ABSTRACT
Invasive candidiasis presents an emerging global public health challenge due to the emergence of resistance to the frontline treatment options, such as fluconazole. Hence, the identification of other compounds capable of pairing with fluconazole and averting azole resistance would potentially prolong the clinical utility of this important group. In an effort to repurpose drugs in the field of antifungal drug discovery, we explored sulfa antibacterial drugs for the purpose of reversing azole resistance in Candida. In this study, we assembled and investigated a library of 21 sulfa antibacterial drugs for their ability to restore fluconazole sensitivity in Candida albicans. Surprisingly, the majority of assayed sulfa drugs (15 of 21) were found to exhibit synergistic relationships with fluconazole by checkerboard assay with fractional inhibitory concentration index (ΣFIC) values ranging from <0.0312 to 0.25. Remarkably, five sulfa drugs were able to reverse azole resistance in a clinically achievable range. The structure-activity relationships (SARs) of the amino benzene sulfonamide scaffold as antifungal agents were studied. We also identified the possible mechanism of the synergistic interaction of sulfa antibacterial drugs with azole antifungal drugs. Furthermore, the ability of sulfa antibacterial drugs to inhibit Candida biofilm by 40% in vitro was confirmed. In addition, the effects of sulfa-fluconazole combinations on Candida growth kinetics and efflux machinery were explored. Finally, using a Caenorhabditis elegans infection model, we demonstrated that the sulfa-fluconazole combination does possess potent antifungal activity in vivo, reducing Candida in infected worms by ∼50% compared to the control.
KEYWORDS: Candida albicans, antifungal, azole resistance, fluconazole resistance, sulfa antibacterial, biofilm, Caenorhabditis elegans
INTRODUCTION
Invasive candidiasis remains the single most important cause of fungal bloodstream infections and the fourth leading cause of nosocomial bloodstream infections in the United States (1). The mortality rate of invasive candidiasis can reach more than 40%, even with the introduction of new antifungal therapies (2). Moreover, the development of new antifungal drugs is currently unable to keep pace with the urgent demand for safe and effective treatment options. Collectively, this points to an urgent need for different strategies to develop antifungal drugs to deal with this emerging scourge.
The Infectious Diseases Society of America has adopted fluconazole as a primary drug of choice for controlling and treating invasive candidiasis (3). Fluconazole has several advantages over other antifungal drugs in terms of the cost, safety, oral bioavailability, and ability to cross the blood-brain barrier (4, 5). However, the extensive use of fluconazole has increased the incidence of resistance to the drug among different fungal strains, especially Candida albicans (6–8). Unfortunately, fluconazole resistance has the potential to cross over to other azole drugs, such as itraconazole and voriconazole (9).
Several studies (10–13) have reported the ability of some drugs and compounds to reverse azole resistance in C. albicans. Unfortunately, the concentrations required for the majority of these drugs to suppress the azole resistance are generally above their clinically achievable concentration. In addition, some of these drugs can result in serious side effects, such as those caused by tacrolimus and cyclosporine (14).
Interestingly, a few reports (15–18) have superficially outlined the ability of some sulfa antibacterial drugs to act synergistically with different antifungals. For example, sulfamethoxazole was found to exhibit synergistic activity with different azole antifungal drugs, such as miconazole, ketoconazole, and clotrimazole, against C. albicans. Also, sulfamethoxazole showed a synergistic interaction with caspofungin against different Aspergillus species, such as Aspergillus fumigatus and Aspergillus niger.
Sulfa antibacterial drugs were the first systemic antimicrobial agents to be discovered and have been used extensively for several decades in human and veterinary medicine to treat bacterial infections (19). Sulfa drugs exert their antibacterial action by inhibiting the folate pathway through a competitive antagonism to the dihydropteroate synthase enzyme (DHPS), which is required for the conversion of para-amino benzoic acid (PABA) into dihydrofolate (20). In fungi, sulfa drugs and dihydrofolate reductase (DHFR) inhibitors, such as methotrexate and pyrimethamine, have been reported to inhibit the folate pathway by the same mechanism, albeit very high concentrations are required to inhibit the growth (21). For instance, sulfamethoxazole and trimethoprim have been shown to have antimycotic activity against A. fumigatus through inhibition of the folate pathway (22). Furthermore, interruption of the folate pathway in C. albicans was found to inhibit ergosterol biosynthesis, which could explain the synergistic activity of folate inhibitors and azole antifungal agents (23, 24).
In an effort to repurpose drugs and explore new leads in the field of antifungal drug discovery, we explored sulfa antibacterial drugs for the purpose of reversing azole resistance in Candida. In this study, we investigated a library of 21 sulfa antibacterial drugs for their ability to restore fluconazole sensitivity in C. albicans.
RESULTS
Antifungal susceptibility testing and identification of azole-resistant Candida strains.
The MICs of fluconazole, itraconazole, voriconazole, amphotericin B, and 5-fluorocytosine were examined against a panel of 53 C. albicans strains. We identified 10 strains as azole resistant (i.e., resistant to fluconazole, itraconazole, and voriconazole) (Table 1). These 10 azole-resistant strains were selected for further studies.
TABLE 1.
Synergistic activity of combinations of fluconazole and sulfonamide antibacterial drugs against different resistant strains of Candida albicans isolates
| Sulfa drug | MIC50 (μg/ml)a |
FIC index | Interpretationc | |||
|---|---|---|---|---|---|---|
| FLC |
Sulfa drug |
|||||
| Alone | In combination (MICc)b | Alone | In combination (MICc) | |||
| 4-Amino phenyl sulfone (dapsone) | 512 | 1 | 512 | 32 | 0.0625 | SYN |
| Sulfadiazine | 512 | 0.5 | 512 | 32 | 0.0625 | SYN |
| Sulfamethazine | 1,024 | 1 | 1024 | 128 | 0.125 | SYN |
| Sulfamethoxazole | 512 | 1 | 512 | 16 | 0.0312 | SYN |
| Sulfanilamide | >1,024 | >128 | >1,024 | >1,024 | 2 | IND |
| Sulfacetamide | >1,024 | >128 | >1,024 | >1,024 | 2 | IND |
| Sulfabenzimide | >1,024 | >128 | >1,024 | >1,024 | 2 | IND |
| Sulfadoxine | 512 | 1 | 512 | 64 | 0.125 | SYN |
| Sulfanitran | >1,024 | >128 | >1,024 | >1,024 | 2 | IND |
| Sulfamerazine | 1,024 | 0.5 | 1,024 | 128 | 0.125 | SYN |
| Sulfamonomethoxine | 512 | 0.5 | 512 | 16 | 0.0312 | SYN |
| Sulfadimethoxine | 1,024 | 0.5 | 1,024 | 64 | 0.0625 | SYN |
| Succinylsulfathiazole | >1,024 | >128 | >1,024 | >1,024 | 2 | IND |
| Sulfathiazole | 1,024 | 0.5 | 1,024 | 128 | 0.125 | SYN |
| Sulfaguanidine | >1,024 | >128 | >1,024 | >1,024 | 2 | IND |
| Sulfapyridine | 512 | 1 | 512 | 64 | 0.125 | SYN |
| Sulfametopyrazine (sulfalene) | 1,024 | 0.5 | 1,024 | 64 | 0.0625 | SYN |
| Sulfametoxydiazine (sulfameter) | 512 | 0.5 | 512 | 64 | 0.125 | SYN |
| Sulfisomidine | 1,024 | 0.5 | 1,024 | 128 | 0.125 | SYN |
| Sulfisoxazole (sulfafurazole) | 1,024 | 1 | 1,024 | 128 | 0.125 | SYN |
| Sulfamethoxypyridazine | 512 | 1 | 512 | 32 | 0.0625 | SYN |
MIC50 is the lowest concentration of the drug that showed at least 80% reduction of the growth of 50% of the tested Candida strains.
MICc is the MIC of the drug when used in combination with fluconazole.
FIC index interpretations: ≤0.5, synergism (SYN); >0.5 to 4, indifferent (IND); >4, antagonism.
Assembly of the sulfa antibacterial drugs and testing of their antifungal activity.
The library of 21 sulfa antibacterial drugs were initially screened at a single concentration of 1,024 μg/ml against C. albicans strains to identify any possible antifungal activity. Eight sulfa antibacterials showed weak antifungal activity at the high concentration of 512 μg/ml (Table 1). The rest of the drugs did not show any antifungal activity up to 1,024 μg/ml.
Sulfa drugs exert potent synergistic activity with fluconazole (checkerboard assay).
We assessed the activity of sulfa drugs in combination with fluconazole against azole-resistant Candida strains using the checkerboard assay (25–28). As shown in Table 1, 15 sulfa drugs were found to exhibit synergistic relationships with fluconazole against 7 of the 10 Candida strains tested with fractional inhibitory concentration (ΣFIC) values ranging from <0.0312 to <0.25. Sulfamethoxazole and sulfamonomethoxine were found to have the most potent synergistic activities. At 1/32× MIC for sulfa, we observed a >128-fold reduction in the MIC for fluconazole (when combined with sulfamethoxazole or sulfamonomethoxine) for all seven clinical Candida strains in which synergy was detected (data not presented). Six drugs showed an additive effect (indifference) with an FIC index (FICI) of >0.5 to 4. Interestingly, five drugs, i.e., sulfamethoxazole (SMX), sulfadiazine (SDZ), sulfadoxine (SDX), sulfadimethoxine (SDM), and sulfamethoxypyridazine (SMP), reversed azole resistance in a clinically achievable range (MIC when combined with fluconazole [MICc] values were below their achievable therapeutic concentrations in plasma).
Growth kinetics of sulfa antibacterial drugs.
After confirming that the sulfa antibacterial drugs showed synergistic relationship with fluconazole against azole-resistant Candida, we next assessed the growth kinetics of C. albicans strain NR 29448 when exposed to the sulfa drugs that showed synergistic activity with fluconazole. As shown in Fig. 1, the combination of sulfamethoxazole (16 μg/ml) and fluconazole (1 μg/ml) significantly inhibited the growth of C. albicans NR 29448. However, neither sulfamethoxazole (256 μg/ml) nor fluconazole (32 μg/ml) was solely able to inhibit the fungal growth at such high concentrations. Similar growth kinetics were observed for all other active sulfa drugs (data not shown).
FIG 1.
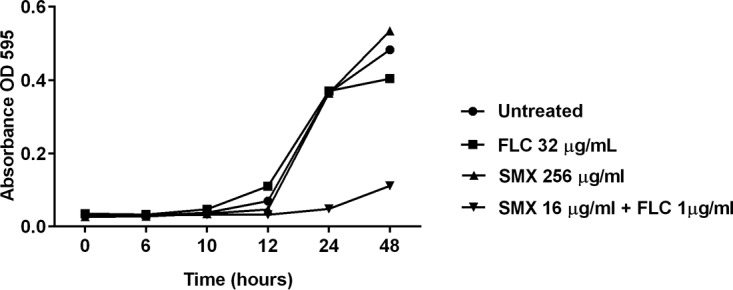
Growth kinetics of C. albicans (NR 29448) when exposed to sulfamethoxazole-fluconazole combination. An overnight culture of the yeast cells was diluted to 1 × 103 CFU/ml in RPMI 1640 medium. Cells were treated with 256 μg/ml sulfamethoxazole (SMX), 32 μg/ml fluconazole (FLC), or a combination of the two drugs at their MICc (16 μg/ml SMX and 1 μg/ml FLC). Cells were grown in the assay medium for 48 h, and OD595 values were measured at different time points (0, 6, 10, 12, 24, and 48 h) and plotted against the values for control (untreated) cells.
Sulfa drug-fluconazole combinations do inhibit biofilm formation.
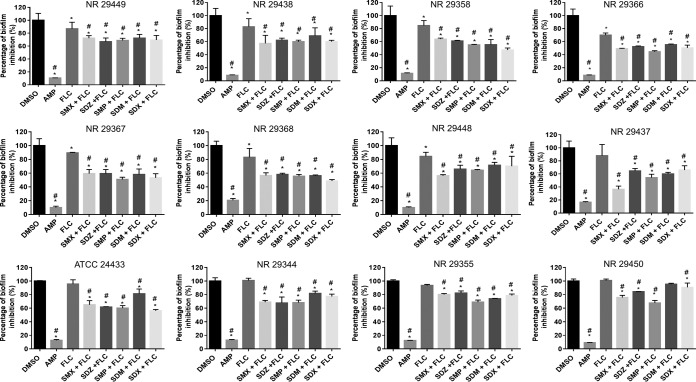
We examined whether the potential therapeutic application of sulfa drugs could be expanded beyond just inhibition of planktonic Candida. As presented in Fig. 2, sulfa drugs at subinhibitory concentrations (0.125× MIC) resulted in a significant reduction (∼40%) of the biofilm-forming ability of 12 C. albicans strains when combined with fluconazole (0.0625 μg/ml).
FIG 2.
Effects of sulfa drug-fluconazole combinations on the biofilm-forming ability of 12 C. albicans clinical isolates. Fresh overnight cultures of C. albicans strains were back diluted 1:100 in RPMI 1640 medium, treated with different combinations of fluconazole (0.0625 μg/ml) and the indicated sulfa drugs (0.125× MIC), and then incubated for 24 h. Amphotericin B, fluconazole, and DMSO treatments served as controls. After 24 h, biofilms were stained by crystal violet and then destained with absolute ethanol. The absorbance of crystal violet at OD595 was measured and compared to that of the controls. *, significant difference from untreated; #, significant difference from fluconazole.
Sulfa drug-fluconazole combinations reduced the fungal burden of infected Caenorhabditis elegans.
In order to validate the in vitro results, the temperature-sensitive sterile mutant C. elegans strain AU37, genotype [glp-4(bn2) I; sek-1(km4) X] was utilized as a whole-animal model to assess the ability of the sulfa drugs to reverse azole resistance in a living system. In comparison to fluconazole alone, sulfa drug-fluconazole combinations produced a significant reduction (∼50%) in the mean fungal burden (Fig. 3). This result was comparable to the effect of the antifungal drug 5-fluorocytosine.
FIG 3.
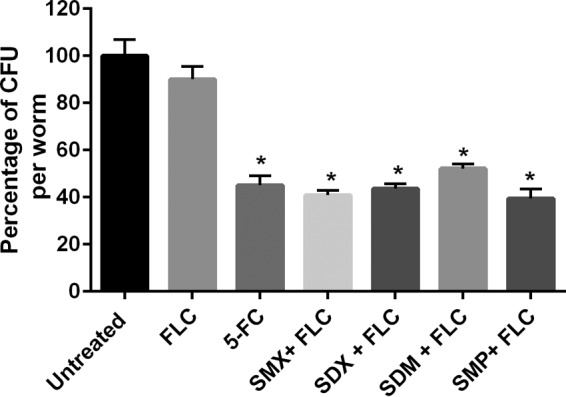
In vivo activity of sulfa drug-fluconazole combinations on C. elegans infected with C. albicans (NR 29448). Synchronized C. elegans worms, strain AU37 genotype [glp-4(bn2) I; sek-1(km4) X], were infected with C. albicans strain NR 29448 for 3 h and then treated with the indicated drugs. Sulfa drugs were used at 10× MICc in combination with fluconazole (10 μg/ml). 5-Fluorocytosine (5-FC) was used as a positive control. After 24 h of treatment, the worms were washed out and disrupted using silica carbide beads, and then the CFU/worm were counted on YPD plates containing streptomycin-penicillin.
Fungal efflux machinery is not inhibited by sulfa drugs.
To test whether the sulfa drugs would have any effect on efflux of drugs in Candida, we assessed the effect of sulfa on rhodamine 123 (Rh123) efflux. Under our experimental conditions, sulfa drugs did not inhibit the efflux pump machinery expressed by C. albicans NR 29448 as indicated by intracellular accumulation of rhodamine dye (Fig. 4). On the other hand, cyclosporine significantly increased the accumulation of intracellular rhodamine dye, in accordance with previous reports (11, 29, 30).
FIG 4.
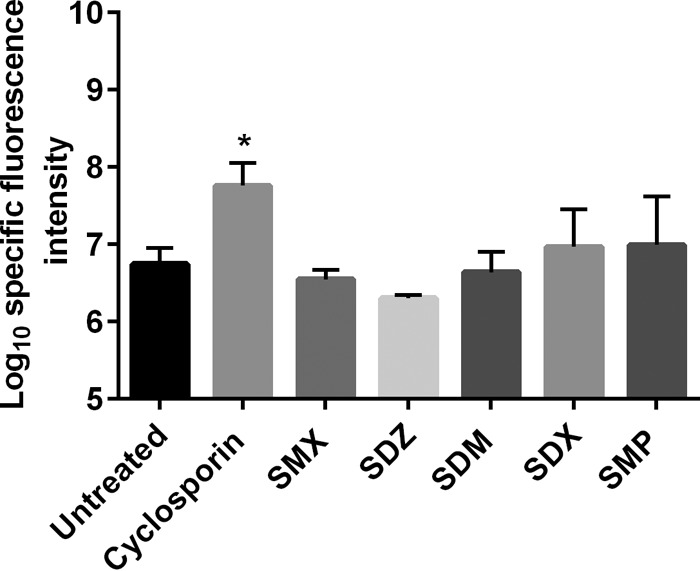
Effects of different sulfa antibacterial drugs on intracellular accumulation of rhodamine 123 by C. albicans (NR 29448). Deenergized pretreated Candida cells with subinhibitory concentrations of the indicated sulfa drugs (128 μg/ml) were loaded with 10 μM rhodamine for 30 min at 35°C. Then, the cells were washed thrice to remove extracellular dye. Pellets were resuspended in PBS, and fluorescence at excitation and emission wavelengths of 485 and 538 nm, respectively, was recorded. Cyclosporine was used as a positive control.
PABA supplementation inhibits the synergistic activity of sulfa drugs.
We asked whether the ability of sulfa antibacterial drugs to reverse azole resistance is due to their ability to disrupt the folate pathway and the related ergosterol biosynthesis in Candida (23, 24). This was tested via supplementation of different concentrations of PABA and calculation of FIC indices. Supplementation of PABA restored Candida growth and reversed the inhibition caused by sulfa drug-fluconazole combinations in a concentration-dependent manner (Table 2). The FIC indices increased up to 64-fold with all sulfa drugs, losing their synergistic activity with fluconazole (except for sulfamonomethoxine).
TABLE 2.
Effects of PABA on the FIC indices of the sulfa drug-fluconazole combinations
| Sulfa drug-fluconazole combination | FIC index under PABA concn (μg/ml) of: |
|||||||
|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 4 | 8 | 16 | 32 | 64 | 128 | |
| 4-Amino phenyl sulfone (dapsone) | 0.0625 | 0.125 | 0.25 | 0.5 | 0.5 | 2 | 2 | >2 |
| Sulfadiazine | 0.0625 | 0.125 | 0.5 | 0.5 | 0.5 | >2 | >2 | >2 |
| Sulfamethazine | 0.125 | 0.5 | 1 | 1 | 2 | 2 | >2 | >2 |
| Sulfamethoxazole | 0.0312 | 0.0312 | 0.0625 | 0.0625 | 0.125 | 0.25 | 2 | 2 |
| Sulfadoxine | 0.125 | 0.125 | 0.25 | 0.25 | 0.5 | 2 | 2 | >2 |
| Sulfamerazine | 0.125 | 0.125 | 0.25 | 0.25 | 0.5 | 2 | >2 | >2 |
| Sulfamonomethoxine | 0.0312 | 0.0625 | 0.125 | 0.125 | 0.125 | 0.25 | 0.5 | 0.5 |
| Sulfadimethoxine | 0.0625 | 0.125 | 0.25 | 0.25 | 0.25 | >2 | >2 | >2 |
| Sulfathiazole | 0.125 | 0.25 | 0.5 | 0.5 | 0.5 | 2 | >2 | >2 |
| Sulfapyridine | 0.25 | 0.5 | 2 | 2 | 2 | 2 | 2 | >2 |
| Sulfametopyrazine (sulfalene) | 0.0625 | 0.0625 | 0.125 | 0.125 | 0.125 | 0.5 | 0.5 | 2 |
| Sulfametoxydiazine (sulfameter) | 0.125 | 0.125 | 0.25 | 0.25 | 0.5 | 2 | >2 | >2 |
| Sulfisomidine | 0.125 | 0.5 | 2 | 2 | 2 | >2 | >2 | >2 |
| Sulfisoxazole (sulfafurazole) | 0.125 | 0.25 | 0.25 | 0.5 | 0.5 | 2 | >2 | >2 |
| Sulfamethoxypyridazine | 0.0625 | 0.125 | 0.25 | 0.25 | 0.5 | 2 | >2 | >2 |
DISCUSSION
Fifteen sulfa drugs were found to exhibit a synergistic relationship with fluconazole against azole-resistant Candida strains, with ΣFIC values ranging from <0.0312 to 0.25. Interestingly, of the 15 drugs with synergistic activity, 5 sulfa drugs (sulfamethoxazole, sulfadiazine, sulfadoxine, sulfadimethoxine, and sulfamethoxypyridazine) showed great promise and were able to reverse azole resistance in Candida in vitro at a clinically applied concentration. The significance of this finding cannot be overstated because of its potential in clinical applications.
We were curious to determine if the synergistic relationship observed between sulfa drugs and fluconazole was limited to fluconazole only or if it could be applied to other azole antifungals drugs too, such as itraconazole and voriconazole. Itraconazole and voriconazole were used to further explore the partnership between sulfa drugs and azole antifungal agents. As expected, sulfamethoxazole at 16 μg/ml was able to reverse resistance to both itraconazole and voriconazole in Candida (data not shown).
We then evaluated the structure-activity relationships (SARs) of the amino benzene sulfonamide scaffold as antifungal agents. In some respects, the SAR was similar to the antibacterial activity of sulfa drugs, whereby all sulfa prodrugs (derivatives with substitutions at the aniline amino group), such as succinylsulfathiazole, are completely inactive in the in vitro assay (31). In contrast, unlike the case with antibacterial activity, the fluconazole-antifungal synergistic activity is highly correlated with the N1 substitution. The simplest unsubstituted derivative, sulfanilamide, does not work synergistically with fluconazole and has to have an aromatic system substituted to synergize the antifungal activity of fluconazole. Furthermore, drugs with N1 aliphatic derivatives, for example, N1-imidinyl and acetyl derivatives (sulfaguanidine and sulfacetamide), lose their synergistic antifungal activity with fluconazole.
On the other hand, aromatic substitution at the N1 position appears to be a requirement for sulfa drugs to exert synergistic activity with fluconazole. We noticed that the 2-pyrinyl and 2-oxazolyl derivatives (sulfapyridine and sulfathiazole) demonstrated potent synergistic activity with fluconazole (FIC indices of 0.25 and 0.125, respectively). In addition, isoxazole and 4-pyrimidine were among the most-active drugs (sulfamethoxazole and sulfamonomethoxine), with an FIC index of 0.0312. Thus, we concluded that the aromatic ring is critical to the synergistic activity of sulfa drugs with fluconazole. In addition, the electron properties of substituents on the N1 ring play a pivotal role in this synergistic activity. Using the example of pyrimidine-containing sulfa drugs, it was observed that a methoxy group at pyrimidine position 6 (sulfamonomethoxine) demonstrated the best synergistic antifungal activity. Adding a second methoxy group at position 5 (sulfadoxine) led to a less potent compound. In the same vein, the replacement of 5,6-dimethoxy groups with more-lipophilic 2,6-dimethyl moieties resulted in sulfisomidine with remarkably decreased antifungal synergistic activity. The same observation was made with sulfisoxazole, where two methyl groups are at isoxazole positions 3 and 4. Sulfisoxazole has a weaker antifungal synergistic activity than that of its 6-methylisoxazole analogue (sulfamethoxazole), which demonstrated the best synergistic antifungal potency. The SAR information presented here will prove critical to the medicinal chemistry community in relation to the development of new sulfa antifungal analogues.
Fungal cells present within biofilms are more resistant to antifungal agents than planktonic cells (32). Thus, there is an unmet need to identify and develop new agents to attack fungal biofilm and circumvent increasing public health concerns about antifungal resistance. To determine whether or not the potential therapeutic application of sulfa drugs could be expanded beyond merely inhibiting planktonic Candida, the ability of a sulfa drug-fluconazole combination to inhibit biofilm was evaluated. This combination proved to be far superior to fluconazole alone, significantly inhibiting biofilm formation in C. albicans (Fig. 2). The biofilm-forming ability of six strains was significantly reduced (∼15%) by fluconazole alone (Fig. 2). The ability of fluconazole to inhibit biofilm formation in fluconazole-resistant Candida is in agreement with previous reports (33). In addition, there was a significant difference in biofilm-forming ability in these six strains between the sulfa drug-fluconazole combination group and fluconazole alone (Fig. 2).
The finding that the sulfa drug-fluconazole combinations have synergistic activity in vitro against numerous resistant Candida strains and inhibit biofilm formation prompted us to investigate the efficacy of this combination in vivo in a C. elegans animal model. Utilizing the C. elegans sensitive strain AU37, which is more sensitive to the effects of pathogens than other strains (34), we confirmed that the sulfa drug-fluconazole combination does possess potent antifungal activity in vivo. As shown in Fig. 3, the sulfa drug-fluconazole combination reduced the presence of Candida in infected worms by ∼50%.
The activity of most azole resensitizing drugs has been attributed mainly to their ability to inhibit the overexpression of efflux pumps in Candida (35–37). Hence, we turned our attention to studying the effects of sulfa drugs on the efflux pump in Candida using a rhodamine 123 accumulation assay (27). The sulfa drugs did not affect the accumulation of rhodamine dye inside the Candida cells and therefore did not result in efflux pump inhibition (Fig. 4). This is in agreement with previously reported findings whereby sulfa drugs, such as sulfamethoxazole, had no effect on multiple transport-related genes (38). The results of our accumulation assay were confirmed using additional dyes, namely, Nile red and calcein AM (data not shown).
After confirming that sulfa drugs did not result in the inhibition of the Candida efflux pump, we were curious to explore the potential mechanism for the synergistic relationship between sulfa drugs and fluconazole. We hypothesized that the inhibition of the fungal dihydropteroate synthase (DHPS) enzyme by sulfa drugs would lead to restriction of the Candida ergosterol biosynthesis pathway, resulting in synergy with the azole drugs (23, 24). To test this hypothesis, we relied on the premise that the mode of antibacterial action by sulfa drugs depends on structural similarity between the sulfa drugs and para-amino benzoic acid (PABA). Hence, the sulfa drugs act as competitive inhibitors of the DHPS enzyme, preventing folic acid synthesis (39). PABA supplementation restored Candida growth and reversed the inhibition caused by sulfa drugs in a concentration-dependent manner (Table 2). Interestingly, the resensitization activity of sulfa drugs is medium dependent. Strong synergetic activity with fluconazole was observed in chemically defined media, such as RPMI 1640 medium, and weaker synergetic activity was observed in complex media, such as yeast extract-peptone-dextrose (YPD) medium. This difference can be attributed to the high content of PABA and folic acid precursors in the complex media. A similar observation about the effect of the medium was reported in a similar study in which the activity of sulfamethoxazole against A. fumigatus was evaluated (22). The results of this study provide critical information that will facilitate development and testing of novel sulfa drugs with potential antifungal activity.
MATERIALS AND METHODS
Chemicals and reagents.
RPMI 1640 broth powder with glutamine but without NaHCO3 was purchased from Thermo Fisher Scientific (Waltham, MA), and yeast extract-peptone-dextrose (YPD) broth medium and agar were obtained from BD (Franklin Lakes, NJ). Fluconazole, rhodamine 123, sulfaguanidine, and 4-aminophenyl sulfone (dapsone) were obtained from Fisher Scientific (Pittsburgh, PA). Itraconazole, voriconazole, Nile red, sulfamonomethoxine, sulfathiazole, 5-fluorocytosine, sulfamethoxypyridazine, sulfisomidine, sulfametopyrazine (sulfalene), sulfapyridine, and succinylsulfathiazole were obtained from TCI America (Portland, OR). Sulfabenzamide, sulfadiazine, sulfadimethoxine, sulfamethazine, sulfanitran, sulfanilamide, sulfisoxazole (sulfafurazole), sulfametoxydiazine (sulfameter), and 3-(N-morpholino) propanesulfonic acid (MOPS) were obtained from Sigma-Aldrich (St. Louis, MO). Sulfacetamide, sulfadoxine, and sulfamerazine were obtained from Alfa Aesar (Tewksbury, MA). FK 506 and cyclosporine were obtained from Biotang Inc., Lexington, MA. Sulfamethoxazole and amphotericin B were obtained from Chem-Impex International (Wood Dale, IL). Penicillin-streptomycin was obtained from Lonza (Walkersville, MD). Calcein AM was obtained from BD Biosciences (San Jose, CA).
Clinical isolates and antifungal susceptibility testing.
A total of 53 Candida albicans clinical isolates (Table 3) were screened against fluconazole, itraconazole, voriconazole, 5-fluorocytosine, and amphotericin B in accordance with the Clinical and Laboratory Standards Institute (CLSI) M27-A3 guidelines for yeast (40) to determine their MICs. All experiments were carried out in triplicates and repeated at least twice.
TABLE 3.
Strain data and susceptibility testing of Candida albicans isolates against standard antifungal agents 5-fluorocytosine (5-FC), fluconazole (FLC), itraconazole (ITC), voriconazole (VOC), and amphotericin B (AMP)
| Strain ID | Designation | Isolation source | Geographical region | MIC (μg/ml) |
||||
|---|---|---|---|---|---|---|---|---|
| 5-FC | FLC | ITC | VOC | AMP | ||||
| ATCC 14053 | NIH 3172 | Bloodstream isolate | Maryland, USA | 0.062 | 0.25 | 0.062 | 0.031 | 1 |
| ATCC 24433 | Wasson | Nail infection | NAa | 1 | 1 | 0.125 | 0.062 | 1 |
| ATCC 26790 | H-29 | Human bronchomycosis | NA | 0.062 | >64 | >16 | >16 | 1 |
| ATCC 10231 | 3147 | Human bronchomycosis | NA | 0.125 | 0.5 | 0.5 | 0.5 | 0.5 |
| ATCC 64124 | Darlington | Oral isolate | NA | 0.5 | >64 | >16 | 8 | 0.5 |
| ATCC 90028 | NCCLS 11 | Bloodstream isolate | Iowa, USA | 1 | 1 | 0.031 | 0.25 | 1 |
| ATCC 90029 | NCCLS 67 | Bloodstream isolate | Iowa, USA | >128 | 0.5 | 0.25 | 0.031 | 0.5 |
| ATCC MYA573 | M4 | Bloodstream isolate | Germany | 0.125 | >64 | 0.5 | 0.25 | 0.5 |
| NR 29339 | 23R | Human isolate | China | 0.062 | 1 | 0.125 | 0.125 | 0.5 |
| NR 29340 | 23B | Human isolate | China | 0.125 | 0.25 | 0.062 | 0.031 | 1 |
| NR 29341 | 23Q | Human isolate | China | 0.125 | 0.25 | 0.062 | 0.031 | 1 |
| NR 29342 | 23P | Human isolate | China | 0.125 | 0.125 | 0.062 | 0.031 | 1 |
| NR 29343 | 25N | Human isolate | China | 0.062 | 0.25 | 0.062 | 0.031 | 1 |
| NR 29344 | 24A | Human isolate | China | 0.062 | 0.25 | 0.062 | 0.031 | 1 |
| NR 29345 | 24C | Human isolate | China | 0.062 | 0.5 | 0.25 | 0.125 | 1 |
| NR 29346 | 11E | Human isolate | China | 0.125 | 2 | 0.25 | 0.5 | 1 |
| NR 29347 | 24F | Human isolate | China | 0.062 | 1 | 0.125 | 0.5 | 0.5 |
| NR 29348 | 23C | Human isolate | China | 0.062 | 1 | 0.0312 | 0.0312 | 0.5 |
| NR 29349 | 22F | Human isolate | China | 0.062 | 2 | 0.25 | 0.125 | 1 |
| NR 29350 | 23K | Human isolate | China | 0.125 | 0.125 | 0.0312 | 0.0312 | 1 |
| NR 29351 | 18 M | Human isolate | China | 0.25 | 0.125 | 0.0312 | 0.0312 | 1 |
| NR 29352 | 22C | Human isolate | China | 0.062 | 0.5 | 0.25 | 0.25 | 0.5 |
| NR 29353 | 18K | Human isolate | China | 0.25 | 0.125 | 0.0312 | 0.0312 | 1 |
| NR 29354 | 18E | Human isolate | China | 0.25 | 0.125 | 0.0312 | 0.0312 | 1 |
| NR 29355 | 24E | Human isolate | China | 0.062 | 1 | 0.0625 | 0.0625 | 1 |
| NR 29356 | 23G | Human isolate | China | 0.125 | 0.25 | 0.0625 | 0.0312 | 1 |
| NR 29357 | 25O | Human isolate | China | 0.062 | 0.25 | 0.0625 | 0.0312 | 1 |
| NR 29358 | 28H | Human isolate | China | 0.125 | 64 | >16 | >16 | 1 |
| NR 29359 | 25 M | Human isolate | China | 0.062 | 0.25 | 0.0625 | 0.0312 | 1 |
| NR 29360 | 22O | Human isolate | China | 0.062 | 0.5 | 0.0625 | 0.0625 | 1 |
| NR 29361 | 22K | Human isolate | China | 0.062 | 0.5 | 0.0625 | 0.25 | 1 |
| NR 29362 | 11C | Human isolate | China | 0.25 | 0.25 | 0.0625 | 0.0312 | 1 |
| NR 29363 | 18J | Human isolate | China | 0.25 | 0.125 | 0.0312 | 0.0312 | 1 |
| NR 29364 | 25P | Human isolate | China | 0.125 | 0.25 | 0.0625 | 0.0312 | 1 |
| NR 29365 | 23F | Human isolate | China | 0.062 | 0.25 | 0.0625 | 0.0312 | 0.5 |
| NR 29366 | 28I | Human isolate | China | 0.062 | >64 | >16 | >16 | 1 |
| NR 29367 | 28A | Human isolate | China | 0.062 | >64 | >16 | >16 | 1 |
| NR 29368 | 28C | Human isolate | China | 0.062 | >64 | >16 | >16 | 1 |
| NR 29434 | P78048 | Bloodstream isolate | Manitoba, Canada | 0.125 | 0.25 | 0.0625 | 0.0312 | 1 |
| NR 29435 | P57072 | Bloodstream isolate | Iowa, USA | 0.062 | 0.25 | 0.031 | 0.031 | 1 |
| NR 29436 | P34048 | Bloodstream isolate | Istanbul, Turkey | 0.062 | 0.25 | 0.031 | 0.031 | 1 |
| NR 29437 | P75010 | Bloodstream isolate | Brussels, Belgium | 0.062 | >64 | >16 | >16 | 1 |
| NR 29438 | P75016 | Bloodstream isolate | Tel-Hashomer, Israel | 0.062 | 0.25 | 0.031 | 0.031 | 1 |
| NR 29444 | 12C | Oral isolate | Michigan, USA | 1 | 0.25 | 0.0625 | 0.0312 | 1 |
| NR 29445 | L26 | Vaginal isolate | Iowa, USA | 2 | 1 | 0.0625 | 0.0625 | 1 |
| NR 29446 | P94015 | Bloodstream isolate | Utah, USA | 0.5 | >64 | 4 | 8 | 0.5 |
| NR 29447 | P37005 | Oral isolate | Florida, USA | 0.5 | 0.125 | 0.0625 | 0.0312 | 1 |
| NR 29448 | P60002 | Bloodstream isolate | Arizona, USA | 0.125 | >64 | >16 | >16 | 2 |
| NR 29449 | 19F | Vaginal isolate | Michigan, USA | >128 | 0.5 | 0.062 | <0.031 | 1 |
| NR 29450 | P37037 | Oral isolate | Wisconsin, USA | 0.5 | 8 | 0.0625 | 0.0312 | 2 |
| NR 29451 | P37039 | Oral isolate | New Jersey, USA | 0.125 | 8 | 0.125 | 0.5 | 1 |
| NR 29452 | GC75 | Oral isolate | Medunsa, South Africa | 0.062 | 0.25 | 0.125 | 0.0312 | 1 |
| NR 29453 | P87 | Oral isolate | Pretoria, South Africa | 0.062 | 0.125 | 0.062 | 0.031 | 0.5 |
NA, not available.
Identification of azole-resistant Candida strains.
Resistance to fluconazole, itraconazole, and voriconazole was identified by following the guidelines of the Clinical and Laboratory Standards Institute (40). Strains with MIC values of >32 μg/ml fluconazole, ≥1 μg/ml itraconazole, and ≥1 μg/ml voriconazole were considered azole resistant (R) (41, 42). Candida albicans ATCC 64124 was used as a reference strain for azole resistance, due to this strain's known genetic mutation in the azole target (Erg11) (43).
Assembly of the sulfa antibacterial drugs and testing of their antifungal activity.
A library of 21 FDA-approved sulfa antibacterial drugs (Table 4) were purchased from commercial sources, and the drugs were prepared as 10 mM stock solutions in dimethyl sulfoxide (DMSO). The MICs of the sulfa antibacterial drugs were determined against azole-resistant C. albicans strains according to the CLSI M27-A3 guidelines as described above (40).
TABLE 4.
Name, indication, and therapeutic concentration in plasma of different sulfa antibacterial drugsa
| Name of sulfa antibacterial drug | Route of administration and indication | Therapeutic concn in plasmab |
|---|---|---|
| 4,Amino phenyl sulfone (dapsone) | Oral treatment for leprosy and Pneumocystis pneumonia | 0.63–1.66 |
| Sulfadiazine | Oral treatment of UTI | 19–40 |
| Sulfamethazine | Potential treatment of Escherichia coli enteritis in veterinary medicine | 64.0–88.4 |
| Sulfamethoxazole | Oral treatment for UTI and prostatitis | 15–65 |
| Sulfanilamide | Topical treatment of vaginal yeast infections | NA |
| Sulfacetamide | Topical treatment of acne and eye infections | NA |
| Sulfabenzamide | Topical intravaginal antibacterial preparation | NA |
| Sulfadoxine | Oral treatment used in combination with pyrimethamine to treat or prevent malaria | 130–200 |
| Sulfanitran | Veterinary use, as an aid in the prevention of coccidiosis | NA |
| Sulfamerazine | In some countries, this medicine may be approved only for veterinary use, for example, in control of acute fowl cholera | NA |
| Sulfamonomethoxine | Used in veterinary medicine for the treatment of toxoplasmosis | NA |
| Sulfadimethoxine | Oral treatment of respiratory, urinary tract, enteric, and soft tissue infections | 120–180 |
| Succinylsulfathiazole | Treatment of intestinal amebiasis | 5% of a dose is absorbed after oral dose |
| Sulfathiazole | Oral and topical antimicrobial | NA |
| Sulfaguanidine | Treatment of bacillary dysentery | 15–40 |
| Sulfapyridine | Help in control of dermatitis herpetiformis (Duhring's disease) | 35–60 |
| Sulfametopyrazine (sulfalene) | Treatment of UTI and chronic bronchitis | 25–33 |
| Sulfametoxydiazine (sulfameter) | Long-acting sulfonamide antibacterial used as a leprostatic agent | 62–90 |
| Sulfisomidine | Treatment of pertussis | NA |
| Sulfisoxazole (sulfafurazole) | Treatment of bladder infections, ear infections, or meningitis | 127–210 |
| Sulfamethoxypyridazine | Treatment of vaginal irritation, severe acute thrush, and dermatitis herpetiformis, where it is an alternative therapy to dapsone | 110–180 |
UTI, urinary tract infection; NA, not available.
Values are in micrograms per milliliter unless otherwise indicated. Data are from reference 55.
Interaction of sulfa antibacterial drugs with fluconazole against azole-resistant Candida.
The interaction between sulfa antibacterial drugs and azole antifungals against azole-resistant C. albicans clinical isolates was investigated using the checkerboard assay, and the fractional inhibitory concentration index (FICI) was calculated as described previously (25, 26, 44–46). An FIC index of ≤0.5 is considered synergism (SYN); an FIC index of >4 is considered antagonism; and a result of >0.5 to 4 is considered indifferent (IND).
Growth kinetics of sulfa antibacterial drugs.
C. albicans strain NR 29448 was used in this experiment because it exhibited rapid resistance (∼16 h) to all azole antifungal drugs used in this study. All sulfa drugs that showed synergetic activity with fluconazole were further studied in a growth kinetic curve to confirm their ability to reverse azole resistance (47). Briefly, an overnight culture of C. albicans strain NR 29448 was adjusted to 2.5 × 103 CFU/ml in RPMI 1640 medium. Then, each drug at its MICc (MIC when combined with fluconazole) was incubated with C. albicans at 35°C, either alone or in combination with 1 μg/ml fluconazole. The growth kinetic was monitored at an optical density at 595 nm (OD595) at 0, 6, 10, 12, 24, and 48 h after incubation.
Effects of sulfa drug-fluconazole combinations on biofilm-forming ability of C. albicans.
Biofilm-forming C. albicans strains were used to study whether sulfa drug-fluconazole combinations can interfere with their abilities to form biofilms. Cells were prepared as previously described (48–50). Briefly, overnight cultures of 12 strains of C. albicans (Fig. 2) in YPD broth were diluted in RPMI 1640 medium to an inoculum size of 1 × 105 CFU/ml. Sulfa drugs, i.e., sulfamethoxazole, sulfadiazine, sulfadoxine, sulfadimethoxine, and sulfamethoxypyridazine, were added to the yeast cell suspension of C. albicans at concentrations of 0.5×, 0.25×, and 0.125× MIC in the presence of a fixed concentration of fluconazole (0.0625 μg/ml). Amphotericin B, at concentrations of 0.5×, 0.25×, and 0.125 × MIC, was used as a positive control. Candida cells were then transferred to the wells of microtiter plates, and the plates were incubated at 35°C for 24 h. The formed biofilms were rinsed twice with phosphate-buffered saline (PBS) and then left to air dry at room temperature. Air-dried biofilms were stained for 10 min with 200 μl crystal violet (0.1%). Stained biofilms were rinsed three times with PBS and air dried for 1 h. The amount of crystal violet was quantitated by destaining the biofilms for 10 min with 200 μl of absolute ethanol, and then the absorbance of the crystal violet solution at OD595 was measured. All experiments were carried out in quadruplicates and repeated at least twice.
Caenorhabditis elegans infection study.
To examine the efficacy of sulfa drugs in reversing azole resistance in vivo, we used the C. elegans animal model (51–54). The biofilm-forming strain NR 29448 was found to colonize the worms effectively, so we used it in this experiment. Briefly, L4 stage worms [strain AU37 genotype glp-4(bn2) I; sek-1(km4) X] were infected with C. albicans NR 29448 for 3 h at room temperature. After infection, worms were washed five times with M9 buffer and transferred into tubes (∼20 worms per tube). Worms were treated for 24 h (in triplicate) with a combination of 10× MICc sulfa drugs and 10 μg/ml fluconazole (SDZ was insoluble at 10× MICc and was excluded from this experiment). DMSO, 5-fluorocytosine at 10× MIC (0.625 μg/ml), and fluconazole (10 μg/ml) served as controls. Posttreatment, worms were examined microscopically to evaluate morphological changes and ensure viability, after which they were washed with M9 five times and then disrupted using silicon carbide particles, and the resulting suspensions were serially diluted and transferred to YPD agar plates containing penicillin (100 μg/ml) and streptomycin (100 μg/ml). Plates were incubated for 48 h at 35°C before the viable Candida CFU per worm was determined.
Effects of sulfa drugs on efflux of rhodamine.
To test if sulfa drugs have inhibitory effects on the efflux pump in Candida, a rhodamine accumulation assay was performed (27). Briefly, C. albicans strain NR 29448 was grown overnight at 35°C in YPD broth and then transferred to a fresh YPD broth and incubated at 35°C for 4 h until the cells reached the mid-log phase. Cells were harvested and adjusted to 1 × 106 CFU/ml in YPD broth. Candida cells were then incubated with sulfa drugs at subinhibitory concentrations (0.25 × MIC) for 1 h. Then, rhodamine 123 dye (Rh123) at a final concentration of 10 μM was added to the cell suspension and incubated at 35°C in a reciprocating shaker for an additional 30 min. After incubation, 1-ml samples were taken and centrifuged at 5,000 × g for 5 min. Supernatants were discarded, and the pellets were washed three times with PBS to remove extracellular Rh123. Fluorescence at excitation and emission wavelengths of 485 and 538 nm, respectively, was recorded for 6 replicates for each drug using a Spectramax-ix3 microplate reader. Rh123 accumulations were expressed as arbitrary fluorescence per OD595 unit (specific fluorescence).
Effects of PABA supplementation on synergistic activity of sulfa antibacterial drugs and fluconazole.
To study the effects of para-amino benzoic acid (PABA) on the synergistic relationship between sulfa drugs and fluconazole, a checkerboard assay was performed as described above, in the presence and absence of different concentrations of PABA (1, 4, 8, 16, 32, 64, and 128 μg/ml). Then, FIC indices for each drug were calculated as described above.
Statistical analysis.
Data are presented as means ± standard deviations. Statistical analyses were performed using GraphPad Prism 6.0 (Graph Pad Software, La Jolla, CA, USA). P values were calculated using one-way analysis of variance (ANOVA). P values of <0.05 were considered significant.
ACKNOWLEDGMENTS
Research reported in this publication was supported by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health under award number 01AI130186 to M.N.S.
We thank BEI resources, NIAID, NIH, for providing the fungal strains used in this study.
REFERENCES
- 1.Diekema D, Arbefeville S, Boyken L, Kroeger J, Pfaller M. 2012. The changing epidemiology of healthcare-associated candidemia over three decades. Diagn Microbiol Infect Dis 73:45–48. doi: 10.1016/j.diagmicrobio.2012.02.001. [DOI] [PubMed] [Google Scholar]
- 2.Bassetti M, Merelli M, Righi E, Diaz-Martin A, Rosello EM, Luzzati R, Parra A, Trecarichi EM, Sanguinetti M, Posteraro B, Garnacho-Montero J, Sartor A, Rello J, Tumbarello M. 2013. Epidemiology, species distribution, antifungal susceptibility, and outcome of candidemia across five sites in Italy and Spain. J Clin Microbiol 51:4167–4172. doi: 10.1128/JCM.01998-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pappas PG, Kauffman CA, Andes DR, Clancy CJ, Marr KA, Ostrosky-Zeichner L, Reboli AC, Schuster MG, Vazquez JA, Walsh TJ, Zaoutis TE, Sobel JD. 2016. Clinical practice guideline for the management of candidiasis: 2016 update by the Infectious Diseases Society of America. Clin Infect Dis 62:e1–e50. doi: 10.1093/cid/civ1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Debruyne D. 1997. Clinical pharmacokinetics of fluconazole in superficial and systemic mycoses. Clin Pharmacokinet 33:52–77. doi: 10.2165/00003088-199733010-00005. [DOI] [PubMed] [Google Scholar]
- 5.Debruyne D, Ryckelynck JP. 1993. Clinical pharmacokinetics of fluconazole. Clin Pharmacokinet 24:10–27. doi: 10.2165/00003088-199324010-00002. [DOI] [PubMed] [Google Scholar]
- 6.Ghannoum MA, Rice LB. 1999. Antifungal agents: mode of action, mechanisms of resistance, and correlation of these mechanisms with bacterial resistance. Clin Microbiol Rev 12:501–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rex JH, Rinaldi MG, Pfaller MA. 1995. Resistance of Candida species to fluconazole. Antimicrob Agents Chemother 39:1–8. doi: 10.1128/AAC.39.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goncalves SS, Souza ACR, Chowdhary A, Meis JF, Colombo AL. 2016. Epidemiology and molecular mechanisms of antifungal resistance in Candida and Aspergillus. Mycoses 59:198–219. doi: 10.1111/myc.12469. [DOI] [PubMed] [Google Scholar]
- 9.Kanafani ZA, Perfect JR. 2008. Antimicrobial resistance: resistance to antifungal agents: mechanisms and clinical impact. Clin Infect Dis 46:120–128. doi: 10.1086/524071. [DOI] [PubMed] [Google Scholar]
- 10.Parkinson T, Falconer DJ, Hitchcock CA. 1995. Fluconazole resistance due to energy-dependent drug efflux in Candida glabrata. Antimicrob Agents Chemother 39:1696–1699. doi: 10.1128/AAC.39.8.1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schuetzer-Muehlbauer M, Willinger B, Egner R, Ecker G, Kuchler K. 2003. Reversal of antifungal resistance mediated by ABC efflux pumps from Candida albicans functionally expressed in yeast. Int J Antimicrob Agents 22:291–300. doi: 10.1016/S0924-8579(03)00213-9. [DOI] [PubMed] [Google Scholar]
- 12.Liu SY, Yue LT, Gu WR, Li XY, Zhang LP, Sun SJ. 2016. Synergistic effect of fluconazole and calcium channel blockers against resistant Candida albicans. PLoS One 11(3):e0150859. doi: 10.1371/journal.pone.0150859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen X, Ren BA, Chen M, Liu MX, Ren W, Wang QX, Zhang LX, Yan GY. 2014. ASDCD: antifungal synergistic drug combination database. PLoS One 9(1):e86499. doi: 10.1371/journal.pone.0086499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rodrigues-Diez R, Gonzalez-Guerrero C, Ocana-Salceda C, Rodrigues-Diez RR, Egido J, Ortiz A, Ruiz-Ortega M, Ramos AM. 2016. Calcineurin inhibitors cyclosporine A and tacrolimus induce vascular inflammation and endothelial activation through TLR4 signaling. Sci Rep 6:27915. doi: 10.1038/srep27915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Beggs WH, Sarosi GA, Steele NM. 1976. Synergistic action of clotrimazole and sulfamethoxazole on Candida albicans and related species. Curr Ther Res Clin E 20:623–629. [Google Scholar]
- 16.Beggs WH, Sarosi GA. 1977. Synergistic action of miconazole and sulfamethoxazole on strains of Candida albicans. Curr Ther Res Clin E 21:547–549. [Google Scholar]
- 17.Beggs WH. 1982. Combined activity of ketoconazole and sulfamethoxazole against Candida albicans. J Antimicrob Chemother 10:539–541. doi: 10.1093/jac/10.6.539. [DOI] [PubMed] [Google Scholar]
- 18.Yekutiel A, Shalit I, Shadkchan Y, Osherov N. 2004. In vitro activity of caspofungin combined with sulfamethoxazole against clinical isolates of Aspergillus spp. Antimicrob Agents Chemother 48:3279–3283. doi: 10.1128/AAC.48.9.3279-3283.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Aminov RI. 2010. A brief history of the antibiotic era: lessons learned and challenges for the future. Front Microbiol 1:134. doi: 10.3389/fmicb.2010.00134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bermingham A, Derrick JP. 2002. The folic acid biosynthesis pathway in bacteria: evaluation of potential for antibacterial drug discovery. Bioessays 24:637–648. doi: 10.1002/bies.10114. [DOI] [PubMed] [Google Scholar]
- 21.Bush K, Freudenberger JS, Slusarchyk DS, Sykes RB, Meyers E. 1982. Activity of sulfa drugs and dihydrofolate reductase inhibitors against Candida albicans. Experientia 38:436–437. doi: 10.1007/BF01952625. [DOI] [PubMed] [Google Scholar]
- 22.Hida S, Yoshida M, Nakabayashi I, Miura NN, Adachi Y, Ohno N. 2005. Anti-fungal activity of sulfamethoxazole toward Aspergillus species. Biol Pharm Bull 28:773–778. doi: 10.1248/bpb.28.773. [DOI] [PubMed] [Google Scholar]
- 23.Navarro-Martinez MD, Cabezas-Herrera J, Rodriguez-Lopez JN. 2006. Antifolates as antimycotics? Connection between the folic acid cycle and the ergosterol biosynthesis pathway in Candida albicans. Int J Antimicrob Agents 28:560–567. [DOI] [PubMed] [Google Scholar]
- 24.Navarro-Martinez MD, Garcia-Canovas F, Rodriguez-Lopez JN. 2006. Tea polyphenol epigallocatechin-3-gallate inhibits ergosterol synthesis by disturbing folic acid metabolism in Candida albicans. J Antimicrob Chemother 57:1083–1092. doi: 10.1093/jac/dkl124. [DOI] [PubMed] [Google Scholar]
- 25.Chen YL, Lehman VN, Averette AF, Perfect JR, Heitman J. 2013. Posaconazole exhibits in vitro and in vivo synergistic antifungal activity with caspofungin or FK506 against Candida albicans. PLoS One 8(3):e57672. doi: 10.1371/journal.pone.0057672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Koselny K, Green J, DiDone L, Halterman JP, Fothergill AW, Wiederhold NP, Patterson TF, Cushion MT, Rappelye C, Wellington M, Krysan DJ. 2016. The celecoxib derivative AR-12 has broad-spectrum antifungal activity in vitro and improves the activity of fluconazole in a murine model of cryptococcosis. Antimicrob Agents Chemother 60:7115–7127. doi: 10.1128/AAC.01061-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sun LM, Liao K, Liang S, Yu PH, Wang DY. 2015. Synergistic activity of magnolol with azoles and its possible antifungal mechanism against Candida albicans. J Appl Microbiol 118:826–838. doi: 10.1111/jam.12737. [DOI] [PubMed] [Google Scholar]
- 28.Stringaro A, Vavala E, Colone M, Pepi F, Mignogna G, Garzoli S, Cecchetti S, Ragno R, Angiolella L. 2014. Effects of Mentha suaveolens essential oil alone or in combination with other drugs in Candida albicans. Evid Based Complement Alternat Med 2014:125904. doi: 10.1155/2014/125904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Marchetti O, Moreillon P, Glauser MP, Bille J, Sanglard D. 2000. Potent synergism of the combination of fluconazole and cyclosporine in Candida albicans. Antimicrob Agents Chemother 44:2373–2381. doi: 10.1128/AAC.44.9.2373-2381.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Egner R, Bauer BE, Kuchler K. 2000. The transmembrane domain 10 of the yeast Pdr5p ABC antifungal efflux pump determines both substrate specificity and inhibitor susceptibility. Mol Microbiol 35:1255–1263. doi: 10.1046/j.1365-2958.2000.01798.x. [DOI] [PubMed] [Google Scholar]
- 31.Kirby WMM, Rantz LA. 1942. The treatment of typhoid and dysentery carriers with succinylsulfathiazole. JAMA 119:615–618. doi: 10.1001/jama.1942.02830250011004. [DOI] [Google Scholar]
- 32.Fanning S, Mitchell AP. 2012. Fungal biofilms. PLoS Pathog 8:e1002585. doi: 10.1371/journal.ppat.1002585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bruzual I, Riggle P, Hadley S, Kumamoto CA. 2007. Biofilm formation by fluconazole-resistant Candida albicans strains is inhibited by fluconazole. J Antimicrob Chemother 59:441–450. doi: 10.1093/jac/dkl521. [DOI] [PubMed] [Google Scholar]
- 34.Abushahba MF, Mohammad H, Seleem MN. 2016. Targeting multidrug-resistant staphylococci with an anti-rpoA peptide nucleic acid conjugated to the HIV-1 TAT cell penetrating peptide. Mol Ther Nucleic Acids 5:e339. doi: 10.1038/mtna.2016.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pina-Vaz C, Rodrigues AG, Costa-de-Oliveira S, Ricardo E, Mardh PA. 2005. Potent synergic effect between ibuprofen and azoles on Candida resulting from blockade of efflux pumps as determined by FUN-1 staining and flow cytometry. J Antimicrob Chemother 56:678–685. doi: 10.1093/jac/dki264. [DOI] [PubMed] [Google Scholar]
- 36.Huang S, Cao YY, Dai BD, Sun XR, Zhu ZY, Cao YB, Wang Y, Gao PH, Jiang YY. 2008. In vitro synergism of fluconazole and baicalein against clinical isolates of Candida albicans resistant to fluconazole. Biol Pharm Bull 31:2234–2236. doi: 10.1248/bpb.31.2234. [DOI] [PubMed] [Google Scholar]
- 37.Holmes AR, Keniya MV, Ivnitski-Steele I, Monk BC, Lamping E, Sklar LA, Cannon RD. 2012. The monoamine oxidase A inhibitor clorgyline is a broad-spectrum inhibitor of fungal ABC and MFS transporter efflux pump activities which reverses the azole resistance of Candida albicans and Candida glabrata clinical isolates. Antimicrob Agents Chemother 56:1508–1515. doi: 10.1128/AAC.05706-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Henry KW, Cruz MC, Katiyar SK, Edlind TD. 1999. Antagonism of azole activity against Candida albicans following induction of multidrug resistance genes by selected antimicrobial agents. Antimicrob Agents Chemother 43:1968–1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Castelli LA, Nguyen NP, Macreadie IG. 2001. Sulfa drug screening in yeast: fifteen sulfa drugs compete with p-aminobenzoate in Saccharomyces cerevisiae. FEMS Microbiol Lett 199:181–184. doi: 10.1111/j.1574-6968.2001.tb10671.x. [DOI] [PubMed] [Google Scholar]
- 40.CLSI. April 2008. Reference method for broth dilution antifungal susceptibility testing of yeast; approved standards, 3rd ed (M27-A3), vol 28, no 14 CLSI, Wayne, PA. [Google Scholar]
- 41.Pfaller MA. 2012. Antifungal drug resistance: mechanisms, epidemiology, and consequences for treatment. Am J Med 125:S3–S13. doi: 10.1016/j.amjmed.2011.11.001. [DOI] [PubMed] [Google Scholar]
- 42.Liu JY, Shi C, Wang Y, Li WJ, Zhao Y, Xiang MJ. 2015. Mechanisms of azole resistance in Candida albicans clinical isolates from Shanghai, China. Res Microbiol 166:153–161. doi: 10.1016/j.resmic.2015.02.009. [DOI] [PubMed] [Google Scholar]
- 43.Sanglard D, Ischer F, Parkinson T, Falconer D, Bille J. 2003. Candida albicans mutations in the ergosterol biosynthetic pathway and resistance to several antifungal agents. Antimicrob Agents Chemother 47:2404–2412. doi: 10.1128/AAC.47.8.2404-2412.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gu WR, Guo DM, Zhang LP, Xu DM, Sun SJ. 2016. The synergistic effect of azoles and fluoxetine against resistant Candida albicans strains is attributed to attenuating fungal virulence. Antimicrob Agents Chemother 60:6179–6188. doi: 10.1128/AAC.03046-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sun S, Li Y, Guo QJ, Shi CW, Yu JL, Ma L. 2008. In vitro interactions between tacrolimus and azoles against Candida albicans determined by different methods. Antimicrob Agents Chemother 52:409–417. doi: 10.1128/AAC.01070-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Scott EM, Tariq VN, Mccrory RM. 1995. Demonstration of synergy with fluconazole and either ibuprofen, sodium salicylate, or propylparaben against Candida albicans in vitro. Antimicrob Agents Chemother 39:2610–2614. doi: 10.1128/AAC.39.12.2610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pinjon E, Sullivan D, Salkin I, Shanley D, Coleman D. 1998. Simple, inexpensive, reliable method for differentiation of Candida dubliniensis from Candida albicans. J Clin Microbiol 36:2093–2095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bendaoud M, Vinogradov E, Balashova NV, Kadouri DE, Kachlany SC, Kaplan JB. 2011. Broad-spectrum biofilm inhibition by Kingella kingae exopolysaccharide. J Bacteriol 193:3879–3886. doi: 10.1128/JB.00311-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mowat E, Butcher J, Lang S, Williams C, Ramage G. 2007. Development of a simple model for studying the effects of antifungal agents on multicellular communities of Aspergillus fumigatus. J Med Microbiol 56:1205–1212. doi: 10.1099/jmm.0.47247-0. [DOI] [PubMed] [Google Scholar]
- 50.Peralta MA, da Silva MA, Ortega MG, Cabrera JL, Paraje MG. 2015. Antifungal activity of a prenylated flavonoid from Dalea elegans against Candida albicans biofilms. Phytomedicine 22:975–980. doi: 10.1016/j.phymed.2015.07.003. [DOI] [PubMed] [Google Scholar]
- 51.Thangamani S, Younis W, Seleem MN. 2015. Repurposing clinical molecule ebselen to combat drug resistant pathogens. PLoS One 10:e0133877. doi: 10.1371/journal.pone.0133877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Alajlouni RA, Seleem MN. 2013. Targeting Listeria monocytogenes rpoA and rpoD genes using peptide nucleic acids. Nucleic Acid Ther 23:363–367. doi: 10.1089/nat.2013.0426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mohamed MF, Brezden A, Mohammad H, Chmielewski J, Seleem MN. 2017. Targeting biofilms and persisters of ESKAPE pathogens with P14KanS, a kanamycin peptide conjugate. Biochim Biophys Acta 1861:848–859. doi: 10.1016/j.bbagen.2017.01.029. [DOI] [PubMed] [Google Scholar]
- 54.Mohamed MF, Brezden A, Mohammad H, Chmielewski J, Seleem MN. 2017. A short D-enantiomeric antimicrobial peptide with potent immunomodulatory and antibiofilm activity against multidrug-resistant Pseudomonas aeruginosa and Acinetobacter baumannii. Sci Rep 7:6953. doi: 10.1038/s41598-017-07440-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Moffat AC, Osselton MD, Widdop B, Watts J (ed). 2011. Clarke's analysis of drugs and poisons in pharmaceuticals, body fluids and postmortem material, 4th ed Pharmaceutical Press, London, United Kingdom. [Google Scholar]