Abstract
Background:
Chronic pain is a common symptom in people with multiple sclerosis (MS) and often requires a multimodal approach to care. The practice of mindfulness has been shown to decrease the experience of pain in other conditions, yet little is known about the relationship between mindfulness and pain in people with MS. The objective of this study was to evaluate the association between pain interference and trait mindfulness in people with MS.
Methods:
In this cross-sectional survey, 132 people with any type of MS completed the Patient-Reported Outcomes Measurement Information System Pain Interference scale and the Five Facet Mindfulness Questionnaire. Linear regression was used to test the association between pain and mindfulness while adjusting for demographic and MS-related characteristics.
Results:
The relationship between pain and mindfulness was clinically meaningful and highly significant (t = −5.52, P < .0001). For every 18-point increase in mindfulness scores, pain interference scores are expected to decrease by 3.96 (95% CI, −2.52 to −5.40) points (β = −0.22, P < .0001). The adjusted model, including age, type of MS, the interaction between mindfulness and age, and the interaction between mindfulness and MS type, explains 26% of the variability in pain interference scores (R2 = 0.26).
Conclusions:
These results suggest a clinically significant association between mindfulness and pain interference in MS and support further exploration of mindfulness-based interventions in the management of MS-related pain.
Up to 80% of people with multiple sclerosis (MS) experience pain,1,2 and up to one-third report that pain is either a significant or the most significant symptom of their disease.3,4 Pain often interferes with activities of daily living and sleep, compromising occupational and social roles for many.5,6 People experience various types of pain, including central neuropathic pain (eg, dysesthetic extremity pain, tonic muscle spasm, or trigeminal neuralgia), musculoskeletal pain (such as low back pain), or mixed neuropathic and nonneuropathic forms of pain (eg, headaches).2 There are limited pharmacological trial data for managing MS-related pain, and many treatment plans are derived from evidence of effect in similar conditions and clinical experience.7,8 In addition to treating pain directly, the effect of pain on mood, sleep, mobility, and social roles often needs to be addressed with a multimodal approach to care.9 A recent survey by Ehde et al.10 found that people with MS used an average of nine methods to manage their pain, medications being the most frequently tried approach, with few treatments providing even moderate relief. Many people with MS report dissatisfaction with their pain management plans,6,10 and there is a compelling need to identify effective interventions that reduce the experience of pain.
Mindfulness is the ability to be wholly present with one's experience.11 Jon Kabat-Zinn first studied the practice of mindfulness as a behavioral intervention for pain in 198212 and describes mindfulness as “paying attention in a particular way; on purpose, in the present moment, and non-judgmentally.”13(p4) Observing without judgment is a distinguishing feature of mindfulness, and this includes one's experience of pain. In mindfulness practice, there is an attempt to let go of defense, resistance, or protection against pain and a movement toward acceptance.12 Mindfulness-based intervention studies have found positive effects for chronic pain in fibromyalgia, low back pain, arthritis, and a variety of somatization disorders,14–18 but very few trials have been conducted in MS. Three small mindfulness-based MS trials have demonstrated significant trends toward pain reduction, yet results are difficult to interpret due to limitations in sample size and study design.19–21 To explore the appropriateness of future mindfulness-based interventions targeting pain in MS, we evaluated the relationship between pain interference and trait mindfulness in 132 people with MS.
Methods
Overview and Study Participants
The methods for this cross-sectional survey have been previously described.22 After approval was received from the Oregon Health & Science University institutional review board, a convenience sample of men and women was recruited during outpatient visits to the MS Center at the university and through MS community events. Participants completed several questionnaires during one study visit. The inclusion criteria comprised any type of MS, the ability to read and write in English, and age 18 to 90 years. The exclusion criteria included a relapse or exacerbation in the previous 90 days. The MS diagnosis was confirmed by medical record review according to the 2010 McDonald criteria.23 Any questions regarding a participant's diagnosis were discussed with their neurologist. From December 1, 2011, through February 28, 2013, 150 people with MS were recruited, gave written consent, and partook in the study.
Dependent Variable
Pain interference is a subjective measure of how much pain limits one's ability to engage in daily and recreational activities. Pain interference, as measured by the Patient-Reported Outcomes Measurement Information System (PROMIS) Pain–Interference computerized adaptive test (National Institutes of Health [NIH], Bethesda, MD), was the dependent variable in all the analyses. Scores are reported on a T-score metric with a mean ± standard deviation (SD) of 50 ± 10, referenced to the mean for the general US population.24 Thus, a score of 60 on PROMIS Pain–Interference is 1 SD above the mean of a normative US sample. Higher scores indicate increased pain interference. When administered to people with MS, PROMIS Pain–Interference is highly correlated with the Medical Outcomes Study Pain Effects Scale (a validated subscale of the Multiple Sclerosis Quality of Life Inventory25; r = 0.86) and demonstrates strong convergent and discriminatory validity compared with the Pain Effects Scale.26 PROMIS Pain–Interference shows no evidence of differential item functioning between people with MS and those with other disabling conditions (spinal cord injury, muscular dystrophy, postpolio syndrome) or across age groups within MS.27
Independent Variables
Trait mindfulness was the primary predictor variable for these analyses and was measured by the total score on the Five Facet Mindfulness Questionnaire (FFMQ).28 The FFMQ is a 39-item, Likert-type questionnaire that measures five elements of trait mindfulness: observing, describing, acting with awareness, nonjudgment, and nonreactivity, providing a total score and five subscale scores. The overall FFMQ score ranges from 39 to 195, with higher scores indicating higher levels of mindfulness. The subscales show adequate-to-good internal consistency, with the coefficient alpha ranging from 0.72 to 0.92,28 and are sensitive to change in participants of mindfulness-based interventions.29 The FFMQ has not yet been validated for specific use with people with MS.
Six demographic characteristics that might affect the relationship between pain and mindfulness were assessed for potential interaction and confounding: age (continuous variable), MS disease-modifying therapy (DMT) use (categorical; yes or no), education (categorical; some college or less vs. bachelor's degree or higher), MS type (categorical; relapsing-remitting, secondary progressive, or primary progressive), sex (categorical; male or female), and self-reported level of disability (continuous; 6-point scale). The disability scale asked participants to identify which of six statements best described their MS; statements ranged from “I have no or minimal MS-related symptoms, no limitations in my walking ability, and no limitations in daily activities” to “I have many severe MS-related symptoms and am restricted to a wheelchair or bed.” This scale is a modified version of the Patient-Determined Disease Steps scale30 and has previously been shown to correlate with the Expanded Disability Status Scale, a clinician-rated, objective measure of disease severity (r = 0.85).31
Statistical Analysis
Exploratory analysis was conducted to ensure that assumptions of linearity and normality were met. Pairwise correlations were used to explore preliminary relationships between all the variables. Continuous variables are described as mean ± SD and categorical variables as frequency (percentage). Linear regression was used to test the association between pain and mindfulness. A P value < .05 was considered statistically significant for the association between pain and mindfulness. All the independent variables described herein were assessed for interaction with the primary predictor (mindfulness). If including a covariate and its interaction with mindfulness yielded P ≤ .10 for the interaction term, then the covariate and the interaction term were retained in the model. Independent variables that demonstrated no interaction with the primary predictor were further assessed for potential confounding. Any variable that changed the unstandardized regression coefficient for the effect of mindfulness on pain by more than 10% (relative to the simple model) was retained as a covariate in the full model. Diagnostic tests to assess normality, linearity, homoscedasticity, collinearity, and the influence of outliers were conducted for all the models. Collinear terms were centralized for any model with a mean variance inflation factor greater than 10. All the analyses were performed using Stata for Mac, version 14 (StataCorp LLC, College Station, TX).
Results
Sample Size and Participant Characteristics
Two hundred fourteen people with MS were assessed for study eligibility (Figure 1). Sixty-four people declined to participate or did not meet the inclusion criteria; 150 people participated in the study visit. Four participants were dropped from the analysis: MS diagnosis could not be verified (n = 3) and relapse within the past 90 days (n = 1). Fourteen participants were unable to complete the entire study visit due to survey fatigue, and because the PROMIS Pain–Interference questionnaire was administered at the end of the visit; these 14 participants are missing the necessary data for inclusion in the present analysis. Missing (n = 14) and excluded (n = 4) data resulted in a final sample size of 132 for this analysis.
Figure 1.
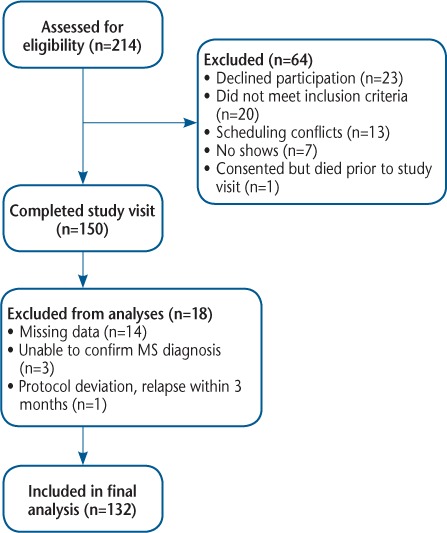
Study flow diagram
MS, multiple sclerosis.
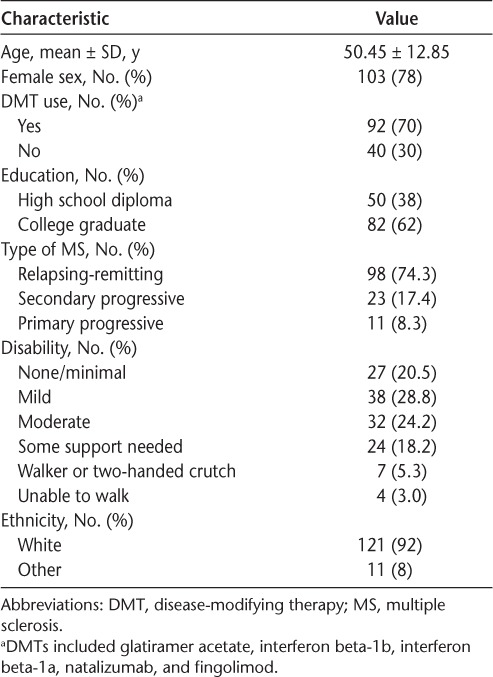
Demographic and clinical characteristics of the study sample are shown in Table 1. Most participants were women (78%), and the mean ± SD age of the study sample was 50.45 ± 12.85 years. Most participants had relapsing-remitting MS (74%), and 70% of participants were taking some kind of MS DMT, including glatiramer acetate, interferon beta-1b, interferon beta-1a, natalizumab, and fingolimod. Seventy-three percent of the participants experienced minimal-to-moderate disability, 23.5% of participants needed an assistive device to walk, and 3% of participants were restricted to a wheelchair. The mean ± SD total mindfulness score was 132 ± 21.5 (respondent range, 74–182; questionnaire range, 39–195). The mean ± SD pain interference score was 52 ± 9.5 (respondent range, 39–72).
Table 1.
Demographic and clinical data (n = 132)
Identification of Potential Covariates, Confounders, and Effect Modifiers
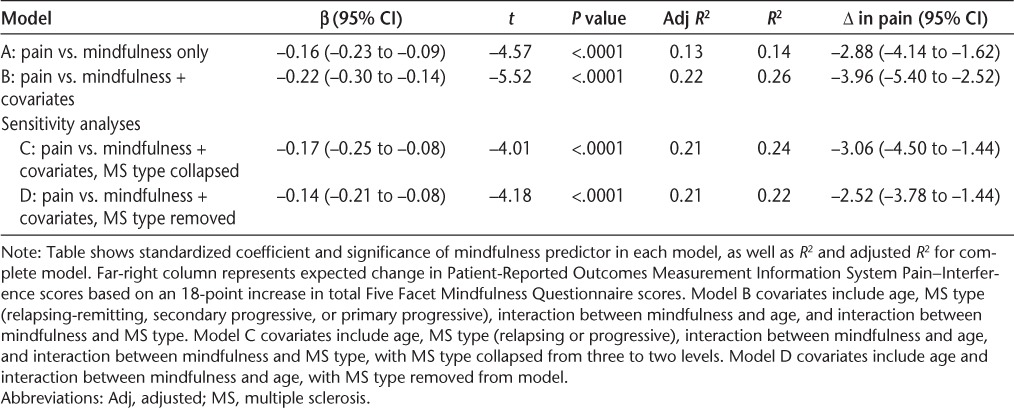
The bivariate Pearson correlation between pain and mindfulness in this sample was r = −0.37 (P < .0001). According to the simple model, for every 18-point increase in the mindfulness score we would expect the Pain–Interference score to decrease by 2.88 points, approximately one-quarter of an SD relative to the general US population (β = −0.16) (Table 2, model A). We chose to look at an 18-point increase in mindfulness scores because, as subsequently discussed, several studies demonstrate a mean increase in the FFMQ of 18 points after mindfulness training. The six demographic characteristics (age, sex, level of disability, type of MS, DMT use, and education) were individually assessed for interaction with the primary predictor, mindfulness. Both MS type (t = 1.84, P = .07) and age (t = 1.92, P = .06) were considered significant for interaction and were retained as predictors in the final model. The four remaining demographic variables were individually assessed for confounding; none of them changed the slope of the relationship between pain and mindfulness by more than 10%. The final association model included mindfulness, age, type of MS, the interaction between mindfulness and age, and the interaction between mindfulness and type of MS (Table 2, model B). Mindfulness and age were centralized to reduce collinearity, bringing the mean variance inflation factor for the model to 1.64.
Table 2.
Crude and adjusted linear regression models for primary relationship between pain interference and trait mindfulness
Adjusted Effects of Mindfulness on Pain–Interference
In the main analysis, adjusted for age, type of MS, the interaction between mindfulness and age, and the interaction between mindfulness and MS type, the relationship between pain and mindfulness was highly significant (t = −5.52, P < .0001). For every 18-point increase in the mindfulness score, the Pain–Interference score is expected to decrease by 3.96 points (β = −0.227, P < .0001). Overall, the model explains 26% of the variability in Pain–Interference scores (R2 = 0.26) (Table 2, model B).
Sensitivity Analyses
Because there was a substantial difference in the number of people with relapsing-remitting MS (n = 98), secondary progressive MS (n = 23), and primary progressive MS (n = 11), we conducted two sensitivity analyses of the variable MS type. In the first analysis we collapsed MS type into two levels (relapsing [n = 98] or progressive [n = 34]) and reran the final association model (Table 2, model C). In the second analysis we removed MS type and the interaction between mindfulness and type and reran the final model (Table 2, model D). When MS type is removed from the model altogether, an 18-point increase in the mindfulness score is expected to result in a 2.5-point decrease in the Pain–Interference score (β = −0.14, P <.0001).
Discussion
Several studies have examined determinants of pain in MS,2,3,6,32 but none have assessed trait mindfulness as a potential predictor of pain interference. These data show a strong and significant association between greater mindfulness and lower levels of pain interference, even after adjusting for age and type of MS. These results echo findings from Schütze et al.,33 who, in a heterogeneous sample of people with chronic pain, found the correlation between pain interference and trait mindfulness to be r = −0.30 (P = .002). In a simple regression model, the authors reported that FFMQ scores accounted for approximately 17% of the variability in pain interference scores, a comparable result to that obtained using the present model A (Table 2). The present analyses suggest that up to 26% of the variability in pain interference could be explained by MS type, age, and mindfulness alone. With the need for more effective approaches to manage MS pain, these findings are notable and warrant further exploration.
In the present study, the raw correlation between pain and disability was r = 0.31 (P = .0002). Some previous studies have reported that MS-related disability is associated with pain,3,6 whereas others have found no association.34,35 Possible explanations for conflicting findings include vast differences in sample sizes (with larger, survey studies more likely to find significant associations), different aspects of pain explored (acute pain, chronic pain, experience of any pain, frequency, intensity, or pain interference), different instruments used to measure different aspects of pain, and differences in how MS-related disability is measured (clinical examination or self-report). Thus, the relationship between pain and disability remains unclear. We did not see a significant association between disability and trait mindfulness (r = −0.012, P = .99), which suggests that interventions intent on building mindfulness skills may enhance trait mindfulness across people with varying degrees of physical abilities.
The primary analysis suggests that with an 18-point increase in the total FFMQ score, one could expect the PROMIS Pain–Interference score to decrease 4 points (95% CI, 2.52- to 5.4-point decrease) (Table 2, model B). The FFMQ is responsive to change, and several trials have demonstrated a mean 18-point increase in total FFMQ scores after mindfulness-based interventions.29,36,37 Our predictions for change are statistically significant and may likewise be clinically meaningful. Minimally important differences have yet to be determined for people with MS, but, for cancer-related pain, Yost et al.38 report that minimally important differences for PROMIS Pain–Interference likely range from 4 to 6 points. The 95% CI for our primary model includes clinically meaningful change, yet the interval is fairly wide, and future studies with larger sample sizes are needed to decrease uncertainty.
Very few mindfulness-based interventions have been conducted with people who have MS,19–21,39,40 and only some of these have assessed the intervention's effect on pain. Three small trials found significant trends toward pain reduction,19–21 but the results are difficult to interpret. Tavee et al.20 conducted an 8-week nonrandomized trial of group meditation training (n = 10 people with MS and 12 people with peripheral neuropathy) versus usual care (n = 7 people with MS and 11 people with peripheral neuropathy). Mean bodily pain scores improved for people with MS in the intervention group (n = 10, P = .03) and remained the same for the control group; between-group analyses were not conducted. Mills and Allen19 conducted a 6-week, one-on-one “mindfulness of movement” intervention using key components of tai chi and qigong (n = 8) compared with usual care (n = 8). The intervention involved “developing a moment to moment awareness of quality of breathing, posture, and movement. Awareness is developed as to whether breathing is shallow or deep, whether posture is aligned or misaligned, and whether movements are integrated with mental preparation or whether there is a sense of mind–body disconnection.”19(p425) Pain was not assessed using a traditional validated measure. Instead, participants completed a 21-symptom questionnaire indicating whether they felt there was improvement or deterioration of symptoms, including pain, on a 5-point scale. Descriptive data seem to indicate that pain improved in the intervention group compared with the control group; however, statistical analysis was conducted for the entire questionnaire only and not for individual symptoms. Both studies had high dropout rates, and intention-to-treat analyses were not conducted. Bogosian et al.21 recently provided online mindfulness training to people with progressive MS, in which mindful-movement components were specifically removed from the program. Participants were randomized to receive the intervention (n = 19) or to a wait list control (n = 21). Pain was assessed using a numerical rating scale from 0 to 10 addressing the average intensity of pain associated with MS. No difference in pain intensity was found immediately after the intervention; however, those who received mindfulness training reported significantly less pain intensity compared with controls 3 months after the intervention.
As interest in mindfulness-based interventions continues to grow, it would be useful for future trials to consistently measure pain, pain interference, and mindfulness. In fact, to our knowledge, none of the mindfulness-based trials in MS have measured mindfulness, limiting our understanding of the change process that occurs with training. We stand to benefit most from this work when researchers use the same patient-reported outcome measures so that meaningful comparisons can be made across studies.
The experience of pain is generated by a combination of experiential and neural cognitive, affective, and sensory processes.41 It is hypothesized that mindfulness meditation modulates the sensory experience of pain by enhancing cognitive control and emotional regulation.42 In his seminal paper on mindfulness and pain, Kabat-Zinn12 described how a meditative state (observing experiences as separate from “self”) can lead to an uncoupling of thoughts (“It's killing me”) and emotions (“I'm scared this will never end”) from the sensory experience of pain, thereby reducing hurt and suffering. In line with this theory, experimental brain imaging studies suggest that the neural networks underlying these experiential processes can also be uncoupled by mindfulness meditation.43 Different meditative practices fall under the umbrella term “mindfulness” (eg, focused attention or open monitoring), each of which activates overlapping yet unique neural networks and can lead to different effects on the pain experience.42–44 Importantly, even brief training in meditative practice can reduce pain by both experiential and neural mechanisms.45
The present data support future investigations of mindfulness-based interventions to reduce pain for people with all types of MS and a wide range of physical abilities. This study has some limitations. The majority of participants were from a single center, and convenience sampling might have led to underrepresentation or overrepresentation of factors in this sample (eg, MS type, MS-related disability); indeed, most of the study sample was white. We did not collect information about socioeconomic status or comorbidities. Fourteen participants experienced fatigue and discontinued the visit before completing the pain interference survey, further complicating our ability to tease out the effect of disability status on the relationship between pain and mindfulness. Nonetheless, our initial findings are closely aligned with other cross-sectional estimates.33 We predicted mindfulness effects on pain interference, but our cross-sectional design precludes determination of the direction of the observed relationships, and alternative models should not be discounted. Regardless of potential limitations, we found highly significant relationships that we believe merit further study.
In conclusion, the present data show a significant relationship between self-reported mindfulness and pain interference scores in people with MS. Levels of mindfulness are malleable and can be increased by mindfulness training. With the need for more effective approaches to manage MS pain and the recent interest in mindfulness-based interventions, these findings are notable and warrant further exploration.
PRACTICE POINTS
Findings from this study suggest a clinically significant association between mindfulness and pain interference in MS.
Community-based mindfulness interventions are safe and relatively low cost and deserve consideration for people with MS in chronic pain.
Financial Disclosures:
The authors have no conflicts of interest to disclose.
Funding/Support:
This work was supported by the Oregon Clinical and Translational Research Institute (grant UL1 RR024140 from the National Center for Research Resources and the National Center for Advancing Translational Sciences of the NIH), the National Center for Complementary and Integrative Health of the NIH (grants AT002688 and AT008211), the Agency for Healthcare Research Quality (grant 5T32HS017582-05), and the Medical Research Foundation of Oregon.
Disclaimer:
The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
References
- 1. Foley PL, Vesterinen HM, Laird BJ, . et al. Prevalence and natural history of pain in adults with multiple sclerosis: systematic review and meta-analysis. Pain. 2013; 154: 632– 642. [DOI] [PubMed] [Google Scholar]
- 2. O'Connor AB, Schwid SR, Herrmann DN, Markman JD, Dworkin RH.. Pain associated with multiple sclerosis: systematic review and proposed classification. Pain. 2008; 137: 96– 111. [DOI] [PubMed] [Google Scholar]
- 3. Ehde DM, Gibbons LE, Chwastiak L, Bombardier CH, Sullivan MD, Kraft GH.. Chronic pain in a large community sample of persons with multiple sclerosis. Mult Scler. 2003; 9: 605– 611. [DOI] [PubMed] [Google Scholar]
- 4. Stenager E, Knudsen L, Jensen K.. Acute and chronic pain syndromes in multiple sclerosis. Acta Neurol Scand. 1991; 84: 197– 200. [DOI] [PubMed] [Google Scholar]
- 5. Amtmann D, Askew RL, Kim J, . et al. Pain affects depression through anxiety, fatigue, and sleep in multiple sclerosis. Rehabil Psychol. 2015; 60: 81– 90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hadjimichael O, Kerns RD, Rizzo MA, Cutter G, Vollmer T.. Persistent pain and uncomfortable sensations in persons with multiple sclerosis. Pain. 2007; 127: 35– 41. [DOI] [PubMed] [Google Scholar]
- 7. Solaro C, Trabucco E, Messmer Uccelli M.. Pain and multiple sclerosis: pathophysiology and treatment. Curr Neurol Neurosci Rep. 2013; 13: 320. [DOI] [PubMed] [Google Scholar]
- 8. Attal N, Cruccu G, Baron R, . et al. EFNS guidelines on the pharmacological treatment of neuropathic pain: 2010 revision. Eur J Neurol. 2010; 17: 1113– e88. [DOI] [PubMed] [Google Scholar]
- 9. Pöllmann W, Feneberg W.. Current management of pain associated with multiple sclerosis. CNS Drugs. 2008; 22: 291– 324. [DOI] [PubMed] [Google Scholar]
- 10. Ehde DM, Alschuler KN, Osborne TL, Hanley MA, Jensen MP, Kraft GH.. Utilization and patients' perceptions of the effectiveness of pain treatments in multiple sclerosis: a cross-sectional survey. Disabil Health J. 2015; 8: 452– 456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ludwig DS, Kabat-Zinn J.. Mindfulness in medicine. JAMA. 2008; 300: 1350– 1352. [DOI] [PubMed] [Google Scholar]
- 12. Kabat-Zinn J. An outpatient program in behavioral medicine for chronic pain patients based on the practice of mindfulness meditation: theoretical considerations and preliminary results. Gen Hosp Psychiatry. 1982; 4: 33– 47. [DOI] [PubMed] [Google Scholar]
- 13. Kabat-Zinn J. Wherever You Go, There You Are: Mindfulness Meditation in Everyday Life. London, England: Piatkus; 2005. [Google Scholar]
- 14. Rosenzweig S, Greeson JM, Reibel DK, Green JS, Jasser SA, Beasley D.. Mindfulness-based stress reduction for chronic pain conditions: variation in treatment outcomes and role of home meditation practice. J Psychosom Res. 2010; 68: 29– 36. [DOI] [PubMed] [Google Scholar]
- 15. Lauche R, Cramer H, Dobos G, Langhorst J, Schmidt S.. A systematic review and meta-analysis of mindfulness-based stress reduction for the fibromyalgia syndrome. J Psychosom Res. 2013; 75: 500– 510. [DOI] [PubMed] [Google Scholar]
- 16. Lakhan SE, Schofield KL.. Mindfulness-based therapies in the treatment of somatization disorders: a systematic review and meta-analysis. PLoS One. 2013; 8: e71834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Morone NE, Greco CM, Moore CG, . et al. A mind-body program for older adults with chronic low back pain: a randomized clinical trial. JAMA Intern Med. 2016; 176: 329– 337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cherkin DC, Sherman KJ, Balderson BH, . et al. Effect of mindfulness-based stress reduction vs cognitive behavioral therapy or usual care on back pain and functional limitations in adults with chronic low back pain: a randomized clinical trial. JAMA. 2016; 315: 1240– 1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mills N, Allen J.. Mindfulness of movement as a coping strategy in multiple sclerosis: a pilot study. Gen Hosp Psychiatry. 2000; 22: 425– 431. [DOI] [PubMed] [Google Scholar]
- 20. Tavee J, Rensel M, Planchon SM, Butler RS, Stone L.. Effects of meditation on pain and quality of life in multiple sclerosis and peripheral neuropathy: a pilot study. Int J MS Care. 2011; 13: 163– 168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bogosian A, Chadwick P, Windgassen S, . et al. Distress improves after mindfulness training for progressive MS: a pilot randomised trial. Mult Scler. 2015; 21: 1184– 1194. [DOI] [PubMed] [Google Scholar]
- 22. Senders A, Bourdette D, Hanes D, Yadav V, Shinto L.. Perceived stress in multiple sclerosis: the potential role of mindfulness in health and well-being. J Evid Based Complementary Altern Med. 2014; 19: 104– 111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Polman CH, Reingold SC, Banwell B, . et al. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann Neurol. 2011; 69: 292– 302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Cella D, Riley W, Stone A, . et al. The Patient-Reported Outcomes Measurement Information System (PROMIS) developed and tested its first wave of adult self-reported health outcome item banks: 2005–2008. J Clin Epidemiol. 2010; 63: 1179– 1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Fischer JS, LaRocca NG, Miller DM, Ritvo PG, Andrews H, Paty D.. Recent developments in the assessment of quality of life in multiple sclerosis (MS). Mult Scler Houndmills Basingstoke Engl. 1999; 5: 251– 259. [DOI] [PubMed] [Google Scholar]
- 26. Senders A, Hanes D, Bourdette D, Whitham R, Shinto L.. Reducing survey burden: feasibility and validity of PROMIS measures in multiple sclerosis. Mult Scler. 2014; 20: 1102– 1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Cook KF, Bamer AM, Amtmann D, Molton IR, Jensen MP.. Six patient-reported outcome measurement information system short form measures have negligible age- or diagnosis-related differential item functioning in individuals with disabilities. Arch Phys Med Rehabil. 2012; 93: 1289– 1291. [DOI] [PubMed] [Google Scholar]
- 28. Baer RA, Smith GT, Lykins E, . et al. Construct validity of the five facet mindfulness questionnaire in meditating and nonmeditating samples. Assessment. 2008; 15: 329– 342. [DOI] [PubMed] [Google Scholar]
- 29. Bränström R, Kvillemo P, Brandberg Y, Moskowitz JT.. Self-report mindfulness as a mediator of psychological well-being in a stress reduction intervention for cancer patients: a randomized study. Ann Behav Med Publ Soc Behav Med. 2010; 39: 151– 161. [DOI] [PubMed] [Google Scholar]
- 30. Hohol MJ, Orav EJ, Weiner HL.. Disease steps in multiple sclerosis: a simple approach to evaluate disease progression. Neurology. 1995; 45: 251– 255. [DOI] [PubMed] [Google Scholar]
- 31. Shinto L, Yadav V, Morris C, Lapidus JA, Senders A, Bourdette D.. Demographic and health-related factors associated with complementary and alternative medicine (CAM) use in multiple sclerosis. Mult Scler. 2006; 12: 94– 100. [DOI] [PubMed] [Google Scholar]
- 32. Harrison AM, McCracken LM, Bogosian A, Moss-Morris R.. Towards a better understanding of MS pain: a systematic review of potentially modifiable psychosocial factors. J Psychosom Res. 2015; 78: 12– 24. [DOI] [PubMed] [Google Scholar]
- 33. Schütze R, Rees C, Preece M, Schütze M.. Low mindfulness predicts pain catastrophizing in a fear-avoidance model of chronic pain. Pain. 2010; 148: 120– 127. [DOI] [PubMed] [Google Scholar]
- 34. Beiske AG, Pedersen ED, Czujko B, Myhr K-M.. Pain and sensory complaints in multiple sclerosis. Eur J Neurol. 2004; 11: 479– 482. [DOI] [PubMed] [Google Scholar]
- 35. Archibald CJ, McGrath PJ, Ritvo PG, . et al. Pain prevalence, severity and impact in a clinic sample of multiple sclerosis patients. Pain. 1994; 58: 89– 93. [DOI] [PubMed] [Google Scholar]
- 36. Carmody J, Baer RA.. Relationships between mindfulness practice and levels of mindfulness, medical and psychological symptoms and well-being in a mindfulness-based stress reduction program. J Behav Med. 2008; 31: 23– 33. [DOI] [PubMed] [Google Scholar]
- 37. Garland SN, Tamagawa R, Todd SC, Speca M, Carlson LE.. Increased mindfulness is related to improved stress and mood following participation in a mindfulness-based stress reduction program in individuals with cancer. Integr Cancer Ther. 2013; 12: 31– 40. [DOI] [PubMed] [Google Scholar]
- 38. Yost KJ, Eton DT, Garcia SF, Cella D.. Minimally important differences were estimated for six Patient-Reported Outcomes Measurement Information System-Cancer scales in advanced-stage cancer patients. J Clin Epidemiol. 2011; 64: 507– 516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Grossman P, Kappos L, Gensicke H, . et al. MS quality of life, depression, and fatigue improve after mindfulness training: a randomized trial. Neurology. 2010; 75: 1141– 1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kolahkaj B, Zargar F.. Effect of mindfulness-based stress reduction on anxiety, depression and stress in women with multiple sclerosis. Nurs Midwifery Stud. 2015; 4: e29655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Melzack R, Katz J.. Pain. Wiley Interdiscip Rev Cogn Sci. 2013; 4: 1– 15. [DOI] [PubMed] [Google Scholar]
- 42. Zeidan F, Grant JA, Brown CA, McHaffie JG, Coghill RC.. Mindfulness meditation-related pain relief: evidence for unique brain mechanisms in the regulation of pain. Neurosci Lett. 2012; 520: 165– 173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Grant JA. Meditative analgesia: the current state of the field: meditative analgesia. Ann N Y Acad Sci. 2014; 1307: 55– 63. [DOI] [PubMed] [Google Scholar]
- 44. Tomasino B, Chiesa A, Fabbro F.. Disentangling the neural mechanisms involved in Hinduism- and Buddhism-related meditations. Brain Cogn. 2014; 90: 32– 40. [DOI] [PubMed] [Google Scholar]
- 45. Zeidan F, Gordon NS, Merchant J, Goolkasian P.. The effects of brief mindfulness meditation training on experimentally induced pain. J Pain. 2010; 11: 199– 209. [DOI] [PubMed] [Google Scholar]


