ABSTRACT
Ten Enterobacteriaceae isolates collected in a Czech hospital carried blaKPC-positive plasmids of different sizes (∼30, ∼45, and ∼80 kb). Sequencing revealed three types of plasmids (A to C) with the Tn4401a transposon. Type A plasmids comprised an IncR backbone and a KPC-2-encoding multidrug resistance (MDR) region. Type B plasmids were derivatives of type A plasmids carrying an IncN3-like segment, while type C plasmids were IncP6 plasmids sharing the same KPC-2-encoding MDR region with type A and B plasmids.
KEYWORDS: Citrobacter freundii, Tn4401a, IncR, ST18, Illumina sequencing
TEXT
KPC-type β-lactamases comprise a distinct group of plasmid-borne enzymes, with carbapenemase activity mainly occurring in Klebsiella pneumoniae. KPC-producing Enterobacteriaceae have emerged as challenging pathogens causing health care-associated infections, due to their extremely drug-resistant phenotypes and ability to cause infections associated with high mortality (1). KPC producers have disseminated worldwide and currently constitute an important public health problem (2). In Europe, Greece and Italy are the most affected countries, with high proportions of KPC-producing K. pneumoniae (3). In the Czech Republic, however, the occurrence of KPC producers has been rare. A sporadic case of KPC-2-producing K. pneumoniae recovered from a patient, who had been previously hospitalized in Greece, was detected in the Czech Republic in 2009 (4). Additionally, in 2011, an outbreak of KPC-3-producing K. pneumoniae was observed in another Czech hospital (5), with the index case being a patient repatriated from Italy.
In the present study, we describe the molecular characterization of KPC-2-producing Enterobacteriaceae isolates, mainly of the species Citrobacter freundii, recovered in the University Hospital of Hradec Kralove (Czech Republic).
From 2014 until 2016, a total of 10 nonrepetitive Enterobacteriaceae isolates showing carbapenemase activity on matrix-assisted laser desorption ionization–time of flight mass spectrometry (MALDI-TOF MS) meropenem hydrolysis assay (6) were detected. Among them, 7 of the isolates were identified to be C. freundii, 1 was identified to be K. pneumoniae, 1 was identified to be Escherichia coli, and 1 was identified to be Morganella morganii (Table 1). Phenotypic testing, PCR screening, and sequencing (7) showed that all isolates were positive for the presence of the blaKPC-2 gene. The 10 KPC-2-producing isolates were recovered from 7 patients, 6 of which were hospitalized in the same unit (Table 1). In addition, 5 of the patients had overlapping stays in several combinations, suggesting transmission of KPC-2 producers.
TABLE 1.
Characteristics of KPC-2-producing Enterobacteriaceae isolates
| Species and isolate | Hospital departmenta | Isolation date | Clinical material | STb | Size of blaKPC plasmid (kb) | Type of plasmid sequence (replicon) | MIC (μg/ml) ofc: |
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ctx | Caz | Fep | Atm | Imp | Mem | Etp | Gen | Amk | Tob | Sxt | Cip | Col | Tgc | Fos | |||||||
| C. freundii | |||||||||||||||||||||
| Cfr-27569 | ICU | 11/2014 | Urine | 18 | 46.826 | A (IncR) | >8 | >16 | >16 | >16 | 16 | >16 | >4 | >32 | 16 | >8 | >64 | >8 | 0.25 | 0.25 | ≤2 |
| Cfr-31260 | ICU | 09/2015 | Urine | 18 | 46.826 | A (IncR) | >8 | >16 | >16 | >16 | >32 | >16 | >4 | >32 | 32 | >8 | >64 | >8 | 0.12 | 0.25 | ≤2 |
| Cfr-31816 | ICU | 10/2015 | Urine | 18 | 46.826 | A (IncR) | >8 | >16 | >16 | >16 | >32 | 16 | 4 | >32 | >32 | >8 | >64 | >8 | 0.12 | 0.12 | ≤2 |
| Cfr-33038d | ICU | 11/2015 | Rectal swab | 18 | 46.826 | A (IncR) | >8 | >16 | >16 | >16 | >32 | >16 | >4 | >32 | 16 | >8 | >64 | >8 | 4 | 1 | 8 |
| Cfr-33795 | ICU | 04/2016 | Urine | 142 | 30.051 | C (IncP6) | >8 | >16 | >16 | >16 | 4 | 8 | 4 | 4 | 32 | >8 | >64 | 4 | 0.12 | 0.5 | ≤2 |
| Cfr-36049 | ICU | 09/2016 | Wounds | 87 | 81.348 | B (IncR and IncN3) | >8 | >16 | >16 | >16 | 8 | 8 | >4 | 1 | 16 | >8 | 1 | ≤0.06 | 1 | 0.06 | ≤2 |
| Cfr-36808 | HD | 11/2016 | Rectal swab | 18 | 46.826 | A (IncR) | >8 | 16 | 16 | >16 | 12 | 16 | >4 | >32 | >32 | >8 | >64 | >8 | 0.25 | 0.12 | ≤2 |
| K. pneumoniae | |||||||||||||||||||||
| Kpn-35786d | ICU | 09/2016 | Catheter | 11 | 46.826 | A (IncR) | >8 | >16 | >16 | >16 | >32 | >16 | 2 | >32 | 16 | >8 | >64 | >8 | >16 | 1 | >128 |
| E. coli | |||||||||||||||||||||
| Eco-36682d | ICU | 11/2016 | Catheter | 216 | 81.348 | B (IncR and IncN3) | 4 | 4 | 2 | >16 | 32 | 4 | 2 | 2 | >32 | >8 | 0.06 | ≤0.06 | ≤0.06 | 0.25 | ≤2 |
| M. morganii | |||||||||||||||||||||
| Mmo-37590d | ICU | 12/2016 | Urine | NA | 30.051 | C (IncP6) | >8 | 8 | >16 | >16 | 16 | 8 | 1 | 2 | 32 | 8 | >64 | 8 | >16 | 0.5 | >128 |
ICU, intensive care unit; HD, hematology department.
ST, sequence type; NA, not applicable.
Ctx, cefotaxime; Caz, ceftazidime; Fep, cefepime; Atm, aztreonam; Imp, imipenem; Mem, meropenem; Etp, ertapenem; Gen, gentamicin; Amk, amikacin; Tob, tobramycin; Sxt, trimethoprim-sulfamethoxazole; Cip, ciprofloxacin; Col, colistin; Tgc, tigecycline; Fos, fosfomycin.
KPC-2-like-producing isolates recovered from the same patient.
Susceptibility to various antimicrobial agents was determined by the broth dilution method (8). MICs, interpreted according to European Committee on Antimicrobial Susceptibility Testing (EUCAST) criteria (http://www.eucast.org/), showed that all KPC-2 producers exhibited resistance to aminopenicillins, aminopenicillin-inhibitor combinations (data not shown), cephalosporins, and aztreonam, and were nonsusceptible to carbapenems. Additionally, KPC-2 producers also exhibited resistance to several non-β-lactam antibiotics, whereas all isolates remained susceptible to tigecycline (Table 1).
The population structure of KPC-2-producing isolates studied by multilocus sequence typing (MLST) (9–11) is shown in Table 1. The C. freundii isolates comprised three sequence types (STs). ST18 was the most prevalent, accounting for five isolates. ST18 was previously found among NDM-1-producing isolates from Denmark and VIM-1-producing isolates from Spain (12, 13). The K. pneumoniae isolate was assigned to the high-risk clone ST11, previously associated with the production of several carbapenemases (14), while the E. coli isolate belonged to ST216.
None of the clinical isolates was capable of transferring the blaKPC-2 gene to the E. coli A15 laboratory strain by conjugation. Plasmid DNAs from clinical isolates were extracted using a Qiagen maxikit (Qiagen, Hilden, Germany) and used to transform E. coli DH5α cells. Transformants were selected on Luria-Bertani agar plates with ampicillin (50 μg/ml), confirmed to be KPC-2 producers by PCR (7), and MALDI-TOF MS meropenem hydrolysis assay (6), and tested for antimicrobial susceptibility (see Table S1 in the supplemental material). The plasmid location of the blaKPC-2 genes was demonstrated by S1 nuclease analysis of clinical and recombinant strains (15), followed by hybridization with a digoxigenin-labeled blaKPC probe. Plasmid analysis revealed the transfer of plasmids, most of which (n = 6) were ∼45 kb in size. The remaining plasmids were ∼80 kb (n = 2) or ∼30 kb (n = 2) in size. Replicon typing showed that eight of the plasmids, including those ∼45 kb and ∼80 kb in size, were positive for the IncR replicon (16) (Table 1), whereas the two remaining plasmids were nontypeable by the PCR-based replicon typing (PBRT) method (17, 18).
Plasmid DNAs from all KPC-2-producing transformants were extracted using a Qiagen large-construct kit (Qiagen, Hilden, Germany). Multiplexed plasmid DNA libraries were prepared using the Nextera XT library preparation kit, and 300-bp paired-end sequencing was performed on the Illumina MiSeq platform (Illumina Inc., San Diego, CA, USA) using the MiSeq v3 600-cycle reagent kit. Initial paired-end reads were quality trimmed using the Trimmomatic tool v0.33 (19) with a sliding window size of 4 bp, required average base quality ≥17, and minimum read length of 48 bases. For assembly of the plasmids, reads were mapped to the reference E. coli strain K-12 substrain MG 1655 genome (GenBank accession no. U00096) using the BWA-MEM algorithm (20), in order to filter out the chromosomal DNA. Then, all of the unmapped reads were assembled by use of the de Bruijn graph-based de novo assembler SPAdes v3.9.1 (21), using k-mer sizes 21, 33, 55, and 77. De novo assembly resulted in sets of contigs with length-weighted average k-mer coverage ranging from 23× to 95×. The sequence gaps were filled by a PCR-based strategy and Sanger sequencing. For sequence analysis and annotation, the BLAST algorithm (www.ncbi.nlm.nih.gov/BLAST), the ISfinder database (www-is.biotoul.fr/), and the ORF (open reading frame) Finder tool (www.bioinformatics.org/sms/) were utilized. Comparative genome alignments were performed using the Mauve (version 2.3.1) program (22).
Plasmid analysis revealed three types of blaKPC-2-carrying plasmid sequences (types A to C; Table 1), with type A being the most prevalent. All plasmids contained the Tn4401a isoform of the Tn4401 transposon, which is similar to that described in plasmid pNYC, lacking 100 bp upstream of blaKPC-2 (23).
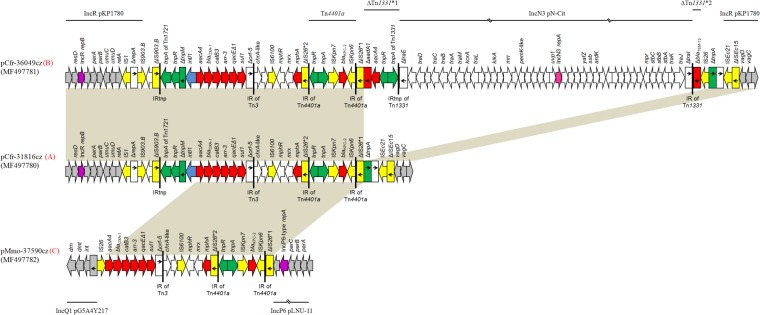
All blaKPC-2-carrying plasmids that were ∼45 kb in size belonged to type A and showed high degrees of similarity to each other. The plasmids included a contiguous segment of 12,036 bp (nucleotide [nt] 1 to 10294 and 45085 to 46826; GenBank accession number MF497780) sharing extensive similarity with the backbone of the recently described IncR plasmids (24). This segment was composed of regions responsible for the replication (repB gene and iteron region), maintenance (resD gene), and stability (parAB, vagCD, and umuDC operons) of the plasmids (Fig. 1).
FIG 1.
Linear maps of the blaKPC-2-carrying plasmids. For each plasmid, the type of plasmid sequence is indicated in red next to the plasmid name. Arrows show the direction of transcription of open reading frames (ORFs), while truncated ORFs appear as rectangles (arrows within rectangles indicate the direction of transcription). Resistance genes, insertion sequence (IS) elements, and transposases are shown in red, yellow, and green, respectively. intI1 genes are shaded blue. Gray arrows or rectangles indicate plasmid scaffold regions; the replication genes are shown in purple. The remaining genes are shown in white. Homologous segments (representing ≥99% sequence identity) are indicated by light gray shading. Thin lines above and below the maps correspond to highly similar sequences from other plasmids.
In the remaining 34,790-bp sequence (nt 10295 to 45084; GenBank accession number MF497780) adjoining the boundaries of the IncR backbone, a multidrug resistance (MDR) region containing the KPC-2-encoding transposon Tn4401a was identified. The Tn4401a transposon was localized within a copy of insertion sequence IS26 (ΔIS26). Target site duplications of 5 bp (ATGCA) at the boundaries of Tn4401a indicated insertion by transposition. Upstream from ΔIS26*1, an ISEc21-like element and a 916-bp fragment of an ISEc15-like element (ΔISEc15) were found. The ISEc21-ΔISEc15 structure was at the boundary of the plasmid backbone, downstream of vagCD, in the same configuration previously described in the IncR MDR plasmid pKP1780 (24).
In the remaining part of the MDR region, an IS1 that was followed by a 674-bp segment of the IncN replication region (ΔrepA) (25) was found at the boundary of the plasmid backbone, downstream of retA. The IS1-ΔrepA structure was also identified in pKP1780 at a similar position (24). Next to this sequence, an intact IS903.B-like element and a second copy of a IS903.B-like element truncated at the 3′ end (ΔIS903.B) were found. The deleted part of IS903.B was occupied by a Tn1721-like fragment (ΔTn1721-like) consisting of the 38-bp inverted repeat (IRtnp) of the transposon, tnpA, tnpR, and tnpM. The Tn1721-like sequence also included an integron similar to In37 from pHSH2, the variable region of which comprised the aacA4, blaOXA-1, catB3, and arr-3 cassettes (26). The IRi of In37 was located within the tnpM gene of the Tn1721-like transposon, while the 3′CS of In37 was truncated 174 bp after the start codon of orf5 (Δorf5). Immediately downstream of Δorf5, a Tn501-like sequence including the 38-bp inverted repeat (IR) of the transposon, a chrA-like gene encoding a chromate ion transporter, an IS6100, a macrolide resistance operon [mph(A)], and the remaining part of IS26 (ΔIS26*2) was found. A similar structure, which confers resistance to ampicillin, streptomycin, sulfonamides and mercury, has also been observed in plasmid pLEW517 from the primate intestinal E. coli strain 517-2H1 (27).
The type B plasmids pCfr-36049cz and pEco-36682cz appeared to be derivatives of type A IncR KPC-2-encoding plasmids characterized during the present study. Type B plasmids differed from type A plasmids by the presence of an additional 34,522-bp sequence (nt 41399 to 75920; GenBank accession number MF497781) upstream of ΔIS26*1. This sequence comprised two fragments of the Tn1331 transposon flanking a central sequence (Fig. 1). The central sequence (nt 46232 to 74780; GenBank accession number MF497781) shared extensive similarity with the sequence of pN-Cit (96% coverage and 95% identity), an IncN3-type plasmid originally described from C. freundii STE strain collected in France from a patient who had been transferred from India (28). The IncN3-derived sequence possessed genes encoding a transfer locus, and a repA gene that was 98% similar to the respective region of pN-Cit. However, a part of the IncN3 transfer system was missing, explaining the inability of pCfr-36049cz and pEco-36682cz to transfer via conjugation.
Plasmids pCfr-33795cz and pMmo-37590cz, which were assigned to type C, included a contiguous segment of 4,062-bp (nt 261 to 4322; GenBank accession number MF497782) containing the partitioning genes, parA, parB, and parC, and the replication gene repA (Fig. 1). The parABC operon of pCfr-33795cz and pMmo-37590cz was identical to those of IncP6-type plasmids like pCOL-1 described from the KPC-2-producing Pseudomonas aeruginosa COL-1 strain isolated in Colombia (29) and to pLNU-11 (GenBank accession number KX863568), which was identified from a C. freundii ATetA strain captured from the sediments of an urban coastal wetland. The putative repA product of pCfr-33795cz and pMmo-37590cz showed high amino acid sequence similarity (99%) with the replication initiation protein of pLNU-11. Additionally, type C plasmids included a 3,835-bp segment (nt 1 to 260 and 26477 to 30051; GenBank accession number MF497782) consisting of genes encoding a DNA invertase/recombinase (int), a deoxymethyltransferase (dmt), and a DNase (drn) of type II restriction module. The int-dmt-drn region has also been observed in IncQ1 blaGES-5-carrying plasmids isolated from E. coli and Serratia marcescens strains persisting in Canada (30). The remaining 22,154-bp sequence of pCfr-33795cz and pMmo-37590cz (nt 4323 to 26476; GenBank accession number MF497782), which contained the KPC-2-encoding transposon Tn4401a, was identical to the MDR region of type A and B plasmids (Fig. 1). In contrast, in plasmid pCOL-1, the blaKPC-2 gene was part of the Tn4401b isoform of the transposon and was located in a different insertion site.
In conclusion, the present study reports the “hidden outbreak” of ST18 KPC-2-producing C. freundii isolates in a Czech hospital. However, the blaKPC-2 gene was also identified in other STs of C. freundii and other species of Enterobacteriaceae. In one of the patients, four different KPC-2 producers were identified during the hospitalization, implying in vivo horizontal transfer of the blaKPC-2-carrying plasmid. Sequencing data confirmed the presence of the same blaKPC-2-carrying plasmid in two of these isolates (Table 1), further supporting this hypothesis. Of note was that, in the remaining two isolates recovered from the same patient, two different types of blaKPC-2-carrying plasmids were identified, indicating the ability of enterobacterial plasmids to further evolve through reshuffling.
Illumina analysis results showed that, in 6 out of the 10 isolates, the KPC-2-encoding transposon Tn4401a was localized on an IncR-type plasmid (type A). To our knowledge, this is the first report on complete sequences of IncR plasmids carrying Tn4401a transposon. However, previous studies have reported the presence of multireplicon IncFIIK2-IncR KPC-2-encoding plasmids from ST101 K. pneumoniae isolated in Italian hospitals (31, 32). In addition, type B plasmids were derivatives of type A IncR blaKPC-2-positive plasmids carrying an IncN3-derived segment. Type C plasmids belonged to the IncP6 group and shared the same KPC-2-encoding MDR region with type A and B plasmids. Therefore, en bloc acquisition of the KPC-2-encoding MDR region by an InpP6-type replicon from type A or B plasmids is a plausible hypothesis regarding the formation of type C blaKPC-2-carrying plasmids. All three types of plasmids were noncapable of transferring the blaKPC-2 gene via conjugation, due to partial deletion or absence of the transfer system genes. Thus, the hypothesis of mobilization in trans of the blaKPC-2-carrying plasmids by a coresident plasmid cannot be excluded.
The data presented here contribute to the current knowledge of KPC-2-producing Enterobacteriaceae. In agreement with the results of previous studies (16, 24, 31, 32), our findings underline the increasing clinical importance of the IncR plasmid family as well as the spreading potential of large MDR segments through reshuffling of enterobacterial plasmids.
Accession number(s).
The nucleotide sequences of pCfr-31816cz, pCfr-36049cz, pMMO-37590cz, pCfr-27569cz, pCfr-31260cz, pCfr-33038cz, pCfr-36808cz, pKpn-35786cz, pEco-36682cz, and pCfr-33795cz have been deposited in GenBank under the accession numbers MF497780, MF497781, MF497782, MG557994, MG557995, MG557996, MG557997, MG557998, MG557999, and MG558000, respectively.
Supplementary Material
ACKNOWLEDGMENTS
We thank Ø. Samuelsen and J. Janice for helpful suggestions. We also thank Dana Kralova for technical assistance.
This work was supported by the Medical Research Foundation of the Czech Republic (grants 15-28663A and 17-29239A), the National Sustainability Program I (NPU I) grant LO1503 provided by the Ministry of Education Youth and Sports of the Czech Republic, the Charles University Research Fund—PROGRES (grant Q39), and the Norwegian Financial Mechanism (grant NF-CZ07-MOP-4-254-2015).
We have no conflicts to declare.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/AAC.02152-17.
REFERENCES
- 1.Tumbarello M, Trecarichi EM, De Rosa FG, Giannella M, Giacobbe DR, Bassetti M, Losito AR, Bartoletti M, Del Bono V, Corcione S, Maiuro G, Tedeschi S, Celani L, Cardellino CS, Spanu T, Marchese A, Ambretti S, Cauda R, Viscoli C, Viale P; ISGRI-SITA. 2015. Infections caused by KPC-producing Klebsiella pneumoniae: differences in therapy and mortality in a multicentre study. J Antimicrob Chemother 70:2133–2143. doi: 10.1093/jac/dkv086. [DOI] [PubMed] [Google Scholar]
- 2.Grundmann H, Livermore DM, Giske CG, Canton R, Rossolini GM, Campos J, Vatopoulos A, Gniadkowski M, Toth A, Pfeifer Y, Jarlier V, Carmeli Y; CNSE Working Group. 2010. Carbapenem-nonsusceptible Enterobacteriaceae in Europe: conclusions from a meeting of national experts. Euro Surveill 15(46):pii=19711. doi: 10.2807/ese.15.46.19711-en. [DOI] [PubMed] [Google Scholar]
- 3.Albiger B, Glasner C, Struelens MJ, Grundmann H, Monnet DL; EuSCAPE working group. 2015. Carbapenemase-producing Enterobacteriaceae in Europe: assessment by national experts from 38 countries, May 2015. Euro Surveill 20(45):pii=30062. doi: 10.2807/1560-7917.ES.2015.20.45.30062. [DOI] [PubMed] [Google Scholar]
- 4.Hrabak J, Niemczykova J, Chudackova E, Fridrichová M, Studentová V, Cervená D, Urbášková P, Zemličková H. 2011. KPC-2-producing Klebsiella pneumoniae isolated from a Czech patient previously hospitalized in Greece and in vivo selection of colistin resistance. Folia Microbiol (Praha) 56:361–365. doi: 10.1007/s12223-011-0057-6. [DOI] [PubMed] [Google Scholar]
- 5.Hrabak J, Papagiannitsis CC, Studentova V, Jakubu V, Fridrichová M, Zemlickova H; Czech Participants of European Antimicrobial Resistance Surveillance Network . 2013. Carbapenemase-producing Klebsiella pneumoniae in the Czech Republic in 2011. Euro Surveill 18(45):pii=20626. doi: 10.2807/1560-7917.ES2013.18.45.20626. [DOI] [PubMed] [Google Scholar]
- 6.Papagiannitsis CC, Studentova V, Izdebski R, Oikonomou O, Pfeifer Y, Petinaki E, Hrabak J. 2015. MALDI-TOF MS meropenem hydrolysis assay with NH4HCO3, a reliable tool for the direct detection of carbapenemase activity. J Clin Microbiol 53:1731–1735. doi: 10.1128/JCM.03094-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Papagiannitsis CC, Izdebski R, Baraniak A, Fiett J, Herda M, Hrabák J, Derde LP, Bonten MJ, Carmeli Y, Goossens H, Hryniewicz W, Brun-Buisson C, Gniadkowski M, Grabowska A, Nikonorow E, Dautzenberg MJ, Adler A, Kazmas M, Navon-Venezia S, Malhotra-Kumar S, Lammens C, Legrand P, Annane D, Chalfine A, Giamarellou H, Petrikkos GL, Nardi G, Balode A, Dumpis U, Stammet P, Arag I, Esteves F, Muzlovic I, Tomic V, Torres Mart A, Lawrence C, Salomon J, Paul M, Lerman Y, Rossini A, Salvia A, Vidal Samso J, Fierro J. 2015. Survey of metallo-β-lactamase-producing Enterobacteriaceae colonizing patients in European ICUs and rehabilitation units, 2008–11. J Antimicrob Chemother 70:1981–1988. doi: 10.1093/jac/dkv055. [DOI] [PubMed] [Google Scholar]
- 8.European Committee on Antimicrobial Susceptibility Testing (EUCAST). 2003. Determination of minimum inhibitory concentrations (MICs) of antibacterial agents by broth dilution. Clin Microbiol Infect 9:ix–xv. doi: 10.1046/j.1469-0691.2003.00790.x. [DOI] [Google Scholar]
- 9.Bai L, Xia S, Lan R, Liu L, Ye C, Wang Y, Jin D, Cui Z, Jing H, Xiong Y, Bai X, Sun H, Zhang J, Wang L, Xu J. 2012. Isolation and characterization of cytotoxic, aggregative Citrobacter freundii. PLoS One 7:e33054. doi: 10.1371/journal.pone.0033054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wirth T, Falush D, Lan R, Colles F, Mensa P, Wieler LH, Karch H, Reeves PR, Maiden MC, Ochman H, Achtman M. 2006. Sex and virulence in Escherichia coli: an evolutionary perspective. Mol Microbiol 60:1136–1151. doi: 10.1111/j.1365-2958.2006.05172.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Diancourt L, Passet V, Verhoef J, Grimont PA, Brisse S. 2005. Multilocus sequence typing of Klebsiella pneumoniae nosocomial isolates. J Clin Microbiol 43:4178–4182. doi: 10.1128/JCM.43.8.4178-4182.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hammerum AM, Hansen F, Nielsen HL, Jakobsen L, Stegger M, Andersen PS, Jensen P, Nielsen TK, Hansen LH, Hasman H, Fuglsang-Damgaard D. 2016. Use of WGS data for investigation of a long-term NDM-1-producing Citrobacter freundii outbreak and secondary in vivo spread of blaNDM-1 to Escherichia coli, Klebsiella pneumoniae and Klebsiella oxytoca. J Antimicrob Chemother 71:3117–3124. doi: 10.1093/jac/dkw289. [DOI] [PubMed] [Google Scholar]
- 13.Villa J, Arana DM, Viedma E, Perez-Montarelo D, Chaves F. 2017. Characterization of mobile genetic elements carrying VIM-1 and KPC-2 carbapenemases in Citrobacter freundii isolates in Madrid. Int J Med Microbiol 307:340–345. doi: 10.1016/j.ijmm.2017.07.001. [DOI] [PubMed] [Google Scholar]
- 14.Woodford N, Turton JF, Livermore DM. 2011. Multiresistant Gram-negative bacteria: the role of high-risk clones in the dissemination of antibiotic resistance. FEMS Microbiol Rev 35:736–755. doi: 10.1111/j.1574-6976.2011.00268.x. [DOI] [PubMed] [Google Scholar]
- 15.Barton BM, Harding GP, Zuccarelli AJ. 1995. A general method for detecting and sizing large plasmids. Anal Biochem 226:235–240. doi: 10.1006/abio.1995.1220. [DOI] [PubMed] [Google Scholar]
- 16.Garcia-Fernandez A, Fortini D, Veldman K, Mevius D, Carattoli A. 2009. Characterization of plasmids harbouring qnrS1, qnrB2 and qnrB19 genes in Salmonella. J Antimicrob Chemother 63:274–281. doi: 10.1093/jac/dkn470. [DOI] [PubMed] [Google Scholar]
- 17.Carattoli A, Bertini A, Villa L, Falbo V, Hopkins KL, Threlfall EJ. 2005. Identification of plasmids by PCR-based replicon typing. J Microbiol Methods 63:219–228. doi: 10.1016/j.mimet.2005.03.018. [DOI] [PubMed] [Google Scholar]
- 18.Johnson TJ, Bielak EM, Fortini D, Hansen LH, Hasman H, Debroy C, Nolan LK, Carattoli A. 2012. Expansion of the IncX plasmid family for improved identification and typing of novel plasmids in drug-resistant Enterobacteriaceae. Plasmid 68:43–50. doi: 10.1016/j.plasmid.2012.03.001. [DOI] [PubMed] [Google Scholar]
- 19.Bolger AM, Lohse M, Usadel B. 2014. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics 30:2114–2120. doi: 10.1093/bioinformatics/btu170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li H. 2013. Aligning sequence reads, clone sequences and assembly contigs with BWA-MEM. arXiv arXiv:1303.3997 [q-bio.GN]. https://arxiv.org/abs/1303.3997.
- 21.Bankevich A, Nurk S, Antipov D, Gurevich AA, Dvorkin M, Kulikov AS, Lesin VM, Nikolenko SI, Pham S, Prjibelski AD, Pyshkin AV, Sirotkin AV, Vyahhi N, Tesler G, Alekseyev MA, Pevzner PA. 2012. SPAdes: a new genome assembly algorithm and its applications to single-cell sequencing. J Comp Biol 19:455–477. doi: 10.1089/cmb.2012.0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Darling AE, Mau B, Perna NT. 2010. progressiveMauve: multiple genome alignment with gene gain, loss and rearrangement. PLoS One 5:e11147. doi: 10.1371/journal.pone.0011147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Naas T, Cuzon G, Villegas MV, Lartigue MF, Quinn JP, Nordmann P. 2008. Genetic structure at the origin of acquisition of the beta-lactamase blaKPC gene. Antimicrob Agents Chemother 52:1257–1263. doi: 10.1128/AAC.01451-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Papagiannitsis CC, Miriagou V, Giakkoupi P, Tzouvelekis LS, Vatopoulos AC. 2013. Characterization of pKP1780, a novel IncR plasmid from the emerging Klebsiella pneumoniae ST147, encoding the VIM-1 metallo-β-lactamase. J Antimicrob Chemother 68:2259–2262. doi: 10.1093/jac/dkt196. [DOI] [PubMed] [Google Scholar]
- 25.Miriagou V, Papagiannitsis CC, Kotsakis SD, Loli A, Tzelepi E, Legakis NJ, Tzouvelekis LS. 2010. Sequence of pNL194, a 79.3-kilobase IncN plasmid carrying the blaVIM-1 metallo-β-lactamase gene in Klebsiella pneumoniae. Antimicrob Agents Chemother 54:4497–4502. doi: 10.1128/AAC.00665-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang M, Tran JH, Jacoby GA, Zhang Y, Wang F, Hooper DC. 2003. Plasmid-mediated quinolone resistance in clinical isolates of Escherichia coli from Shanghai, China. Antimicrob Agents Chemother 47:2242–2248. doi: 10.1128/AAC.47.7.2242-2248.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Williams LE, Detter C, Barry K, Lapidus A, Summers AO. 2006. Facile recovery of individual high-molecular-weight, low-copy-number natural plasmids for genomic sequencing. Appl Environ Microbiol 72:4899–4906. doi: 10.1128/AEM.00354-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Villa L, Carattoli A, Nordmann P, Carta C, Poirel L. 2013. Complete sequence of the IncT-type plasmid pT-OXA-181 carrying the blaOXA-181 carbapenemase gene from Citrobacter freundii. Antimicrob Agents Chemother 57:1965–1957. doi: 10.1128/AAC.01297-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Naas T, Bonnin RA, Cuzon G, Villegas MV, Nordmann P. 2013. Complete sequence of two KPC-harbouring plasmids from Pseudomonas aeruginosa. J Antimicrob Chemother 68:1757–1762. doi: 10.1093/jac/dkt094. [DOI] [PubMed] [Google Scholar]
- 30.Boyd D, Taylor G, Fuller J, Bryce E, Embree J, Gravel D, Katz K, Kibsey P, Kuhn M, Langley J, Mataseje L, Mitchell R, Roscoe D, Simor A, Thomas E, Turgeon N, Mulvey M; Canadian Nosocomial Infection Surveillance Program. 2015. Complete sequence of four multidrug-resistant MOBQ1 plasmids harboring blaGES-5 isolated from Escherichia coli and Serratia marcescens persisting in a hospital in Canada. Microb Drug Resist 21:253–260. doi: 10.1089/mdr.2014.0205. [DOI] [PubMed] [Google Scholar]
- 31.Frasson I, Lavezzo E, Franchin E, Toppo S, Barzon L, Cavallaro A, Richter SN, Palù G. 2012. Antimicrobial treatment and containment measures for an extremely drug-resistant Klebsiella pneumoniae ST101 isolate carrying pKPN101-IT, a novel fully sequenced blaKPC-2 plasmid. J Clin Microbiol 50:3768–3772. doi: 10.1128/JCM.01892-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Papagiannitsis CC, Di Pilato V, Giani T, Giakkoupi P, Riccobono E, Landini G, Miriagou V, Vatopoulos AC, Rossolini GM. 2016. Characterization of KPC-encoding plasmids from two endemic settings, Greece and Italy. J Antimicrob Chemother 71:2824–2830. doi: 10.1093/jac/dkw227. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.