Abstract
Objectives
Cefotaxime-resistant Enterobacteriaceae (CE) infections are intractable, with limited treatment options. Though carbapenems are frequently prescribed for CE infections, the emergence of carbapenem-resistant Enterobacteriaceae is of huge concern. Flomoxef is effective against CE in vitro, and some clinical data on its demonstrated effectiveness against CE bloodstream infections (BSIs) exists.
Patients and methods
We conducted a retrospective study on adults with BSI caused by flomoxef-susceptible CE to investigate the efficacy of flomoxef compared with that of ertapenem. The outcome was evaluated with propensity score-based matching and logistic regression analysis.
Results
Demographic and clinical characteristics of patients treated with flomoxef (n = 58) or ertapenem (n = 188) were compared. In the multivariate analysis, severe sepsis (adjusted odds ratio [AOR] = 3.84; 95% confidence interval [CI], 1.16–12.78; p = 0.03), high BSI mortality score (AOR = 5.59; 95% CI, 2.37–13.21; p < 0.01), ultimately or rapidly fatal comorbidity (AOR = 10.60; 95% CI, 3.43–32.75; p < 0.01), and pneumonia (AOR = 10.11; 95% CI, 3.43–29.81; p < 0.01) were independently associated with 28-day mortality. Using propensity scores, 58 flomoxef-treated patients were matched to 116 ertapenem-treated patients. There were no intergroup differences in BSI severity, comorbidity, or BSI sources. The 28-day mortality rates (20.7% vs 13.8%, p = 0.28) did not differ significantly. However, hospitalization length was shorter in the ertapenem group (10.2 ± 8.5 vs. 14.6 ± 9.4 days, p < 0.01).
Conclusion
Although similar outcomes were observed between the groups, ertapenem therapy was associated with a shorter hospitalization time in adults after CE BSI.
Keywords: bacteremia, cephamycin, ESBL, flomoxef, outcomes, MIC
Introduction
In 2010, the Clinical and Laboratory Standards Institute (CLSI) lowered the minimum inhibitory concentration (MIC) breakpoints for many β-lactam antibiotics, including extended-spectrum cephalosporins, to enhance the detection of known resistance among Enterobacteriaceae. The CLSI Antibiotic Subcommittee asserted that routine extended-spectrum β-lactamase (ESBL) testing was no longer necessary and that treatment decisions can be solely based on MICs.1 Escherichia coli (E. coli), Klebsiella species, and Proteus mirabilis (P. mirabilis) have not, since, been routinely evaluated for ESBL production. Of note, the new breakpoint MIC defining ceftriaxone or cefotaxime susceptibility (1 mg/L) is lower than the corresponding new breakpoint for ceftazidime (4 mg/L).1 High rates of susceptibility to ceftazidime have been found among prevalent CTX-M-producing E. coli, Klebsiella species, and P. mirabilis.2,3 This suggests that the CLSI ceftazidime breakpoint might be too high to be a reliable ESBL marker. Cefotaxime non-susceptibility afforded the detection of >95% of ESBL true-positive isolates.3 Thus, evaluating cefotaxime susceptibility may be the optimal strategy to predict ESBL presence in Enterobacteriaceae.
Carbapenems remain the mainstay for treatment of infections with high-inoculum ESBL-producers.4,5 However, their use should be restricted, considering the emergence of carbapenem-resistant organisms.6 Alternative treatments are urgently needed to relieve the selective pressure for carbapenem.7 Thus, over the past decades, numerous studies have been conducted to determine possible alternatives. Flomoxef – classified as a 1-oxacephem cephamycin – was found to be stable in the presence of β-lactamase and active against cephalosporin-resistant strains.8 While flomoxef has the potential to be effective against β-lactamase–producing strains, clinical data with regard to its potential value for the treatment of ESBL-associated infections are currently limited.9 Some researchers are reluctant to use cephamycins as first-line therapy for infections with ESBL-producing organisms.10 Furthermore, the updated CLSI breakpoints obviate ESBL screening, suggesting that the new CLSI breakpoint for cefotaxime susceptibility could be safely used to predict ESBL presence in Enterobacteriaceae. However, no clinical studies have verified flomoxef’s efficacy through using ertapenem as the comparator in patients with bacteremia caused by cefotaxime-resistant Enterobacteriaceae. Therefore, we aimed to compare therapeutic efficacies of flomoxef and ertapenem therapy in the management of bacteremia caused by cefotaxime-resistant Enterobacteriaceae.
Patients and methods
Setting
This study was conducted at Kaohsiung Chang Gung Memorial Hospital, a tertiary-care medical center in southern Taiwan. The Institutional Review Board of the Chang Gung Memorial Hospital approved the study (No. 201601503B0) and waived the need for patient consent due to the retrospective nature of the study. The patient data was anonymized to maintain confidentiality.
During 2011–2015, blood culture records from adult patients (age >18 years) who had at least one positive result for cefotaxime-resistant Enterobacteriaceae were collected. In cases where the patient experienced more than one bacteremia episode, only the first episode was included. Patients who fulfilled both of the following criteria were enrolled: 1) mono-microbial bacteremia confirmed by the isolation of cefotaxime-resistant Enterobacteriaceae from blood cultures of patients showing sepsis syndrome and 2) definitive parenteral therapy with flomoxef or ertapenem for more than 48 h until termination of the antimicrobial therapy or death. Definitive therapy was defined as the circumstance where ertapenem or flomoxef monotherapy was used to treat a cefotaxime-resistant Enterobacteriaceae bloodstream infection (BSI), and the strain showed in vitro susceptibility to the prescribed drug.1 Antimicrobial susceptibility was determined using a standard broth micro-dilution method, and interpreted according to the breakpoints suggested by the CLSI.1 The MICs of cefotaxime and flomoxef were ≤1 and ≤8 mg/L, respectively.1,11 Selection of the appropriate antimicrobial regimen was at the discretion of the attending physician. The following intravenous doses or adjusted equivalents for renal insufficiency were prescribed: ertapenem (1 g/24 h) or flomoxef (1 g/8 h). Through the antimicrobial stewardship system in the hospital,12 all ertapenem and flomoxef prescriptions had been approved by infectious disease specialists for their indications and dosages.
Definitions
Medical records of flomoxef (Shionogi, Osaka, Japan) - or ertapenem (MSD, Kenilworth, NJ, USA) -treated patients were reviewed and compared. Blood samples for the first positive cultures collected within 48 h of admission were defined as community-onset infections, whereas those obtained after 48 h were categorized as hospital-onset infections. The severity of underlying medical conditions was classified as nonfatal, fatal, and ultimately fatal.13 Pitt bacteremia scores were used to evaluate the severity of bacteremia on the day of onset.14 Furthermore, enrolled patients were stratified by BSI mortality risk score; patients with guarded prognoses had scores ≥5, and those with good prognoses had scores <5.15 Inappropriate empirical antibiotic therapy was defined as the first dose of appropriate antimicrobial agent that was not administered within the first 24 h after blood samples were drawn.16 Antibiotic therapy was considered appropriate if the route and dosage of the antimicrobial agent was administered as recommended by the Sanford Guide,17 and isolated pathogens were susceptible in vitro to the prescribed agent with reference to contemporary CLSI breakpoints.1 BSI sources were determined clinically based on the presence of an active infection site coincident with bacteremia or the isolation of a microorganism from other clinical specimens before or on the same date of BSI onset. If the BSI source could not be assigned to a specific site, it was classified as primary bacteremia. Adequate source control was defined as having a timely 1) percutaneous, or surgical, intervention to drain infected fluid collections, debride infected tissues, and control ongoing enteric or other drainage producing intra-abdominal source of infection eliciting the bacteremia, 2) central venous catheter removal for catheter-related bacteremia, and 3) urinary catheter removal for BSI secondary to catheter-related urinary tract infection. Severe sepsis was defined as the coexistence of sepsis and signs or symptoms of acute organ dysfunction and/or hypoperfusion. Septic shock was defined as the presence of a systemic inflammatory response syndrome and systolic blood pressure ≤90 mmHg following a crystalloid-fluid challenge or a high blood lactate concentration (>4 mmol/L).18 The early clinical response was assessed on Day 7 after initiating flomoxef or ertapenem therapy, while the evaluation upon completion of fomoxef or ertapenem therapy was referred to as a final clinical response. Patients were evaluated on Day 7 for the presence of 1) fatality resulting from cefotaxime-resistant Enterobacteriaceae bacteremia, 2) septic shock, 3) persistent bacteremia, 4) persistent fever, and 5) persistent leukocytosis, and favorable early clinical responses referred to the absence of any of these findings. The final clinical response was categorized as cure, improvement, or failure. Cure was defined as the resolution of clinical signs and symptoms and a negative culture report at the end of therapy. Improvement was defined as a partial resolution of clinical signs and symptoms based on clinical adjustment, with a need for additional antibiotic therapy. Patients with 1) clinical progression or relapse of sepsis that had previously improved clinically, 2) fatality, and/or 3) culture of blood sampled at the end of flomoxef or ertapenem treatment remaining positive for cefotaxime-resistant Enterobacteriaceae were defined as treatment failures. The clinical responses of cure and improvement were classified as favorable outcomes. The primary outcome was the 28-day crude mortality, which was defined as all-cause mortality occurring within 28 days of hospitalization after the onset of cefotaxime-resistant Enterobacteriaceae bacteremia. Furthermore, we traced the incidence of recurrent BSI caused by cefotaxime-resistant Enterobacteriaceae within 6 months post treatment among these patients.
To control confounding variables associated with the choice of definitive antimicrobial agents, a propensity score (estimated probability of mortality) was used to assess each case on the basis of final parameter estimates in the multivariate model. Each patient in the flomoxef group was then matched to two patients in the ertapenem group (ratio of 1:2) who had a similar propensity score (<5% difference between scores). If two or more candidates were found to have identical propensity scores, the one with a similar BSI mortality risk score ≥5 points (initial secondary matching variable) and similar McCabe classification (backup secondary matching variable) would have a higher priority in the matching process.
Statistical analyses
Statistical analyses were conducted with SPSS version 11.5 (SPSS Inc., Chicago, IL, USA). Categorical variables were expressed as percentages of total patients and compared using the χ2 or Fisher’s exact test, as appropriate. Continuous variables were expressed as mean values ± standard deviations and compared using the Mann–Whitney U or Student’s t-test. Independent predictors of mortality were identified using logistic regression analysis. Variables with a p-value ≤ 0.1 in the univariate analysis were included in the logistic regression model to identify variables with either a negative or positive impact on the 28-day crude mortality. Goodness-of-fit was assessed by the Hosmer–Lemeshow statistic. Receiver operating characteristic (ROC) curve analysis was used to evaluate the predictive performance of the logistic regression model. Kaplan–Meier analysis was used to examine the length of hospital stay following cefotaxime-resistant Enterobacteriaceae BSI. Patients were followed from the time of collection of the index blood culture until hospital discharge. The permitted censoring of patients who died prior to hospital discharge was done to avoid accounting for early death as a favorable outcome. A log-rank test was used to assess the difference in length of hospital stay between patients who received flomoxef compared with ertapenem as definitive antimicrobial therapy. Variables with a two-tailed p-value <0.05 were considered statistically significant.
Results
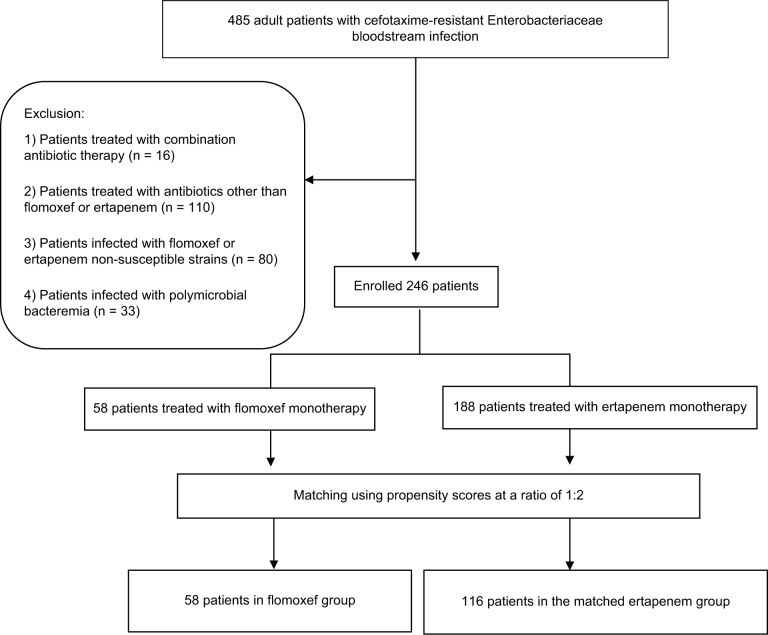
A total of 485 cases of cefotaxime-resistant Enterobacteriaceae bacteremia were identified during the 5-year study period, including 33 (6.8%) of polymicrobial BSI, 80 (16.5%) of bacteremia caused by strains not susceptible to flomoxef or ertapenem, 16 (3.3%) treated with combined antimicrobial agents, and 110 (22.7%) treated with antibiotics other than flomoxef or ertapenem. Overall, 246 (50.7%) patients (major causative microorganisms were E. coli, n = 128 [52.0%], Klebsiella species, n = 74 [30.1%], and P. mirabilis, n = 22 [8.9%]) were eligible for inclusion. Definitive flomoxef therapy was administered in 58 patients, and ertapenem therapy was administered to the remaining 188 patients (Figure 1). Between the groups, no patient received the other study drug for empiric antibiotic therapy. Age, gender, comorbidity, disease severity, BSI source, inappropriate empiric antibiotic, duration of administration empiric/total antibiotic, infectious diseases physicians’ consultation, and adequate source control did not differ significantly between the two groups (Table 1). In view of clinical outcome, favorable outcome at the short-term time point, favorable outcome at the end of treatment, duration of defervescence, recurrent BSI caused by cefotaxime-resistant Enterobacteriaceae within 6 months post treatment, and crude mortality did not differ significantly between the two groups (Table 1). There were no emergences of carbapenem-resistant Enterobacteriaceae in the recurrent BSI post treatment among these patients. However, the flomoxef group had prolonged hospital stay, compared to that of the ertapenem group (14.6 ± 9.4 vs 10.8 ± 7.4 days, p < 0.01; Table 1). In addition, the enrolled patients were stratified by the BSI mortality risk score. In the subgroups of patients with guarded prognoses (BSI mortality risk score ≥5) and patients with good prognoses (BSI mortality risk score <5), the 28-day crude mortality did not differ significantly between those who received flomoxef, compared with ertapenem, as definitive therapy. The hospital stay remained longer in the flomoxef group than in the ertapenem group, regardless of whether these patients had guarded or good prognoses (Table 1).
Figure 1.
Patient enrollment.
Table 1.
Demographics, clinical characteristics, and outcomes of 246 adults with cefotaxime-resistant Enterobacteriaceae bloodstream infections treated with flomoxef or ertapenem, categorized by critical (BSI mortality score ≥5) and less critical (BSI mortality score <5) illnesses
| Characteristics | Patient number (%)
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Total
|
BSI mortality score ≥5
|
BSI mortality score <5
|
|||||||
| Flomoxef N = 58 |
Ertapenem N = 188 |
p | Flomoxef N = 18 |
Ertapenem N = 48 |
p | Flomoxef N = 40 |
Ertapenem N = 140 |
p | |
| Gender, female | 29 (50.0) | 96 (51.1) | >0.99 | 8 (44.4) | 25 (52.1) | 0.59 | 21 (52.5) | 71 (50.7) | 0.86 |
| Old age ≥65 years | 38 (65.5) | 117 (62.2) | 0.76 | 12 (66.7) | 24 (50.0) | 0.27 | 26 (65.0) | 93 (66.4) | 0.85 |
| Community-onset bacteremia | 18 (31.0) | 56 (29.8) | 0.87 | 8 (44.4) | 25 (52.1) | 0.59 | 10 (25.0) | 31 (22.1) | 0.68 |
| Initial syndrome | |||||||||
| Severe sepsis | 29 (50.0) | 94 (50.0) | >0.99 | 17 (94.4) | 35 (72.9) | 0.09 | 12 (30.0) | 59 (42.1) | 0.20 |
| Septic shock | 10 (17.2) | 38 (20.2) | 0.71 | 8 (44.4) | 24 (50.0) | 0.79 | 2 (5.0) | 14 (10.0) | 0.53 |
| Major causative microorganisms | |||||||||
| Escherichia coli | 30 (52.0) | 98 (52.1) | >0.99 | 8 (44.4) | 18 (37.5) | 0.78 | 22 (55.0) | 80 (57.1) | 0.86 |
| Klebsiella species | 20 (34.5) | 54 (28.7) | 0.42 | 5 (27.8) | 16 (33.3) | 0.77 | 15 (37.5) | 38 (27.1) | 0.24 |
| Proteus mirabilis | 6 (10.3) | 16 (8.5) | 0.61 | 3 (16.7) | 7 (14.6) | >0.99 | 3 (7.5) | 9 (6.4) | 0.73 |
| Ultimately or rapidly fatal comorbidity (McCabe classification) | 11 (18.9) | 45 (23.9) | 0.48 | 5 (27.8) | 10 (20.8) | 0.53 | 6 (15.0) | 35 (25.0) | 0.21 |
| Major comorbidities | |||||||||
| Hypertension | 31 (53.4) | 87 (46.3) | 0.37 | 10 (55.5) | 16 (33.3) | 0.16 | 21 (50.0) | 71 (50.7) | 0.86 |
| Diabetes mellitus | 17 (29.3) | 70 (37.2) | 0.35 | 8 (44.4) | 14 (29.2) | 0.26 | 9 (22.5) | 56 (40.0) | 0.06 |
| Malignancy | 15 (25.9) | 49 (26.1) | >0.99 | 8 (44.4) | 10 (20.8) | 0.07 | 7 (17.5) | 39 (27.9) | 0.22 |
| Chronic kidney disease | 12 (20.7) | 30 (15.9) | 0.43 | 3 (16.7) | 5 (10.4) | 0.67 | 9 (22.5) | 25 (17.9) | 0.50 |
| Liver cirrhosis | 7 (12.1) | 35 (18.6) | 0.32 | 3 (16.7) | 6 (12.5) | 0.69 | 4 (10.0) | 29 (20.7) | 0.16 |
| Major source of bacteremia* | |||||||||
| Urinary tract infection | 22 (37.9) | 88 (46.8) | 0.29 | 6 (33.3) | 12 (25.0) | 0.54 | 16 (40.0) | 76 (54.3) | 0.15 |
| Catheter-related infection | 9 (5.5) | 24 (12.8) | 0.66 | 1 (5.6) | 6 (12.5) | 0.67 | 8 (20.0) | 18 (12.9) | 0.31 |
| Intra-abdominal infection | 8 (13.8) | 34 (18.1) | 0.55 | 2 (11.1) | 14 (29.2) | 0.19 | 6 (15.0) | 20 (14.3) | >0.99 |
| Pneumonia | 10 (17.2) | 28 (14.9) | 0.68 | 8 (44.4) | 10 (20.8) | 0.07 | 2 (5.0) | 18 (12.9) | 0.25 |
| Primary bacteremia | 13 (22.4) | 28 (14.9) | 0.23 | 5 (27.8) | 8 (16.7) | 0.32 | 8 (20.0) | 20 (14.3) | 0.46 |
| Inappropriate empiric antibiotic | 15 (25.9) | 56 (29.8) | 0.51 | 5 (27.8) | 14 (29.2) | >0.99 | 10 (25.0) | 42 (30.0) | 0.69 |
| Administration empiric antibiotic duration, day, mean ± SD | 4.0 ± 2.2 | 4.5 ± 1.6 | 0.78 | 3.6 ± 2.4 | 4.4 ± 1.6 | 0.88 | 4.4 ± 2.4 | 4.6 ± 1.8 | 0.67 |
| Administration total antibiotic duration, day, mean ± SD | 9.6 ± 6.6 | 8.6 ± 7.4 | 0.85 | 10.4 ± 7.2 | 9.2 ± 8.4 | 0.76 | 8.8 ± 6.2 | 8.0 ± 6.8 | 0.63 |
| ID physician consultation | 13 (22.4) | 38 (20.2) | 0.71 | 5 (27.8) | 14 (29.2) | >0.99 | 8 (20.0) | 24 (17.1) | 0.65 |
| Adequate source control+, A/B | 26/30 | 88/108 | 0.60 | 5/7 | 20/25 | 0.63 | 21/23 | 68/83 | 0.35 |
| Clinical outcome | |||||||||
| Favorable outcome at short-term pointa | 38 (65.5) | 117 (62.2) | 0.76 | 12 (66.7) | 24 (50.0) | 0.27 | 26 (65.0) | 93 (66.4) | 0.85 |
| Favorable outcome at the end of treatmentb | 50 (86.2) | 166 (88.3) | 0.65 | 14 (77.8) | 41 (85.4) | 0.47 | 36 (90.0) | 125 (89.3) | 0.99 |
| Time to defervescence, day, mean ± SD++ | 4.8 ± 3.4 | 4.2 ± 3.6 | 0.40 | 5.8 ± 3.8 | 5.6 ± 4.2 | 0.48 | 4.2 ± 3.2 | 3.8 ± 3.2 | 0.44 |
| Length of hospitalization, day, mean ± SD | 14.6 ± 9.4 | 10.8 ± 7.4 | <0.01 | 16.4 ± 5.4 | 14.2 ± 8.8 | 0.04 | 11.8 ± 7.4 | 9.4 ± 6.2 | 0.01 |
| Recurrent BSI caused by cefotaxime-resistant Enterobacteriaceae within 6 months post treatment | 5 (9.8) | 13 (6.9) | 0.77 | 2 (11.1) | 3 (6.3) | 0.61 | 3 (7.5) | 10 (7.1) | >0.99 |
| Crude mortality | |||||||||
| 3-day | 4 (6.9) | 8 (4.3) | 0.48 | 2 (11.1) | 6 (12.5) | >0.99 | 2 (5.0) | 2 (1.4) | 0.21 |
| 14-day | 6 (10.3) | 18 (9.6) | 0.80 | 4 (22.2) | 8 (16.7) | 0.72 | 2 (5.0) | 10 (7.1) | >0.99 |
| 28-day | 12 (20.7) | 29 (15.4) | 0.42 | 8 (44.4) | 14 (29.2) | 0.26 | 4 (10.0) | 15 (10.7) | 0.79 |
Notes:
Some patients may have more than one bacteremia source.
Surgical intervention, drainage, central venous catheter removal, and urinary catheter removal were defined as source control. Patients with pneumonia or primary bacteremia were excluded. A/B ratio; A: adequate and timely removal or debridement of the source of bacteremia, B: source of bacteremia needs to be removed or debrided.
52 afebrile patients (flomoxef, 16 patients; ertapenem, 36) at admission were excluded.
Assessment on Day 7 after starting flomoxef or ertapenem therapy.
Evaluation at completion of flomoxef or ertapenem therapy.
Abbreviations: BSI, bloodstream infection; ID, infectious disease.
Risk factors for the 28-day crude mortality in the unadjusted univariate analysis included BSI mortality score ≥5 at onset, severe sepsis, septic shock, ultimately or rapid fatal comorbidity, and both urinary tract infection and pneumonia as the source of bacteremia (Table 2). After adjustments were made using multivariate analysis, we found that BSI mortality score ≥5 at onset (adjusted odds ratio [AOR] = 5.59; 95% confidence interval [CI], 2.37–13.21; p < 0.001), severe sepsis (AOR = 3.84; 95% CI, 1.16–12.78; p = 0.028), pneumonia as the source of bacteremia (AOR = 10.11; 95% CI, 3.43–29.81; p < 0.001), and the presence of ultimately or rapid fatal comorbidity (AOR = 10.60; 95% CI, 3.43–32.75; p < 0.001) were independently associated with the 28-day crude mortality (Table 2). In the subgroup analysis of the flomoxef group, the 28-day crude mortality rate was significantly higher in patients with bacteremia caused by Klebsiella isolates (40.0%, 8/20) than in those with bacteremia caused by E. coli (10.0%, 3/30; Figure 2). Of the 74 patients infected with Klebsiella species, patients who received flomoxef therapy had a significantly higher mortality rate than those who received ertapenem therapy (40.0% [8/20] vs 12.9% [7/54], p = 0.02; Figure 2).
Table 2.
Risk factor of the 28-day crude mortality in 246 patients with cefotaxime-resistant Enterobacteriaceae bloodstream infection
| Variables at bacteremia onset | Patients number (%)
|
Univariate analysis
|
Multivariate analysis
|
|||
|---|---|---|---|---|---|---|
| Death, n = 41 |
Survival, n = 205 |
OR (95% CI) | p | Adjusted OR (95% CI) |
p | |
| BSI mortality risk score ≥5 at onset | 22 (53.7) | 44 (21.5) | 1.69 (1.21–2.38) | <0.001 | 5.59 (2.37–13.21) | <0.001 |
| Severe sepsis | 29 (70.7) | 94 (45.9) | 1.54 (1.21–1.97) | 0.006 | 3.84 (1.16–12.78) | 0.028 |
| Septic shock | 16 (39.0) | 32 (15.6) | 2.50 (1.52–4.11) | 0.002 | NS | NS |
| Ultimately or rapidly fatal comorbidity (McCabe classification) | 19 (46.3) | 39 (19.0) | 2.57 (1.65–3.99) | <0.001 | 10.60 (3.43–32.75) | <0.001 |
| Bacteremia source | ||||||
| Pneumonia | 17 (41.4) | 21 (10.2) | 4.05 (2.35–6.98) | <0.001 | 10.11 (3.43–29.81) | <0.001 |
| Urinary tract infection | 11 (26.8) | 99 (48.3) | 0.56 (0.33–0.94) | 0.015 | NS | NS |
Notes: There was adequate goodness of fit (Hosmer–Lemeshow test, χ2 = 6.89, p = 0.23). Receiver operating characteristic analysis indicated that predictive performance of logistic regression model was adequate (area under the curve = 0.82).
Abbreviations: BSI, bloodstream infection; CI, confidence interval; NS, no significance; OR, odds ratio.
Figure 2.
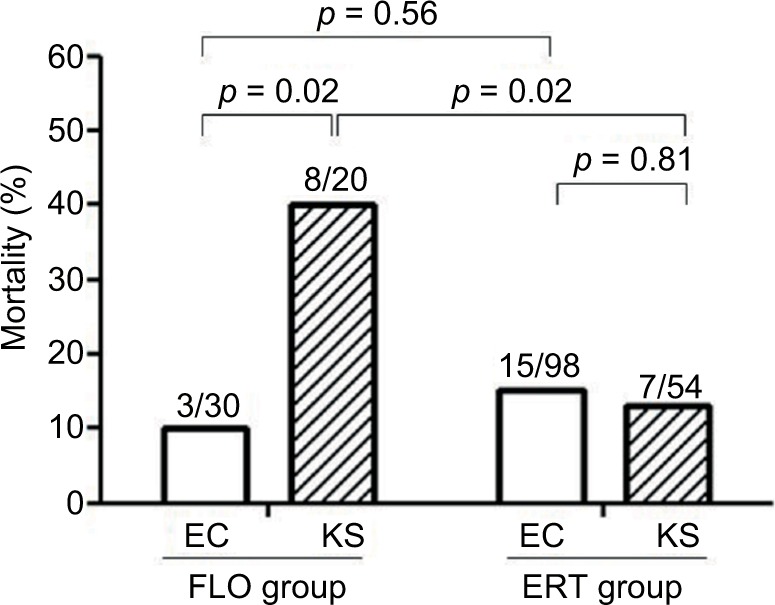
The 28-day crude mortality for patients with cefotaxime-resistant Enterobacteriaceae bacteremia. Cases are grouped according to treatment with flomoxef (FLO) or ertapenem (ERT) and are further stratified by infection by Escherichia coli (EC) or Klebsiella species (KS). Number of cases with mortality among total cases in the respective group are indicated. Comparison between groups was undertaken using the c2 or Fisher’s exact test, as appropriate. The results are indicted as two-tailed p < 0.05 indicating statistical significance.
Furthermore, based on propensity scores, the 58 patients who received definitive flomoxef therapy could be matched with 116 patients who received ertapenem. There were no significant differences in terms of BSI severity, comorbidity severity, BSI sources, inappropriate empiric antibiotic, duration of administration empiric/total antibiotic, infectious diseases physicians’ consultation, or adequate source control between these two groups (Table 3). We found that, compared with those receiving ertapenem, patients receiving flomoxef had a significantly longer hospital length of stay following cefotaxime-resistant Enterobacteriaceae bacteremia (14.6 ± 9.4 vs 10.2 ± 8.5 days, p < 0.01). Overall, flomoxef therapy was associated with prolonged hospital stay following cefotaxime-resistant Enterobacteriaceae BSIs in the Kaplan–Meier analysis (log-rank p < 0.001; Figure 3).
Table 3.
Demographics, clinical characteristics, and outcome of patients with cefotaxime-resistant Enterobacteriaceae bloodstream infection treated with flomoxef or ertapenem, matched by propensity scores (1:2)
| Characteristics | Patients number (%)
|
p | |
|---|---|---|---|
| Flomoxef, n = 58 | Ertapenem, n = 116 | ||
| Gender, female | 29 (50.0) | 65 (56.0) | 0.52 |
| Age, year, mean ± SD | 65.7 ± 16.5 | 69.1 ± 15.5 | 0.22 |
| Community-onset bacteremia | 18 (31.0) | 36 (31.0) | >0.99 |
| Bloodstream infection mortality risk score at onset (point allocation)a | |||
| Guarded progression (≥5) | 18 (31.0) | 36 (31.0) | >0.99 |
| Malignancy (3) | 15 (25.9) | 30 (25.9) | >0.99 |
| Liver cirrhosis (4) | 7 (12.1) | 19 (16.4) | 0.51 |
| Source of infection other than urinary tract or central venous catheter (4) | 27 (46.6) | 60 (51.7) | 0.53 |
| Pitt bacteremia scoreb | |||
| 0–1 (0) | 14 (24.1) | 25 (21.6) | 0.70 |
| 2–3 (2) | 36 (62.1) | 70 (60.3) | 0.87 |
| ≥4 (5) | 8 (13.8) | 21 (18.1) | 0.52 |
| Major causative microorganisms | |||
| Escherichia coli | 30 (51.7) | 64 (55.2) | 0.75 |
| Klebsiella species | 20 (34.5) | 46 (39.7) | 0.62 |
| Proteus mirabilis | 6 (10.3) | 6 (5.2) | 0.22 |
| Ultimately or rapidly fatal comorbidity (McCabe classification) | 11 (18.9) | 24 (20.7) | 0.84 |
| Major comorbidities | |||
| Hypertension | 31 (53.4) | 55 (47.4) | 0.52 |
| Diabetes mellitus | 17 (29.3) | 44 (37.9) | 0.31 |
| Malignancy | 15 (25.9) | 30 (25.9) | >0.99 |
| Chronic kidney disease | 12 (20.7) | 18 (15.5) | 0.40 |
| Liver cirrhosis | 7 (12.1) | 19 (16.4) | 0.51 |
| Major source of bacteremia* | |||
| Urinary tract infection | 22 (37.9) | 46 (39.7) | 0.87 |
| Catheter-related infection | 9 (15.5) | 14 (12.1) | 0.64 |
| Intra-abdominal infection | 8 (13.8) | 18 (15.5) | 0.83 |
| Pneumonia | 10 (17.2) | 15 (12.9) | 0.49 |
| Primary bacteremia | 13 (22.4) | 36 (31.0) | 0.28 |
| Inappropriate empiric antibiotic | 15 (25.9) | 36 (31.0) | 0.60 |
| Administration empiric antibiotic duration, day, mean ± SD | 4.0 ± 2.2 | 4.3 ± 1.8 | 0.72 |
| Administration total antibiotic duration, day, mean ± SD | 9.6 ± 6.6 | 8.8 ± 7.2 | 0.78 |
| ID physician consultation | 13 (22.4) | 24 (20.6) | 0.85 |
| Adequate source control+, A/B | 26/30 | 50/60 | 0.77 |
| Clinical outcome | |||
| Favorable outcome at short-term pointc | 38 (65.5) | 82 (70.7) | 0.49 |
| Favorable outcome at the end of treatmentd | 50 (86.2) | 105 (91.3) | 0.44 |
| Time to defervescence, day, mean ± SD++ | 4.8 ± 3.4 | 4.2 ± 3.2 | 0.58 |
| Length of hospitalization, day, mean ± SD | 14.6 ± 9.4 | 10.2 ± 8.5 | <0.01 |
| Recurrent BSI caused by cefotaxime-resistant Enterobacteriaceae within 6 months post treatment | 5 (9.8) | 10 (8.6) | >0.99 |
| Crude mortality | |||
| 3-day | 4 (6.9) | 4 (3.4) | 0.44 |
| 14-day | 6 (10.3) | 10 (8.6) | 0.78 |
| 28-day | 12 (20.7) | 16 (13.8) | 0.28 |
Notes:
A patient might have more than one bacteremia source.
Surgical intervention, drainage, central venous catheter removal, urinary catheter removal were defined as source control. Patients with pneumonia or primary bacteremia were excluded. A/B ratio, A: adequate and timely removal or debridement of the source of bacteremia, B: source of bacteremia need to be removed or debrided.
42 afebrile patients (flomoxef, 16 patients; ertapenem, 26) at admission were excluded.
Data from a previous study.15
Pitt bacteremia score is calculated based on: 1) oral temperature, 2 points for a temperature of ≤35°C or ≥40°C, 1 point for a temperature of 35.1–36.0°C or 39.0–39.9°C, and 0 points for a temperature of 36.1–38.9°C; 2) hypotension, 2 points for an acute hypotensive event with decreases in systolic (>30 mmHg) and diastolic (>20 mmHg) blood pressures, use of intravenous vasopressor agents, or a systolic blood pressure of <90 mmHg; 3) mechanical ventilation, 2 points; 4) cardiac arrest, 4 points; and 5) mental status, 0 points for alert, 1 point for disoriented, 2 points for stuporous, and 4 points for comatose. Scores were recorded on the day the index blood culture was obtained.
Assessment on Day 7 after starting flomoxef or ertapenem therapy.
Evaluation at completion of flomoxef or ertapenem therapy.
Abbreviations: BSI, bloodstream infection; ID, infectious diseases.
Figure 3.
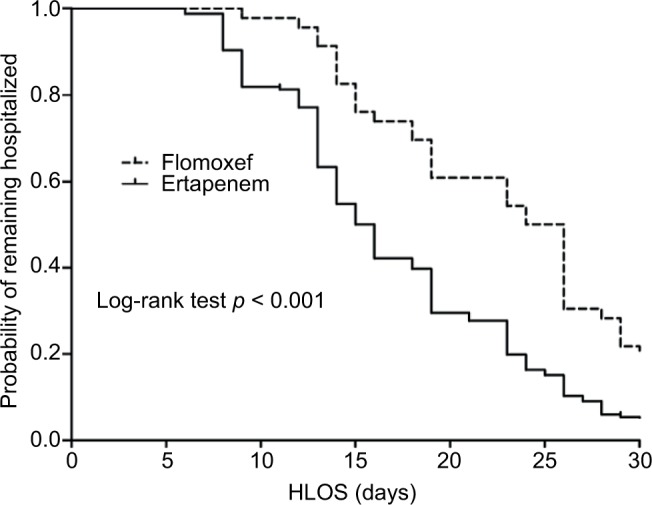
Kaplan–Meier curves of the hospital length of stay per receiving flomoxef or ertapenem therapy in 205 survivors* within 28 days after cefotaxime-resistant Enterobacteriaceae bacteremia onset. *Of 246 patients, 12 patients with flomoxef therapy and 29 patients with ertapenem therapy who died within the 28 days were excluded.
Abbreviation: HLOS, hospital length of stay.
Discussion
The 2010 updated CLSI breakpoints obviated ESBL screening.1 Previous studies have suggested that flomoxef was effective in the treatment of bacteremia caused by ESBL producers when in vitro tests indicated susceptibility.9,19 Therefore, it is important to determine whether flomoxef can be used to treat BSIs caused by cefotaxime-resistant Enterobacteriaceae without ESBL screening. We found that the 28-day crude mortality rate was 16.7% among patients with flomoxef-susceptible cefotaxime-resistant Enterobacteriaceae bacteremia treated with flomoxef or ertapenem. Patients with severe sepsis, high BSI mortality scores, ultimately or rapid fatal comorbidity, and pneumonia were independent predictors of the 28-day crude mortality. Propensity scores used for matching BSI severity, comorbidity, and BSI sources between patients in the flomoxef and ertapenem groups did not suggest a significant difference in the 28-day crude mortality rate. However, the length of hospitalization was shorter in patients who received ertapenem than in those who received flomoxef treatment (10.2 ± 8.5 vs 14.6 ± 9.4 days, p < 0.01).
Our findings show that, in the presence of high-inoculum infections such as pneumonia, the 28-day crude morality was reported for nine patients treated with ertapenem (n = 28) and eight treated with flomoxef (n = 10); additionally, a higher mortality rate was noted in pneumonic BSI patients in the flomoxef group (p = 0.02, Fisher’s exact test). When patients were stratified by prognosis, the mortality rate in patients treated with ertapenem (6 of 10) did not differ from patients treated with flomoxef (6/8) in the subgroup with BSI mortality score ≥5 (p = 0.64, Fisher’s exact test). However, a significant difference in mortality rates between patients treated with ertapenem (3/18) and patients treated with flomoxef (2/2), was found in the subgroup with BSI mortality score <5 (p < 0.01, Fisher’s exact test). The clinical difference between patients who received flomoxef and ertapenem therapy may be explained by the fact that flomoxef and ertapenem behave differently against the challenge of high bacterial load using ESBL-producing and ESBL–non-producing bacterial isolates. Our data suggest that ertapenem, rather than flomoxef, was the preferred treatment for BSIs caused by cefotaxime-resistant Enterobacteriaceae with high inoculum size. It is noteworthy that the flomoxef breakpoint for susceptibility in this study was ≤8 mg/L, which may have overestimated the susceptibility rate. This was in contrast with our recent study suggesting a flomoxef breakpoint of ≤1 mg/L, which resulted in a lower rate of treatment failure.8 Increasing trends of mortality with increased MICs of flomoxef were also observed among patients with BSIs related to high inocula (e.g. non-urinary tract sources).8 Further investigations are needed to determine factors affecting the bactericidal activity related to high inocula and their clinical consequences.
For Enterobacteriaceae including ESBL-producers, antibacterial effects were observed for β-lactam agents when free drug concentrations (f) in the serum were above the MIC (T>MIC) for as little as 35–40% of the dosing interval, and this effect appeared to plateau at T>MIC for 60–70% of the dosing interval.20 For ertapenem, the T>MIC was ≥70% (for isolates with ertapenem MICs ≤0.5 mg/L) and occurred in all patients at a dose of 1 g ertapenem (CLSI susceptibility ≤0.5 mg/L) per day.21 The probability of a T>MIC ≥70% (for isolates with flomoxef MIC90 = 0.5 mg/L) occurred in 67.3% of patients who received flomoxef (suggested susceptibility ≤8 mg/L) at a dose of 1 g every 8 h.22 These data suggest that flomoxef may not be effectively bactericidal against Enterobacteriaceae, particularly for isolates with higher flomoxef MICs. According to our previous study, the proportion of flomoxef MIC >1 mg/L in ESBL-producing K. pneumoniae were higher than those in ESBL-producing E. coli.8 Furthermore, we found that flomoxef treatment in ESBL-producing bacteremia might not effectively eliminate the infected reservoir during the first episode, thereby increasing the risk of recurrent bacteremia caused by identical strains. Strains resistant to cephamycins via impermeability were reported more frequently in K. pneumoniae than in E. coli.23 In the current study, the poorer clinical outcomes observed in patients who received flomoxef to treat cefotaxime-resistant Enterobacteriaceae bacteremia were due to the Klebsiella species. Pharmacodynamic studies as well as measurements of flomoxef MICs are warranted with regard to the treatment of such infections.
In our recent study, patients who received flomoxef for ESBL-producing Enterobacteriaceae bacteremia due to strains with higher flomoxef MICs (2–8 mg/L) had worse clinical outcome than patients who received carbapenem therapy.8 In one multicenter, retrospective study of cefmetazole and flomoxef for treatment of ESBL-producing E. coli bacteremia, definitive cefmetazole and flomoxef therapy represented an effective alternative to carbapenem treatment.19 However, the higher flomoxef MICs (2–8 mg/L) of ESBL-E. coli isolates were only found in five (8.6%) of the 58 isolates with available MICs data.19 Furthermore, cephamycins have been used more frequently in urinary tract infections and in patients with lower acute severity of illness.19,24 Nearly 40% of our patients in the flomoxef group had cefotaxime-resistant Enterobacteriaceae BSIs related to the urinary tract. Because flomoxef concentrates in the urine to a high degree,22 the use of this agent for cefotaxime-resistant Enterobacteriaceae may be associated with positive clinical outcomes. Nevertheless, this is an important contribution, as it provides support for the reestablishment of flomoxef as a potential alternative for the treatment of infections due to cefotaxime-resistant Enterobacteriaceae. Flomoxef might be a potential alternative for specific clinical circumstances, such as BSIs with cefotaxime-resistant E. coli in the urinary tract.
Receipt of inappropriate antimicrobial therapy may lead to delayed clinical response to treatment in these patients such as persistence of fever or other symptoms. It is conceivable that some patients may remain hospitalized following completion of antimicrobial therapy due to either complications of bacteremia, such as respiratory, renal, or other end organ failure, or due to factors unrelated to the infection. Our study was in agreement with another study, wherein most of the individual components of guarded prognoses were associated with prolonged duration of hospitalization.15 Reporting hospital length of stay after bacteremia onset in the current study showed ertapenem therapy was associated with reduction in stay when compared with flomoxef therapy in patients with either guarded or good prognosis. In future studies, it would be interesting to define the factors associated with prolonged time of hospitalization in patients receiving flomoxef therapy.
Several limitations are inherent in the study design. Firstly, different bacterial strains were grouped together, although they varied in MICs data and infection characteristics. This study lacked the detection of flomoxef or ertapenem MIC values against specific pathogens. Our study found that the 28-day crude mortality rate of patients receiving flomoxef therapy for bacteremia caused by Klebsiella species was statistical higher than those who received ertapenem. Therefore, discrepancies in the therapeutic efficacy of flomoxef among different pathogens should not be disregarded. Secondly, as this was a retrospective study, the decision-making process that clinicians took to choose either ertapenem or flomoxef for the treatment of cefotaxime-resistant Enterobacteriaceae bacteremia would not necessarily be reflected in the medical records. Although we conducted propensity score matching in the case–control investigation, this study could be inherently limited by several identified or unidentified confounding factors. All our analyses led to the same finding that flomoxef treatment was significantly associated with longer length of hospitalization. The use of a propensity score likely helped to mitigate this imbalance, but was likely insufficient in controlling for the effects of all potential confounders and interactions that could not be investigated. Therefore, more studies are needed to assess whether flomoxef is as effective as ertapenem, particularly in patients with high inocula infections, such as pneumonia, or in patients infected by different Enterobacteriaceae strains such as E. coli or Klebsiella species. Third, the sample size was relatively small, which might preclude the identification of other independent predictors of outcomes in this study. However, there was adequate goodness of fit and the ROC analysis indicated that the predictive performance of our logistic regression model was adequate. Finally, the assessment of the outcomes of interest was conducted in an un-blinded manner, thus raising the potential for misclassification, although the outcome measure (28-day crude mortality) was a hard endpoint.
Conclusion
Flomoxef might provide a reasonable carbapenem-sparing option for cefotaxime-resistant Enterobacteriaceae bacteremia. Data were more robust for BSIs of the urinary tract. Ertapenem therapy is associated with reduced length of hospitalization compared to flomoxef therapy following cefotaxime-resistant Enterobacteriaceae bacteremia in the absence of ESBL screening.
Acknowledgments
This study was partially supported by grants from Chang Gung Memorial Hospital, Taiwan (CMRPG 8F1801). The funding source played no role in study design and conduct, data collection, analysis or interpretation, writing of the manuscript, or the decision to submit for publication. The authors thank Dr Chien-Ching Hung at the Department of Internal Medicine, National Taiwan University Hospital, Taipei, for his critical review of this manuscript. The authors also appreciate the Biostatistics Center, Kaohsiung Chang Gung Memorial Hospital, for their work on the statistical analysis.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Clinical and Laboratory Standards Institute . Performance standards for antimicrobial susceptibility testing: 20th informational supplement, CLSI document M100-S20. Wayne, PA: Clinical and Laboratory Standards Institute; 2010. [Google Scholar]
- 2.Williamson D, Roberts SA, Smith M, et al. High rates of susceptibility to ceftazidime among globally prevalent CTX-M-producing Escherichia coli: potential clinical implications of the revised CLSI interpretive criteria. Eur J Clin Microbiol Infect Dis. 2012;31(5):821–824. doi: 10.1007/s10096-011-1380-1. [DOI] [PubMed] [Google Scholar]
- 3.Morrissey I, Bouchillon SK, Hackel M, et al. Evaluation of the Clinical and Laboratory Standards Institute phenotypic confirmatory test to detect the presence of extended-spectrum β-lactamases from 4005 Escherichia coli, Klebsiella oxytoca, Klebsiella pneumoniae and Proteus mirabilis isolates. J Med Microbiol. 2014;63(Pt 4):556–561. doi: 10.1099/jmm.0.068981-0. [DOI] [PubMed] [Google Scholar]
- 4.Cheng WL, Hsueh PR, Lee CC, et al. Bacteremic pneumonia caused by extended spectrum beta-lactamase-producing Escherichia coli and Klebsiella pneumoniae: Appropriateness of empirical treatment matters. J Microbiol Immunol Infect. 2016;49(2):208–215. doi: 10.1016/j.jmii.2014.05.003. [DOI] [PubMed] [Google Scholar]
- 5.Vardakas KZ, Tansarli GS, Rafailidis PI, Falagas ME. Carbapenems versus alternative antibiotics for the treatment of bacteraemia due to Enterobacteriaceae producing extended-spectrum β-lactamases: a systematic review and meta-analysis. J Antimicrob Chemother. 2012;67(12):2793–2803. doi: 10.1093/jac/dks301. [DOI] [PubMed] [Google Scholar]
- 6.Nordmann P, Naas T, Poirel L. Global spread of Carbapenemase-producing Enterobacteriaceae. Emerg Infect Dis. 2011;17(10):1791–1798. doi: 10.3201/eid1710.110655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guh AY, Limbago BM, Kallen AJ. Epidemiology and prevention of carbapenem-resistant Enterobacteriaceae in the United States. Expert Rev Anti-Infect Ther. 2014;12(5):565–580. doi: 10.1586/14787210.2014.902306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee CH, Su LH, Chen FJ, et al. Comparative effectiveness of flomoxef versus carbapenems in the treatment of bacteraemia due to extended-spectrum β-lactamase-producing Escherichia coli or Klebsiella pneumoniae with emphasis on minimum inhibitory concentration of flomoxef: a retrospective study. Int J Antimicrob Agents. 2015;46(6):610–615. doi: 10.1016/j.ijantimicag.2015.07.020. [DOI] [PubMed] [Google Scholar]
- 9.Lee CH, Su LH, Tang YF, Liu JW. Treatment of ESBL-producing Klebsiella pneumoniae bacteraemia with carbapenems or flomoxef: a retrospective study and laboratory analysis of the isolates. J Antimicrob Chemother. 2006;58(5):1074–1077. doi: 10.1093/jac/dkl381. [DOI] [PubMed] [Google Scholar]
- 10.Paterson DL, Bonomo RA. Extended-spectrum beta-lactamases: a clinical update. Clin Microbiol Rev. 2005;18(4):657–686. doi: 10.1128/CMR.18.4.657-686.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grimm H. Interpretive criteria of antimicrobial disk susceptibility tests with flomoxef. Infection. 1991;19(Suppl 5):S258–S263. doi: 10.1007/BF01645537. [DOI] [PubMed] [Google Scholar]
- 12.Chen IL, Lee CH, Su LH, Tang YF, Chang SJ, Liu JW. Antibiotic consumption and healthcare-associated infections caused by multidrug-resistant gram-negative bacilli at a large medical center in Taiwan from 2002 to 2009: implicating the importance of antibiotic stewardship. PLoS One. 2013;8(5):e65621. doi: 10.1371/journal.pone.0065621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 14.Chow JW, Yu VL. Combination antibiotic therapy versus monotherapy for gram-negative bacteraemia: a commentary. Int J Antimicrob Agents. 1999;11(1):7–12. doi: 10.1016/s0924-8579(98)00060-0. [DOI] [PubMed] [Google Scholar]
- 15.Battle SE, Bookstaver PB, Justo JA, Kohn J, Albrecht H, Al-Hasan MN. Association between inappropriate empirical antimicrobial therapy and hospital length of stay in Gram-negative bloodstream infections: stratification by prognosis. J Antimicrob Chemother. 2017;72(1):299–304. doi: 10.1093/jac/dkw402. [DOI] [PubMed] [Google Scholar]
- 16.Retamar P, Portillo MM, López-Prieto MD, et al. SAEI/SAMPAC Bacteremia Group Impact of inadequate empirical therapy on the mortality of patients with bloodstream infections: a propensity score-based analysis. Antimicrob Agents Chemother. 2012;56(1):472–478. doi: 10.1128/AAC.00462-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gilbert ND, Moellering RC, Jr, Eliopoulos GM, Chambers HF, Saag MS. The Sanford Guide to Antimicrobial Therapy. Sperryville, VA: Antimicrobial Therapy; 2009. Selected pharmacologic features of antimicrobial agents; pp. 78–82. [Google Scholar]
- 18.Brun-Buisson C, Doyon F, Carlet J, et al. Incidence, risk factors, and outcome of severe sepsis and septic shock in adults. A multicenter prospective study in intensive care units. French ICU Group for Severe Sepsis. JAMA. 1995;274(12):968–974. [PubMed] [Google Scholar]
- 19.Matsumura Y, Yamamoto M, Nagao M, et al. Multicenter retrospective study of cefmetazole and flomoxef for treatment of extended-spectrum-β-lactamase-producing Escherichia coli bacteremia. Antimicrob Agents Chemother. 2015;59(9):5107–5113. doi: 10.1128/AAC.00701-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Craig WA. Interrelationship between pharmacokinetics and pharmacodynamics in determining dosage regimens for broad-spectrum cephalosporins. Diagn Microbiol Infect Dis. 1995;22(1–2):89–96. doi: 10.1016/0732-8893(95)00053-d. [DOI] [PubMed] [Google Scholar]
- 21.Zhanel GG, Denisuik A, Vashisht S, Yachison C, Adam HJ, Hoban DJ. Pharmacodynamic activity of ertapenem versus genotypically characterized extended-spectrum β-lactamase (ESBL)-, KPC- or NDM-producing Escherichia coli with reduced susceptibility or resistance to ertapenem using an in vitro model. J Antimicrob Chemother. 2014;69(9):2448–2452. doi: 10.1093/jac/dku149. [DOI] [PubMed] [Google Scholar]
- 22.Ito A, Tatsumi YM, Wajima T, Nakamura R, Tsuji M. Evaluation of antibacterial activities of flomoxef against ESBL producing Enterobacteriaceae analyzed by Monte Carlo simulation. Jpn J Antibiot. 2013;66(2):71–86. [PubMed] [Google Scholar]
- 23.Lee CH, Su LH, Chen FJ, Tang YF, Chien CC, Liu JW. Clinical and microbiologic characteristics of adult patients with recurrent bacteraemia caused by extended-spectrum β-lactamase-producing Escherichia coli or Klebsiella pneumoniae. Clin Microbiol Infect. 2015;21(12):1105.e1–e8. doi: 10.1016/j.cmi.2015.07.025. [DOI] [PubMed] [Google Scholar]
- 24.Fukuchi T, Iwata K, Kobayashi S, Nakamura T, Ohji G. Cefmetazole for bacteremia caused by ESBL-producing Enterobacteriaceae comparing with carbapenems. BMC Infect Dis. 2016;16(1):427. doi: 10.1186/s12879-016-1770-1. [DOI] [PMC free article] [PubMed] [Google Scholar]