ABSTRACT
Amikacin, kanamycin, and capreomycin are among the most important second-line drugs for multidrug-resistant tuberculosis. Although amikacin and kanamycin are administered at the same dose and show the same pharmacokinetics, they have different WHO breakpoints, suggesting that the two drugs have different MICs. The aim of this study was to investigate possible differences in MICs between the aminoglycosides and capreomycin. Using the direct concentration method, a range of concentrations of amikacin, kanamycin, and capreomycin (0.25, 0.50, 1.0, 2.0, 4.0, 8.0, 16.0, 32.0, and 64.0 mg/liter) were tested against 57 clinical Mycobacterium tuberculosis strains. The 7H10 agar plates were examined for mycobacterial growth after 14 days. At 2 mg/liter, 48 strains (84%) were inhibited by amikacin and only 5 strains (9%) were inhibited by kanamycin (P < 0.05, Wilcoxon signed-rank test). The median MICs of amikacin, kanamycin, and capreomycin were 2, 4, and 8 mg/liter, respectively. No difference in amikacin, kanamycin, and capreomycin MIC distributions was observed between multidrug-resistant strains and fully susceptible strains. The results indicate that amikacin is more active than kanamycin and capreomycin against M. tuberculosis with the absolute concentration method. Determination of the impact of this difference on clinical outcomes in daily practice requires a prospective study, including pharmacokinetic and pharmacodynamic evaluations.
KEYWORDS: Mycobacterium tuberculosis, amikacin, aminoglycosides, capreomycin, glycopeptides, kanamycin, susceptibility testing, tuberculosis
INTRODUCTION
Tuberculosis (TB), caused by Mycobacterium tuberculosis, affects over 12 million people worldwide. More than 9 million new cases and about 1.3 million deaths are documented each year (1). Multidrug-resistant (MDR)-TB, defined by resistance to at least isoniazid and rifampin, requires treatment with second-line drugs. MDR-TB is treated with a combination of a drug from group A (fluoroquinolones), a drug from group B (aminoglycosides and capreomycin), and other second-line agents (groups C and D) (1). Aminoglycosides such as amikacin and kanamycin and the glycopeptide antibiotic capreomycin can be used without a clear preference and have identical dosing schedules, according to WHO guidelines (1).
In daily practice, drug susceptibility is usually tested using breakpoints of 1 mg/liter for amikacin and 2.5 mg/liter for kanamycin and capreomycin, with a MGIT 960 system (2–4). The difference in breakpoints for amikacin, kanamycin, and capreomycin is supported by literature findings, indicating that the MIC of amikacin in vitro is lower than the MICs of kanamycin and capreomycin (5). This could imply that kanamycin is less effective than amikacin in vitro, since a higher concentration is needed to inhibit the growth of the same strain. It is suggested that this difference in MICs may be caused by the butyric acid moiety at the R3 position of kanamycin, reducing its activity against M. tuberculosis (6).
This difference in MICs might be clinically relevant, since the effectiveness of aminoglycosides is likely to depend on the maximum drug concentration (Cmax)/MIC ratio (7, 8). Using a pharmacokinetic (PK)/pharmacodynamic (PD) approach, this suggests that the dosing of amikacin and kanamycin should be adjusted according to their Cmax and MIC values to reach optimal efficacy. From an earlier PK study, it is known that Cmax values do not differ between amikacin and kanamycin when the drugs are given at the same dose of 15 mg/kg (9). More recently, it was noted that the chance of developing hearing loss is lower with reduced aminoglycoside dosing based on peak and trough levels (21). Therapeutic drug monitoring of aminoglycosides seems promising (10) and feasible, as bioanalytical immunoassays and mass spectrometric methods have been made available for amikacin and kanamycin (11, 12). Obviously, to reach the same Cmax/MIC ratio for amikacin and kanamycin, which have different MIC values, a difference in dosing should be employed.
Apparently, there is an inconsistency between available data on the amikacin and kanamycin Cmax and MIC values for M. tuberculosis from a PK/PD point of view and the current WHO dosing recommendations. However, there is a paucity of data on the difference in MIC values for amikacin, kanamycin, and capreomycin using the same panel of strains. Therefore, we tested the in vitro susceptibility of clinical non-MDR and MDR isolates of M. tuberculosis to amikacin, kanamycin, and capreomycin.
RESULTS
Susceptibility testing.
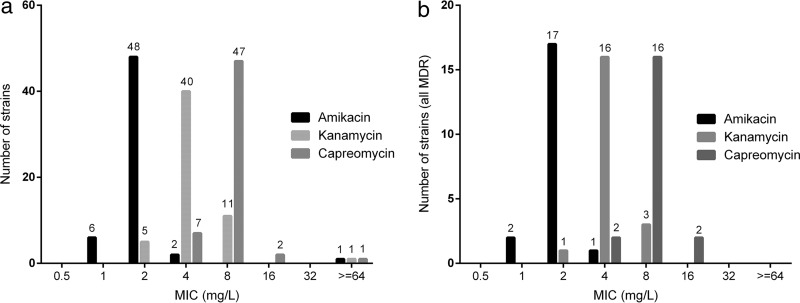
In total, 57 available clinical M. tuberculosis strains and two control strains (ATCC 27294 [H37Rv], which is sensitive, and ATCC 35827, which is resistant to kanamycin) were tested using the direct concentration method. The MICs measured with the H37Rv reference strain were as follows: amikacin, 2 mg/liter; capreomycin, 4 mg/liter; kanamycin, 2 mg/liter. The amikacin, kanamycin, and capreomycin MIC distributions for the clinical isolates are shown in Fig. 1. Individual results per strain can be found in Data Set S1 in the supplemental material.
FIG 1.
MIC distributions for amikacin, kanamycin, and capreomycin. (a) MIC distributions for amikacin, kanamycin, and capreomycin in all strains. (b) MIC distributions for amikacin, kanamycin, and capreomycin in all MDR strains.
At 2 mg/liter, 48 strains (84%) were inhibited by amikacin and 5 strains (9%) by kanamycin. At 8.0 mg/liter, all strains were inhibited by both aminoglycosides. The difference in MICs for amikacin and kanamycin is presented in Table 1. A Wilcoxon signed-rank test showed that the MICs differed significantly between amikacin and kanamycin (Z = −6.6, P < 0.05). The median amikacin and kanamycin MICs were 2 and 4 mg/liter, respectively, and the median capreomycin MIC was 8 mg/liter. The MIC of capreomycin differed significantly from the MICs of amikacin (Z = −6.9, P < 0.05) and kanamycin (Z = −6.2, P < 0.05).
TABLE 1.
Susceptibility of M. tuberculosis strains to amikacin, kanamycin, and capreomycin
| MIC findingsa | No. of strains (%) |
|---|---|
| Amikacin > kanamycin | 0 |
| Kanamycin > amikacin | 12 (21) |
| Amikacin = kanamycin | 45 (79) |
| Amikacin > capreomycin | 0 |
| Capreomycin > amikacin | 51 (89) |
| Amikacin = capreomycin | 6 (11) |
| Kanamycin > capreomycin | 0 |
| Capreomycin > kanamycin | 2 (4) |
| Kanamycin = capreomycin | 55 (96) |
| Total | 57 |
MICs with one or more intermediate dilution steps were considered different.
Comparing individual strains, the MIC of amikacin was more than one dilution step lower than the MIC of kanamycin in 21% of all strains. No strains were more susceptible to kanamycin than to amikacin. The capreomycin MIC was higher than the amikacin MIC in 51 (89%) of all tested strains. In the other 6 strains, the MICs were comparable (within one dilution step). In 2 strains (4%), the capreomycin MICs were more than one dilution step higher than the kanamycin MICs. All other strains showed similar MICs (within one dilution step) for kanamycin and capreomycin. The MICs of amikacin, kanamycin, and capreomycin did not differ between MDR-TB and non-MDR-TB strains (P = 0.98, 0.38, and 0.74, respectively; Mann-Whitney test).
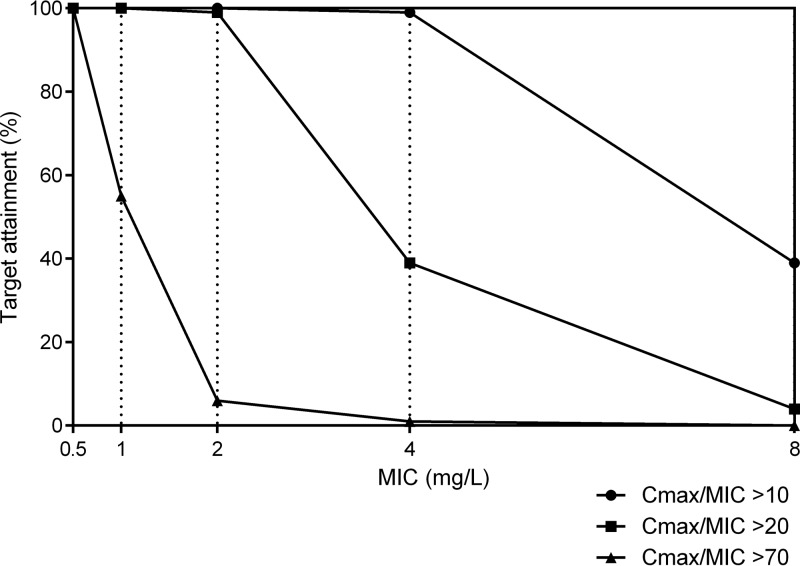
Probability of target attainment.
The probability of target attainment is pictured in Fig. 2, based on a dose of 15 mg/kg. The probability of achieving a Cmax/MIC ratio of 10 at a MIC of 2 mg/liter is 100% for both amikacin and kanamycin. At a MIC of 4 mg/liter, the probability of target attainment is 99%. The probability of target attainment with a MIC of 8 mg/liter is 39%. With a target Cmax/MIC ratio of >20, the probability of target attainment is still 99% at a MIC of 2 mg/liter. Targeting a higher Cmax/MIC ratio of >70, the probability of target attainment at a MIC of 2 mg/liter falls to 6%. The cumulative fraction of response (CFR) of each aminoglycoside at different Cmax/MIC targets is presented in Table 2.
FIG 2.
Target attainment analysis for amikacin and kanamycin at doses of 15 mg/kg, at various Cmax/MIC ratios.
TABLE 2.
Cumulative fraction of response
| Cmax/MIC | CFR (%) |
|
|---|---|---|
| Amikacin | Kanamycin | |
| >10 | 98 | 86 |
| >20 | 96 | 37 |
| >70 | 11 | 1 |
DISCUSSION
This is, to our knowledge, the first systematic study relating the MICs of amikacin, kanamycin, and capreomycin to the probability of target attainment. At least one dilution step in growth inhibition between drugs was required for a significant difference in MICs. We showed that, in general, amikacin was more active than kanamycin and capreomycin in killing M. tuberculosis in vitro. The difference in MICs between amikacin and kanamycin was confirmed in other reports. In all three reports (6, 13, 14), the MICs of amikacin were lower than those of kanamycin. In a more recent study with 207 MDR-TB isolates, the median MICs of amikacin and kanamycin, determined with the MGIT system, were 4 mg/liter and 2 mg/liter, respectively (15); however, large proportions of the strains were above the WHO breakpoints (62.8% and 48.3% for kanamycin and amikacin, respectively). The median MIC of capreomycin was 1 mg/liter, with 62.8% of the strains being above the WHO breakpoint of 2.5 mg/liter (15). These findings indicate that there is a large variation in susceptibility among individual cultures, emphasizing the need to individualize treatments based on quantitative susceptibility.
The difference in MICs between amikacin and kanamycin was illustrated by other studies. Reported epidemiological cutoff (ECOFF) values were 1 mg/liter for amikacin and 4 mg/liter for kanamycin and capreomycin (5). In addition, a large comparative study showed that amikacin MICs were equal to or lower than kanamycin MICs, supporting our findings (16). It should also be noted that kanamycin-resistant strains are commonly amikacin susceptible (17). Based on these findings, it can be concluded that amikacin is more effective than kanamycin in killing M. tuberculosis in vitro. However, this finding has to be confirmed with clinical studies, as in vitro data cannot be extrapolated to clinical efficacy without further clinical study.
This difference could be caused by mutations in the eis promoter gene (18, 19). This gene is responsible for low-level aminoglycoside resistance through the production of acetyltransferase, which inactivates aminoglycosides. However, this enzyme has a higher affinity for kanamycin than for amikacin (18, 19). Therefore, a mutation in the eis promoter gene could result in reduced susceptibility to kanamycin, with only a minor impact on the amikacin MIC, depending on the specific mutation. After mutation of the eis promoter gene, the MICs of amikacin were 0.25 to 2 mg/liter (wild type, 0.25 to 0.5 mg/liter), while the MICs of kanamycin were greatly affected; the MICs of mutants ranged from 5 to 20 mg/liter (wild type, 0.6 to 2.5 mg/liter) (20). However, it should be noted that different mutations in the eis promoter gene result in different phenotypic MICs.
It is generally assumed that the efficacy of aminoglycosides depends on the Cmax/MIC ratio (7). This relationship has only recently been established for M. tuberculosis, however (8). Based on the differences in MICs, it can be debated whether amikacin and kanamycin are equally effective at the same dose. In situations in which MIC and Cmax data were available and therapeutic drug monitoring was applied, preliminary results showed that, in the presence of other active drugs, a lower dose of amikacin was adequate (21).
The method used in this study has some limitations, which are related to the slow multiplication rate of mycobacteria. More modern techniques, such as the Bactec MGIT 960 system, use oxygen consumption to measure growth (22). However, these methods are relatively expensive, in comparison with the direct concentration method.
The absence of genotypic testing in this study is another limitation. With genotypic data, the MIC distribution can be divided for genotypically resistant and sensitive isolates. This prevents the inclusion of resistant isolates in the MIC distributions, which may affect the interpretation of the distributions. However, the MIC distributions shown here provide the actual Cmax/MIC ratios for aminoglycoside treatment and could forecast the efficacy of this treatment.
In most parts of the world, drug susceptibility testing (DST) for second-line drugs is not integrated into standard care. According to a recent WHO report, isolates in 24% of all new cases throughout the world were subjected to DST for rifampin and 53% of isolates from previously treated patients were tested for drug susceptibility (1). Therefore, it is important to provide information on the wild-type MIC distributions to determine the optimal dosing schedule for MDR-TB treatment when DST is not available. With the Sensititre MycoTB plate system, it is also possible to determine the MICs of various anti-TB drugs against TB. This method could also be applied in low-resource settings (23).
In addition to the earlier data used to identify the Cmax/MIC target for M. tuberculosis (8), it is of interest to repeat these experiments in the presence of other anti-TB drugs. This information may help tailor the dose needed to reach a sufficient Cmax/MIC ratio that would likely translate into treatment success (21).
In conclusion, the MIC of amikacin in clinical isolates in vitro appears to be slightly but significantly lower than the MICs of kanamycin and capreomycin. Determination of the impact on clinical outcomes requires a prospective study, including PK and PD evaluations.
MATERIALS AND METHODS
Susceptibility testing.
The absolute concentration method we used in this study is a widely used method to test drug susceptibility (24). The method is historically called a direct concentration method, but an additional control diluted 1/100 was added for the analyses. In brief, 7H10 medium with different concentrations of amikacin, kanamycin, or capreomycin (0.25, 0.50, 1.0, 2.0, 4.0, 8.0, 16.0, 32.0, or 64.0 mg/liter) was sterilized for 10 min at 121°C. All compounds have been shown to be stable in medium after sterilization (25). After sterilization, the bottles were cooled to 50°C and oleic acid-dextrose-catalase (OADC) (Becton Dickinson and Company) was added. The pH was set at 6.6 ± 0.2 after the addition of OADC. Twenty-five-well plates were prepared and filled with 2.5 ml of the sterilized medium containing different concentrations of the drugs (or no addition).
A small loop of bacteria was suspended in 40 ml distilled water and homogenized. The concentration of bacteria was adjusted to between 2 × 105 and 10 × 105 CFU/ml. In total, 10 μl of this suspension was added to the 25-well plates. The two control wells were inoculated with 10 μl of this suspension and 10 µl of a 1/100 dilution of the suspension. Inhibition of >99% of the growth was considered prevention of growth. In addition, the control M. tuberculosis strain H37Rv (ATCC 27294) was tested in duplicate. The growth of the bacilli was checked after 14 days. The majority of the plates showed sufficient growth after 14 days and were read; however, some plates showed insufficient growth and were read 21 days after inoculation.
Target attainment analysis.
PK/PD parameters were based on published data (26). The volume of distribution was calculated for 1,000 virtual patients on the basis of the volume of distribution (mean ± standard deviation) in MDR-TB patients, with a normal distribution, using the random number generator of SPSS version 23 (IBM, Armonk, NY). The volume of distribution and the recommended aminoglycoside dose of 15 mg/kg were used to calculate the Cmax (Cmax = dose/volume of distribution), and the attainable Cmax/MIC was calculated based on this Cmax. Target attainment was calculated for the classic Cmax/MIC target of >10 and the suggested Cmax/MIC targets of >20 (21) and >70 (8). In addition, the CFR was calculated (27). The target attainment analysis was performed for both amikacin and kanamycin, since the PK profiles are highly similar (26). This analysis was not performed for capreomycin, since the PK profile is largely unknown.
Supplementary Material
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/AAC.01724-17.
REFERENCES
- 1.World Health Organization. 2016. WHO treatment guidelines for drug-resistant tuberculosis, 2016 update. World Health Organization, Geneva, Switzerland: http://apps.who.int/iris/bitstream/10665/250125/1/9789241549639-eng.pdf. [Google Scholar]
- 2.Pfyffer GE, Bonato DA, Ebrahimzadeh A, Gross W, Hotaling J, Kornblum J, Laszlo A, Roberts G, Salfinger M, Wittwer F, Siddiqi S. 1999. Multicenter laboratory validation of susceptibility testing of Mycobacterium tuberculosis against classical second-line and newer antimicrobial drugs by using the radiometric BACTEC 460 technique and the proportion method with solid media. J Clin Microbiol 37:3179–3186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rusch-Gerdes S, Pfyffer GE, Casal M, Chadwick M, Siddiqi S. 2006. Multicenter laboratory validation of the BACTEC MGIT 960 technique for testing susceptibilities of Mycobacterium tuberculosis to classical second-line drugs and newer antimicrobials. J Clin Microbiol 44:688–692. doi: 10.1128/JCM.44.3.688-692.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.World Health Organization. 2012. Updated interim critical concentrations for first-line and second-line DST (as of May 2012). World Health Organization, Geneva, Switzerland: http://www.stoptb.org/wg/gli/assets/documents/Updated%20critical%20concentration%20table_1st%20and%202nd%20line%20drugs.pdf. [Google Scholar]
- 5.Jureen P, Angeby K, Sturegard E, Chryssanthou E, Giske CG, Werngren J, Nordvall M, Johansson A, Kahlmeter G, Hoffner S, Schon T. 2010. Wild-type MIC distributions for aminoglycoside and cyclic polypeptide antibiotics used for treatment of Mycobacterium tuberculosis infections. J Clin Microbiol 48:1853–1858. doi: 10.1128/JCM.00240-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rastogi N, Labrousse V, Goh KS. 1996. In vitro activities of fourteen antimicrobial agents against drug susceptible and resistant clinical isolates of Mycobacterium tuberculosis and comparative intracellular activities against the virulent H37Rv strain in human macrophages. Curr Microbiol 33:167–175. doi: 10.1007/s002849900095. [DOI] [PubMed] [Google Scholar]
- 7.Craig WA. 2001. Does the dose matter? Clin Infect Dis 33(Suppl 3):S233–S237. doi: 10.1086/321854. [DOI] [PubMed] [Google Scholar]
- 8.Srivastava S, Modongo C, Siyambalapitiyage Dona CW, Pasipanodya JG, Deshpande D, Gumbo T. 2016. Amikacin optimal exposure targets in the hollow-fiber system model of tuberculosis. Antimicrob Agents Chemother 60:5922–5927. doi: 10.1128/AAC.00961-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Peloquin CA, Berning SE, Nitta AT, Simone PM, Goble M, Huitt GA, Iseman MD, Cook JL, Curran-Everett D. 2004. Aminoglycoside toxicity: daily versus thrice-weekly dosing for treatment of mycobacterial diseases. Clin Infect Dis 38:1538–1544. doi: 10.1086/420742. [DOI] [PubMed] [Google Scholar]
- 10.Zuur MA, Bolhuis MS, Anthony R, den Hertog A, van der Laan T, Wilffert B, de Lange W, van Soolingen D, Alffenaar JW. 2016. Current status and opportunities for therapeutic drug monitoring in the treatment of tuberculosis. Expert Opin Drug Metab Toxicol 12:509–521. doi: 10.1517/17425255.2016.1162785. [DOI] [PubMed] [Google Scholar]
- 11.Dijkstra JA, Sturkenboom MG, Hateren K, Koster RA, Greijdanus B, Alffenaar JW. 2014. Quantification of amikacin and kanamycin in serum using a simple and validated LC-MS/MS method. Bioanalysis 6:2125–2133. doi: 10.4155/bio.14.191. [DOI] [PubMed] [Google Scholar]
- 12.Dijkstra JA, Voerman AJ, Greijdanus B, Touw DJ, Alffenaar JW. 2016. Immunoassay analysis of kanamycin in serum using the tobramycin kit. Antimicrob Agents Chemother 60:4646–4651. doi: 10.1128/AAC.03025-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ho YI, Chan CY, Cheng AF. 1997. In-vitro activities of aminoglycoside-aminocyclitols against mycobacteria. J Antimicrob Chemother 40:27–32. doi: 10.1093/jac/40.1.27. [DOI] [PubMed] [Google Scholar]
- 14.Sanders WE Jr, Hartwig C, Schneider N, Cacciatore R, Valdez H. 1982. Activity of amikacin against mycobacteria in vitro and in murine tuberculosis. Tubercle 63:201–208. doi: 10.1016/S0041-3879(82)80031-7. [DOI] [PubMed] [Google Scholar]
- 15.Zheng X, Zheng R, Hu Y, Werngren J, Forsman LD, Mansjo M, Xu B, Hoffner S. 2016. Determination of MIC breakpoints for second-line drugs associated with clinical outcomes in multidrug-resistant tuberculosis treatment in China. Antimicrob Agents Chemother 60:4786–4792. doi: 10.1128/AAC.03008-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Engstrom A, Perskvist N, Werngren J, Hoffner SE, Jureen P. 2011. Comparison of clinical isolates and in vitro selected mutants reveals that tlyA is not a sensitive genetic marker for capreomycin resistance in Mycobacterium tuberculosis. J Antimicrob Chemother 66:1247–1254. doi: 10.1093/jac/dkr109. [DOI] [PubMed] [Google Scholar]
- 17.Kruuner A, Jureen P, Levina K, Ghebremichael S, Hoffner S. 2003. Discordant resistance to kanamycin and amikacin in drug-resistant Mycobacterium tuberculosis. Antimicrob Agents Chemother 47:2971–2973. doi: 10.1128/AAC.47.9.2971-2973.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zaunbrecher MA, Sikes RD Jr, Metchock B, Shinnick TM, Posey JE. 2009. Overexpression of the chromosomally encoded aminoglycoside acetyltransferase eis confers kanamycin resistance in Mycobacterium tuberculosis. Proc Natl Acad Sci U S A 106:20004–20009. doi: 10.1073/pnas.0907925106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Georghiou SB, Magana M, Garfein RS, Catanzaro DG, Catanzaro A, Rodwell TC. 2012. Evaluation of genetic mutations associated with Mycobacterium tuberculosis resistance to amikacin, kanamycin and capreomycin: a systematic review. PLoS One 7:e33275. doi: 10.1371/journal.pone.0033275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chakravorty S, Lee JS, Cho EJ, Roh SS, Smith LE, Lee J, Kim CT, Via LE, Cho SN, Barry CE III, Alland D. 2015. Genotypic susceptibility testing of Mycobacterium tuberculosis isolates for amikacin and kanamycin resistance by use of a rapid sloppy molecular beacon-based assay identifies more cases of low-level drug resistance than phenotypic Lowenstein-Jensen testing. J Clin Microbiol 53:43–51. doi: 10.1128/JCM.02059-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.van Altena R, Dijkstra JA, van der Meer ME, Borjas Howard JF, Kosterink JG, van Soolingen D, van der Werf TS, Alffenaar JW. 2017. Reduced chance of hearing loss associated with therapeutic drug monitoring of aminoglycosides in the treatment of multidrug-resistant tuberculosis. Antimicrob Agents Chemother 61:e01400–16. doi: 10.1128/AAC.01400-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Said HM, Kock MM, Ismail NA, Baba K, Omar SV, Osman AG, Hoosen AA, Ehlers MM. 2012. Comparison between the BACTEC MGIT 960 system and the agar proportion method for susceptibility testing of multidrug resistant tuberculosis strains in a high burden setting of South Africa. BMC Infect Dis 12:369. doi: 10.1186/1471-2334-12-369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Heysell SK, Pholwat S, Mpagama SG, Pazia SJ, Kumburu H, Ndusilo N, Gratz J, Houpt ER, Kibiki GS. 2015. Sensititre MycoTB plate compared to Bactec MGIT 960 for first- and second-line antituberculosis drug susceptibility testing in Tanzania: a call to operationalize MICs. Antimicrob Agents Chemother 59:7104–7108. doi: 10.1128/AAC.01117-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.van Klingeren B, Dessens-Kroon M, van der Laan T, Kremer K, van Soolingen D. 2007. Drug susceptibility testing of Mycobacterium tuberculosis complex by use of a high-throughput, reproducible, absolute concentration method. J Clin Microbiol 45:2662–2668. doi: 10.1128/JCM.00244-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Griffith ME, Bodily HL. 1992. Stability of antimycobacterial drugs in susceptibility testing. Antimicrob Agents Chemother 36:2398–2402. doi: 10.1128/AAC.36.11.2398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dijkstra JA, van Altena R, Akkerman OW, de Lange WC, Proost JH, van der Werf TS, Kosterink JG, Alffenaar JW. 2015. Limited sampling strategies for therapeutic drug monitoring of amikacin and kanamycin in patients with multidrug-resistant tuberculosis. Int J Antimicrob Agents 46:332–337. doi: 10.1016/j.ijantimicag.2015.06.008. [DOI] [PubMed] [Google Scholar]
- 27.Mouton JW, Dudley MN, Cars O, Derendorf H, Drusano GL. 2005. Standardization of pharmacokinetic/pharmacodynamic (PK/PD) terminology for anti-infective drugs: an update. J Antimicrob Chemother 55:601–607. doi: 10.1093/jac/dki079. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.