Abstract
Among the diverse clades of highly pathogenic avian influenza (HPAI) H5N1 viruses of the goose/Guangdong lineage, only a few have been able to spread across continents: clade 2.2 viruses spread from China to Europe and into Africa in 2005–2006, clade 2.3.2.1 viruses spread from China to Eastern Europe in 2009–2010 and clade 2.3.4.4 viruses of the H5Nx subtype spread from China to Europe and North America in 2014/2015. While the poultry trade and wild-bird migration have been implicated in the spread of HPAI H5N1 viruses, it has been proposed that robust virus-shedding by wild ducks in the absence of overt clinical signs may have contributed to the wider dissemination of the clade 2.2, 2.3.2.1 and 2.3.4.4 viruses. Here we determined the phenotype of two divergent viruses from clade 2.3.2.1, a clade that spread widely, and two divergent viruses from clade 2.3.4, a clade that was constrained to Southeast Asia, in young (ducklings) and adult (juvenile) mallard ducks. We found that the virus-shedding magnitude and duration, transmission pattern and pathogenicity of the viruses in young and adult mallard ducks were largely independent of the virus clade. A clade-specific pattern could only be detected in terms of cumulative virus shedding, which was higher with clade 2.3.2.1 than with clade 2.3.4 viruses in juvenile mallards, but not in ducklings. The ability of clade 2.3.2.1c A/common buzzard/Bulgaria/38 WB/2010-like viruses to spread cross-continentally may, therefore, have been strain-specific or independent of phenotype in wild ducks.
Keywords: influenza virus, duck, host, transmission
Introduction
Highly pathogenic avian influenza (HPAI) H5N1 of the goose/Guangdong lineage was first recognized in 1996 during an outbreak in domestic geese in southern China [1], which was followed by an outbreak of a reassortant HPAI H5N1 virus in 1997 in Hong Kong [2–4]. After several years of low-level circulation in aquatic poultry in southern China, HPAI H5N1 re-emerged with outbreaks in domestic chickens and was associated with human infections from 2003 onwards [5–9]. Due to their rapid diversification, HPAI H5N1 viruses have been classified into clades (0 to 9) and sub-clades on the basis of their haemagglutinin (HA) gene sequence [10], and also by genotypes according to their internal gene constellations [11–13].
During spring 2005 an outbreak of HPAI H5N1 was observed in wild birds at Qinghai Lake in western China and subsequently classified as clade 2.2 (various genotypes) [14–16]. From late 2005 the virus spread westward through Russia, Kazakhstan, Mongolia and Turkey to Eastern and Western Europe, the United Kingdom, the Middle East, and eventually Africa [10, 16–19]. This outbreak was eventually stamped out/ceased, with the notable exception of Egypt, where clade 2.2 viruses have become entrenched in poultry and have further diversified [16, 20]. The manner of cross-continental transmission of clade 2.2 virus remains controversial in large part due to a lack of sustained surveillance activities immediately before the first outbreaks in 2005 in many of the affected countries, but virologically and epizootologically, the outbreak in Qinghai Lake (2005) has been linked to European and African HPAI H5N1 cases [21–24]. Wild-bird migration and poultry movement through trade have both been implicated in the cross-continental spread of the pathogen [25–29]. Among wild migratory birds, mallard ducks have been identified as possible vectors in the spread of HPAI H5N1 [30–33]. From 2008 onwards a new subclade of HPAI H5N1, clade 2.3.2.1, gained high prevalence in China and Southeast Asia [10, 34], subsequently expanding from China to Mongolia, Russia and Eastern Europe in early 2010 [35–37], and also to South Korea and Japan and into Southeast Asia (http://www.oie.int/en/animal-health-in-the-world/update-on-avian-influenza/2016/). Clade 2.3.2.1 viruses have been particularly associated with wild birds, suggesting that some inherent characteristics of this virus subgroup may enhance its ability to establish and spread in some specie(s) of the wild-bird host population [10]. This is predicated on the observation that clade 2.3.4 viruses were also widespread in Southeast Asia over the same period of time (2008–2010), but appeared to be less associated with wild birds and did not expand outside of the region [37, 38].
From 2009 onwards, reassortment events of HPAI H5N1 with LPAI viruses were detected in China, resulting in the generation of multiple subtypes, including H5N2, H5N3, H5N6 and H5N8 [39–42]. Subtypes H5N6 and H5N8 of what were now termed clade 2.3.4.4 viruses spread from China into other Asian countries in 2013/2014 [43, 44]. Further dissemination to North America and Europe followed [45–48] and additional reassortment events were documented in North America with North American LPAI lineages [45]. HPAI H5Nx viruses of clade 2.3.4.4 were detected in wild migratory birds in North America, Europe and Asia [49–51].
In this study we examined co-circulating clade 2.3.2.1 and clade 2.3.4 viruses isolated in 2008–2010 using the mallard duck model. We aimed to determine whether a general replicative advantage exists, in the absence of severe symptoms, for clade 2.3.2.1 viruses that may be consistent with their wider geographic dispersal.
Results
Phylogenetic relationship between four HPAI H5N1 viruses and residue markers of interest
Two viruses each from clades 2.3.4 and 2.3.2.1 (both progeny of genotype Z.3) were selected for this study: A/chicken/Hong Kong/AP156/2008 (AP156/2.3.4), A/chicken/Lao/LH2/2010 (LH2/2.3.4.1), A/barn swallow/Hong Kong/1161/2010 (HK1161/2.3.2.1b) and A/common buzzard/Bulgaria/38 WB/2010 (Bulg/2.3.2.1c). Bulg/2.3.2.1c had been isolated from a dead wild buzzard in Eastern Europe in early 2010 and represents viruses that spread out of Asia during that time [10, 36]. Within clade 2.3.4 two divergent viruses were chosen: the HA segment of AP156/2.3.4 is situated closer to the root of the clade 2.3.4 tree than LH2/2.3.4.1, which has been classified as belonging to sub-clade 2.3.4.1 [52, 53]. Subsequent to our virus selection, clade 2.3.2.1 sub-categories a, b, and c were recognized in acknowledgement of their divergent evolution. Two relatively divergent viruses of clade 2.3.2.1 were chosen: HK1161/2.3.2.1b serves as the type strain for sub-clade 2.3.2.1b, while Bulg/2.3.2.1c is closely related to the type strain of sub-clade 2.3.2.1c, A/Hong Kong/6841/2010 [54].
The multi-basic cleavage site between HA1 and HA2 contained six basic amino acids immediately preceding the cleavage position for HK1161/2.3.2.1b and five for the other three viruses. We examined the HA protein for the presence of N-linked glycosylation motifs: using the N-{P}-[S/T]-{P} motif as the indicator of a potential N-linked glycosylation site, we detected seven sites in AP156/2.3.4 and Bulg/2.3.2.1c (H5 numbering with A/Vietnam/1203/2004 as the reference strain: positions 10 or 11, 23, 140, 165, 286, 484, 543), and eight sites in LH2/2.3.4.1 and HK1161/2.3.2.1b (positions 10 or 11, 23, 140, 165, 273, 286, 484, 543).
In the HA receptor binding site we identified the following molecular markers: 133A and 189R in AP156/2.3.4, HK1161/2.3.2.1b and Bulg/2.3.2.1c (133S and 189K for LH2/2.3.4.1), and 188I in LH2/2.3.4.1 (188T for the three other viruses). All four viruses harboured H275 and R293 in NA, indicating sensitivity to oseltamivir and an NA stalk deletion of 20 residues (position 49–68). In the M1 protein all four viruses harboured 30D and 215A, and in M2 the 27A determinant was present in AP156/2.3.4, indicating resistance to adamantanes. LH2/2.3.4.1 and HK1161/2.3.2.1b harboured truncated PB1-F2 open reading frames with 25 and 57 residues, respectively. AP156/2.3.4 harboured the 66D marker in PB1-F2. All but LaoLH2/2.3.4.1 (25 residue length) harboured 51M and 56V. The full-length PB1-F2 proteins of Bulg/2.3.2.1c and AP156/2.3.4 harboured 87E. The open reading frame for PA-X was present in all four viruses, predicting a protein with a 252-residue length. In the NS1 protein we observed a D92E substitution for HK1161/2.3.2.1b and a five-residue deletion (positions 80–84) in AP156/2.3.4, LH2/2.3.4.1 and HK1161/2.3.2.1b. The C-terminal motif at residue positions 227–230 was GSEV for AP156/2.3.4 and ESEV for LH2/2.3.4.1, HK1161/2.3.2.1b and Bulg/2.3.2.1c.
Virulence and replication of four HPAI H5N1 in inoculated ducklings
Young (3–4-week-old) mallard ducks (ducklings) were inoculated with 104 EID50 (50 % embryo infectious dose) of virus via the natural route, as described in Methods. All inoculated ducklings became productively infected as determined by virus shedding and showed clinical signs, while the survivors showed sero-conversion.
Clinical symptoms including death were observed significantly earlier for HK1161/2.3.2.1b-inoculated animals (2.8 days post-inoculation (p.i.)) compared to Bulg/2.3.2.1c-inoculated animals (4.3 days p.i.; P=0.004) and LH2/2.3.4.1-inoculated animals (4.5 days p.i.; P=0.005). Only one animal inoculated with AP156/2.3.4 showed clinical signs (4 days p.i.; not significantly different from any other group) (Table 1). All ducklings inoculated with Bulg/2.3.2.1c or LH2/2.3.4.1 developed conjunctivitis (4/4), as did one inoculated with AP156/2.3.4 (1/4). Neurological signs (ataxia, seizures, disorientation) developed in five of 20 inoculated ducklings, and were present in at least one bird in each virus group. Overall, 100 % of donor birds inoculated with HK1161/2.3.2.1b and 75 % of donor birds inoculated with LH2/2.3.4.1, succumbed to infection, while one mallard inoculated with AP156/2.3.4 died, and all birds inoculated with Bulg/2.3.2.1c survived (Tables 1 and 2).
Table 1. Virulence and transmission of four HPAI H5N1 viruses in young mallard ducks.
| Duck group* | Challenge virus† | H5 clade | Morbidity/ mortality‡ | Mean day of shedding onset§ | Mean day of symptom onset|| | Day of death¶ | Mean shedding duration# | Mean peak titre** | Cumulative mean virus shedding†† | HI titre‡‡ | Productively. infected |
|---|---|---|---|---|---|---|---|---|---|---|---|
| D | HK1161 | 2.3.2.1b | 25/100 | 1.0±0.0 | 2.8±0.3 | 2, 3, 3, 4 | 2.0±0.4 | 7.5±0.1 (3) | 11.4±2.5 | na | 4/4 |
| Bulg38WB | 2.3.2.1c | 100/0 | 1.0±0.0 | 4.3±0.3 | –, –, –, – | 7.0±0.5 | 4.8±0.5 (3) | 26.3±2.8 | 55±15 (4/4) | 4/4 | |
| LaoLH2 | 2.3.4.1 | 100/75 | 1.0±0.0 | 4.5±0.3 | 5, 5, 7, – | 5.1±0.7 | 6.0±0.4 (3) | 24.2±2.2 | 80 (1/1) | 4/4 | |
| HKAP156 | 2.3.4 | 33/33 | 2.0±0.7 | 4 | 7, –, – | 3.8±2.1 | 2.6±1.0 (3) | 9.4±2.1 | 53±13 (3/3) | 3/3 | |
| DC | HK1161 | 2.3.2.1b | 50/100 | 2.3±0.3 | 4.5±0.6 | 3, 4, 5, 7 | 2.5±0.7 | 6.1±1.4 (5) | 12.8±3.0 | na | 4/4 |
| Bulg38WB | 2.3.2.1c | 100/25 | 3.0±0.7 | 6.5±0.6 | 9, –, –, – | 7.6±0.7 | 4.9±0.3 (7) | 29.4±1.2 | 25±15 (2/3) | 4/4 | |
| LaoLH2 | 2.3.4.1 | 75/75 | 2.5±0.5 | 6.0±0.4 | 5, 8, 8, – | 4.8±0.8 | 5.1±0.3 (5) | 19.7±2.1 | 40 (1/1) | 4/4 | |
| HKAP156 | 2.3.4 | 25/0 | 3.7±0.3# | 3 | –, –, –, – | 1.7±0.6 | 1.6±0.9 (5) | 4.0±2.0 | 40±0 (2/4) | 3/4 | |
| AC | HK1161 | 2.3.2.1b | 25/100 | 3.3±0.5 | 5.3±0.5 | 4, 6, 6, 6 | 2.3±0.6 | 5.5±0.7 (6) | 9.9±1.7 | na | 4/4 |
| Bulg38WB | 2.3.2.1c | 75/25 | 4.0±1.0# | 10.5±2.5 | 9, –, –, – | 5.1±1.8 | 5.0±0.5 (9) | 18.9±6.4 | 170±150 (2/3) | 3/4 | |
| LaoLH2 | 2.3.4.1 | 50/50 | 5.3±1.2 | 7.8±0.3 | 10, 14, –, – | 3.4±1.1 | 4.4±0.6 (9) | 15.1±5.0 | 40 (1/2) | 4/4 | |
| HKAP156 | 2.3.4 | 0/0 | 5.3±0.3# | na | –, –, –, – | 2.3±1.2 | 1.6±0.9 (7) | 4.8±2.3 | 20 (1/4) | 3/4 | |
| D | HK1161 Bulg38WB | 2.3.2.1 | 63/50 | 1.0±0.0 | – | – | 4.5±1.0 | 6.0±0.6 (3) | 18.9±3.3 | – | 8/8 |
| LaoLH2 HKAP156 | 2.3.4 | 63/50 | 1.5±0.4 | – | – | 3.9±1.0 | 4.3±0.8 (3) | 16.8±3.1 | – | 7/7 | |
| DC | HK1161 Bulg38WB | 2.3.2.1 | 75/63 | 2.6±0.4 | – | – | 5.1±1.1 | 4.8±0.7 (5) | 21.1±3.5 | – | 8/8 |
| LaoLH2 HKAP156 | 2.3.4 | 50/38 | 3.0±0.4 | – | – | 2.9±0.8 | 3.4±0.8 (5) | 11.9±3.3 | – | 7/8 | |
| AC | HK1161 Bulg38WB | 2.3.2.1 | 50/63 | 3.6±0.5 | – | – | 3.7±1.0 | 3.8±1.3 (9) | 14.4±3.5 | – | 7/8 |
| LaoLH2 HKAP156 | 2.3.4 | 25/25 | 5.3±0.6 | – | – | 2.3±0.7 | 2.1±0.7 (7) | 9.9±3.2 | – | 7/8 |
*D, donor (inoculated) animals; DC, direct-contact animals; AC, aerosol-contact animals.
†Abbreviations as in Methods.
‡In per cent; morbidity as detected via conjunctivitis, depression, or neurological symptoms (ataxia, seizure, disorientation); some animals died/were found dead without symptoms having been observed.
§Mean day p.i. ± standard error. Limit of detection was 0.75 log10 EID50 ml−1. # one animal in the group did not shed.
||Mean day p.i. ± standard error. For mean day of symptom onset, death was included as a symptom.
¶Either found dead in cage or euthanized due to moribund state.
#Shown are days ± standard error for oropharyngeal shedding. Swabs were titrated every day or every other day; we considered that a bird that shed from day 1 to day 5 but not on day 7 and with no data for day 6 shed 4.5 days. Birds that did not shed were excluded from the shedding duration calculation.
**In log10 EID50 ml−1. Limit of detection was 0.75 log10 EID50 ml−1. (Day of peak titre p.i.)
††Cumulative mean virus shedding over time (area under the curve) as log10 EID50 of oropharyngeal swabs.
‡‡Mean haemagglutination inhibition titre to homologous antigen ± standard error (animals with HI titre ≥10/surviving animals).
§§By virus shedding and/or seroconversion.
Table 2. Virulence and replication of four HPAI H5N1 viruses in adult mallards.
| Challenge virus* | No. ducks | Clade | Mortality/morbidity† | Mean day of shedding onset‡ | Mean day of symptom onset§ | Day of death|| | Mean shedding duration¶ | Mean shedding duration until death¶ | Mean peak titre## | Cumulative mean virus shedding** | HI titre†† | Productively infected‡‡ |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HK1161 | 4 | 2.3.2.1b | 75/100 | 1.0±0.0 | 4.8±0.6 | 4, 6, 8, – | 6.0±1.2 | 5.0±1.0 | 4.6±0.5 (5) | 21.1±3.3 | 15 (1/1) | 4/4 |
| Bulg38WB | 4 | 2.3.2.1c | 0/50 | 1.0±0.0 | 4.5±0.5 | –, –, –, – | 4.8±0.5 | na | 4.3±0.5 (1) | 17.1±1.7 | 43±14 (4/4) | 4/4 |
| LaoLH2 | 4 | 2.3.4.1 | 0/0 | 1.0±0.0 | na | –, –, –, – | 3.8±0.8 | na | 3.5±0.5 (1) | 11.5±3.9 | 60±12 (4/4) | 4/4 |
| HKAP156 | 3 | 2.3.4 | 0/0 | 1.0±0.0# | na | –, –, – | 2.5±0.4 | na | 2.0±1.0 (1) | 4.7#±2.3 | 73±7 (3/3) | 3/3 |
| HK1161 Bulg38WB | 8 | 2.3.2.1 | 75/38 | 1.0±0.0 | – | – | 5.4±0.6 | – | 3.5±0.4 (5) | 19.1±1.9 | – | 8/8 |
| LaoLH2 HKAP156 | 7 | 2.3.4 | 0/0 | 1.0±0.0# | – | – | 3.3±0.6 | – | 3.3±0.4 (1) | 8.6±2.7 | – | 7/7 |
*Abbreviations as in Methods.
†In per cent; morbidity as detected via conjunctivitis, depression, or neurological symptoms (ataxia, seizure, disorientation). Some animals died/were found dead without symptoms having been observed.
‡Mean day p.i. ± standard error. Oropharyngeal shedding. Limit of detection was 0.75 log10 EID50 ml−1. #One animal did not shed.
§Mean day p.i. ± standard error. For mean day of symptom onset, death was included as a symptom.
||Either found dead in cage or euthanized due to moribund state.
¶Shown are mean days ± standard error of oropharyngeal shedding. Swabs were titrated every day or every other day; we considered that a bird that shed from day 1 to day 5 but not on day 7 and with no data for day 6 shed 4.5 days. The bird in the HKAP156 group that did not shed was excluded from the shedding duration calculation. na, not applicable.
##In log10 EID50 ml−1. Limit of detection was 0.75 log10 EID50 ml−1. (Day of peak titre p.i.)
**Cumulative mean virus shedding over time (area under the curve) as log10 EID50 of oropharyngeal swabs.
††Mean haemagglutination inhibition titre to homologous antigen ± standard error (animals with HI titre ≥10/surviving animals).
‡‡By virus shedding and/or seroconversion.
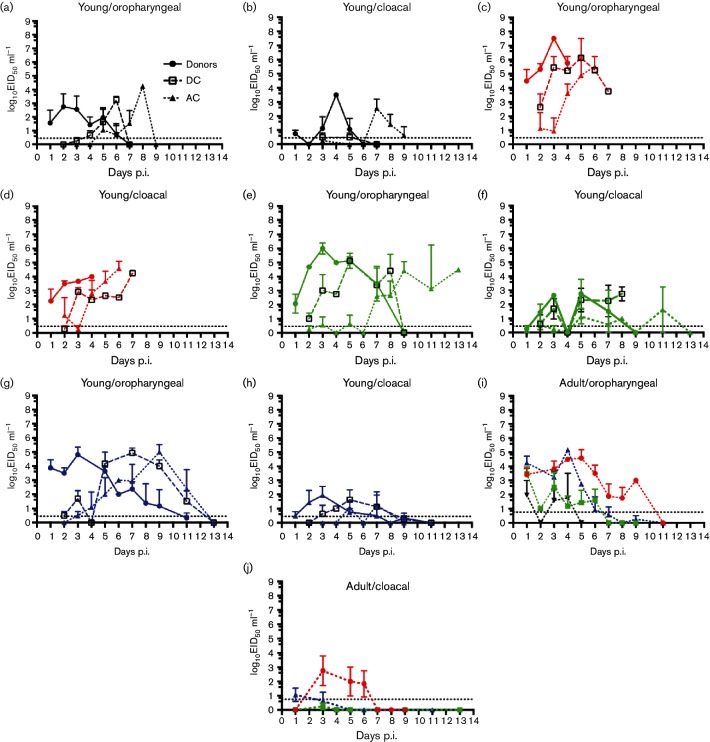
Oropharyngeal virus shedding was higher than cloacal shedding in all groups, although bird D-4 of the AP156-shedding animals showed higher titres in the cloacal sample (Fig. 1). There was no discernable difference in the relative magnitude of oropharyngeal and cloacal shedding between any of the viruses. Oropharyngeal viral titres were used in all comparisons of virus titres and shedding duration (Fig. 1).
Fig. 1.
Shedding patterns of four HPAI H5N1 viruses in young and juvenile mallards. Young (a–h) or juvenile (i, j) donor mallards were inoculated with 104 EID50 of virus via the natural route. (a-h) Direct-contact mallards and aerosol-contact mallards were introduced to donors 24 h p.i. (a-h). Virus titres in oropharyngeal swabs (a, c, e, g, i) and cloacal swabs (b, d, f, h, j) are shown. Legend in (a) applies to (a–h). Virus groups are coloured as: black – HKAP156/2.3.4; red – HK1161/2.3.2.1b; green – LaoLH2/2.3.4.1; blue – Bulg/2.3.2.1c.
The mean peak titres in all virus groups were reached 3 days p.i., with HK1161/2.3.2.1b having significantly higher titres than any other group (P-values 0.008–0.026) (Table 1). Shedding onset (oropharyngeal) was rapid with virus detected 1 day p.i. and virus replication apparent by 2 days p.i. for all groups (Fig. 1). Bulg/2.3.2.1c- and LH2/2.3.4.1-inoculated birds shed virus for similar periods of time. Bulg/2.3.2.1c-inoculated birds shed virus significantly longer than those inoculated with HK1161/2.3.2.1b or AP156/2.3.4 (P-values 0.003 and 0.01, respectively). LH2/2.3.4.1-inoculated birds shed virus for significantly longer than HK1161/2.3.2.1b-inoculated birds (P-value 0.007), with the difference in shedding duration compared to AP156/2.3.4 not reaching significance (Table 1).
Overall, Bulg/2.3.2.1c and LH2/2.3.4.1 showed similar duration of virus shedding and peak virus titres in the inoculated (donor) mallards. HK1161/2.3.2.1b- and AP156/2.3.4-infected birds shed for a shorter duration and had a lower peak titre (Table 1). Using cumulative mean virus shedding over time as a measure of virus produced, we found that Bulg/2.3.2.1c and LH2/2.3.4.1 donor mallards produced significantly more virus during the course of infection than either HK1161/2.3.2.1b or AP156/2.3.4 (P-values 0.003–0.009).
All surviving ducklings exhibited haemagglutination inhibition (HI) titres >10 using chicken red-blood cells (CRBCs) and homologous antigen with no differences between groups (Table 1).
Overall, a clade-specific pattern of virus shedding or clinical symptoms was not apparent in inoculated young (donor) mallards.
Direct contact and airborne transmission of four HPAI H5N1 viruses in ducklings
Mallard ducks have been implicated in the cross-continental dissemination of HPAI H5N1 during 2005–2006 and 2009–2010 [25, 26, 28, 29], requiring multiple rounds of transmission between birds. Transmission could have been mediated through various modes: direct contact or indirect contact with infected animals, via shared food and water, through fomites, droplets, or aerosols. In order to determine whether the transmission dynamics differed between the viruses under investigation, two different modes of transmission were assessed: direct contact (including shared food and water) and airborne contact (food and water not shared; birds physically separated but within the same airspace).
On 1 day p.i. one donor mallard was placed with one direct-contact and one airborne-contact mallard, with four repetitions for each virus (four donors, four direct contacts and four airborne contacts in total for each virus), as described in Method.
All four HPAI H5N1 viruses transmitted to direct-contact animals, with only one direct-contact mallard in the AP156/2.3.4 group remaining uninfected as determined by virus shedding, clinical signs and seroconversion (Table 1, Fig. 1).
Additionally, all four HPAI H5N1 viruses transmitted to airborne-contact animals, with only one airborne-contact mallard in the AP156/2.3.4 group and one airborne-contact mallard in the Bulg/2.3.2.1c group remaining uninfected, as determined by virus shedding, clinical signs and seroconversion (Table 1, Fig. 1).
Clinically, in the direct-contact animals, all viruses caused disease comparable to that caused in their respective inoculated donor birds. Shedding onset was rapid post-contact (1.3–2.7 days), though it was significantly delayed for AP156/2.3.4 compared to HK1161/2.3.2.1b (P-value 0.02) (Table 1).
The duration and magnitude of viral shedding was consistent between donor mallards and direct-contact mallards for each virus. Consistent with this observation, among the direct-contact mallards, Bulg/2.3.2.1c was shed for a significantly longer duration than the other three viruses [P-values of 0.001 (HK1161/2.3.2.1b), 0.03 (LH2/2.3.4.1) and 0.0002 (AP156/2.3.4)]. LH2/2.3.4.1 was shed for significantly longer than AP156/2.3.4 (P-value 0.007) (Table 1).
Among the direct-contact mallards, the AP156/2.3.4-group had statistically significantly lower mean peak titres than the LH2/2.3.4.1- and Bulg/2.3.2.1c-groups (P-values of 0.01 and 0.02, respectively) (Table 1).
Not all surviving direct-contact mallards seroconverted by HI using homologous antigen: half of the AP156/2.3.4 direct contacts did not seroconvert, and of these one did not shed detectable virus and one shed virus at one time point at the limit of detection. One of three surviving Bulg/2.3.2.1c-direct-contact mallards did not seroconvert, even though this animal shed intermediate amounts of virus. Among the mallards that did seroconvert, there were no significant differences in HI titres between donor mallards and direct contact or between different virus groups among the direct-contact mallards (Table 1).
Overall, no clade-specific pattern of virus replication could be detected between the four viruses. Additionally, when using combined datasets to further investigate any differences between clades 2.3.2.1 and 2.3.4 in the direct-contact animals, no differences in the magnitude or duration of virus shedding or cumulative virus shedding over time were observed.
In the airborne-contact animals the clinical symptoms and mortality rates remained largely consistent with the direct-contact and donor groups, with little difference in morbidity and mortality (Table 1). The onset of symptoms occurred significantly faster in the HK1161/2.3.2.1b group compared to the LH2/2.3.4.1 group (P-value 0.04) and the Bulg/2.3.2.1c group (P-value 0.004), while the AP156/2.3.4-group did not display any symptoms (Table 1).
In terms of shedding duration there were no significant differences between the four airborne-contact virus groups and their matched groups among direct-contact or donor mallards, but also no significant differences of shedding duration between the different virus groups (Table 1). The significant differences observed in the donor and direct-contact groups were hence lost. There were no significant differences of peak virus titres between the airborne-contact groups and their donor and direct-contact groups (Table 1). Significantly lower mean peak titres were observed in the AP156/2.3.4 airborne contacts compared to HK1161/2.3.2.1b airborne contacts (P-value of 0.03) and Bulg/2.3.2.1c airborne contacts (P-value of 0.03), consistent with the patterns observed in the donor and direct-contact mallards.
When using combined datasets to further investigate any significant differences between clades 2.3.2.1 and 2.3.4 in the airborne-contact animals, no differences in magnitude or duration of virus shedding, or in cumulative virus shedding over time were observed (Table 1).
Not all surviving airborne-contact mallards seroconverted by HI using CRBC and homologous antigen (Table 1). Those animals that did not seroconvert tended to shed low amounts of virus or started to shed late during the experiment and therefore may have had insufficient time to seroconvert at the end of the experiment. Among the mallards that did seroconvert, there were no significant differences in HI titres between airborne contacts and donors or direct contacts, or between different virus groups among the airborne-contact mallards (Table 1).
Virulence and replication of four HPAI H5N1 viruses in juvenile mallards
Surveillance studies on LPAI viruses have identified first-season birds to be particularly receptive to avian influenza virus (AIV) infection, although changes of the dominant AIV subtype between different years and the infection of older adult birds have also been observed [55–58]. Mallards fledge at 50–60 days post-hatch and are considered juvenile until about 14 months of age. First-season birds or mallards during their first migration would therefore be considered juveniles.
We assessed the phenotypes of the four distinct HPAI H5N1 viruses of this investigation in AIV-naïve 3–4 month-old mallard ducks. These juvenile mallards were inoculated with 104 EID50 via the natural route as described in Methods. Overall, the clinical signs in juvenile mallards were milder than in ducklings and symptoms could only be detected in the clade 2.3.2 virus groups: three of four ducks inoculated with HK1161/2.3.2.1b developed conjunctivitis, two animals developed neurological symptoms and 75 % succumbed to the infection or were euthanized due to moribund state (Table 2). These mallards showed symptoms on average 4.8 days p.i. and died on average 6 days p.i. Of the ducks inoculated with Bulg/2.3.2.1c, half developed conjunctivitis (4.5 days p.i.), but all animals had fully recovered by the end of the experiment (14 days) (Table 2). No morbidity was observed in the juvenile mallards inoculated with either AP156/2.3.4 or LH2/2.3.4.1.
Virus shedding was higher in oropharyngeal samples than in cloacal samples for all groups (Fig. 1(i,j)) and commenced early after inoculation for all virus groups (Table 2).
The mean peak titres of virus shedding were reached 1 day p.i. for all but the HK1161/2.3.2.1b-inoculated group, where the mean peak titre was reached 5 days p.i. (Table 2). There were no statistically significant differences between the mean peak titres of each virus group.
The average duration of virus shedding (oropharyngeal) was the longest for those mallards inoculated with HK1161/2.3.2.1b (6 days) and statistically similar for Bulg/2.3.2.1c (4.8 days) and LH2/2.3.4.1 (3.8 days). AP156/2.3.4 was shed on average for 2.5 days, which was statistically shorter than Bulg/2.3.2.1c (P-value 0.04) (Table 2).
Cumulative mean virus shedding was significantly greater in the two clade 2.3.2.1 virus groups, HK1161/2.3.2.1b and Bulg/2.3.2.1c, compared to the AP156/2.3.4 group (P-values 0.007 and 0.013, respectively), but with no significant difference observed compared to the LH2/2.3.4.1-group (Table 2).
We then examined virus shedding according to clade using combined datasets and found no statistical differences in the mean peak titres between the clade 2.3.2.1-inoculated and the clade 2.3.4-inoculated juvenile mallards. However, the duration of virus shedding was significantly longer in mallards inoculated with clade 2.3.2.1 viruses (5.4 days) than in clade 2.3.4 viruses (3.3 days; P=0.038), as was cumulative virus shedding (P-value=0.006). All survivors exhibited HI titres >10 using CRBC and homologous antigens (Table 2). There were no statistically significant differences in HI titres between the four groups.
Comparison of virulence and virus replication observed in young and juvenile mallards
Both young and juvenile mallards were exposed to the same dose of virus (104 EID50). Clinically, ducklings displayed greater morbidity and mortality than juvenile mallards (Table S1, available in the online Supplementary Material).
Onset of virus shedding was rapid in both young and juvenile birds, with no significant differences observed (Tables 1 and 2). The mean peak titres (oropharyngeal) were significantly higher in ducklings inoculated with LH2/2.3.4.1 (P=0.007) and HK1161/2.3.2.1b (P=0.009) compared with juvenile mallards. This trend was not observed in ducklings inoculated with Bulg/2.3.2.1c and AP156/2.3.4 (Tables 1 and 2).
In terms of duration of virus shedding, ducklings inoculated with HK1161/2.3.2.1b shed for a significantly shorter period of time than the juvenile mallards (P-value 0.018), due to more rapid progression to death. Ducklings inoculated with Bulg/2.3.2.1c shed for a significantly longer period of time (P-value 0.021) compared to juvenile mallards (Tables 1 and 2; Fig. 1). LH2/2.3.4.1- or AP156/2.3.4-inoculated mallards showed no significant differences in shedding duration between young and juvenile birds (Table S1).
When comparing the cumulative virus shedding of adult and young donor mallards, ducklings inoculated with LH2/2.3.4.1 or Bulg/2.3.2.1c shed significantly more virus over time than juvenile mallards (P-values 0.039 and 0.038, respectively). There was no statistical difference in cumulative virus shedding in the AP156/2.3.4 or HK1161/2.3.2.1b groups (Table S1). We then examined virus shedding according to clade using combined datasets and found no statistical differences in the cumulative virus shedding between clade 2.3.2.1-inoculated and clade 2.3.4-inoculated young and juvenile mallards.
Discussion
The goose/Guangdong (Gs/Gd) lineage of HPAI H5N1 has become an extremely successful virus lineage since its genesis in the 1990s: different clades and sub-clades of HPAI H5N1 have disseminated over vast geographical distances and established stable host interactions with domestic waterfowl, with frequent spillover infections into poultry and wild birds [1, 12, 14, 38, 59].
To date, three large expansions of Gs/GD-HPAI H5N1 have been observed: clade 2.2 in 2006/2007, clade 2.3.2.1 in 2009/2010 and clade 2.3.4.4 from 2014/2015 [36, 38, 48, 60]. While these viruses have all been derived from the Gs/Gd-lineage HA, their genotypic constellations have been somewhat more complicated. Genotype Z (A/duck/Guangxi/50/01-like) viruses were the most predominant by the early 2000s, eventually giving rise to genotypes Z.1 (the HA clade 2.2 precursor) and Z.3 (precursor to clades 2.1 and 2.3.4) [61]. The 2.2 lineage further evolved through mutations and was sub-divided into clades 2.2 and 2.2.1 [62]. Clade 2.3.2.1 genotypes have also evolved into 2.3.2.1 a/b/c sub-clades and undergone multiple intra-2.3.2.1 reassortment events (such as in Vietnam in 2012, as described by Creanga et al. in 2013 [54]), as well as inter-clade reassortments, such as the 2.3.2.1/2.3.4.4 reassortants observed in Bangladesh in 2011–2014 [63]. A/buzzard/Bulgaria/38 WB/2010 (clade 2.3.2.1c) is a 2.3.2.1c virus harbouring an M-gene from an H5N1 clade 2.3.4 virus [64]. In addition, clade 2.3.4 viruses have further evolved into sub-sub-clades as well [62]. The 2.3.4.4 viruses have diversified through reassortment in multiple ways, including: (i) in North America in 2014–2015, where reassortment of clade 2.3.4.4 H5N8 viruses with avian viruses of the North-American lineage resulted in the generation of novel H5N2 viruses [65]; and (ii) in Europe in 2015–2017, where HPAI H5N8 viruses of clade 2.3.4.4 were generated through reassortment between Asian HPAI H5N8 viruses and Asian LPAI viruses [66]. Among the four viruses we used in the present study, A/buzzard/Bulgaria/38 WB/2010 (clade 2.3.2.1c) is actually a 2.3.2.1c virus harbouring an M-gene from H5N1 clade 2.3.4 [64], A/chicken/AP156/2008 (clade 2.3.4) contains a PB1 gene segment from H9N2 viruses [closest hit by Blast: A/chicken/Shandong/LY/2008(H9N2)], and the other two viruses harbour full 2.3.2.1 or 2.3.4 gene constellations [64].
In 2006, the HPAI H5N1 viruses of clade 2.2 were detected in Asia, Europe and Africa, and clade 2.2 became established in poultry in Egypt, disappearing from Europe and the remaining African countries [38]. In late 2009 to early 2010, clade 2.3.2.1c expanded from China to Siberia, Bangladesh, Nepal, Bhutan and eventually Eastern Europe, but not further [36, 60–63]. In August 2011 a second, seemingly independent introduction of clade 2.3.2.1c was detected in northern Iran [64]. Furthermore, clade 2.3.2.1c viruses also reached Java, Indonesia, by unknown means [65]. In 2014/2015 clade 2.3.4.4 viruses spread from China into other Asian countries, and to Europe and North America [43–48]. Outbreaks in North America and Europe seemed transient in 2014–2015, while various subtypes of clade 2.3.4.4 continued to circulate in China and Southeast Asia (EMPRES-i, http://empres-i.fao.org/, data recovered 22 March 2017, [66]). It is interesting to note that while the 2.3.4 viruses, represented by A/chicken/Hong Kong/AP156/2008 and A/chicken/Lao/LH2/2010 in this study, were localized geographically, descendants of this clade, specifically 2.3.4.4 viruses, have spread globally, aided by wild birds. Such an event is consistent with our conclusion that association with wild birds is a virus characteristic rather than a clade-specific phenomenon. It is unclear what drove the dissemination of the clade 2.3.4.4 viruses, but it is clear they have been a major influence on the impact of the Gs/Gd-lineage viruses. The circulation of clade 2.3.4.4 viruses, the majority of which did not contain the N1 gene segment predominant in the Gs/Gd-lineage HPAI H5N1 viruses, increased in China from 2010 to 2017. The Influenza Research database (https://www.fludb.org/brc/home.spg?decorator=influenza) contained 17 HA sequences of clade 2.3.4.4 H5 between 2008 and 2010 (all originating from China, all from H5N1 viruses), while 71 2.3.4.4 HA sequences were available for the 2011–2013 period (68 of them from China; 3 H5N1) and 464 were available for 2014–2017 (as of 22 March 2017; 154 of them from China; 15 H5N1 only). While it is risky to directly interpret the increase in 2.3.4.4 virus sequences in public databases as representing the actual increase in the virus in the field, it is clear that these viruses have been successful. Human cases of HPAI H5 caused by 2.3.4.4 HA viruses have all been of the H5N6 subtype, [62] and long-distance spread has been observed with H5N6 and H5N8 viruses. Both the increase in virus circulation and the changes in gene constellation may have favoured the geographical spread of clade 2.3.4.4 viruses.
In this study (carried out before 2.3.4.4 viruses spread outside Asia), we investigated the putative role of mallard ducks in the spread of one of the virus clades that has been spread intercontinentally, HPAI H5N1 clade 2.3.2.1. We hypothesized that clade 2.3.2.1 HPAI H5N1 viruses induce a phenotype in mallard ducks that would be (1) characterized by robust virus replication in the absence of overt symptoms, (2) universal for the clade and (3) readily distinguishable from the phenotype of a contemporary clade that did not spread beyond Asia (clade 2.3.4). Using two divergent viruses of clade 2.3.4 (LH2/2.3.4.1 and AP156/2.3.4) and two divergent viruses of clade 2.3.2.1 (Bulg/2.3.2.1c and HK1161/2.3.2.1b), we found that all four viruses were able to infect young (4-week-old) and adult (3-month-old) mallards efficiently. Pathogenicity varied greatly between the four viruses, from mildly pathogenic AP156/2.3.4 to lethal HK1161/2.3.2.1b, with ducklings experiencing more severe symptoms than juvenile mallards. A deletion in the stalk of the NA protein was present in all four viruses and has been associated with both adaptation to terrestrial poultry and increased virulence in mallards [67, 68]. This molecular signature has been a hallmark of Gs/Gd-lineage HPAI H5N1 viruses since the early 2000s [6, 38, 68].
Previous observational and experimental studies have implicated migratory waterfowl as possible vectors of HPAI H5N1 during long-distance spread [25–29, 69]. Using experimental infections, several wild waterfowl species have been shown to shed different HPAI H5N1 viruses robustly in the absence of overt clinical symptoms [70, 71]. It has been proposed that this phenotype (robust shedding and few-to-no clinical signs) was necessary for migratory waterfowl to be able to contribute to the cross-continental spread of HPAI H5N1 [27, 28, 37].
Of late, the extensive spread of clade 2.3.4.4 viruses has led to several investigations of virus shedding from mallard ducks: Kang et al. [72] determined the pathogenicity of three clade 2.3.4.4 H5N8 viruses, one clade 2.2 H5N1 virus and one clade 2.3.2.1 H5N1 virus in intranasally inoculated wild-captured mallard ducks [72]. Mallard ducks showed no mortality and little morbidity when challenged with any of the study viruses. Virus replication was detected in donor mallards and in direct-contact cage mates, with virus shedding higher from the trachea than the cloaca [72]. Zhao et al. [39] reported generally mild-to-intermediate symptoms in mallard ducks inoculated intranasally with H5N5 and H5N8. Viruses were shed both from the trachea and cloacally, with higher titres detected in tracheal swabs [39].
We found that in donor (inoculated) ducklings the peak titre, duration of virus shedding and cumulative virus shedding varied between the four study viruses, but that no clade-specific phenotype could be identified. In juvenile mallards we detected significant differences between clade 2.3.2.1 and clade 2.3.4 viruses in shedding duration and cumulative virus shedding. Clade-2.3.2.1-infected birds shed for longer, resulting in greater cumulative virus shedding than their clade-2.3.4-infected counterparts. Of note, the clade 2.3.2.1c virus that represents the strains capable of cross-continental spread (Bulg/2.3.2.1c) was consistently shed for a statistically longer duration than the other viruses in both adult and young donor (inoculated) mallards and also in young direct-contact mallards. Previous studies have detected shedding of HPAI H5N1 viruses from ducks for 2–5 days p.i. and sometimes for much longer, both in the absence of overt symptoms [70, 73]. In Hulse-Post et al. [74] A/mallard/Vietnam/16D/03 and A/chicken/Vietnam/48C/04 were shed for 17 days by experimentally inoculated ducks, but direct-contact transmission was only observed with A/mallard/Vietnam/16D/03 and not with A/chicken/Vietnam/48C/04. Therefore the duration of virus shedding was not the sole determinant for virus transmission in this instance [74].
The mode of transmission of HPAI H5N1 among migratory birds necessarily has a large effect on the propensity of these hosts to spread these viruses over large distances. LPAI transmission between wild waterfowl is thought to be largely mediated by the faecal–oral route [75]. However, HPAI H5N1 is predominantly shed via the respiratory system [70] and therefore airborne transmission in addition to shared food/water may be an important mode of transmission between wild waterfowl. We therefore examined the transmission of our four viruses of interest in both a direct-contact and an airborne-contact setting.
All four study viruses were transmitted to direct-contact mallards rapidly and efficiently, with clinical symptoms and virus shedding consistent with the donor groups for each virus. Not all surviving direct-contact mallards seroconverted, which was associated with low or no detectable viral replication (AP156/2.3.4), except for one animal in the Bulg38/2.3.2.1c direct-contact group, which showed intermediate levels of virus shedding. Among the mallards that did seroconvert, there were no significant differences in HI titres between direct-contact mallards and donor mallards.
In our setting, almost all airborne-contact mallards were productively infected. Clinical symptoms and virus shedding were consistent with donor and direct-contact groups. No significant differences in magnitude or duration of virus shedding, or in cumulative virus shedding over time, were observed between clades 2.3.2.1 and 2.3.4 in the airborne group. We conclude that all four viruses were well able to transmit to airborne contacts, causing disease comparable to that in donor and direct-contact animals.
In this study we hypothesized that highly related viruses, as defined by HA clade, would display a common phenotype; in this case ability or inability to be spread via mallards. While this is a possibility, it is also possible that mixed phenotypes are present in similar viruses within a clade. In this light, a limitation of our study was that only two divergent representative viruses of each clade were examined. We were able to identify only limited evidence of a clade-specific phenotype in juvenile mallards, but not in ducklings. In the context of potential transmission during bird migration, the virus phenotype in juvenile mallards rather than in ducklings may be more informative. Furthermore, the mallard duck species may not be the optimal model system for assessing the capability of clade 2.3.2.1 or other HPAI H5N1 viruses to spread from Asia to Europe: other duck species or migratory geese may be the preferred vector for this wild-bird-mediated transmission [76–78].
Further phenotype assessment of HPAI H5N1 viruses, particularly of those more frequently isolated from wild birds, is warranted to identify fitness markers in mallards and potentially in other wild waterfowl species. Comparative molecular analyses between pre-2014 2.3.4, 2014–2016 2.3.4.4 (that reached Europe, Africa and North America in 2014/2015) and 2.3.2.1c gene sequences were carried out in an attempt to link phenotypes of viruses spreading over long distances and molecular determinants, but no specific markers could be identified. Further studies of clade 2.2 viruses (that reached Europe and Africa in 2005/2006) and clade 2.3.4.4 viruses (that reached Europe, Africa and North America in 2014/2015 and 2016/2017) in comparison to other sub-clades circulating in Asia during 2005–2006 and 2014–2015, respectively, would be especially informative to better understand the factors involved in the potential for inter-continental spread of HPAI H5Nx strains by wild birds. Taken together, post-2014 2.3.4.4 HPAI H5 viruses have shown a high degree of reassortment and this ongoing evolution may indicate that the viruses have not yet reached a stable equilibrium with their host(s).
Methods
Viruses
Four HPAI H5N1 viruses were used: clade 2.3.2.1b virus A/barn swallow/Hong Kong/1161/2010 (HK1161/2.3.2.1b), clade 2.3.2.1c virus A/buzzard/Bulgaria/38 WB/2010 (Bulg/2.3.2.1c), clade 2.3.4 virus A/chicken/Hong Kong/AP156/2008 (AP156/2.3.4) and clade 2.3.4.1 virus A/duck/Lao/LH2/2.3.4.1/2010 (LH2/2.3.4.1). The viruses were propagated in 10-day-old embryonated chicken eggs. All experiments were carried out at the St Jude Children's Research Hospital (St Jude) enhanced biosafety level 3 (ABSL3+) facility approved by the US Department of Agriculture. Viruses were sequenced by the Hartwell Center for Bioinformatics and Biotechnology at St Jude, and analysed with BioEdit and mega software [79, 80]. The full genome sequences are available in GenBank under the following accession numbers: CY110851 to CY110858 for Bulg/2.3.2.1c; CY098304, CY098305, KF735635 to KF735640 for LH2/2.3.4.1; KC436130, KC357320, KF735641 to KF735646 for HK1161/2.3.2.1b; and KF735634 to KF735634 for AP156/2.3.4. Before infection, virus titre was determined by calculating the 50 % embryo infectious dose (EID50) by the method of Reed and Muench [81].
Assessment of virus pathogenicity and transmission in ducks
Mixed-sex mallard ducks (Anas platyrhynchos) were either purchased as 1-day-old hatchlings from Ideal Poultry (Cameron, TX, USA) or as fertilized eggs from Duckeggs.com (Corona, CA, USA), incubated, hatched, and brought up to the required age [3–4 weeks (ducklings) or 3–4 months (juvenile)] at the Animal Resource Center at St Jude Children’s Research Hospital. The ducks were wing-banded, and provided feed and water ad libitum. All animal experiments were approved by the Animal Care and Use Committee of St Jude and complied with institutional, National Institutes of Health and Animal Welfare Act policies and regulations. For all experiments, mallards were inoculated by instillation of 104 EID50 of virus in a total volume of 1 ml via the natural route (500 µL intranasal and intraocular; 500 µL oral). One day post-infection (p.i.), each inoculated (donor) bird was moved to a cage with one naïve duck [direct-contact (DC) birds], adjacent to a cage with one other naïve duck [airborne-contact (AC) birds]. Donors and DC birds were separated from AC birds by 7.5 cm (3 in.). For the adult mallard study, four donors were inoculated for each virus with the exception of AP156, which had three donors. For the studies in young birds we used four donors, four DC and four AC ducks for each virus. All birds were observed at least once daily for morbidity and mortality. Moribund state was defined as inability to feed or drink, or apparent neurological symptoms (tremors, ataxia, seizures, torticollis, disorientation). Cloacal and oropharyngeal swabs were collected daily (experiments in juvenile and ducklings) for 14 days p.i. All surviving ducks were bled 14–18 days p.i.
Virus titration
Virus in oropharyngeal and cloacal swabs was titrated in embryonated chicken eggs, and the log10 EID50 ml−1 was calculated by the method of Reed and Muench [81]. The lower limit of virus detection was 0.75 log10 EID50 ml−1. A value of zero (log101) was assigned to titres below the lower limit of detection.
Serology assays
Convalescent duck sera (sampled 14–18 days p.i.) were screened by ELISA and/or HI assay. ELISAs were performed by using the IDEXX FlockChek AI MultiS-Screen Ab Test kit according to the manufacturer’s instructions (Westbrook, ME, USA). For HI assays, duck sera were treated with receptor-destroying enzyme (Denka Seiken Co., Japan) overnight at 37 °C, heat inactivated at 56 °C for 30 min, diluted 1 : 10 with PBS and tested using 0.5 % packed CRBCs as previously described [82].
Statistical analyses
Statistically significant differences between experimental groups were determined using one-way ANOVA and Student’s t-test of means (unpaired, two tailed) with Graph Pad Prism version 5.03 or MS Office Excel version 14.3.9. P-value<0.05 was considered statistically significant.
Prediction of N-linked glycosylation
To determine N-linked glycosylation we used the ScanProsite server (http://prosite.expasy.org/prosite.html) on 8 June 2014 [83]. ScanProsite uses the motif N-{P}-S/T-{P}, where proline is not accepted in positions 2 and 4 and either serine or threonine is accepted in position 3, to detect potential N-linked glycosylation motifs.
Funding information
This study was supported by the National Institute of Allergy and Infectious Diseases, National Institutes of Health, Department of Health and Human Services under contract no. HHSN266200700005C, by ALSAC,and by the French Ministry of Agriculture.
Acknowledgements
We thank Patrick Seiler, Beth Little, Lisa Kercher and David Carey for assistance with animal work in the ABSL3+ laboratory. We thank Vani Shanker for editorial assistance and the Hartwell Center for Bioinformatics and Biotechnology at St Jude Children’s Research Hospital for assistance with the sequencing.
Conflicts of interest
The authors declare that there are no conflicts of interest.
Supplementary Data
Footnotes
Abbreviations: AC, aerosol contact; AIV, avian influenza virus; CBRC, chicken red-blood cell; D, donor; DC, direct contact; EID50, 50% embryo infectious dose; Gs/Gd, goose/Guangdong; HA, haemagglutinin; HI, haemagglutination inhibition; HPAI, highly pathogenic avian influenza; LPAI, low pathogenic avian influenza; p.i., post inoculation.
The GenBank[/EMBL/DDBJ] accession number for the influenza virus sequences are CY110851 to CY110858; CY098304, CY098305, KF735635 to KF735640; KC436130, KC357320, KF735641 to KF735646; and KF735634 to KF735634.
One supplementary table is available with the online Supplementary Material.
References
- 1.Xu X, Subbarao CNJ, Cox NJ, Guo Y. Genetic characterization of the pathogenic influenza A/Goose/Guangdong/1/96 (H5N1) virus: similarity of its hemagglutinin gene to those of H5N1 viruses from the 1997 outbreaks in Hong Kong. Virology. 1999;261:15–19. doi: 10.1006/viro.1999.9820. [DOI] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention (CDC) Update: isolation of avian influenza A(H5N1) viruses from humans—Hong Kong, 1997–1998. MMWR Morb Mortal Wkly Rep. 1998;46:1245–1247. [PubMed] [Google Scholar]
- 3.Claas EC, Osterhaus AD, van Beek R, de Jong JC, Rimmelzwaan GF, et al. Human influenza A H5N1 virus related to a highly pathogenic avian influenza virus. Lancet. 1998;351:472–477. doi: 10.1016/S0140-6736(97)11212-0. [DOI] [PubMed] [Google Scholar]
- 4.Shortridge KF, Zhou NN, Guan Y, Gao P, Ito T, et al. Characterization of avian H5N1 influenza viruses from poultry in Hong Kong. Virology. 1998;252:331–342. doi: 10.1006/viro.1998.9488. [DOI] [PubMed] [Google Scholar]
- 5.Cauthen AN, Swayne DE, Schultz-Cherry S, Perdue ML, Suarez DL. Continued circulation in China of highly pathogenic avian influenza viruses encoding the hemagglutinin gene associated with the 1997 H5N1 outbreak in poultry and humans. J Virol. 2000;74:6592–6599. doi: 10.1128/JVI.74.14.6592-6599.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guan Y, Peiris JS, Lipatov AS, Ellis TM, Dyrting KC, et al. Emergence of multiple genotypes of H5N1 avian influenza viruses in Hong Kong SAR. Proc Natl Acad Sci USA. 2002;99:8950–8955. doi: 10.1073/pnas.132268999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guan Y, Peiris M, Kong KF, Dyrting KC, Ellis TM, et al. H5N1 influenza viruses isolated from geese in Southeastern China: evidence for genetic reassortment and interspecies transmission to ducks. Virology. 2002;292:16–23. doi: 10.1006/viro.2001.1207. [DOI] [PubMed] [Google Scholar]
- 8.Webster RG, Guan Y, Peiris M, Walker D, Krauss S, et al. Characterization of H5N1 influenza viruses that continue to circulate in geese in Southeastern China. J Virol. 2002;76:118–126. doi: 10.1128/JVI.76.1.118-126.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen H, Deng G, Li Z, Tian G, Li Y, et al. The evolution of H5N1 influenza viruses in ducks in Southern China. Proc Natl Acad Sci USA. 2004;101:10452–10457. doi: 10.1073/pnas.0403212101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.WHO/OIE/FAO H5N1 Evolution Working Group Continued evolution of highly pathogenic avian influenza A (H5N1): updated nomenclature. Influenza Other Respir Viruses. 2012;6:1–5. doi: 10.1111/j.1750-2659.2011.00298.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Duan L, Bahl J, Smith GJ, Wang J, Vijaykrishna D, et al. The development and genetic diversity of H5N1 influenza virus in China, 1996-2006. Virology. 2008;380:243–254. doi: 10.1016/j.virol.2008.07.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vijaykrishna D, Bahl J, Riley S, Duan L, Zhang JX, et al. Evolutionary dynamics and emergence of panzootic H5N1 influenza viruses. PLoS Pathog. 2008;4:e1000161. doi: 10.1371/journal.ppat.1000161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen H, Smith GJ, Li KS, Wang J, Fan XH, et al. Establishment of multiple sublineages of H5N1 influenza virus in Asia: implications for pandemic control. Proc Natl Acad Sci USA. 2006;103:2845–2850. doi: 10.1073/pnas.0511120103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen H, Smith GJ, Zhang SY, Qin K, Wang J, et al. Avian flu: H5N1 virus outbreak in migratory waterfowl. Nature. 2005;436:191–192. doi: 10.1038/nature03974. [DOI] [PubMed] [Google Scholar]
- 15.Liu J, Xiao H, Lei F, Zhu Q, Qin K, et al. Highly pathogenic H5N1 influenza virus infection in migratory birds. Science. 2005;309:1206. doi: 10.1126/science.1115273. [DOI] [PubMed] [Google Scholar]
- 16.WHO/OIE/FAO H5N1 Evolution Working Group Toward a unified nomenclature system for highly pathogenic avian influenza virus (H5N1) Emerg Infect Dis. 2008;14:e1. doi: 10.3201/eid1407.071681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Saad MD, Ahmed LS, Gamal-Eldein MA, Fouda MK, Khalil F, et al. Possible avian influenza (H5N1) from migratory bird, Egypt. Emerg Infect Dis. 2007;13:1120–1121. doi: 10.3201/eid1307.061222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ducatez MF, Olinger CM, Owoade AA, de Landtsheer S, Ammerlaan W, et al. Multiple introductions of H5N1 in Nigeria — phylogenetic analysis reveals that this deadly virus first arrived in Africa from different sources. Nature. 2006;442:37. doi: 10.1038/442037a. [DOI] [PubMed] [Google Scholar]
- 19.Ducatez MF, Olinger CM, Owoade AA, Tarnagda Z, Tahita MC, et al. Molecular and antigenic evolution and geographical spread of H5N1 highly pathogenic avian influenza viruses in western Africa. J Gen Virol. 2007;88:2297–2306. doi: 10.1099/vir.0.82939-0. [DOI] [PubMed] [Google Scholar]
- 20.Arafa A, Suarez D, Kholosy SG, Hassan MK, Nasef S, et al. Evolution of highly pathogenic avian influenza H5N1 viruses in Egypt indicating progressive adaptation. Arch Virol. 2012;157:1931–1947. doi: 10.1007/s00705-012-1385-9. [DOI] [PubMed] [Google Scholar]
- 21.Gauthier-Clerc M, Lebarbenchon C, Thomas F. Recent expansion of highly pathogenic avian influenza H5N1: a critical review. Ibis. 2007;149:202–214. doi: 10.1111/j.1474-919X.2007.00699.x. [DOI] [Google Scholar]
- 22.Gilbert M, Xiao X, Domenech J, Lubroth J, Martin V, et al. Anatidae migration in the western Palearctic and spread of highly pathogenic avian influenza H5NI virus. Emerg Infect Dis. 2006;12:1650–1656. doi: 10.3201/eid1211.060223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kilpatrick AM, Chmura AA, Gibbons DW, Fleischer RC, Marra PP, et al. Predicting the global spread of H5N1 avian influenza. Proc Natl Acad Sci USA. 2006;103:19368–19373. doi: 10.1073/pnas.0609227103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Normile D. Avian influenza. evidence points to migratory birds in H5N1 spread. Science. 2006;311:1225. doi: 10.1126/science.311.5765.1225. [DOI] [PubMed] [Google Scholar]
- 25.Gaidet N, Dodman T, Caron A, Balança G, Desvaux S, et al. Avian influenza viruses in water birds, Africa. Emerg Infect Dis. 2007;13:626–629. doi: 10.3201/eid1304.061011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Prosser DJ, Cui P, Takekawa JY, Tang M, Hou Y, et al. Wild bird migration across the Qinghai-Tibetan plateau: a transmission route for highly pathogenic H5N1. PLoS One. 2011;6:e17622. doi: 10.1371/journal.pone.0017622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Feare CJ. The role of wild birds in the spread of HPAI H5N1. Avian Dis. 2007;51:440–447. doi: 10.1637/7575-040106R1.1. [DOI] [PubMed] [Google Scholar]
- 28.Feare CJ. Role of wild birds in the spread of highly pathogenic avian influenza virus H5N1 and implications for global surveillance. Avian Dis. 2010;54:201–212. doi: 10.1637/8766-033109-ResNote.1. [DOI] [PubMed] [Google Scholar]
- 29.Gilbert M, Newman SH, Takekawa JY, Loth L, Biradar C, et al. Flying over an infected landscape: distribution of highly pathogenic avian influenza H5N1 risk in South Asia and satellite tracking of wild waterfowl. Ecohealth. 2010;7:448–458. doi: 10.1007/s10393-010-0672-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Globig A, Staubach C, Beer M, Köppen U, Fiedler W, et al. Epidemiological and ornithological aspects of outbreaks of highly pathogenic avian influenza virus H5N1 of Asian lineage in wild birds in Germany, 2006 and 2007. Transbound Emerg Dis. 2009;56:57–72. doi: 10.1111/j.1865-1682.2008.01061.x. [DOI] [PubMed] [Google Scholar]
- 31.Nagy A, Vostinakova V, Pindova Z, Hornickova J, Cernikova L, et al. Molecular and phylogenetic analysis of the H5N1 avian influenza virus caused the first highly pathogenic avian influenza outbreak in poultry in the Czech Republic in 2007. Vet Microbiol. 2009;133:257–263. doi: 10.1016/j.vetmic.2008.07.013. [DOI] [PubMed] [Google Scholar]
- 32.Brown JD, Stallknecht DE, Beck JR, Suarez DL, Swayne DE. Susceptibility of North American ducks and gulls to H5N1 highly pathogenic avian influenza viruses. Emerg Infect Dis. 2006;12:1663–1670. doi: 10.3201/eid1211.060652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Keawcharoen J, van Riel D, van Amerongen G, Bestebroer T, Beyer WE, et al. Wild ducks as long-distance vectors of highly pathogenic avian influenza virus (H5N1) Emerg Infect Dis. 2008;14:600–607. doi: 10.3201/eid1404.071016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.World Health Organization H5N1 avian influenza: timeline of major event. 2011. http://www.who.int/influenza/human_animal_interface/avian_influenza/H5N1_avian_influenza_update.pdf
- 35.World Organisation for Animal Health Update on highly pathogenic avian influenza in animals (type H5 and H7) 2012. http://www.oie.int/en/animal-health-in-the-world/update-on-avian-influenza/2012/
- 36.Reid SM, Shell WM, Barboi G, Onita I, Turcitu M, et al. First reported incursion of highly pathogenic notifiable avian influenza A H5N1 viruses from clade 2.3.2 into European poultry. Transbound Emerg Dis. 2011;58:76–78. doi: 10.1111/j.1865-1682.2010.01175.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Squires RB, Noronha J, Hunt V, García-Sastre A, Macken C, et al. Influenza research database: an integrated bioinformatics resource for influenza research and surveillance. Influenza Other Respir Viruses. 2012;6:404–416. doi: 10.1111/j.1750-2659.2011.00331.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brown IH. Summary of avian influenza activity in Europe, Asia, and Africa, 2006–2009. Avian Dis. 2010;54:187–193. doi: 10.1637/8949-053109-Reg.1. [DOI] [PubMed] [Google Scholar]
- 39.Zhao K, Gu M, Zhong L, Duan Z, Zhang Y, et al. Characterization of three H5N5 and one H5N8 highly pathogenic avian influenza viruses in China. Vet Microbiol. 2013;163:351–357. doi: 10.1016/j.vetmic.2012.12.025. [DOI] [PubMed] [Google Scholar]
- 40.Zhao G, Gu X, Lu X, Pan J, Duan Z, et al. Novel reassortant highly pathogenic H5N2 avian influenza viruses in poultry in China. PLoS One. 2012;7:e46183. doi: 10.1371/journal.pone.0046183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Qi X, Cui L, Yu H, Ge Y, Tang F. Whole-genome sequence of a reassortant H5N6 avian Influenza virus isolated from a live poultry market in China, 2013. Genome Announc. 2014;2:e00706-14. doi: 10.1128/genomeA.00706-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Su S, Bi Y, Wong G, Gray GC, Gao GF, et al. Epidemiology, evolution, and recent outbreaks of avian influenza virus in China. J Virol. 2015;89:8671–8676. doi: 10.1128/JVI.01034-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lee YJ, Kang HM, Lee EK, Song BM, Jeong J, et al. Novel reassortant influenza A(H5N8) viruses, South Korea, 2014. Emerg Infect Dis. 2014;20:1087–1089. doi: 10.3201/eid2006.140233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.de Vries E, Guo H, Dai M, Rottier PJ, van Kuppeveld FJ, et al. Rapid emergence of highly pathogenic avian influenza subtypes from a subtype H5N1 hemagglutinin variant. Emerg Infect Dis. 2015;21:842–846. doi: 10.3201/eid2105.141927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lee DH, Bahl J, Torchetti MK, Killian ML, Ip HS, et al. Highly pathogenic avian influenza viruses and generation of novel reassortants, United States, 2014–2015. Emerg Infect Dis. 2016;22:1283–1285. doi: 10.3201/eid2207.160048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hanna A, Banks J, Marston DA, Ellis RJ, Brookes SM, et al. Genetic characterization of highly pathogenic avian influenza (H5N8) virus from domestic ducks, England, November 2014. Emerg Infect Dis. 2015;21:879–882. doi: 10.3201/eid2105.141954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bouwstra R, Heutink R, Bossers A, Harders F, Koch G, et al. Full-genome sequence of influenza A(H5N8) virus in poultry linked to sequences of strains from Asia, the Netherlands, 2014. Emerg Infect Dis. 2015;21:872–874. doi: 10.3201/eid2105.141839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Claes F, Morzaria SP, Donis RO. Emergence and dissemination of clade 2.3.4.4 H5Nx influenza viruses: how is the Asian HPAI H5 lineage maintained. Curr Opin Virol. 2016;16:158–163. doi: 10.1016/j.coviro.2016.02.005. [DOI] [PubMed] [Google Scholar]
- 49.Harder T, Maurer-Stroh S, Pohlmann A, Starick E, Höreth-Böntgen D, et al. Influenza A(H5N8) Virus similar to strain in Korea causing highly pathogenic avian influenza in Germany. Emerg Infect Dis. 2015;21:860–863. doi: 10.3201/eid2105.141897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ozawa M, Matsuu A, Tokorozaki K, Horie M, Masatani T, et al. Genetic diversity of highly pathogenic H5N8 avian influenza viruses at a single overwintering site of migratory birds in Japan, 2014/15. Euro Surveill. 2015;20:21132. doi: 10.2807/1560-7917.ES2015.20.20.21132. [DOI] [PubMed] [Google Scholar]
- 51.Bevins SN, Dusek RJ, White CL, Gidlewski T, Bodenstein B, et al. Widespread detection of highly pathogenic H5 influenza viruses in wild birds from the Pacific flyway of the United States. Sci Rep. 2016;6:28980. doi: 10.1038/srep28980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sonnberg S, Phommachanh P, Naipospos TS, Mckenzie J, Chanthavisouk C, et al. Multiple introductions of avian influenza viruses (H5N1), Laos, 2009-2010. Emerg Infect Dis. 2012;18:1139–1143. doi: 10.3201/eid1807.111642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Xu L, Bao L, Yuan J, Li F, Lv Q, et al. Antigenicity and transmissibility of a novel clade 2.3.2.1 avian influenza H5N1 virus. J Gen Virol. 2013;94:2616–2626. doi: 10.1099/vir.0.057778-0. [DOI] [PubMed] [Google Scholar]
- 54.Creanga A, Thi Nguyen D, Gerloff N, Thi do H, Balish A, et al. Emergence of multiple clade 2.3.2.1 influenza A (H5N1) virus subgroups in Vietnam and detection of novel reassortants. Virology. 2013;444:12–20. doi: 10.1016/j.virol.2013.06.005. [DOI] [PubMed] [Google Scholar]
- 55.Hinshaw VS, Wood JM, Webster RG, Deibel R, Turner B. Circulation of influenza viruses and paramyxoviruses in waterfowl originating from two different areas of North America. Bull World Health Organ. 1985;63:711–719. [PMC free article] [PubMed] [Google Scholar]
- 56.Webster RG, Bean WJ, Gorman OT, Chambers TM, Kawaoka Y. Evolution and ecology of influenza A viruses. Microbiol Rev. 1992;56:152–179. doi: 10.1128/mr.56.1.152-179.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Krauss S, Walker D, Pryor SP, Niles L, Chenghong L, et al. Influenza A viruses of migrating wild aquatic birds in North America. Vector Borne Zoonotic Dis. 2004;4:177–189. doi: 10.1089/vbz.2004.4.177. [DOI] [PubMed] [Google Scholar]
- 58.Fouchier RA, Munster VJ. Epidemiology of low pathogenic avian influenza viruses in wild birds. Rev Sci Tech. 2009;28:49–58. doi: 10.20506/rst.28.1.1863. [DOI] [PubMed] [Google Scholar]
- 59.Alexander DJ. Summary of avian influenza activity in Europe, Asia, Africa, and Australasia, 2002–2006. Avian Dis. 2007;51:161–166. doi: 10.1637/7602-041306R.1. [DOI] [PubMed] [Google Scholar]
- 60.Islam MR, Haque ME, Giasuddin M, Chowdhury EH, Samad MA, et al. New introduction of clade 2.3.2.1 avian influenza virus (H5N1) into Bangladesh. Transbound Emerg Dis. 2012;59:460–463. doi: 10.1111/j.1865-1682.2011.01297.x. [DOI] [PubMed] [Google Scholar]
- 61.Marinova-Petkova A, Georgiev G, Seiler P, Darnell D, Franks J, et al. Spread of influenza virus A (H5N1) clade 2.3.2.1 to Bulgaria in common buzzards. Emerg Infect Dis. 2012;18:1596–1602. doi: 10.3201/eid1810.120357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Khan SU, Berman L, Haider N, Gerloff N, Rahman MZ, et al. Investigating a crow die-off in January-February 2011 during the introduction of a new clade of highly pathogenic avian influenza virus H5N1 into Bangladesh. Arch Virol. 2014;159:509–518. doi: 10.1007/s00705-013-1842-0. [DOI] [PubMed] [Google Scholar]
- 63.Marinova-Petkova A, Feeroz MM, Rabiul Alam SM, Kamrul Hasan M, Akhtar S, et al. Multiple introductions of highly pathogenic avian influenza H5N1 viruses into Bangladesh. Emerg Microbes Infect. 2014;3:e11. doi: 10.1038/emi.2014.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kord E, Kaffashi A, Ghadakchi H, Eshratabadi F, Bameri Z, et al. Molecular characterization of the surface glycoprotein genes of highly pathogenic H5N1 avian influenza viruses detected in Iran in 2011. Trop Anim Health Prod. 2014;46:549–554. doi: 10.1007/s11250-013-0528-7. [DOI] [PubMed] [Google Scholar]
- 65.Dharmayanti NL, Hartawan R, Pudjiatmoko H, Wibawa H, Hardiman A, et al. Genetic characterization of clade 2.3.2.1 avian influenza A(H5N1) viruses, Indonesia, 2012. Emerg Infect Dis. 2014;20:677–680. doi: 10.3201/eid2004.130517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Martin V, de Simone L, Lubroth J. Geographic information systems applied to the international surveillance and control of transboundary animal diseases, a focus on highly pathogenic avian influenza. Vet Ital. 2007;43:437–450. [PubMed] [Google Scholar]
- 67.Li Y, Chen S, Zhang X, Fu Q, Zhang Z, et al. A 20-amino-acid deletion in the neuraminidase stalk and a five-amino-acid deletion in the NS1 protein both contribute to the pathogenicity of H5N1 avian influenza viruses in mallard ducks. PLoS One. 2014;9:e95539. doi: 10.1371/journal.pone.0095539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Li J, Zu Dohna H, Cardona CJ, Miller J, Carpenter TE. Emergence and genetic variation of neuraminidase stalk deletions in avian influenza viruses. PLoS One. 2011;6:e14722. doi: 10.1371/journal.pone.0014722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Newman SH, Hill NJ, Spragens KA, Janies D, Voronkin IO, et al. Eco-virological approach for assessing the role of wild birds in the spread of avian influenza H5N1 along the Central Asian flyway. PLoS One. 2012;7:e30636. doi: 10.1371/journal.pone.0030636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sturm-Ramirez KM, Hulse-Post DJ, Govorkova EA, Humberd J, Seiler P, et al. Are ducks contributing to the endemicity of highly pathogenic H5N1 influenza virus in Asia? J Virol. 2005;79:11269–11279. doi: 10.1128/JVI.79.17.11269-11279.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Brown JD, Stallknecht DE, Swayne DE. Experimental infection of swans and geese with highly pathogenic avian influenza virus (H5N1) of Asian lineage. Emerg Infect Dis. 2008;14:136–142. doi: 10.3201/eid1401.070740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kang HM, Lee EK, Song BM, Jeong J, Choi JG, et al. Novel reassortant influenza A(H5N8) viruses among inoculated domestic and wild ducks, South Korea, 2014. Emerg Infect Dis. 2015;21:298–304. doi: 10.3201/eid2102.141268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wibawa H, Bingham J, Nuradji H, Lowther S, Payne J, et al. Experimentally infected domestic ducks show efficient transmission of Indonesian H5N1 highly pathogenic avian influenza virus, but lack persistent viral shedding. PLoS One. 2014;9:e83417. doi: 10.1371/journal.pone.0083417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hulse-Post DJ, Sturm-Ramirez KM, Humberd J, Seiler P, Govorkova EA, et al. Role of domestic ducks in the propagation and biological evolution of highly pathogenic H5N1 influenza viruses in Asia. Proc Natl Acad Sci USA. 2005;102:10682–10687. doi: 10.1073/pnas.0504662102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hénaux V, Samuel MD. Avian influenza shedding patterns in waterfowl: implications for surveillance, environmental transmission, and disease spread. J Wildl Dis. 2011;47:566–578. doi: 10.7589/0090-3558-47.3.566. [DOI] [PubMed] [Google Scholar]
- 76.Kim JK, Negovetich NJ, Forrest HL, Webster RG. Ducks: the 'Trojan horses' of H5N1 influenza. Influenza Other Respir Viruses. 2009;3:121–128. doi: 10.1111/j.1750-2659.2009.00084.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Cui P, Hou Y, Xing Z, He Y, Li T, et al. Bird migration and risk for H5N1 transmission into Qinghai Lake, China. Vector Borne Zoonotic Dis. 2011;11:567–576. doi: 10.1089/vbz.2009.0240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sonnberg S, Webby RJ, Webster RG. Natural history of highly pathogenic avian influenza H5N1. Virus Res. 2013;178:63–77. doi: 10.1016/j.virusres.2013.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hall TA. BioEdit: a user-friendly biological sequence alignment editor and analysis for Windows 95/98/NT. Nucleic Acids SympSer. 1999;41:95–98. doi: 10.1128/JVI.79.7.4201-4212.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Tamura K, Peterson D, Peterson N, Stecher G, Nei M, et al. MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol. 2011;28:2731–2739. doi: 10.1093/molbev/msr121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Reed LJ, Muench H. A simple method of estimating fifty per cent endpoints. Am J Epidemiol. 1938;27:493–497. doi: 10.1093/oxfordjournals.aje.a118408. [DOI] [Google Scholar]
- 82.World Health Organization WHO Manual on Animal Influenza Diagnosis and Surveillance. 2011. http://www.who.int/csr/resources/publications/influenza/en/whocdscsrncs20025rev.pdf
- 83.de Castro E, Sigrist CJ, Gattiker A, Bulliard V, Langendijk-Genevaux PS, et al. ScanProsite: detection of PROSITE signature matches and ProRule-associated functional and structural residues in proteins. Nucleic Acids Res. 2006;34:W362–W365. doi: 10.1093/nar/gkl124. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.