ABSTRACT
The treatment of drug-susceptible tuberculosis (TB) is long and cumbersome. Mismanagement of TB treatment can lead to the emergence of drug resistance in patients, so shortening the treatment duration could significantly improve TB chemotherapy and prevent the development of drug resistance. We previously discovered that high concentrations of vitamin C sterilize cultures of drug-susceptible and drug-resistant Mycobacterium tuberculosis. Here, we tested subinhibitory concentration of vitamin C in combination with TB drugs against M. tuberculosis in vitro and in a mouse model of M. tuberculosis infection. In vivo, we showed that the vitamin C level in mouse serum can be increased by intraperitoneal injection of vitamin C to reach vitamin C levels close to the concentrations required for activity in vitro. Although vitamin C had no activity by itself in M. tuberculosis-infected mice, the combination of vitamin C with the first-line TB drugs isoniazid and rifampin reduced the bacterial burden in the lungs of M. tuberculosis-infected mice faster than isoniazid and rifampin combined in two independent experiments. These experiments suggest that the addition of vitamin C to first-line TB drugs could shorten TB treatment. Vitamin C, an inexpensive and nontoxic compound, could easily be added to the TB pharmacopeia to substantially improve chemotherapy outcome, which would have a significant impact on the worldwide TB community.
KEYWORDS: isoniazid, mice, rifampicin, tuberculosis, vitamin C
INTRODUCTION
Tuberculosis (TB), caused by the bacillus Mycobacterium tuberculosis, remains a serious global health issue, with more than a million deaths worldwide due to this disease. The World Health Organization has set a deadline to end the TB epidemic by 2035, which includes a 90 to 95% reduction in TB incidence and in the number of TB-related deaths (1). A major obstacle to achieving this goal remains the long treatment time for TB (1). The treatment of drug-susceptible TB lasts 6 months and requires the use of four drugs to achieve a cure. Multidrug-resistant TB (MDR-TB) cases can emerge in patients initially infected with drug-susceptible TB whose treatment is mismanaged. The treatment of MDR-TB, which is resistant to the two most effective first-line TB drugs isoniazid (INH) and rifampin (RIF), is lengthier and involves the use of toxic second-line TB drugs with severe side effects (2). New drugs to fight drug-susceptible and drug-resistant TB have been developed and are in different phases of clinical trials (3), but development and approval are long processes, and these drugs have not been shown to shorten effective therapy. Another challenge in TB chemotherapy is to identify drugs that will eliminate persisters, a fraction of the M. tuberculosis population that is refractory to killing by drugs or immune effectors. The difficulties in eradicating these persisters leads to the establishment of a latent M. tuberculosis infection and drug-resistant mutants (4). We have previously demonstrated that high concentrations of vitamin C sterilize drug-susceptible, MDR, and extensively drug-resistant M. tuberculosis cultures and prevent the emergence of drug persisters (5). This sterilization activity relies on the iron-dependent Fenton/Haber-Weiss reactions, producing reactive oxygen species, DNA damage, lipid modifications, and redox unbalance (5), as well as an increase in M. tuberculosis respiration (6), which prevents persister formation (7). This pleiotropism led us to investigate whether the addition of vitamin C to TB chemotherapy could decrease the time to sterilization in vitro and in a mouse model of M. tuberculosis infection.
RESULTS
Vitamin C combined with TB drugs sterilizes M. tuberculosis cultures faster than drugs alone in vitro.
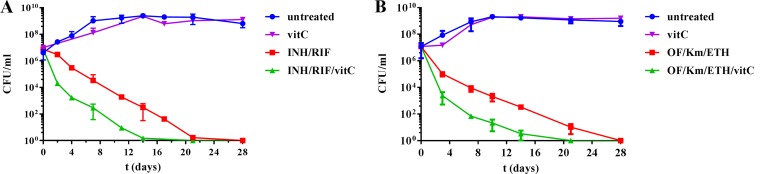
We had previously observed that cultures of M. tuberculosis were sterilized faster with a combination of vitamin C (1 or 4 mM) and INH than with vitamin C alone (5). Since TB treatment is based on the use of multiple drugs and not monotherapy, and a dose of 4 mM vitamin C is difficult to achieve in vivo, we tested a subinhibitory concentration of vitamin C (1 mM) added to a combination of INH and RIF. While INH and RIF combined sterilized a culture of M. tuberculosis within 3 to 4 weeks, the addition of 1 mM vitamin C to this treatment shortened the sterilization time by 7 days (Fig. 1A). This suggests that vitamin C could be used as an adjunct therapy. Shortening chemotherapy for MDR-TB, which requires at least 2 years of treatment, would also be beneficial. The treatment of MDR-TB is selected based on a combination of second-line TB drugs containing at least one fluoroquinolone, one injectable, and one conventional second-line drug, such as a thioamide. We therefore chose to test vitamin C with the fluoroquinolone ofloxacin (OF), the injectable kanamycin (Km), and the thioamide ethionamide (ETH). Again, the addition of vitamin C to the OF-Km-ETH treatment shortened the sterilization time by 9 days compared to OF-Km-ETH treatment alone (Fig. 1B), indicating that vitamin C does potentiate the action of TB drugs in vitro. Based on these in vitro data, we decided to test the combination of vitamin C with INH and RIF in a mouse model of M. tuberculosis infection.
FIG 1.
The combinations of vitamin C and TB drugs sterilize M. tuberculosis cultures. M. tuberculosis H37Rv was grown to mid-log (OD600, 0.6 to 0.8), as described in Materials and Methods, diluted 1:100, and treated for 4 weeks with vitamin C (vitC, 1 mM), a combination of INH (7 μM) and RIF (1.2 μM), or the combination INH (7 μM)/RIF (1 μM)/vitC (1 mM) (A); or vitamin C (vitC, 1 mM), a combination of ofloxacin (OF, 14 μM), kanamycin (Km, 41 μM), and ethionamide (ETH, 150 μM), or the combination OF (14 μM)-Km (41 μM)-ETH (150 μM)-vitC (1 mM) (B). INH, RIF, OF, Km, and ETH were used at concentrations equivalent to 10 times their MIC. Aliquots were taken at indicated times and plated to determine the CFU. The plates were incubated at 37°C for 4 to 6 weeks. The mean and standard deviation are plotted (n = 3).
Vitamin C level in mice is increased by intraperitoneal injection of vitamin C.
To test vitamin C in a mouse model of M. tuberculosis infection, we first had to determine how to administer and which concentration of vitamin C to administer to mice in order to increase the levels of vitamin C in mouse serum. Previous studies reported that vitamin C was best absorbed in mice when given by intravenous (i.v.) or intraperitoneal (i.p.) injections rather than orally (8). i.v. and i.p. administration of vitamin C (1 g/kg of body weight) in mice gave maximum plasma concentrations of 15 mM and 7 mM, respectively, whereas the addition of vitamin C to the drinking water of the mice at a dose of 6g/liter only afforded a plasma concentration of 40 μM after 4 weeks (8). The administration of 4.5 g/kg of vitamin C by i.p. injection was shown to be detrimental to mice, whereas 3 g/kg was well tolerated (9). In view of these published data, we tested two aqueous solutions of vitamin C (1.8 and 3 g/kg), buffered to pH 7, and injected the solutions i.p. into uninfected C57BL/6 mice (two mice per concentration). In the control experiment, one mouse received phosphate-buffered saline (PBS). One hour postinjection with vitamin C, serum was collected and vitamin C was quantified by high-performance liquid chromatography (10). The mouse receiving PBS had a vitamin C concentration in its serum of 0.08 mM. The two mice injected intraperitoneally with 1 M vitamin C (1.8-g/kg dose) had vitamin C concentrations in their serum of 0.4 and 0.7 mM, whereas i.p. injection of 1.7 M vitamin C (3-g/kg dose) resulted in serum vitamin C concentrations of 2 and 6 mM. Although these data were obtained from a small set of mice and the variation within each group was substantial, the results showed that the vitamin C level in mouse serum could be increased by i.p. injection of vitamin C. In subsequent experiments, mice received vitamin C as a buffered aqueous 1.7 M solution by i.p. injection, corresponding to a dose of 3 g/kg.
High-dose vitamin C in combination with INH and RIF reduces lung bacterial burden in mice.
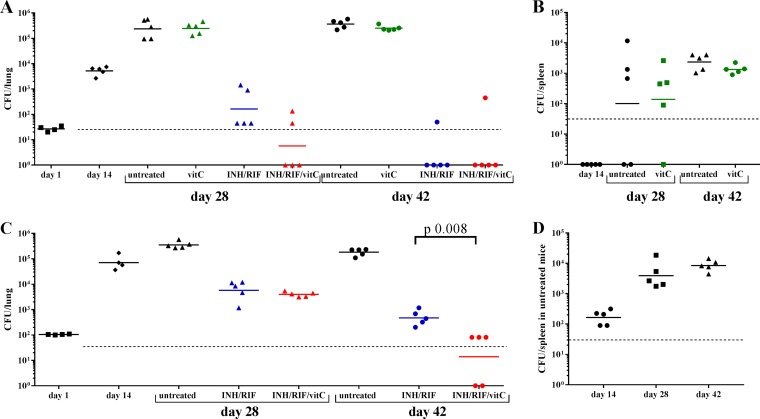
For our initial mouse experiment, we set up an acute model of M. tuberculosis infection in which mice were infected with a low dose of M. tuberculosis H37Rv (Table 1) via the aerosol route. Two weeks postinfection, treatment was started for 4 weeks (Fig. 2A). Mice were divided into four groups: untreated, treated with INH and RIF, treated with vitamin C, and treated with INH, RIF, and vitamin C. Although vitamin C had been shown to sterilize M. tuberculosis cultures in vitro, in vivo, untreated mice, and those treated with vitamin C had similar M. tuberculosis lung burdens over the course of the experiment (Fig. 2A and Table 1); similar spleen burdens were also observed at 4 and 6 weeks postinfection in the vitamin C-treated group and in the untreated group (Fig. 2B and Table 1). The mice treated with the combination of INH-RIF-vitamin C had a lung bacterial burden about one log lower than that of mice treated with INH-RIF on average at 4 weeks postinfection. At 6 weeks postinfection, no M. tuberculosis colonies were detected in the lungs of 4/5 mice treated with either INH-RIF-vitamin C or INH-RIF, although M. tuberculosis colonies were isolated from the lungs of one mouse in each of these two groups. No M. tuberculosis colonies were detected in the spleens of M. tuberculosis-infected mice treated with INH-RIF or with INH-RIF-vitamin C at 4 or 6 weeks postinfection.
TABLE 1.
Average CFU counts in the lungs and spleens of M. tuberculosis-infected mice
| Organ sample | Day | Avg CFU counta |
|||
|---|---|---|---|---|---|
| No treatment | VitC | INH-RIF | INH-RIF-VitC | ||
| Expt 1 | |||||
| Lung | 1 | 28 | |||
| 14 | 5.5 × 103 | ||||
| 28 | 3.1 × 105 | 2.7 × 105 | 5.0 × 102 | 36 | |
| 42 | 3.9 × 105 | 2.6 × 105 | NDb | NDb | |
| Spleen | 14 | ND | |||
| 28 | 2.8 × 103 | 9.2 × 102 | ND | ND | |
| 42 | 2.7 × 103 | 1.4 × 103 | ND | ND | |
| Expt 2 | |||||
| Lung | 1 | 105 | |||
| 14 | 8.4 × 104 | ||||
| 28 | 3.7 × 105 | 7.5 × 103 | 4.1 × 103 | ||
| 42 | 1.9 × 105 | 5.7 × 102 | 44 | ||
| Spleen | 14 | 1.9 × 102 | ND | ND | |
| 28 | 6.1 × 103 | ND | ND | ||
| 42 | 9.1 × 103 | ND | ND | ||
VitC, vitamin C; ND, no detectable bacteria.
Four out of five mice had no detectable bacteria in their lungs.
FIG 2.
Vitamin C potentiates the activity of INH and RIF in mice. CBA/J mice were infected via the aerosol route with 28 CFU (A and B) or 105 CFU (C and D) of M. tuberculosis H37Rv. Treatment started 2 weeks postinfection. One group was left untreated. The mice received INH-RIF in their drinking water ad libitum. Vitamin C was given by i.p. injection at a concentration of 3 g/kg, 5 days a week, for 4 weeks. At the indicated times, mice were euthanized, and lungs and spleens were homogenized to quantify bacterial burdens. The CFU counts in lungs (A and C) and spleen (B and D) are shown for each individual animal, and the geometric mean is indicated for each group. The limit of detection is indicated by a dotted line. No colonies were isolated in the spleens of M. tuberculosis-infected mice treated with INH-RIF or INH-RIF-vitamin C in both experiments and are therefore not shown on panels C and D to simplify the figure. When the difference between two groups is significant, the P value is indicated based on a two-tailed unpaired nonparametric Mann-Whitney test.
In a second experiment (Fig. 2C and D and Table 1), mice were infected with a higher dose of M. tuberculosis H37Rv via the aerosol route. The vitamin C group was omitted in view of the poor results with this group in the previous experiment. The treatment started 2 weeks postinfection, as previously done. The lung burden at the beginning of the treatment was a log higher than in the previous experiment (Table 1). In the lungs of treated mice, the difference in bacterial burden was not statistically significant at 4 weeks postinfection but became significant at 6 weeks postinfection (P = 0.008), by which time the mice treated with the INH-RIF-vitamin C combination had on average 1 log lower M. tuberculosis burden in their lungs than the mice treated with INH-RIF (Fig. 2C). In the mice treated with INH-RIF or INH-RIF-vitamin C, no colonies were obtained on plates from the spleen lysates, whereas M. tuberculosis could be detected in the spleens of untreated mice (Fig. 2D and Table 1).
Concluding remarks.
These experiments demonstrate that vitamin C alone has no activity against M. tuberculosis in mice but has the potential to boost the efficacy of INH-RIF treatment, thus increasing the elimination rate of M. tuberculosis in mice compared to that with INH-RIF alone. Previously, M. tuberculosis-infected mice cotreated with vitamin C (1 g/kg, daily subcutaneous injection) and INH (25 mg/kg, daily subcutaneous injection) for 74 days were found to have no sign of infection at the end of treatment, whereas the mice treated with INH had visible lesions (11). These data suggest that it is worth revisiting the effect of vitamin C on M. tuberculosis-infected patients. Although the daily dose of vitamin C in a normal human diet is far below the levels needed for optimal activity in vitro against M. tuberculosis, megadoses of vitamin C have been administered to human patients, leading to high vitamin C plasma concentrations (up to 49 mM) (12, 13), which were shown to be safe (14). In an earlier study, terminally ill TB patients were given daily high doses (15 g/day) of vitamin C orally for 6 to 8 months, with no side effects. Although the author did not observe any regression of the TB lesions, the effects on the bedridden patients were described as remarkable; they had regained appetite and physical activity (15). A 1946 study showed that TB patients who received a daily supplement of vitamin C with their routine diet and treatment had a better hematological response than the TB patients who did not get the vitamin C supplement, suggesting a better prognosis, even though at the time, none of the first-line TB drugs were available and treatment options were limited (16). Although no radiological improvement in the disease was observed, the authors concluded that the addition of vitamin C to TB treatment might improve patients' resistance to infection.
Additional studies would also merit analysis of the natural resistance-associated macrophage protein (Nramp) and vitamin C transporters alleles in TB patients. We had previously shown that, in vitro, vitamin C activity was dependent on the iron concentration present in the media. When a low concentration of ferric ion or when the iron chelator deferoxamine was used, vitamin C had no activity in vitro (5). Nramp transports iron and regulates iron levels within the phagosome of macrophages where M. tuberculosis resides (17). Human Nramp1 polymorphisms, which have been associated with TB resistance (18), as well as polymorphisms found in the vitamin C transporters encoded by SLC23A1 and SLC23A2 (19), may indicate that the host genotype could impact the efficacy of vitamin C as an adjunct therapy. The addition of vitamin C to a TB drug treatment regimen should be tested in TB patients to assess whether vitamin C is a means to achieving faster sputum smear-negative conversion, which would indicate a quicker reduction in mycobacterial burden. We therefore hypothesize that the addition of vitamin C to TB chemotherapy might shorten TB treatment, leading to a reduction in the incidences of both drug resistance development and disease relapse. If this hypothesis is proven correct, vitamin C could improve TB chemotherapy.
MATERIALS AND METHODS
Bacterial strains and reagents.
The M. tuberculosis H37Rv strain used in this study was obtained from laboratory stocks and was grown at 37°C in Middlebrook 7H9 medium (Difco, Sparks, MD) supplemented with 10% (vol/vol) oleic acid-albumin-dextrose-catalase (OADC) enrichment (Difco), 0.2% (vol/vol) glycerol, and 0.05% (vol/vol) tyloxapol. Plating was done on Middlebrook 7H10 medium (Difco) supplemented with 10% (vol/vol) OADC enrichment and 0.2% (vol/vol) glycerol. Plates were incubated at 37°C for four to 6 weeks. Phosphate-buffered saline (PBS) was obtained from Corning cellgro (Manassas, VA). All other chemicals were obtained from Sigma (St. Louis, MO).
Mouse experiment.
CBA/J female mice (6 to 8 weeks old) were obtained from Envigo (Indianapolis, IN). The animal protocol no. 20150502 “Using vitamin C to augment TB chemotherapy in mouse model” was approved by the Einstein Animal Institute, which is accredited by the American Association for the Use of Laboratory Animals, and accepts as mandatory the NIH Principles for the Use of Animals. The M. tuberculosis H37Rv culture was grown to an optical density at 600 nm (OD600) of ≈0.7, washed twice with PBS, sonicated, and diluted in PBS prior to aerosol infection. The mice were infected with M. tuberculosis H37Rv via the aerosol route. The mice were left to rest for 2 weeks before treatment was begun. Vitamin C was dissolved in water, and the pH was adjusted to 7 with an aqueous solution of 10 M sodium hydroxide. The vitamin C solution was given by i.p. injection at a concentration of 3 g/kg (1.7 M, 0.2 ml) 5 days a week for 4 weeks. Mice were given INH (100 mg/liter) and RIF (40 mg/liter) ad libitum in their drinking water, which was changed twice a week. At specific times, mice were euthanized, and their spleen and right lung were collected and homogenized in PBS. The organ lysates were then serially diluted in PBS and plated on Middlebrook 7H10 plates (see media described above) to obtain the number of CFU per organ.
Vitamin C quantification in serum.
Blood was collected from the mice by retro-orbital bleeding and dispensed in a microtube Z-gel. The microtubes were then centrifuged at 10,000 rpm for 10 min, and the serum was removed from the tubes and stored at −20°C until ready for use. The serum was mixed with an equal volume of 0.56% metaphosphoric acid solution in water, vortexed for 30 s, and then centrifuged. The supernatant was collected and analyzed by high-performance liquid chromatography (HPLC) on a Hewlett-Packard model HP1100 gradient chromatograph equipped with an HP1100 series thermostated column compartment, and an HP1100 series diode array detector. The data were collected and processed with the HP ChemStation software. The column was a reverse-phase C18 column (4.6 by 150 mm; 3-mm column diameter; Alltima C18 [Alltech]) set at 50°C. The wavelength was set at 254 nm. The mobile phases were A, 0.5 mM KH2PO4 (pH 5.0); and B, isopropanol-acetonitrile (1/4 [vol/vol]). The conditions used were 0 to 1.5 min, 5% B; 5 min, 15% B; 10 min, 90% B; and 15 min, 90% B. The flow rate was set at 0.8 ml/min, and the injection volume was set at 80 μl. Standards of vitamin C were run on the HPLC at different concentrations to obtain a calibration curve, allowing for vitamin C quantification in the samples.
ACKNOWLEDGMENTS
We are grateful to Mei Chen and Laura Cole for technical assistance with the animal work.
W.R.J. acknowledges generous support from the NIH Centers for AIDS Research (CFAR) grant AI-051519 at the Albert Einstein College of Medicine. This work was supported by the National Institutes of Health grants AI026170 and U19AI111276 (to W.R.J.).
C.V. and W.R.J. conceived, designed, and analyzed the experiments. C.V. and J.K. performed the experiments. C.V. and W.R.J. wrote the manuscript.
We declare no competing financial interests.
REFERENCES
- 1.Lienhardt C, Lonnroth K, Menzies D, Balasegaram M, Chakaya J, Cobelens F, Cohn J, Denkinger CM, Evans TG, Kallenius G, Kaplan G, Kumar AM, Matthiessen L, Mgone CS, Mizrahi V, Mukadi YD, Nguyen VN, Nordstrom A, Sizemore CF, Spigelman M, Squire SB, Swaminathan S, Van Helden PD, Zumla A, Weyer K, Weil D, Raviglione M. 2016. Translational research for tuberculosis elimination: priorities, challenges, and actions. PLoS Med 13:e1001965. doi: 10.1371/journal.pmed.1001965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Törün T, Gungor G, Ozmen I, Bolukbasi Y, Maden E, Bicakci B, Atac G, Sevim T, Tahaoglu K. 2005. Side effects associated with the treatment of multidrug-resistant tuberculosis. Int J Tuberc Lung Dis 9:1373–1377. [PubMed] [Google Scholar]
- 3.Zumla AI, Gillespie SH, Hoelscher M, Philips PP, Cole ST, Abubakar I, McHugh TD, Schito M, Maeurer M, Nunn AJ. 2014. New antituberculosis drugs, regimens, and adjunct therapies: needs, advances, and future prospects. Lancet Infect Dis 14:327–340. doi: 10.1016/S1473-3099(13)70328-1. [DOI] [PubMed] [Google Scholar]
- 4.Sebastian J, Swaminath S, Nair RR, Jakkala K, Pradhan A, Ajitkumar P. 2016. De novo emergence of genetically resistant mutants of Mycobacterium tuberculosis from the persistence phase cells formed against antituberculosis drugs in vitro. Antimicrob Agents Chemother 61:e01343-. doi: 10.1128/AAC.01343-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vilchèze C, Hartman T, Weinrick B, Jacobs WR Jr. 2013. Mycobacterium tuberculosis is extraordinarily sensitive to killing by a vitamin C-induced Fenton reaction. Nat Commun 4:1881. doi: 10.1038/ncomms2898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vilchèze C, Hartman T, Weinrick B, Jain P, Weisbrod TR, Leung LW, Freundlich JS, Jacobs WR Jr. 2017. Enhanced respiration prevents drug tolerance and drug resistance in Mycobacterium tuberculosis. Proc Natl Acad Sci U S A 114:4495–4500. doi: 10.1073/pnas.1704376114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hartman T, Weinrick B, Vilchèze C, Berney M, Tufariello J, Cook GM, Jacobs WR Jr. 2014. Succinate dehydrogenase is the regulator of respiration in Mycobacterium tuberculosis. PLoS Pathog 10:e1004510. doi: 10.1371/journal.ppat.1004510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Verrax J, Calderon PB. 2009. Pharmacologic concentrations of ascorbate are achieved by parenteral administration and exhibit antitumoral effects. Free Radic Biol Med 47:32–40. doi: 10.1016/j.freeradbiomed.2009.02.016. [DOI] [PubMed] [Google Scholar]
- 9.Sato T, Kinoshita M, Yamamoto T, Ito M, Nishida T, Takeuchi M, Saitoh D, Seki S, Mukai Y. 2015. Treatment of irradiated mice with high-dose ascorbic acid reduced lethality. PLoS One 10:e0117020. doi: 10.1371/journal.pone.0117020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Romeu-Nadal M, Morera-Pons S, Castellote AI, Lopez-Sabater MC. 2006. Rapid high-performance liquid chromatographic method for vitamin C determination in human milk versus an enzymatic method. J Chromatogr B Analyt Technol Biomed Life Sci 830:41–46. doi: 10.1016/j.jchromb.2005.10.018. [DOI] [PubMed] [Google Scholar]
- 11.Venulet F, Czerski K. 1955. Effect of isonicotinic acid hydrazide and of vitamin C on experimental tuberculosis. Med Dosw Mikrobiol 7:311–314. (In Polish.) [PubMed] [Google Scholar]
- 12.Hoffer LJ, Levine M, Assouline S, Melnychuk D, Padayatty SJ, Rosadiuk K, Rousseau C, Robitaille L, Miller WH Jr. 2008. Phase I clinical trial of i.v. ascorbic acid in advanced malignancy. Ann Oncol 19:1969–1974. doi: 10.1093/annonc/mdn377. [DOI] [PubMed] [Google Scholar]
- 13.Stephenson CM, Levin RD, Spector T, Lis CG. 2013. Phase I clinical trial to evaluate the safety, tolerability, and pharmacokinetics of high-dose intravenous ascorbic acid in patients with advanced cancer. Cancer Chemother Pharmacol 72:139–146. doi: 10.1007/s00280-013-2179-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hathcock JN, Azzi A, Blumberg J, Bray T, Dickinson A, Frei B, Jialal I, Johnston CS, Kelly FJ, Kraemer K, Packer L, Parthasarathy S, Sies H, Traber MG. 2005. Vitamins E and C are safe across a broad range of intakes. Am J Clin Nutr 81:736–745. [DOI] [PubMed] [Google Scholar]
- 15.Charpy J. 1948. La vitamine C (acide ascorbique) à très hautes doses, administrée seule ou associée à la vitamine D2, dans la tuberculose. Bull Acad Natl Med 132:421–423. [PubMed] [Google Scholar]
- 16.Rudra MN, Roy SK. 1946. Haematological study in pulmonary tuberculosis and the effect upon it of large doses of vitamin C. Tubercle 27:93. doi: 10.1016/S0041-3879(43)80036-2. [DOI] [PubMed] [Google Scholar]
- 17.Goswami T, Bhattacharjee A, Babal P, Searle S, Moore E, Li M, Blackwell JM. 2001. Natural-resistance-associated macrophage protein 1 is an H+/bivalent cation antiporter. Biochem J 354:511–519. doi: 10.1042/bj3540511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li X, Yang Y, Zhou F, Zhang Y, Lu H, Jin Q, Gao L. 2011. SLC11A1 (NRAMP1) polymorphisms and tuberculosis susceptibility: updated systematic review and meta-analysis. PLoS One 6:e15831. doi: 10.1371/journal.pone.0015831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Eck P, Erichsen HC, Taylor JG, Yeager M, Hughes AL, Levine M, Chanock S. 2004. Comparison of the genomic structure and variation in the two human sodium-dependent vitamin C transporters, SLC23A1 and SLC23A2. Hum Genet 115:285–294. doi: 10.1007/s00439-004-1167-x. [DOI] [PubMed] [Google Scholar]