Abstract
The aim of this case series was to reveal the difficulties in diagnosing fibro-osseous lesions with radiological and histopathological examinations and quantify the potential risk of infection to fibro-osseous legions. To analyze the concordance between radiological and histopathological diagnoses, this retrospective case series included patients who were clinically diagnosed with fibro-osseous lesions via radiological findings and excluded the patients who did not undergo histopathological examinations. This study also included the patients in whom histopathological results confirmed fibro-osseous legions when preoperative radiological diagnosis did not include fibro-osseous legions. Eleven patients (three men, eight women; median age 24.5 years, range 15–57 years) were enrolled. Although radiological diagnoses of fibrous dysplasia (FD) corresponded with histopathological diagnoses in seven patients, mismatches between radiological findings and histopathological results were found in three patients. In one patient, suspected diagnosis with radiological examinations was malignant lymphoma or FD. In two patients, the histopathological differentiation between FD and ossifying fibroma (OF) was difficult. One patient had lesion recurrence which was suspected to be OF with surgical findings and postoperative course after the initial surgery. In three patients, infections of FD were found. Preoperative diagnosis of OF with radiographic feature of unilocular radiolucency is difficult. In cases in which histopathological differentiation between FD and OF is difficult, operative findings should be used because OF is often found to be well-encapsulated and easily enucleated. Bone in FD showing mixed radiolucent-radiopaque may be vulnerable to infection.
Keywords: Fibro-osseous legion, Fibrous dysplasia, Ossifying fibroma, Recurrence, Infection
INTRODUCTION
A simple classification of fibro-osseous lesions of the maxillofacial region by Speight and Carlos in 2006 [1], which is based on the World Health Organization classification [2] and published literature [3–6], divides legions into fibrous dysplasia (FD), ossifying fibroma (OF), and osseous dysplasia (OD). There is a previous review in 2008 which classified benign fibro-osseous lesions into bone dysplasia, cemento-osseous dysplasia, an inflammatory/reactive process, metabolic disease, and neoplastic lesions [7].
OD is characterized by reactive lesions present only in the jaw bones and therefore no specific treatment is necessary [8]. FD growth tends to stabilize when skeletal maturity is attained; therefore, surgical intervention in children and adolescents should be delayed as long as possible [9]. When FD stabilizes, the optimal treatment is a contouring surgery to correct facial deformity [8]. However, it has been reported that the progression of FD may continue during adulthood, resulting in facial deformity and functional problems [10–12]. In contrast, OF requires surgical interventions such as complete enucleation from the surrounding bone because of its growth pattern and risk for recurrence [9].
Typical cases of FD and OF can be distinguished by radiological findings. In a previous study, more than 60% of FD cases had characteristic radiographic features (e.g., ground-glass appearance), about 20% of FD showed mixed radiolucent-radiopacity, and about 60% of OF had radiographic features including unilocular radiolucency or mixed radiolucent-radiopaque appearance [8]. In contrast, accurate histopathological distinctions between FD and OF are often difficult unless the alpha subunit of the stimulatory G protein gene (GNAS) mutation analysis is performed [9,13].
As mentioned above, there are difficulties in diagnosing fibro-osseous legions. This retrospective study primarily intended to show the discrepancy between the radiological and histopathological diagnosis of fibro-osseous legions of the jaws. In addition, we also quantified the potential risk of infection to fibro-osseous legions.
MATERIAL AND METHODS
To analyze concordance between radiological and histopathological diagnoses, this retrospective study included patients who were clinically diagnosed with fibro-osseous lesions with radiological findings by experienced oral and maxillofacial surgeons at the Department of Oral and Maxillofacial Surgery at the Kobe University Hospital and Kakogawa East City Hospital between July 2007 and August 2015. We excluded patients who did not undergo histopathological examination. This study also excluded the lesions diagnosed as OD because OD has no need for surgical treatments. Moreover, this study included patients in whom postoperative histopathological results diagnosed fibro-osseous legions despite a preoperative radiological diagnosis that did not indicate fibro-osseous legions.
Clinical data, including patients’ age, sex, lesion locations, and radiological diagnosis, treatment, and recurrence were collected from medical records. Panoramic x-rays and computed tomography (CT) scans were taken for all patients. Radiographic parameters analyzed were the periphery of the lesion (well- or ill-defined); internal structure (unilocular radiolucency, mixed radiolucent-radiopaque, or ground-glass appearance); effects on adjacent teeth (displacement or root resorption), as mentioned in previous studies [8,14]. In patients who underwent contouring, the part of resected specimen was submitted for pathological analysis. In patients who underwent debridement, the part of debrided granulation tissue and adjacent hard tissue was submitted for pathological analysis. In patients with suspected tumor preoperatively, the excision specimen was submitted for pathological analysis. Microscopic examination of each case was performed by experienced pathologists without clinical information.
RESULTS
Eleven patients (three men, eight women; median age 24.5 years, range 15–57 years) were enrolled. Nine patients were excluded because they did not undergo histopathological examination. These nine patients were clinically suspected as FD. A summary of eleven patients is shown in Table 1. Although radiological diagnoses of FD corresponded with histopathological diagnoses in seven patients (No. 1, 2, 5, 6, 7, 8, and 9), mismatches between radiological diagnoses and histopathological results were found in four patients (No. 3, 4, and 10).
Table 1.
Patients’ characteristics Summary of 11 patients
| No. | Sex | Age (years) | Location | Radiological features | Radiological diagnosis | Treatment | Histopathological diagnosis | ||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Internal structure | Periphery | Effect on adjacent tooth | |||||||
| 1 | M | 19 | Mandible | Ground-glass appearance | Ill defined | Slightly displacement | FD | Contouring under general anesthesia | FD |
| 2 | F | 57 | Maxilla | Mixed radiolucent-radiopaque | Ill defined | Extrusion | FD | Tooth extraction and debridement under local anesthesia | FD |
| 3 | F | 43 | Mandible | Ground-glass appearance | Ill defined | None | FD | Incisional biopsy under general anesthesia | Calcifying epithelial odontogenic tumor |
| 4 | F | 20 | Condyle | Unilocular radiolucency | Well defined | - | Unilocular radiolucency not otherwise specified | Complete resection under general anesthesia | Fibro-osseous lesion |
| 5 | F | 17 | Mandible | Ground-glass appearance | Ill defined | Slightly displacement | FD | Contouring under general anesthesia | FD |
| 6 | M | 41 | Maxilla | Mixed radiolucent-radiopaque | Ill defined | None | FD | Contouring under general anesthesia | FD |
| 7 | F | 20 | Maxilla | Mixed radiolucent-radiopaque | Ill defined | None | FD | Partial resection under general anesthesia | FD |
| 8 | F | 24 | Mandible | Mixed radiolucent-radiopaque | Ill defined | None | FD | Partial resection under general anesthesia | FD |
| 9 | F | 35 | Mandible | Ground-glass appearance | Ill defined | None | FD | Debridement under general anesthesia | FD |
| 10 | M | 25 | Mandible | Unilocular radiolucency | Well defined | Root resorption | Ameloblastoma | Extirpation under general anesthesia | Fibro-osseous lesion |
| 11 | F | 15 | Mandible | Mixed radiolucent-radiopaque | Ill defined | None | ML or FD | Incisional biopsy, lymph node excision, and bone marrow aspiration under general anesthesia | FD |
FD = Fibrous dysplasia, ML = Malignant lymphoma.
Radiographic features of No. 1, 3, and 5 were typical of FD, namely ground-glass appearance (Figure 1). No. 3 was diagnosed as FD because of ground-glass appearance (Figure 2). However, the histopathological examination revealed a calcifying epithelial odontogenic tumor.
Figure 1.

Case No. 5.
A typical radiographic feature of fibrous dysplasia showing ground-glass appearance (arrow).
Figure 2.

Case No. 3.
A patient in whom a mismatch between radiological finding (fibrous dysplasia) and histopathological result (calcifying epithelial odontogenic tumor) was found. Arrow indicates ground-glass appearance.
No. 4, in whom the lesion was located at the condyle (Figure 3) and No. 10 (Figure 4) were diagnosed as tumors with radiographic examinations. However, the histopathological diagnoses were fibrous-osseous legions. Both radiographic features were unilocular radiolucency.
Figure 3.

Case No. 4.
A well-defined and unicystic radiolucency in the right condyle (arrow). The histopathological result was fibro-osseous lesion.
Figure 4.
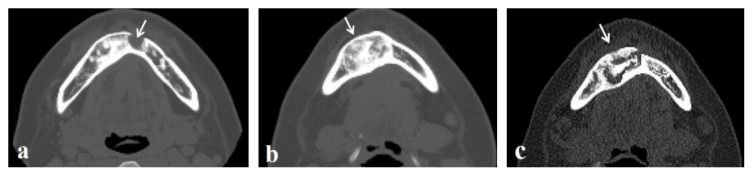
Case No. 10.
A patient in whom a recurrence was found in this study. (a, b) A well-defined, unicystic, round-shaped radiolucency before the initial surgery (arrows). (c, d) An extensive bone resorption involving the mandibular body and ramus two years after the initial surgery (arrows). Axial CT image showing relatively ill-defined border of lesion (c). Notice the root resorption of the first molar (arrowhead). (e–f) Results of hematoxylin and eosin staining (original magnification ×40) showing fibrous tissue composed of spindle-shaped cells (e), irregular trabeculae (f), and bone without apparent osteoblastic rimming (g). Bars, 50μm.
In No. 10, extensive bone resorption in the mandibular molar region was found preoperatively (Figures 4a, 4b), and an enucleation was performed with suspicion of a benign tumor, such as an ameloblastoma. Unexpectedly, the histopathological result was FD. Two years after the initial surgery, routine follow-up CT revealed bone resorption extending to the ramus and root resorption of the first molar (Figures 4c, 4d). Extirpation was performed with suspicion of recurrence of the lesion. In both the initial and second surgery, lesions were easily removed. Histopathological examination showed fibrous tissue composed of spindle-shaped cells (Figure 4e), irregular trabeculae (Figure 4f), and bone without apparent osteoblastic rimming (Figure 4g). Similar histological findings were found in case 4. They were histopathologically diagnosed as fibro-osseous legions.
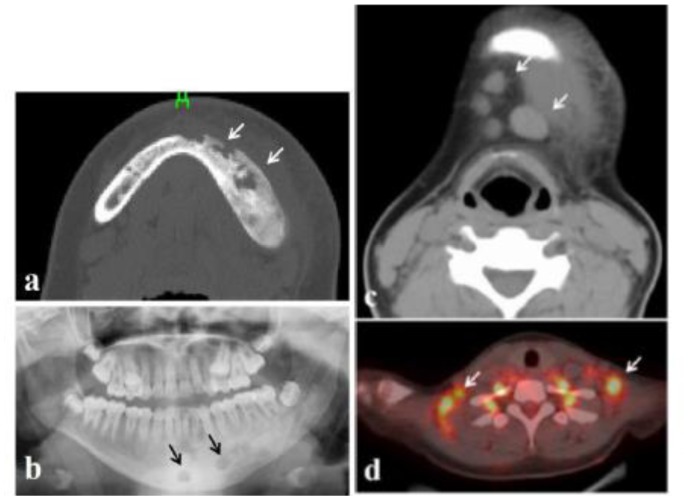
No. 11 was a 15-year-old girl, whose chief complaint was a severely swollen cheek and jaw pain. In other hospital, she received the intravenous administration of antibiotic with suspicion of infection. CT and panoramic x-ray images revealed irregular bone resorption surrounded by radiopaque lesion (Figures 5a, 5b) and was consequently introduced to our department. CT (Figure 5c) and positron emission tomography (Figure 5d) revealed the enlarged submental, submandibular, supraclavicular, and axillary lymph nodes with the fluorodeoxyglucose uptake. Incisional biopsy of the mandibular lesion, excision of the enlarged submental lymph nodes, and bone marrow aspiration for diagnosis under general anesthesia was performed with suspicion of malignant lymphoma or infection of FD. Result of hematoxylin and eosin staining of mandibular lesion showed irregular trabeculae and inflammatory cell infiltration into the fibrous tissue. The histological diagnosis was FD with inflammation.
Figure 5.
Case No. 11. (a, b) Sporadic radiolucency in the left mandible (arrows) surrounded by radiopaque lesion. (c) CT image showing enlargement submental lymph nodes (arrows). (d) PET image showing supraclavicular lymph nodes with fluorodeoxyglucose uptake (arrows).
Cases No. 2 and 9 had also FD infections. The chief complaint of No. 2 was dull pain in the maxillary molar. The radiographic features of the CT image were mixed radiolucent-radiopaque appearance and localized bone resorption around the maxillary second molar (Figures 6a, 6b). Tooth extraction of causal tooth, debridement of infected lesion, and incisional biopsy were performed under local anesthesia. After irrigation, wound was primarily closed. The recurrence of infection was not found one year postoperatively.
Figure 6.
Case No. 2.
(a, b) Axial and coronal CT images showing a typical radiographic feature of fibrous dysplasia with mixed radiolucent-radiopaque appearance, concomitant with localized bone resorption due to periodontitis of maxillary second molar (arrows).
Case No. 9 had a keratocystic odontogenic tumor (KCOT) in the left anterior mandible (Figure 7a) and FD in the right anterior mandible (Figure 7b). She received the extirpation of the left KCOT and the incisional biopsy of the right FD. The secondary intension healing of open wound following KOCT extirpation was achieved without problems. Five years after the initial surgery, she had dull pain and pus discharge in the right anterior mandible and was consequently introduced to our department. Preoperative CT images revealed sequestrum formation was probably owing to FD infection via the bone defect after the extirpation of KCOT (Figure 7c). Debridement under general anesthesia was performed and the postoperative course was good.
Figure 7.
Case No. 9. A patient who had keratocystic odontogenic tumor (KCOT) in the left anterior mandible (arrow) (a) and fibrous dysplasia (FD) in the right anterior mandible (arrow) (b). CT image five years after the initial surgery for KCOT showing the sequestrum formation and bone resorption (arrow) in FD probably due to the infection of FD (c).
DISCUSSION
In this retrospective study, we show the difficulties of radiographic and histopathologic diagnosis of fibro-osseous lesions. The differentiation of FD and OF is important because the appropriate clinical intervention is quite different for these lesions. OF is neoplasm in the true sense, exhibiting progressive proliferative capability with bony expansion [7]. Moreover, surgery, infection, dental extraction, and trauma could serve as a proliferative factors of OF [15,16]. In this study, two out of eleven fibro-osseous lesions could not be diagnosed as fibro-osseous legions using only radiological means (Nos. 4 and 10). Their radiographic features were unilocular radiolucency. A previous publication reported that the rate of mismatch between radiographic and histopathological diagnoses was 25% [15].
Importantly, OF often exhibits well-defined margins with corticated lines radiologically and often shows cystic (unicystic 75%, multicystic 25%) or mixed-density appearances [7,15]. In contrast to FD, the radiological shape of OF is round or oval. The specific radiological finding of OF is a radiolucent rim around a mixed or radiopaque lesion [17]. Liu et al. [15] described that a radiolucent band of capsule at the periphery separated the more mature internal radiopaque portion from the surrounding normal bone in OF. OF may present as irregular in shape, especially if the tumor recurs or grows quickly in a short time period [15,18]. Root resorption of the adjacent tooth is also known to be a pathological effect of OF [1,15,19]. The clinical findings after the initial surgery in No.10 (i.e., rapid growth and root resorption) suggests OF. However, histopathologically differentiating FD and OF was difficult which was also true in No. 4 and 10. OF shows three histologic patterns (“ossifying”, “cementifying”, and “storiform”); and the most common ossifying forms shows patterns that may be indistinguishable from FD, with small, irregular osteoid trabeculae typically rimmed by osteoblasts [7]. The stromal element is hyper-celluar and the fibroblastic cells are devoid of atypical cytologic features. Osteoblastic rimming is minimal and the irregular trabeculae are often lamellar when the lesions mature [7]. Although peritrabecular clefting may be a hallmark of FD and a valuable microscopic feature for distinguishing FD from OF [20], mutational analysis of the GNAS gene may be only a reliable adjunct to differentiate FD and OF [21]. Despite the difficulties of radiographic and histopathological diagnosis mentioned above, surgical findings in FD and OF are distinctly different. OF is often found to be well-encapsulated [19], because a thin fibrous capsule demarcates the lesion from the adjoining normal bone [22]. Although radiographic and histopathological diagnosis in fibro-osseous lesions especially with radiographic feature of radiolucency is sometimes difficult, oral and maxillofacial surgeons should distinguish OF from FD by referring to their own operative findings. Considering operative findings (lesion was well-encapsulated, therefore, easily removed) and the recurrence, the lesion in No.10 was clinically suspected to be OF. In No.4, osteoblastic rimming was observed in just a part of the excised specimen. Therefore, it was impossible to completely exclude the possibility of OF histopathologically. Unlike FD, since OF has a risk of recurrence, careful follow-up now is necessary in No.4.
In this study, there were three patients with FD infections (Nos. 2, 9, and 11). Some authors have reported fibro-osseous lesions with infectious diseases, such as mandibular chronic osteomyelitis (OM) [23] and chronic maxillary sinusitis [24]. Fibro-osseous legion of the mandible can mimic OM clinically and radiographically [23,25]. A previous, retrospective, large-sample-sized study including 68 patients reported that only one patient treated with bone graft reconstruction (1.5%) had postoperative infection for craniomaxillofacial FD [26]. A case report about FD with chronic osteomyelitis of the clavicle mentioned that the structural integrity of the bone affected by FD is weakened and bowing of weight-bearing bones is common [27]. Most pathologic fractures in FD occur in weight-bearing long bones at young ages [28] and FD is not typically associated with facial fractures [29]. The only report of an association between monostotic FD and facial fractures by Pinto et al. mentions that the association between FD and facial fractures is rare, likely because the irregular deposition of trabecular bone increases the thickness and resiliency of the involved bones, thus increasing their fracture resistance [29]. They also noted that areas of bone modification were avoided for implant placement because of the unknown response of dysplastic bone to the presence of screws [29].
We experienced a rare case (No. 9) in which KCOT and FD coincided in the anterior mandible. Bone resorption, probably due to chronic infection via the bone defect after KCOT removal, was found in CT images five years postoperatively. In contrast, there was no infection in cases that received contouring for FD with primary closure (No. 1, 5, and 6). Their radiographic features were ground-glass appearance, probably indicating the stiffness of bone. In contrast, bone resorption caused by infection of FD was extensive in No. 9. Importantly, her radiographic feature was mixed radiolucent-radiopaque. The radiographic feature in No. 2 was also mixed radiolucent-radiopaque, however, the lesion adjacent to the bone resorption probably due to the periodontitis of the maxillary second molar appeared radiopaque (see Fig. 6b), indicating the stiffness of bone. Taken together, the bone characteristics of areas showing ground-glass appearance perhaps have stiffness, but the areas showing mixed radiolucent-radiopaque may be vulnerable to local infection, especially in radiolucent region.
In conclusion, preoperative diagnosis of OF with radiographic feature of radiolucency is often difficult. For cases in which histopathological differentiation between FD and OF is unclear, operative findings should be used because OF is often found to be well-encapsulated and easily enucleated. Careful observation after surgery for OF is essential because OF often recurs. Although bone in FD showing mixed radiolucent-radiopaque may be vulnerable to infection, the further investigations about infection tolerance of fibro-osseous lesions is needed.
ACKNOWLEDGMENTS AND DISCLOSURE STATEMENTS
The authors report no conflicts of interest related to this study.
REFERENCES
- 1.Speight PM, Carlos R. Maxillofacial fibro-osseous lesions. Current Diagnostic Pathology. 2006;12:1–10. [Google Scholar]
- 2.Barnes L, Eveson JW, Reichart P, Sidransky D, editors. Pathology and genetics of head and neck tumours. Lyon: ARC Press; 2005. WHO classification of tumours. [Google Scholar]
- 3.Waldron CA. Fibro-osseous lesions of the jaws. J Oral Maxillofac Surg. 1993;51:828–835. doi: 10.1016/s0278-2391(10)80097-7. [DOI] [PubMed] [Google Scholar]
- 4.Slootweg PJ. Maxillofacial fibro-osseous lesions: classification and differential diagnosis. Semin Diagn Pathol. 1996;13:104–112. [PubMed] [Google Scholar]
- 5.Brannon RB, Fowler CB. Benign fibro-osseous lesions: a review of current concepts. Adv Anat Pathol. 2001;8:126–143. doi: 10.1097/00125480-200105000-00002. [DOI] [PubMed] [Google Scholar]
- 6.El-Mofty S. Psammomatoid and trabecular juvenile ossifying fibroma of the craniofacial skeleton: two distinct clinicopathologic entities. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2002;93:296–304. doi: 10.1067/moe.2002.121545. [DOI] [PubMed] [Google Scholar]
- 7.Eversole R, Su L, ElMofty S. Benign fibro-osseous lesions of the craniofacial complex. A review. Head Neck Pathol. 2008;2:177–202. doi: 10.1007/s12105-008-0057-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Phattarataratip E, Pholjaroen C, Tiranon P. A Clinicopathologic Analysis of 207 Cases of Benign Fibro-Osseous Lesions of the Jaws. Int J Surg Pathol. 2004;22:326–333. doi: 10.1177/1066896913511985. [DOI] [PubMed] [Google Scholar]
- 9.Toyosawa S, Yuki M, Kishino M, Ogawa Y, Ueda T, Murakami S, Konishi E, Iida S, Kogo M, Komori T, Tomita Y. Ossifying fibroma vs fibrous dysplasia of the jaw: molecular and immunological characterization. Mod Pathol. 2007;20:389–396. doi: 10.1038/modpathol.3800753. [DOI] [PubMed] [Google Scholar]
- 10.Posnick JC. Fibrous dysplasia of the craniomaxillofacial region: current clinical perspectives. Br J Oral Maxillofac Surg. 1998;36:264–273. doi: 10.1016/s0266-4356(98)90710-0. [DOI] [PubMed] [Google Scholar]
- 11.Park BY, Cheon YW, Kim YO, Pae NS, Lee WJ. Prognosis for craniofacial fibrous dysplasia after incomplete resection: age and serum alkaline phosphatase. Int J Oral Maxillofac Surg. 2010;39:221–226. doi: 10.1016/j.ijom.2009.12.008. [DOI] [PubMed] [Google Scholar]
- 12.Ma J, Liang L, Gu B, Zhang H, Wen W, Liu H. A retrospective study on craniofacial fibrous dysplasia: preoperative serum alkaline phosphatase as a prognostic marker? J Craniomaxillofac Surg. 2013;41:644–647. doi: 10.1016/j.jcms.2012.12.007. [DOI] [PubMed] [Google Scholar]
- 13.Tabareau-Delalande F, Collin C, Gomez-Brouchet A, Decouvelaere AV, Bouvier C, Larousserie F, Marie B, Delfour C, Aubert S, Rosset P, de Muret A, Pagès JC, de Pinieux G. Diagnostic value of investigating GNAS mutations in fibro-osseous lesions: a retrospective study of 91 cases of fibrous dysplasia and 40 other fibro-osseous lesions. Mod Pathol. 2013;26:911–921. doi: 10.1038/modpathol.2012.223. [DOI] [PubMed] [Google Scholar]
- 14.Nityasri V, Haris PS, Bose T, Balan A. Fibrous dysplasia--a 13-year retrospective radiographic analysis in a south Indian population. Dentomaxillofac Radiol. 2011;40:282–289. doi: 10.1259/dmfr/32556437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu Y, Wang H, You M, Yang Z, Miao J, Shimizutani K, Koseki T. Ossifying fibromas of the jaw bone: 20 cases. Dentomaxillofac Radiol. 2010;39:57–63. doi: 10.1259/dmfr/96330046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.MacDonald-Jankowski DS. Fibro-osseous lesions of the face and jaws. Clin Radiol. 2004;59:11–25. doi: 10.1016/j.crad.2003.07.003. [DOI] [PubMed] [Google Scholar]
- 17.Mortazavi H, Baharvand M, Rahmani S, Jafari S, Parvaei P. Radiolucent rim as a possible diagnostic aid for differentiating jaw lesions. Imaging Sci Dent. 2015;45:253–261. doi: 10.5624/isd.2015.45.4.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wenig BL, Sciubba JJ, Cohen A, Goldstein MN, Abramson AL. A destructive maxillary cemento-ossifying fibroma following maxillofacial trauma. Laryngoscope. 1984;94:810–815. doi: 10.1288/00005537-198406000-00015. [DOI] [PubMed] [Google Scholar]
- 19.Vura NG, Gaddipati R, Ramisetti S, Kumara R, Reddy R, Kanchi U. Surgical Management of Ossifying Fibroma in Maxilla: Report of Two Cases. J Int Oral Health. 2015;7:115–118. [PMC free article] [PubMed] [Google Scholar]
- 20.Prado Ribeiro AC, Carlos R, Speight PM, Hunter KD, Santos-Silva AR, de Almeida OP, Vargas PA. Peritrabecular clefting in fibrous dysplasia of the jaws: an important histopathologic feature for differentiating fibrous dysplasia from central ossifying fibroma. Oral Surg Oral Med Oral Pathol Oral Radiol. 2012;114:503–508. doi: 10.1016/j.oooo.2012.06.014. [DOI] [PubMed] [Google Scholar]
- 21.Shi RR, Li XF, Zhang R, Chen Y, Li TJ. GNAS mutational analysis in differentiating fibrous dysplasia and ossifying fibroma of the jaw. Mod Pathol. 2013;26:1023–1031. doi: 10.1038/modpathol.2013.31. [DOI] [PubMed] [Google Scholar]
- 22.Eversole LR, Leider AS, Nelson K. Ossifying fibroma: a clinicopathologic study of sixty-four cases. Oral Surg Oral Med Oral Pathol. 1985;60:505–511. doi: 10.1016/0030-4220(85)90239-7. [DOI] [PubMed] [Google Scholar]
- 23.Chang CY, Wu KG, Tiu CM, Hwang B. Fibrous dysplasia of mandible with chronic osteomyelitis in a child: report of one case. Acta Paediatr Taiwan. 2002;43:354–357. [PubMed] [Google Scholar]
- 24.Barat M, Rybak LP, Mann JL. Fibrous dysplasia masquerading as chronic maxillary sinusitis. Ear Nose Throat J. 1989;68:42, 44–6. [PubMed] [Google Scholar]
- 25.Jacobsson S, Hallén O, Hollender L, Hansson CG, Lindström J. Fibro-osseous lesion of the mandible mimicking chronic osteomyelitis. Oral Surg Oral Med Oral Pathol. 1975;40:433–444. doi: 10.1016/0030-4220(75)90239-x. [DOI] [PubMed] [Google Scholar]
- 26.Valentini V, Cassoni A, Marianetti TM, Terenzi V, Fadda MT, Iannetti G. Craniomaxillofacial fibrous dysplasia: conservative treatment or radical surgery? A retrospective study on 68 patients. Plast Reconstr Surg. 2009;123:653–660. doi: 10.1097/PRS.0b013e318196bbbe. [DOI] [PubMed] [Google Scholar]
- 27.Weerasuriya T, Nashi S, Morgan A, Ebizie A. A dilemma of fibrous dysplasia versus chronic osteomyelitis of the clavicle. BMJ Case Rep. 2012 doi: 10.1136/bcr.12.2011.5319. pii: bcr1220115319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.DiCaprio MR, Enneking WF. Fibrous dysplasia. Pathophysiology, evaluation, and treatment. J Bone Joint Surg Am. 2005;87:1848–1864. doi: 10.2106/JBJS.D.02942. [DOI] [PubMed] [Google Scholar]
- 29.Pinto LC, Ribeiro AL, Aquime JR, Carreira AS, Alves-Junior SM, Pinheiro JJ. Zygomaticomaxillary complex fracture in a zygomatic bone affected by monostotic fibrous dysplasia: a very rare association. J Craniofac Surg. 2013;24:219–22. doi: 10.1097/SCS.0b013e318286984e. [DOI] [PubMed] [Google Scholar]