Abstract
Background
The inherent challenges of selecting an acceptable donor for the increasing number and acuity of recipients has forced programs to take increased risks, including accepting donors with a cancer history (DWCH). Outcomes of organ transplantation using organs from DWCH must be clarified. We assessed transplant outcomes of recipients of organs from DWCH.
Material/Methods
Retrospective analysis of the Scientific Registry of Transplant Recipients data from January 1, 2000 to December 31, 2014 identified 8385 cases of transplants from DWCH. A Cox-proportional hazard regression model and log-rank test were used to compare patient survival and hazard levels of various cancer types.
Results
DWCH was an independent risk factor of 5-year patient survival (HR=1.089, 95% CI: 1.009–1.176, P=0.03) and graft survival (HR=1.129, 95% CI: 1.056–1.208, P<0.01) in liver and heart transplantation (patient survival: HR=1.112, 95% CI: 1.057–1.170, P<0.01; graft survival: HR=1.244, 95% CI: 1.052–1.472, P=0.01). There was no remarkable difference between the 2 groups in kidney and lung transplantation. Donors with genitourinary and gastrointestinal cancers were associated with inferior outcomes in kidney transplantation. Transplantation from donors with central nervous system cancer resulted in poorer survival in liver transplant recipients. Recipients of organs from donors with hematologic malignancy and otorhinolaryngologic cancer had poorer survival following heart transplantation.
Conclusions
Under the current donor selection criteria, recipients of organs from DWCH had inferior outcomes in liver and heart transplantation, whereas organs from DWCH were safely applied in kidney and lung transplantation. Specific cancer types should be cautiously evaluated before performing certain types of organ transplantation.
MeSH Keywords: Organ Transplantation, Survival Analysis, Tissue Donors
Background
The waiting list for organs continues to grow with increasing improvements in organ transplantation, making shortages in organ supplies an enormous obstacle. The number of transplantations has not met the demand for organs from candidates added to the wait list every year [1]. This challenge highlights the importance of expanding the organ donor pool by including organs from marginal donors who potentially carry transmissible diseases, such as cancers [2]. Transplanted organs carry the risk of donor-transmitted cancer (DTC), which is tremendously controversial, although the risk of DTC is believed to have been exaggerated [2]. Donors with a cancer history (DWCH) are becoming more and more important as an organ source [3]. Since the low risk of DTC cannot be easily assessed or foreseen, it is crucial to formulate allocation strategies that put DWCH to good use while avoiding other disadvantages.
In this study, we collected and analyzed the outcomes of organ recipients from DWCH and donors with no cancer history (DWNCH) from January 1, 2000 to December 31, 2014 in the Scientific Registry of Transplant Recipients (SRTR) database. The primary aim of this study was to compare patient survival, graft survival, and cancer-free survival between patients who received organs from DWCH and those receiving organs from DWNCH. Additionally, we attempted to evaluate the hazard level of various donor cancer types in different organ transplants, aiming to formulate allocation strategies to minimize the risk to recipients while maximizing the utility of organs from DWCH.
Material and Methods
Identification of donors and recipients
This study is a retrospective analysis using data from the SRTR, which includes data on all donors, wait-listed candidates, and transplant recipients in the United States submitted by the members of the Organ Procurement and Transplantation Network (OPTN), and was supplemented by mortality ascertainment from the Social Security Death Master File. The SRTR has reviewed and approved this study. Single organ transplant cases (kidney, liver, heart, lung, pancreas, and intestine transplantation) from January 1, 2000 to December 31, 2014 in the SRTR were examined in this study. Cases with a missing or unknown record of the donor’s cancer history were excluded. Donors were classified into the DWCH and DWNCH groups according to their cancer history. DWCH were patients with a specific history of malignancy in the registry. In the DWNCH group, patients who met the expanded criteria in the SRTR donor record data were defined as the expanded donor (ECD) group.
Statistical analysis
For descriptive analyses, categorical variables are presented as frequencies. The Kolmogorov-Smirnov test was used to verify normal distributions. Comparisons between groups of categorical variables were conducted using Pearson’s chi-squared test. The variables compared between 2 groups were the basic characteristics of donors and recipients, including age, race, gender, race, donor and recipient blood type and body mass index (BMI), and history of malignancy in recipients.
Survival analysis was performed for kidney, liver, heart, and lung transplantation. Pancreas and intestine transplantation were excluded because only a small number of DWCH were involved in these procedures. Patient survival was defined as the time between transplantation and death. Graft survival was from transplantation to graft failure or death. The cumulative probability of malignancy and cancer-free survival were defined as the time between transplantation to the first event(s) of malignancy during follow-up. The survival rate was analyzed by the Kaplan-Meier method, and group comparisons were conducted with the log-rank test. A Cox-proportional hazard regression model and the log-rank test were used to compare the survival, hazard ratios and P values between different groups with adjustment for the variables described above. The results are presented as adjusted hazard ratios (AHR) with 95% confidence intervals (CI). The variables used for adjustment were those with statistically significant differences between the DWCH and DWNCH groups. Variables used for adjustment in the risk analysis of kidney transplantation were donor age, gender, race, and blood type and recipient age, gender, blood type, BMI, and previous malignancy. Variables used for adjustment in the risk analysis of liver transplantation were donor age, gender, and race and recipient age, gender, BMI and primary diagnosis. Variables used for adjustment in the risk analysis of heart transplantation were donor age, gender, race, and blood type and recipient age, primary diagnosis and previous malignancy. Variables used for adjustment in the risk analysis of lung transplantation were donor age, gender, and race and recipient age and previous malignancy. Expected cases of malignancy were calculated by referencing the incidence in the whole transplant population. Binary logistic regression analysis was utilized to compute odds ratios (OR) and 95% CI between the malignancy incidence of the DWCH and DWNCH groups during follow-up.
All P values were 2-sided, and P<0.05 was accepted as statistically significant. All analyses were carried out by the Statistical Package for the Social Science (SPSS) 22.0 (IBM, USA).
Results
Trends of donation from DWCH
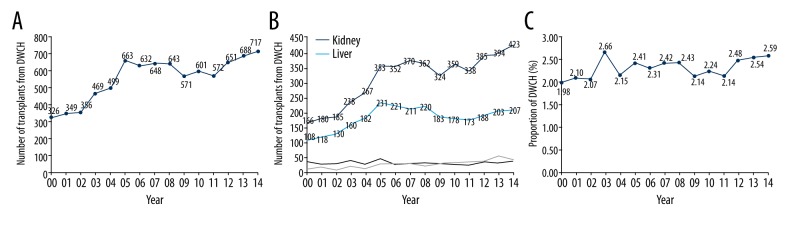
Among all donors included in this study, 8385 donors (2.28%) had a history of cancer from January 1, 2000 through December 31, 2014 in the SRTR database. A total of 326 patients with a cancer history underwent organ donation in 2000. The number of patients with a cancer history who underwent donation kept rising until 2005, when it reached 663. Then, the number increased gradually to 717 in 2014 (Figure 1A). Since 2005, more than 300 kidney transplants and approximately 200 liver transplants came from DWCH annually. In 2014, 423 kidney transplants and 207 liver transplants came from these donors (Figure 1B). The proportion of DWCH in the whole population of donors remained nearly same even though the number of donors grew annually (Figure 1C). The proportion peaked in 2003 at 2.66%. In 2014, the rate was only 2.59%. A total of 4696 (2.24%) kidney transplants were from DWCH. Liver transplants included 2713 (3.12%) cases, which was the highest rate of organ use from DWCH in all solid-organ transplants. There were 504 (1.52%), 413 (1.93%), 50 (0.81%) and 9 (0.43%) cases involving DWCH in heart, lung, pancreas, and intestine transplants, respectively (Supplementary Table 1). No more than 5% of donor cancer history records in the database were missing for all types of transplantation during 2000 to 2014. The main difference of clinical demographics was shown in Supplementary Table 2.
Figure 1.
(A) The number of transplants from DWCH by year from January 1, 2000, to December 31, 2014. (B) Number of transplants from DWCH in different types of organ transplants yearly. (C) Proportion of DWCH in all donors by each year.
Distribution of donor cancer types in various transplants
Table 1 shows the distribution of different cancer types of all cases included in this study. We conducted a systematic classification of donor cancer types into 11 groups, including non-melanoma skin cancer (NMSC), melanoma, central nervous system (CNS) cancer, genitourinary cancer, breast cancer, thyroid cancer, hematologic malignancy, gastrointestinal cancer, lung cancer, and other types. NMSC (n=2785), consisting of squamous and basic cell skin cancer, constituted the largest group and was followed by genitourinary cancer (n=1816). CNS cancer (n=1337) represented the third largest group. Other types, such as breast cancer (n=481), melanoma (n=299) and thyroid cancer (n=191), also contributed to the total number of donors with cancer. Glioblastoma multiform (n=275) and astrocytoma (n=264) were the 2 most common types of CNS tumors in these donors. Uterine cervical cancer (UCC, n=937) was the most frequent type in genitourinary cancer. NMSC and UCC were the 2 most common cancer types associated with kidney, liver, heart, and lung transplant. Interestingly, unlike liver transplants, which came from a small number of donors with a gastrointestinal cancer history, or lung transplants, which did not come from donors with a lung cancer history, kidney transplant recipients received a large number (n=994) of organs from donors with a genitourinary cancer history, even with a kidney cancer history. Both pancreas and intestine transplantation included a small number of DWCH.
Table 1.
Distribution of donor cancer types in various transplants from 2000 to 2014 in the SRTR.
| Cancer type of donor | Kidney TX | Liver TX | Heart TX | Lung TX | Pancreas TX | Intestine TX | Total |
|---|---|---|---|---|---|---|---|
| Non-melanoma skin cancer | 1642 | 898 | 114 | 127 | 4 | 0 | 2785 |
| Melanoma | 183 | 90 | 13 | 12 | 1 | 0 | 299 |
| Cns cancer | 641 | 391 | 180 | 108 | 14 | 3 | 1337 |
| Glioblastoma multiforme | 106 | 99 | 43 | 22 | 4 | 1 | 275 |
| Astrocytoma | 135 | 71 | 39 | 13 | 6 | 0 | 264 |
| Meningioma | 131 | 71 | 26 | 28 | 4 | 1 | 261 |
| Medulloblastoma | 7 | 9 | 3 | 2 | 0 | 0 | 21 |
| Neuroblastoma | 4 | 3 | 2 | 2 | 0 | 0 | 11 |
| Angioblastoma | 4 | 3 | 0 | 0 | 0 | 0 | 7 |
| Tumor – other | 254 | 135 | 67 | 41 | 0 | 1 | 498 |
| Genitourinary cancer | 994 | 629 | 101 | 72 | 19 | 1 | 1816 |
| Uterine cervical | 546 | 290 | 59 | 35 | 6 | 1 | 937 |
| Prostate | 113 | 130 | 2 | 9 | 0 | 0 | 254 |
| Ovarian | 116 | 68 | 7 | 7 | 1 | 0 | 199 |
| Penis, testicular | 68 | 31 | 13 | 3 | 3 | 0 | 118 |
| Uterine body endometrial | 69 | 38 | 0 | 8 | 0 | 0 | 115 |
| Bladder | 20 | 21 | 3 | 3 | 8 | 0 | 55 |
| Kidney | 9 | 24 | 5 | 4 | 1 | 0 | 43 |
| Vulva | 19 | 7 | 1 | 1 | 0 | 0 | 28 |
| Uterine body choriocarcinoma | 10 | 5 | 7 | 1 | 0 | 0 | 23 |
| Genitourinary, unknown | 24 | 15 | 4 | 1 | 0 | 0 | 44 |
| Breast cancer | 295 | 165 | 7 | 13 | 1 | 0 | 481 |
| Thyroid cancer | 125 | 51 | 4 | 11 | 0 | 0 | 191 |
| Hematologic malignancy | 59 | 36 | 5 | 6 | 0 | 0 | 106 |
| Gastrointestinal cancer | 80 | 59 | 5 | 7 | 0 | 0 | 151 |
| Colo-rectal | 61 | 48 | 4 | 5 | 0 | 0 | 118 |
| Stomach | 4 | 7 | 1 | 2 | 0 | 0 | 14 |
| Small intestine | 5 | 4 | 0 | 0 | 0 | 0 | 9 |
| Pancreas | 6 | 0 | 0 | 0 | 0 | 0 | 6 |
| Esophageal | 2 | 0 | 0 | 0 | 0 | 0 | 2 |
| Liver/biliary tract | 2 | 0 | 0 | 0 | 0 | 0 | 2 |
| Lung cancer | 15 | 15 | 0 | 0 | 0 | 0 | 30 |
| Otorhinolaryngologic cancer | 30 | 29 | 1 | 2 | 0 | 0 | 62 |
| Tongue/throat | 21 | 21 | 0 | 0 | 0 | 0 | 42 |
| Larynx | 9 | 8 | 1 | 2 | 0 | 0 | 20 |
| Other, specify | 632 | 350 | 74 | 55 | 11 | 5 | 1127 |
| Total | 4696 | 2713 | 504 | 413 | 50 | 9 | 8385 |
Survival outcomes of transplants from DWCH versus ECD
As a first attempt to evaluate transplants from DWCH, we compared the survival outcomes between the DWCH group and ECD group. Table 2 shows that the DWCH group had a lower 5-year patient survival rate (82% versus 75%, P<0.001), 5-year graft survival rate (72% versus 62%, P<0.001) and 10-year cancer-free survival rate (82% versus 80%, P=0.03) than the ECD group in kidney transplantation. The DWCH group also had a better 5-year patient survival rate (56% versus 50%, P=0.04) and 5-year graft survival days (54% versus 47%, P=0.04) in lung transplantation than the ECD group. There was no significant difference in survival outcomes between the DWCH group and ECD group in liver and heart transplantation.
Table 2.
Survival outcomes of transplants from DWCH versus ECD.
| TX type | 5-year patient survival rate | 5-year graft survival rate | 10-year cancer-free survival rate | ||||||
|---|---|---|---|---|---|---|---|---|---|
| ECD | DWCH | P | ECD | DWCH | P | ECD | DWCH | P | |
| Kidney | 75% | 82% | <0.01 | 62% | 72% | <0.01 | 80% | 82% | 0.03 |
| Liver | 66% | 66% | 0.86 | 62% | 61% | 0.26 | 50% | 48% | 0.25 |
| Heart | 68% | 69% | 0.97 | 67% | 68% | 0.95 | 67% | 70% | 0.37 |
| Lung | 50% | 56% | 0.04 | 47% | 54% | 0.04 | 42% | 43% | 0.25 |
DWCH – donors with a cancer history; ECD – expanded criteria donor, TX – treatment
Patient and graft survival of transplants from DWCH versus DWNCH
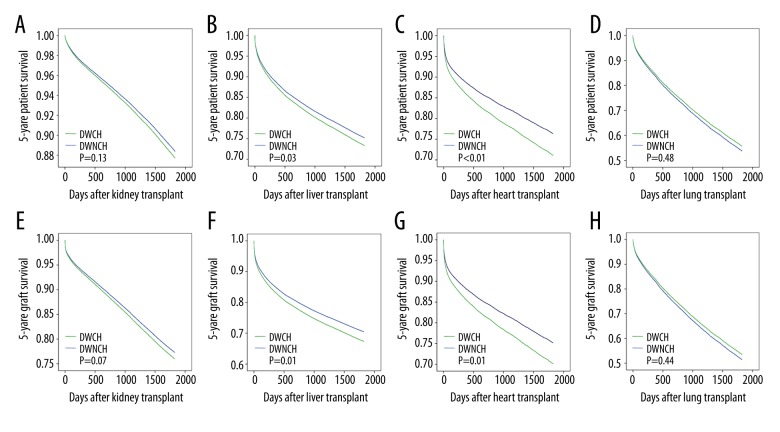
After comparing outcomes between the DWCH and ECD groups, we attempted further comparisons between the DWCH and DWNCH groups by multivariate Cox-proportional hazards regression analysis. DWCH was an independent risk factor for liver and heart transplantation. The adjusted patient and graft survival in the DWCH group were significantly lower than those in the DWNCH group in liver and heart transplantation (Figure 2). Liver transplants from DWCH were associated with significantly poorer adjusted 5-year patient survival (HR=1.089, 95% CI: 1.009–1.176, P=0.03) and graft survival (HR=1.129, 95% CI: 1.056–1.208, P<0.01) than those from DWNCH (Figure 2B, 2F). Furthermore, heart transplant recipients receiving organs from DWCH had a lower adjusted 5-year patient survival (HR=1.112, 95% CI: 1.057–1.170, P<0.01) and adjusted 5-year graft survival (HR=1.244, 95% CI: 1.052–1.472, P=0.01) than those receiving organs from DWNCH (Figure 2C, 2G). Figure 2A and 2E show that DWCH was not an independent risk factor for kidney transplantation (P=0.13 and P=0.07 for patient and graft survival, respectively). There was no statistical significance between the 2 groups in the adjusted 5-year patient or graft survival after lung transplantation (P=0.48 and P=0.44, respectively; Figure 2D, 2H).
Figure 2.
Adjusted patient and graft survival analysis between the two groups of transplantation from donors with or without a cancer history. (A, E) Comparison of patient and graft survival in kidney transplant. (B, F) Comparison of patient and graft survival in liver transplant. (C, G) Comparison of patient and graft survival in heart transplant. (D, H) Comparison of patient and graft survival in lung transplant.
Incidence and cumulative probability of malignancy during follow-up
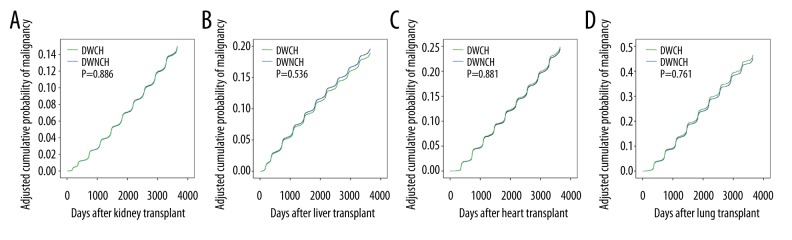
The cancer burden for organ transplant recipients is a widely acknowledged issue. Recipients are confronted with a remarkably higher risk of de novo cancer than the general population [4,5]. As shown in Table 3, recipients of kidney transplants from DWCH had a significantly higher incidence of malignancy at 5 years and 10 years of follow-up than did those receiving transplants from DWNCH. The ORs were 1.18 (95%CI 1.04–1.33, P=0.01) and 1.14 (95%CI 1.03–1.26, P=0.01) for the DWCH and DWNCH groups, respectively. Otherwise, the DWCH group did not have a higher incidence of malignancy in association with liver, heart, or lung transplantation, and the adjusted cumulative probability of malignancy was not significantly different between the DWCH group and DWNCH group for these 4 transplant types (Figure 3A–3D).
Table 3.
Malignancy cases identified at follow-up in recipients from DWCH.
| TX type | Following years | Observed cases | Expected cases | OR | 95% CI | P |
|---|---|---|---|---|---|---|
| Kidney | 5 | 285 | 245.13 | 1.18 | 1.04–1.33 | 0.01 |
| 10 | 397 | 353.28 | 1.14 | 1.03–1.26 | 0.01 | |
| Liver | 5 | 223 | 208.84 | 1.12 | 0.96–1.30 | 0.15 |
| 10 | 284 | 282.16 | 1.11 | 0.96–1.27 | 0.11 | |
| Heart | 5 | 46 | 42.75 | 1.16 | 0.86–1.57 | 0.32 |
| 10 | 75 | 67.76 | 1.13 | 0.88–1.44 | 0.34 | |
| Lung | 5 | 43 | 54.47 | 0.79 | 0.58–1.09 | 0.15 |
| 10 | 71 | 75.64 | 0.97 | 0.75–1.25 | 0.79 |
DWCH – donors with a cancer history; TX – treatment; OR – odds ratio; 95% CI – 95% confidence interval.
Figure 3.
(A–D) The adjusted cumulative probability of cancer after kidney, liver, heart and lung transplant, respectively.
Overall hazard assessment of donor’s cancer type in relation to recipient’s survival
Apparently, not all types of cancer were negative factors for survival. An adjusted Cox-regression analysis was carried out in order to determine specific cancer types with significant disadvantages in each transplant type. As shown in Table 4, the regression analysis showed that kidney transplant recipients from donors with genitourinary cancer and donors with a gastrointestinal cancer history had lower patient [HR, genitourinary: 1.20 (1.04–1.38) and gastrointestinal: 1.70 (1.13–2.56)] and graft survival [genitourinary: 1.25 (1.13–1.39) and gastrointestinal: 1.55 (1.12–2.15)]. Donors with NMSC were associated with poorer cancer-free survival [1.22 (1.05–1.42)] in kidney transplantation. Recipients of liver transplants from donors with a CNS cancer history and donors with NMSC had a higher risk of graft failure [1.23 (1.03–1.46) and 1.14 (1.02–1.28), respectively]. The analysis showed that recipients of heart transplants from donors with a hematologic malignancy and donors with an otorhinolaryngologic cancer history had inferior patient [hematologic: 7.06 (2.64–18.90) and otorhinolaryngologic: 8.29 (1.17–58.92)] and graft survival [hematologic: 6.71 (2.51–17.94) and otorhinolaryngologic: 8.03 (1.13–57.08)].
Table 4.
Overall hazard assessment of donor cancer type on recipient survival.
| Donor cancer types | Patient survival | Graft survival | Cancer-free survival | ||||||
|---|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | HR | 95% CI | P | |
| Kidney transplantation | |||||||||
| No cancer | 1.00 | – | – | 1.00 | – | – | 1.00 | – | – |
| CNS cancer | 1.18 | 0.99–1.40 | 0.06 | 1.14 | 1.00–1.30 | 0.06 | 0.67 | 0.49–0.93 | 0.02 |
| Non-melanoma skin cancer | 1.09 | 0.98–1.22 | 0.11 | 1.04 | 0.96–1.14 | 0.34 | 1.22 | 1.05–1.42 | 0.01 |
| Melanoma | 1.09 | 0.81–1.47 | 0.57 | 0.96 | 0.75–1.23 | 0.75 | 0.79 | 0.47–1.31 | 0.35 |
| Genitourinary cancer | 1.20 | 1.04–1.38 | 0.01 | 1.25 | 1.13–1.39 | 0.00 | 0.92 | 0.73–1.16 | 0.47 |
| Thyroid cancer | 1.00 | 0.65–1.55 | 1.00 | 0.89 | 0.621.26 | 0.51 | 1.41 | 0.82–2.44 | 0.21 |
| Hematologic malignancy | 0.89 | 0.45–1.78 | 0.74 | 1.22 | 0.78–1.92 | 0.38 | 0.00 | – | 0.56 |
| Lung cancer | 2.54 | 1.06–6.10 | 0.04 | 1.78 | 0.80–3.96 | 0.16 | 1.29 | 0.18–9.16 | 0.80 |
| Otorhinolaryngologic cancer | 0.67 | 0.28–1.60 | 0.37 | 0.56 | 0.27–1.17 | 0.12 | 0.98 | 0.32–3.05 | 0.98 |
| Breast cancer | 0.96 | 0.73–1.26 | 0.76 | 1.03 | 0.84–1.26 | 0.80 | 0.93 | 0.621.41 | 0.75 |
| Gastrointestinal cancer | 1.70 | 1.13–2.56 | 0.01 | 1.55 | 1.12–2.15 | 0.01 | 0.92 | 0.41–2.05 | 0.84 |
| Other, specify | 1.03 | 0.86–1.23 | 0.77 | 0.96 | 0.83–1.11 | 0.59 | 0.99 | 0.76–1.29 | 0.94 |
| Liver transplantation | |||||||||
| No cancer | 1.00 | – | – | 1.00 | – | – | 1.00 | – | – |
| CNS cancer | 1.19 | 0.98–1.45 | 0.08 | 1.23 | 1.03–1.46 | 0.02 | 1.36 | 1.01–1.83 | 0.05 |
| Non-melanoma skin cancer | 1.10 | 0.97–1.25 | 0.14 | 1.14 | 1.02–1.28 | 0.02 | 1.19 | 0.96–1.46 | 0.11 |
| Melanoma | 1.22 | 0.83–1.79 | 0.32 | 1.21 | 0.85–1.71 | 0.29 | 0.67 | 0.28–1.60 | 0.36 |
| Genitourinary cancer | 1.01 | 0.86–1.19 | 0.86 | 1.07 | 0.93–1.23 | 0.33 | 1.38 | 1.09–1.74 | 0.01 |
| Thyroid cancer | 1.12 | 0.65–1.93 | 0.69 | 1.25 | 0.79–1.99 | 0.34 | 1.31 | 0.59–2.93 | 0.50 |
| Hematologic malignancy | 1.57 | 0.89–2.77 | 0.12 | 1.51 | 0.90–2.55 | 0.12 | 1.81 | 0.73–4.34 | 0.19 |
| Lung cancer | 1.04 | 0.39–2.76 | 0.94 | 1.05 | 0.44–2.52 | 0.92 | 2.08 | 0.67–6.46 | 0.20 |
| Otorhinolaryngologic cancer | 1.22 | 0.63–2.34 | 0.56 | 1.21 | 0.67–2.19 | 0.52 | 1.60 | 0.60–4.26 | 0.35 |
| Breast cancer | 0.96 | 0.70–1.32 | 0.78 | 1.06 | 0.81–1.38 | 0.69 | 1.51 | 0.97–2.34 | 0.07 |
| Gastrointestinal cancer | 0.51 | 0.26–1.02 | 0.06 | 1.45 | 0.24–1.87 | 0.52 | 1.04 | 0.43–2.49 | 0.94 |
| Other, specify | 1.14 | 0.93–1.40 | 0.21 | 1.18 | 0.98–1.41 | 0.08 | 0.98 | 0.48–1.42 | 0.92 |
| Heart transplantation | |||||||||
| No cancer | 1.00 | – | – | 1.00 | – | – | 1.00 | – | – |
| CNS cancer | 1.21 | 0.91–1.62 | 0.19 | 1.20 | 0.91–1.60 | 0.20 | 1.11 | 0.78–1.59 | 0.56 |
| Non-melanoma skin cancer | 1.17 | 0.82–1.66 | 0.40 | 1.19 | 0.84–1.68 | 0.33 | 1.11 | 0.71–1.74 | 0.65 |
| Melanoma | 1.24 | 0.47–3.31 | 0.67 | 1.18 | 0.44–3.16 | 0.74 | 0.91 | 0.23–3.65 | 0.90 |
| Genitourinary cancer | 1.29 | 0.88–1.90 | 0.20 | 1.24 | 0.84–1.82 | 0.28 | 0.65 | 0.35–1.21 | 0.17 |
| Thyroid cancer | 1.15 | 0.168.16 | 0.89 | 1.10 | 0.16–7.85 | 0.92 | 7.73 | 1.92–31.04 | 0.00 |
| Hematologic malignancy | 7.06 | 2.64–18.90 | 0.00 | 6.71 | 2.51–17.94 | 0.00 | 0.00 | – | 0.93 |
| Otorhinolaryngologic cancer | 8.29 | 1.17–58.92 | 0.03 | 8.03 | 1.13–57.08 | 0.04 | 0.00 | – | 1.00 |
| Breast cancer | 2.81 | 0.91–8.72 | 0.07 | 2.67 | 0.86–8.29 | 0.09 | 0.00 | – | 0.91 |
| Gastrointestinal cancer | 2.29 | 0.57–9.18 | 0.24 | 2.20 | 0.558.82 | 0.26 | 0.00 | – | 0.92 |
| Other, specify | 1.14 | 0.72–1.81 | 0.59 | 1.08 | 0.681.72 | 0.73 | 1.11 | 0.63–1.96 | 0.71 |
HR – hazard ratio; 95% CI – 95% confidence interval.
Discussion
A severe organ shortage has forced transplant teams to expand donor pools to marginal donors with extended criteria. Donors with malignancy history are marginal donors because they are possibly associated with higher risks than reference donors [6,7]. Marginal grafts could be more frequently accepted by patients in critical medical situations or patients who perceive their situation as critical [8]. In turn, this acceptance may help reduce mortality in those on transplant waiting lists, benefiting the largest number of recipients [9]. Cancer in donors is considered as a transmissible disease that seriously threatens the recipient’s life, which is the main reason why these organs are applied with great caution. Therefore, we should weigh the benefits and threats of transplanted organs from DWCH. Careful evaluation and vigilant screening of potential DWCH can provide valuable data for transplant teams and may identify additional candidates [10,11].
In the present study, we found that the number of transplants from DWCH gradually increased yearly. However, we observed no increase in the proportion of DWCH, as they were a low percentage of all donors (Figure 1C). A total of 4696 (2.24%) kidney transplants and 2713 (3.12%) liver transplants came from DWCH from January 1, 2000 through December 31, 2014 in the SRTR database. NMSC, genitourinary cancer, and CNS cancer were the 3 most frequent cancer types associated with transplants. Notably, liver and heart transplant recipients in the DWCH group had similar outcomes to recipients of these transplants in the ECD group, whereas kidney and lung transplant recipients in the DWCH group had even better outcomes than recipients of these transplants in the ECD group. All these results suggest that DWCH are a qualified source for organ transplantation.
In the literature, attention is always paid to the weaknesses of DWCH, such as DTC. For the first time, we focused on the long-term outcomes of recipients who were transplanted with organs from DWCH in comparison to those receiving organs from DWNCH based on analysis of a nationwide database. In an adjusted survival analysis, the data demonstrated that liver and heart transplant recipients in the DWCH group had lower patient and graft survival than those in the DWNCH group. According to our study, DWCH had the greatest impact on heart transplantation. Recipients of organs from DWCH had lower adjusted 5-year patient survival rates and graft survival rates in heart transplantation, especially for those receiving organs from donors with hematologic malignancy and otorhinolaryngologic cancer. The treatment for these cancers, chemotherapy or radiotherapy, might lead to cardiotoxicity or radioactive myocardial damage [12–14]. Therefore, heart grafts from these donors should be carefully evaluated before their use in heart transplantation.
Our results showed that liver transplantation using organs from DWCH was associated with significantly poorer patient and graft survival. It has been reported that liver transplants from ECD, including DWCH, have comparable outcomes to those from referenced donors [15]. Even though grafts from DWCH affect overall survival, apparently not all types of cancer are risk factors for survival. A single-center study came to the conclusion that there is no difference in survival between recipients of grafts from donors with CNS tumors and recipients of grafts from donors without CNS tumors in liver transplantation [16]. Similarly, Warrens et al. showed that life expectancy is not shorter for recipients of a liver from a donor with a primary CNS tumor than for recipients of a liver from a donor without a primary CNS tumor [17]. However, the present study showed that the graft survival and cancer-free survival of recipients of organs from donors with a CNS cancer history were poorer in liver transplantation than the graft and cancer-free survival of recipients of organs from DWNCH. The reason for this association is largely unknown because we were unable to obtain detailed cancer characteristics from the database. As a result, livers from donors with CNS cancer should be cautiously assessed.
Even though the adjusted 5-year graft survival was a significantly different in kidney transplantation between the 2 groups, this slight difference is worth neglecting owing to the benefit to candidates. Lung transplantation was an exception in that no difference was documented between the 2 groups. These survival analysis results should be provided to transplant candidates for decision-making when organs from DWCH become available.
It is well-known that solid-organ transplant recipients are at increased risk of developing cancer compared with the general population [18]. In this study, we found that kidney transplant recipients in the DWCH group had a significantly higher incidence of malignancy than those in the DWNCH group. However, after adjustment for potential confounders, the cumulative probability of malignancy was not significantly different in the 4 transplant types. Therefore, under the current practice, the use of organs from DWCH does not increase the long-term incidence of malignancy. Notably, information is absent in the SRTR database concerning whether the malignancy is transmitted, recurrent, or de novo. We recommend that these data be added to the SRTR database to better assess the transmission risk of using organs from DWCH.
The current analysis showed comparable outcomes in kidney and lung transplantation using allografts from DWCH versus DWNCH. However, when considering donor cancer type, adjusted hazard assessment suggested that genitourinary and gastrointestinal cancer histories in donors are risk factors for patient and graft survival in kidney transplantation. Carcinogenesis is a multistep process and a multi-systemic disease, indicating that donors with genitourinary and gastrointestinal cancer histories have poorer kidney quality [19]. Cancer patients experience kidney injury from multiple sources, including the tumor itself, diagnostic procedures, hypovolemia, infection, and drug exposure, which is superimposed upon baseline chronic damage [20]. It is worth noting that 994 kidney transplants were from donors with a genitourinary cancer history, which represented more than 1/5 of all cancer donors involved in kidney transplantation during the study period. Studies have shown that kidney injury is commonly a frequent and significant complication of cancer and cancer therapy, especially in genitourinary cancers, such as prostate cancer and bladder cancer [21–23]. This tendency in organ allocation should be reevaluated. Moreover, our analysis showed that recipients of organs from donors with a CNS cancer history had equivalent patient and graft survival and better cancer-free survival in kidney transplantation than recipients of organs from DWNCH, which is consistent with the results reported by Tatar et al. in a single-center study [24].
Although we have provided a comprehensive assessment of how using organs from DWCH affects transplant outcomes, there are some limitations in this study. In the SRTR database, some variables with high missing value rates were excluded from the analysis. The exclusion of these variables might result in selection bias. Moreover, the SRTR database lacks complete documentation of donor cancer history and detailed follow-up of malignancy in recipients, which might lead to underestimates of the incidence of DWCH and development of post-transplant malignancy. Another limitation is that outcomes were not analyzed in pancreas and intestine transplantation due to the small number of DWCH involved in these cases. These limitations call for a well-designed cohort study concerning the use of organs from DWCH to provide high-level evidence for guiding clinical practice.
Conclusions
The current comprehensive analysis of the outcomes suggests that under the current guidelines, DWCH are a qualified source for expanding the donor pool. Organs from DWCH might distinctly affect transplant outcomes in different organ transplant types. Interestingly, transplant outcomes are specifically affected by certain categories of donor cancers in different organ transplantations. Therefore, careful risk and benefit assessments of using organs from DWCH should be made before transplantation. A prospective, multi-center, cohort study is required to provide reliable guidance for clinical practice.
Supplementary Tables
Supplementary Table 1.
Donor amount with or without a cancer history from 2000 to 2014 in the SRTR.
| Donor type | Kidney TX | Liver TX | Heart TX | Lung TX | Pancreas TX | Intestine TX | Total |
|---|---|---|---|---|---|---|---|
| DWCH | 4696 | 2713 | 504 | 413 | 50 | 9 | 8385 |
| DWNCH | 209236 | 86891 | 33144 | 21411 | 6180 | 2098 | 372010 |
| Percentage (%) | 2.24 | 3.12 | 1.52 | 1.93 | 0.81 | 0.43 | 2.28 |
Supplementary Table 2.
Clinical characteristics of donors and recipients included in the study.
| Variables | Kidney transplantation | Liver transplantation | Heart transplantation | Lung transplantation | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DWCH | DWNCH | P | DWCH | DWNCH | P | DWCH | DWNCH | P | DWCH | DWNCH | P | ||
| Characteristics of donor | |||||||||||||
| Age | <18 | 18345 | 77 | <0.01 | 11665 | 55 | <0.01 | 23787 | 353 | <0.01 | 2815 | 11 | <0.01 |
| 18–50 | 135387 | 1767 | 48076 | 760 | 6783 | 27 | 15070 | 213 | |||||
| >50 | 55504 | 2852 | 27150 | 1898 | 2574 | 124 | 3526 | 189 | |||||
| Gender | Male | 113302 | 1870 | <0.01 | 51671 | 1228 | <0.01 | 22979 | 258 | <0.01 | 12873 | 161 | <0.01 |
| Female | 95934 | 2826 | 35220 | 1485 | 10165 | 246 | 8538 | 252 | |||||
| Race | White | 173869 | 4396 | <0.01 | 69660 | 2507 | <0.01 | 27079 | 457 | <0.01 | 16470 | 374 | <0.01 |
| Black | 27680 | 230 | 14474 | 168 | 5211 | 34 | 3995 | 27 | |||||
| Asian | 5498 | 44 | 1969 | 29 | 542 | 7 | 500 | 9 | |||||
| Other/unknown | 2155 | 24 | 788 | 9 | 305 | 6 | 172 | 3 | |||||
| Blood type | A | 70690 | 1761 | <0.01 | 31739 | 1009 | 0.19 | 11788 | 211 | 0.03 | 7634 | 166 | 0.30 |
| B | 22302 | 446 | 9935 | 302 | 3468 | 42 | 2273 | 41 | |||||
| AB | 5693 | 120 | 2577 | 62 | 665 | 10 | 441 | 8 | |||||
| O | 110551 | 2369 | 42640 | 1340 | 17223 | 241 | 11063 | 198 | |||||
| Characteristics of recipient | |||||||||||||
| Age (years) | <18 | 9783 | 68 | <0.01 | 8034 | 48 | <0.01 | 9376 | 129 | <0.01 | 711 | 7 | 0.05 |
| 18–50 | 86848 | 1403 | 23432 | 677 | 4964 | 21 | 5905 | 101 | |||||
| >50 | 112605 | 3225 | 55425 | 2713 | 18804 | 354 | 14795 | 305 | |||||
| Gender | Male | 127093 | 2923 | 0.04 | 56555 | 1801 | 0.16 | 23893 | 348 | 0.13 | 12131 | 222 | 0.24 |
| Female | 82143 | 1773 | 30336 | 912 | 9251 | 156 | 9280 | 191 | |||||
| Race | White | 140790 | 3205 | 0.55 | 73164 | 2316 | 0.12 | 25699 | 395 | 0.98 | 19285 | 378 | 0.30 |
| Black | 53994 | 1169 | 8853 | 244 | 6085 | 89 | 1718 | 32 | |||||
| Asian | 11014 | 245 | 3800 | 126 | 956 | 14 | 272 | 1 | |||||
| Other/unknown | 3426 | 77 | 1074 | 27 | 404 | 6 | 135 | 2 | |||||
| Blood type | A | 77749 | 1837 | <0.01 | 32354 | 1071 | <0.01 | 13668 | 227 | 0.33 | 8566 | 184 | 0.30 |
| B | 27150 | 535 | 11716 | 299 | 4659 | 68 | 2381 | 45 | |||||
| AB | 10208 | 226 | 4317 | 124 | 1701 | 21 | 847 | 14 | |||||
| O | 94129 | 2098 | 38504 | 1219 | 13116 | 168 | 9617 | 170 | |||||
| BMI | <25 | 86774 | 1771 | <0.01 | 31046 | 825 | <0.01 | 14771 | 223 | 0.96 | 10994 | 191 | 0.16 |
| 25–<30 | 63203 | 1473 | 26445 | 863 | 10528 | 162 | 7071 | 154 | |||||
| 30–<35 | 39796 | 968 | 15543 | 567 | 5436 | 81 | 3049 | 60 | |||||
| ≥35 | 19463 | 484 | 9164 | 316 | 1581 | 27 | 297 | 8 | |||||
| Unknown | 0 | 0 | 4693 | 142 | 828 | 11 | 0 | 0 | |||||
| Primary diagnosis* | A | 47450 | 1183 | <0.01 | 5013 | 140 | <0.01 | 27645 | 453 | 0.00 | 799 | 17 | 0.97 |
| B | 49590 | 1303 | 65473 | 1999 | 1447 | 22 | 8280 | 121 | |||||
| C | 30353 | 598 | 11657 | 432 | 3101 | 18 | 7701 | 150 | |||||
| D | 80177 | 1583 | 4748 | 142 | 951 | 11 | 6631 | 125 | |||||
| Malignancy history | No | 191219 | 4196 | <0.01 | 78235 | 1901 | 0.33 | 30832 | 447 | <0.01 | 19717 | 371 | 0.04 |
| Yes | 10273 | 310 | 4259 | 144 | 1770 | 45 | 1259 | 27 | |||||
| Unknown | 7310 | 184 | 1879 | 586 | 542 | 12 | 418 | 15 | |||||
| Primary diagnosis* | |||||||||||||
| A | Hypertension | ALF | Cardiomyopathy | Alpha-1 | |||||||||
| B | Diabetes | CLD | CAD | COPD | |||||||||
| C | GN | Malignancy | Congenital | IPF | |||||||||
| D | Other | Other | Other | Other | |||||||||
Acknowledgments
Thanks for SRTR for approving this study.
Footnotes
Source of support: This study was supported by the National Natural Science Foundation of China (81373156, 81471583 and 81570587), the Special Fund for Science Research by the Ministry of Health (201302009), the Key Clinical Specialty Construction Project of National Health and Family Planning Commission of the People’s Republic of China, the Guangdong Provincial Key Laboratory Construction Projection on Organ Donation and Transplant Immunology (2013A061401007), Guangdong Provincial International Cooperation Base of Science and Technology (Organ Transplantation) (2015B050501002), Guangdong Provincial Natural Science Funds for Major Basic Science Culture Project (2015A030308010), Guangdong Provincial Natural Science Funds for Distinguished Young Scholars (2015A030306025), Special Support Program for Training High-level Talent in Guangdong Province (2015TQ01R168), Pearl River Nova Program of Guangzhou (201506010014), and the Science and Technology Program of Guangzhou (201704020150)
Conflicts of interest
None.
References
- 1.OPTN/SRTR 2015 annual data report: Introduction. Am J Transplant. 2017;17(Suppl 1):11–20. doi: 10.1111/ajt.14123. [DOI] [PubMed] [Google Scholar]
- 2.Schold JD, Segev DL. Increasing the pool of deceased donor organs for kidney transplantation. Nat Rev Nephrol. 2012;8:325–31. doi: 10.1038/nrneph.2012.60. [DOI] [PubMed] [Google Scholar]
- 3.Engels EA, Castenson D, Pfeiffer RM, et al. Cancers among US organ donors: A comparison of transplant and cancer registry diagnoses. Am J Transplant. 2014;14:1376–82. doi: 10.1111/ajt.12683. [DOI] [PubMed] [Google Scholar]
- 4.Garrett GL, Blanc PD, Boscardin J, et al. Incidence of and risk factors for skin cancer in organ transplant recipients in the United States. JAMA Dermatol. 2017;153:296–303. doi: 10.1001/jamadermatol.2016.4920. [DOI] [PubMed] [Google Scholar]
- 5.Chapman JR, Lynch SV. Donor-transmitted, donor-derived, and de novo cancer after liver transplant. Exp Clin Transplant. 2014;12:50–54. doi: 10.6002/ect.25liver.l49. [DOI] [PubMed] [Google Scholar]
- 6.Harring TR, O’Mahony CA, Goss JA. Extended donors in liver transplantation. Clin Liver Dis. 2011;15:879–900. doi: 10.1016/j.cld.2011.08.006. [DOI] [PubMed] [Google Scholar]
- 7.Yanik EL, Nogueira LM, Koch L, et al. Comparison of cancer diagnoses between the US solid organ transplant registry and linked central cancer registries. Am J Transplant. 2016;16:2986–93. doi: 10.1111/ajt.13818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kamran S, Conti F, Pomey MP, et al. Patients’ preferences in transplantation from marginal donors: Results of a discrete choice experiment. Transpl Int. 2017;30:589–602. doi: 10.1111/tri.12944. [DOI] [PubMed] [Google Scholar]
- 9.Studer SM, Orens JB. Cadaveric donor selection and management. Respir Care Clin N Am. 2004;10:459–71. doi: 10.1016/j.rcc.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 10.Buell JF, Alloway RR, Woodle ES. How can donors with a previous malignancy be evaluated? J Hepatol. 2006;45:503–7. doi: 10.1016/j.jhep.2006.07.019. [DOI] [PubMed] [Google Scholar]
- 11.Nickkholgh A, Frey E, Krenzel C, et al. The need for vigilance in extended criteria donors with a past history of malignancy: A case report and review of literature. Ann Transplant. 2011;16:75–79. [PubMed] [Google Scholar]
- 12.Di Lisi D, Leggio G, Vitale G, et al. Chemotherapy cardiotoxicity: Cardioprotective drugs and early identification of cardiac dysfunction. J Cardiovasc Med. 2016;17:270–75. doi: 10.2459/JCM.0000000000000232. [DOI] [PubMed] [Google Scholar]
- 13.Kiscsatari L, Sarkozy M, Kovari B, et al. High-dose radiation induced heart damage in a rat model. In Vivo. 2016;30:623–31. [PubMed] [Google Scholar]
- 14.Eldabaje R, Le DL, Huang W, Yang LX. Radiation-associated cardiac injury. Anticancer Res. 2015;35:2487–92. [PubMed] [Google Scholar]
- 15.Heilman RL, Khamash HA, Huskey JL, et al. Kidney transplant program at the mayo clinic in Arizona. Clin Transpl. 2014:61–68. [PubMed] [Google Scholar]
- 16.Kashyap R, Ryan C, Sharma R, et al. Liver grafts from donors with central nervous system tumors: A single-center perspective. Liver Transpl. 2009;15:1204–8. doi: 10.1002/lt.21838. [DOI] [PubMed] [Google Scholar]
- 17.Warrens AN, Birch R, Collett D, et al. Advising potential recipients on the use of organs from donors with primary central nervous system tumors. Transplantation. 2012;93:348–53. doi: 10.1097/TP.0b013e31823f7f47. [DOI] [PubMed] [Google Scholar]
- 18.Campistol JM, Cuervas-Mons V, Manito N, et al. New concepts and best practices for management of pre- and post-transplantation cancer. Transplant Rev (Orlando) 2012;26:261–79. doi: 10.1016/j.trre.2012.07.001. [DOI] [PubMed] [Google Scholar]
- 19.Sugimura T. Multistep carcinogenesis: A 1992 perspective. Science. 1992;258:603–7. doi: 10.1126/science.1411570. [DOI] [PubMed] [Google Scholar]
- 20.Troxell ML, Higgins JP, Kambham N. Antineoplastic treatment and renal injury: An update on renal pathology due to cytotoxic and targeted therapies. Adv Anat Pathol. 2016;23:310–29. doi: 10.1097/PAP.0000000000000122. [DOI] [PubMed] [Google Scholar]
- 21.Campbell GA, Hu D, Okusa MD. Acute kidney injury in the cancer patient. Adv Chronic Kidney Dis. 2014;21:64–71. doi: 10.1053/j.ackd.2013.08.002. [DOI] [PubMed] [Google Scholar]
- 22.Crawford ED, Moul JW. ADT risks and side effects in advanced prostate cancer: Cardiovascular and acute renal injury. Oncology. 2015;29:55–58. [PubMed] [Google Scholar]
- 23.Kwon T, Jeong IG, Lee C, et al. Acute kidney injury after radical cystectomy for bladder cancer is associated with chronic kidney disease and mortality. Ann Surg Oncol. 2016;23:686–93. doi: 10.1245/s10434-015-4886-4. [DOI] [PubMed] [Google Scholar]
- 24.Tatar E, Turan MN, Firat O, et al. Use of kidney donors with hepatitis B, hepatitis C, or brain tumor: A single-center experience. Transplant Proc. 2012;44:1601–3. doi: 10.1016/j.transproceed.2012.04.028. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table 1.
Donor amount with or without a cancer history from 2000 to 2014 in the SRTR.
| Donor type | Kidney TX | Liver TX | Heart TX | Lung TX | Pancreas TX | Intestine TX | Total |
|---|---|---|---|---|---|---|---|
| DWCH | 4696 | 2713 | 504 | 413 | 50 | 9 | 8385 |
| DWNCH | 209236 | 86891 | 33144 | 21411 | 6180 | 2098 | 372010 |
| Percentage (%) | 2.24 | 3.12 | 1.52 | 1.93 | 0.81 | 0.43 | 2.28 |
Supplementary Table 2.
Clinical characteristics of donors and recipients included in the study.
| Variables | Kidney transplantation | Liver transplantation | Heart transplantation | Lung transplantation | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DWCH | DWNCH | P | DWCH | DWNCH | P | DWCH | DWNCH | P | DWCH | DWNCH | P | ||
| Characteristics of donor | |||||||||||||
| Age | <18 | 18345 | 77 | <0.01 | 11665 | 55 | <0.01 | 23787 | 353 | <0.01 | 2815 | 11 | <0.01 |
| 18–50 | 135387 | 1767 | 48076 | 760 | 6783 | 27 | 15070 | 213 | |||||
| >50 | 55504 | 2852 | 27150 | 1898 | 2574 | 124 | 3526 | 189 | |||||
| Gender | Male | 113302 | 1870 | <0.01 | 51671 | 1228 | <0.01 | 22979 | 258 | <0.01 | 12873 | 161 | <0.01 |
| Female | 95934 | 2826 | 35220 | 1485 | 10165 | 246 | 8538 | 252 | |||||
| Race | White | 173869 | 4396 | <0.01 | 69660 | 2507 | <0.01 | 27079 | 457 | <0.01 | 16470 | 374 | <0.01 |
| Black | 27680 | 230 | 14474 | 168 | 5211 | 34 | 3995 | 27 | |||||
| Asian | 5498 | 44 | 1969 | 29 | 542 | 7 | 500 | 9 | |||||
| Other/unknown | 2155 | 24 | 788 | 9 | 305 | 6 | 172 | 3 | |||||
| Blood type | A | 70690 | 1761 | <0.01 | 31739 | 1009 | 0.19 | 11788 | 211 | 0.03 | 7634 | 166 | 0.30 |
| B | 22302 | 446 | 9935 | 302 | 3468 | 42 | 2273 | 41 | |||||
| AB | 5693 | 120 | 2577 | 62 | 665 | 10 | 441 | 8 | |||||
| O | 110551 | 2369 | 42640 | 1340 | 17223 | 241 | 11063 | 198 | |||||
| Characteristics of recipient | |||||||||||||
| Age (years) | <18 | 9783 | 68 | <0.01 | 8034 | 48 | <0.01 | 9376 | 129 | <0.01 | 711 | 7 | 0.05 |
| 18–50 | 86848 | 1403 | 23432 | 677 | 4964 | 21 | 5905 | 101 | |||||
| >50 | 112605 | 3225 | 55425 | 2713 | 18804 | 354 | 14795 | 305 | |||||
| Gender | Male | 127093 | 2923 | 0.04 | 56555 | 1801 | 0.16 | 23893 | 348 | 0.13 | 12131 | 222 | 0.24 |
| Female | 82143 | 1773 | 30336 | 912 | 9251 | 156 | 9280 | 191 | |||||
| Race | White | 140790 | 3205 | 0.55 | 73164 | 2316 | 0.12 | 25699 | 395 | 0.98 | 19285 | 378 | 0.30 |
| Black | 53994 | 1169 | 8853 | 244 | 6085 | 89 | 1718 | 32 | |||||
| Asian | 11014 | 245 | 3800 | 126 | 956 | 14 | 272 | 1 | |||||
| Other/unknown | 3426 | 77 | 1074 | 27 | 404 | 6 | 135 | 2 | |||||
| Blood type | A | 77749 | 1837 | <0.01 | 32354 | 1071 | <0.01 | 13668 | 227 | 0.33 | 8566 | 184 | 0.30 |
| B | 27150 | 535 | 11716 | 299 | 4659 | 68 | 2381 | 45 | |||||
| AB | 10208 | 226 | 4317 | 124 | 1701 | 21 | 847 | 14 | |||||
| O | 94129 | 2098 | 38504 | 1219 | 13116 | 168 | 9617 | 170 | |||||
| BMI | <25 | 86774 | 1771 | <0.01 | 31046 | 825 | <0.01 | 14771 | 223 | 0.96 | 10994 | 191 | 0.16 |
| 25–<30 | 63203 | 1473 | 26445 | 863 | 10528 | 162 | 7071 | 154 | |||||
| 30–<35 | 39796 | 968 | 15543 | 567 | 5436 | 81 | 3049 | 60 | |||||
| ≥35 | 19463 | 484 | 9164 | 316 | 1581 | 27 | 297 | 8 | |||||
| Unknown | 0 | 0 | 4693 | 142 | 828 | 11 | 0 | 0 | |||||
| Primary diagnosis* | A | 47450 | 1183 | <0.01 | 5013 | 140 | <0.01 | 27645 | 453 | 0.00 | 799 | 17 | 0.97 |
| B | 49590 | 1303 | 65473 | 1999 | 1447 | 22 | 8280 | 121 | |||||
| C | 30353 | 598 | 11657 | 432 | 3101 | 18 | 7701 | 150 | |||||
| D | 80177 | 1583 | 4748 | 142 | 951 | 11 | 6631 | 125 | |||||
| Malignancy history | No | 191219 | 4196 | <0.01 | 78235 | 1901 | 0.33 | 30832 | 447 | <0.01 | 19717 | 371 | 0.04 |
| Yes | 10273 | 310 | 4259 | 144 | 1770 | 45 | 1259 | 27 | |||||
| Unknown | 7310 | 184 | 1879 | 586 | 542 | 12 | 418 | 15 | |||||
| Primary diagnosis* | |||||||||||||
| A | Hypertension | ALF | Cardiomyopathy | Alpha-1 | |||||||||
| B | Diabetes | CLD | CAD | COPD | |||||||||
| C | GN | Malignancy | Congenital | IPF | |||||||||
| D | Other | Other | Other | Other | |||||||||