ABSTRACT
Alalevonadifloxacin (WCK 2349) is a novel l-alanine ester prodrug of levonadifloxacin that is being developed as an oral fluoroquinolone antibiotic. The primary objective of this study was to determine and compare plasma, epithelial lining fluid (ELF), and alveolar macrophage (AM) concentrations of levonadifloxacin following oral administration of alalevonadifloxacin to healthy adult subjects. Levonadifloxacin concentrations in plasma, ELF, and AM samples from 30 healthy subjects were measured by liquid chromatography-tandem mass spectrometry (LC-MS/MS) following oral dosing of alalevonadifloxacin (1,000 mg twice daily for 5 days). Six subjects were assigned to each bronchoalveolar lavage (BAL) fluid sampling time, i.e., 2, 4, 6, 8, or 12 h after the ninth oral dose. Noncompartmental pharmacokinetic (PK) parameters were determined from serial total plasma concentrations collected over a 12-h interval following the first and ninth oral doses. Penetration ratios were calculated from the areas under the concentration-time curves from 0 to 12 h (AUC0–12) for plasma, ELF, and AM by using mean (and median) concentrations at each BAL sampling time. Unbound plasma concentrations (∼85% plasma protein binding) were used to determine site-to-plasma penetration ratios. Plasma PK parameter values for levonadifloxacin were similar after the first and ninth doses. The respective AUC0–12 values based on mean ELF and AM concentrations were 172.6 and 35.3 mg · h/liter, respectively. The penetration ratios for ELF and AM levonadifloxacin concentrations to unbound plasma levonadifloxacin concentrations were 7.66 and 1.58, respectively. Similar penetration ratios were observed with median concentrations. The observed plasma, ELF, and AM concentrations of levonadifloxacin support further studies of alalevonadifloxacin for treatment of lower respiratory tract bacterial infections caused by susceptible pathogens. (This study has been registered at ClinicalTrials.gov under identifier NCT02253342.)
KEYWORDS: levonadifloxacin, alalevonadifloxacin, pharmacokinetics, epithelial lining fluid, alveolar macrophages
INTRODUCTION
Nadifloxacin (OPC-7251; Otsuka Pharmaceuticals, Tokyo, Japan) is a potent, broad-spectrum benzoquinolizine quinolone approved in Japan for topical use of skin infections and acne vulgaris (1). Levonadifloxacin, the S-(−)-isomer of nadifloxacin, has been formulated into a parenteral product as the l-arginine salt (WCK 771) and into alalevonadifloxacin as the l-alanine ester prodrug for oral administration. Levonadifloxacin has greater in vitro potency than that of the R-(+)-enantiomer of nadifloxacin (2, 3). The in vitro spectrum of activity of levonadifloxacin includes commonly encountered respiratory organisms, such as Streptococcus pneumoniae, Haemophilus influenzae, and Moraxella catarrhalis (4–7). In addition, levonadifloxacin has been found to be highly active against methicillin-resistant Staphylococcus aureus (MRSA), including fluoroquinolone-resistant strains (4, 5, 8).
Since levonadifloxacin is poorly soluble in water, the l-arginine salt formulation was developed for the parenteral administration of levonadifloxacin (WCK 771). Alalevonadifloxacin (WCK 2349), a highly water-soluble l-alanine ester mesylate salt of levonadifloxacin, achieves high oral bioavailability (∼89%) and is being developed as the oral prodrug formulation of levonadifloxacin (2, 3, 9). Both formulations are being developed for the treatment of acute bacterial skin and skin structure infections (ABSSSI), community-acquired bacterial pneumonia (CABP), and hospital-acquired bacterial pneumonia (HABP) caused by MRSA. Alalevonadifloxacin has completed several phase 1 studies, including studies of the safety and pharmacokinetics of single and multiple ascending doses, hepatic impairment, and an evaluation of electrocardiographic effects at supratherapeutic doses (9, 10–14). Alalevonadifloxacin and levonadifloxacin have completed open-label phase 2 trials for the treatment of ABSSSI. Alalevonadifloxacin is currently in phase 3 of development and undergoing a registration study for treatment of ABSSSI in India.
For pulmonary infections, concentrations of antibiotics in epithelial lining fluid (ELF) (for extracellular pathogens) and alveolar macrophages (AM) (for intracellular pathogens) are thought to reflect relevant infection sites of acute bacterial pneumonia (15–18). The primary objective of this study was to compare plasma, ELF, and AM concentrations of levonadifloxacin and to determine the tolerability and safety of oral administration of alalevonadifloxacin in healthy adult male and female subjects (ClinicalTrials registration no. NCT02253342).
(This work was presented in part at the 26th European Congress of Clinical Microbiology and Infectious Disease [ECCMID], Amsterdam, Netherlands, April 2016.)
RESULTS
Subjects.
A total of 31 subjects were enrolled in this study. Twelve subjects (38.7%) experienced a total of 17 treatment-emergent adverse events (TEAEs) over the course of the study. All TEAEs were mild in severity, and no serious adverse events were observed. Among 17 adverse events, 4 were considered not related to study medication and 13 were considered related to study medicine. The most frequently occurring TEAEs included photophobia in four subjects and dysgeusia (i.e., altered sense of taste) in four subjects. The other adverse events considered to be related to the study drug were leukopenia, back pain, headache, and skin papule. No clinically significant changes were observed in physical examination findings, vital signs, or electrocardiograms (ECGs).
One subject was not included in the pharmacokinetic analysis because administration of the study drug was discontinued after the eighth dose. The characteristics of the remaining 30 subjects, who completed the entire study protocol, are reported in Table 1. The most notable difference in demographic characteristics was in gender, as only male subjects were enrolled in the 8- and 12-h sampling times.
TABLE 1.
Characteristics of study subjects receiving alalevonadifloxacin (1,000 mg) every 12 h for 10 dosesa
| Sampling time (h)b | Sex of patients | Age (yr) | ht (cm) | wt (kg) | eCLCR (ml/min) | Total cell count in BAL fluid (cells/mm3) | Macrophages (%) |
|---|---|---|---|---|---|---|---|
| 2 | 2 M, 4 F | 39 ± 6 | 172 ± 7 | 78.3 ± 12.2 | 123 ± 24 | 106 ± 22 | 84 ± 8 |
| 4 | 3 M, 3 F | 37 ± 9 | 172 ± 10 | 74.6 ± 7.0 | 105 ± 13 | 163 ± 92 | 86 ± 6 |
| 6 | 4 M, 2 F | 31 ± 8 | 171 ± 9 | 70.5 ± 9.0 | 106 ± 20 | 96 ± 25 | 84 ± 7 |
| 8 | 6 M | 41 ± 12 | 175 ± 7 | 83.0 ± 9.6 | 119 ± 24 | 105 ± 35 | 85 ± 11 |
| 12 | 6 M | 36 ± 9 | 178 ± 6 | 85.7 ± 11.6 | 123 ± 26 | 118 ± 65 | 88 ± 6 |
Data are expressed as means ± 1 SD, except for the data on sex. Abbreviations: M, males; F, females; eCLCR, estimated creatinine clearance.
Six subjects were examined at each sampling time.
Pharmacokinetics.
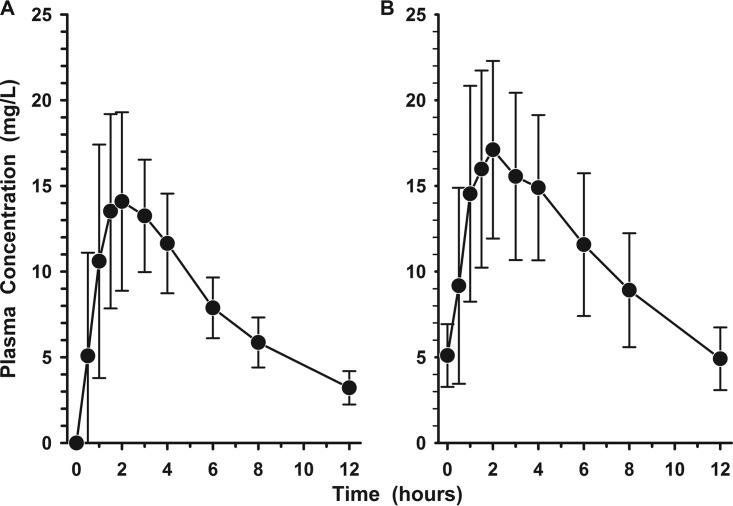
The plasma concentration-time profiles of levonadifloxacin after the first and ninth oral doses of alalevonadifloxacin are shown in Fig. 1A and B, respectively. Trough concentrations (Cmin) progressively increased (i.e., means ± standard deviations [SD] of 3.22 ± 0.97, 4.60 ± 1.57, 5.21 ± 1.91, and 5.35 ± 2.15 mg/liter 12 h after the first, second, fourth, and sixth doses, respectively), and steady state was being achieved by the fifth dose of alalevonadifloxacin. Table 2 lists the mean (±SD) pharmacokinetic parameters of levonadifloxacin in plasma during these two study periods. The average increases in maximum concentration of drug in serum (Cmax) and area under the concentration-time curve (AUC) for the first and ninth doses were 26.9% and 12.4%, respectively.
FIG 1.
Mean (±SD) plasma concentration-versus-time profiles of levonadifloxacin before and in the 12-h interval following the first (A) and ninth (B) oral doses of alalevonadifloxacin (1,000 mg every 12 h).
TABLE 2.
Pharmacokinetic parameters of levonadifloxacin in plasma following the first and ninth oral doses of 1,000 mg
| Dose no. | Mean ± SD |
|||||
|---|---|---|---|---|---|---|
| Cmax (mg/liter) | Tmax (h) | AUCa (mg · h/liter) | t1/2 (h) | V/F (liters) | CL/F (liters/h) | |
| 1 | 16.5 ± 5.1 | 1.8 ± 0.7 | 116.2 ± 28.7 | 4.5 ± 0.9 | 58.0 ± 14.7 | 9.11 ± 2.23 |
| 9 | 20.0 ± 4.3 | 2.1 ± 1.4 | 129.8 ± 31.6 | 5.1 ± 1.3 | 59.2 ± 16.0 | 8.17 ± 2.05 |
AUC for the first dose was AUC0–∞, and AUC for the ninth dose was AUC0–12.
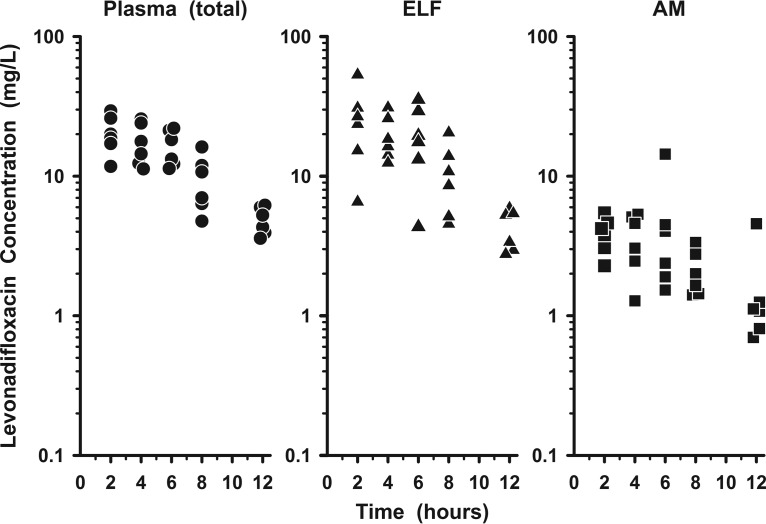
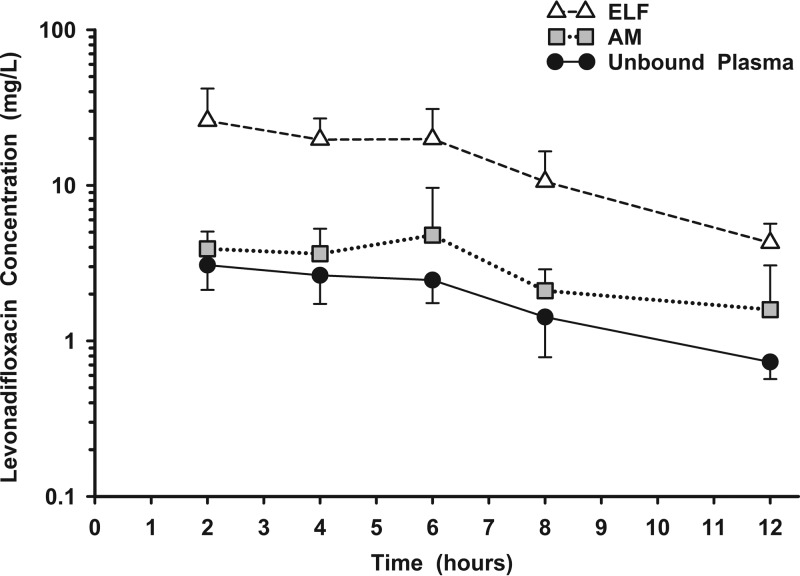
Figure 2 displays the measured plasma (total), ELF, and AM concentrations of levonadifloxacin for each subject following the ninth oral dose of alalevonadifloxacin. The mean (±SD) concentrations of levonadifloxacin after the ninth dose in plasma, ELF, and AM at the five bronchopulmonary sampling times are reported in Table 3. Concentrations of levonadifloxacin in ELF tended to be similar to or higher than simultaneous total plasma concentrations, whereas concentrations in AM tended to be lower than those in the other two matrices. Once plasma concentrations were corrected for protein binding, the mean (±SD) concentrations in both ELF and AM were higher than the unbound plasma concentrations of levonadifloxacin (Fig. 3). The mean (±SD) ratios of ELF or AM concentrations to the simultaneous unbound plasma concentrations of levonadifloxacin are reported in Table 4. The variabilities of these site-to-unbound-plasma ratios ranged from 17% to 53% for ELF and from 32% to 84% for AM.
FIG 2.
Individual concentrations of levonadifloxacin in plasma (total) (circles), epithelial lining fluid (ELF) (triangles), and alveolar macrophages (AM) (squares) 2, 4, 6, 8, and 12 h after the ninth oral dose of alalevonadifloxacin (1,000 mg every 12 h). The y axis is on the log scale.
TABLE 3.
Levonadifloxacin concentrations in plasma (total), ELF, and AM at time of bronchoscopy and BALa
| Sampling time (h)b | Levonadifloxacin concn (mg/liter) in: |
||
|---|---|---|---|
| Plasma | ELF | AM | |
| 2 | 20.5 ± 6.3 | 26.0 ± 15.9 | 3.91 ± 1.14 |
| 4 | 17.6 ± 6.1 | 19.7 ± 7.2 | 3.64 ± 1.63 |
| 6 | 16.4 ± 4.7 | 19.9 ± 11.1 | 4.79 ± 4.85 |
| 8 | 9.49 ± 4.25 | 10.6 ± 6.0 | 2.10 ± 0.79 |
| 12 | 4.88 ± 1.09 | 4.28 ± 1.40 | 1.59 ± 1.47 |
Data are expressed as means ± 1 SD.
Six concentrations per matrix were examined at each sampling time.
FIG 3.
Mean (±SD) concentration-versus-time profiles of levonadifloxacin in plasma (unbound) (circles), epithelial lining fluid (ELF) (triangles), and alveolar macrophages (AM) (squares) 2, 4, 6, 8, and 12 h after the ninth oral dose of alalevonadifloxacin (1,000 mg every 12 h). The y axis is on the log scale.
TABLE 4.
Ratios of ELF and AM to unbound plasma concentrations at time of bronchoscopy and BALa
| Sampling time (h)b | Concn ratio |
|
|---|---|---|
| ELF to plasma (unbound) | AM to plasma (unbound) | |
| 2 | 7.94 ± 3.15 | 1.40 ± 0.61 |
| 4 | 7.54 ± 1.25 | 1.37 ± 0.47 |
| 6 | 8.01 ± 4.25 | 1.79 ± 1.40 |
| 8 | 7.79 ± 3.45 | 1.60 ± 0.52 |
| 12 | 5.83 ± 1.38 | 2.18 ± 1.83 |
Data are expressed as means ± 1 SD.
Six subjects were examined per sampling time.
The values for the AUC from 0 to 12 h (AUC0–12) based on mean and median ELF concentrations were 172.6 and 161.2 mg · h/liter, respectively. The ratios of ELF to unbound plasma levonadifloxacin concentrations based on the mean and median AUC0–12 values were 7.66 and 7.58, respectively. The AUC0–12 values based on mean and median drug concentrations in AM were 35.3 and 30.6 mg · h/liter, respectively. The ratios of AM to unbound plasma levonadifloxacin concentrations based on the mean and median AUC0–12 values were 1.58 and 1.44, respectively.
DISCUSSION
The observed plasma concentration-time profile of levonadifloxacin and parameter values for Cmax, time to maximum concentration of drug in serum (Tmax), and AUC0–12 in this study were similar to those in previous pharmacokinetic studies of alalevonadifloxacin in healthy subjects (9, 10–12). A population pharmacokinetic analysis of three phase 1 studies reported mean clearance (CL/F) and volume of distribution (V/F) values of 7.2 liters/h and 54.5 liters, respectively, which are comparable to our mean values after multiple doses (CL/F, 8.17 liters/h; and V/F, 59.2 liters) (13). The largest observed differences occurred with elimination half-life (t1/2), with higher values being reported in other phase 1 studies (ranging from 6.35 to 9.26 h). The most likely explanation for this is the extensive blood sampling scheme (e.g., up to 48 h after dosing) used in other phase 1 studies compared to our restricted sampling times during the 12-h dosing interval.
The mean percent plasma protein binding of levonadifloxacin has been reported to be 86.7%, 85.4%, and 81.3% after multiple oral doses of 800, 1,000, and 1,200 mg, respectively, administered twice daily for 5 days (Wockhardt Ltd., personal communication on 1 July 2015). For determination of penetration ratios in this study, the AUC0–12 values based on total plasma concentrations were transformed to unbound AUC0–12 values (assuming an average plasma protein binding level of 85%), since only unbound drug in plasma is considered to cross into the lungs. Estimations of protein binding in the intrapulmonary compartments are unknown, and we assumed that the measured concentrations in ELF and AM represented unbound drug concentrations.
Penetration into ELF has been considered an important characteristic of antibiotics used to treat extracellular pathogens. Similar to those of other fluoroquinolones, such as levofloxacin and moxifloxacin, the ELF exposure (measured by AUC) of levonadifloxacin was >7-fold higher than unbound plasma concentrations (17–19). Concentrations of levonadifloxacin in ELF were significantly higher than simultaneous unbound plasma concentrations throughout the 12-h dosing interval after the ninth dose (Fig. 3). Potent in vitro activity of levonadifloxacin has been demonstrated against extracellular pathogens commonly associated with community-acquired pneumonia. The MIC90 values for levonadifloxacin against large numbers of isolates of Streptococcus pneumoniae (n = 1,196), Haemophilus influenzae (n = 1,002), and Moraxella catarrhalis (n = 504) were 0.5, 0.03, and 0.015 mg/liter, respectively (4). In addition, Staphylococcus aureus has a MIC90 value of 1 mg/liter, with MIC90 values for MRSA strains ranging from 0.5 to 2.0 mg/liter (4, 8).
The pharmacokinetic-pharmacodynamic parameter that correlates with microbiological and clinical outcomes of fluoroquinolones has been the unbound AUC0–24/MIC ratio for plasma (20, 21). Preliminary analyses suggested an unbound AUC0–24/MIC pharmacodynamic target ratio of 20 for levonadifloxacin in plasma, based on static and 1-log10 bactericidal activities derived from thigh and lung infections in the neutropenic mouse model (22). Combining the mean ELF exposure (e.g., AUC0–12 of 172.6 mg · h/liter × 2 = AUC0–24) with the MIC90 values, the estimated AUC0–24/MIC90 ratios for ELF range from 23,013 for Moraxella catarrhalis (MIC90, 0.015 mg/liter) down to 173 for MRSA (MIC90, 2 mg/liter). The high ELF exposure along with the low MICs will likely be a significant advantage for levonadifloxacin over older fluoroquinolone agents against these extracellular pathogens. Clinical studies are needed to determine if ELF and unbound plasma AUC0–24/MIC ratios are predictive of the efficacy of levonadifloxacin.
Penetration into macrophages has been considered an important characteristic of antibiotics used to treat intracellular pathogens, such as Legionella pneumophila and Chlamydophila pneumoniae (15, 16, 23). Atypical pneumonias caused by these pathogens and Mycoplasma pneumoniae have been treated effectively with fluoroquinolones (23–25). Concentrations of levonadifloxacin in AM ranged from 0.70 to 14.4 mg/liter during the 12-h dosing interval of our study, with 90% of these values being between 1.0 and 5.55 mg/liter (Fig. 2). Although the magnitude of AM concentrations that is needed to ensure clinical efficacy against atypical pathogens is unknown, the constant level of levonadifloxacin in AM throughout the 12-h dosing interval should be valuable for providing in vivo activity against these atypical respiratory pathogens.
In summary, oral administration of alalevonadifloxacin at 1,000 mg twice daily for 5 days was observed to be safe and well tolerated. The most frequently occurring TEAEs included photophobia in four subjects and dysgeusia in four subjects. Levonadifloxacin concentrations in ELF were equal to or higher than total plasma concentrations after the ninth oral dose of alalevonadifloxacin. The ratios of ELF to total plasma levonadifloxacin concentrations based on the mean and median AUC0–12 values were 1.15 and 1.14, respectively. Plasma protein binding of levonadifloxacin is significantly higher (∼85%) than that of the commonly used fluoroquinolones (e.g., ciprofloxacin, levofloxacin, or moxifloxacin), and the unbound plasma concentrations should be considered in determining ratios of site penetration. The ratios of ELF to unbound plasma levonadifloxacin concentrations increased to 7.66 and 7.58 based on the mean and median AUC0–12 values, respectively. The concentrations of levonadifloxacin in AM were lower than the total plasma and ELF concentrations. The ratios of AM to total plasma levonadifloxacin concentrations based on the mean and median AUC0–12 values were 0.24 and 0.22, respectively. These ratios increased to 1.58 and 1.44, respectively, when unbound plasma levonadifloxacin concentrations were considered. These data support further study of alalevonadifloxacin for treatment of lower respiratory tract bacterial infections caused by susceptible extracellular and intracellular pathogens.
MATERIALS AND METHODS
Study design and subjects.
This was a phase 1, open-label pharmacokinetic study at one center and was conducted in accordance with the principles of good clinical practice. The protocol was reviewed and approved by the Institutional Review Board at Quorum Review (Seattle, WA, USA). Written informed consent was obtained from each subject before any protocol-related activities were conducted.
Adult male and female subjects between 18 and 55 years of age were evaluated as healthy based on a detailed medical history, physical examination, clinical laboratory tests, pulmonary function test, and 12-lead electrocardiogram (ECG). Eligible subjects had to have no significant medical history, evidence of organ dysfunction, or clinically significant laboratory abnormalities. Subjects were required to have a body weight between 55 and 100 kg and a body mass index between 18.5 and 30 kg/m2. Two highly effective methods of birth control (as defined in the protocol) were required for female subjects of childbearing potential, from the screening visit until 30 days following the last oral dose of alalevonadifloxacin. Male subjects engaging in sexual activity were required to use two highly effective methods of birth control from the time of the screening visit until 90 days following the last oral dose of alalevonadifloxacin. Male subjects were also not allowed to donate sperm during the same period.
Exclusion criteria included a history or presence of clinically significant medical disorders, known hypersensitivity to any quinolone antibiotics, and a history of allergic or other serious adverse reactions to benzodiazepines or lidocaine. Subjects were excluded if they had a clinically significant pulmonary or other disease that would place them at increased risk to undergo a standardized bronchoscopy and bronchoalveolar lavage (BAL). Subjects with a baseline QT interval using Fridericia's correction method (QTcF) of >450 ms or a history of prolonged QT syndrome at the screening visit were excluded. Subjects must not have had a history of using tobacco or other smoking materials (defined as smoking or use of snuff chewing tobacco or other nicotine or nicotine-containing products) within 6 months of the screening visit. The remaining exclusion criteria were similar to those commonly used in our previous intrapulmonary studies (19, 26, 27).
Subjects received 10 oral doses of 1,000 mg of alalevonadifloxacin (two 400-mg tablets and one 200-mg tablet), administered every 12 h for a total of 5 days. The tablets were administered with 240 ml of water within 2 h after a standard meal and under direct observation at the study site. Blood samples were collected to measure concentrations of levonadifloxacin in plasma within 15 min prior to and 0.5, 1, 1.5, 2, 3, 4, 6, 8, 12, and 24 h following the first and ninth doses of alalevonadifloxacin. The 24-h sample after the first dose was collected prior to the third dose on day 2. Blood samples were also collected 15 min prior to the fifth and seventh doses of alalevonadifloxacin.
Each subject underwent one standardized bronchoscopy with BAL 2, 4, 6, 8, or 12 h following the ninth dose of alalevonadifloxacin. Samples of BAL fluid were obtained to assay study drug concentrations, to determine urea concentrations, and for cell counts. A blood sample was collected at the time of bronchoscopy to determine the plasma urea concentration. Methods for sample handling, bronchoscopy and BAL procedures, and storage conditions have been described previously (19, 26, 27).
Determination of plasma concentrations of levonadifloxacin.
Levonadifloxacin plasma concentrations were determined by use of a high-performance liquid chromatography-tandem mass spectrometry (LC-MS/MS) method that was developed and validated at the Clinical Pharmacokinetics and Biopharmaceutics Department of Wockhardt Ltd., Maharashtra, India (internal reports CPB-AM-062-00 and MVP-LNSL-001-11). Seventeen analytical runs were used to measure levonadifloxacin in 734 plasma samples between 27 January 2015 and 3 February 2015. Fifty-six samples (7.6%) were reassayed to assess the incurred sample reanalysis (ISR) of previously analyzed samples.
A previously published LC-MS/MS procedure was modified to include a solid-phase extraction technique, with carbamazepine as the internal standard (28). Plasma standard curves were linear (r2 > 0.990) over the concentration range of 0.127 to 60.007 μg/ml for levonadifloxacin. Precision (i.e., the percent coefficient of variation) and accuracy (i.e., the percent bias) from analysis of quality control (QC) plasma samples at four concentrations (0.365, 12.317, 24.635, and 49.271 μg/ml) were in the range of 5.53% to 6.53% and 0.63% to 3.35%, respectively. The lower limit of quantification (LLOQ) in plasma was 0.127 μg/ml for levonadifloxacin. All of the samples selected for reproducibility were within a 20.0% difference between the original and ISR concentration values for levonadifloxacin.
Determination of BAL fluid and cell pellet concentrations of levonadifloxacin.
Determination of levonadifloxacin concentrations in the BAL fluid supernatant and cell pellet was performed using validated LC-MS/MS methods at Keystone Bioanalytical (North Wales, PA) (internal reports 150102 and 150103, respectively). In brief, the method used protein precipitation (with acetonitrile used as a solvent) to isolate levonadifloxacin (batch WK7/A-445/116; Wockhardt) and the internal standard (carbamazepine) (lot FN03241402; Cerilliant) for both matrices. The blank control matrix used for calibration standards and QC samples was human BAL fluid (BioreclamationIVT).
A total of 30 BAL fluid samples and 30 cell pellet suspensions were assayed for levonadifloxacin in two analytical runs for each matrix. The standard curves for levonadifloxacin were linear over the concentration range of 1.0 to 1,000 ng/ml for both BAL fluid (r2 ≥ 0.998) and cell suspensions (r2 ≥ 0.996). The respective precision and accuracy for levonadifloxacin QC samples in BAL fluid were 26.47% and 10.43% at 3 ng/ml, 1.68% and −5.64% at 200 ng/ml, and 0.38% and −6.47% at 750 ng/ml. The respective precision and accuracy for levonadifloxacin QC samples in cell suspensions were 3.80% and −4.30% at 3 ng/ml, 0.93% and −5.14% at 200 ng/ml, and 1.55% and −6.45% at 750 ng/ml. The LLOQ for levonadifloxacin in human BAL fluid and cell suspensions was 1 ng/ml. A total of 10 samples for both BAL fluid and cell pellets were selected for ISR testing, and the calculated assay variability values were below 20%.
Determination of urea concentrations.
Concentrations of urea in plasma and BAL fluid supernatant were determined by an LC-MS/MS method that was developed and validated at Keystone Bioanalytical (North Wales, PA) (internal reports 150105 and 150106, respectively). A total of 30 human plasma and BAL fluid samples were assayed for urea concentrations. In brief, the method used protein precipitation with methanol to isolate urea and the internal standard ([13C,15N2]urea) (lot MBBB2767V; Sigma-Aldrich) for both matrices. The blank control matrix for calibration standards was phosphate-buffered saline (PBS) for plasma assay and 0.9% sodium chloride for BAL fluid assay. Human K2EDTA-plasma and blank controls were used to prepare QC samples.
The assay for urea in human plasma was linear (r2 = 0.9983) over the range of concentrations from 100 to 3,000 μg/ml. The precision and accuracy for urea QC samples in PBS at 300 μg/ml were 1.37% and 2.97%, respectively. The respective precision and accuracy for urea QC samples in plasma were 4.51% and 4.40% at 285 μg/ml, 1.36% and −0.83% at 1,000 μg/ml, and 1.05% and −6.91% at 2,250 μg/ml. The LLOQ for urea in human plasma was 100 μg/ml.
The assay for urea in BAL fluid was linear (r2 = 0.9989) over the range of concentrations from 0.2 to 10 μg/ml. The respective precision and accuracy for urea QC samples in human BAL fluid were 3.40% and −2.00% at 0.6 μg/ml, 2.60% and 0.03% at 3.0 μg/ml, and 2.12% and 4.47% at 7.5 μg/ml. The LLOQ for urea in human BAL fluid was 0.2 μg/ml. A total of 10 samples for both plasma and BAL fluid were selected for ISR testing, and the calculated assay variability values were below 20%.
Calculations of concentrations of levonadifloxacin in ELF and AM.
The apparent volume of ELF in BAL fluid was determined by the urea dilution method described by Rennard and colleagues (29). The estimated concentration of levonadifloxacin (LNFELF) in ELF was calculated as follows: LNFELF = LNFBAL × (UreaPlasma/UreaBAL), where LNFBAL is the measured levonadifloxacin concentration in BAL fluid and UreaPlasma and UreaBAL are the measured concentrations of urea in plasma and BAL fluid, respectively. The absolute cell numbers and differential cell count percentages were determined from the BAL fluid. The estimated concentration of levonadifloxacin in AM (LNFAM) was calculated as follows: LNFAM = LNFS/VAM, where LNFS and VAM are the measured concentration of levonadifloxacin and the volume of alveolar cells in the cell suspension, respectively. The calculations for VAM used a mean macrophage cell volume of 2.42 μl/106 cells (30, 31).
Pharmacokinetic analysis.
Pharmacokinetic parameters for levonadifloxacin were determined by noncompartmental methods, using WinNonlin software (version 6.3; Pharsight Corporation, Cary, NC). The maximum plasma concentration (Cmax), time to Cmax (Tmax), and minimum plasma concentration (Cmin) were read from the observed plasma concentration-time profile following the first and ninth oral doses of alalevonadifloxacin. The 12-h plasma concentration represented Cmin after a specific dose. The areas under the plasma concentration-time curves (AUC) after the first and ninth doses were calculated with the linear-log trapezoidal rule. The AUC for the first dose was extrapolated to infinity (AUC0–∞), and the AUC for the ninth dose was determined for the 12-h dosing interval (AUC0–12). The elimination rate constant (β) was determined by nonlinear least-squares regression, and the elimination half-life (t1/2) was calculated by dividing the natural logarithm of 2 by β. The apparent volume of distribution (V/F) and clearance (CL/F) for extravascular administration were calculated; F is the fraction of bioavailability and was assumed to equal a value of 1 (100%).
Mean and median plasma, ELF, and AM concentration values at the BAL fluid sampling times were used to estimate AUC0–12 by the linear-log trapezoidal method. The levonadifloxacin concentration at the 12-h sampling time after the ninth dose also served as the time zero value for determining the AUC0–12 for each matrix. The intrapulmonary penetration ratios of levonadifloxacin were estimated from the ratio of AUC0–12 for ELF or AM to the AUC0–12 for unbound plasma. The unbound plasma levonadifloxacin concentration was calculated from the total concentration by assuming 85% plasma binding (Wockhardt Ltd., personal communication).
Safety and laboratory assessments.
All enrolled subjects who had received at least one dose of alalevonadifloxacin were included in the safety analyses. Safety was determined by evaluating physical examinations, vital signs, standard 12-lead ECGs, clinical laboratory tests (serum chemistry, liver function tests, hematology, coagulation, and urinalysis), and recording of adverse events. The investigators assessed each subject for observed and reported adverse events throughout the duration of the study (i.e., from the time of signed informed consent until the final visit was completed). The common terminology criteria for adverse events (CTCAE) (U.S. National Cancer Institute) were used as a reference for all adverse events and for the grading of severity (i.e., mild, moderate, severe, life-threatening, or fatal). Causality assessment of adverse events was defined and included the following categories: certain, probable/likely, possible, unlikely, not related, and unknown.
ACKNOWLEDGMENTS
This study was supported by Wockhardt Bio Ag.
Regarding conflicts of interest, K.A.R. and M.H.G. have been consultants to Wockhardt Bio Ag, M.H.G. has received research support from Wockhardt Bio Ag, and R.C., M.G., R.Y., A.P., and A.B. are current employees of Wockhardt Bio Ag.
REFERENCES
- 1.Jacobs MR, Appelbaum PC. 2006. Nadifloxacin: a quinolone for topical treatment of skin infections and potential for systemic use of its active isomer, WCK 771. Expert Opin Pharmacother 7:1957–1966. doi: 10.1517/14656566.7.14.1957. [DOI] [PubMed] [Google Scholar]
- 2.de Souza Mendes C, de Souza Antunes AM. 2013. Pipeline of known chemical classes of antibiotics. Antibiotics 2:500–534. doi: 10.3390/antibiotics2040500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bush K, Page MGP. 2017. What we may expect from novel antibacterial agents in the pipeline with respect to resistance and pharmacodynamics principles. J Pharmacokinet Pharmacodyn 44:113–132. doi: 10.1007/s10928-017-9506-4. [DOI] [PubMed] [Google Scholar]
- 4.Flamm RK, Farrell DJ, Sader HS, Rhomberg PR, Jones RN. 2016. In vitro activity of WCK 771, a benzoquinolizine fluoroquinolone (levonadifloxacin) when tested against contemporary Gram-positive and -negative bacteria from a global surveillance program, abstr Sunday-456. Abstr ASM Microbe, Boston, MA American Society for Microbiology, Washington, DC. [Google Scholar]
- 5.Hackel M, Bhagwat S, Khande H, Joshi P, Patel M, Sahm D. 2015. In vitro activity of WCK 771, a new benzoquinolizine quinolone in development, against key bacterial groups from the USA and Europe, abstr F-1196. Abstr 55th Intersci Conf Antimicrob Agents Chemother, San Diego, CA American Society for Microbiology, Washington, DC. [Google Scholar]
- 6.Appelbaum PC, Pankuch GA, Bozdogan B, Lin G, Jacobs MR, Patel MV, Gupte SV, Jafri MA, De Sourza NJ, Korahiwala HF. 2004. Activity of the new quinolone WCK 771 against pneumococci. Clin Microbiol Infect Dis 11:9–14. doi: 10.1111/j.1469-0691.2004.01017.x. [DOI] [PubMed] [Google Scholar]
- 7.Al-Lahham A, De Souza NJ, Patel M, Reinert RR. 2005. Activity of the new quinolones WCK 771, WCK 1152 and WCK 1153 against clinical isolates of Streptococcus pneumoniae and Streptococcus pyogenes. J Antimicrob Chemother 56:1130–1133. doi: 10.1093/jac/dki361. [DOI] [PubMed] [Google Scholar]
- 8.Bhagwat SS, McGhee P, Kosowska-Shick K, Patel MV, Appelbaum PC. 2009. In vitro activity of the quinolone WCK 771 against recent U.S. hospital and community-acquired Staphylococcus aureus pathogens with various resistance types. Antimicrob Agents Chemother 53:811–813. doi: 10.1128/AAC.01150-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chugh R, Lakdavla F, Bhagwat S, Patel LM, Bhatia A. 2015. Food effect and absolute bioavailability study of WCK 2349 and WCK 771 in healthy adult human volunteers in U.S., abstr A-039. Abstr 55th Intersci Conf Antimicrob Agents Chemother, San Diego, CA American Society for Microbiology, Washington, DC. [Google Scholar]
- 10.Niu J, Ivaturi V, Niu J, Gobburu J, Chugh R, Bhatia A. 2015. Pharmacokinetics of levonadifloxacin administered as intravenous WCK 771 and oral WCK 2349 in healthy Indian male adults, abstr A-032. Abstr 55th Intersci Conf Antimicrob Agents Chemother, San Diego, CA American Society for Microbiology, Washington, DC. [Google Scholar]
- 11.Chugh R, Lakdavala F, Bhatia A. 2016. Safety and pharmacokinetics of multiple ascending doses of WCK 771 and WCK 2349, abstr P1268. Abstr 26th Eur Congr Clin Microbiol Infect Dis, Amsterdam, Netherlands European Society of Clinical Microbiology and Infectious Diseases, Basel, Switzerland. [Google Scholar]
- 12.Preston RA, Chugh R, Mastim M, Bhatia A. 2016. Single-center evaluation of the pharmacokinetics and safety of the novel fluoroquinolone WCK 2349 in hepatic impairment, abstr P1270. Abstr 26th Eur Congr Clin Microbiol Infect Dis, Amsterdam, Netherlands European Society of Clinical Microbiology and Infectious Diseases, Basel, Switzerland. [Google Scholar]
- 13.Ivaturi V, Niu J, Chugh R, Bhatia A, Chavan R, Patel A, Takalkar S, Patel M, Bhagwat S, Gobburu J. 2015. Population pharmacokinetics of levonadifloxacin administered as WCK 2349, a novel oral benzoquinolizine quinolone prodrug, abstr A-033. Abstr 55th Intersci Conf Antimicrob Agents Chemother, San Diego, CA American Society for Microbiology, Washington, DC. [Google Scholar]
- 14.Mason JW, Chugh R, Lakdavala F, Bhatia A. 2016. Electrocardiographic effects of WCK 2349, abstr P1269. Abstr 26th Eur Congr Clin Microbiol Infect Dis, Amsterdam, Netherlands European Society of Clinical Microbiology and Infectious Diseases, Basel, Switzerland. [Google Scholar]
- 15.Cazzola M, Blasi F, Terzano C, Matera MG, Marsico SA. 2002. Delivering antibacterials to the lungs: considerations for optimizing outcomes. Am J Respir Med 1:261–272. doi: 10.1007/BF03256617. [DOI] [PubMed] [Google Scholar]
- 16.Baldwin DR, Honeybourne D, Wise R. 1992. Pulmonary disposition of antimicrobial agents: in vivo observations and clinical relevance. Antimicrob Agents Chemother 36:1176–1180. doi: 10.1128/AAC.36.6.1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nix DE. 1998. Intrapulmonary concentrations of antimicrobial agents. Infect Dis Clin North Am 12:631–646. doi: 10.1016/S0891-5520(05)70202-6. [DOI] [PubMed] [Google Scholar]
- 18.Rodvold KA, George JM, Yoo L. 2011. Penetration of anti-infective agents into pulmonary epithelial lining fluid: focus on antibacterial agents. Clin Pharmacokinet 50:637–664. doi: 10.2165/11594090-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 19.Gotfried MH, Danziger LH, Rodvold KA. 2001. Steady-state plasma and intrapulmonary concentrations of levofloxacin and ciprofloxacin in healthy adult subjects. Chest 119:1114–1122. doi: 10.1378/chest.119.4.1114. [DOI] [PubMed] [Google Scholar]
- 20.Craig WA. 1998. Pharmacokinetic/pharmacodynamics parameters: rationale for antibacterial dosing of mice and men. Clin Infect Dis 26:1–10. doi: 10.1086/516284. [DOI] [PubMed] [Google Scholar]
- 21.Ambrose PG, Bhavnani SM, Rubino CM, Louie A, Gumbo T, Forrest A, Drusano GL. 2007. Pharmacokinetics-pharmacodynamics of antimicrobial therapy: it's not just for mice anymore. Clin Infect Dis 44:79–86. doi: 10.1086/510079. [DOI] [PubMed] [Google Scholar]
- 22.Ivaturi V, Chugh R, Bhatia A, Chavan R, Patel A, Takalkar S, Patel M, Bhagwat S, Gobburu J. 2015. WCK 771/WCK 2349 pharmacokinetic-pharmacodynamic target attainment analyses, abstr A-034. Abstr 55th Intersci Conf Antimicrob Agents Chemother, San Diego, CA American Society for Microbiology, Washington, DC. [Google Scholar]
- 23.Cunha CB, Cunha BA. 2017. Antimicrobial therapy for Legionnaire's disease: antibiotic stewardship implications. Infect Dis Clin North Am 31:179–191. doi: 10.1016/j.idc.2016.10.013. [DOI] [PubMed] [Google Scholar]
- 24.Musher DM, Thorner AR. 2014. Community-acquired pneumonia. N Engl J Med 371:1619–1628. doi: 10.1056/NEJMra1312885. [DOI] [PubMed] [Google Scholar]
- 25.File TM, Marrie TJ. 2013. Does empiric therapy for atypical pathogens improve outcomes for patients with CAP? Infect Dis Clin North Am 27:99–114. doi: 10.1016/j.idc.2012.11.005. [DOI] [PubMed] [Google Scholar]
- 26.Rodvold KA, Gotfried MH, Chugh R, Gupta M, Friedland HD, Bhatia A. 2017. Comparison of plasma and intrapulmonary concentrations of nafithromycin (WCK 4873) in healthy adult subjects. Antimicrob Agents Chemother 61:e01096-17. doi: 10.1128/AAC.01096-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rodvold KA, Gotfried MH, Still JG, Clark K, Fernandes P. 2012. Comparison of plasma, epithelial lining fluid, and alveolar macrophage concentrations of solithromycin (CEM-101) in healthy adult subjects. Antimicrob Agents Chemother 56:5076–5081. doi: 10.1128/AAC.00766-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yeole RD, Kulkarni VL, Latad SB, Chavan RP, Chugh Y, Patel MV, Khorakiwala HF. 2007. Simple liquid chromatography-tandem spectrometry method for determination of novel anti-methicillin-resistant Staphylococcus aureus fluoroquinolone WCK 771 in human serum. J Chromatogr B 846:306–312. doi: 10.1016/j.jchromb.2006.09.022. [DOI] [PubMed] [Google Scholar]
- 29.Rennard SI, Basset G, Lecossier D, O'Donnell KM, Pinkston P, Martin PG, Crystal RG. 1986. Estimation of volume of epithelial lining fluid recovered by lavage using urea as marker of dilution. J Appl Physiol 60:532–538. doi: 10.1152/jappl.1986.60.2.532. [DOI] [PubMed] [Google Scholar]
- 30.Baldwin DR, Honeybourne D, Wise R. 1992. Pulmonary disposition of antimicrobial agents: methodological considerations. Antimicrob Agents Chemother 36:1171–1175. doi: 10.1128/AAC.36.6.1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Baldwin DR, Wise R, Andrews JM, Ashby JP, Honeybourne D. 1990. Azithromycin concentrations at the site of pulmonary infections. Eur Respir J 3:886–890. [PubMed] [Google Scholar]