Abstract
BACKGROUND
Spinal muscular atrophy (SMA) is one of the most common autosomal recessive disorders. The symptoms are caused by defects of lower motor neurons in the spinal cord. More than 95% of SMA patients are homozygous for survival motor neuron 1 (SMN1) deletion. We previously developed a screening system for SMN1 deletion based on a modified competitive oligonucleotide priming-PCR (mCOP-PCR) technique using dried blood spot (DBS) on filter paper. This system is convenient for mass screening in the large population and/or first-tier diagnostic method of the patients in the remote areas. However, this system was still time-consuming and effort-taking, because it required pre-amplification procedure to avoid non-specific amplification and gel-electrophoresis to detect the presence or absence of SMN1 deletion. When the fresh blood samples are used instead of DBS, or when the gel-electrophoresis is replaced by real-time PCR, we may have a simpler and more rapid diagnostic method for SMA.
AIM
To establish a simpler and more rapid diagnostic method of SMN1 deletion using fresh blood DNA.
METHODS
DNA samples extracted from fresh blood and stored at 4 °C for 1 month. The samples were assayed using a real-time mCOP-PCR system without pre-amplification procedures. DNA samples had already been genotyped by PCR-restriction fragment length polymorphism (PCR-RFLP), showing the presence or absence of SMN1 exon 7. The DNA samples were directly subjected to the mCOP-PCR step. The amplification of mCOP-PCR was monitored in a real-time PCR apparatus.
RESULTS
The genotyping results of the real-time mCOP-PCR system using fresh blood DNA were completely matched with those of PCR-RFLP. In this real-time mCOP-PCR system using fresh blood-DNA, it took only four hours from extraction of DNA to detection of the presence or absence of SMN1 deletion, while it took more than 12 hours in PCR-RFLP.
CONCLUSION
Our real-time mCOP-PCR system using fresh blood DNA was rapid and accurate, suggesting it may be useful for the first-tier diagnostic method of SMA.
Keywords: spinal muscular atrophy, SMN1, SMN2, mCOP-PCR, real-time PCR
INTRODUCTION
Spinal Muscular Atrophy (SMA) is a common autosomal recessive neuromuscular disorder with an incidence of 1 in 10,000 newborns [9]. SMA patients show muscle weakness and progressive loss of motor function because of the defects in lower motor neurons. The severity of the disease varies from patient to patient [6], but infantile SMA is a leading genetic cause of infantile death [10].
In 1990, SMA locus was mapped to chromosome 5q11[1, 8]. In 1995, the survival motor neuron (SMN) gene was found in the SMA locus [7]. According to Lefebvre et al., SMN exists in two nearly identical copies, SMN1 (telomeric copy) and SMN2 (centromeric copy) [7]. Although SMN1 is present in all healthy individuals, SMN1 is absent in more than 95% of SMA patients. They are homozygous for SMN1 deletion, and the rest may harbor some deleterious mutations in SMN1 [7].
Contrarily, SMN2 was previously considered to be dispensable because ~5% of normal individuals do not carry the gene [7]. But, now, SMN2 is considered to be an SMA-modifying gene, because SMN2 can also produce a small amount of SMN protein [2, 11], and a high copy number of SMN2 is related to the milder phenotype of SMA [3]. It should also be noted that all SMA patients with a homozygous SMN1 deletion carry at least one copy of the SMN2 gene [12].
For the diagnosis of SMA, SMN1 deletion test should come first. However, high similarity in the nucleotide sequence between SMN1 and SMN2 makes it difficult to detect SMN1 deletion by conventional PCR methods. Therefore, a more advanced technique to separately amplify SMN1 or SMN2 has been requested.
In 2014, we developed a screening system for SMN1 deletion using dried blood spot (DBS) on filter paper. This system was based on a modified competitive oligonucleotide priming-PCR (mCOP-PCR) technique, which separately amplified SMN1 exon 7 and SMN2 exon 7 [5]. But non-specific amplification products of unexpected sizes often appeared in mCOP-PCR, especially when using DBS on filter paper. To overcome this problem, we added a targeted pre-amplification step prior to the mCOP-PCR step [13].
The current form of mCOP-PCR screening system for SMN1 deletion using DBS may be convenient for mass screening in the large population and/or first-tier diagnostic method of the patients in the remote areas. But we thought that the system was still time-consuming and effort-taking, because it required pre-amplification procedure to avoid non-specific amplification and gel-electrophoresis to detect the presence or absence of SMN1 deletion. If the fresh blood samples could be used instead of DBS, and if the gel-electrophoresis could be replaced by real-time PCR, we would have a simpler and more rapid diagnostic method for SMA. In this study, we established a simpler and more rapid diagnostic method for SMN1 deletion using fresh blood DNA.
MATERIAL AND METHODS
Patient and control samples
Twelve DNA samples from 8 controls and 4 SMA patients were assayed in this study. Each DNA sample was extracted from fresh whole blood by a DNA extraction kit, SepaGene (EIDIA, Tokyo, Japan). The samples had already been genotyped by PCR-restriction fragment length polymorphism (PCR-RFLP) [14], showing the presence or absence of SMN1 exon 7. Prior to analysis, informed consent was obtained from study participants. The study was approved by the Ethics Committee of Kobe University Graduate School of Medicine.
Gene-specific amplification of SMN1 and SMN2 exon 7 by mCOP-PCR
SMN1/SMN2 specific amplification was performed by real-time PCR using the LightCycler® 96 system (Roche Applied Science). 50ng of DNA (in 2 μl of TE buffer) was added to PCR mixture (total volume, 30 μl) containing 1× PCR buffer, 2 mM of MgCl2, 0.2 mM of each dNTP, 0.3 μM of each primer, 1.0 U Fast Start Taq DNA polymerase, and 1.5 μl of 20× EvaGreen® Dye (Biotium, Hayward, CA, USA). The sequences of the primers are as follows: R111 (5′-AGA CTA TCA ACT TAA TTT CTG ATC A-3′), SMN1-COP (5′-TGT CTG AAA CC-3′) and SMN2-COP (5′-TTG TCT AAA ACC-3′). The PCR conditions were: (1) initial denaturation at 94°C for 7 min; (2) 40 cycles of denaturation at 94°C for 1 min, annealing at 37°C for 1 min, and extension at 72°C for 1 min; and (3) melting analysis. Fluorescence signals were detected at the end of each extension procedure. Melting curve analysis was performed after PCR amplification, with 10 sec of denaturation at 95°C, 1 min of renaturation at 60°C, and then melting, which consisted of a continuous fluorescence reading from 65°C to 97°C at the rate of five data acquisitions per °C.
RESULTS
We succeeded in gene-specific amplification of SMN1 exon 7 and/or SMN2 exon 7 in real-time PCR using fresh blood DNA, without the targeted pre-amplification step. We analyzed 12 samples that were assigned to each of the three genotype groups. Group 1 contained four samples from four control individuals with SMN1 (+) and SMN2 (+). Group 2 contained four samples from four control individuals with SMN1 (+) and SMN2 (−). Group 3 contained four samples from four patients with SMN1 (−) and SMN2 (−).
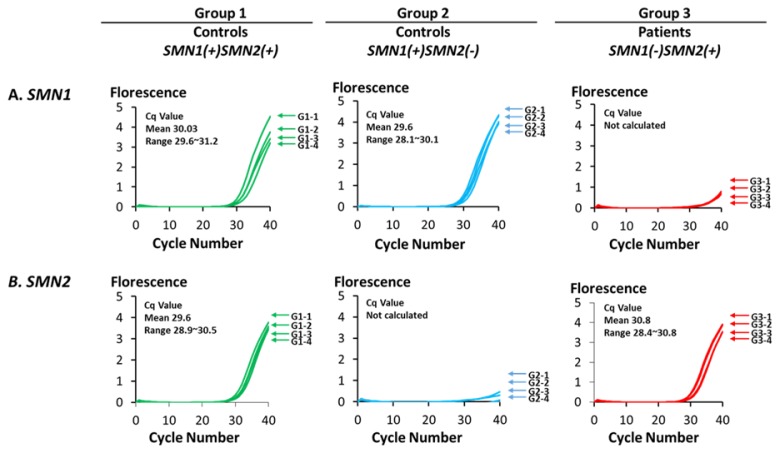
As shown in Figure 1, real-time PCR with SMN1-COP primer efficiently amplified SMN1 exon 7 in the samples from control individuals of Groups 1 and 2, but not in the patients of Group 3 samples. On the other hand, real-time PCR with an SMN2-COP primer efficiently amplified SMN2 exon 7 in the samples from control individuals of Group 1 and the samples from patients of Group 3, but not in the samples from control individuals of Group 2.
Figure 1.
Selective amplification of SMN1 and SMN2 by real-time mCOP-PCR.
Gene-specific amplification of SMN1/SMN2 exon 7 by real-time mCOP-PCR. (A) Amplification curves of SMN1 for the three different groups. The numbers shown next to each curve represents the sample number in each group. The samples with SMN1(+) showed significant amplification, while the samples with SMN1(−) showed no significant amplification. (B) Amplification curves of SMN2 for the three different groups. The numbers shown next to each curve represents the sample number in each group. The samples with SMN2(+) showed significant amplification, while the samples with SMN2(−) showed no significant amplification.
The amplification efficiency of SMN1 or SMN2 was assessed from the quantification cycle (Cq) values. In this study, a Cq value of less than 32 was judged to indicate the presence of SMN1 or SMN2. At this Cq value, positive and negative amplification can be clearly distinguished. The results based on the Cq values were completely matched to the results of PCR-RFLP.
In addition, the total procedures of extraction of DNA to detection of the presence or absence of SMN1 took only 4 hours, while it took more than 12 hours in PCR-RFLP using fresh blood DNA.
DISCUSSION
SMA has been considered an incurable disease. However, in 2016, clinical trial results of intrathecal administration of an antisense-oligo, nusinersen, demonstrated encouraging clinical efficacy of the drug [4]. We are now about to enter an era with the possibility that SMA can be treated and cured. Thus, diagnosis of SMA or detection of SMN1-deletion will become much more important in the near future. By foreseeing the future requirements, we have already engaged in the development of rapid and accurate SMN1-deletion detection system. In this study, we established a real-time mCOP-PCR system for detection of SMN1 deletion using fresh blood DNA.
PCR-RFLP method, which was reported by van der Steege et al., 1995 has been widely used as the first-tier diagnostic method of SMA, but it is a time-consuming and effort-taking method, because it needs enzyme-digestion process and gel-electrophoresis. Compared with the conventional PCR-RFLP method, our new system is much more rapid: the genotyping results can be obtained in a short time.
It is difficult to discuss the superiority between the new real-time mCOP-PCR system in this study and the current form of mCOP-PCR system using DBS as a DNA source and gel-electrophoresis for the detection of amplified products [13]. The new mCOP-PCR system needs an expensive machine for real-time PCR, while the current form of mCOP-PCR system using DBS and gel-electrophoresis does not. However, the new system enables us to obtain the genotyping results in a shorter time. Replacement of DBS by fresh blood DNA can eliminate the pre-amplification step which is essential for preventing non-specific amplification. In addition, real-time PCR does not require gel-electrophoresis.
In conclusion, our real-time mCOP-PCR system using fresh blood DNA was rapid and accurate, suggesting it may be useful for the first-tier diagnostic method of SMA. To prove the practicability of our method, we are now planning to assay more than 100 samples including SMA patients.
ACKNOWLEDGMENTS
This research was supported in part by the Practical Research Project for Rare/Intractable Diseases from the Japan Agency for Medical Research and Development, Grant No. 16ek0109086h0002 (title “Practical study for multicenter cooperative and investigator initiated clinical trial using valproic acid in childhood onset spinal muscular atrophy”).
Footnotes
DECLARATION OF INTEREST
The authors declare that there is no conflict of interests regarding the publication of this paper.
REFERENCES
- 1.Brzustowicz LM, Lehner T, Castilla LH, et al. Genetic mapping of chronic childhood-onset spinal muscular atrophy to chromosome 5q11.2-13.3. Nature. 1990;344:540–541. doi: 10.1038/344540a0. [DOI] [PubMed] [Google Scholar]
- 2.Burghes AH, Beattie CE. Spinal muscular atrophy: why do low levels of survival motor neuron protein make motor neurons sick? Nat Rev Neurosci. 2009;10:597–609. doi: 10.1038/nrn2670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Feldkötter M, Schwarzer V, Wirth R, et al. Quantitative analyses of SMN1 and SMN2 based on real-time lightCycler PCR; fast and highly reliable carrietest and prediction of spinal muscular atrophy. Am J Hum Genet. 2002;70:358–368. doi: 10.1086/338627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Finkel RS, Chiribaga CA, Vajsar J, Day JW, Montes J, De Vivo DC, et al. Treatment of infantile-onset spinal muscular atrophy with nusinersen: a phase 2, open-label, dose-escalation study. Lancet. 2016;388:3017–26. doi: 10.1016/S0140-6736(16)31408-8. [DOI] [PubMed] [Google Scholar]
- 5.Kato N, Sa’Adah N, Ar Rochmah M, et al. SMA screening system using dried blood spots on filter paper: application of COP-PCR to the SMN1 deletion test. Kobe J Med Sci. 2014;60:E78–85. [PubMed] [Google Scholar]
- 6.Kolb SJ, Kissel JT. Spinal muscular atrophy: a timely review. Arch Neurol. 2011;68:979–84. doi: 10.1001/archneurol.2011.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lefebvre S, Bürglen L, Reboullet S, et al. Identification and characterization of a Spinal Muscular Atrophy-determining gene. Cell. 1995;80:155–165. doi: 10.1016/0092-8674(95)90460-3. [DOI] [PubMed] [Google Scholar]
- 8.Melki J, Abdelhak S, Sheth P, et al. Gene for chronic proximal spinal muscular atrophies maps to chromosome 5q. Nature. 1990;344:767–768. doi: 10.1038/344767a0. [DOI] [PubMed] [Google Scholar]
- 9.Nurputra DK, Lai PS, Harahap NI, et al. Spinal Muscular Atrophy: From Gene Discovery to Clinical Trials. Ann Hum Genet. 2013;77:435–463. doi: 10.1111/ahg.12031. [DOI] [PubMed] [Google Scholar]
- 10.Oskoui M, Levy G, Garland CJ, et al. The changing natural history of spinal muscular atrophy type 1. Neurology. 2007;69:1931–6. doi: 10.1212/01.wnl.0000290830.40544.b9. [DOI] [PubMed] [Google Scholar]
- 11.Prior TW. Perspectives and diagnostic considerations in spinal muscular atrophy. Genet Med. 2011;12:145–152. doi: 10.1097/GIM.0b013e3181c5e713. [DOI] [PubMed] [Google Scholar]
- 12.Schrank B, Götz R, Gunnersen JM, et al. Inactivation of the survival motor neuron gene, a candidate gene for human spinal muscular atrophy, leads to massive cell death in early mouse embryos. Proc Natl Acad Sci. 1997;94:9920–9925. doi: 10.1073/pnas.94.18.9920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shinohara M, Rochmah AM, Nakanishi K, Harahap NIF, Niba ETE, et al. New, Improved Version of the mCOP-PCR Screening System for Detection of Spinal Muscular Atrophy Gene (SMN1) Deletion. Kobe J Med Sci. 2017;63:E37–40. [PMC free article] [PubMed] [Google Scholar]
- 14.Van der Steege G, Grootscholten PM, Van der Vlies P, et al. PCR-based DNA test to confirm clinical diagnosis of autosomal recessive spinal muscular atrophy. The Lancet. 1995;345:985–986. [PubMed] [Google Scholar]