Abstract
Iodinated contrast media (iodinated CM) have increased ability to absorb x-rays and to visualize structures that normally are impossible to observe in a radiological examination. The use of iodinated CM may destory renal function, commonly known as contrast-induced nephropathy (CIN), which can result in acute renal failure (ARF). This review article mainly focuses on the following areas: (1) classifications of iodinated CM: ionic or non-ionic, high-osmolarity contrast media (HOCM), low-osmolarity contrast media (LOCM) and iso-osmolarity contrast media (IOCM); (2) an introduction to the physical and chemical properties of the non-ionic iodinated CM; (3) the management of anaphylactic reaction by iodinated CM; (4) a suggested single injection of adult doses and maximum dose for non-ionic iodinated CM; (5) the molecular mechanism of contrast-induced nephropathy (CIN); (6) In vitro studies on iodinated CM. Based on above information, this review article provide an insight for understanding the drug safety of iodinated CM.
Keywords: Iodinated contrast media, The management of anaphylactic reaction by iodinated contrast media, Dose for non-ionic contrast media, Contrast-induced nephropathy (CIN), In vitro studies on iodinated contrast media
1. Introduction
Iodinated contrast media (iodinated CM) absorb x-rays and visualize structures that are normally hard to observe in a radiological examination [1-4]. It has been used widely for many years. Contrast media provide an ability to enhance normal structures or pathological lesions, which makes these places look different from surrounding. The mechanism of iodinated contrast media is based on shielding effect: high energy x-ray penetrates substances and yields a dark place in a plane image. Iodine, the content of iodinated contrast media, absorbs the energy of x-ray; that is to say, iodinated CM “shield” x-ray from detector and lead to a high density, white “shadow” appearing. Iodinated CM elevate the sensitivity and diagnostic accuracy in radiological examination [1, 5-7].
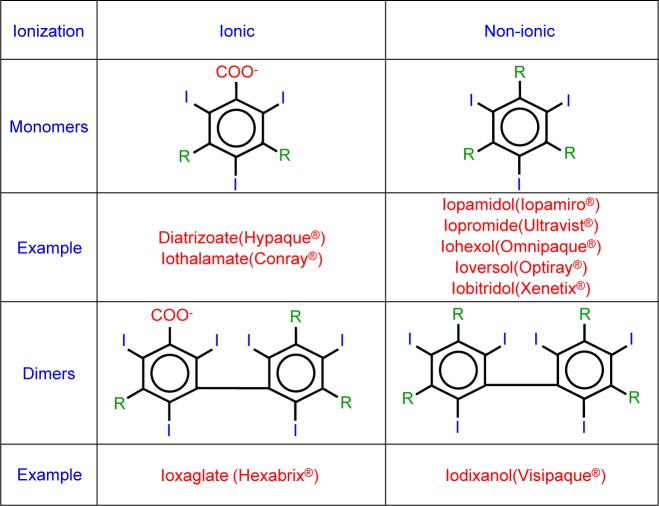
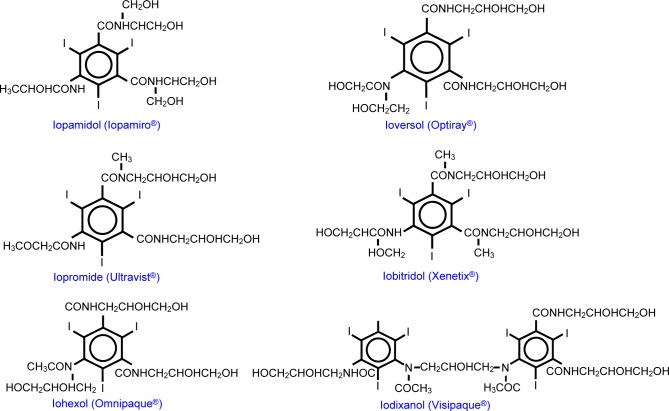
Based on the solubility, iodinated CM are divided into three groups: oily iodinated CM, water-soluble iodinated CM and water-insoluble iodinated CM [8-10]. Iodinated CM are usually classified into ionic iodinated CM and non-ionic iodinated CM [10, 11]. Generally, ionic contrast media have higher osmolality, higher toxicity and higher anaphylactic reaction. Non-ionic contrast media possess lower osmolality, lower toxicity and lower anaphylactic reaction [12, 13]. Based on the structure, iodinated CM are divided into four groups: ionic monomer, ionic dimer, nonionic monomer and nonionic dimer (Fig. 1). Based on the osmolality, iodinated CM are classified into high-osmolar contrast media (HOCM), low-osmolar contrast media (LOCM) and isoosmolar contrast media (IOCM). High-osmolar contrast media (HOCM) is characterized by osmolarity of above 1500 mOsm/kg H2O. Low osmolar contrast media (LOCM) is characterized by osmolarities within a relatively wide range of 300-900 mOsm/kg H2O. The iso-osmolar contrast media (IOCM) is characterized by osmolarity level similar to that of blood (290 mOsm/kg H2O) [14, 15]. The osmolarity of high-osmolar contrast media (HOCM) is up to 7 or 8 fold greater than blood and has been associated with high risk of adverse drug reactions (ADR) and renal toxicity. Since the late 1960s, the nonionic low-osmolar contrast media (LOCM) have been developed to better safety and replace ionic iodinated CM for clinical uses. In 1996, the US Food and Drug Administration (FDA) approved the iso-osmolar contrast media (IOCM), iodixanol (Visipaque®), to have a better safety profile [14]. Furthermore, discomfort such as pain and heat associated with the injection site was found to be lower when using iso-osmolarity contrast media (IOCM) than low osmolar contrast media (LOCM) [14]. It is low neuro-toxicity and low osmolality that are important to intrathecal route injected contrast media, such as Iopamidol (Iopamiro®) 300 and Iohexol (Omnipaque®) 300 [16, 17]. Table 1 shows the biologic adverse drug reaction (ADR) difference between ionic iodinated CM and non-ionic iodinated CM. Currently used non-ionic iodinated CM in Taiwan and their chemical properties are summarized in Table 2. The chemical structures of non-ionic iodinated CM are shown in Fig. 2 [3, 11, 18-31]. In Table 3, we summarized the suggested single injection of adult doses and maximum dose for non-ionic iodinated CM by intra-arterial route. In Table 4, we summarized the suggested single injection of adult doses and maximum dose for non-ionic iodinated CM by intravenous route. In Table 5, we summarized the suggested single injection of adult doses and maximum dose for non-ionic iodinated CM by intrathecal route.
Fig. 1.
Water-soluble iodinated CM are divided into four groups based on the structure. They are ionic monomer, ionic dimer, nonionic monomer and nonionic dimer.
Table 1.
The biologic adverse reaction between ionic and non-ionic contrast media.
| Biologic adverse reaction | Ionic contrast media | Non-ionic contrast media |
|---|---|---|
| Thermal effect | Moderate | Mild to less |
| Pain during injection | Moderate | Mild to less |
| Nausea and vomiting | Moderate | Mild to less |
| Toxicity to kidney | Higher | Lower |
| Tissue necrosis when extravasation occurs | More severe | Less severe |
| Other allergic effects | Often (around 10%) | Seldom (lower than 5%) |
Table 2.
The chemistry and physical properties of non-ionic contrast media in Taiwan [31].
| Brand name | Iopamiro | Ultravist | Omnipaque | Optiray | Xenetix | Visipaque |
| Generic name | Iopamidol | Iopromide | Iohexol | Ioversol | Iobitridol | Iodixanol |
| Iodine concentration (mgI/ml) |
200 250 300 (Taiwan) 370 (Taiwan) |
150 240 300 (Taiwan) 370 (Taiwan) |
140 180 210 240 300 (Taiwan) 350 (Taiwan) |
240 300 320 (Taiwan) 350 (Taiwan) |
250 300 (Taiwan) 350 (Taiwan) |
270 320 (Taiwan) |
| Osmolality (mOsmo/kg H2O, 37°C) |
413 524 616 (Taiwan) 796 (Taiwan) |
328 483 607 (Taiwan) 774 (Taiwan) |
322 408 460 520 672 (Taiwan) 844 (Taiwan) |
502 651 702 (Taiwan) 792 (Taiwan) |
585 695 (Taiwan) 915 (Taiwan) |
290 (Taiwan) |
| Low osmolality | Low osmolality | Low osmolality | Low osmolality | Low osmolality | Iso-osmolality | |
| Viscosity (mPa-s, 37°C) | 2.0 3.0 4.7 (Taiwan) 9.4 (Taiwan) |
1.5 2.8 4.9 (Taiwan) 10.0 (Taiwan) |
1.5 2.0 2.5 3.4 6.3 (Taiwan) 10.4 (Taiwan) |
3.0 5.5 5.8 (Taiwan) 9.0 (Taiwan) |
4.0 6.0 (Taiwan) 10.0 (Taiwan) |
11.8 (Taiwan) |
| Median lethal dose (LD50) | 21.8 g I/Kg | 18.5 g I/Kg | 18.5 g I/Kg | 17.0 g I/Kg | 15.9 g I/Kg | 17.9 g I/Kg |
| Expiration duration | 5 years | 3 years | 3 years | 3 years | 3 years | 3 years |
| National Health Insurance in Taiwan (NHI), 2017 |
Cover | Cover | Cover | Cover | Cover | Self-paid |
| Administration | Intravenous injection; intra-arterial injection; Intrathecal injection (Iopamiro 300, Omnipaque 300); Oral | Intravenous injection |
||||
| Uses | Computed tomography (CT); Angiocardiography; Arteriography of cerebral arteries; Pyelography; Peripheral angiography | Angiocardiography Computed tomography (CT) | ||||
Fig. 2.
The chemical structures of currently used non-ionic iodinated CM.
Table 3.
Suggested single injection of adult doses and maximum total dose for non-ionic contrast media by intra-arterial injection [31].
| Non-ionic contrast media | Angiography of arteries of extremity | Femoral arteriography | Aortography | Arteriography | Arteriography of cerebral arteries | Cardiac ventriculography, Left (FDA Dosage) | Cardiac ventriculography, Left (Off label Dosage) | Coronary angiography (FDA Dosage) | Coronary angiography (Off label Dosage) | Inferior vena cavogram | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Iopromide (Ultravist) (300 mgI/ml) | Adult doses suggestion | 5-40 ml for subclavian or femoral artery | 65 ml | 3-12 ml for carotid arteries | |||||||
| 25-50 ml for aortic bifurcation | 4-12 ml for vertebral arteries | ||||||||||
| 20 to 50 ml for aortic arch injection | |||||||||||
| Maximum dose | 250 ml | 150 ml | |||||||||
| Iopromide (Ultravist) (370 mgI/ml) | Adult doses suggestion | Blood flow and vascular and pathological nature of the vessels of interest | 30-60 ml | 44-60 ml | 3-14 ml for right or left coronary artery | 7 to 10 ml (4-5 injections) -left coronary artery | Blood flow and vascular and pathological nature of the vessels of interest | ||||
| 7-10 ml (2 to 3 injections)- right coronary artery | |||||||||||
| Maximum dose | 225 ml | 225 ml | 225 ml | 225 ml | |||||||
| Ioversol (Optiray) (320 mgI/ml) | Adult doses suggestion | 2-12 ml | 40 ml (30-50 ml) | 45 ml (10-80 ml) | |||||||
| Maximum dose | 200 ml | ||||||||||
| Iobitridol (Xenetix) (350 mgI/ml) | Adult doses suggestion | 10-80 ml | 30-60 ml | ||||||||
| Maximum dose | 250 ml | ||||||||||
| Carotid arteries: 10-14 ml | 10-14 ml | ||||||||||
| Verterbral arteries: 10-12 ml | |||||||||||
| Right coronary artery: 3-8 ml | |||||||||||
| Iodixanol (Visipaque) (320 mgI/ml) | Adult doses suggestion | Left coronary artery: 3-10 ml | |||||||||
| Left ventricle: 20-45 ml | |||||||||||
| Renal arteries: 8-18 ml | |||||||||||
| Aortography: 30-70 ml | |||||||||||
| Major aorta branch: 10-70 ml | |||||||||||
| Peripheral arteries: 15-30 ml | |||||||||||
| Aortofermoral runoffs: 20-90 ml | |||||||||||
| Maximum dose | 250 ml (80 gI) | 175 ml (80 gI) | |||||||||
Table 4.
Suggested single injection of adult doses and maximum total dose for non-ionic contrast media by Intravenous injection [31].
| Non-ionic contrast media | Computerized axial tomography, Body | Computerized axial tomography of head (brain) | Computerized axial tomography of abdomen | Intravenous pyelogram (urography) | Angiocardiography-Coronary Arteriography/Ventriculography | Angiocardiography-ventriculography or nonselective opacification of multiple coronary arteries | Aortography | Arteriography, peripheral | Arteriography, selective visceral | Arteriography of cerebral arteries | Renal arteriography | Venography | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Iopromide (Ultravist) (300 mgI/ml) | Adult doses suggestion | 50-200 ml for bolus IV injection | 50-200 ml | 300 mgI/kg | |||||||||
| 100-200 ml for rapid IV infusion | |||||||||||||
| Maximum dose | 200 ml (60 gI) | 200 ml (60 gI) | 100 ml (30 gI) | ||||||||||
| Iopromide (Ultravist) (370 mgI/ml) | Adult doses suggestion | 41-162 ml for bolus IV injection | 41-162 ml | ||||||||||
| 81-162 ml for rapid IV infusion | |||||||||||||
| Maximum dose | 162 ml (60 gI) | 162 ml (60 gI) | |||||||||||
| Iopamiro (Iopamidol) (300 mgI/ml) | Adult doses suggestion | 100-200 ml | 100-200 ml | 2.0-2.5 ml/Kg | 50 ml | 5-40 ml for femoral or subclavian | 8-12 ml | ||||||
| 25-50 ml for aorta for a distal runoff | |||||||||||||
| Maximum dose | 200 ml (60 gI) | 200 ml (60 gI) | 250 ml | 90 ml | |||||||||
| Iopamiro (Iopamidol) (370 mgI/ml) | Adult doses suggestion | 81-162 ml | 40 ml | 2-10 ml | 25-50 ml | 50 ml | 50 ml-larger vessels | ||||||
| 81-162 ml | 10 ml-renal arteries | ||||||||||||
| Maximum dose | 200 ml (60 gI) | 200 ml (60 gI) | 200 ml | 225 ml | 225 ml | ||||||||
| Omnipaque (Iohexol) (300 mgI/ml) | Adult doses suggestion | 50-200 ml | 75-150 ml | 200-350 mgI/Kg | 30-90 ml | 50-80 ml-aorta, 30- 60 mlbranches, 5 -15 ml- renal arteries. | 6-12 ml-Common carotid artery; 8-10 ml-Internal carotid artery; 6-9 ml-External carotid artery; 6-10 ml Vertebral artery. | ||||||
| Maximum dose | 291 ml | ||||||||||||
| Omnipaque (Iohexol) (350 mgI/ml) | 60-100 ml | 350 ml | 200-350 mgI/Kg | 5 ml (3-14 ml) | 40 ml (30-60 ml) | 20-70 ml | 50-80 ml-aorta, 30- 60 mlbranches,5 -15 ml- renal arteries. | ||||||
| Maximum dose | Total combined-250 ml | 250 ml | |||||||||||
| Ioversol (Optiray) (320 mgI/ml) | 25-75 ml (bolus injection) | 50-150 ml | 50-75 ml | 8 ml (2-10 ml) for the left coronary; 6 ml (1-10 ml) for for the right coronary artery. | 9 ml (6-15 ml) | ||||||||
| Maximum dose | 150 ml | 250 ml | 250 ml | ||||||||||
| Ioversol (Optiray) (350 mgI/ml) | 25-75 ml (bolus injection) | 50-75 ml | 50-100 ml | ||||||||||
| Maximum dose | 150 ml | 250 ml | |||||||||||
| Iobitridol (Xenetix) (300 mgI/ml) |
50-100 ml | 30-60 ml (3-5 ml/Kg) | |||||||||||
| Maximum dose | |||||||||||||
| Iobitridol (Xenetix) (350 mgI/ml) | Depend on the organs under investigation, the diagnostic problem and, in particular, the different scan and image-reconstruction times of the scanners in use | 1-1.5 ml/Kg | 155-330 ml | 30-60 ml (3-5 ml/Kg) | 10-80 ml | 105-205 ml | |||||||
| Maximum dose | 1-1.5 ml/Kg | 250 ml | |||||||||||
| Iodixanol (Visipaque) (320 mgI/ml) | 75-150 ml | 75-150 ml | 1 ml/Kg | 20 ml | |||||||||
| Maximum dose | 150 ml (80 gI) | 150 ml (80 gI) | 100 ml (80 gI) | ||||||||||
Table 5.
Suggested single injection of adult doses and maximum total dose for non-ionic contrast media by Intrathecal route injection [31].
| Non-ionic contrast media | Myelogram - cervical myelogram (via lumbar injection) | Myelogram - total columnar myelography | Myelogram -thoracic | Myelogram -spinal cord | |
|---|---|---|---|---|---|
| Iopamiro (Iopamidol) (300 mgI/ml) | Adult doses suggestion | 10 ml | 10 ml | ||
| Maximum total dose | |||||
| Iohexol (Omnipaque) (300 mgI/ml) | Adult doses suggestion | 4-10 ml | 6-10 ml | 6-10 ml | |
| Maximum total dose | 3060 mgI | 3060 mgI | 3060 mgI | ||
2. The adverse drug reaction (ADR) of iodinated contrast media and management
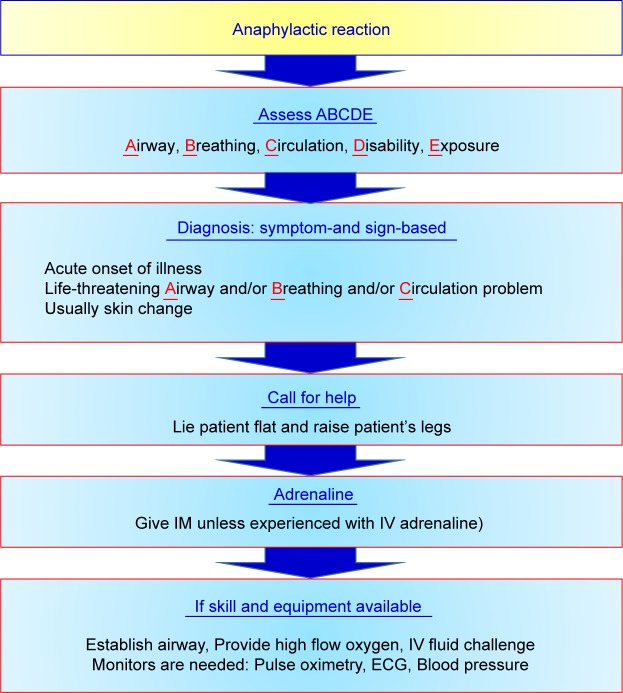
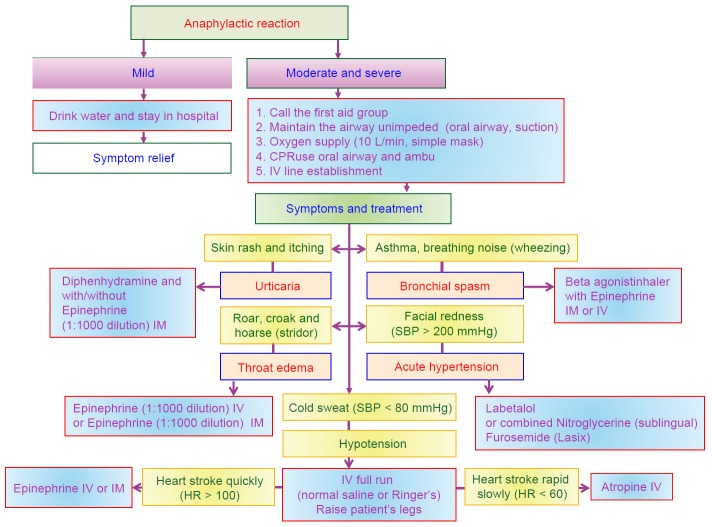
ADR caused by iodinated CM includes chemical and constitutional effects. Chemical effects are mainly referred as contrast-induced nephropathy (CIN) and will be discussed later. Anaphylactic reaction is the most common situation in constitutional effect and may cause mild symptom such as nausea and vomiting, dizziness, rash and itch, or chest discomfort, shock in more severe situation, or even death [21, 23, 28, 29, 32]. Iodinated contrast media cause little allergic reactions, especially for low-osmolar contrast media (LOCM). The incidence of adverse effect to LOCM is 2 to 7/1000, that of severe allergic reaction to LOCM is lower 1 to 4/100,000, and that of lethal rate to LOCM is around 2-9/1000,000 [33, 34]. We should recognize adverse effects and receive early intervene to reverse bad situation. The management and treatment of adverse effects on anaphylactic reaction by Advanced Cardiovascular Life Support (ACLS) guideline is shown in Fig. 3. The Fig. 4 shows that management and treatment of anaphylactic reaction by iodinated CM is proposed in 2017 RSROC Contrast Media Manual [33]. There are several affecting factors for anaphylactic reaction by iodinated CM such as particularly allergy (arising from consuming sea foods or drugs), previous adverse reactions, history of asthma or bronchospasm, history of allergy, cardiac disease, dehydration, haematological and metabolic conditions (sickle cell anaemia, patients with thrombotic tendency), renal disease, neonates, old patients, anxiety and apprehension medications (β-blockers, interleukin-2 (IL-2), aspirin, NSAIDs) [33]. In addition, IOCM (ie, iodixanol (Visipaque®)) are associated with the highest risk of causing a delayed hypersensitivity reactions. The incidence of delayed hypersensitivity reactions to IOCM is 10.9% and 5%-6% for LOCM [33, 35, 36]. Lasser et al. suggested that two doses of corticosteroid prophylaxis (32 mg of methyl prednisolone, orally 12 and 24 h before iodinated CM injection signification reduced the iodinated CM-induce anaphylactic reaction [13, 34].
Fig. 3.
Advanced Cardiovascular Life Support (ACLS) guideline for the management and treatment of adverse effects on anaphylactic reaction.
Fig. 4.
Management and treatment of anaphylactic reaction by iodinated CM is proposed in 2017 RSROC Contrast Media Manual.
3. Molecular mechanism of contrast-induced nephropathy (CIN)
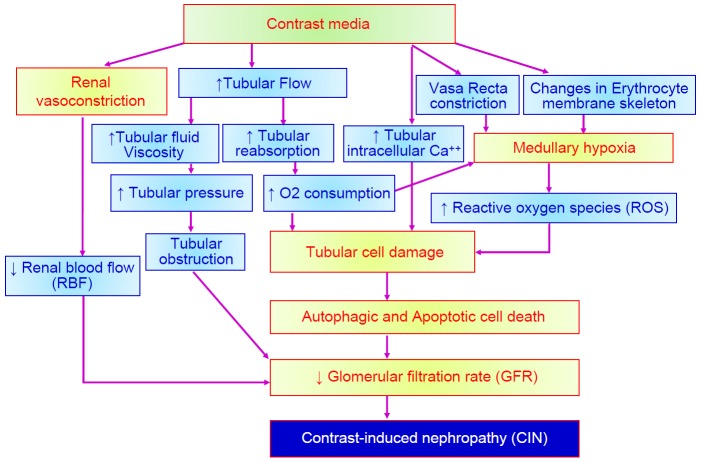
CIN is one of chemical adverse effects of iodinated CM. The pathophysiology of CIN is related to hemodynamic changes caused by vasoconstriction which makes a decrease of glomerular filtration rate (GFR) and a renal ischemia. Direct cytotoxicity to renal tubular cell is another pathway leading to kidney damage [37-49]. Norbert H. et al. medullary ischemia and direct cytotoxicity to renal tubular cell are two main mechanism to result in CIN. Medullary ischemia is a complex result of vasoconstriction, lower oxygen delivery and higher oxygen demand. In Fig. 5, there are three factors such as increasing oxidative stress, enhancing renal vasoconstriction and inducing tubular cell damage responsible for CIN [50, 51]. Several factors including renal ischemia, particularly in the medulla, reactive oxygen species (ROS) formation, reduction of nitric oxide production, tubular epithelial and vascular endothelial injury may be implicated in CIN. Many studies demonstrated that iodinated CM exert cytotoxic effects and renal tubular epithelial cells present severe cell death by autophagy and/or apoptosis [6]. Iodinated CM induces renal vasoconstriction by increase of adenosine and endothelin, and changes the blood flow from the medulla to the cortex and GFR are reduced. Reduction in renal blood flow can increase ROS release by oxidative stress. In tubular cells, iodinated CM directly caused osmotic necrosis or vacuolization leading to acute tubular cell death [15, 37-39]. Several antioxidant compounds have been demonstrated prevention effects by CIN, including sodium bicarbonate, N-acetylcysteine (NAC), ascorbic acid, statins, and recently, phosphodiesterase type 5 inhibitors [4-7]. The detailed molecular mechanisms of CIN are described in Fig. 6.
Fig. 5.
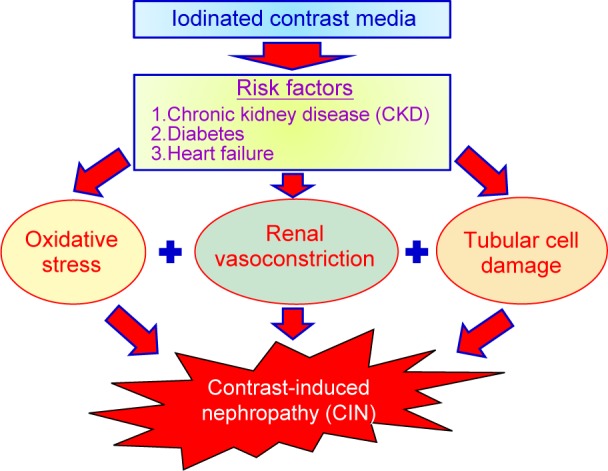
Three factors are responsible for contrast-induced nephropathy.
Fig. 6.
The detailed molecular mechanisms of contrast-induced nephropathy.
4. In-vitro studies on contrast-induced nephropathy (CIN) by iodinated CM.
In 2017 year, Charalampos Mamoulakis et al. summarize recent in vivo studies on oxidative stress related to CIN in animal models and humans [6]. Hereby, we summarize recent in vitro studies on the mechanisms in contrast-induced nephropathy (CIN). Direct damage, a risk factor of CIN, induces cell death to renal tubular cells caused by iodinated CM. Table 6 is a summary of the manifestation of CIN which is collected from in vitro studies. Inhibiting cell proliferation and inducing cell death are found in renal cell lines including KRK52-E, LLC-PK1, HKCS, HK-2 at the concentration higher than 75 mgI/ml. Importantly, iodinated CM induced cell death no matter whether in LOCM or IOCM. Apoptosis and/or autophagy are two cell types in cell death [52- 58]. Readers refer to our previous article for detailed molecular mechanisms of apoptosis and autophagy [59].
Table 6.
In vitro studies of mechanisms on contrast-induced nephropathy (CIN) in iodinated contrast media.
| In-vitro cell lines | Iodinated contrast media | Dose | Time of treatment | Results | References |
|---|---|---|---|---|---|
| KRK52-E (Rat kidney epithelial cell) | Iodixanol (Visipaque) Ioversol (Optiray) Iohexol (Omnipaque) Iopromide (Ultravist) |
150 mgI/ml | 0.5 h, 1h , 3 h , 6 h , 12 h , 24 h. | 1. Decreasing cell proliferation by MTT assay. 2. Induce cells death by Trypan blue assay. 3. Increasing apoptosis by hematoxylin-stained. |
[60] |
| NRK52-E (Rat tubular cells) | Iohexol (Omnipaque) | 100 mgI/ml | 24 h | 1. Decreasing cell proliferation by MTT assay. 2. Increasing apoptotic cells by TUNEL assay. 3. Increasing caspase-3, caspase-9 and cytochrome c protein levels by western. 4. Decreasing cell viability by iohexol was aggravated with 3-MA pretreatment. |
[61] |
| LLC-PK1 (Pig renal tubular epithelial cells) | Iohexol (Omnipaque) Iodixanol (Visipaque) |
100 mgI/ml | 24 h | 1. Decreasing cell proliferation by MTT assay. 2. Increasing apoptotic cells by TUNEL assay. 3. Increasing caspase-8, caspase-9 and caspase-3 protein levels by western. |
[62] |
| HK-2 (human embryonic proximal tubule) | Iopamiro (Iopamidol) | 200 mgI/ml | 0 h 12 h 24 h |
1. Decreasing cell proliferation by MTT assay. 2. Increasing apoptotic cells by TUNEL assay. 3. The mRNA level of Bax was increased and Bcl-2 was decreased by qPCR. 4. Increasing Bax, caspase-3 protein levels and decreasing Bcl-2, HSP70 protein levels by western. |
[63] |
| LLC-PK1 (Pig renal tubular epithelial cells) | Iodixanol (Visipaque) | 4.7-75 mgI/ml | 2h , 24h | 1. Decreasing cell proliferation by MTT assay. | [58] |
| HK-2 (human embryonic proximal tubule) | Iopromide (Ultravist) | 40 mgI/ml 20 mgI/ml 10 mgI/ml |
24-72 h | 1. Caused the breaking of intercellular connections and cell migration by scratch assay. 2. Increasing SGK, SNAIL1, CTGE, COL1A1 mRNA levels by qPCR |
[64] |
| LLC-PK1 (Pig renal tubular epithelial cells) | Ioversol (Optiray) | 100 mgI/ml | 24 h | 1. Increasing caspase-3 protein activity by caspase-3 activity assay | [56] |
| HK-2 (human embryonic proximal tubule) | Ioversol (Optiray) | 100 μL/ml 200 μL/ml |
24 h | 1. Decreasing cell proliferation by MTT and LDH assay. | [55] |
| HK-2 (human embryonic proximal tubule) | Iodixanol (Visipaque) | 25 mgI/ml 50 mgI/ml 100 mgI/ml 200 mgI/ml |
2 h, 4 h, 8 h, 24h | 1. Decreasing cell proliferation by CellTiter 96 assay. | [53] |
| LLC-PK1 (Pig renal tubular epithelial cells) | Iodixanol (Visipaque) | 18.75-75 mgI/ ml | 24 h | 1. Decreasing cell proliferation by BrdU assay 2. Increasing apoptotic cells by cytoplasmic oligonucleosomes ELISA assay. |
[52] |
5. Conclusion
Autophagy and apoptosis were associated with the pathophysiology of CIN in in vitro reports. In conclusion, in vitro studies showed that increased cell death by apoptosis and/or autophagy was demonstrated in the kidney cell lines after the administration of iodinated CM. Inhibition of autophagy induced cell apoptosis suggested the protective role of autophagy in CIN. In the future, studies about how to reduce cellular stress and cell death by new methods or new compounds and understanding the details molecular mechanisms may be helpful for the development of new therapeutic strategies for the treatment of CIN.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Acknowledgments
This work was supported by the grant from China Medical University Hospital, Taichung, Taiwan (DMR-107-123). The authors also would like to express our gratitude to Miss Huei-Min Chen for drug information supports.
References
- 1.La Grutta L, Toia P, Maffei E, Cademartiri F, Lagalla R, Midiri M. Infarct characterization using CT. Cardiovasc Diagn Ther. 2017; 7: 171-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rybicki FJ, Piazzo K, Prior R, Wake N, Dill KE. Iodinated contrast injection data from a new technology. Radiol Technol. 2012; 84: 120-5. [PubMed] [Google Scholar]
- 3.Meunier B, Joskin J, Damas F, Meunier P. Iodinated contrast media and iodine allergy: myth or reality? Rev Med Liege. 2013; 68: 465-9. [PubMed] [Google Scholar]
- 4.Goodwill PW, Saritas EU, Croft LR, Kim TN, Krishnan KM, Schaffer DV, et al. X-space MPI: magnetic nanoparticles for safe medical imaging. Adv Mater. 2012; 24: 3870-7. [DOI] [PubMed] [Google Scholar]
- 5.Harbron RW, Ainsbury EA, Bouffler SD, Tanner RJ, Eakins JS, Pearce MS. Enhanced radiation dose and DNA damage associated with iodinated contrast media in diagnostic x-ray imaging. Br J Radiol. 201720170028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mamoulakis C, Tsarouhas K, Fragkiadoulaki I, Heretis I, Wilks MF, Spandidos DA, et al. Contrast-induced nephropathy: Basic concepts, pathophysiological implications and prevention strategies. Pharmacol Ther. 2017. [DOI] [PubMed] [Google Scholar]
- 7.Prezzi D, Khan A, Goh V. Perfusion CT imaging of treatment response in oncology. Eur J Radiol. 2015; 84: 2380-5. [DOI] [PubMed] [Google Scholar]
- 8.Price DB, Ortiz AO. Myelography: From Lipid-Based to Gadolinium-Based Contrast Agents. Magn Reson Imaging Clin N Am. 2017; 25: 713-24. [DOI] [PubMed] [Google Scholar]
- 9.Cheng KT. Lipiodol-loaded poly(oxyethylene)-block-poly(oxypropylene)-block-poly(oxyethylene) triblock copolymers/ polyethylene glycol-nanoparticles. In. Molecular Imaging and Contrast Agent Database (MICAD). ed. Bethesda (MD): 2004. [PubMed] [Google Scholar]
- 10.Cheng KT. Ioxilan carbonate particles. In. Molecular Imaging and Contrast Agent Database (MICAD). ed. Bethesda (MD): 2004. [Google Scholar]
- 11.Lee SY, Rhee CM, Leung AM, Braverman LE, Brent GA, Pearce EN. A review: Radiographic iodinated contrast media-induced thy-roid dysfunction. J Clin Endocrinol Metab. 2015; 100: 376-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Costa N. Understanding contrast media. J Infus Nurs. 2004; 27: 302-12. [DOI] [PubMed] [Google Scholar]
- 13.Bettmann MA, Morris TW. Recent advances in contrast agents. Radiol Clin North Am. 1986; 24: 347-57. [PubMed] [Google Scholar]
- 14.Spampinato MV, Abid A, Matheus MG. Current Radiographic Iodinated Contrast Agents. Magn Reson Imaging Clin N Am. 2017; 25: 697-704. [DOI] [PubMed] [Google Scholar]
- 15.Schrader R. Contrast material-induced renal failure: an overview. J Interv Cardiol. 2005; 18: 417-23. [DOI] [PubMed] [Google Scholar]
- 16.Pugh ND, Griffith TM, Karlsson JO. Effects of iodinated contrast media on peripheral blood flow. Acta Radiol Suppl. 1995; 399: 155-63. [DOI] [PubMed] [Google Scholar]
- 17.Stolberg HO, McClennan BL. Ionic versus nonionic contrast use. Curr Probl Diagn Radiol. 1991; 20: 47-88. [DOI] [PubMed] [Google Scholar]
- 18.ten Dam MA, Wetzels JF. Toxicity of contrast media: an update. Neth J Med. 2008; 66: 416-22. [PubMed] [Google Scholar]
- 19.Campbell KL, Hud LM, Adams S, Andrel J, Ballas SK, Feldman AM, et al. Safety of iodinated intravenous contrast medium administration in sickle cell disease. Am J Med. 2012; 125: 100 e11-6. [DOI] [PubMed] [Google Scholar]
- 20.Katzberg RW, Barrett BJ. Risk of iodinated contrast material-induced nephropathy with intravenous administration. Radiology. 2007; 243: 622-8. [DOI] [PubMed] [Google Scholar]
- 21.Runge VM. A review of contrast media research in 1999-2000. Invest Radiol. 2001; 36: 123-30. [DOI] [PubMed] [Google Scholar]
- 22.McCullough PA. Renal safety of iodixanol. Expert Rev Cardiovasc Ther. 2006; 4: 655-61. [DOI] [PubMed] [Google Scholar]
- 23.Mruk B. Renal Safety of Iodinated Contrast Media Depending on Their Osmolarity - Current Outlooks. Pol J Radiol. 2016; 81: 157-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dawson P. The non-ionic isotonic contrast agents. Perspectives and controversies. Eur Radiol. 1996; 6 Suppl 2: S20-4. [DOI] [PubMed] [Google Scholar]
- 25.Konen E, Apter S, Morag B, Itzchak Y. Iodinated contrast media: adverse reactions, prevention and treatment. Harefuah. 1995; 128: 719-23. [PubMed] [Google Scholar]
- 26.Singh J, Daftary A. Iodinated contrast media and their adverse reactions. J Nucl Med Technol. 2008; 36: 69-74; quiz 76-7. [DOI] [PubMed] [Google Scholar]
- 27.Weisbord SD, Palevsky PM. Iodinated contrast media and the role of renal replacement therapy. Adv Chronic Kidney Dis. 2011; 18: 199-206. [DOI] [PubMed] [Google Scholar]
- 28.Weisbord SD. Iodinated contrast media and the kidney. Rev Cardiovasc Med. 2008; 9 Suppl 1: S14-23. [PubMed] [Google Scholar]
- 29.Pintassilgo Santos A, Mascarenhas Gaivao A, Tavares A, Ferreira S. Iodinated contrast agents. Acta Med Port. 2009; 22: 261-74. [PubMed] [Google Scholar]
- 30.Erley C. Iodinated contrast agent-induced nephropathy. Radiologe. 2007; 47: 761-7. [DOI] [PubMed] [Google Scholar]
- 31.https://www.micromedexsolutions.com/home/dispatch/ssl/true. Published 2017. Updated Accessed.
- 32.Hu XH, Gong XY, Hu P. Transient small bowel angioedema due to intravenous iodinated contrast media. World J Gastroenterol. 2012; 18: 999-1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.2017 RSROC Contrast Media Manual (https://www.rsroc.org.tw/DB/Info/file/123-1.pdf.) https://www.rsroc.org.tw/DB/Info/file/123-1.pdf. Published 2017. Updated Accessed.
- 34.Thomsen HS, Morcos SK. Radiographic contrast media. BJU Int 2000; 86 Suppl 1:1-10. [DOI] [PubMed] [Google Scholar]
- 35.Almen T. The etiology of contrast medium reactions. Invest Radiol. 1994; 29 Suppl 1: S37-45. [PubMed] [Google Scholar]
- 36.Beckett K.R, MAK, Langer J.M. Safe Use of Contrast Media: What the Radiologist Needs to Know. Radiographics. 2015; 35: 1738-50. [DOI] [PubMed] [Google Scholar]
- 37.Modi K, Dulebohn SC. Contrast Induced Nephropathy. In. Stat-Pearls. ed. Treasure Island (FL): 2017. [Google Scholar]
- 38.Ursta AA, Kharkov EI, Petrova MM, Ursta OV, Kotikov AR, Kiselev AN. Contrast induced nephropathy in the older age group patients. Adv Gerontol. 2017; 30: 306-10. [PubMed] [Google Scholar]
- 39.Wong GT, Lee EY, Irwin MG. Contrast induced nephropathy in vas-cular surgery. Br J Anaesth 2016; 117 Suppl 2: ii63-ii73. [DOI] [PubMed] [Google Scholar]
- 40.Davenport MS, Cohan RH, Ellis JH. Contrast media controversies in 2015: imaging patients with renal impairment or risk of contrast reaction. AJR Am J Roentgenol. 2015; 204: 1174-81. [DOI] [PubMed] [Google Scholar]
- 41.Homma K. Contrast-induced Acute Kidney Injury. Keio J Med. 2016; 65: 67-73. [DOI] [PubMed] [Google Scholar]
- 42.McCullough PA, Choi JP, Feghali GA, Schussler JM, Stoler RM, Vallabahn RC, et al. Contrast-Induced Acute Kidney Injury. J Am Coll Cardiol. 2016; 68: 1465-73. [DOI] [PubMed] [Google Scholar]
- 43.Genovesi E, Romanello M, De Caterina R. Contrast-induced acute kidney injury in cardiology. G Ital Cardiol (Rome) 2016; 17: 984- 1000. [DOI] [PubMed] [Google Scholar]
- 44.Ozkok S, Ozkok A. Contrast-induced acute kidney injury: A review of practical points. World J Nephrol. 2017; 6: 86-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chalikias G, Drosos I, Tziakas DN. Contrast-Induced Acute Kidney Injury: An Update. Cardiovasc Drugs Ther. 2016; 30: 215-28. [DOI] [PubMed] [Google Scholar]
- 46.Mohammed NM, Mahfouz A, Achkar K, Rafie IM, Hajar R. Contrast-induced Nephropathy. Heart Views. 2013; 14: 106-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wichmann JL, Katzberg RW, Litwin SE, Zwerner PL, De Cecco CN, Vogl TJ, et al. Contrast-Induced Nephropathy. Circulation. 2015; 132: 1931-6. [DOI] [PubMed] [Google Scholar]
- 48.Contrast-Induced Nephropathy (CIN): Current State of the Evidence on Contrast Media and Prevention of CIN. In. Comparative Effectiveness Review Summary Guides for Clinicians. ed. Rockville (MD): 2007. [Google Scholar]
- 49.Katsiki N, Athyros VG, Karagiannis A, Mikhailidis DP. Contrast-Induced Nephropathy: An “All or None” Phenomenon? Angiology. 2015; 66: 508-13. [DOI] [PubMed] [Google Scholar]
- 50.Lameire NH. Contrast-induced nephropathy--prevention and risk reduction. Nephrol Dial Transplant. 2006; 21: i11-23. [DOI] [PubMed] [Google Scholar]
- 51.Bagshaw SM, Culleton BF. Contrast-induced nephropathy: epidemiology and prevention. Minerva Cardioangiol. 2006; 54: 109-29. [PubMed] [Google Scholar]
- 52.Heinrich MC, Scheer M, Heckmann M, Kuefner MA, Uder M. Iodixanol induces apoptotic and antiproliferative effects but no necrotic cell death in renal proximal tubular cells in vitro. Rofo 2009; 181: 349-54. [DOI] [PubMed] [Google Scholar]
- 53.Yao L, Kolluru GK, Kevil CG, Zhang WW. Intravascular radiocontrast iodixanol increases permeability of proximal tubule epithelium: a possible mechanism of contrast-induced nephropathy. Vasc Endovascular Surg. 2013; 47: 632-8. [DOI] [PubMed] [Google Scholar]
- 54.Lerch M, Keller M, Britschgi M, Kanny G, Tache V, Schmid DA, et al. Cross-reactivity patterns of T cells specific for iodinated contrast media. J Allergy Clin Immunol. 2007; 119: 1529-36. [DOI] [PubMed] [Google Scholar]
- 55.Zager RA, Johnson AC, Hanson SY. Radiographic contrast mediainduced tubular injury: evaluation of oxidant stress and plasma membrane integrity. Kidney Int. 2003; 64: 128-39. [DOI] [PubMed] [Google Scholar]
- 56.Yokomaku Y, Sugimoto T, Kume S, Araki S, Isshiki K, Chin-Kanasaki M, et al. Asialoerythropoietin prevents contrast-induced nephropathy. J Am Soc Nephrol. 2008; 19: 321-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Duan S, Zhou X, Liu F, Peng Y, Chen Y, Pei Y, et al. Comparative cytotoxicity of high-osmolar and low-osmolar contrast media on HKCs in vitro. J Nephrol. 2006; 19: 717-24. [PubMed] [Google Scholar]
- 58.Heinrich M, Scheer M, Heckmann M, Bautz W, Uder M. Reversibility and time-dependency of contrast medium induced inhibition of 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyl-tetrazolium bromide (MTT) conversion in renal proximal tubular cells in vitro: comparison of a monomeric and a dimeric nonionic iodinated contrast medium. Invest Radiol. 2007; 42: 732-8. [DOI] [PubMed] [Google Scholar]
- 59.Yang JS, Lu CC, Kuo SC, Hsu YM, Tsai SC, Chen SY, et al. Autophagy and its link to type II diabetes mellitus. Biomedicine. (Taipei) 2017; 7: 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jensen H, Doughty RW, Grant D, Myhre O. The effects of the iodinated X-ray contrast media iodixanol, iohexol, iopromide, and ioversol on the rat kidney epithelial cell line NRK 52-E. Ren Fail. 2011; 33: 426-33. [DOI] [PubMed] [Google Scholar]
- 61.Ko GJ, Bae SY, Hong YA, Pyo HJ, Kwon YJ. Radiocontrast-induced nephropathy is attenuated by autophagy through regulation of apoptosis and inflammation. Hum Exp Toxicol. 2016; 35: 724-36. [DOI] [PubMed] [Google Scholar]
- 62.Kolyada AY, Liangos O, Madias NE, Jaber BL. Protective effect of erythropoietin against radiocontrast-induced renal tubular epithelial cell injury. Am J Nephrol. 2008; 28: 203-9. [DOI] [PubMed] [Google Scholar]
- 63.He X, Yang J, Li L, Tan H, Wu Y, Ran P, et al. Atorvastatin protects against contrast-induced nephropathy via anti-apoptosis by the upregulation of Hsp27 in vivo and in vitro. Mol Med Rep. 2017; 15: 1963-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sayarlioglu H, Okuyucu A, Bedir A, Salis O, Yenen E, Bekfilavioglu G, et al. Is there any role of epithelial to mesenchymal transition in the pathogenesis of contrast nephropathy? Ren Fail. 2016; 38: 1249-55. [DOI] [PubMed] [Google Scholar]