Abstract
Background:
Sedentary behavior is a pervasive public health concern in the general population. To date, little is known regarding the possible health risks associated with sedentary behavior in patients with multiple sclerosis (MS), although this population has increased risks of comorbidities such as hypertension.
Methods:
This cross-sectional study examined the association between sedentary behavior and blood pressure (BP) in 31 patients with MS and 31 matched controls. Self-reported sitting time, one form of sedentary behavior, was measured using the International Physical Activity Questionnaire. Using an automated oscillometric monitor, systolic BP, diastolic BP, and mean arterial pressure were measured in the supine position after 10 minutes of rest lying down in a quiet room.
Results:
There were significant correlations between International Physical Activity Questionnaire–measured sitting time and systolic BP (r = 0.365, P = .044, 95% CI, 0.013–0.636), diastolic BP (r = 0.382, P = .034, 95% CI, 0.032–0.648), and mean arterial pressure (r = 0.425, P = .017, 95% CI, 0.084–0.677) in patients with MS but not in controls (P > .05). The associations in patients with MS were unchanged even after adjusting for body mass index in linear regression analyses.
Conclusions:
This study identified a significant association between sitting time and BP outcomes in patients with MS, supporting the need for additional examinations of sitting time and its possible health consequences in patients with MS.
There is increased awareness of the prevalence and impact of comorbidity in patients with multiple sclerosis (MS).1,2 Hypertension is one of the five leading causes of disability in the general population,3 and hypertension is one comorbid condition that is prevalent and impactful among patients with MS.4 Indeed, hypertension is the third most prevalent comorbidity in MS,5 and, based on self-report, medical records, or formal evaluation, it affects 17.1% to 30.1% of patients with MS in North America.4,6–8 Patients with MS have an increased relative risk of cardiovascular disease, and this risk varies based on disease course.9 Cardiovascular comorbidities, such as hypertension, are linked to more rapid progression of ambulatory disability and increased risk of cane use in patients with MS.1,8 Pulse pressure (ie, the difference between systolic and diastolic pressures) also has been associated with ambulatory functioning in patients with MS.10 Based on this literature, it is clear that hypertension and high blood pressure (BP) are prevalent and problematic conditions for patients with MS. Understanding factors such as lifestyle behaviors that may correlate with BP in patients with MS is important. One recent systematic review identified the importance of research examining common risk factors, such as sedentary behavior, that may influence the risk of comorbidities, such as hypertension.11
Sedentary behavior, defined as any behavior involving sitting or lying down that does not increase energy expenditure during waking hours,12 might be associated with higher BP in MS. Patients with MS spend approximately 7.5 hours per day sitting.13 Sedentary behavior has been associated with putative outcomes of disability status and walking function, but not with cognition, in patients with MS.14 We are unaware of research on the association between sedentary behavior and BP in MS, although sedentary time (eg, hours of sitting in front of the television) has been associated with higher BP15,16 and increased risk of hypertension15 in adults in the general population.
The present study examined the association between sedentary behavior and BP in patients with MS and matched controls. We hypothesized that greater amounts of sedentary behavior would be associated with higher systolic BP (SBP), diastolic BP (DBP), and mean arterial pressure (MAP) in patients with MS based on the frequencies of sedentary behavior and hypertension in patients with MS4 and the relationship between hypertension and sedentary behavior in the general population.15–18 We further hypothesized that the association would be stronger in persons with MS compared with controls considering the possible role of a chronic disease condition.
Methods
Study Sample
The data were secondary outcomes from a previous investigation of vascular dysfunction and physical activity in MS.19 The sample was based on convenience and included 31 patients with MS and 31 persons without MS who were matched on age, sex, height, and weight. The patients with MS were recruited from a laboratory-specific database of participants who were involved in previous research studies of physical activity and who resided within 50 miles of the University of Illinois at Urbana-Champaign campus. The controls were recruited from the university community using an electronic listserv. Participants were contacted by telephone by a member of our research laboratory and underwent a brief screening interview for the following inclusion criteria: 1) being ambulatory with or without a cane, 2) having the visual ability to read 14-point font, 3) being aged 18 to 64 years, 4) being willing to undergo cardiovascular and walking function testing, 5) being abstinent from smoking for a minimum of 6 months, and 6) being willing to abstain from caffeine for 4 hours before testing. Persons with MS were further required to be relapse free for the previous 30 days.
Sitting Time
Sitting time, a form of sedentary behavior, was measured using item 7 from the abbreviated version of the International Physical Activity Questionnaire (IPAQ),20 and scores from this item have been validated by accelerometry.21 This item reads, “During the last 7 days, how much time did you spend sitting on a weekday?” The item further has instructions regarding the location and opportunity for sitting time, such as at work or at home and while doing course work or during leisure time. The item includes examples of sitting activities, such as sitting at a desk, visiting friends, reading, or watching television.
Blood Pressure
Resting SBP, DBP, and MAP were measured using an automated oscillometric monitor (model HEM-907XL; Omron Healthcare Co Ltd, Muko, Kyoto, Japan). All BP measurements were collected after 10 minutes of quiet rest lying down and were recorded in the supine position, rather than sitting or standing, to yield a stabilized, steady-state value.22 Blood pressure can be measured in either the seated or supine position. We measured BP in the supine position because other outcomes were measured concurrently. Furthermore, the supine position removes postural and body positions that can influence BP measures and standardizes these parameters across all participants. We recorded two BP measurements and averaged the values for data analysis. The presence of hypertension was defined according to the World Health Organization guidelines as SBP greater than 140 mm Hg, DBP greater than 90 mm Hg, or taking antihypertensive medications.23 This method has been used in a previous publication.15
Possible Covariates of Sitting Time and Hypertension
Body mass index (BMI), physical activity level, disability status, and the use of antihypertensive medication were collected as covariates in the statistical analyses. Height to the nearest 0.1 cm and weight were measured using a scale-stadiometer unit (model 3P7044; Detecto Scale Co, Webb City, MO). Then, BMI was calculated as weight in kilograms divided by height in meters squared. Physical activity, including but not limited to walking, dancing, gardening, hiking, swimming, or cycling, was measured using the Godin Leisure-Time Exercise Questionnaire (GLTEQ).24 This questionnaire measures the frequency of strenuous, moderate, and mild leisure-time physical activity performed for periods of 15 minutes or more during a typical week. A continuous measure of leisure-time physical activity (arbitrary units) was calculated using responses from the GLTEQ by multiplying the frequencies of strenuous, moderate, and mild activities by 9, 5, and 3 metabolic equivalent units, respectively.25 Disability status was measured using the Patient-Determined Disease Steps (PDDS) scale.26,27 The PDDS scale is a self-reported, single-item measure with scores that range from 0 (normal) to 8 (bedridden). The PDDS scale has been recommended as an alternative to the physician-rated Expanded Disability Status Scale, as PDDS scale scores have been strongly and linearly associated with Expanded Disability Status Scale scores (r = 0.783).27 Using a study-specific demographic questionnaire that captured sex and age, we further identified the use of antihypertensive medication through a single item (“Are you currently taking any prescribed medications?”) that further required the participant to list the medication(s) and dosage(s).
Procedure
The research was approved for the use of human subjects by the University of Illinois at Urbana-Champaign institutional review board. The research study was explained on arrival, and if willing to volunteer, the participants provided written informed consent. Participants then completed a demographic scale, the GLTEQ as a measure of physical activity, the PDDS scale as a measure of disability status, and question 7 of the IPAQ as a measure of sitting time during the past 7 days. Next, participants had their height and weight measured. Finally, participants were taken to a separate, quiet room and underwent 10 minutes of supine rest. We then measured BP metrics in the supine position.
Statistical Analyses
Data analyses were conducted on de-identified data using IBM SPSS Statistics for Windows, version 22.0 (IBM Corp, Armonk, NY). Descriptive statistics are provided in the text and tables as means with standard deviations unless otherwise noted (eg, medians or percentages). We first compared the MS and control groups for differences in sedentary time and BP measures using independent-samples t tests. We then examined the association between sitting time and the BP measures using Pearson product moment correlations in persons with MS and controls separately. Pearson correlations were further calculated between measures of BP and other possible correlates of sitting time and hypertension (ie, antihypertensive medications, BMI, the GLTEQ, and the PDDS scale) for identifying possible confounding variables. These correlations were compared between the MS and control groups using a Fisher Z-transformation and Z-test.28 The final analyses involved multiple hierarchical linear regressions of sitting time in only the patients with MS wherein we used direct entry of all BP-related variables in step 1 (ie, SBP, DBP, and MAP) and all the covariate variables in step 2. All the variables were considered random in the regression equations. We regressed sitting time on the BP outcomes in step 1 and adjusted for the possible covariates of antihypertensive medications, BMI, physical activity levels (GLTEQ), and the PDDS scale through direct entry in step 2. Only variables that demonstrated significant bivariate correlations with sitting time or BP measures were entered in step 2. We examined the independent association between sitting time with BP measures by comparing the standardized β coefficient between steps 1 and 2, and examining the change in R2. Finally, we calculated incremental associations between sitting time and the BP measures based on the unstandardized regression coefficients.
Results
Sample Characteristics
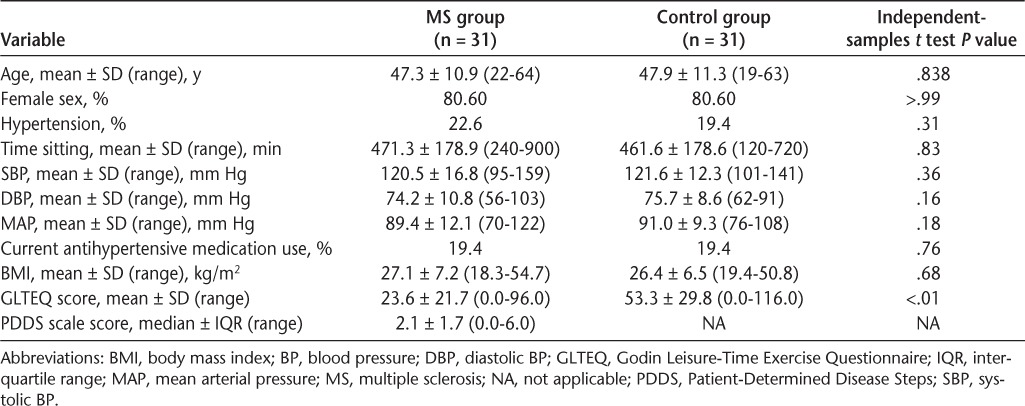
Participants in the MS and control samples were matched based on age, sex, height, and weight (Table 1). The MS sample consisted mostly of persons with relapsing-remitting MS (85%) who had a disease diagnosis for less than 10 years (58%). The median PDDS scale score was 2.1 (interquartile range, 1.7), indicating minimal disability. Six participants (19.4%) in each group were taking antihypertensive medications, and an additional patient with MS (3.2%) was classified as hypertensive based on BP. Thus, 22.6% and 19.4% of participants with MS and control participants, respectively, were hypertensive based on BP and/or taking antihypertensive medications. These differences were not significant (P > .05). There were two patients with MS and one control participant whose BP readings were within the hypertensive range, despite taking medication. These differences were not significant (P > .05).
Table 1.
Descriptive statistics for sitting time and BP outcomes of patients with MS and controls
Descriptive Statistics for Sitting Time and BP Outcomes
Table 1 displays the descriptive statistics for sitting time and BP outcomes. The independent-samples t test did not identify a significant difference in minutes of sitting time between patients with MS and controls (t = 0.21, P = .83, df = 60). The independent-samples t test further did not identify any significant differences between patients with MS and controls for SBP (t = −0.93, P = .36, df = 60), DBP (t = −1.41, P = .16, df = 60), and MAP (t = −1.37, P = .18, df = 60). There was a significant difference in GLTEQ scores between patients with MS and controls based on the independent-samples t test (t = −4.49, P < .01, df = 60), and controls (mean ± SD GLTEQ score = 53.3 ± 29.8) reported higher levels of physical activity than those with MS (mean ± SD GLTEQ score = 23.6 ± 21.7).
Correlations Between Sitting Time and BP Outcomes
The correlations between sitting time and BP outcomes are presented in Table 2. Of note, there were significant correlations between IPAQ-measured sitting time and SBP (r = 0.365, P = .044, 95% CI, 0.013–0.636) and DBP (r = 0.382, P = .034, 95% CI, 0.032–0.648) in those with MS. There also was a significant correlation between sitting time and MAP (r = 0.425, P = .017, 95% CI, 0.084–0.677). There were no significant correlations between the IPAQ measure of sitting time and SBP, DBP, or MAP in controls (P > .05). There were no significant differences between groups (P > .05) when comparing the correlations between the IPAQ measure of sitting time and SBP, DBP, or MAP. Sitting time was not correlated with antihypertensive medications (0 = not taking medication, 1 = taking medication), BMI, or GLTEQ (arbitrary units) in patients with MS or controls; sitting time was unrelated to the PDDS scale (arbitrary units) in MS.
Table 2.
Correlations between sitting time and BP measures in patients with MS and controls
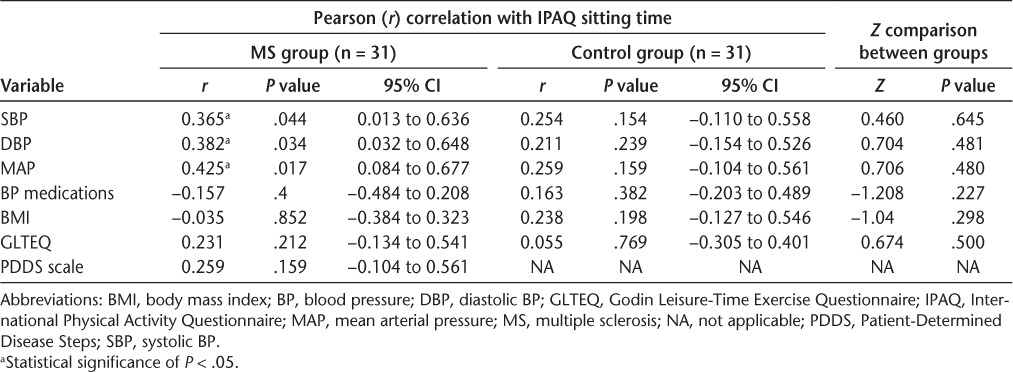
Antihypertensive medications, BMI, and GLTEQ were not significantly correlated with SBP, DBP, or MAP in controls (P > .05). Body mass index had nearly significant correlations with SBP (r = 0.339, P = .062) and MAP (r = 0.326, P = .073) but not with DBP (r = 0.291, P = .112) in patients with MS. Antihypertensive medications, GLTEQ, and PDDS scale were not associated with BP variables in patients with MS (P > .05).
Multiple Hierarchical Linear Regressions
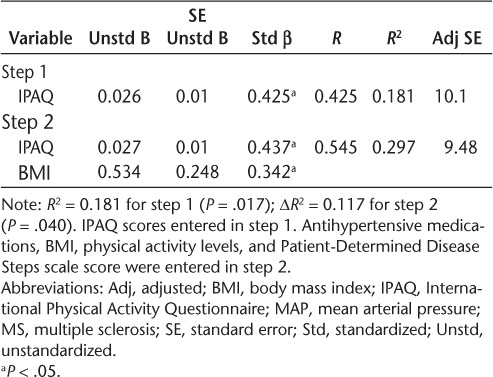
We conducted regression analyses to examine whether the relationships between sitting time and BP measures were independent of BMI in patients with MS (Tables 3–5). The IPAQ measure of sitting time was a significant predictor of SBP in step 1 and explained 13% of the variance (R2 = 0.133, P = .044). The addition of BMI in step 2 explained 12% of the variance beyond IPAQ scores (ΔR2 = 0.124, P = .040). The IPAQ measure of sitting time was a significant predictor of DBP in step 1 and explained 15% of the variance (R2 = 0.146, P = .034). The addition of BMI in step 2 did not explain additional, significant variance (ΔR2 = 0.093, P = .075). The IPAQ measure of sitting time was a significant predictor of MAP in step 1 and explained 18% of the variance (R2 = 0.181, P = .017). The addition of BMI in step 2 explained 12% of the variance beyond IPAQ scores (ΔR2 = 0.117, P = .040). Based on the unstandardized β coefficients from the regression, every 60-minute increase in sitting time would be associated with 2.0–, 1.3–, and 1.6–mm Hg increases in SBP, DBP, and MAP, respectively.
Table 3.
Summary of hierarchical regression analysis for variables predicting SBP in patients with MS
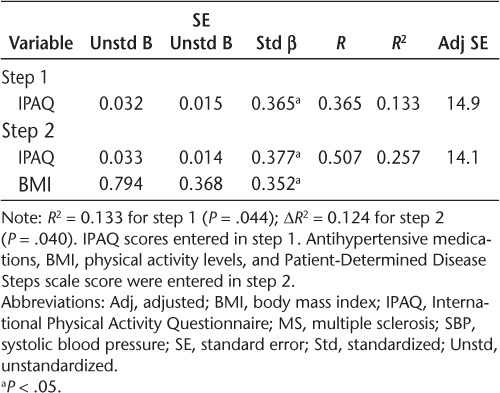
Table 4.
Summary of hierarchical regression analysis for variables predicting DBP in patients with MS
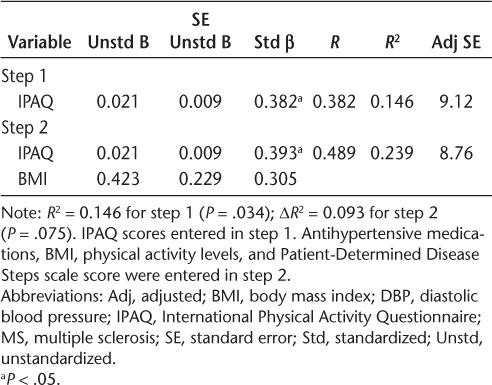
Table 5.
Summary of hierarchical regression analysis for variables predicting MAP in patients with MS
Discussion
Sedentary behavior is prevalent and problematic in developed society12 and worldwide,29 yet we know very little about its associations with comorbidities such as BP in persons with chronic diseases such as MS. To that end, the present study examined the association of self-reported sitting time with BP outcomes in patients with MS versus controls. Overall, there were no significant differences between the two groups for sitting time, SBP, DBP, and MAP. Importantly, both groups spent approximately 7.5 hours per day sitting, and this is generally consistent with previous studies.30,31 Approximately 22.6% of our patients with MS had hypertension based on either taking antihypertensive medications or the presence of elevated BP, and this is consistent with estimates from previous research that range from 17.1% to 30.1% of patients with MS in North America.4,6–8 The BP metrics indicated that participants in both samples were generally not hypertensive according the World Health Organization's guidelines.23 Interestingly, the present data identified moderate, statistically significant correlations (r = 0.365–0.425) between all BP outcomes and sitting time for the patients with MS but not for the controls, although these correlations did not significantly differ between groups. Such results indicate that a higher amount of time sitting was moderately associated with higher values for SBP, DBP, and MAP in patients with MS, and this was independent of BMI. We are intrigued by these findings that identify sitting time as a correlate of BP in MS.
We considered BMI and disability status, in particular, as possible explanations for the association between sitting time and BP measures in MS. This was based on two previous studies that identified BMI as a possible mediator for the relationship between television viewing and BP-related outcomes in healthy adult populations.15,17 We further note that disability is associated with sitting time and increased risk of cardiovascular comorbidities in MS.1 Our correlational analyses between BP and other possible correlates (ie, BMI, PDDS scale, GLTEQ, and BP medications) identified nearly significant correlations between only BMI and two of the three BP measures (SBP and MAP) in patients with MS. To that end, we calculated multiple hierarchical linear regressions between sitting time and BP measures, adjusting for BMI only in patients with MS. The IPAQ measure of sitting time accounted for 13%, 15%, and 18% of the variance in SBP, DBP, and MAP, respectively. The association between sitting time and DBP was unaffected in the additional analyses adjusting for BMI (ΔR2 = 0.093, P = .075). Interestingly, the present analyses indicated that BMI was a significant predictor of additional variance for SBP (ΔR2 = 0.124, P = .040) and MAP (ΔR2 = 0.117, P = .040). These data suggest that BMI is not the likely explanation for the association between sitting time and BP metrics in MS, but there may be an interaction between BMI and sitting time in MS that should be researched further.
We additionally considered leisure-time physical activity as a possible covariate of the association between sitting time and BP outcomes. Leisure-time physical activity includes activities such as walking, dancing, gardening, hiking, swimming, and cycling. The present analyses demonstrated a significant and large difference in physical activity between patients with MS and controls. The literature supports this finding32–35 and suggests that people with MS do not engage in enough physical activity to acquire its benefits. However, GLTEQ scores were not significantly related to sitting time or the BP measures and were not included in the multiple hierarchical linear regressions. Note that sedentary behavior and leisure-time physical activity are two separate constructs,12 instead of the absence or presence of one or the other. Indeed, there is a general weak association between sedentary behavior and physical activity,36,37 and these represent separate and distinct risk factors for chronic, noncommunicable diseases.12 Thus, we are not inclined to offer leisure-time physical activity as a possible explanation for the correlation between sitting time and BP metrics in MS.
Sitting time was not associated with any of the BP measures in the control sample without MS. These results are consistent with previous literature identifying significant associations between objectively measured sitting time and waist circumference, triglyceride levels, and C-reactive protein level but not SBP and DBP in adults from the National Health and Nutrition Examination Survey cohort.38 Other data on adults from Canada indicated significant but inconsistent relationships between sedentary time variables and BP, such that sitting time was associated with DBP but not with SBP and sedentary breaks were associated with SBP but not with DBP.39
There are other possibilities that might explain the association between sitting time and BP in persons with MS but not in controls. One possibility is other health behaviors, such as diet and smoking. Dietary sodium intake has been linked to disability status in persons with MS.40 Smoking has been associated with a progressive disease course, increased progression in clinical disability, increased lesion volumes, and brain atrophy in MS.41,42 These findings suggest that health behaviors exert profound effects in MS. Perhaps those with MS who are sedentary also have poor dietary behaviors and smoke, and both of those health behaviors may be associated with BP. However, we do not have data on dietary intake, and none of our participants were current smokers. Other environmental factors, such as living in an urban versus a residential neighborhood, pet ownership, and employment status and environment, may affect this relationship. It is also possible that the stronger relationship between sitting time and BP may be associated with something unique about MS and its pathophysiology. Neuroanatomical changes, including lesions in the brainstem and upper spinal cord, might interfere with autonomic control and influence BP and perhaps sedentary behavior.9 Some research demonstrated that brain lesion load in MS was correlated with cardiovascular autonomic dysfunction.43 Others have reported that the metaboreflex and carotid baroreflex are altered in MS, affecting BP control.44,45 These should be considered as possible mechanisms in future research. These possible explanations warrant further investigations in persons with MS regarding sitting time and BP that include behavioral, environmental, and pathophysiologic outcomes.
Sitting time may be a new target for reducing cardiovascular comorbid conditions in patients with MS, and the associated impact in MS could be great. Sedentary behavior and hypertension, individually, may contribute to the disease process of advancing MS.46 We identified significant associations between sitting time and BP in the present sample of patients with MS, although our sample had relatively mild disability, primarily presented with a relapsing-remitting disease course, and was slightly overweight based on BMI. This is promising as an initial examination and clearly addresses our research question, and one might expect even stronger associations in persons with moderate or severe MS disability wherein there is more sitting time13 and possibility more cardiovascular comorbidity.47 Such a possibility requires verification in future research. Future research might be strengthened by conducting 24-hour ambulatory monitoring of BP when considering this association with sedentary behavior. Nevertheless, we observed significant associations between sitting time and BP metrics even in this sample with relatively mild MS disability, and this suggests that BP might be an important cardiovascular disease outcome of sedentary behavior change interventions. Importantly, conditions such as hypertension are reasonably prevalent and associated with enough morbidity in the MS population to warrant specific prevention efforts,11 and behavioral modifications might be one such approach for reducing sitting time. To that end, prescriptions that decrease sitting time in patients with MS could possibly reduce cardiovascular comorbid conditions and disability progression associated with cardiovascular comorbidities.8,46 Such results as ours and those from the general population literature29 suggest that sedentary time may have consequences in patients with MS and further support the assessment of sedentary time as a target for reducing comorbidities and secondary consequences.
This study is not without limitations. The present study is a secondary analysis of data collected for another study. Sedentary behavior was measured by self-report of sitting time, and we did not include an objective assessment of all forms of sedentary behaviors (eg, lying down or stationary standing). However, the self-report measure used has been validated previously21 and yielded sitting time similar to that of other previous investigations in MS.21,30 The present sample size was relatively small and homogenous, with minimal disability. The small sample size has limited power and may be responsible for the nonsignificance of many correlations reported herein. We did not collect data on smoking history or physical therapy participation. The sample was mostly female, married, employed, and white and had children. On average, the participants with MS had relapsing-remitting MS for less than 10 years. The present results are not necessarily generalizable to those with more severe disability or who are nonambulatory, with progressive MS, or who are black, and may not be representative of the entire MS population. The black community, in particular, typically has a more severe disease progression and exhibits higher rates of hypertension,48 and this warrants a similar study for persons in this demographic group. There is a potential for selection bias and cohort effect bias in the present study, and physically active participants may have preferentially volunteered for this study; however, this does not seem to be the case considering the difference in GLTEQ scores between patients with MS and controls. We used self-report measures, which have the potential for recall bias. Our recruitment methods differed between groups, which may attenuate the comparability of the results between persons with MS and controls. Nonetheless, the strengths of this study include the novel association between sedentary time and BP metrics using age- and weight-matched patients with MS and controls.
In conclusion, overall this study identifies a significant association between increased sedentary time and increased BP outcomes in MS. These data prompt the need for additional examinations of sitting time and its possible consequences in the risk of cardiovascular comorbidity in patients with MS and behavioral interventions designed to combat sedentary time in patients with MS. The reduction of sitting time might yield desirable consequences for outcomes in MS, especially in persons who are ambulatory. For example, future research could include cardiovascular outcomes and objectively measured sedentary time. Future behavioral interventions could include reducing or breaking up sedentary time through intermittent activity breaks or a walking program. We await additional research in this area that examines sedentary behavior and its role in MS.
PRACTICE POINTS
Cardiovascular comorbidities such as hypertension are linked to more rapid progression of ambulatory disability and increased risk of cane use in patients with MS.
This study observed significant correlation between increased sitting time and increased blood pressure metrics in patients with MS.
Sitting time may be a new target for reducing cardiovascular comorbidity in patients with MS.
Financial Disclosures:
The authors have no conflicts of interest to disclose.
Funding/Support:
None.
References
- 1. Marrie RA, Horwitz RI.. Emerging effects of comorbidities on multiple sclerosis. Lancet Neurol. 2010; 9: 820– 828. [DOI] [PubMed] [Google Scholar]
- 2. Culpepper WJ 2nd. . The incidence and prevalence of comorbidity in multiple sclerosis. Mult Scler. 2015; 21: 261– 262. [DOI] [PubMed] [Google Scholar]
- 3. Centers for Disease Control and Prevention (CDC) Prevalence of disabilities and associated health conditions—United States, 1991–1992. MMWR Morb Mortal Wkly Rep. 1994; 43: 730– 731, 737– 739. [PubMed] [Google Scholar]
- 4. Marrie RA, Yu BN, Leung S, . et al. Rising prevalence of vascular comorbidities in multiple sclerosis: validation of administrative definitions for diabetes, hypertension, and hyperlipidemia. Mult Scler. 2012; 18: 1310– 1319. [DOI] [PubMed] [Google Scholar]
- 5. Marrie RA, Cohen J, Stuve O, . et al. A systematic review of the incidence and prevalence of comorbidity in multiple sclerosis: overview. Mult Scler. 2015; 21: 263– 281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Pohar SL, Jones CA, Warren S, Turpin KVL, Warren K.. Health status and health care utilization of multiple sclerosis in Canada. Can J Neurol Sci. 2007; 34: 167– 174. [DOI] [PubMed] [Google Scholar]
- 7. Marrie RA, Horwitz R, Cutter G, Tyry T, Campagnolo D, Vollmer T.. Comorbidity, socioeconomic status and multiple sclerosis. Mult Scler. 2008; 14: 1091– 1098. [DOI] [PubMed] [Google Scholar]
- 8. Marrie RA, Rudick R, Horwitz R, . et al. Vascular comorbidity is associated with more rapid disability progression in multiple sclerosis. Neurology. 2010; 74: 1041– 1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Roshanisefat H, Bahmanyar S, Hillert J, Olsson T, Montgomery S.. Multiple sclerosis clinical course and cardiovascular disease risk: Swedish cohort study. Eur J Neurol. 2014; 21: 1353– e88. [DOI] [PubMed] [Google Scholar]
- 10. Heffernan KS, Ranadive S, Weikert M, . et al. Pulse pressure is associated with walking impairment in multiple sclerosis. J Neurol Sci. 2011; 309: 105– 109. [DOI] [PubMed] [Google Scholar]
- 11. Marrie RA, Reider N, Cohen J, . et al. A systematic review of the incidence and prevalence of cardiac, cerebrovascular, and peripheral vascular disease in multiple sclerosis. Mult Scler. 2015; 21: 318– 331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sedentary Behaviour Research Network Letter to the Editor: Standardized use of the terms “sedentary” and “sedentary behaviours.”. Appl Physiol Nutr Metab. 2012; 37: 540– 542. [DOI] [PubMed] [Google Scholar]
- 13. Hubbard EA, Motl RW, Manns PJ.. The descriptive epidemiology of daily sitting time as a sedentary behavior in multiple sclerosis. Disabil Health J. 2015; 8: 594– 601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hubbard EA, Motl RW.. Sedentary behavior is associated with disability status and walking performance, but not cognitive function, in multiple sclerosis. Appl Physiol Nutr Metab. 2014; 40: 203– 206. [DOI] [PubMed] [Google Scholar]
- 15. Pouliou T, Ki M, Law C, Li L, Power C.. Physical activity and sedentary behaviour at different life stages and adult blood pressure in the 1958 British cohort. J Hypertens. 2012; 30: 275– 283. [DOI] [PubMed] [Google Scholar]
- 16. Celis-Morales CA, Perez-Bravo F, Ibañez L, Salas C, Bailey MES, Gill JMR.. Objective vs. self-reported physical activity and sedentary time: effects of measurement method on relationships with risk biomarkers. PLoS One. 2012; 7: e36345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Pinto Pereira SM, Ki M, Power C.. Sedentary behaviour and biomarkers for cardiovascular disease and diabetes in mid-life: the role of television-viewing and sitting at work. PLoS One. 2012; 7: e31132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chase JM, Lockhart CK, Ashe MC, Madden KM.. Accelerometer-based measures of sedentary behavior and cardiometabolic risk in active older adults. Clin Invest Med. 2014; 37: E108– E116. [DOI] [PubMed] [Google Scholar]
- 19. Ranadive S, Yan H, Weikert M, . et al. Vascular dysfunction and physical activity in multiple sclerosis. Med Sci Sports Exerc. 2012; 44: 238– 243. [DOI] [PubMed] [Google Scholar]
- 20. Craig CL, Marshall AL, Sjostrom M, . et al. International physical activity questionnaire: 12-country reliability and validity. Med Sci Sports Exerc. 2003; 195: 1381– 1395. [DOI] [PubMed] [Google Scholar]
- 21. Rosenberg DE, Bull FC, Marshall AL, Sallis JF, Bauman AE.. Assessment of sedentary behavior with the International Physical Activity Questionnaire. J Phys Act Health. 2008; 5: 30S– 44S. [DOI] [PubMed] [Google Scholar]
- 22. Keselbrener L, Akselrod S, Ahiron A, Eldar M, Barak Y, Rotstein Z.. Is fatigue in patients with multiple sclerosis related to autonomic dysfunction? Clin Auton Res Off J Clin Auton Res Soc. 2000; 10: 169– 175. [DOI] [PubMed] [Google Scholar]
- 23. Guidelines Subcommittee: 1999 World Health Organization–International Society of Hypertension Guidelines for the Management of Hypertension. J Hypertens. 1999; 17: 151– 183. [PubMed] [Google Scholar]
- 24. Godin G, Shephard RJ.. A simple method to assess exercise behavior in the community. Can J Appl Sport Sci. 1985; 10: 141– 146. [PubMed] [Google Scholar]
- 25. Gosney JL, Scott JA, Snook EM, Motl RW.. Physical activity and multiple sclerosis: validity of self-report and objective measures. Fam Community Health. 2007; 30: 144– 150. [DOI] [PubMed] [Google Scholar]
- 26. Rizzo MA, Hadjimichael OC, Preiningerova J, Vollmer TL.. Prevalence and treatment of spasticity reported by multiple sclerosis patients. Mult Scler. 2004; 10: 589– 595. [DOI] [PubMed] [Google Scholar]
- 27. Learmonth YC, Motl RW, Sandroff BM, Pula JH, Cadavid D.. Validation of Patient Determined Disease Steps (PDDS) scale scores in persons with multiple sclerosis. BMC Neurol. 2013; 13: 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Cohen J, Cohen P, West SG, Aiken LS.. Applied Multiple Regression/Correlation Analysis for the Behavioral Sciences. New York, NY: Routledge; 2013. [Google Scholar]
- 29. Bauman A, Ainsworth BE, Sallis JF, . et al. The descriptive epidemiology of sitting: a 20-country comparison using the International Physical Activity Questionnaire (IPAQ). Am J Prev Med. 2011; 41: 228– 235. [DOI] [PubMed] [Google Scholar]
- 30. Cavanaugh JT, Gappmaier VO, Dibble LE, Gappmaier E.. Ambulatory activity in individuals with multiple sclerosis. J Neurol Phys Ther. 2011; 35: 26– 33. [DOI] [PubMed] [Google Scholar]
- 31. Matthews CE, Chen KY, Freedson PS, . et al. Amount of time spent in sedentary behaviors in the United States, 2003–2004. Am J Epidemiol. 2008; 167: 875– 881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Motl RW, McAuley E, Snook EM.. Physical activity and multiple sclerosis: a meta-analysis. Mult Scler. 2005; 11: 459– 463. [DOI] [PubMed] [Google Scholar]
- 33. Klaren RE, Motl RW, Dlugonski D, Sandroff BM, Pilutti LA.. Objectively quantified physical activity in persons with multiple sclerosis. Arch Phys Med Rehabil. 2013; 94: 2342– 2348. [DOI] [PubMed] [Google Scholar]
- 34. Motl RW. Lifestyle physical activity in persons with multiple sclerosis: the new kid on the MS block. Mult Scler. 2014; 20: 1025– 1029. [DOI] [PubMed] [Google Scholar]
- 35. Rietberg MB, van Wegen EEH, Kollen BJ, Kwakkel G.. Do patients with multiple sclerosis show different daily physical activity patterns from healthy individuals? Neurorehabil Neural Repair. 2014; 28: 516– 523. [DOI] [PubMed] [Google Scholar]
- 36. Biddle SJ, Gorely T, Marshall SJ, Murdey I, Cameron N.. Physical activity and sedentary behaviours in youth: issues and controversies. J R Soc Promot Health. 2004; 124: 29– 33. [DOI] [PubMed] [Google Scholar]
- 37. Ekelund U, Brage S, Froberg K, . et al. TV viewing and physical activity are independently associated with metabolic risk in children: the European Youth Heart Study. PLoS Med. 2006; 3: e488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Healy GN, Matthews CE, Dunstan DW, Winkler EAH, Owen N.. Sedentary time and cardio-metabolic biomarkers in US adults: NHANES 2003-06. Eur Heart J. 2011; 32: 590– 597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Carson V, Wong SL, Winkler E, Healy GN, Colley RC, Tremblay MS.. Patterns of sedentary time and cardiometabolic risk among Canadian adults. Prev Med. 2014; 65: 23– 27. [DOI] [PubMed] [Google Scholar]
- 40. Farez MF, Fiol MP, Gaitán MI, Quintana FJ, Correale J.. Sodium intake is associated with increased disease activity in multiple sclerosis. J Neurol Neurosurg Psychiatry. 2015; 86: 26– 31. [DOI] [PubMed] [Google Scholar]
- 41. Pittas F, Ponsonby A-L, van der Mei IAF, . et al. Smoking is associated with progressive disease course and increased progression in clinical disability in a prospective cohort of people with multiple sclerosis. J Neurol. 2009; 256: 577– 585. [DOI] [PubMed] [Google Scholar]
- 42. Zivadinov R, Weinstock-Guttman B, Hashmi K, . et al. Smoking is associated with increased lesion volumes and brain atrophy in multiple sclerosis. Neurology. 2009; 73: 504– 510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Saari A, Tolonen U, Pääkkö E, . et al. Cardiovascular autonomic dysfunction correlates with brain MRI lesion load in MS. Clin Neurophysiol. 2004; 115: 1473– 1478. [DOI] [PubMed] [Google Scholar]
- 44. Huang M, Allen DR, Keller DM, Fadel PJ, Frohman EM, Davis SL.. Impaired carotid baroreflex control of arterial blood pressure in multiple sclerosis. J Neurophysiol. 2016; 116: 81– 87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Marongiu E, Olla S, Magnani S, . et al. Metaboreflex activity in multiple sclerosis patients. Eur J Appl Physiol. 2015; 115: 2481– 2490. [DOI] [PubMed] [Google Scholar]
- 46. Motl RW. Physical activity and irreversible disability in multiple sclerosis. Exerc Sport Sci Rev. 2010; 38: 186– 191. [DOI] [PubMed] [Google Scholar]
- 47. Marrie RA, Horwitz R, Cutter G, Tyry T, Campagnolo D, Vollmer T.. Comorbidity delays diagnosis and increases disability at diagnosis in MS. Neurology. 2009; 72: 117– 124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Ferdinand KC, Nasser SA.. Understanding the importance of race/ethnicity in the care of the hypertensive patient. Curr Hypertens Rep. 2015; 17: 15. [DOI] [PubMed] [Google Scholar]


