ABSTRACT
Treatment recommendations for Plasmodium malariae and Plasmodium ovale malaria are largely based on anecdotal evidence. The aim of this prospective study, conducted in Gabon, was to systematically assess the efficacy and safety of artemether-lumefantrine for the treatment of patients with uncomplicated P. malariae or P. ovale species monoinfections or mixed Plasmodium infections. Patients with microscopically confirmed P. malariae, P. ovale, or mixed-species malaria with at least one of these two Plasmodium species were treated with an oral, fixed-dose combination of artemether-lumefantrine for 3 consecutive days. The primary endpoints were per-protocol PCR-corrected adequate clinical and parasitological response (ACPR) on days 28 and 42. Tolerability and safety were recorded throughout the follow-up period. Seventy-two participants (42 male and 30 female) were enrolled; 62.5% of them had PCR-corrected mixed Plasmodium infections. Per protocol, PCR-corrected ACPR rates were 96.6% (95% confidence interval [CI], 91.9 to 100) on day 28 and 94.2% (95% CI, 87.7 to 100) on day 42. Considering Plasmodium species independently from their coinfecting species, day 42 ACPR rates were 95.5% (95% CI, 89.0 to 100) for P. falciparum, 100% (exact CI, 84.6 to 100) for P. malariae, 100% (exact CI, 76.8 to 100) for P. ovale curtisi, and 90.9% (95% CI, 70.7 to 100) for P. ovale wallikeri. Study drug-related adverse events were generally mild or moderate. In conclusion, this clinical trial demonstrated satisfying antimalarial activity of artemether-lumefantrine against P. ovale wallikeri, P. ovale curtisi, P. malariae, and mixed Plasmodium infections, with per-protocol efficacies of 90% to 100% and without evident tolerability or safety concerns. (This trial was registered in the clinical study database ClinicalTrials.gov under the identifier NCT02528279.)
KEYWORDS: Plasmodium malariae, Plasmodium ovale, artemether-lumefantrine, mixed Plasmodium malaria
INTRODUCTION
Whereas a plethora of treatment trials have been conducted for Plasmodium falciparum and, to a lesser extent, for Plasmodium vivax malaria over the past decades, there is a virtual dearth of interventional trials for Plasmodium ovale and Plasmodium malariae malaria (1, 2). Although drug resistance is of lesser concern for these Plasmodium species, high-quality evidence is nevertheless needed for informed treatment recommendations. In addition, P. malariae and P. ovale may cause severe diseases and death in some patients, highlighting the necessity for fast and efficacious treatment (1).
Oral chloroquine has long been the single recommended treatment for uncomplicated malaria caused by P. malariae and P. ovale parasites. Although declining, resistance to chloroquine is still widespread in P. falciparum populations in many parts of the world, including Gabon (3, 4). Chloroquine treatment of missed P. falciparum coinfections could thus harbor the danger of treatment failure. In order to increase treatment efficacy and reduce the risk of development of drug resistance, the World Health Organization (WHO) advocates the use of artemisinin-based combination therapies (ACTs). At present, chloroquine and ACTs are suggested for use in uncomplicated P. malariae and P. ovale infections in areas where the organisms are chloroquine susceptible, whereas the recommendation for areas with chloroquine-resistant infections exclusively shifted to ACTs (5). A uniform treatment strategy excluding the risk of ineffective drug administration seems even more beneficial, as the ongoing development of molecular methods reveals that the composition of species in a malaria infection is more diverse than detected by microscopy alone (6).
To date, ACTs dominate treatment policies in sub-Saharan Africa (7). Many clinical trials have proven that ACTs have been very efficacious and tolerable against P. falciparum malaria in the African population over the past years (8–10). Treatment recommendations for P. malariae and P. ovale malaria are, however, mainly based on considered efficacy (5). Evidence about the therapeutic effects of ACTs is scarce, and sample sizes are small (1, 2). The aim of this study thus was to evaluate the efficacy of artemether-lumefantrine in a high-transmission setting for patients with P. malariae or P. ovale malaria or mixed Plasmodium malaria.
RESULTS
Clinical characteristics.
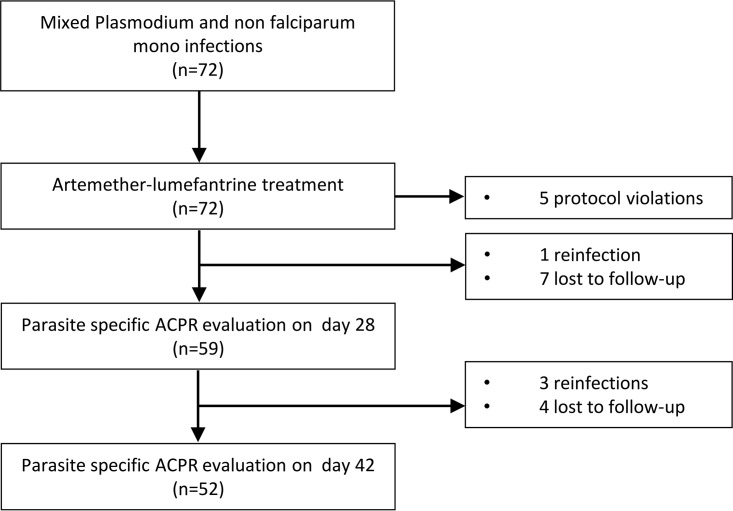
During the screening between October 2014 and April 2016, 72 patients were included in this study (intention-to-treat [ITT] population). An overview of the study flow is provided in Fig. 1.
FIG 1.
Study flow chart of the PP population.
Forty-two (58.3%) participants were male and 30 (41.7%) were female. Baseline characteristics of the study population are outlined in Table 1.
TABLE 1.
Baseline characteristics of the ITT population
| Parameter | No. of patients (%) | Value |
|---|---|---|
| Median age (yr) (IQR) | 72 | 6 (3–13) |
| <5 | 27 (37.5) | |
| 5–18 | 31 (43.1) | |
| >18 | 14 (19.4) | |
| Median wt (kg) (IQR) | 58a | 17.5 (13–26) |
| Mean overall BMI (SD) | 54 | 16.7 (13.2) |
| BMI adapted to sex and age | ||
| Severely underweight | 4 (7.4) | |
| Underweight | 4 (7.4) | |
| Normal wt | 34 (63) | |
| Overweight | 8 (14.8) | |
| Obese | 4 (7.4) | |
| Median asexual parasitemia (parasites/μl) (IQR) | 68b | 932 (334.3–4,126) |
| Mean hemoglobin (mmol/liter) (SD) | 62 | 6.1 (1.4) |
| Mean hematocrit (%) (SD) | 62 | 31.7 (6) |
| Mean systolic blood pressure (mm Hg) (SD) | 64 | 104.1 (16) |
| Mean diastolic blood pressure (mm Hg) (SD) | 64 | 69.4 (13.3) |
| Mean heart rate (beats/min) (SD) | 66 | 101.4 (21.8) |
Out of the values missing, the weights of 2 children were calculated with the following formula: weight = (2 × age) + 8; the other 12 missing weights were for adults and received an adult dose of artemether-lumefantrine.
For missing values only positivity and detection of non-P. falciparum species were documented.
Baseline parasitemias ranged from 11/μl to 24,807/μl, with a median of 932/μl. P. vivax was not detected by microscopy or by PCR. Species determination of baseline blood samples shown listed in Table 2. Microscopic asexual parasitemia cleared for all evaluable participants during the first week after treatment initiation.
TABLE 2.
Plasmodium species composition at baseline
| Microscopy results |
Real-time PCR results |
||
|---|---|---|---|
| Plasmodium species | No. of patients infected | Plasmodium species | No. of patients infected |
| Monoinfection | Monoinfection | ||
| P. falciparum | 0 | P. falciparum | 16 |
| P. malariae | 18 | P. malariae | 1 |
| P. ovale | 13 | P. ovale curtisi | 2 |
| —a | P. ovale wallikeri | 2 | |
| Mixed infection | Mixed infection | ||
| P. falciparum + P. malariae | 12 | P. falciparum + P. malariae | 21 |
| P. falciparum + P. ovale | 19 | P. falciparum + P. ovale curtisi | 6 |
| — | P. falciparum + P. ovale wallikeri | 4 | |
| — | P. falciparum + P. ovale curtisi + P. ovale wallikeri | 2 | |
| P. falciparum + P. malariae + P. ovale | 4 | P. falciparum + P. malariae + P. ovale curtisi | 5 |
| — | P. falciparum + P. malariae + P. ovale wallikeri | 5 | |
| — | P. falciparum + P. malariae + P. ovale curtisi + P. ovale wallikeri | 2 | |
| P. malariae + P. ovale | 2 | — | |
| Positive non-falciparum | 4 | No result | 6 |
| Total | 72 | Total | 72 |
—, not applicable.
The median parasite clearance time (PCT) was 24 h (interquartile range [IQR], 24 to 48 h). Five participants presented with a temperature of ≥38°C prior to drug administration, whereas all other patients reported a history of fever within the past 3 days. The median fever clearance time (FCT) was 24 h (IQR, 8 to 24 h). PCR-corrected adequate clinical and parasitological response (ACPR) for the per-protocol (PP) and ITT populations on days 28 and 42 are outlined in Table 3. In Table 4, the ACPRs of the PP populations are stratified by age, describing drug efficacy separately for the high-risk (<5 years) and low-risk (≥5 years) populations.
TABLE 3.
PCR-corrected ACPR in PP and crude ACPR in ITT populations
| Parameter | No. of patients (%) | 95% CI |
|---|---|---|
| PCR-corrected ACPR in PP population | ||
| ACPR28 (n = 59) | 57 (96.6) | 91.9–100 |
| ACPR42 (n = 52) | 49 (94.2) | 87.7–100 |
| Non-PCR-corrected ACPR in ITT population | ||
| ACPR28 (n = 72) | 57 (79.2) | 69.6–88.8 |
| ACPR42 (n = 72) | 44 (61.1) | 49.6–72.6 |
TABLE 4.
PCR-corrected ACPR in the PP population stratified by age
| Parameter | No. of patients (%) | 95% CI |
|---|---|---|
| ACPR28 | ||
| <5 yrs (n = 21) | 20 (95.2) | 85.3–100 |
| ≥5 yrs (n = 38) | 37 (97.4) | 92.0–100 |
| ACPR42 | ||
| <5 yrs (n = 18) | 16 (88.9) | 72.8–100 |
| ≥5 yrs (n = 34) | 33 (97.1) | 91.1–100 |
Fifty-three participants (85.5%; n = 62) were anemic at the time of inclusion, and 6 (9.7%) were severely anemic. Hemoglobin levels of patients with severe anemia were above the limit of classification as severe malaria (>5 g/dl or 3.11 mmol/liter). Most cases of baseline anemia occurred in the 13- to 59-month age group (95.6%; n = 22). The lowest proportion occurred in the oldest age group, ≥15 years, with 50% (n = 7) anemic cases. Preceding blood transfusion was not reported in the patients' medical history. Mean hemoglobin rose from a predose level (mean ± standard deviation [SD]) of 6.1 ± 1.4 mmol/liter to 6.7 ± 1.2 mmol/liter on day 28 further to 6.8 ± 1.3 mmol/liter on day 42. On day 42, 18 participants (36.7%) presented with normal hemoglobin levels. None of the participants with a normal baseline value had severe anemia on day 42.
Adverse events.
One event of severe anemia arose in the course of a day 42 P. falciparum recrudescence and was thus not considered to be a severe adverse event (SAE) resulting from the primary malaria event. Otherwise, no SAEs occurred during the observation period. One participant, however, developed appendicitis on the 10th day after treatment initiation and was hospitalized for surgery. This SAE was rated to be unrelated to the study drug.
One pregnancy was reported on day 42 posttreatment. Thus, this participant had received artemether-lumefantrine during the first trimester of her pregnancy. The gestational age at delivery was 37 weeks plus 1 day. Follow-up after pregnancy revealed no complications during labor, an adequate APGAR score (9 after 1 min and 10 after 10 min), and neither congenital anomalies nor birth defects. The birth weight was 3.3 kg, with a length of 51 cm and a head circumference of 35 cm. Yet 5 months after birth, the child suffered from diarrhea and was malnourished, conditions judged unrelated to the drug exposure in utero. He was thus transferred to the pediatric ward. During the last patient contact 8 months after birth, the infant's medical condition had improved.
Reappearance of parasites.
Ten participants had reappearing parasitemia during the 42 days of follow-up. Seven of these events were reinfections, two were late parasitological failures (days 7 and 42), and one was a P. falciparum reinfection mixed with a reappearing P. ovale wallikeri strain. In order to avoid overestimation of the efficacy that was observed in this study, the last was regarded as late parasitological failure in ACPR analyses due to the present-day lack of more precise methods of analysis. No early treatment failures or late clinical failures were observed. Further details are displayed in Table 5.
TABLE 5.
Molecular classification of reappearing parasitemia during the 42-day follow-up period
| No.a | Species | Method of determination | Comment | Result |
|---|---|---|---|---|
| 7* | P. falciparum | glurp and msp-2 nPCRb | One recurrent coinfection with P. ovale wallikeri (see below) | Reinfection |
| 1 | Different PCR-corrected species at predose and recurrence time points | Reinfection | ||
| 1* | P. ovale wallikeri | PCR correction of microscopic result | Coinfection; reinfection of P. falciparum parasite determined by genotyping; no adequate genotyping method available to further analyze reappearing P. ovale wallikeri parasitemia | Reappearance of a P. ovale wallikeri strain (day 28) |
| 2 | P. falciparum | Microscopic result | No sample available for PCR/genotyping | Late parasitological failure (day 7, day 42) |
The number of events with the given results. *, multiple counting.
glurp encodes glutamate-rich protein; msp-2 encodes merozoite surface protein 2. nPCR, nested PCR.
Subgroup analysis of the Plasmodium species-specific efficacy of artemether-lumefantrine.
In the respective study populations, artemether-lumefantrine showed PP efficacies of 100% in P. malariae and P. ovale curtisi infections on days 28 and 42. Species-specific efficacies against P. falciparum and P. ovale wallikeri exceeded 95% and 90% at both time points (Table 6).
TABLE 6.
PCR corrected efficacy of artemether-lumefantrine for each Plasmodium species observed in the PP populationa
| Parameter |
P. falciparum |
P. malariae |
P. ovale curtisi |
P. ovale wallikeri |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total no. of patients | No. of patients with ACPR (%) | 95% CI | Total no. of patients | No. of patients with ACPR (%) | 95% CI | Total no. of patients | No. of patients with ACPR (%) | 95% CI | Total no. of patients | No. of patients with ACPR (%) | 95% CI | |
| ACPR28 | 50 | 49 (98.0) | 94.0–100 | 27 | 27 (100) | 87.2–100* | 16 | 16 (100) | 79.4–100* | 11 | 10 (90.9) | 70.7–100 |
| ACPR42 | 44 | 42 (95.5) | 89.0–100 | 22 | 22 (100) | 84.6–100* | 14 | 14 (100) | 76.8–100* | 11 | 10 (90.9) | 70.7–100 |
In this table, the efficacy of artemether-lumefantrine is depicted for each infecting species allowing for multiple counting of single patients: e.g., in the “P. malariae” column, all P. malariae cases that occurred either as monoinfection or as mixed infection are considered. Every malaria event is split up into its causative species and likewise counted for each of these Plasmodium species. For example, a coinfection with P. falciparum and P. malariae is depicted both in the P. falciparum group and in the P. malariae group. This applies similarly to all other depicted groups. *, exact confidence interval computed with R binom package.
DISCUSSION
According to the WHO and Medicines for Malaria Venture (MMV) guidelines for testing antimalarial drug efficacy, artemether-lumefantrine was very efficacious against acute uncomplicated non-P. falciparum monoinfections and mixed Plasmodium malaria (11). This result is in accordance with an earlier study from this area indicating similarly high cure rates (12). Proportions of non-falciparum malaria species in sub-Sahara Africa are not well described. However, it appears that they are more widespread than initially assumed (6, 13). A recent study performed on a malaria-asymptomatic population (n = 850) in Gabon found a microscopic presence of non-falciparum malaria pathogens in 8.5% of all obtained blood smears (14). Another publication on the sympatric occurrence of P. ovale demonstrated population prevalences of up to 1.4% in Equatorial Guinean centers and up to 4.3% in the Ugandan centers (15).
Artemether-lumefantrine was highly efficacious in this study for non-falciparum and mixed-species malaria, with an estimated day 28 efficacy of 94% and the lower 95% confidence interval (CI) above 85%. The study drug was equally efficacious in high-risk (children <5 years) and low-risk populations as well as against all Plasmodium species, although confidence intervals for effect estimates were larger due to the smaller sample size in subgroups. The 9% difference in ACPR rates on day 42 (ACPR42) between children younger than 5 years and older than or equal to 5 years was not statistically significant, which is demonstrated by the overlapping 95% confidence intervals of the two subgroups. Overlapping 95% confidence intervals were also present for respective ACPRs in the Plasmodium species-specific subgroup analysis. Although subtle differences may not be completely ruled out based on the limited sample size, these data provide strong evidence that artemether-lumefantrine is very efficacious against P. malariae and both P. ovale species in all relevant patient populations in Gabon. Since specific drug resistance is not a problem in malariae and ovale malaria, these data are considered to be of high external validity for other regions with these non-falciparum malaria species.
Light microscopy is the current “gold standard” for the diagnosis of malaria in clinical routine practice due to its practicability and availability also in resource-poor settings. Its sensitivity is, however, inferior to those of molecular methods. Additionally, species differentiation is challenging especially in low-parasitemia infections and a known and frequently reported shortcoming of this diagnostic technique (12, 16–18). This study confirmed this shortcoming of microscopy and indicated a tendency of underreporting of the coinfecting Plasmodium species, mainly P. falciparum, when correcting the microscopic results by PCR. This misclassification is a potential hazard for the clinical management of patients and highlights the advantage of a unified treatment approach for all uncomplicated malaria cases with an ACT.
Monitoring the continuous therapeutic efficacy of presently used antimalarial therapies to ensure absence of clinically relevant antimalarial drug resistance is an important task of the scientific community. In this study, patients microscopically misdiagnosed as having non-falciparum malaria cases which were then confirmed by molecular methods as P. falciparum monoinfections constituted a control group for the evaluation of P. falciparum susceptibility. This analysis demonstrated a 98% PP efficacy on day 28 and a 96% PP efficacy on day 42 (both species specific), indicating that artemether-lumefantrine remains efficacious against P. falciparum. This result is consistent with findings from neighboring Congo (19), Cameroon (20), and other countries in this region (21–23).
Safety data about drug exposure during pregnancy are usually scarce. However, artemether-lumefantrine and other antimalarials have been recently evaluated in studies that were exclusively designed for pregnant women (24, 25). One article was published by the Pregact Study Group, which carried out a multicenter randomized open-label trial on P. falciparum-infected women with 2nd- and 3rd-trimester pregnancies. They found no differences in the birth outcomes between the tested ACTs. Artemether-lumefantrine was, however, associated with fewest AEs (25). Manyando et al. performed a prospective cohort study on women who had been exposed to antimalarials during their first trimester. They described that patients exposed to only artemether-lumefantrine during their first trimester of pregnancy did not have a greater risk for adverse birth outcomes and had preterm deliveries similar to those of the others. Gestational age, length, and head circumference were similar to those of babies of women that did not receive an antimalarial (24). This outcome is concordant with our case.
Apart from one not-treatment-related SAE, AEs were generally mild or moderate, which was concordant with observations made in other studies (25, 26). Yet an intriguingly high number of participants were and remained anemic during the observation period, indicating the potential impact of non-falciparum malaria as a cause of anemia, as also previously observed in P. malariae-infected patients, as well as the interplay with other chronic conditions (27).
To conclude, artemether-lumefantrine was an efficacious and well-tolerated treatment for uncomplicated P. ovale curtisi, P. ovale wallikeri, and P. malariae monoinfections as well as mixed Plasmodium malaria. The high efficacy in the P. falciparum subgroup indicates the absence of resistance against artemether or lumefantrine in the Central African region.
MATERIALS AND METHODS
This prospective, single-arm, open-label study was conducted at the Centre de Recherches Médicales de Lambaréné (CERMEL) and at the Centre de Recherches Médicales de la Ngounié (CRMN) in Gabon, Central Africa (14, 28). Patients were included at the above-mentioned research centers, which are both situated in areas where malaria is highly endemic, with perennial malaria transmission (29). The trial was registered in the clinical study database ClinicalTrials.gov under the identifier NCT02528279.
The present study was a repeated conduct carried out with the intention to target an increased number of PCR-corrected non-P. falciparum monoinfections and mixed Plasmodium infections in a larger overall sample size and with a prolonged observation period (12). To attain increased sensitivity for the molecular diagnosis of P. malariae and P. ovale, which was discussed to be a potential drawback in the aforementioned publication, frozen blood samples were used instead of dried blood spots and an ultrasensitive reverse transcription-PCR (RT-PCR) assay was applied. Additionally, an analysis of species-specific treatment efficacy was performed for all detected species.
Ethical conduct.
The clinical trial was conducted in accordance with the International Conference on Harmonisation's Guideline for Good Clinical Practice, the Declaration of Helsinki, and applicable national and international standards. The study protocol was approved by the Institutional Ethics Committee of CERMEL. Written informed consent was provided by study participants or legal representatives prior to inclusion. In case of illiteracy, witnessed consent was permitted.
Inclusion and exclusion criteria.
Patients older than 1 year with microscopically confirmed uncomplicated malaria with fever or a history of fever in the preceding 3 days were considered for inclusion. Presence of a microscopic diagnosis of P. malariae, P. ovale subspecies, or a mixed Plasmodium species infection, including either P. malariae or P. ovale subspecies, with an asexual parasite count between 10 and 200,000 parasites per microliter was required for inclusion. P. ovale wallikeri and P. ovale curtisi infections were both eligible, as they cannot be distinguished microscopically (30). Patients with an intake of any antimalarial or antibiotic with known antimalarial activity in the preceding 72 h, or intake of an 8-aminoquinoline or atovaquone-proguanil in the preceding 28 days, were not eligible for the study. Further exclusion criteria were presence of microscopic P. falciparum monoinfection, presence of severe malaria according to the WHO criteria or other febrile conditions, known history of hypersensitivity or allergic or adverse reactions to artemether or lumefantrine, and pregnancy in the first trimester (31). Patients were followed up for 42 days to determine the efficacy of the artemether-lumefantrine treatment.
Study drug administration.
Oral artemether-lumefantrine (Coartem or Coartem Dispersible, Novartis, Switzerland) was administered as a six-dose regimen for 3 consecutive days according to the manufacturer's recommendations. Participants were encouraged to stay for 3 days at the research unit where the study medication was administered by trained personnel under observation with fatty food or milk. In the case that participants were unable to stay at the research unit for 3 days, participants or guardians received a treatment plan and were trained in when and how to take the medication. Adherence to the regimen was inquired during the following visits, which took place daily during the treatment period, followed by a visit on day 7. Concomitant antimalarial drug intake during the study led to exclusion from the PP population. Antirelapse therapy with an 8-aminoquinoline is not recommended in national treatment policies in Gabon and was therefore not administered during the study.
Efficacy and safety assessments.
Primary efficacy endpoints were ACPR on day 28 and day 42. Secondary efficacy endpoints were PCT and FCT. For the assessment of PCT and FCT, the first negative event observed was taken as an endpoint.
Medical history, body weight, and height were obtained at recruitment. Body mass index (BMI) was normalized for sex and age. For children younger than 19 years, BMI was evaluated according to the percentiles of the WHO simplified field tables (http://www.who.int/childgrowth/standards/bmi_for_age_field/en/, http://www.who.int/growthref/who2007_bmi_for_age_field/en/, and http://apps.who.int/bmi/index.jsp). Physical examination was performed on days 0, 14, 28, and 42 and as clinically indicated. Hematology testing was done on days 0, 28, and 42 and as part of unscheduled visits. Severity of anemia was evaluated according to the WHO recommendations (http://www.who.int/vmnis/indicators/haemoglobin.pdf). Vital signs were measured on days 0, 1, and 2 and on every visit thereafter. Concomitant medication was recorded throughout the whole study. Thick and thin blood smears were collected prior to dosing, on days 1, 2, 7, 14, 21, 28, 35, and 42 and when clinically indicated. Parasitological diagnosis was performed with light microscopy by trained microscopists following the Lambaréné method (32, 33). Venous blood was taken prior to dosing, on days 14, 28, 42, and in the case of microscopic recurrence and stored for further molecular analysis. Temperature and clinical signs and symptoms of malaria were assessed at each visit during the study.
Safety was assessed considering nature, incidence, drug relationship, and severity of adverse events (AEs) and serious adverse events (SAEs). Concomitant diseases and AEs requiring medical care were treated according to local practice.
Molecular methods.
Microscopically confirmed non-falciparum malaria was further evaluated by molecular methods to confirm species distribution at recruitment and to perform PCR correction for recrudescence or reinfection in case of reappearing parasitemia.
Sample collection and nucleic acid extraction.
The collected blood samples were mixed with RNAlater stabilization solution (Thermo Fisher Scientific) and stored at −20°C for total nucleic acid extraction. Purification of nucleic acids was performed automated in the QIAsymphony SP (Qiagen) using a QIAsymphony DSP DNA Midi kit and DNA Blood 400 V6 DSP protocol as per the manufacturer's instructions. Before purification, blood samples were thawed at room temperature. RNAlater was removed by centrifugation at ∼16,000 × g for 1 min. The packed red blood cells were resuspended with 1× phosphate-buffered saline (PBS; Thermo Fisher Scientific) to a final volume of 420 μl and loaded onto the QIAsymphony SP. Nucleic acids were eluted in 100 μl of elution buffer and stored in a 96-well plate (Qiagen) at −20°C until use.
Detection of Plasmodium infections by ultrasensitive RT-qPCR.
Screening for Plasmodium infections was performed by pan-Plasmodium reverse transcription-quantitative PCR (RT-qPCR) assay as described previously (34). In brief, the assay was performed using a TaqMan RNA-to-CT 1-Step kit (Thermo Fisher Scientific) according to the manufacturer's recommendations. These primers amplify a 100-bp fragment of the small-subunit rRNA gene (18S) which is conserved across the human malaria pathogens. A quantification cycle (Cq) threshold of 40 was used as a cutoff to define sample positivity (Cq < 40). The assay setup and pipetting were performed in a sterile PCR workstation and with the QIAgility (Qiagen).
Differential diagnosis of Plasmodium species infections by nested quantitative real-time PCR.
Samples that were positive by pan-Plasmodium RT-qPCR assay were first subjected to conventional PCR amplification using genus-specific primers by Snounou et al. (35) for further utilization as the template for quantitative real-time PCR assay. These so-called preamplifications were performed in a 50-μl reaction volume containing 7.5 μl of nucleic acid extract, 300 μM each primer (PLU5 and PLU6), 1× Qiagen PCR buffer with 1.5 mM MgCl2, a 250 μM concentration of each deoxynucleoside triphosphate (dNTP), and 1 U of Taq DNA polymerase (Qiagen; catalog no. 201223). The cycling conditions were initial denaturation at 95°C for 5 min followed by 20 cycles of 95°C for 30 s, 58°C for 30 s, and 72°C for 1 min 20 s and a final extension at 72°C for 5 min.
Diagnosis of Plasmodium infections was carried out by nested qPCR assay using primers and probes specifically developed for this study, as well as primers described in previous publications (35–37) (see Table S1 in the supplemental material). Primers and probes for the detection of P. malariae and the two P. ovale subspecies were designed using OligoArchitect online (Sigma-Aldrich) after comparing the 18S rRNA gene sequences from GenBank. The singleplex qPCR assay was performed for five human malaria pathogens (P. falciparum, P. malariae, P. ovale curtisi, P. ovale wallikeri, and P. vivax) on the LightCycler 480 Instrument II (Roche Applied Science). Each of the species-specific qPCR assays consisted of 2.5 μl of the preamplified product, 1× SensiMix II Probe No-ROX (Bioline), 300 μM each primer pair, and 150 μM each probe (see Table S1 in the supplemental material for further details) per 10-μl final reaction volume. The cycling conditions were polymerase activation at 94°C for 10 min followed by 45 cycles of 95°C for 10 s and 60°C for 60 s. Each of the samples was tested in duplicates, and all the assays included a nontemplate control (NTC) and a positive control in duplicates. The quantification cycle values were automatically calculated by the second derivative maximum method integrated in the LightCycler 480 software (version 1.5.1.62). Positivity was considered after visual assessment of the amplification curves for variability between each sample replicate (standard deviation ≤ 1 cycle) and the quantification threshold value of less than 40. All the assays were tested and validated in accordance with the Minimum Information for Publication of Quantitative Real-Time PCR Experiments (MIQE) guidelines (38) for their specificity and showed no cross-species amplification.
Subgroup analysis.
This study was designed as an uncontrolled trial based on the fact that chloroquine—the alternative standard treatment for non-falciparum malaria—must not be used in cases of mixed-species infection due to the high prevalence of chloroquine-resistant P. falciparum. However, in order to highlight the efficacy of artemether-lumefantrine for each of the observed Plasmodium species, a subgroup analysis was performed to determine the ACPR of P. falciparum, P. malariae, P. ovale curtisi, and P. ovale wallikeri regardless of whether they occurred in a monoinfection or a mixed infection, allowing for multiple counting of patients for respective Plasmodium species as applicable. In this analysis, each malaria event was split up in its causative species and likewise counted for each Plasmodium species (e.g., a P. falciparum and P. malariae coinfection was considered for P. falciparum and P. malariae analyses). Impacts on the species-specific efficacy by the coinfecting Plasmodium parasite could, however, not be ruled out.
Plasmodium falciparum genotyping.
In microscopically reappearing P. falciparum infections, genotyping techniques were used to distinguish between recrudescence and reinfection employing glurp and msp-2 nested PCR assays as described previously (11, 39, 40). Bands on agarose gels were interpreted manually on digitized images. Reinfections were not counted as treatment failures and were removed from the PCR-corrected efficacy analysis in the PP population upon their occurrence. In the case of other recurrent Plasmodium species, microscopic species determination was corrected with RT-qPCR and reappearance of the same Plasmodium species was classified as recrudescence. In the case of missing blood samples, the microscopic results were used.
Statistical evaluation and data management.
This descriptive study was not designed to test a statistical hypothesis. Therefore, based on an estimated 95% efficacy on day 28, a sample size of 60 participants was required to allow for the lower 95% confidence interval to remain above 85%. Assuming 20% of loss to follow-up and nonanalyzable participants, a total of 72 patients were recruited into this study.
The ITT population comprised all included patients. Loss to follow-up and exclusions for any reason before day 42 were classified as failures in this analysis. Participants that received a full course of the study medication and were followed up until the respective endpoint were included into the PP analysis. Data were collected on case report forms and transferred anonymized to an electronic standardized Microsoft Excel 2013 datasheet. IBM SPSS Statistics 20 and Stata 13 were used to perform descriptive statistics and to compute 95% confidence intervals. The last-observation-carried-forward method was applied unless missing data were due to subjects dropping out of the study.
Supplementary Material
ACKNOWLEDGMENTS
We acknowledge the support with study activities from Fleuramie M. Boukoumba and Solveig Zöller.
We thank the study participants and the family members accompanying the young participants for their cooperation. We also thank the field workers, nurses, and other study personnel for their commitment.
This work was financially supported by the Karl Landsteiner Gesellschaft and the Federal Ministry of Science, Research, and Economy of Austria as part of the EDCTP-2 program, supported by the European Union.
The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
We have no conflicts of interest to declare.
M.G., L.V., C.C., G.M.-N., R.Z.-M., J.M., L.E., A.K., J.K., T.N., J.R., and L.F. contributed substantially to the acquisition of data. A.L., M.G., and J.M. contributed substantially to the analysis. M.G., J.M., and M.R. contributed substantially to the interpretation of data. M.R., G.M.-N., M.G., B.M., P.G.K., A.A.A., S.T.A., and P.-B.M. contributed substantially to the conception. M.G., A.L., J.M., M.R., and L.V. drafted the manuscript. G.M.-N., P.G.K., C.C., A.K., R.Z.-M., L.F., B.M., A.A.A., S.T.A., P.-B.M., L.E., J.K., T.N., and J.R. revised the work critically for important intellectual content. All authors gave final approval of the version to be published and are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/AAC.01758-17.
REFERENCES
- 1.Groger M, Fischer HS, Veletzky L, Lalremruata A, Ramharter M. 2017. A systematic review of the clinical presentation, treatment and relapse characteristics of human Plasmodium ovale malaria. Malar J 16:112. doi: 10.1186/s12936-017-1759-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Visser BJ, Wieten RW, Kroon D, Nagel IM, Belard S, van Vugt M, Grobusch MP. 2014. Efficacy and safety of artemisinin combination therapy (ACT) for non-falciparum malaria: a systematic review. Malar J 13:463. doi: 10.1186/1475-2875-13-463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Binder RK, Borrmann S, Adegnika AA, Missinou MA, Kremsner PG, Kun JF. 2002. Polymorphisms in the parasite genes for pfcrt and pfmdr-1 as molecular markers for chloroquine resistance in Plasmodium falciparum in Lambarene, Gabon. Parasitol Res 88:475–476. doi: 10.1007/s00436-001-0546-7. [DOI] [PubMed] [Google Scholar]
- 4.Borrmann S, Binder RK, Adegnika AA, Missinou MA, Issifou S, Ramharter M, Wernsdorfer WH, Kremsner PG. 2002. Reassessment of the resistance of Plasmodium falciparum to chloroquine in Gabon: implications for the validity of tests in vitro vs. in vivo. Trans R Soc Trop Med Hyg 96:660–663. doi: 10.1016/S0035-9203(02)90345-7. [DOI] [PubMed] [Google Scholar]
- 5.WHO. 2015. Guidelines for the treatment of malaria, 3rd ed WHO, Geneva, Switzerland: http://apps.who.int/iris/bitstream/10665/162441/1/9789241549127_eng.pdf. [Google Scholar]
- 6.Mueller I, Zimmerman PA, Reeder JC. 2007. Plasmodium malariae and Plasmodium ovale—the “bashful” malaria parasites. Trends Parasitol 23:278–283. doi: 10.1016/j.pt.2007.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.WHO. 2016. World malaria report 2016. WHO, Geneva, Switzerland: http://apps.who.int/iris/bitstream/10665/252038/1/9789241511711-eng.pdf?ua=1. [Google Scholar]
- 8.Bouyou-Akotet MK, Ramharter M, Ngoungou EB, Mamfoumbi MM, Mihindou MP, Missinou MA, Kurth F, Belard S, Agnandji ST, Issifou S, Heidecker JL, Trapp S, Kremsner PG, Kombila M. 2010. Efficacy and safety of a new pediatric artesunate-mefloquine drug formulation for the treatment of uncomplicated falciparum malaria in Gabon. Wien Klin Wochenschr 122:173–178. doi: 10.1007/s00508-010-1317-1. [DOI] [PubMed] [Google Scholar]
- 9.Four Artemisinin-Based Combinations (4ABC) Study Group. 2011. A head-to-head comparison of four artemisinin-based combinations for treating uncomplicated malaria in African children: a randomized trial. PLoS Med 8(11):e1001119. doi: 10.1371/journal.pmed.1001119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.WWARN AL Dose Impact Study Group. 2015. The effect of dose on the antimalarial efficacy of artemether-lumefantrine: a systematic review and pooled analysis of individual patient data. Lancet Infect Dis 15:692–702. doi: 10.1016/S1473-3099(15)70024-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.WHO, MMV. 2008. Methods and techniques for clinical trials on antimalarial drug efficacy: genotyping to identify parasite populations. WHO, Geneva, Switzerland: http://apps.who.int/iris/bitstream/10665/43824/1/9789241596305_eng.pdf. [Google Scholar]
- 12.Mombo-Ngoma G, Kleine C, Basra A, Wurbel H, Diop DA, Capan M, Adegnika AA, Kurth F, Mordmuller B, Joanny F, Kremsner PG, Ramharter M, Belard S. 2012. Prospective evaluation of artemether-lumefantrine for the treatment of non-falciparum and mixed-species malaria in Gabon. Malar J 11:120. doi: 10.1186/1475-2875-11-120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Collins WE, Jeffery GM. 2005. Plasmodium ovale: parasite and disease. Clin Microbiol Rev 18:570–581. doi: 10.1128/CMR.18.3.570-581.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Manego RZ, Mombo-Ngoma G, Witte M, Held J, Gmeiner M, Gebru T, Tazemda B, Mischlinger J, Groger M, Lell B, Adegnika AA, Agnandji ST, Kremsner PG, Mordmuller B, Ramharter M, Matsiegui PB. 2017. Demography, maternal health and the epidemiology of malaria and other major infectious diseases in the rural department Tsamba-Magotsi, Ngounie Province, in central African Gabon. BMC Public Health 17:130. doi: 10.1186/s12889-017-4045-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Oguike MC, Betson M, Burke M, Nolder D, Stothard JR, Kleinschmidt I, Proietti C, Bousema T, Ndounga M, Tanabe K, Ntege E, Culleton R, Sutherland CJ. 2011. Plasmodium ovale curtisi and Plasmodium ovale wallikeri circulate simultaneously in African communities. Int J Parasitol 41:677–683. doi: 10.1016/j.ijpara.2011.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Alemu A, Fuehrer HP, Getnet G, Kassu A, Getie S, Noedl H. 2014. Comparison of Giemsa microscopy with nested PCR for the diagnosis of malaria in North Gondar, north-west Ethiopia. Malar J 13:174. doi: 10.1186/1475-2875-13-174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cao Y, Wang W, Liu Y, Cotter C, Zhou H, Zhu G, Tang J, Tang F, Lu F, Xu S, Gu Y, Zhang C, Li J, Cao J. 2016. The increasing importance of Plasmodium ovale and Plasmodium malariae in a malaria elimination setting: an observational study of imported cases in Jiangsu Province, China, 2011–2014. Malar J 15:459. doi: 10.1186/s12936-016-1504-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chavatte JM, Tan SB, Snounou G, Lin RT. 2015. Molecular characterization of misidentified Plasmodium ovale imported cases in Singapore. Malar J 14:454. doi: 10.1186/s12936-015-0985-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.van den Broek I, Kitz C, Al Attas S, Libama F, Balasegaram M, Guthmann JP. 2006. Efficacy of three artemisinin combination therapies for the treatment of uncomplicated Plasmodium falciparum malaria in the Republic of Congo. Malar J 5:113. doi: 10.1186/1475-2875-5-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nji AM, Ali IM, Moyeh MN, Ngongang EO, Ekollo AM, Chedjou JP, Ndikum VN, Evehe MS, Froeschl G, Heumann C, Mansmann U, Ogundahunsi O, Mbacham WF. 2015. Randomized non-inferiority and safety trial of dihydroartemisin-piperaquine and artesunate-amodiaquine versus artemether-lumefantrine in the treatment of uncomplicated Plasmodium falciparum malaria in Cameroonian children. Malar J 14:27. doi: 10.1186/s12936-014-0521-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Denoeud-Ndam L, Dicko A, Baudin E, Guindo O, Grandesso F, Diawara H, Sissoko S, Sanogo K, Traore S, Keita S, Barry A, de Smet M, Lasry E, Smit M, Wiesner L, Barnes KI, Djimde AA, Guerin PJ, Grais RF, Doumbo OK, Etard JF. 2016. Efficacy of artemether-lumefantrine in relation to drug exposure in children with and without severe acute malnutrition: an open comparative intervention study in Mali and Niger. BMC Med 14:167. doi: 10.1186/s12916-016-0716-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Getnet G, Fola AA, Alemu A, Getie S, Fuehrer HP, Noedl H. 2015. Therapeutic efficacy of artemether-lumefantrine for the treatment of uncomplicated Plasmodium falciparum malaria in Enfranze, north-west Ethiopia. Malar J 14:258. doi: 10.1186/s12936-015-0775-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ursing J, Rombo L, Rodrigues A, Kofoed PE. 2016. Artemether-lumefantrine versus dihydroartemisinin-piperaquine for treatment of uncomplicated Plasmodium falciparum malaria in children aged less than 15 years in Guinea-Bissau—an open-label non-inferiority randomised clinical trial. PLoS One 11(9):e0161495. doi: 10.1371/journal.pone.0161495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Manyando C, Njunju EM, Virtanen M, Hamed K, Gomes M, Van Geertruyden JP. 2015. Exposure to artemether-lumefantrine (Coartem) in first trimester pregnancy in an observational study in Zambia. Malar J 14:77. doi: 10.1186/s12936-015-0578-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pregact Study Group. 2016. Four artemisinin-based treatments in African pregnant women with malaria. N Engl J Med 374:913–927. doi: 10.1056/NEJMoa1508606. [DOI] [PubMed] [Google Scholar]
- 26.Makanga M, Bassat Q, Falade CO, Premji ZG, Krudsood S, Hunt P, Walter V, Beck HP, Marrast AC, Cousin M, Rosenthal PJ. 2011. Efficacy and safety of artemether-lumefantrine in the treatment of acute, uncomplicated Plasmodium falciparum malaria: a pooled analysis. Am J Trop Med Hyg 85:793–804. doi: 10.4269/ajtmh.2011.11-0069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Langford S, Douglas NM, Lampah DA, Simpson JA, Kenangalem E, Sugiarto P, Anstey NM, Poespoprodjo JR, Price RN. 2015. Plasmodium malariae infection associated with a high burden of anemia: a hospital-based surveillance study. PLoS Negl Trop Dis 9:4195. doi: 10.1371/journal.pntd.0004195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ramharter M, Adegnika AA, Agnandji ST, Matsiegui PB, Grobusch MP, Winkler S, Graninger W, Krishna S, Yazdanbakhsh M, Mordmuller B, Lell B, Missinou MA, Mavoungou E, Issifou S, Kremsner PG. 2007. History and perspectives of medical research at the Albert Schweitzer Hospital in Lambarene, Gabon. Wien Klin Wochenschr 119:8–12. doi: 10.1007/s00508-007-0857-5. [DOI] [PubMed] [Google Scholar]
- 29.Sylla EH, Kun JF, Kremsner PG. 2000. Mosquito distribution and entomological inoculation rates in three malaria-endemic areas in Gabon. Trans R Soc Trop Med Hyg 94:652–656. doi: 10.1016/S0035-9203(00)90219-0. [DOI] [PubMed] [Google Scholar]
- 30.Sutherland CJ, Tanomsing N, Nolder D, Oguike M, Jennison C, Pukrittayakamee S, Dolecek C, Hien TT, Vedo Rosario Arez AP, Pinto J, Michon P, Escalante AA, Nosten F, Burke M, Lee R, Blaze M, Otto TD, Barnwell JW, Pain A, Williams J, White NJ, Day NP, Snounou G, Lockhart PJ, Chiodini PL, Imwong M, Polley SD. 2010. Two nonrecombining sympatric forms of the human malaria parasite Plasmodium ovale occur globally. J Infect Dis 201:1544–1550. doi: 10.1086/652240. [DOI] [PubMed] [Google Scholar]
- 31.WHO. 2012. Management of severe malaria: a practical handbook, 3rd ed WHO, Geneva, Switzerland: http://apps.who.int/iris/bitstream/10665/79317/1/9789241548526_eng.pdf. [Google Scholar]
- 32.Kremsner PG, Zotter GM, Feldmeier H, Graninger W, Rocha RM, Wiedermann G. 1988. A comparative trial of three regimens for treating uncomplicated falciparum malaria in Acre, Brazil. J Infect Dis 158:1368–1371. doi: 10.1093/infdis/158.6.1368. [DOI] [PubMed] [Google Scholar]
- 33.Planche T, Krishna S, Kombila M, Engel K, Faucher JF, Ngou-Milama E, Kremsner PG. 2001. Comparison of methods for the rapid laboratory assessment of children with malaria. Am J Trop Med Hyg 65:599–602. doi: 10.4269/ajtmh.2001.65.599. [DOI] [PubMed] [Google Scholar]
- 34.Mordmüller B, Surat G, Lagler H, Chakravarty S, Ishizuka AS, Lalremruata A, Gmeiner M, Campo JJ, Esen M, Ruben AJ, Held J, Calle CL, Mengue JB, Gebru T, Ibanez J, Sulyok M, James ER, Billingsley PF, Natasha KC, Manoj A, Murshedkar T, Gunasekera A, Eappen AG, Li T, Stafford RE, Li M, Felgner PL, Seder RA, Richie TL, Sim BK, Hoffman SL, Kremsner PG. 2017. Sterile protection against human malaria by chemoattenuated PfSPZ vaccine. Nature 542:445–449. doi: 10.1038/nature21060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Snounou G, Viriyakosol S, Zhu XP, Jarra W, Pinheiro L, VEdo Rosario Thaithong S, Brown KN. 1993. High sensitivity of detection of human malaria parasites by the use of nested polymerase chain reaction. Mol Biochem Parasitol 61:315–320. [DOI] [PubMed] [Google Scholar]
- 36.Kamau E, Alemayehu S, Feghali KC, Saunders D, Ockenhouse CF. 2013. Multiplex qPCR for detection and absolute quantification of malaria. PLoS One 8(8):e71539. doi: 10.1371/journal.pone.0071539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Véron V, Legrand E, Yrinesi J, Volney B, Simon S, Carme B. 2009. Genetic diversity of msp3alpha and msp1_b5 markers of Plasmodium vivax in French Guiana. Malar J 8:40. doi: 10.1186/1475-2875-8-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bustin SA, Benes V, Garson JA, Hellemans J, Huggett J, Kubista M, Mueller R, Nolan T, Pfaffl MW, Shipley GL, Vandesompele J, Wittwer CT. 2009. The MIQE guidelines: Minimum Information for Publication of Quantitative Real-Time PCR Experiments. Clin Chem 55:611–622. doi: 10.1373/clinchem.2008.112797. [DOI] [PubMed] [Google Scholar]
- 39.WHO, MMV. 2008. Recommended genotyping procedures to identify parasite populations. SOP initiated after informal consultation of MMV and WHO. WHO, Geneva, Switzerland. [Google Scholar]
- 40.WWARN Clinical Group. 10 March 2015. Molecular testing for malaria standard operating procedure (SOP). Msp2 PCR v1.1. https://www.google.at/url?sa=t&rct=j&q=&esrc=s&source=web&cd=1&ved=0ahUKEwj5vIenv8nRAhUGbxQKHWYzBZMQFggdMAA&url=http%3A%2F%2Fwww.wwarn.org%2Fsites%2Fdefault%2Ffiles%2Fattachments%2Fprocedures%2Fmsp2-pcr-v1.1.doc&usg=AFQjCNEg2oxcmCYFoEwJH7vJqVhz227Lqw&bvm=bv.144224172, d.d24&cad=rja.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.