Abstract
Background:
Atrial fibrillation is increasingly prevalent as the US population ages and is associated with significant morbidity and mortality. Care for patients with atrial fibrillation can be costly, US health care costs are comparatively high, and there are few cost estimates available that incorporate detailed measurement of comorbidities and their effects on costs.
Methods and Results:
In the Cardiovascular Health Study and the Framingham Heart Study, participants aged 65 years or older with newly diagnosed atrial fibrillation were matched on age and follow-up time to referents free of atrial fibrillation. The total clinical and hospital medical costs paid by Medicare Parts A and B (drug costs from Medicare Part D costs were not included) in the year prior to diagnosis (or matching) were compared with costs in the following year. Estimates were adjusted for other medical conditions and adjusted to 2009 dollars. In the Cardiovascular Health Study, 513 participants were diagnosed with new-onset atrial fibrillation and survived 30 days post-atrial fibrillation diagnosis, and 513 referents (as a control cohort) were identified, with a mean age of 77 years. In the Framingham Heart Study, we identified 336 participants diagnosed with atrial fibrillation, who survived 30 days post-atrial fibrillation diagnosis and matched these participants to 336 referents. We compared these new-onset atrial fibrillation participants with referents, using a difference in difference design to account for both time trends and differences between the two groups. The adjusted incremental cost for participants with atrial fibrillation, compared with referents, was US$18,060 (95% confidence interval: US$14,965–US$21,155) in the Cardiovascular Health Study and US$20,012 (95% confidence interval: US$15,057–US$24,966) in the Framingham Heart Study. The pooled estimate was US$18,601 (95% confidence interval: US$15,981–US$21,234).
Conclusion:
Atrial fibrillation was associated with increased costs in the year after diagnosis in two community-based cohorts, even after careful accounting for age, time period, and systematically measured comorbidities.
Keywords: Atrial fibrillation, health care costs, Medicare, epidemiology, cohort
Atrial fibrillation (AF) prevalence has been increasing over the past few decades in the United States and AF is expected to affect as many as 10 million Americans by 2020.1–3 The underlying reasons for the increase in the observed prevalence of AF are not fully understood but have been attributed to some combination of higher burden of risk factors, increase in prevalence of heart failure, longer survival times with cardiovascular diseases, and aging of the population.4
AF can be a devastating condition that is associated with decreased functional status and several cardiovascular comorbidities, including heart failure, stroke, and death.5,6 Novel therapies to manage AF and to prevent stroke in AF patients are effective, but they also carry the risk of substantial increases in costs and complications, including death.7–10 Thus, AF shares important factors that are believed to be related to health care cost inflation in general, such as aging of the population and the introduction of innovative therapies.
AF has been shown to be related to increased costs,11,12 especially in the period immediately after diagnosis, in the context of both private insurance11 and European public insurance.12 These claims-based approaches lack the detailed and systematic assessment of the individual covariates of the participants that would have been present in cohort studies. For example, it is well known that medical claims data often lack routine assessment of smoking, blood biomarkers, or body size, which need to be inferred from the relevant diagnostic codes and are only collected at times of patient contact. The use of these irregularly recorded codes in place of systematic measurements could be a source of residual confounding, although the seriousness of this concern is dependent on the specific research question and the strength of these variables as potential confounders. Given the potential economic importance of AF as a driver of increased health care costs, we examined the hospital and clinical care costs related to AF in the setting of two large cohort studies of cardiovascular disease. We included adjustment for systematically measured clinical factors that are known to be associated with cost as possible confounders and are generally unavailable or poorly measured on most or all participants in larger administrative database-based studies (e.g. blood pressure, smoking). Furthermore, we designed our analyses to discriminate the incremental cost after accounting for the burden of other medical comorbidities in participants with AF, to address the possibility that AF was merely a marker for other diseases that increase costs. The goal of our analysis was to compare the hospital and clinical care costs during 1 year after the initial AF diagnosis versus 1 year before the initial AF diagnosis, under the payer perspective. We compared individuals who developed AF with age- and follow-up time-matched referents without AF to account for increases in costs that can be attributed to underlying changes in medical costs as the cohort ages, above and beyond those accounted for by changes in the medical care component of the consumer price index.
Methods
Cohorts
The Cardiovascular Health Study (CHS) is a population-based, multi-center prospective cohort study of cardiovascular risk factors in community-dwelling older adults who were randomly recruited from the Medicare eligibility lists. The CHS cohort includes four US communities: Forsyth County, North Carolina; Sacramento County, California; Washington County, Maryland; and Allegheny County, Pennsylvania. It originated in 1989 and included 5888 participants who were eligible for Medicare (5201 participants recruited in 1989, with 687 African American participants added in 1992).13 Those eligible to participate met the following inclusion criteria: (1) age ≥65 years, (2) non-institutionalized, (3) expected to remain in the area for at least 3 years, and (4) able to give informed consent and had no need for proxy respondent at CHS study entry. For the present analysis, we required that participants were still alive and under follow-up in 1992–1993 (our study baseline) at the time of the match to Medicare enrollment data (n = 351 excluded) and were enrolled in Medicare Parts A and B (n = 797 excluded), leaving 4740 participants available for selection into the analysis. Only participants who were eligible for Medicare by age (65 years or older) were included.
The participant characteristics were assessed at entry into the CHS cohort and at annual follow-up visits to the CHS study clinic. Participants were contacted every 6 months and were asked about all hospitalizations; medical records, including International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) discharge diagnosis codes, were obtained for each hospitalization. The CHS design and recruitment details are described elsewhere.13–15 The CHS cohort data were matched to Medicare enrollment files starting with the 1992/1993 examination, and Medicare claims data were available through the end of 2009. Since Medicare data were not available at the CHS study entry in 1989–1990, this analysis begins on the date of the annual study examination in 1992–1993, to prevent left censoring of cost data.
The Framingham Heart Study (FHS) is a community-based cohort study.16 The Original FHS cohort began enrollment in 1948 and 1952, with subsequent examinations at roughly 2-year intervals, and continued with the descendants (and their spouses) of the Original FHS, referred to as the Offspring cohort, examined at roughly 4- to 8-year intervals.17 Both Original and Offspring cohorts contributed participants to this analysis, using the 1998–2001 cycle as baseline. We required that participants were still alive and under follow-up in 1998–2003 at the time of the match to Medicare enrollment data, were enrolled in Medicare Parts A and B, and were 65 years of age or older. Details of the exclusion criteria for the FHS participants are presented in Supplementary Table 1.
Atrial fibrillation diagnosis
In CHS, incident AF (including AF or atrial flutter) was ascertained from three sources: ECGs from annual study examinations through 1999, hospital discharge diagnoses ascertained from participant report or from Medicare claims data, and diagnoses of AF from Medicare outpatient or physician service claims. For AF identified according to hospital discharge or Medicare claims data, a diagnosis of AF was based on a single inpatient claim or hospital discharge diagnosis, or two outpatient or physician claims within 365 days (ICD-9-CM code 427.31 or 427.32 in any position). Individuals who experienced AF that occurred during the same hospitalization as coronary artery bypass graft or heart valve surgery were excluded from the analysis. The date of AF diagnosis, which we designated as the index date for the present analysis, was the earliest of the date of ECG indicating AF, the admission date of the qualifying inpatient claim or hospital discharge diagnosis, or the service date of the second qualifying outpatient or physician claim.
In FHS, AF was defined by AF or atrial flutter on an electrocardiogram obtained at a research study visit or during an encounter with an external clinician, by Holter monitoring, or noted in hospital records. All newly diagnosed AF cases in FHS were reviewed by E.J.B.
Referents free of AF
We individually matched participants with newly identified AF to incidence density-sampled referents based on baseline age (within 1 year) and follow-up time (within 1 day) in CHS and FHS. To accomplish the matching, for each participant who developed AF, we randomly selected a matched comparator from the set of all remaining participants of the same age who were still active in the cohort but free of AF at the index date of the AF case. The index date for each AF case was assigned to his or her comparator. Incidence density sampling is a standard approach for selection of referents when careful control of a select set of confounders, age, and calendar time in this case, is desired.18–21 Wang et al.18 have shown that the method we used, incidence density sampling with replacement, limits bias compared with incidence density sampling without replacement and is the preferred method when sample size is limited, as was the case in this study.
Cost calculations
Costs were calculated from Medicare payments under Parts A and B and all costs were reported in 2009 dollars, to account for underlying inflation. Costs from previous years (1992–2008) were adjusted using the Medical Care Component of the Consumer Price Index, as reported in December in those years.22 Costs included all sources of payment from Medicare Parts A and B for expenses incurred in treating participants, including inpatient hospitalizations, physician visits, skilled nursing facility care, and hospice care. We used the actual costs for participants who died during the follow-up year, and did not adjust for the reduced person-time at risk to accumulate medical costs, which would have induced bias by up-weighting costs associated with death and would not have reflected the actual costs that were billed. Because data on Medicare costs for prescription medications (Medicare Part D), implemented in the United States starting in 2006, were not available for either cohort for the entire time frame of observation, we did not include Medicare costs for prescription drugs in our cost analyses.
Covariates
Participants who developed AF and referents without AF were matched on age (within 1 year) and follow-up time. We adjusted for possible confounding factors including age, sex, race/ethnicity, baseline values of body mass index, smoking, diabetes mellitus, hypertension, ischemic heart disease (angina, unstable angina, or myocardial infarction), peripheral vascular disease, heart failure, stroke, transient ischemic attack, any cancer, and time-varying covariates for chronic obstructive pulmonary disease, chronic kidney disease, dementia, pacemaker/automated implantable cardioverter-defibrillator (AICD) implantation, and valve replacement.13,16,23 Information on these characteristics came from questionnaires and standardized measurements made at study visits. These results were supplemented by a medical record review of key outcomes and ICD-9-CM codes from the Medicare claims data. This allowed us to adjust for high quality and systematically measured potential confounders, to reduce the potential for unmeasured and residual confounding.
Statistical analysis
We estimated the incremental cost for participants who developed AF compared with the matched referents, by estimating the difference in medical billing costs in the year after the index date (which we designate as the “follow-up year”) compared with the year before the index date (which we designate as the “pre-event year.” We excluded the first 30 days of the follow-up year for all participants, to avoid inclusion of high end-of-life costs for those and the costs of initial hospitalization at which AF was diagnosed, and added 30 days at the end to make the period precisely 1 year. We then adjusted for suspected confounding variables, giving us additional control for any confounding due to remaining differences in the matched participants. The design is known as a difference in differences approach, also known as a pre-post quasi-experimental study design (Figure 1). Analysis was done using linear regression with robust standard errors to handle the skewed distribution of medical costs and the repeated measure of some referents. In CHS, data were missing in 10% for cholesterol, in 9% for body mass index, and in less than 7% for all other variables. We used single imputation to handle this low rate of missing data. In a sensitivity analysis, we used multiple imputation with 10 imputation replicates.
Figure 1.
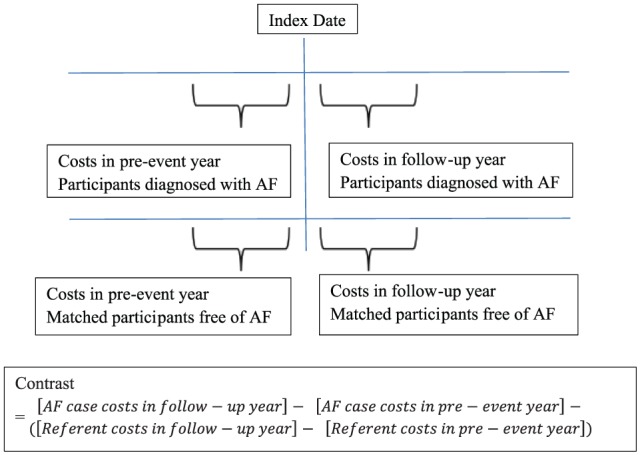
The pre-post quasi-experimental study design (also known as the difference in differences design) is graphically displayed.
Our declared, primary endpoint was the 1-year increase in medical costs billed to Centers for Medicare & Medicaid Services (CMS) in the presence of AF among participants who survived at least 30 days post-diagnosis. We used meta-analysis to combine the CHS and FHS study estimates to a single pooled estimate.23 Heterogeneity was assessed with I2, which we used to estimate the percentage of variance attributable to inter-study heterogeneity between CHS and FHS.
In a sensitivity analysis for CHS, we updated covariates to the most recent measure available at the time of AF diagnosis or matching. Higher rates of missing data were present for AF cases, so we used the last value carried forward to minimize missingness in the time-updated covariate analysis (we also explored combining this time-updated analysis with multiple imputation, but the results were similar). Since medical practice has evolved over the study time period, in a sensitivity analysis, we re-did the CHS analysis limited to the latter portion of the follow-up period, from 1999 to 2009. We also graphed the total medical costs by a quarter, with an exclusion period of the event in which AF was diagnosed and all costs billed in the 30 days following, as a supplementary figure.
The statistical code used to analyze CHS data was shared with the FHS team and the same analysis plan was followed in FHS as in CHS. Local Institutional Review Boards reviewed the CHS and FHS protocols and participants provided informed consent. A two-tailed p-value < 0.05 was considered statistically significant. Analyses were conducted using SAS version 9.4 (SAS Institute, Cary, NC) and STATA version 14. This article followed the CHEERS guidelines24 throughout and a checklist is attached as a supplementary file.
Results
The mean age of CHS participants identified with AF was 77 years, which was similar to that of FHS participants (79 years). CHS participants diagnosed with AF were more likely to be men, current smokers, have baseline clinical cardiovascular disease, and were less likely to use statin medications (Table 1) relative to matched referents. In FHS, the same general patterns were present (Table 2), although the use of statin medications was relatively well balanced between the participants with AF and the referents. Of note, hypertension did not appear to vary significantly between the cases and the referents, nor did blood pressure, in either cohort (Tables 1 and 2), although current smoking was far more common among participants with AF. Furthermore, in the CHS cohort, 138 participants with incident AF developed incident heart failure between the baseline and the index date; the same was true for only 41 comparators.
Table 1.
In Cardiovascular Health Study participants, cardiovascular risk factors in participants diagnosed with atrial fibrillation (AF) and in referents free of AF, matched on age and follow-up time.
| Diagnosed with AF, survived 30 days thereafter (n = 513) | Time- and age-matched participants without AF (n = 513) | |
|---|---|---|
| Age, years, mean (SD) | 77.1 (5.7) | 77.1 (5.7) |
| Men | 48% | 39% |
| African American | 12% | 11% |
| Body mass index, kg/m2, mean (SD) | 26.9 (4.9) | 26.9 (4.6) |
| Current smoker | 16% | 3% |
| Impaired glucose or diabetes | 28% | 22% |
| Systolic blood pressure, mmHg, mean (SD) | 139 (23) | 137 (22) |
| Diastolic blood pressure, mmHg, mean (SD) | 71 (12) | 70 (12) |
| Hypertensiona | 45% | 42% |
| Total cholesterol, mg/dL, mean (SD) | 202 (38) | 209 (37) |
| HDL cholesterol, mg/dL, mean (SD) | 52 (15) | 53 (14) |
| Statin medication use | 2% | 5% |
| Angina at baseline | 29% | 19% |
| MI at baseline | 16% | 9% |
| Heart failure at baseline | 12% | 5% |
| Stroke at baseline | 9% | 4% |
SD: standard deviation; HDL: high-density lipoprotein; MI: myocardial infarction.
Hypertension defined by systolic blood pressure ≥140 or diastolic blood pressure ≥90, or use of antihypertensive medication use plus a physician diagnosis of hypertension.
Table 2.
In Framingham Heart Study participants, cardiovascular risk factors in participants diagnosed with atrial fibrillation (AF) and in referents free of AF, matched on age and follow-up time.
| Diagnosed with AF, survived 30 days thereafter (n = 336) | Time- and age-matched participants without AF (n = 336) | |
|---|---|---|
| Age, years, mean (SD) | 79.7 (7.3) | 79.7 (7.4) |
| Male sex | 46% | 34% |
| African American | b | b |
| Body mass index, kg/m2, mean (SD) | 28.2 (4.8) | 26.7 (4.5) |
| Current smoker | 16% | 3% |
| Impaired glucose or diabetes | 20% | 10% |
| Systolic blood pressure, mmHg, mean (SD) | 137 (23) | 135 (18) |
| Diastolic blood pressure, mmHg, mean (SD) | 70 (11) | 71 (10) |
| Hypertensiona | 74% | 70% |
| Total cholesterol, mg/dL, mean (SD) | 188 (35) | 198 (37) |
| HDL cholesterol, mg/dL, mean (SD) | 50 (11) | 54.5 (16) |
| Statin use | 31% | 28% |
| MI at baseline | 12% | 4.5% |
| Angina at baseline | 21% | 12% |
| Stroke at baseline | 10% | 5.0% |
| Heart failure at baseline | b | 5.6% |
SD: standard deviation; HDL: high-density lipoprotein; MI: myocardial infarction.
Hypertension defined by elevated clinic blood pressure or by antihypertensive medication use plus physician diagnosis of hypertension.
Medicare-enhanced data are not presented in these cells due to a small sample size.
In the combined data from CHS and FHS, the primary endpoint of difference in cost in the presence of AF with adjustment for age, follow-up time, and pre-event year costs, the pooled adjusted incremental cost for participants with AF who survived at least 30 days, compared to referents, was US$18,601; 95% confidence interval (CI): US$15,981–US$21,234, with no significant heterogeneity (p = 0.51) and I2 = 0%.
The individual studies showed similar differences in costs: in CHS, the difference was US$18,060 (95% CI US$14,965–US$21,155), and in FHS, US$20,012 (95% CI: US$15,057–US$24,966; Tables 3 and 4), without using time-updated covariates.
Table 3.
In Cardiovascular Health Study participants, estimates of difference in cost between pre-event year and follow-up year for participants diagnosed with atrial fibrillation (AF) compared with matched referents free of AF.
| Exposure | Adjustment | No. of AF cases/referents | Estimate of difference in cost (US$) | 95% Confidence interval (US$) | p-value |
|---|---|---|---|---|---|
| AF, 1993–2009 | Model 1a | 513/513 | 18,060 | 14,965–21,155 | <0.0001 |
| Model 2b | 513/513 | 19,070 | 15,236–22,904 | <0.0001 | |
| AF, 1999–2009 | Model 2b | 151/151 | 25,340 | 17,472–33,207 | <0.0001 |
Model 1: Adjusted for age, sex, race/ethnicity, and baseline body mass index, smoking, diabetes mellitus, hypertension, ischemic heart disease (angina, unstable angina, myocardial infarction), peripheral vascular disease, heart failure, stroke, transient ischemic attack, chronic obstructive pulmonary disease, chronic kidney disease, dementia, any cancer, pacemaker/automated implantable cardioverter-defibrillator (AICD), and valve replacement.
Model 2: Adjusted for the same characteristics as in Model 1, except that all characteristics were updated to the most recent measure available at the time of AF diagnosis or matching.
Table 4.
In Framingham Heart Study participants, estimates of difference in cost between pre-event year and follow-up year for participants diagnosed with atrial fibrillation (AF) compared with matched referents free of AF.
| Exposure | Adjustment | No. of AF cases/referents | Estimate of difference in cost (US$) | 95% Confidence interval (US$) | p-value |
|---|---|---|---|---|---|
| AF, 1999–2009 | Model 1a | 336/336 | 20,717 | 15,389–26,045 | <0.0001 |
Model 1: Adjusted for age, sex, race/ethnicity, and baseline body mass index, smoking, diabetes mellitus, hypertension, ischemic heart disease (angina, unstable angina, myocardial infarction), peripheral vascular disease, heart failure, stroke, transient ischemic attack, chronic obstructive pulmonary disease, chronic kidney disease, dementia, any cancer, pacemaker/automated implantable cardioverter-defibrillator (AICD), and valve replacement.
In the analysis of CHS participants further adjusted for time-updated covariates, medical costs were again statistically significantly higher in the year after AF diagnosis for all participants who survived at least 30 days after the diagnosis of AF relative to matched referents (US$25,340; 95% CI: US$17,472–US$33,207; Table 3). In the analysis limited to the latter part of the follow-up period, 1999–2009, the results were similar to those for the entire follow-up period, although the difference in costs was slightly higher (Table 3). The results were very similar in the FHS participants overall (Table 4).
Unadjusted costs were higher among the participants with incident AF than in their matched comparators in the pre-event year, suggesting a higher burden of morbidity in these participants (Table 5). In addition, the largest proportion of the increase in costs associated with AF was due to inpatient costs, more than any other category of health expenses analyzed (Table 5).
Table 5.
Crude average medical costs by CMS category, for the 513 cases and comparators in the Cardiovascular Health Study, during the pre-event and follow-up years.
| Variable | Incident AF | Comparator | ||
|---|---|---|---|---|
| Mean (US$) | SD (US$) | Mean (US$) | SD (US$) | |
| Inpatient costs, pre-event year | 2378.68 | 3343.69 | 1359.90 | 1923.38 |
| Outpatient costs, pre-event year | 930.11 | 2835.41 | 348.90 | 703.13 |
| Carrier cost, pre-event year | 5879.11 | 14260.67 | 2421.55 | 7675.73 |
| Home health costs, pre-event year | 520.73 | 1560.95 | 245.16 | 1300.51 |
| Skilled nursing facility, pre-event year | 695.25 | 2964.36 | 311.72 | 2266.11 |
| Hospice costs, pre-event year | 5.20 | 117.82 | 39.72 | 625.33 |
| Total costs, pre-event year | 10,409.09 | 19,603.12 | 4726.94 | 10,405.78 |
| Inpatient costs, follow-up year | 4158.99 | 4384.87 | 1420.76 | 1867.44 |
| Outpatient costs, follow-up year | 1309.28 | 4175.03 | 445.09 | 1079.09 |
| Carrier cost, follow-up year | 16,379.17 | 24,066.98 | 2510.21 | 7098.31 |
| Home health costs, follow-up year | 1107.74 | 2781.70 | 284.40 | 1346.62 |
| Skilled nursing facility, follow-up year | 2279.05 | 4819.82 | 423.11 | 2016.47 |
| Hospice costs, follow-up year | 440.70 | 3043.37 | 74.90 | 721.89 |
| Total costs, follow-up year | 25,674.93 | 30,216.71 | 5158.47 | 10,130.82 |
CMS: Centers for Medicare & Medicaid Services; AF: atrial fibrillation; SD: standard deviation.
We conducted several sensitivity analyses. The use of multiple imputation to address missing data made no appreciable difference in the results in either cohort, as expected when the missing data rates were all less than 10%. We considered time trends (Supplementary Table 1) and saw that most of the costs occurred in the quarter after the AF diagnosis, although participants with AF always had higher costs than comparators, and costs had declined about a year out (justifying the 1-year cost window as the period of main interest).
Discussion
Among older individuals with and without AF of the same age, during the same follow-up period, and after statistical adjustment for clinically measured covariates, AF diagnosis was associated with an increase in Medicare costs for hospital and clinical care in the follow-up year. The increase in 1-year costs was similar in magnitude in two well-characterized cohorts. These estimates of increased cost post-AF diagnosis are comparable to those from studies that relied on medical claims data, which also showed an increase in costs due to AF post-diagnosis (US$9001 in 2009 dollars).11 As expected, those who developed AF had more comorbidity than their matched referents, and higher medical care costs in the pre-event year were strongly associated with greater costs in the follow-up year.
How much of the observed increase in costs is unavoidably due to the costs of treating the underlying disease state and how much might be mitigated with more aggressive therapy (e.g. careful anticoagulation) is difficult to estimate. Information on medication use is collected only once a year in CHS, and even less frequently in FHS, and Medicare prescription medication (Part D) claims data were not available in either cohort for the entire duration, so we are unable to assess the influence of anticoagulation and other medications on costs. The 1-year time window after AF diagnosis we chose to examine is comparable to the time window in which other studies have found increased costs,12 is consistent with other pre-post designs,25 and the use of a longer time window would increase the risk of survivors becoming unrepresentative of the underlying population.
The 1-year cost increase for AF was high, even relative to the 1-year cost of other known costly conditions such as heart failure, estimated at an incremental US$12,924 in 2006 dollars (US$14,423 in 2009 dollars) in the CHS26 or macular degeneration (US$5197 in 2009 dollars);27 but the total hospital and clinical costs were still higher among participants dying of or surviving with prostate cancer28 (US$45,053 in 2009 dollars in medical costs, compared to US$25,675 in the year post-AF diagnosis).
One issue that is challenging to disentangle is the burden of comorbidities that are related to the presence of AF. For example, heart failure and AF are strongly associated.29 In the CHS cohort, 138 participants with incident AF also had incident heart failure between the baseline and the AF diagnosis. The same was true for only 41 comparators. Furthermore, it is clear that the unadjusted costs are higher in the year prior to the AF diagnosis in participants who developed AF than in their comparators, although our matched pre-post design is intended to address this issue. While heart failure could account for only part of the total cost increase,29 it is unclear to what extent these increased costs are being driven by the complexity of the population presenting with AF. It is quite possible that these estimates represent an average burden of disease and may overestimate the cost burden for an AF patient with no other comorbid conditions.
There are some limitations to the interpretation of our results. Medication costs could not be included. It is possible that the small amount (up to 10%) of missing data could have induced some degree of bias. Whereas we observed that AF was associated with an increase in costs in the follow-up year, we cannot definitively conclude that AF itself, as opposed to coexisting medical conditions, was the cause of the increased cost. We studied individuals from CHS and FHS who had Medicare claims data and, therefore, it is uncertain whether health care costs associated with AF can be applied to younger populations, Medicare patients in managed care, or in the context of private health care plans.11,12,30 There are regional variations in health care expenditures in the United States and our study covered only five communities (four CHS, one FHS); it is unclear whether our data generalize to all regions in the United States. Different medical systems may experience quite different costs associated with AF, depending on the culture of care and the mechanism of reimbursement, which makes it difficult to generalize our results to other countries. Whereas the African American race did not appear to be a major confounder, much more work remains to be done to assess costs in AF across the full range of races/ethnicities present in the United States. Finally, although the CHS and FHS cohorts are quite similar in design and measures, they are not identical, and some study-specific differences may still be present. For example, the 30-day mortality rate among those with a new diagnosis of AF differed considerably in CHS (18%) and FHS (5%), suggesting that CHS AF ascertainment may have been more sensitive to identifying AF that occurred near the end of life or that CHS participants had higher comorbidity. In our analysis, we excluded the first 30 days after the index date from the cost accrual period, to avoid inclusion of high end-of-life costs and the costs of initial hospitalization at which AF was diagnosed. The limitation to participants over the age of 65 years (eligible for Medicare) are an important group from a policy perspective (as they are separately funded), but may well include participants with a much higher burden of comorbidity than those with AF diagnosed at earlier ages. We also lacked medication cost data and any information on indirect costs (other than those billed to a Medicare payer), and it may well be the case that ongoing cost differences would be even higher if pharmacotherapy and indirect costs were included. There may be some censoring due to changes in insurance between the types of insurance (fee for service Medicare versus managed care), which may lead to a slight underestimation of the second-year costs (making this a conservative estimate).
The key strengths of our study are the detailed information on comorbidity, assessed in a standardized manner in all participants, and the inclusion of a replication cohort (FHS). Previous work31 has been based on economic modeling32,33 or based on medical claims data alone,11,12,34,35 rather than in the setting of an epidemiological cohort study. The systematic measurement of covariates such as diabetes, smoking, and blood pressure made it possible to determine the potential increase in risk due to comorbidities that are not captured perfectly in medical claims data. While careful analytic techniques can make medical claims data a powerful source of information on health events25 and costs, there is always an advantage to additional data when it can be obtained. Our study found higher costs than those in Kim et al.,11 which found a 1-year increase of US$8705 (US$9001 in 2009 dollars) in a medical claims study, although their cohort included much younger participants. They also used propensity score matching, which provided better covariate balance between the incident AF participants and referents, but less exact matching on calendar time and age. Our study is a unique contribution due to the systematic measurement of confounders in clinical examinations—providing a great deal of extra evidence that this cost increase is not confounded by comorbid conditions. It also provides strong evidence that the difference in differences approach used in our study is able to greatly reduce the confounding present in administrative databases, making the final adjusted estimates quite similar to the unadjusted estimates. Overall, it is notable that a wide range of approaches show evidence of increased costs among patients diagnosed with AF.
From a health policy perspective, our results suggest that reducing AF costs is an attractive option, given that these costs are large even in the context of other costly health conditions. Although the available data do not put us in the position to make specific treatment recommendations, they do imply that improving adherence to standard therapies may be useful in reducing the outcomes that typically drive costs.36 Furthermore, it is plausible that there may be a role for improving screening for AF to reduce future costs,37 as it is possible that some of these large medical costs may be mitigated with earlier detection and intervention. An expensive disease state makes screening much more likely to be cost effective. The ultimate benefit of these strategies needs to be evaluated in the context of careful experiments, as the CHS and FHS data studied in this article demonstrate the cost consequences of AF in an earlier era of therapy and cannot evaluate the possible cost-effectiveness of interventions to treat AF in older adults. Rather, we can only highlight that the large costs associated with AF, in this carefully controlled study, create the potential for significant cost savings from improved management of AF.
Overall, we found that a new AF diagnosis in older adults was associated with large incremental hospital and clinical care costs, even after adjusting for a broad range of systematically measured covariates. Our results were validated in an independent cohort and were robust to various sensitivity analyses. Of interest was that the difference in differences design showed very similar costs in unadjusted, compared to the fully adjusted models, suggesting that this study design may be of great use in data contexts where systematically measured individual clinical data are unavailable. The observed increase in costs for participants diagnosed with AF suggests that the expected increase in AF prevalence over time may also bring an increase in Medicare expenses.
Supplementary Material
Footnotes
Compliance with ethical standards: The IRB at the University of Washington approved the study, namely, the University of Washington Human Subjects Division (Review Group E/J, approval #37714-EG). CHS and FHS cohort data are also publicly available as fully de-identified data at https://biolincc.nhlbi.nih.gov/
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical approval: The IRB at the University of Washington approved the study, namely, the University of Washington Human Subjects Division (Review Group E/J, approval #37714-EG).
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This research was supported by R01HL102214, R01 HL127659, and contracts HHSN268201200036C, HHSN268200800007C, N01HC55222, N01HC85079, N01HC85080, N01HC85081, N01HC85082, N01HC85083, N01HC85086, 2R01HL092577, 1R01HL128914, HHSN268201500001I, N01-HC25195, R01HL127659, and grant U01HL080295 from the National Heart, Lung, and Blood Institute (NHLBI), with additional contribution from the National Institute of Neurological Disorders and Stroke (NINDS). Additional support was provided by R01AG023629, and R56 AG057262 from the National Institute on Aging (NIA). A full list of principal CHS investigators and institutions can be found at CHS-NHLBI.org. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Informed consent: Written informed consent was obtained from all subjects before the cohort study data were collected. These analyses were conducted on de-identified secondary data.
ORCID iDs: Joseph AC Delaney  https://orcid.org/0000-0003-1771-1776
https://orcid.org/0000-0003-1771-1776
Bradley G Hammill  https://orcid.org/0000-0002-0389-6434
https://orcid.org/0000-0002-0389-6434
Emelia J Benjamin  https://orcid.org/0000-0003-4076-2336
https://orcid.org/0000-0003-4076-2336
References
- 1. Miyasaka Y, Barnes ME, Gersh BJ, et al. Secular trends in incidence of atrial fibrillation in Olmsted County, Minnesota, 1980 to 2000, and implications on the projections for future prevalence. Circulation 2006; 114: 119–125. [DOI] [PubMed] [Google Scholar]
- 2. Go AS, Hylek EM, Phillips KA, et al. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the AnTicoagulation and risk factors in Atrial Fibrillation (ATRIA) Study. JAMA 2001; 285: 2370–2375. [DOI] [PubMed] [Google Scholar]
- 3. Piccini JP, Hammill BG, Sinner MF, et al. Incidence and prevalence of atrial fibrillation and associated mortality among medicare beneficiaries,1993-2007. Circ Cardiovasc Qual Outcomes 2012; 5: 85–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Schnabel RB, Yin X, Gona P, et al. 50 year trends in atrial fibrillation prevalence, incidence, risk factors, and mortality in the Framingham Heart Study: a cohort study. Lancet 2015; 386(9989): 154–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Benjamin EJ, Wolf PA, D’Agostino RB, et al. Impact of atrial fibrillation on the risk of death: the Framingham Heart Study. Circulation 1998; 98: 946–952. [DOI] [PubMed] [Google Scholar]
- 6. Wang TJ, Larson MG, Levy D, et al. Temporal relations of atrial fibrillation and congestive heart failure and their joint influence on mortality: the Framingham Heart Study. Circulation 2003; 107: 2920–2925. [DOI] [PubMed] [Google Scholar]
- 7. Connolly SJ, Ezekowitz MD, Yusuf S, et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med 2009; 361: 1139–1151. [DOI] [PubMed] [Google Scholar]
- 8. Patel MR, Mahaffey KW, Garg J, et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med 2011; 365: 883–891. [DOI] [PubMed] [Google Scholar]
- 9. Granger CB, Alexander JH, McMurray JJ, et al. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med 2011; 365: 981–992. [DOI] [PubMed] [Google Scholar]
- 10. Oral H, Pappone C, Chugh A, et al. Circumferential pulmonary-vein ablation for chronic atrial fibrillation. N Engl J Med 2006; 354: 934–941. [DOI] [PubMed] [Google Scholar]
- 11. Kim MH, Johnston SS, Chu BC, et al. Estimation of total incremental health care costs in patients with atrial fibrillation in the United States. Circ Cardiovasc Qual Outcomes 2011; 4: 313–320. [DOI] [PubMed] [Google Scholar]
- 12. Reinhold T, Lindig C, Willich SN, et al. The costs of atrial fibrillation in patients with cardiovascular comorbidities—a longitudinal analysis of German health insurance data. Europace 2011; 13: 1275–1280. [DOI] [PubMed] [Google Scholar]
- 13. Fried LP, Borhani NO, Enright P, et al. The Cardiovascular Health Study: design and rationale. Ann Epidemiol 1991; 1: 263–276. [DOI] [PubMed] [Google Scholar]
- 14. Tell GS, Fried LP, Hermanson B, et al. Recruitment of adults 65 years of age and older as participants in the Cardiovascular Health Study. Ann Epidemiol 1993; 3: 358–366. [DOI] [PubMed] [Google Scholar]
- 15. Psaty BM, Lee M, Savage PJ, et al. Assessing the use of medications in the elderly: method and initial experience in the Cardiovascular Health Study. J Clin Epidemiol 1992; 45: 683–692. [DOI] [PubMed] [Google Scholar]
- 16. Dawber TR, Meadors GF, Moore FE, et al. Epidemiological approaches to heart disease: the Framingham Study. Am J Public Health Nations Health 1951; 41: 279–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Feinleib M, Kannel WB, Garrison RJ, et al. The Framingham Offspring Study: design and preliminary data. Prev Med 1975; 4: 518–525. [DOI] [PubMed] [Google Scholar]
- 18. Wang MH, Shugart YY, Cole SR, et al. A simulation study of control sampling methods for nested case-control studies of genetic and molecular biomarkers and prostate cancer progression. Cancer Epidemiol Biomarkers Prev 2009; 18(3): 706–711. [DOI] [PubMed] [Google Scholar]
- 19. Richardson DB. An incidence density sampling program for nested case-control analyses. Occup Environ Med 2004; 61: e59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Delaney JA, Daskalopoulou SS, Brophy JM, et al. Lifestyle variables and the risk of myocardial infarction in the general practice research database. BMC Cardiovasc Disord 2007; 7: 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Beaumont JJ, Steenland K, Minton A, et al. A computer program for incidence density sampling of controls in case-control studies nested within occupational cohort studies. Am J Epidemiol 1989; 129(1): 212–920. [DOI] [PubMed] [Google Scholar]
- 22.https://www.bls.gov/cpi/ (accessed 25 November 2017).
- 23. Harris RJ, Bradburn MJ, Deeks JJ, et al. Metan: fixed- and random-effects meta-analysis. Stata J 2008; 8: 3–28. [Google Scholar]
- 24. Husereau D, Drummond M, Petrou S. CHEERS Task Force. Consolidated Health Economic Evaluation Reporting Standards (CHEERS) statement. BMJ 2013; 346: f1049. [DOI] [PubMed] [Google Scholar]
- 25. Delaney JA, Moodie EE, Suissa S. Modeling blood pressures changes after drug treatment in the general practice research database. Pharmacoepidemiol Drug Saf 2008; 17: 535–545. [DOI] [PubMed] [Google Scholar]
- 26. Liao L, Anstrom KJ, Gottdiener JS, et al. Long-term costs and resource use in elderly participants with congestive heart failure in the Cardiovascular Health Study. Am Heart J 2007; 153: 245–252. [DOI] [PubMed] [Google Scholar]
- 27. Qualls LG, Hammill BG, Wang F, et al. Costs of newly diagnosed neovascular age-related macular degeneration among medicare beneficiaries, 2004-2008. Retina 2013; 33(4): 854–861. [DOI] [PubMed] [Google Scholar]
- 28. Dinan MA, Li Y, Zhang Y, et al. Resource use in the last year of life among patients who died with versus of prostate cancer. Clin Genitourin Cancer 2016; 14(1): 28–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Anter E, Jessup M, Callans DJ. Atrial fibrillation and heart failure: treatment considerations for a dual epidemic. Circulation 2009; 119: 2516–2525. [DOI] [PubMed] [Google Scholar]
- 30. Miller EA, Decker SL, Parker JD. Characteristics of medicare advantage and fee-for-service beneficiaries upon enrollment in Medicare at age 65. J Ambul Care Manage 2016; 39(3): 231–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sheikh A, Patel NJ, Nalluri N, et al. Trends in hospitalization for atrial fibrillation: epidemiology, cost, and implications for the future. Prog Cardiovasc Dis 2015; 58(2): 105–116. [DOI] [PubMed] [Google Scholar]
- 32. Moeremans K, Aliot E, de Chillou C, et al. Second line pharmacological management of paroxysmal and persistent atrial fibrillation in France: a cost analysis. Value Health 2000; 3: 407–416. [DOI] [PubMed] [Google Scholar]
- 33. Stewart S, Murphy NF, Walker A, et al. Cost of an emerging epidemic: an economic analysis of atrial fibrillation in the UK. Heart 2004; 90: 286–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Coyne KS, Paramore C, Grandy S, et al. Assessing the direct costs of treating nonvalvular atrial fibrillation in the United States. Value Health 2006; 9: 348–356. [DOI] [PubMed] [Google Scholar]
- 35. Patel NJ, Deshmukh A, Pant S, et al. Contemporary trends of hospitalization for atrial fibrillation in the United States, 2000 through 2010: implications for healthcare planning. Circulation 2014; 129: 2371–2379. [DOI] [PubMed] [Google Scholar]
- 36. Morillo CA, Banerjee A, Perel P, et al. Atrial fibrillation: the current epidemic. J Geriatr Cardiol 2017; 14(3): 195–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Freedman B, Camm J, Calkins H, et al. Screening for atrial fibrillation: a report of the AF-SCREEN International Collaboration. Circulation 2017; 135(19): 1851–1867. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


