shepard (shep) gene in Drosophila melanogaster. Genetics 197, 1267-1283. 10.1534/genetics.114.166181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen D., Gu T., Pham T. N., Zachary M. J. and Hewes R. S. (2017). Regulatory mechanisms of metamorphic neuronal remodeling revealed through a genome-wide modifier screen in drosophila melanogaster. Genetics 206, 1429-1443. 10.1534/genetics.117.200378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cocchi E., Drago A. and Serretti A. (2016). Hippocampal pruning as a new theory of schizophrenia etiopathogenesis. Mol. Neurobiol. 53, 2065-2081. 10.1007/s12035-015-9174-6 [DOI] [PubMed] [Google Scholar]
- Dale R. K., Matzat L. H. and Lei E. P. (2014). metaseq: a Python package for integrative genome-wide analysis reveals relationships between chromatin insulators and associated nuclear mRNA. Nucleic Acids Res. 42, 9158-9170. 10.1093/nar/gku644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funakoshi Y., Minami M. and Tabata T. (2001). mtv shapes the activity gradient of the Dpp morphogen through regulation of thickveins. Development 128, 67-74. [DOI] [PubMed] [Google Scholar]
- Gao P.-P., Yue Y., Cerretti D. P., Dreyfus C. and Zhou R. (1999). Ephrin-dependent growth and pruning of hippocampal axons. Proc. Natl. Acad. Sci. USA 96, 4073-4077. 10.1073/pnas.96.7.4073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garin-Aguilar M. E., Diaz-Cintra S., Quirarte G. L., Aguilar-Vázquez A., Medina A. C. and Prado-Alcalá R. A. (2012). Extinction procedure induces pruning of dendritic spines in CA1 hippocampal field depending on strength of training in rats. Front. Behav. Neurosci. 6, 12 10.3389/fnbeh.2012.00012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh D., Gerasimova T. I. and Corces V. G. (2001). Interactions between the Su(Hw) and Mod(mdg4) proteins required for gypsy insulator function. EMBO J. 20, 2518-2527. 10.1093/emboj/20.10.2518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu T., Zhao T. and Hewes R. S. (2014). Insulin signaling regulates neurite growth during metamorphic neuronal remodeling. Biol. Open 3, 81-93. 10.1242/bio.20136437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadžić T., Park D., Abruzzi K. C., Yang L., Trigg J. S., Rohs R., Rosbash M. and Taghert P. H. (2015). Genome-wide features of neuroendocrine regulation in Drosophila by the basic helix-loop-helix transcription factor DIMMED. Nucleic Acids Res. 43, 2199-2215. 10.1093/nar/gku1377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamanaka Y., Park D., Yin P., Annangudi S. P., Edwards T. N., Sweedler J., Meinertzhagen I. A. and Taghert P. H. (2010). Transcriptional orchestration of the regulated secretory pathway in neurons by the bHLH protein DIMM. Curr. Biol. 20, 9-18. 10.1016/j.cub.2009.11.065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harzer H., Berger C., Conder R., Schmauss G. and Knoblich J. A. (2013). FACS purification of Drosophila larval neuroblasts for next-generation sequencing. Nat. Protoc. 8, 1088-1099. 10.1038/nprot.2013.062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hewes R. S., Park D., Gauthier S. A., Schaefer A. M. and Taghert P. H. (2003). The bHLH protein Dimmed controls neuroendocrine cell differentiation in Drosophila. Development 130, 1771-1781. 10.1242/dev.00404 [DOI] [PubMed] [Google Scholar]
- Iguchi-Ariga S. M., Okazaki T., Itani T., Ogata M., Sato Y. and Ariga H. (1988). An initiation site of DNA replication with transcriptional enhancer activity present upstream of the c-myc gene. EMBO J. 7, 3135-3142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jao C. Y. and Salic A. (2008). Exploring RNA transcription and turnover in vivo by using click chemistry. Proc. Natl. Acad. Sci. U.S.A. 105, 15779-15784. 10.1073/pnas.0808480105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayasena C. S. and Bronner M. E. (2012). Rbms3 functions in craniofacial development by posttranscriptionally modulating TGF-beta signaling. J. Cell Biol. 199, 453-466. 10.1083/jcb.201204138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karim F. D., Guild G. M. and Thummel C. S. (1993). The Drosophila Broad-Complex plays a key role in controlling ecdysone-regulated gene expression at the onset of metamorphosis. Development 118, 977-988. [DOI] [PubMed] [Google Scholar]
- Kirilly D., Gu Y., Huang Y., Wu Z., Bashirullah A., Low B. C., Kolodkin A. L., Wang H. and Yu F. (2009). A genetic pathway composed of Sox14 and Mical governs severing of dendrites during pruning. Nat. Neurosci. 12, 1497-1505. 10.1038/nn.2415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C.-Y., Wendel D. P., Reid P., Lam G., Thummel C. S. and Baehrecke E. H. (2000). E93 directs steroid-triggered programmed cell death in Drosophila. Mol. Cell 6, 433-443. 10.1016/S1097-2765(00)00042-3 [DOI] [PubMed] [Google Scholar]
- Li T.-R. and White K. P. (2003). Tissue-specific gene expression and ecdysone-regulated genomic networks in Drosophila. Dev. Cell 5, 59-72. 10.1016/S1534-5807(03)00192-8 [DOI] [PubMed] [Google Scholar]
- Loncle N. and Williams D. W. (2012). An interaction screen identifies headcase as a regulator of large-scale pruning. J. Neurosci. 32, 17086-17096. 10.1523/JNEUROSCI.1391-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luan H., Lemon W. C., Peabody N. C., Pohl J. B., Zelensky P. K., Wang D., Nitabach M. N., Holmes T. C. and White B. H. (2006). Functional dissection of a neuronal network required for cuticle tanning and wing expansion in Drosophila. J. Neurosci. 26, 573-584. J. Neurosci. 26, 573-584. 10.1523/JNEUROSCI.3916-05.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matzat L. H., Dale R. K., Moshkovich N. and Lei E. P. (2012). Tissue-specific regulation of chromatin insulator function. PLoS Genet. 8, e1003069 10.1371/journal.pgen.1003069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milošević N. T. and Ristanović D. (2007). The Sholl analysis of neuronal cell images: semi-log or log-log method? J. Theor. Biol. 245, 130-140. 10.1016/j.jtbi.2006.09.022 [DOI] [PubMed] [Google Scholar]
- Mou X., Duncan D. M., Baehrecke E. H. and Duncan I. (2012). Control of target gene specificity during metamorphosis by the steroid response gene E93. Proc. Natl. Acad. Sci. U.S.A. 109, 2949-2954. 10.1073/pnas.1117559109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newton F. G., Harris R. E., Sutcliffe C. and Ashe H. L. (2015). Coordinate post-transcriptional repression of Dpp-dependent transcription factors attenuates signal range during development. Development 142, 3362-3373. 10.1242/dev.123273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niki T., Izumi S., Saegusa Y., Taira T., Takai T., Iguchi-Ariga S. M. and Ariga H (2000). MSSP promotes ras/myc cooperative cell transforming activity by binding to c-Myc. Genes Cells 5, 127-141. 10.1046/j.1365-2443.2000.00311.x [DOI] [PubMed] [Google Scholar]
- Olesnicky E. C., Bhogal B. and Gavis E. R. (2012). Combinatorial use of translational co-factors for cell type-specific regulation during neuronal morphogenesis in Drosophila. Dev. Biol. 365, 208-218. 10.1016/j.ydbio.2012.02.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park D., Han M., Kim Y.-C., Han K.-A. and Taghert P. H. (2004). Ap-let neurons--a peptidergic circuit potentially controlling ecdysial behavior in Drosophila. Dev. Biol. 269, 95-108. 10.1016/j.ydbio.2004.01.015 [DOI] [PubMed] [Google Scholar]
- Peabody N. C., Diao F. Q., Luan H. J., Wang H., Dewey E. M., Honegger H.-W. and White B. H. (2008). Bursicon Functions within the Drosophila CNS to Modulate Wing Expansion Behavior, Hormone Secretion, and Cell Death. J. Neurosci. 28, 14379-14391. 10.1523/JNEUROSCI.2842-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray D., Kazan H., Cook K. B., Weirauch M. T., Najafabadi H. S., Li X., Gueroussov S., Albu M., Zheng H., Yang A. et al. (2013). A compendium of RNA-binding motifs for decoding gene regulation. Nature 499, 172-177. 10.1038/nature12311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schachtner L. T., Sola I. E., Forand D., Antonacci S., Postovit A. J., Mortimer N. T., Killian D. J. and Olesnicky E.C. (2015). Drosophila Shep and C. elegans SUP-26 are RNA-binding proteins that play diverse roles in nervous system development. Dev. Genes Evol. 225, 319-330. 10.1007/s00427-015-0514-3 [DOI] [PubMed] [Google Scholar]
- Schubiger M., Wade A. A., Carney G. E., Truman J. W. and Bender M. (1998). Drosophila EcR-B ecdysone receptor isoforms are required for larval molting and for neuron remodeling during metamorphosis. Development 125, 2053-2062. [DOI] [PubMed] [Google Scholar]
- Scott J. A., Williams D. W. and Truman J. W. (2011). The BTB/POZ zinc finger protein Broad-Z3 promotes dendritic outgrowth during metamorphic remodeling of the peripheral stretch receptor dbd. Neural Dev. 6, 39 10.1186/1749-8104-6-39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekar A., Bialas A. R., de Rivera H., Davis A., Hammond T. R., Kamitaki N., Tooley K., Presumey J., Baum M., Van Doren V. et al. , (2016). Schizophrenia risk from complex variation of complement component 4. Nature 530: 177-183. 10.1038/nature16549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi W., Chen Y., Gan G., Wang D., Ren J., Wang Q., Xu Z., Xie W. and Zhang Y. Q. (2013). Brain tumor regulates neuromuscular synapse growth and endocytosis in Drosophila by suppressing mad expression. J. Neurosci. 33, 12352-12363. 10.1523/JNEUROSCI.0386-13.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas M. S. C., Davis R., Karmiloff-Smith A., Knowland V. C. P. and Charman T. (2016). The over-pruning hypothesis of autism. Dev. Sci. 19, 284-305. 10.1111/desc.12303 [DOI] [PubMed] [Google Scholar]
- Tunstall N. E., Herr A., de Bruyne M. and Warr C. G. (2012). A screen for genes expressed in the olfactory organs of Drosophila melanogaster identifies genes involved in olfactory behaviour. PLoS ONE 7, e35641 10.1371/journal.pone.0035641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veverytsa L. and Allan D. W. (2011). Retrograde BMP signaling controls Drosophila behavior through regulation of a peptide hormone battery. Development 138, 3147-3157. 10.1242/dev.064105 [DOI] [PubMed] [Google Scholar]
- Watts R. J., Hoopfer E. D. and Luo L. (2003). Axon pruning during Drosophila metamorphosis: evidence for local degeneration and requirement of the ubiquitin-proteasome system. Neuron 38, 871-885. 10.1016/S0896-6273(03)00295-2 [DOI] [PubMed] [Google Scholar]
- Wegener C., Herbert H., Kahnt J., Bender M. and Rhea J. M. (2011). Deficiency of prohormone convertase dPC2 (AMONTILLADO) results in impaired production of bioactive neuropeptide hormones in Drosophila. J. Neurochem. 118, 581-595. 10.1111/j.1471-4159.2010.07130.x [DOI] [PubMed] [Google Scholar]
- Yaniv S. P. and Schuldiner O. (2016). A fly's view of neuronal remodeling. Wiley Interdiscip. Rev. Dev. Biol. 5, 618-635. 10.1002/wdev.241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaniv S. P., Issman-Zecharya N., Oren-Suissa M., Podbilewicz B. and Schuldiner O. (2012). Axon regrowth during development and regeneration following injury share molecular mechanisms. Curr. Biol. 22, 1774-1782. 10.1016/j.cub.2012.07.044 [DOI] [PubMed] [Google Scholar]
- Zhao T., Gu T., Rice H. C., McAdams K. L., Roark K. M., Lawson K., Gauthier S. A., Reagan K. L. and Hewes R. S. (2008). A Drosophila gain-of-function screen for candidate genes involved in steroid-dependent neuroendocrine cell remodeling. Genetics 178, 883-901. 10.1534/genetics.107.082487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng X., Wang J., Haerry T. E., Wu A. Y.-H., Martin J., O'Connor M. B., Lee C.-H. J. and Lee T. (2003). TGF-beta signaling activates steroid hormone receptor expression during neuronal remodeling in the Drosophila brain. Cell 112, 303-315. 10.1016/S0092-8674(03)00072-2 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.