Abstract
The abnormal phosphatase and tensin homolog expression and activated phosphoinositide-3 kinase/Protein kinase B (AKT)/mammalian target of rapamycin signaling pathway are involved in the progression of esophageal squamous cell carcinoma. By assessing the expression pattern of key components in the phosphoinositide-3 kinase/AKT/mammalian target of rapamycin signaling pathway by immunohistochemistry in tumor and nontumor esophageal mucosa from patients with esophageal squamous cell carcinomas, we aimed to carefully explore the relationship between the various protein expressions and clinicopathological factors, as well as patient outcome. A total of 145 tumor and 145 nontumor samples from patients with esophageal squamous cell carcinoma, collected from HuaShan Hospital (Shanghai, China) were evaluated. Clinical characteristics, the targeted protein expressions (including phosphatase and tensin homolog, phosphoinositide-3 kinase, AKT, p-AKT, mammalian target of rapamycin, p-mTOR, p70S6 kinase 1, p-P70S6K1, elongation initiation factor 4E binding protein-1, and p-4E-BP1, and survival rate were analyzed. Among them, phosphoinositide-3 kinase, AKT, p-AKT, mammalian target of rapamycin, p-mTOR, elongation initiation factor 4E binding protein-1, p70S6 kinase 1, and p-P70S6K1 proteins were significantly upregulated in tumor tissue. Conversely, phosphatase and tensin homolog was largely downregulated in tumor tissue, notably in pT3-T4 tumors. Low expression of phosphatase and tensin homolog whereas high expression of mammalian target of rapamycin signaling components in tumors was closely related to the presence of lymph node metastases and advanced TNM stage (all P < .05). Moreover phosphatase and tensin homolog, mammalian target of rapamycin, and p70S6 kinase 1 were correlated with overall survival as well as p-mTOR was correlated with progression-free survival (all P < .05). Overexpression of mammalian target of rapamycin was proved to be an independent adverse prognostic factor for overall survival in esophageal squamous cell carcinomas. Our results suggest that the phosphoinositide-3 kinase/AKT/mammalian target of rapamycin signaling pathway is activated in esophageal squamous cell carcinoma, with the low expression of phosphatase and tensin homolog and the high expression of the mammalian target of rapamycin component proteins (both total and phosphorylated) in tumor tissue. Our result might offer a new strategy for specific targeted therapy and prognostic assessment in esophageal cancer.
Keywords: esophageal squamous cell carcinoma, PI3K/AKT/mTOR signaling pathway, PTEN, Prognosis
Introduction
Esophageal cancer is the eighth common cancer and the sixth leading cause of cancer mortality in the worldwide.1 Esophageal squamous cell carcinoma (ESCC) is the dominant histological subtype in East Asian countries.2 More than 250 000 new diagnosed esophageal cancer cases were reported in China per year, accounting for half of the world.3 In recent years, although the development of multiple therapy approaches includes surgery, chemotherapy, and radiotherapy, the prognosis remains poor for patients with ESCC who undergo esophagectomy and lymph node dissections.4,5 The limited improvement in outcomes achieved by these conventional therapies urges us to seek new strategies, especially the possibility on novel oncogene signaling pathways that affect the development of ESCC.
Encouragingly, genomics profiling studies of esophageal cancer have revealed that the phosphoinositide-3 kinase (PI3K)-AKT-mammalian target of rapamycin (mTOR) pathway is a key signal pathway involved in the regulation of diverse key cellular processes, which could stimulate cell proliferation, apoptosis, and migration.6 Mammalian target of rapamycin is the central protein which interacts with several proteins to form 2 multiprotein complexes, known as mTOR complex 1 (mTORC1) and complex 2 (mTORC2).7 Activation of mTOR regulates a number of its downstream effectors important in cellular growth, such as p70S6 kinase (p70S6K) and elongation initiation factor 4E binding protein-1 (4E-BP1), resulting in enhanced translation of subset of genes that are required for protein synthesis and cell growth, inhibition of cell apoptosis, and acceleration of cell proliferation, which finally lead to a tumorigenesis.8-10 Phosphatase and tensin homolog (PTEN) is the primary negative regulator of PI3K/Akt/mTOR signaling pathway.
Previous studies suggested that the abnormal expression of PTEN and the activation of PI3K/AKT/mTOR signaling pathway were involvedin tumorigenesis and affect ESCC patient prognosis.11,12 However, the limited number of key components of mTOR signaling pathway (less than 3) were examined in these previous studies. In this study, we aimed to investigate the expression level of most well-known components of proteins in the PI3K/AKT/mTOR signaling pathway including PTEN, PI3K, and both total and phosphorylated fraction of AKT, mTOR, P70S6K1, and 4E-BP1 and expect to reveal and pick up the various biomarker proteins correlated closely with clinicopathologic factors and patient prognosis.
Materials and Methods
Patients and Tissue Samples
We selected an ESCC tissue and a paired nontumor esophageal mucosa sample from 145 patients who had advanced ESCC and underwent esophagectomy and lymph node dissections at the department of cardiothoracic surgery of the Huashan Hospital during January 1, 2006, to December 31, 2008. Patients, who had palliative resections, underwent neoadjuvant or R1/R2 resections were excluded from the analysis. Written informed consent was obtained from all participants. The institutional review board of Huashan hospital, Fudan University, reviewed and approved the study.
Clinical Data and Follow-Up
Routine preoperative staging included fibrogastroscopy, computed tomography of the chest and abdomen, ultrasound imaging of the abdomen and neck, and positron emission tomography (PET)-computed tomography (CT) scanning. Preoperative nutritional risk assessment, cardiac function tests, and pulmonary function test were also performed in all patients to make sure they had the physiological ability to undergo esophagectomy.
Patients with esophageal cancer were staged according to the seventh edition American Joint Committee on Cancer (AJCC) staging system. The standard follow-up schedule contained the following: patients were followed every 3 months for 2 years, every 6 months for the next 3 years, and then annually. The outcome is all-cause mortality and defined as time from the data of surgery to the data of death. Routine postoperative tests contained fibrogastroscopy, ultrasound, CT/PET-CT, and radionuclide bone scan.
Reagents
Antibodies recognizing PTEN (9559S), mTOR, phospho-mTOR (Ser2448), PI3K P110a (C73F8), AKT, phospho-Akt (Thr308), p70S6K1, phospho-p70S6K1 (Thr389), p4E-BP1, phospho-p4E-BP1 (Thr70) were purchased from Cell Signaling Technology (Danvers, Massachusetts). All were rabbit polyclonal antibodies.
Immunohistochemistry
Was performed immunohistochemical staining using the rabbit or mouse DAKO ChemMate EnVision system and a Peroxidase/DAB kit (DAKO, Carpinteria, California). The sample containing paraffin was sliced into serial sections with a width of 5.0 μm. Each section was deparaffinized for 1 hour at 60°C in xylene and rehydrated in serial-graded ethanol before being stored overnight in citrate buffer (0.01 M; pH 6.0) at 75°C for antigen retrieval. Endogenous peroxidase activity was blocked with 0.3% hydrogen peroxide in methanol. The sections were incubated at 4°C overnight with a primary antibody and then recovered at 37°C for 20 minutes. The slides were incubated with the secondary antibody for 30 minutes at room temperature and then were developed by DAB. Nuclear staining was carried out with hematoxylin. Phosphate-buffered saline was added as the primary antibody in the negative control. Normal esophageal tissue, serving as the negative controls, and esophageal cancer tissue (known to express PI3K/AKT/mTOR signaling pathway proteins) serving as positive controls were processed in the same way. However, normal esophageal tissue serves as the positive controls, and esophageal cancer tissue serves as negative controls for PTEN.
Evaluation of Immunohistochemistry staining
The results were interpreted by 2 independent pathologists who were blinded to the specific diagnosis and prognosis for each case. If there was disagreement, the final conclusion was reached through discussion. For each sample, at least 5 fields were chosen randomly and at least 500 cells were required for scoring in each field. Staining extent of immunostaining was scored according to the percentage of positive-stained cells as follows: 0, none; 1, up to 10% positive cells; 2, 10% to 50% positive cells; 3, 51% to 80% positive cells; and 4, positive cells > 80%. The intensity of staining was as follows: 0, none; 1, low intensity (light yellow); 2, moderate intensity (yellow); and 3, strong intensity (reddish brown). The score was calculated as grade in stain intensity × grade in coloration rate, which ranges from 0 to 12. The scores ≥4 were defined as positive overexpression. Low-expression group included negative expression or low-expression samples with overall score of 0 to 3. Both the esophageal cancer tissue and the normal esophageal tissue use the same scoring criteria.
Statistical Analysis
All statistical analysis was performed with SPSS 16.0. The associations between immunohistochemistry (IHC) expression and clinicopathological variables were examined using χ2 test and Fisher exact test for categorical variables and the Student t test for continuous variables. The Kaplan-Meier survival curve and log-rank tests were performed to estimate the survival function across groups. Cox proportional hazard regression model for multivariate analysis was performed to identify the independent prognostic factors. The level of significance was set to P < .05.
Results
The Expression of the PI3K/AKT/mTOR Signaling Pathway Proteins in ESCC and Normal Esophageal Mucosa Tissues
The expression of the PI3K/AKT/mTOR signaling pathway proteins were detected by IHC in the study. The proteins investigated were PTEN, PI3K, AKT, p-AKT, mTOR, p-mTOR, P70S6K1, p-P70S6K1, 4E-BP1, and p-4E-BP1. The staining intensity of upregulated and downregulated proteins is shown in Table 1. The significantly upregulated proteins in ESCC were PI3K (x2 = 5.354, P = .021), AKT (x2 = 7.256, P = .007), p-AKT (x2 = 5.747, P = .017), mTOR (x2 = 4.064, P = .044; Figure 1), p-mTOR (x2 = 9.425, P = .002), 4E-BP1 (x2 = 4.994, P = .025), P70S6K1 (x2 = 7.670, P = .006), and p-P70S6K1 (x2 = 4.945, P = .026). In contrast, the expression of PTEN was largely downregulated in tumor tissues (x2 = 24.756, P < .001).
Table 1.
The Staining Scores of Proteins of the mTOR Signaling Pathway Expression in ESCC and Normal Esophageal Mucosa Tissues.
| Factors | ESCC, N (%) | Normal Mucosa Tissue, N (%) | X2 | P |
|---|---|---|---|---|
| PTEN | ||||
| Low | 84 (57.9) | 42 (29.0) | 24.756 | .000 |
| High | 61 (42.1) | 103 (71.0) | ||
| PI3K | ||||
| Low | 124 (85.5) | 116 (93.8) | 5.354 | .021 |
| High | 21 (14.5) | 9 (6.2) | ||
| AKT | ||||
| Low | 82 (56.6) | 104 (71.7) | 7.256 | .007 |
| High | 63 (43.4) | 41 (28.3) | ||
| p-AKT | ||||
| Low | 77 (53.1) | 97 (66.9) | 5.747 | .017 |
| High | 68 (46.9) | 48 (33.1) | ||
| mTOR | ||||
| Low | 74 (51.0) | 91 (62.8) | 4.064 | .044 |
| High | 71 (49.0) | 54 (37.2) | ||
| p-mTOR | ||||
| Low | 67 (46.2) | 93 (64.1) | 9.425 | .002 |
| High | 78 (53.8) | 52 (35.9) | ||
| 4E-BP1 | ||||
| Low | 87 (60.0) | 105 (72.4) | 4.994 | .025 |
| High | 58 (40.0) | 40 (27.6) | 3.639 | .056 |
| p-4E-BP1 | ||||
| Low | 93 (64.1) | 77 (53.1) | ||
| High | 52 (35.9) | 68 (58.6) | ||
| P70S6K1 | ||||
| Low | 77 (53.1) | 100 (69.0) | 7.670 | .006 |
| High | 68 (46.9) | 45 (31.0) | ||
| p-P70S6K1 | ||||
| Low | 86 (59.3) | 104 (71.7) | 4.945 | .026 |
| High | 59 (40.7) | 41 (28.3) |
Abbreviations: ESCC, esophageal squamous cell carcinoma; 4E-BP1, elongation initiation factor 4E binding protein-1; mTOR, mammalian target of rapamycin; P70S6K1, P70S6 kinase 1; PI3K, phosphoinositide-3 kinase; PTEN, phosphatase and tensin homolog.
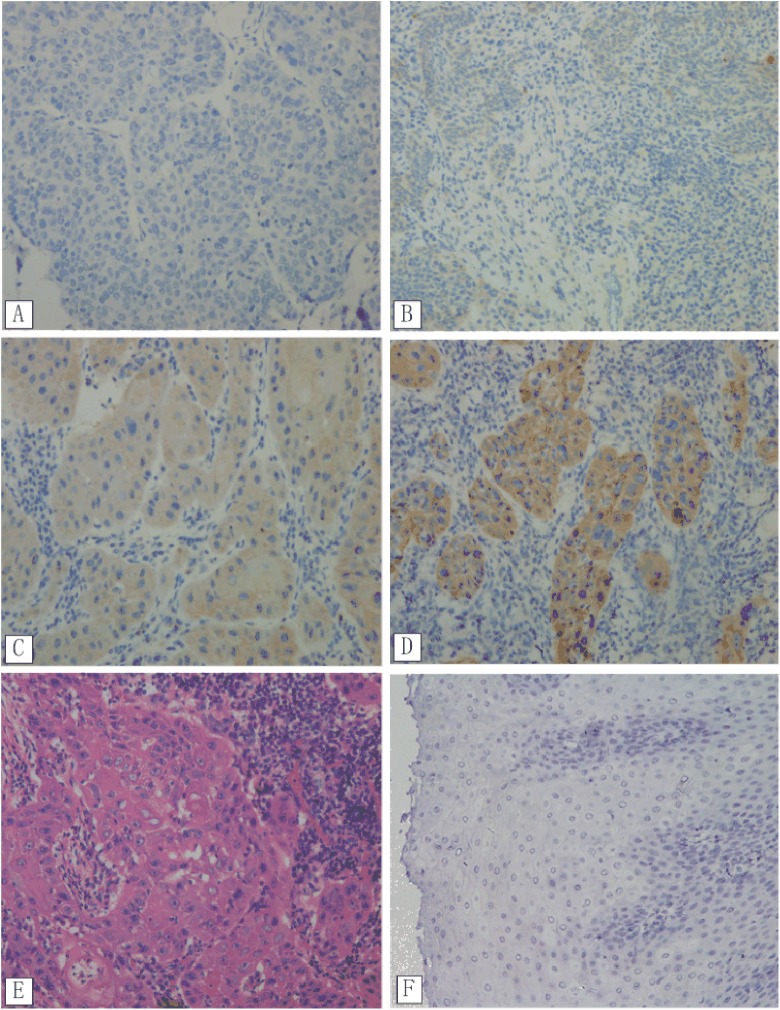
Figure 1.
Immunohistochemistry analysis of mTOR expression in ESCC tissues. (A) Nearly negative expression, (B) low expression, (C) moderate expression, (D) high expression, (E) Hematoxylin and Eosin (H&E) staining of ESCC tissue, and (F) mTOR negative expression in normal esophageal mucosa tissues; all figures ×200. ESCC denotes esophageal squamous cell carcinoma; mTOR, mammalian target of rapamycin.
Patients’ Clinical Characteristics, Survival Analysis, and Subgroup Analysis
The clinical characteristics of the 145 patients are summarized in Table 2. There were 111 men and 34 women with the median age of 59 years (range 36-81 years). The median tumor length was 4.0 cm (ranged 0.4-9.0 cm). The 5-year overall and progression-free survival rates of these 145 patients were 36.1% and 31.4%, respectively.
Table 2.
Patient Characteristics and Univariate Analysis.
| Factors | N | OS | PFS | ||
|---|---|---|---|---|---|
| 5-Year OS (%) | P | 5-Year PFS (%) | P | ||
| Age, year | |||||
| ≤60 | 78 | 46.2 | .102 | 36.8 | .140 |
| >60 | 67 | 33.2 | 25.9 | ||
| Sex | |||||
| Male | 111 | 32.8 | .165 | 27.9 | .240 |
| Female | 34 | 48.0 | 44.1 | ||
| Tumor length | |||||
| <3 cm | 37 | 53.5 | .002 | 61.5 | .002 |
| ≥3 cm | 108 | 30.0 | 23.9 | ||
| Differentiation | |||||
| Well | 17 | 40.3 | .006 | 38.6 | .032 |
| Moderately | 90 | 39.4 | 30.7 | ||
| Poorly | 38 | 24.0 | 24.7 | ||
| T stage | |||||
| T1 | 13 | 82.5 | .000 | 72.9 | .001 |
| T2 | 34 | 48.9 | 37.2 | ||
| T3 | 64 | 31.7 | 28.5 | ||
| T4 | 34 | 18.8 | 19.1 | ||
| N stage | |||||
| N0 | 55 | 47.4 | .000 | 52.1 | .000 |
| N1 | 55 | 33.5 | 32.5 | ||
| N2 | 30 | 5.3 | 5.8 | ||
| N3 | 5 | 0 | 0 | ||
| PTEN | |||||
| Low | 84 | 29.7 | .044 | 24.2 | .140 |
| High | 61 | 45.7 | 43.7 | ||
| PI3K | |||||
| Low | 124 | 34.4 | .932 | 31.5 | .800 |
| High | 21 | 45.5 | 35.1 | ||
| AKT | |||||
| Low | 82 | 38.2 | .250 | 31.5 | .168 |
| High | 63 | 33.3 | 30.6 | ||
| p-AKT | |||||
| Low | 77 | 39.1 | .064 | 34.5 | .072 |
| High | 68 | 32.8 | 27.8 | ||
| mTOR | |||||
| Low | 74 | 46.3 | .011 | 39.2 | .010 |
| High | 71 | 25.6 | 23.2 | ||
| p-mTOR | |||||
| Low | 67 | 47.6 | .053 | 49.2 | .015 |
| High | 78 | 28.9 | 21.3 | ||
| 4E-BP1 | |||||
| Low | 87 | 41.3 | .074 | 33.1 | .159 |
| High | 58 | 28.5 | 27.9 | ||
| p-4E-BP1 | |||||
| Low | 93 | 35.7 | .373 | 37.8 | .279 |
| High | 52 | 35.8 | 24.9 | ||
| P70S6K1 | |||||
| Low | 77 | 43.3 | .009 | 40.7 | .007 |
| High | 68 | 27.8 | 21.4 | ||
| p-P70S6K1 | |||||
| Low | 86 | 41.7 | .106 | 38.4 | .171 |
| High | 59 | 27.5 | 21.7 | ||
Abbreviations: 4E-BP1, elongation initiation factor 4E binding protein-1; mTOR, mammalian target of rapamycin; OS, overall survival; P70S6K1, P70S6 kinase 1; PFS, progression-free survival; PI3K, phosphoinositide-3 kinase; PTEN, phosphatase and tensin homolog. Bold values mean significant difference.
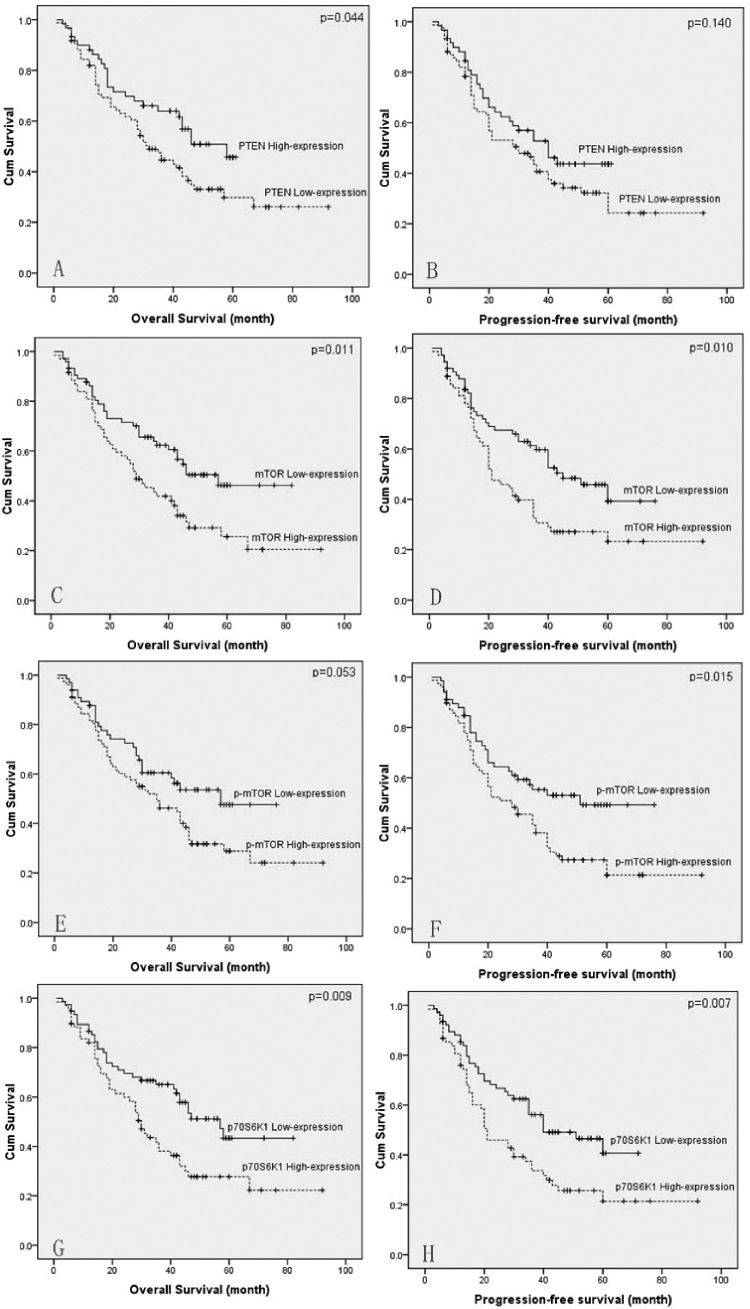
Correlations of the clinical characteristics and the PI3K/AKT/mTOR signaling pathway protein expression levels with overall survival and progression-free survival are shown in Table 2. Univariate analysis demonstrated the prognostic factors for overall survival (OS) were tumor length (P = .002), differentiation (P = .006), T-stage (P < .001), N-stage (P < .001), PTEN expression (P = .044), mTOR expression (P = .011), and P70S6K1 expression (P = .009). The prognostic factors of the PI3K/AKT/mTOR pathway proteins for progression-free survival were mTOR expression (P = .010), p-mTOR expression (P = .015), and P70S6K1 expression (P = .007). Kaplan-Meier survival curves are shown in Figure 2.
Figure 2.
Kaplan-Meier survival curve of patients with advanced ESCC after radical esophageal resection. Overall survival of patients with PTEN expression (A), mTOR expression (C), p-mTOR expression (E), and p70s6k1 expression (G); progression-free survival of patients with PTEN expression (B), mTOR expression (D), p-mTOR expression (F), and p70s6k1 expression (H). ESCC denotes esophageal squamous cell carcinoma; mTOR, mammalian target of rapamycin; PTEN, phosphatase and tensin homolog
The multivariate analyses indicated differentiation, T-stage, N-stage, and mTOR expression (hazard ratio = 1.662; 95% confidence interval: 1.030-2.681; P = .037) were independently associated with overall survival (Table 3). For progression-free survival, none of these pathway proteins had proved to be an independent prognostic factor.
Table 3.
Multivariate Prognostic Analyses of Overall Survival.
| Prognostic Factors | P values | HR | 95% CI |
|---|---|---|---|
| Tumor length | .348 | 1.388 | 0.701-2.749 |
| Differentiation | .042 | 1.519 | 1.015-2.274 |
| T-stage | .017 | 1.489 | 1.074-2.605 |
| N-stage | .009 | 1.523 | 1.109-2.092 |
| PTEN | .585 | 0.824 | 0.412-1.649 |
| mTOR | .037 | 1.662 | 1.030-2.681 |
| P70S6K1 | .566 | 1.200 | 0.644-2.237 |
Abbreviations: CI, confidence interval; HR, hazard ratio; mTOR, mammalian target of rapamycin; P70S6K1, P70S6 kinase 1; PTEN, phosphatase and tensin homolog.
In order to evaluate the impact of prognostic protein biomarkers expressions (PTEN, mTOR, p-mTOR, and P70S6K1) on different tumor stages, we divided patients into subgroups on the basis of N-stage (N0 vs N1-3). In the subgroup of N0, patients with high expression of mTOR (X2 = 12.354, P < .001) and p-mTOR (X2 = 4.549, P = .033) had significantly worse OS, but no statistical significance was found in the subgroup of N1 to N3. And there were no statistical significance of PTEN and P70S6K1 expressions in each subgroup.
Relationship Between Clinical Characteristics and the Expression of PI3K/AKT/mTOR Signaling Pathway Proteins
It was interesting to explore the relationship between clinical characteristics and the prognostic protein biomarkers of the PI3K/AKT/mTOR signaling pathway (Table 4). We found a low expression of PTEN (P = .001) in pT3 and pT4 tumors, whereas the high expression of P70S6K1 (P = .003) in pT3 and pT4 tumors. Patients with a high expression of p-mTOR (P = .008) or P70S6K1 (P = .005) tended to have tumors of more than 3 cm in maximum diameter. The high expression of mTOR (P = .026), p-mTOR (P = .002), and P70S6K1 (P < .001) were related to lymph node metastases. Consistently low expression of PTEN (P = .003) was related to lymph node metastases in ESCC. Especially, the low expression of PTEN (P = .017) or the high expression of mTOR (P = .022), p-mTOR (P = .010), and P70S6K1 (P = .028) were correlated with advanced TNM stage.
Table 4.
Associations Between Clinical Characteristics and the Expression of mTOR Signaling Pathway Proteins.
| Factors | N | PTEN Expression | mTOR Expression | p-mTOR Expression | P70S6K1 Expression | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Low | High | P | Low | High | P | Low | High | P | Low | High | P | ||
| Age, years | .198 | .788 | .989 | .888 | |||||||||
| ≤60 | 78 | 49 | 29 | 39 | 39 | 36 | 42 | 41 | 37 | ||||
| >60 | 67 | 35 | 32 | 35 | 32 | 31 | 36 | 36 | 31 | ||||
| Sex | .360 | .518 | .025 | .420 | |||||||||
| Male | 111 | 62 | 49 | 55 | 56 | 57 | 54 | 61 | 50 | ||||
| Female | 34 | 22 | 12 | 19 | 15 | 10 | 24 | 16 | 18 | ||||
| Tumor length | .185 | .447 | .008 | .005 | |||||||||
| <3 cm | 37 | 18 | 19 | 23 | 14 | 24 | 13 | 27 | 10 | ||||
| ≥3 cm | 108 | 66 | 42 | 51 | 57 | 43 | 65 | 50 | 58 | ||||
| Differentiation | .100 | .282 | .059 | .160 | |||||||||
| Well | 17 | 9 | 8 | 10 | 7 | 9 | 8 | 12 | 5 | ||||
| Moderately | 90 | 54 | 36 | 52 | 38 | 47 | 43 | 44 | 46 | ||||
| Poorly | 38 | 21 | 17 | 12 | 26 | 11 | 27 | 21 | 17 | ||||
| T stage | .001 | .308 | .422 | .003 | |||||||||
| T1 | 13 | 4 | 9 | 9 | 4 | 8 | 5 | 9 | 4 | ||||
| T2 | 34 | 12 | 22 | 16 | 18 | 18 | 16 | 23 | 11 | ||||
| T3 | 64 | 42 | 22 | 35 | 29 | 26 | 38 | 40 | 24 | ||||
| T4 | 34 | 26 | 8 | 14 | 20 | 15 | 19 | 10 | 24 | ||||
| N stage | .003 | .026 | .002 | .000 | |||||||||
| N0 | 55 | 24 | 31 | 35 | 20 | 36 | 19 | 39 | 16 | ||||
| N1 | 55 | 31 | 24 | 28 | 27 | 22 | 33 | 31 | 24 | ||||
| N2 | 30 | 25 | 5 | 9 | 21 | 8 | 22 | 4 | 26 | ||||
| N3 | 5 | 4 | 1 | 2 | 3 | 1 | 4 | 3 | 2 | ||||
| TNM stage | .017 | .022 | .010 | .028 | |||||||||
| I | 12 | 3 | 9 | 9 | 3 | 8 | 4 | 10 | 2 | ||||
| II | 53 | 28 | 25 | 32 | 21 | 31 | 22 | 31 | 22 | ||||
| III + IV | 80 | 53 | 27 | 33 | 47 | 28 | 52 | 36 | 44 | ||||
| PTEN | .085 | .000 | |||||||||||
| Low | 84 | – | – | – | 48 | 36 | – | – | – | 25 | 59 | ||
| High | 61 | – | – | 26 | 35 | – | – | 52 | 9 | ||||
| p-AKT | .000 | .036 | .071 | .000 | |||||||||
| Low | 77 | 36 | 41 | 33 | 44 | 41 | 36 | 52 | 25 | ||||
| High | 68 | 48 | 20 | 41 | 27 | 26 | 42 | 25 | 43 | ||||
| p-mTOR | .544 | .000 | .601 | ||||||||||
| Low | 67 | 37 | 30 | 45 | 22 | – | – | – | 34 | 33 | |||
| High | 78 | 47 | 31 | 29 | 49 | – | – | 43 | 35 | ||||
| p-4E-BP1 | .088 | .056 | .992 | .000 | |||||||||
| Low | 93 | 49 | 44 | 53 | 40 | 43 | 50 | 61 | 32 | ||||
| High | 52 | 35 | 17 | 21 | 31 | 24 | 28 | 16 | 36 | ||||
| p-P70S6K1 | .019 | .167 | .272 | .000 | |||||||||
| Low | 86 | 43 | 43 | 48 | 38 | 43 | 43 | 57 | 29 | ||||
| High | 59 | 41 | 18 | 26 | 33 | 24 | 35 | 20 | 39 | ||||
Abbreviations: mTOR, mammalian target of rapamycin; P70S6K1, P70S6 kinase 1; PTEN, phosphatase and tensin homolog. Bold values mean significant difference.
The presence of PTEN and other PI3K/AKT/mTOR signaling pathway proteins had significant differences. There were significant linear relations between proteins and their phosphorylated forms. Phosphatase and tensin homolog expression was negative associated with p-AKT (P < .001), P70S6K1 (P < .001), and p-P70S6K1 (P = .019). Mammalian target of rapamycin expression was positive associated with p-AKT (P = .036) and p-mTOR expression (P < .001). P70S6K1 expression had a positive correlation with p-P70S6K1 (P < .001), p-AKT (P < .001), and p-4E-BP1 (P < .001) expression, as shown in Table 3.
Discussion
The PI3K/AKT/mTOR signaling pathway plays a crucial role in the regulation of multiple cellular functions including cell growth, proliferation, and angiogenesis in numerous solid tumors.13,14 Particularly, activation of this pathway has been linked to various prognostic clinicopathological parameters, such as the stage of tumor, the degree of tumor differentiation, tumor size, nodal involvement, distant metastasis, and chemosensitivity, which could result a poor survival in gastrointestinal tumors, such as gastric cancer, colon cancer, and so on.15-16 Previous studies have suggested the activation of PI3K/AKT/mTOR signaling pathway in ESCC specimens and revealed the PI3K pathway activation contributes to the proliferation and survival of esophageal cancer, and mTOR is a key downstream protein kinase of PI3K/AKT signaling pathway.17-20 For example, Hirashima et al examined 167 patients with ESCC and found that 116 (69.5%) patients had the overexpression of p-mTOR. Suppression of p-mTOR could inhibit proliferation and invasion and induce apoptosis in ESCC.21 Lu et al found 94 (63.5%) of 148 patients have high expression of mTOR. Instead, 102 (69.9%) of the 148 patients have low expression of PTEN in ESCC and suggested that mTOR was an independent prognostic factor by multivariate survival analysis.22 Hou et al demonstrated that the expression of mTOR has important clinical significance and inhibition of mTOR pathway by mTOR siRNA can improve the chemotherapy sensitivity of ESCC cells.23 Although these studies revealed the importance of PI3K/AKT/mTOR signaling pathway in the dissemination of esophageal cancer, the main mTOR signaling components were not included, retaining the question of the significance of mTOR signaling in esophageal cancer. Thus, we systematically determined the expression level of the key proteins in PI3K/AKT/mTOR signaling pathway.
In our study, statistically significant differences were observed for 9 of the 10 proteins investigated. Compared to nontumor tissues, PI3K, AKT, p-AKT, mTOR, p-mTOR, 4E-BP1, P70S6K1, and p-P70S6K1 were all upregulated in tumor tissues. Conversely, PTEN was found to be downregulated in ESCC tumor tissue. Our results, as well as the previous studies, confirmed that the PI3K/AKT/mTOR signaling pathway plays a key role in the process of ESCC carcinogenesis. Moreover, our study strongly suggested that the main components of PI3K/AKT/mTOR signaling pathway proteins are all involved in the process of oncogenesis.
Our study also found the low expression of PTEN was closely related to advanced T-stage, the presence of lymph node metastases, and advanced TNM stage. Phosphatase and tensin homolog is a tumor suppressor and the previous studies have demonstrated its value as an important marker of good prognosis in some gastrointestinal solid tumors, which contains ESCC.24-26 In our study, we found that PTEN is a major negative regulator of the PI3K/AKT/mTOR signaling pathway. The loss of PTEN expression was inversely correlated with the accumulated expression of the majority of the mTOR signaling pathway proteins. Patients with the loss of PTEN expression had significantly worse overall survival.
In contrast to PTEN that acts as a suppressor of this signaling pathway, activation of mTOR could increase growth signals and increase protein synthesis through the phosphorylation and inactivation of 4E-BP1 and P70S6K1. And both the total and phosphorylated proteins had important clinical implications. Our study found patients with a high expression of p-mTOR or P70S6K1 tended to have tumors of more than 3 cm in maximum diameter. Meanwhile, the overexpression of mTOR, p-mTOR, and P70S6K1 in tumors was closely related to the presence of lymph node metastases, advanced TNM stage. p-mTOR expression was correlated with progression-free survival as well as mTOR expression was correlated with both progression-free survival and OS. The above results indicated that the PI3K/AKT/mTOR signaling pathway played a key role in promoting esophageal cancer and might offer new therapeutic avenues for esophageal cancer.
In the future, it will be valuable to increase the sample size to firmly confirm our observation and build up the solid correlation between PI3K/AKT/mTOR signaling and ESCC tumors. Since we only examined the expression levels of the PI3K/AKT/mTOR signaling components were by the IHC, it will be of great interest to further investigate the related gene alterations, transcriptions, and mutations. Finally, by combination of the mTOR signaling specific inhibitors, it will be possible to identify the precise signaling pathway that is involved in the pathogenesis of ESCC and its value for optimizing individual molecular target therapy.
Conclusions
In summary, our study demonstrated that most advanced ESCC tumors showed the activated PI3K/AKT/mTOR signaling pathway with the low expression of PTEN but accumulated expression of the majority of the mTOR signaling pathway proteins (both total and phosphorylated). The level of expression of PTEN, mTOR, p-mTOR, and P70S6K1 were closely related to the presence of lymph node metastases. The expression of PTEN, mTOR, and P70S6K1 were correlated to the TNM stage and overall survival. Therefore, the PI3K/AKT/mTOR signaling pathway had potential value for both the prognostic marker and therapy of ESCCs.
Abbreviations
- 4E-BP1
elongation initiation factor 4E binding protein-1
- ESCC
esophageal squamous cell carcinoma
- IHC
immunohistochemistry
- mTOR
mammalian target of rapamycin
- p70S6K
p70S6 kinase
- PET
positron emission tomography
- PI3K
phosphoinositide-3 kinase
- PTEN
phosphatase and tensin homolog.
Footnotes
Authors’ Note: Written informed consent was obtained from all individual participated included in the study. Ning Wu and Zunguo Du were co-first authors.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by grant from the Shanghai Municipal Health & Family Planning Commission (no 20174Y0238) and the National Natural Science Foundation of China (no 81602079).
ORCID iD: Ning Wu  http://orcid.org/0000-0002-5458-8327
http://orcid.org/0000-0002-5458-8327
Zhiming Chen  http://orcid.org/0000-0002-0951-6535
http://orcid.org/0000-0002-0951-6535
References
- 1. Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics. CA Cancer J Clin. 2015;65(2):87–108. [DOI] [PubMed] [Google Scholar]
- 2. Kamangar F, Dores GM, Anderson WF. Patterns of cancer incidence, mortality, and prevalence across five continents: defining priorities to reduce cancer disparities in different geographic regions of the world. J Clin Oncol. 2006;24(14):2137–2150. [DOI] [PubMed] [Google Scholar]
- 3. Chen W, Zheng R, Zhang S, et al. Esophageal cancer incidence and mortality in China, 2010. Thorac Cancer. 2011;5(4):343–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wu N, Chen Z, Pang L, Ma Q, Chen G. Prognostic significance of lymph node characteristics on survival in esophageal squamous cell carcinomas. Wien Klin Wochenschr. 2013;125(1-2):26–33. [DOI] [PubMed] [Google Scholar]
- 5. Wu N, Pang LW, Chen ZM, Ma QY, Chen G. Tumour length is an independent prognostic factor of esophageal squamous cell carcinomas. Chin Med J. 2012;125(24):4445–4448. [PubMed] [Google Scholar]
- 6. Vivanco I, Sawyers CL. The phosphatidylinositol 3-kinase AKT pathway in human cancer. Nat Rev Cancer. 2002;2(7):489–501. [DOI] [PubMed] [Google Scholar]
- 7. Yoon MS. The role of mammalian target of rapamycin (mTOR) in insulin signaling. Nutrients. 2017;9(11):1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Paquette M, El-Houjeiri L, Pause A. . mTOR pathways in cancer and autophagy. Cancers (Basel). 2018;10(1):18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sukhbaatar N, Hengstschläger M, Weichhart T. . mTOR-mediated regulation of dendritic cell differentiation and function. Trends Immunol. 2006;37(11):778–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ma XM, Blenis J. Molecular mechanisms of mTOR-mediated translational control. Nat Rev Mol Cell Biol. 2009;10(5):307–318. [DOI] [PubMed] [Google Scholar]
- 11. Zhu J, Wang M, Zhu M, et al. Associations of PI3KR1 and mTOR polymorphisms with esophageal squamous cell carcinoma risk and gene-environment interactions in Eastern Chinese populations. Sci Rep. 2015;5:8250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hildebrandt MA, Yang H, Hung MC, et al. Genetic variations in the PI3K/PTEN/AKT/mTOR pathway are associated with clinical outcomes in esophageal cancer patients treated with chemoradiotherapy. J Clin Oncol. 2009;27(6):857–871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Strimpakos AS, Karapanagiotou EM, Saif MW, Syrigos KN. The role of mTOR in the management of solid tumors: an overview. Cancer Treat Rev. 2009;35(2):148–159. [DOI] [PubMed] [Google Scholar]
- 14. Ocana A, Vera-Badillo F, Al-Mubarak M, et al. Activation of the PI3K/mTOR/AKT pathway and survival in solid tumors: systematic review and meta-analysis. PLoS One. 2014;9(4):e95219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Malinowsky K, Nitsche U, Janssen KP, et al. Activation of the PI3K/AKT pathway correlates with prognosis in stage II colon cancer. Br J Cancer. 2014;110(8):2081–2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ying J, Xu Q, Liu B, Zhang G, Chen L, Pan H. The expression of the PI3K/AKT/mTOR pathway in gastric cancer and its role in gastric cancer prognosis. Onco Targets Ther. 2015;8:2427–2433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Li B, Xu WW, Lam AKY, et al. Significance of PI3K/AKT signaling pathway in metastasis of esophageal squamous cell carcinoma and its potential as a target for anti-metastasis therapy. Oncotarget. 2017;8(24):38755–38766. doi:10.18632/oncotarget.16333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lin JW, Li X, Qiu ML, et al. PI3K overexpression and PIK3CA mutations are associated with age, tumor staging, and other clinical characteristics in Chinese patients with esophageal squamous cell carcinoma. Genet Test Mol Biomarkers. 2017;21:236–241. [DOI] [PubMed] [Google Scholar]
- 19. Zhang HF, Wu C, Alshareef A, et al. The PI3K/AKT/c-MYC axis promotes the acquisition of cancer stem-like features in esophageal squamous cell carcinoma. Stem Cells. 2016;34(8):2040–2051. [DOI] [PubMed] [Google Scholar]
- 20. Lin DC, Hao JJ, Nagata Y, et al. Genomic and molecular characterization of esophageal squamous cell carcinoma. Nat Genet. 2014;46(5):467–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hirashima K, Baba Y, Watanabe M, et al. Aberrant activation of the mTOR pathway and anti-tumour effect of everolimus on oesophageal squamous cell carcinoma. Br J Cancer. 2012;106(5):876–882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lu J, Pan Y, Xia X, Gu Y, Lei Y. Prognostic significance of mTOR and PTEN in patients with esophageal squamous cell carcinoma. Biomed Res Int. 2015;2015:417210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hou G, Yang S, Zhou Y, Wang C, Zhao W, Lu Z. Targeted inhibition of mTOR signaling improves sensitivity of esophageal squamous cell carcinoma cells to cisplatin. J Immunol Res. 2014;2014:845763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zhang X, Park JS, Park KH, et al. PTEN deficiency as a predictive biomarker of resistance to HER2-targeted therapy in advanced gastric cancer. Oncology. 2015;88(2):76–85. [DOI] [PubMed] [Google Scholar]
- 25. Waniczek D, Śnietura M, Młynarczyk-Liszka J, et al. PTEN expression profiles in colorectal adenocarcinoma and its precancerous lesions. Pol J Pathol. 2013;64(1):15–20. [DOI] [PubMed] [Google Scholar]
- 26. Tachibana M, Shibakita M, Ohno S, et al. Expression and prognostic significance of PTEN product protein in patients with esophageal squamous cell carcinoma. Cancer. 2002;94(7):1955–60. [DOI] [PubMed] [Google Scholar]