Abstract
Corticosteroids are the mainstay of therapy for immune reconstitution inflammatory syndrome (IRIS). However, little is known about how to treat IRIS unresponsive to steroids. We report a patient with HIV-TB coinfection who was unresponsive to first prednisolone and then infliximab but whose IRIS resolved with adalimumab.
Keywords: adalimumab, HIV, immune reconstitution, steroid, tuberculosis
Immune reconstitution inflammatory syndrome (IRIS) is commonly described in patients with HIV and opportunistic infections such as tuberculosis (TB), particularly after the initiation of antiretroviral therapy (ART) and subsequent CD4 cell count recovery. IRIS may have a broad range of manifestations, and diagnosis relies on ruling out an infectious cause. IRIS may involve the central nervous system, manifesting as enlarging space-occupying lesions, meningitis, leptomeningeal involvement, vasculopathy, or radiculomyelopathy. IRIS is most frequently associated with cryptococcosis, TB, and JC virus [1]. In addition to difficulties in defining IRIS, there is a lack of clear evidence to guide its management. Corticosteroids are generally used as firstline therapy, but a small proportion of patients fail to respond to this treatment. In this situation, either the diagnosis is wrong (ie, there is an untreated infection) or the immune activation is so potent and established that corticosteroid therapy fails to attenuate it. There is no clear guideline on how to treat patients with corticosteroid-refractory IRIS. We here describe successful treatment using adalimumab in an HIV-infected patient with steroid-refractory tuberculosis-associated systemic and neurological IRIS.
CASE REPORT
A 51-year-old man was diagnosed with HIV infection in 2007, with a CD4 count of 680/microL, and was offered but declined treatment. He presented in December 2016 with a 4-week history of night sweats, fever, cough, and weight loss and several days of worsening confusion and headache. On presentation, his CD4 count was low at 172/microL, and his HIV viral load was 2 million copies/mL. Pulmonary and brain imaging (Figure 2A and B) was suggestive of miliary TB and 2 frontal cerebral tuberculomas. Cerebrospinal fluid polymerase chain reaction (GeneXpert) and subsequent culture demonstrated Mycobacterium tuberculosis. He underwent bilateral frontal craniotomies for diagnostic and therapeutic purposes, and culture of tissue obtained intraoperatively confirmed pan-sensitive Mycobacterium tuberculosis. Rifampicin 600 mg, pyrazinamide 2000 mg, isoniazid 300 mg, and ethambutol 900 mg were initiated, along with prednisolone 60 mg as prophylaxis against IRIS. Three weeks after TB treatment commenced, abacavir 600 mg, lamivudine 300 mg daily, and dolutegravir 50 mg twice daily were initiated. Over the following 3 weeks, his symptoms improved significantly with resolution of chest radiograph (CXR) changes as well as immune recovery with an increase in CD4 count to 400/microL.
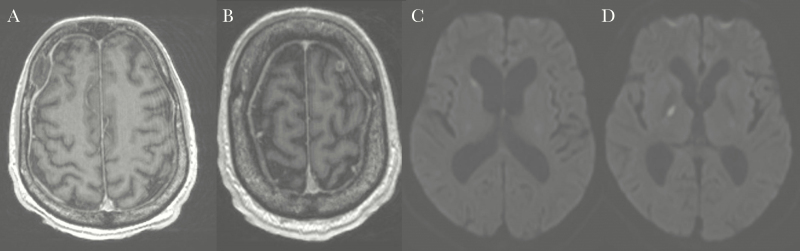
Figure 2.
A and B, Initial cerebral magnetic resonance images (MRIs) suggestive of 2 frontal tuberculous cerebral abscesses. C and D, Repeated cerebral MRIs showing high signal focus in the right basal ganglia and right caudate nucleus with restricted diffusion suggestive of focal infarct.
After 6 weeks of TB treatment and 3 weeks of antiretroviral therapy (Figure 1), while on a tapering dose of prednisolone (25 mg daily), he re-presented with fevers up to 40°C (104°F), headache, and photophobia. Investigations including multiple blood cultures, cerebrospinal fluid (CSF) mycobacterial cultures, cryptococcal antigen, and virology tests showed no other causes of meningitis. A diagnosis of TB IRIS was made after exclusion of concomitant infections. The prednisolone dose was increased to 60 mg daily, but daily fevers up to 40°C persisted for a further 3 weeks. Following a thorough re-evaluation for a missed diagnosis, tumor necrosis factor (TNF)–alpha blockade with infliximab 300 mg by intravenous infusion was commenced. There was no significant improvement after 2 doses of infliximab 2 weeks apart, with ongoing daily high fevers. The patient then developed new-onset dysarthria, diplopia, and nystagmus. A cerebral magnetic resonance imaging (MRI) scan (Figure 2C and D) showed focal basal ganglia infarcts, interpreted as intracranial vasculitis due to progressive neurologic IRIS. Repeat cultures and polymerase chain reaction testing of blood and CSF revealed no pathogen. He was pulsed with intravenous methylprednisolone 500 mg daily for 3 days with no improvement. Infliximab was then discontinued, and adalimumab 40 mg subcutaneous injection was commenced. Two days following the first dose of adalimumab, the patient’s fever settled. His neurological symptoms and signs resolved over the following 48 hours. Adalimumab injections were continued every 2 weeks for a total of 3 months, and prednisolone was slowly withdrawn. At follow-up 7 months after the initial presentation, the patient remains well, off prednisolone, while continuing rifampicin, isoniazid, and his initial ART regimen.
Figure 1.
Clinical course of the patient. *Tuberculosis therapy HRZE. Abbreviations: HRZE, isoniazid, ethambutol, rifampicin, pyrazinamide; TB, tuberculosis.
DISCUSSION
The incidence of TB-IRIS is estimated in different studies to range from 2% to 23% of patients in non-HIV patients and 15.7% in HIV patients on ART treatment [2]. Neurologic TB-IRIS accounts for 12% of all TB -IRIS cases in a single center in South Africa. It is potentially a life-threatening condition and associated with high mortality: 12%–25% [1]. Risk factors for the development of IRIS include early initiation of antiretroviral therapy (2–4 weeks after commencing antituberculosis treatment), marked recovery of CD4 count, or rapid suppression of HIV viral load with ART therapy or disseminated TB, all of which were present in our case [2]. The current World Health Organization guideline supports early initiation of ART in patients with CD4+ T-cell counts of less than 50 per cubic millimeter due to increased AIDS-free survival. For higher CD4+ T-cell counts, deferral of the initiation of ART after completion of intensive 2-month tuberculosis therapy should be considered because it reduces the risks of developing IRIS and the side effects due to ART with no increase in mortality [3].
We report 1 of the few cases in the literature of steroid-refractory IRIS treated successfully with adalimumab. Adalimumab use has been previously reported in 3 cases of IRIS: 1 in the context of TB in an HIV-negative patient [4] and 2 in HIV-related cryptococcal infection [5, 6]. Infliximab use is also reported, with 4 TB cases and 1 MAC-IRIS [7–9]. Duration of therapy varied from 1 dose to 6 months. Like 2 previously described neurological TB-IRIS cases [4, 7], our patient’s IRIS settled with anti-TNF therapy. Our case did not respond to the first TNF antagonist, infliximab, but responded rapidly to adalimumab. Most likely this relates to the delay in onset of clinical improvement after starting TNF therapy (generally 3–6 weeks) [10]. His response occurred 4 weeks after starting TNF blockade first with infliximab and then with adalimumab. Both are effective agents, though infliximab is a chimeric murine/human monoclonal given by intravenous infusion, with a higher rate of infusion and febrile reactions. His therapy was switched to adalimumab as we could not be sure whether his continued fevers and arthralgia, which flared after his second dose of infliximab, were related to the chimeric antibody. The timing of his response to anti-TNF therapy at 3–4 weeks is consistent with previous reports of the time to effect of TNF therapy in other conditions [10]. It is also possible that our patient had antibodies to the active site of infliximab, blocking its efficacy, but such antibodies are rare in previously untreated patients.
The pathogenesis of mycobacterial IRIS requires an excessive or paradoxical inflammatory response characterized by increased levels of gamma interferon and TNF- α [11]. Granuloma formation is a key component of the host response to TB, sequestering TB bacteria within active inflammatory sites and maintaining latency. TNF is vital to that process, and blocking TNF leads to high rates of TB reactivation in normal hosts. TNF-α inhibitors are often withheld in patients who develop active tuberculosis. However, withholding TNF-α inhibitors may lead to paradoxical reactions, and improvement with the resumption of these agents has been extensively reported [12]. In our patient, interrupting very active inflammation and granuloma formation by TNF blockade may have discouraged the exaggerated immune response, which is important to the development of IRIS, while enhancing bacteria killing by maximizing drug penetration into the granulomas [13]. Our case demonstrates that adalimumab is not only effective in controlling IRIS, but that it does so without interfering with mycobacterial or HIV infection control.
Other immunomodulatory agents such as hydroxychloroquine, pentoxifylline, and thalidomide have been reported as therapies for IRIS in anecdotal case reports. Pentoxifylline, a nonspecific phosphodiesterase inhibitor and TNF inhibitor, has been used in treating IRIS and shown to be safe in TB-HIV coinfected patients [14]. Thalidomide has shown in vitro TNF-inhibiting effects and has been used successfully for steroid refractory immune reconstitution syndrome. However, there is limited evidence of efficacy in severe disease, and lack of potency seems a major limitation. For example, COX inhibitors have TNF-blocking effects and can decrease macrophage activation, but they are ineffective for the treatment of chronic granulomatous inflammatory conditions. In addition, drug-to-drug interaction with antituberculous medications is important to consider [15].
In conclusion, TNF antagonism is an effective alternative strategy for steroid-refractory TB-IRIS in HIV-infected patients. Our case highlights the therapeutic effect on IRIS without negative impact on immunological and virological control of HIV infection in short-term follow-up. Long-term adverse reactions, however, need to be carefully monitored. This warrants additional studies to explore the role of anti-TNF agents as salvage therapy in IRIS.
Acknowledgments
Potential conflicts of interest. All authors: no reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Pepper DJ, Marais S, Maartens G et al. Neurologic manifestations of paradoxical tuberculosis-associated immune reconstitution inflammatory syndrome: a case series. Clin Infect Dis 2009; 48:e96–107. [DOI] [PubMed] [Google Scholar]
- 2. Lanzafame M, Vento S. Tuberculosis-immune reconstitution inflammatory syndrome. J Clin Tuberc Other Mycobact Dis 2016; 3:6–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. Department of Health and Human Services; Available at: http://aidsinfo.nih.gov/contentfiles/lvguidelines/AdultandAdolescentGL.pdf. Accessed 16 September 2017. [Google Scholar]
- 4. Lee HS, Lee Y, Lee SO et al. Adalimumab treatment may replace or enhance the activity of steroids in steroid-refractory tuberculous meningitis. J Infect Chemother 2012; 18:555–7. [DOI] [PubMed] [Google Scholar]
- 5. Gaube G, De Castro N, Gueguen A et al. Treatment with adalimumab for severe immune reconstitution inflammatory syndrome in an HIV-infected patient presenting with cryptococcal meningitis. Med Mal Infect 2016; 46:154–6. [DOI] [PubMed] [Google Scholar]
- 6. Sitapati AM, Kao CL, Cachay ER et al. Treatment of HIV-related inflammatory cerebral cryptococcoma with adalimumab. Clin Infect Dis 2010; 50:e7–10. [DOI] [PubMed] [Google Scholar]
- 7. Blackmore TK, Manning L, Taylor WJ, Wallis RS. Therapeutic use of infliximab in tuberculosis to control severe paradoxical reaction of the brain and lymph nodes. Clin Infect Dis 2008; 47:e83–5. [DOI] [PubMed] [Google Scholar]
- 8. Richaud C, Ghosn J, Amazzough K et al. Anti-tumor necrosis factor monoclonal antibody for steroid-dependent TB-IRIS in AIDS. AIDS 2015; 29:1117–9. [DOI] [PubMed] [Google Scholar]
- 9. Hsu DC, Faldetta KF, Pei L et al. A paradoxical treatment for a paradoxical condition: infliximab use in three cases of mycobacterial IRIS. Clin Infect Dis 2016; 62:258–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Nast A, Sporbeck B, Rosumeck S et al. Which antipsoriatic drug has the fastest onset of action? Systematic review on the rapidity of the onset of action. J Invest Dermatol 2013; 133:1963–70. [DOI] [PubMed] [Google Scholar]
- 11. Ravimohan S, Tamuhla N, Steenhoff AP et al. Immunological profiling of tuberculosis-associated immune reconstitution inflammatory syndrome and non-immune reconstitution inflammatory syndrome death in HIV-infected adults with pulmonary tuberculosis starting antiretroviral therapy: a prospective observational cohort study. Lancet Infect Dis 2015; 15:429–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wallis RS, van Vuuren C, Potgieter S. Adalimumab treatment of life-threatening tuberculosis. Clin Infect Dis 2009; 48:1429–32. [DOI] [PubMed] [Google Scholar]
- 13. Wallis RS. Reconsidering adjuvant immunotherapy for tuberculosis. Clin Infect Dis 2005; 41:201–8. [DOI] [PubMed] [Google Scholar]
- 14. Wallis RS, Johnson JL, Okwera A et al. Pentoxifylline in human immunodeficiency virus- positive tuberculosis: safety at 4 years. J Infect Dis 1998; 178:1861. [DOI] [PubMed] [Google Scholar]
- 15. Wallis RS, Hafner R. Advancing host-directed therapy for tuberculosis. Nat Rev Immunol 2015; 15:255–63. [DOI] [PubMed] [Google Scholar]