ABSTRACT
Invasive candidiasis (IC) is a major cause of morbidity and mortality despite antifungal treatment. Azoles and echinocandins are used as first-line therapies for IC. However, their efficacy is limited by yeast tolerance and the emergence of acquired resistance. Tolerance is a reversible stage created due to the yeast's capacity to counter antifungal drug exposure, leading to persistent growth. For Candida albicans, multiple stress signaling pathways have been shown to contribute to this adaptation. Among them, the pH-responsive Rim pathway, through its transcription factor Rim101p, was shown to mediate azole and echinocandin tolerance. The Rim pathway is fungus specific, is conserved among the members of the fungal kingdom, and plays a key role in pathogenesis and virulence. The present study aimed at confirming the role of Rim101p and investigating the implication of the other Rim proteins in antifungal tolerance in C. albicans, as well as the mechanisms underlying it. Time-kill curve experiments and colony formation tests showed that genetic inhibition of all the Rim factors enhances echinocandin and azole antifungal activity. Through RNA sequencing analysis of a rim101−/− mutant, a strain constitutively overexpressing RIM101, and control strains, we discovered novel Rim-dependent genes involved in tolerance, including HSP90, encoding a major molecular chaperone, and IPT1, involved in sphingolipid biosynthesis. Rim mutants were also hypersensitive to pharmacological inhibition of Hsp90. Taken together, these data suggest that Rim101 acts upstream of Hsp90 and that targeting the Rim pathway in combination with existing antifungal drugs may represent a promising antifungal strategy to indirectly but specifically target Hsp90 in yeasts.
KEYWORDS: Candida albicans, HSP90, antifungal agents, pH signaling, sphingolipid, stress adaptation, tolerance
INTRODUCTION
Invasive candidiasis (IC) is a major cause of morbidity and mortality in immunocompromised patients and other critically ill patients, such as those hospitalized in intensive care units. Its incidence has increased over the past 3 decades, and despite antifungal treatment, its mortality remains high, with rates ranging from 46 to 75% (1). Candida albicans is responsible for approximately half of cases of invasive candidiasis worldwide (2).
Prophylaxis and treatment of IC rely mainly on two classes of antifungal drugs: azoles and echinocandins (3). The use of these drugs has increased dramatically over the past years because of their interesting pharmacokinetic and safety profiles and the increasing number of patients requiring prophylactic, preemptive, empirical, or curative antifungal treatment. As a predictable consequence, acquired resistance to both classes has emerged and become a large concern in medical settings. As many as 25% of C. glabrata isolates were found to be nonsusceptible to echinocandins in given medical centers in the United States between 2008 and 2014 (4). In addition, multidrug-resistant C. glabrata isolates resistant to both echinocandins and azoles have been isolated, leaving very few therapeutic options in these cases. In C. albicans, resistance rates are lower: around 0.5% for azoles and <1% for echinocandins (5, 6).
Apart from microbiological resistance, which can be defined as an increase of the MIC of an antifungal compound for a given strain, tolerance has also been described for Candida spp. Tolerance reflects the yeast's capacity to adapt to the presence of the antifungal drug and allows persistent growth at antifungal concentrations above the MIC (7). As a result, yeasts are not completely eradicated in the presence of the drug, leading to a condition that enables the selection of resistant mutants. Tolerance has been described for both fungistatic (azoles) and fungicidal (echinocandins) drugs (8). The mechanisms underlying this phenomenon are not completely elucidated. Multiple stress signaling pathways, activated in response to cell wall or membrane damages, have been shown to contribute to azole and/or echinocandin tolerance in C. albicans. Among these are the calcium-calcineurin, protein kinase C cell integrity, high-osmolarity glycerol, and sphingolipid biosynthesis pathways (9–17). Hsp90, a molecular chaperone interacting with about 10% of the C. albicans proteome, also plays a crucial role in antifungal tolerance: genetic or pharmacological inhibition of Hsp90 enhances azole and echinocandin activities in C. albicans (11, 18–20). Lysine deacetylases, such as Hos2, Hda1, Rpd3, and Rpd31, have also been shown to play a role in antifungal tolerance through Hsp90 deacetylation (21).
In addition to these mechanisms, a role of environmental pH in tolerance toward azole antifungal drugs has been described. Trailing growth, consisting of persistent growth at azole concentrations above the MIC, is inhibited at acidic pH in Candida spp. (22). In yeasts, pH signaling is mediated by the Rim pathway. This pathway is fungus specific and well conserved in the fungal kingdom (23). In C. albicans, external pH is sensed by a complex of 2 transmembrane proteins, Rim9 and Rim21/Dfg16, and the arrestin-like protein Rim8. Under neutral-alkaline conditions, Rim8 becomes hyperphosphorylated, leading to endocytosis of this membrane complex and recruitment of the endosomal sorting complexes required for transport (ESCRT) I, II, and III. Two other Rim proteins, Rim20 and the signaling protease Rim13, are then recruited, leading to cleavage of the C-terminal inhibitory domain of Rim101, the final transcription factor of the Rim pathway. Once activated, Rim101 migrates to the nucleus and regulates the expression of target genes involved in multiple cellular processes, including growth, iron metabolism, cell wall structure, yeast-to-hypha transition, adhesion, and biofilm formation (Fig. 1) (23). As such, this pathway is involved in both pathogenesis and virulence of C. albicans (24–27). The implication of the Rim pathway in tolerance to antifungal drugs was first investigated in the model yeast Saccharomyces cerevisiae; all Rim protein mutants were found to be hypersensitive to azoles (28). In C. albicans, genetic disruption of RIM101 leads to hypersusceptibility to both echinocandins and azoles, but implication of other Rim proteins in antifungal tolerance has not yet been assessed (29).
FIG 1.
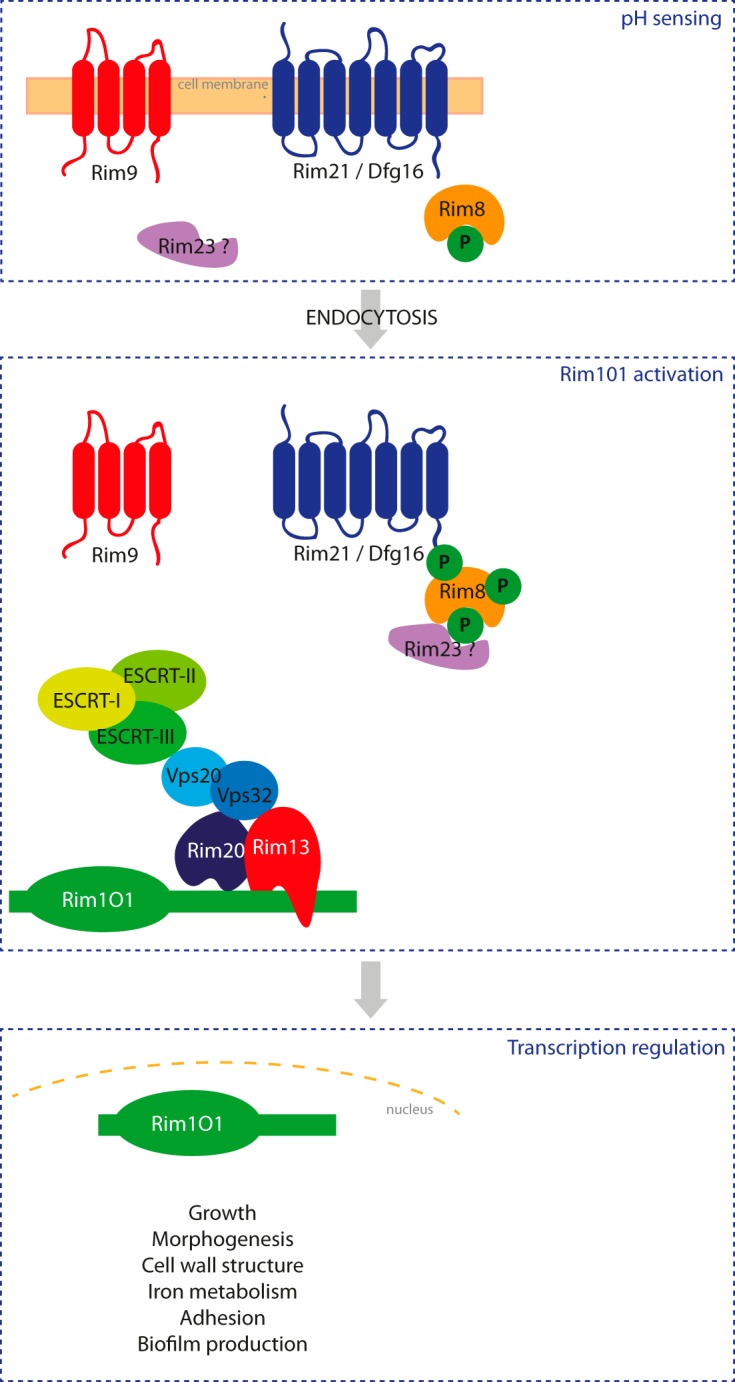
Schematic representation of the Rim pathway in C. albicans. External pH is sensed by a complex of 2 transmembrane proteins, Rim9 and Rim21/Dfg16, and the arrestin-like protein Rim8. Under neutral-alkaline conditions, Rim8 is hyperphosphorylated, leading to endocytosis of this membrane complex and recruitment of the endosomal sorting complexes required for transport (ESCRT) I, II, and III. Two other Rim proteins, Rim20 and the signaling protease Rim13, are then recruited (via Rim8 or an orthologue of S. cerevisiae Rim23, which has not yet been identified in C. albicans), leading to cleavage of the C-terminal inhibitory domain of Rim101, the final transcription factor of the Rim pathway. Once activated, Rim101p migrates to the nucleus and regulates the expression of target genes involved in multiple cellular processes, including growth, iron metabolism, cell wall structure, yeast-to-hypha transition, adhesion, and biofilm formation.
Here we show that the whole Rim pathway is involved in azole and echinocandin tolerance in C. albicans. Transcriptome sequencing (RNA-Seq) and phenotypic analyses suggest that its role in tolerance may be mediated through Hsp90, offering interesting perspectives for new antifungal strategies.
RESULTS
The Rim factors are involved in azole and echinocandin tolerance in C. albicans.
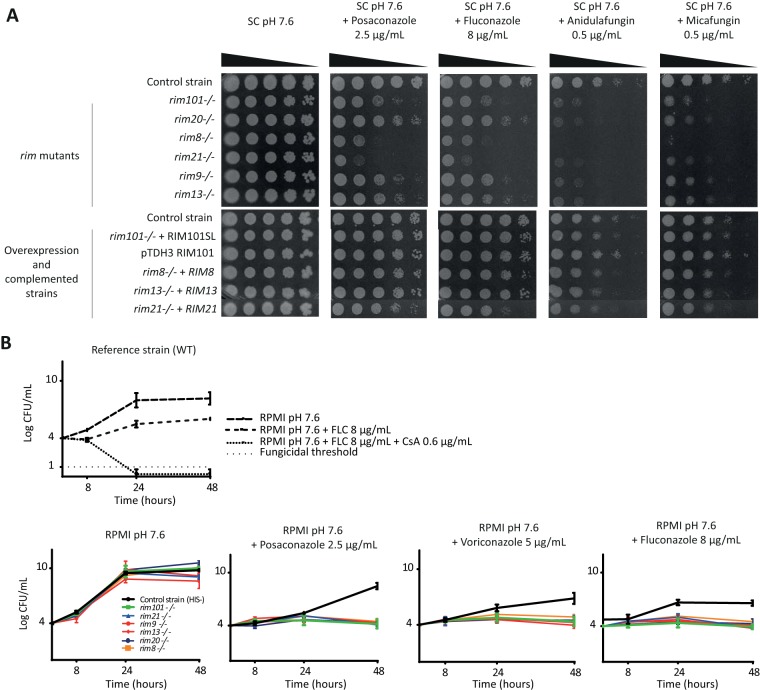
To investigate if all the Rim factors are involved in the response to antifungal drugs, rim mutants as well as complemented strains and strains constitutively overexpressing RIM101 (Table 1) were subjected to colony formation assays at alkaline pH (7.6) in the presence of fluconazole (FLC), voriconazole (VRC), posaconazole (POS), micafungin (MFG), or anidulafungin (AFG). As shown in Fig. 2A, all the rim mutants were hypersensitive to the five antifungal drugs tested, whereas complemented and RIM101-overexpressing strains displayed phenotypes similar to those of the control strains.
TABLE 1.
Strains used in this study
| Strain | Genotype or description | Reference |
|---|---|---|
| SC5314 | Reference strain | |
| CAI4 | ura3Δ::λimm434/ura3Δ::λimm434 | 46 |
| DAY185 | ura3Δ::λimm434/ura3Δ::λimm434 pHIS1::his1::hisG/his1::hisG pARG4::URA3::arg4::hisG/arg4::hisG | 47 |
| DAY25 | ura3Δ::λimm434/ura3Δ::λimm434 pHIS1::his1::hisG/his1::hisG arg4::hisG/arg4::hisG rim101::ARG4/rim101::URA3 | 47 |
| DAY286 | ura3Δ::λimm434/ura3Δ::λimm434 his1::hisG/his1::hisG pARG4::URA3::arg4::hisG/arg4::hisG | 48 |
| DAY5 | ura3Δ::λimm434/ura3Δ::λimm434 his1::hisG/his1::hisG arg4::hisG/arg4::hisG rim101::ARG4/rim101::URA3 | 49 |
| DAY23 | ura3Δ::λimm434/ura3Δ::λimm434 his1::hisG/his1::hisG arg4::hisG/arg4::hisG rim20::URA3/rim20::ARG4 | 49 |
| DAY61 | ura3Δ::λimm434/ura3Δ::λimm434 his1::hisG/his1::hisG arg4::hisG/arg4::hisG rim8::URA3/rim8::ARG4 | 50 |
| GKO88 | ura3Δ::λimm434/ura3Δ::λimm434 his1::hisG/his1::hisG arg4::hisG/arg4::hisG rim13::Tn7-UAU1/rim13::Tn7-URA3 | 51 |
| MC21 | ura3Δ::λimm434/ura3Δ::λimm434 his1::hisG/his1::hisG arg4::hisG/arg4::hisG rim21::URA3/rim21::URA3 | 52 |
| MC23 | ura3Δ::λimm434/ura3Δ::λimm434 his1::hisG/his1::hisG arg4::hisG/arg4::hisG rim9::URA3/rim9::ARG4 | 52 |
| MC21C | ura3Δ::λimm434/ura3Δ::λimm434 pHIS1::RIM21::his1::hisG/his1::hisG arg4::hisG/arg4::hisG rim21::URA3/rim21::URA3 | 52 |
| MC23C | ura3Δ::λimm434/ura3Δ::λimm434 pHIS1::RIM9::his1::hisG/his1::hisG arg4::hisG/arg4::hisG rim9::URA3/rim9::ARG4 | 52 |
| DAY106 | ura3Δ::λimm434/ura3Δ::λimm434 his1::hisG/his1::hisG arg4::hisG/arg4::hisG rim8::URA3/rim8::ARG4::pRIM8::HIS1 | 47 |
| DAY226 | ura3Δ::λimm434/ura3Δ::λimm434 pHIS1::RIM13::his1::hisG/his1::hisG arg4::hisG/arg4::hisG rim13::URA3/rim13::ARG4 | 51 |
| DAY5SL (MC13) | ura3Δ::λimm434/ura3Δ::λimm434 pRIM101SL::HIS′rim101/RIM101 arg4::hisG/arg4::hisG rim101::ARG4/rim101::URA3 | 53 |
| CGY1 | ura3Δ::λimm434/ura3Δ::λimm434 RPS1/RPS1::pTDH3-RIM101 | This study |
FIG 2.
The Rim pathway is involved in antifungal tolerance in C. albicans. (A) Colony formation assays. Cells were plated in 10-fold dilutions in the presence of fluconazole (8 μg/ml), posaconazole (2.5 μg/ml), anidulafungin (0.5 μg/ml), or micafungin (0.5 μg/ml) in SC medium buffered at pH 7.6. The 6 rim mutants were hypersensitive to the different antifungal drugs. (B) (Top) Time-kill curves for the SC5314 reference strain in the presence of fluconazole (FLC; 8 μg/ml) or a combination of fluconazole and the calcineurin inhibitor cyclosporine (CsA; 0.6 μg/ml) at pH 7.6. Inhibition of antifungal tolerance by CsA restored the fungicidal effect of FLC. (Bottom) Time-kill curves for the rim mutants and a control strain in the presence of posaconazole (2.5 μg/ml), voriconazole (5 μg/ml), or fluconazole (8 μg/ml) at pH 7.6. Growth rates of the mutants at 48 h were significantly lower than those of the control strain with the three azole compounds (P < 0.001).
To further characterize the hypersensitive phenotype of the rim mutants, time-kill curve experiments were performed, albeit only for azoles, as previous studies reported that these assays are unsuitable for echinocandins because of a clumping effect (11). Yeast strains were cultured in liquid medium in the presence of azole antifungal drugs and sampled at different time points on solid agar medium to quantify the CFU. As depicted in Fig. 2B, the growth of the rim mutants after 48 h of incubation was significantly reduced in the presence of azole antifungal drugs compared to the growth of the control strain for the three compounds tested (FLC, VRC, and POS) (P < 0.001). In contrast, growth of complemented strains was similar to that of the control strain (data not shown). In our experiments, which used a lower initial inoculum than the recommended one, no significant reduction of the initial inoculum was evident for any mutant strain, impeding confirmation that Rim inhibition restores a fungicidal effect.
Finally, azole and echinocandin MICs at neutral pH were determined as recommended by the EUCAST microdilution method (30, 31). MICs did not differ between control and mutant strains, suggesting that the Rim factors are involved in tolerance rather than resistance to echinocandins and azoles (see Table S1 in the supplemental material).
Taken together, these data show that genetic inhibition of all the Rim factors enhances the antifungal activity of the azoles and echinocandins in C. albicans, which appears to result from inhibition of tolerance toward these antifungal drugs.
Transcriptomic analysis reveals new Rim-dependent genes involved in antifungal tolerance.
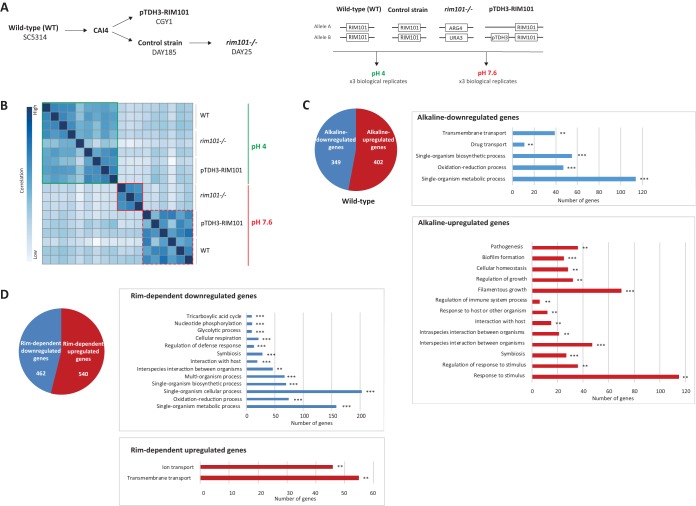
In order to assess the mechanism by which the Rim pathway contributes to antifungal tolerance, deep RNA-Seq analysis of the SC5314 reference strain (wild type [WT]), the DAY185 control strain, a rim101−/− disruption strain (DAY25), and a strain constitutively overexpressing RIM101 (CGY1) was performed. Samples were collected after growth at pH 4 (Rim pathway inactivated) and pH 7.6 (Rim pathway activated). Analysis was performed using biological triplicates (Fig. 3A). RNAs were extracted, prepared, and sequenced using standard RNA-Seq methods. Transcriptomic analysis was performed following the pipeline described in Materials and Methods and presented in Fig. S1A. The transcription programs of control strains SC5314 and DAY185 at alkaline and acidic pHs did not differ significantly (Fig. S1B), and no differentially expressed genes could be identified. For this reason, we chose to use strain SC5314 as a reference in the following differential analysis.
FIG 3.
Transcriptome analysis of a rim101Δ disruption strain (DAY25), a strain overexpressing RIM101 (CGY1), and the SC5314 reference strain. (A) (Left) Strain lineage. (Right) Experimental design of RNA-Seq experiments. (B) Unsupervised hierarchical clustering of the distances between the SC5413 reference strain, the rim101Δ disruption strain (DAY25), and the RIM101-overexpressing strain (CGY1). Data for all replicates are presented. (C) Alkaline pH-upregulated and -downregulated genes in the SC5413 reference strain. The graphs show the results of GO term enrichment analysis of biological processes among alkaline pH-upregulated and -downregulated genes. The number of genes is expressed on the x axis. **, P < 0.01; ***, P < 0.001. (D) Rim-dependent upregulated and downregulated genes in the rim101Δ disruption strain (DAY25) compared to those in the SC5413 reference strain at pH 7.6. The graphs show the results of GO term enrichment analysis of biological processes among Rim-dependent upregulated and downregulated genes. The number of genes is expressed on the x axis. **, P < 0.01; ***, P < 0.001.
Intersample distance clustering was performed to assess the similarity between replicates across each experimental condition (Fig. 3B). Biological replicates clustered together, confirming the reproducibility of the experimental conditions. Moreover, at pH 4, the transcription profiles were similar between the reference and mutant strains. The transcription programs of the reference strain and the RIM101-overexpressing strain also appeared to cluster together at alkaline pH. In contrast, the rim101−/− transcription profile at pH 7.6 differed significantly from both previous clusters (Fig. 3B). These observations were confirmed using another statistical analysis, i.e., principal component analysis (Fig. S1C). These data confirm that environmental pH crucially affects gene expression in C. albicans and that RIM101 expression is essential to the physiological adaptation of C. albicans to alkaline pH conditions.
(i) Acidic pH response.
No statistically significant difference in transcription profiles was evidenced between the rim101Δ disruption strain and the SC5314 reference strain at pH 4 (Fig. S2), suggesting that RIM101 is not required for the transcriptional response under acidic conditions. In contrast, 965 genes were significantly differentially expressed between the RIM101-overexpressing strain and the wild-type strain under such conditions (log2 fold change below −1.5 or above 1.5; adjusted P value of <0.05) (Table S2). GO term analysis of these genes identified significant enrichment in three biological process categories: transmembrane transport, drug transport, and serine family amino acid metabolic processes (Fig. S3).
(ii) Alkaline pH response of the SC5314 reference strain.
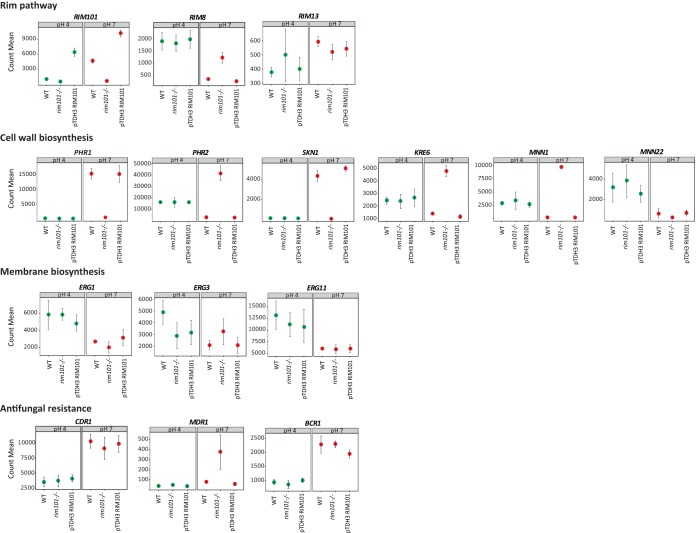
A total of 751 genes were differentially expressed at pH 7.6 versus pH 4 for the SC5314 reference strain, including 402 alkaline pH-upregulated and 349 alkaline pH-downregulated genes (Fig. 3C; Table S4). RIM101 and RIM13 were upregulated at alkaline pH, consistent with the activation of the Rim pathway under such alkaline conditions (Fig. 4). In contrast, RIM8 was downregulated, in accordance with its implication in negative feedback of the Rim pathway and its preferential expression at acidic pH (23, 32) (Fig. 4). Expression of the other RIM genes was not significantly different at pH 7.6 from that at pH 4.
FIG 4.
Normalized gene counts obtained by RNA-Seq at both acidic and alkaline pHs for various genes in the SC5413 reference strain, the rim101Δ disruption strain (DAY25), and the RIM101-overexpressing strain (CGY1).
Interestingly, GO term analysis revealed that the alkaline pH-upregulated genes were mostly enriched for genes involved in pathogenesis, such as filamentous growth, biofilm formation, response to host, and response to stimulus. The alkaline pH-downregulated genes were significantly enriched in the transmembrane and drug transport, metabolic process, biosynthetic process, and oxidation-reduction process categories (Fig. 3C).
Among genes potentially involved in antifungal tolerance and/or resistance (Table S3), 33 were differentially expressed at alkaline and acidic pHs. Among those with the highest fold changes were genes involved in membrane biosynthesis. Expression of IPT1, a gene coding for an enzyme responsible for the synthesis of the most abundant sphingolipid, mannose-(inositol-P)2-ceramide, was induced by alkaline pH (Fig. 5A), whereas expression of ERG11, ERG1, and ERG3, genes involved in biosynthesis of ergosterol, a major component of the fungal cell membrane, was repressed by alkaline pH (Fig. 4; Table S4). Expression of several genes involved in cell wall biosynthesis was also pH dependent. SKN1, coding for a protein involved in β-1,6-glucan synthesis, was strongly induced at alkaline pH (fold change = 24.25). Similarly, PHR1 and PHR2, two genes coding for cell wall transglycosidases, were alkaline pH-upregulated and -downregulated genes, respectively. In addition, MNN1 and MNN22, both coding for mannosyltransferases, were downregulated at alkaline pH. All the above-mentioned pH-dependent genes involved in cell wall synthesis, except for MNN22, are known to be regulated by Rim101. Surprisingly, KRE6, known to be induced by RIM101, was found to be downregulated at alkaline pH in our study. Interestingly, CDR1 and MDR1, coding for transporters involved in azole antifungal drug efflux, and BCR1, coding for a transcription factor potentially involved in fluconazole resistance, were induced at alkaline pH (Fig. 4).
FIG 5.
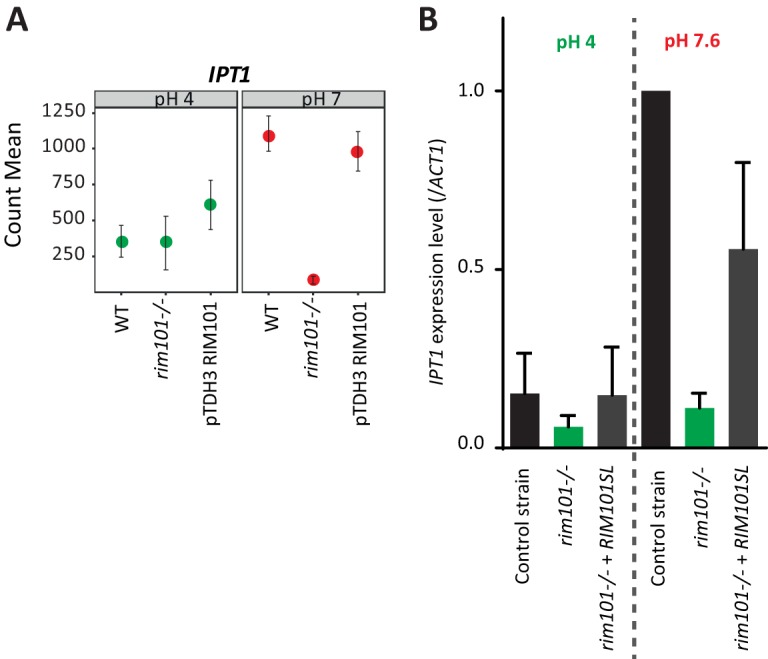
IPT1 is a pH- and Rim-dependent gene. (A) Normalized gene counts obtained by RNA-Seq at both acidic and alkaline pHs for IPT1 in the SC5413 reference strain, the rim101Δ disruption strain (DAY25), and the RIM101-overexpressing strain (CGY1). (B) IPT1 expression levels obtained by RT-qPCR at acidic and alkaline pHs for the rim101Δ disruption strain DAY25, the complemented strain DAY5SL, and the control strain DAY185, normalized to the ACT1 level.
As expected, transcriptomic analysis of the SC5314 reference strain revealed that genes upregulated at alkaline pH are mostly involved in pathogenesis. In addition, several genes involved in antifungal tolerance were differentially expressed at acidic and alkaline pHs, confirming that pH influences this phenomenon.
(iii) Alkaline pH response in the rim101Δ disruption strain.
In order to identify RIM101-dependent genes, transcription profiles at pH 7.6 for the rim101Δ disruption strain and the SC5314 reference strain were compared. A total of 1,002 differentially expressed genes were retrieved, among which 462 were downregulated and 540 were upregulated (Fig. 3D; Table S5).
Rim-dependent genes upregulated in the rim101−/− mutant at alkaline pH were mainly enriched in the ion and transmembrane transport categories, whereas Rim-dependent downregulated genes were more diverse and mostly enriched in metabolic, cellular, biosynthesis, and oxidation-reduction process categories, as well as interaction and response to host (GO term analysis) (Fig. 3D).
As for alkaline pH-responsive genes, a focus was given to genes potentially involved in antifungal tolerance and resistance (Table S3). Thirty-seven of these genes were differentially expressed in the mutant and the wild-type strain, among which 15 were also identified as alkaline pH-responsive genes by transcriptomic analysis of the wild-type strain. As expected, SKN1 and PHR1, known to be induced by RIM101, were significantly downregulated in the rim101−/− mutant compared to those in the wild type, whereas the RIM101-repressed genes PHR2 and MNN1 were upregulated (Fig. 4). Many other genes coding for proteins involved in cell wall biosynthesis were upregulated in the rim101Δ disruption strain. Consistent with our previous observation in the wild-type strain, KRE6 was induced in the rim101−/− mutant (Fig. 4), which confirms that this gene might be repressed rather than induced by RIM101.
Interestingly, IPT1, involved in sphingolipid biosynthesis, was expressed at a significantly lower level in the mutant than in the wild type (fold change = 8.98) (Fig. 5A); its expression therefore appears to be both Rim and pH dependent.
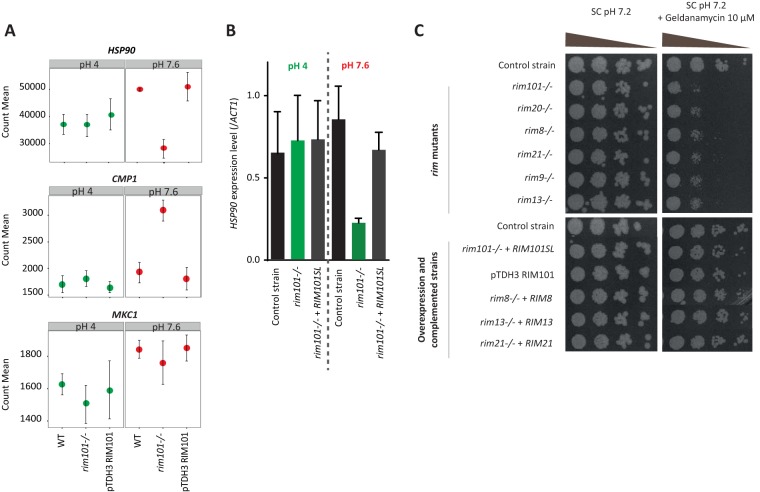
Another gene, HSP90, was found to be downregulated significantly in the rim101−/− mutant (fold change = 1.79) compared to that in the wild type at alkaline pH (Fig. 6A). This gene encodes a molecular chaperone that interacts with about 10% of the C. albicans proteome and plays a key role in echinocandin and azole tolerance in this species (11, 18, 19). HSP90 therefore appears to be a downstream effector of RIM101. Intriguingly, although HSP90 seemed to be upregulated at alkaline pH compared to its level at acidic pH in the wild-type strain (Fig. 6A), this difference was not statistically significant, and HSP90 was not identified as a pH-dependent gene by differential expression analysis.
FIG 6.
HSP90 is a Rim-dependent gene. (A) Normalized gene counts obtained by RNA-Seq at both acidic and alkaline pHs for the HSP90, CMP1, and MKC1 genes in the SC5413 reference strain, the rim101Δ disruption strain (DAY25), and the RIM101-overexpressing strain (CGY1). (B) HSP90 expression levels obtained by RT-qPCR at acidic and alkaline pHs for the rim101Δ disruption strain DAY25, the complemented strain DAY5SL, and the control strain DAY185, normalized to the ACT1 level. (C) Colony formation assays in the presence of the pharmacological Hsp90 inhibitor geldanamycin. Cells were plated in 10-fold dilutions in the presence of geldanamycin (10 μM) in SC medium buffered at pH 7.2. The rim mutants were hypersensitive to pharmacological inhibition of Hsp90.
Hsp90 and Ipt1 may participate in Rim-dependent antifungal tolerance in C. albicans.
Considering the RNA-Seq results and that IPT1 and HSP90 are known to be involved in antifungal tolerance, we therefore hypothesized that the role of the Rim pathway in antifungal tolerance may be mediated partly by these genes.
In parallel to the RNA-Seq analysis, expression levels of IPT1 and HSP90 in the DAY185 control strain, the rim101Δ disruption strain (DAY25), and a complemented strain constitutively expressing the active form of Rim101 (DAY5SL) were assessed by reverse transcription-quantitative PCR (RT-qPCR). These experiments confirmed the Rim- and pH-dependent expression of IPT1 shown in the RNA-Seq analysis (Fig. 5B). Considering HSP90, its expression was significantly reduced in the rim101Δ disruption strain compared to that in the control strain and the complemented strain at pH 7.6, whereas its expression levels were similar in all strains at pH 4 (Fig. 6B). As for RNA-Seq, these results suggest that RIM101 acts upstream of HSP90.
To further characterize the interaction between Hsp90 and the Rim pathway, susceptibility to geldanamycin, a pharmacological inhibitor of Hsp90, was assessed by colony formation assays of rim mutant, wild-type, RIM101-overexpressing, and complemented strains. Geldanamycin binds to the N-terminal ATP/ADP-binding domain of Hsp90 and inhibits its ATPase activity (which is essential for its chaperone function) (33, 34). The combination of both a high pH (7.6) and geldanamycin resulted in a high stress which strikingly inhibited yeast growth of WT strains. For this reason, the experiment was performed at a pH (7.2) lower than that of the previous experiments yet sufficient to activate the Rim pathway. As depicted in Fig. 6C, all rim mutants were hypersensitive to geldanamycin, while the RIM101-overexpressing and complemented strains displayed a phenotype similar to that of the control strain. These data suggest that Rim101 and Hsp90 are chemical-genetic interactors, consistent with the RNA-Seq results. Taken together, the transcriptomic and phenotypic results therefore differ from those of previous studies suggesting that Rim101 is a client protein of Hsp90 (20, 35).
The implication of Hsp90 in antifungal tolerance is known to be mediated by at least two client proteins: calcineurin (Cmp1) and Mkc1 (11, 18, 19). As opposed to HSP90, CMP1 was upregulated in the rim101−/− mutant compared to that in the wild type (Fig. 6A). In addition, no difference in sensitivity to cyclosporine, a pharmacological calcineurin inhibitor, was noticed between the rim101−/− mutant, a strain overexpressing RIM101, and the control strain (Fig. S4). This suggests that Hsp90 may be involved in Rim-dependent antifungal tolerance independent of calcineurin. In addition, no difference in the MKC1 level of expression was evidenced by RNA-Seq analysis of the mutant and the wild-type strain at alkaline pH (Fig. 6A). Thus, the implication of Hsp90 in antifungal tolerance mediated by the Rim pathway may involve additional mechanisms and requires further investigation.
DISCUSSION
This study explored the implication of the Rim pathway in response to azoles and echinocandins in C. albicans by phenotypic and transcriptomic analyses. It implicated the Rim pathway in both azole and echinocandin tolerance. Moreover, it showed that in addition to Rim101p, the terminal transcription factor of the pathway, all the Rim proteins are involved in this tolerance. This study also identified new Rim101 downstream effectors, such as Hsp90, a major molecular chaperone, and Ipt1, involved in sphingolipid biosynthesis, which may contribute to the Rim-mediated antifungal tolerance.
First, we showed that all rim mutants were hypersensitive to azoles and echinocandins by colony formation assays, suggesting an involvement of the Rim pathway in tolerance and/or resistance to these antifungal drugs. We then performed time-kill curve experiments, recommended as a key method for exploring antifungal tolerance, which confirmed the enhanced activity of azoles toward the rim mutants (8). However, this assay presents severe limitations with C. albicans due to a clumping effect: it has previously been shown to be inaccurate for echinocandins, which are still one of the most widely prescribed antifungal classes (11). In this study, we showed that the clumping effect also limits the use of this assay at alkaline pH, a condition known to induce filamentation. Indeed, severe clumping occurred with the usual initial inoculum, especially for the control strain, rendering CFU counts on agar plates inaccurate. We therefore had to use a lower initial inoculum (104 CFU/ml) than the recommended one (at least 105 CFU/ml). Evaluation of antifungal tolerance at alkaline pH therefore remains challenging. However, azole and echinocandin MICs, which did not differ between the control strain and the rim mutants, suggest that the Rim pathway is involved in tolerance rather than resistance to these antifungal drugs.
RNA sequencing of both a wild-type strain and a strain disrupted for RIM101 was then performed at alkaline and acidic pHs to identify potential candidates underlying involvement of the Rim pathway in antifungal tolerance. Two previous studies performed transcriptomic profiling of a C. albicans wild-type strain under both alkaline (pH 8) and acidic (pH 4) conditions. Bensen et al. used microarrays and described 514 pH-responsive genes in the DAY185 strain, using a criterion of a ≥2-fold change (36). More recently, Bruno et al. performed RNA-Seq analysis of the SC5314 reference strain at pH 4 and pH 8 in order to proceed to the comprehensive annotation of the C. albicans transcriptome; in that study, 1,381 genes were found to be differentially expressed at alkaline versus acidic pH (37). Using the criterion of a ≥2-fold change, our study identified only 346 differentially expressed genes. Half of them were also identified as such in the study of Bruno et al. However, direct comparison of results is not accurate because different experimental conditions were used; notably, incubation time, medium, and pH conditions varied between the 3 studies. Bensen et al. also studied the transcriptomic profiles of the DAY25 rim101−/− mutant at acidic and alkaline pHs. However, our study is the first to investigate it by RNA sequencing.
Several genes potentially involved in antifungal tolerance and resistance were identified as Rim dependent. Among them, two particularly retained our interest.
First, HSP90 was identified as a Rim-dependent gene. Although the decrease of its expression in the rim101Δ disruption strain compared to that in the SC5314 reference strain at alkaline pH was not drastic (1.79-fold), it is statistically significant and was confirmed by RT-qPCR (Fig. 6B). In addition, HSP90 expression was also decreased in the rim101−/− mutant strain compared to that in the SC5314 reference strain at alkaline pH in the presence of fluconazole or anidulafungin (see Fig. S5 in the supplemental material). Moreover, rim mutants were hypersensitive to pharmacological Hsp90 inhibition. RIM101 was previously identified as a genetic interactor of HSP90 in C. albicans (38, 39). Another study suggested that Rim101p was a client protein of the chaperone Hsp90 in yeasts, based on the results of a genome-wide genetic screen in S. cerevisiae (20, 35). In contrast, our transcriptomic and phenotypic results support the hypothesis that Rim101 acts upstream of Hsp90.
Second, IPT1 was shown to be downregulated in the rim101−/− mutant compared to that in the wild-type strain at alkaline pH. Importantly, this downregulation was conserved in the presence of fluconazole or anidulafungin (Fig. S5). To our knowledge, this is the first study to identify this gene as a Rim-dependent gene. IPT1 encodes an enzyme responsible for the biosynthesis of the most abundant membrane sphingolipid, mannose-(inositol-P)2-ceramide (16). It was previously shown that disruption of this gene was associated with hypersensitivity to fluconazole in C. albicans (16). In addition, membrane sphingolipids may influence the interaction between the echinocandins and their target, Fks1p, and therefore modulate their antifungal activity (17). IPT1 was also identified as a pH-dependent gene: its expression was significantly induced at alkaline pH versus acidic pH in the wild-type strain. All these data suggest that IPT1 may contribute to Rim-mediated antifungal tolerance.
These results open novel perspectives. Hsp90 has been shown to play a crucial role in antifungal tolerance in C. albicans (11, 18, 19). It has also been involved in development of resistance in this species (19). Moreover, under stress conditions, it has been shown to induce genetic instability in S. cerevisiae, leading to aneuploidy and the occurrence of antifungal resistance (40). Efungumab, a recombinant human antibody fragment targeting fungal Hsp90, has been evaluated as a new antifungal strategy in combination with amphotericin B in vitro and in vivo. In a phase III clinical trial involving patients with invasive candidiasis, efungumab in combination with lipid-associated amphotericin B decreased the Candida-attributable mortality, improved the overall clinical response rate, and increased the rate of culture-confirmed clearance of the infection (41). However, despite its good antifungal activity, its development was stopped due to quality and safety issues. As Hsp90 is not a fungus-specific protein, developing anti-Hsp90 antifungal strategies may expose patients to toxicity. In contrast, the Rim pathway is fungus specific and relatively well conserved among the members of the fungal kingdom. As it appears to act upstream of Hsp90, targeting this pathway would be an interesting way to indirectly target Hsp90 and avoid toxicity in humans. Moreover, as this pathway is also involved in pathogenesis and virulence as well as in antifungal tolerance, inhibiting the Rim pathway in combination with commercially available antifungal drugs would have great potential as a new therapeutic strategy. Such a strategy would therefore be limited to the treatment of infections at anatomical sites displaying neutral or alkaline pH, that is, conditions under which the Rim pathway is activated. However, this is the case for most cases of invasive candidiasis, and notably for candidemia or intra-abdominal candidiasis. It would also be interesting to investigate the Rim pathway in other pathogenic Candida species, especially C. glabrata, which is the second most frequent agent of invasive candidiasis and displays a high genetic distance from C. albicans. Indeed, resistance and therapeutic failures are more challenging with C. glabrata than with C. albicans, although until now the Rim pathway and the mechanisms of antifungal drug tolerance have been described more thoroughly for the latter species (2, 6).
In conclusion, this study shows that the 6 Rim proteins are involved in azole and echinocandin tolerance in C. albicans. Transcriptomic and phenotypic analyses suggest that this implication in tolerance may be mediated partly through Hsp90 and Ipt1. Further studies are warranted to characterize the interactions between Rim101 and Hsp90 as well as to describe the role of Ipt1 in antifungal tolerance. Targeting the Rim pathway in combination with existing antifungal drugs may therefore represent a promising antifungal strategy.
MATERIALS AND METHODS
Strains and antifungal drugs.
All strains used in the study are listed in Table 1. The pTDH3-RIM101 overexpression strain was constructed from strain CAI4 by use of Gateway technology (Gateway LR clonase kit; Thermo Fisher Scientific) and a CIp-pTDH3-GTW expression vector, kindly given by Christophe d'Enfert.
Antifungal drug powders were obtained from the following pharmaceutical companies: fluconazole, voriconazole, and anidulafungin from Pfizer, posaconazole from MSD, and micafungin from Astellas.
Time-kill curve experiments.
Time-kill curve experiments were performed in RPMI medium buffered with HEPES at pH 7.6. Fluconazole, voriconazole, and posaconazole were used at 8, 5, and 2.5 μg/ml, respectively. Anidulafungin and micafungin were used at 0.5 μg/ml. Controls without any antifungal drug were performed in parallel. An initial inoculum of 104 or 106 CFU/ml, obtained from overnight preculture of each strain in liquid yeast extract-peptone-dextrose (YPD) medium, was added to each tube and further incubated at 30°C with agitation for 48 h. After 8, 24, and 48 h of incubation, 10 μl of each suspension was sampled and serially diluted in 0.9% NaCl, and 20-μl aliquots of the diluted suspension were plated in duplicate on YPD agar. If the growth was very limited after 24 or 48 h of incubation, 100 μl of suspension was directly plated in duplicate on YPD agar. Under these conditions, the minimal accurately countable number of CFU per milliliter was 10. Determination of the number of CFU was performed after 24 to 48 h of incubation at 30°C. A minimum of 2 replicates was performed for each time-kill curve experiment. A reduction of the initial inoculum of ≥3 log (≥99.9%) defined fungicidal activity. The ability of such a protocol to evidence a fungicidal effect resulting from inhibition of antifungal tolerance was controlled by the use of a combination of cyclosporine (0.6 μg/ml) and fluconazole compared to the use of fluconazole alone for the reference strain SC5314 (Fig. 2B).
Colony formation assays.
Each strain was precultured overnight in liquid YPD medium at 30°C with agitation and then diluted to an optical density at 600 nm (OD600) of 5. Yeasts were plotted on SC medium buffered at pH 7.6, SC medium buffered at pH 7.6 and containing anidulafungin (0.5 μg/ml), micafungin (0.5 μg/ml), fluconazole (8 μg/ml), or posaconazole (2.5 μg/ml), or SC medium buffered at pH 7.2 and containing geldanamycin (10 μM) in 10-fold dilution series. Plates were incubated at 30°C for 48 h to 6 days prior to imaging.
RNA extraction.
Each strain (DAY185, DAY25, and CGY1 for RNA-Seq and DAY185, DAY25, and DAY5SL for RT-qPCR) (Table 1) was precultured overnight in liquid YPD medium at 30°C with agitation and then diluted to an OD600 of 0.1 in SC medium buffered at pH 7.6 or pH 4 with HEPES or citrate buffer, respectively. Cells were incubated at 30°C (to be consistent with the other experiments of the study and to avoid intense yeast-to-hypha transition) with agitation until an OD600 of 0.6 to 0.8 was reached and then were collected by centrifugation. RNA extraction was performed on the pellet by use of a MasterPure Yeast RNA purification kit (Epicentre) according to the manufacturer's instructions. Three biological replicates were performed for each strain and condition.
RT-qPCR.
RT-qPCR was performed using Kapa Probe Fast qPCR master mix (Clinisciences). Primers and probes are listed in Table S6 in the supplemental material. Gene expression levels were normalized to the ACT1 expression level.
RNA-Seq analysis.
Library preparation and RNA sequencing were performed by GATC Biotech (European Genome and Diagnostics Centre, Constance, Germany). The bioinformatics analysis pipeline can be summarized as follows (Fig. S1A). Reads were quality checked by use of FastQC before and after cleaning by Trimmomatic, applying default parameters (42). Reads were then aligned to C. albicans genome assembly 22 (allele A; available from the Candida Genome Database) by use of Bowtie2 (43). Raw read counts for each gene were calculated using HTSeqCount, using default parameters (44). Read count data were normalized using the DESeq2 R package (45). DESeq2-normalized read counts were used to identify differentially expressed genes with adjusted P values of <0.05 and fold changes below −1.5 or above 1.5 (or below −2 or above 2 for comparison with previously published data). Gene ontology analysis was performed using the GO Slim Mapper available from the Candida Genome Database.
Statistical analysis.
Statistical analysis was performed by two-way analysis of variance (ANOVA) (for antifungal and strain, with antifungal-strain interaction) and Tukey-Kramer post hoc analysis. A secondary analysis was performed for each antifungal.
Accession number(s).
Data from the RNA-Seq analysis are currently deposited in the GEO repository under accession number GSE109291.
Supplementary Material
ACKNOWLEDGMENTS
This study was partly funded by a grant from the Grenoble Alpes University to M. Cornet (grant CSVSB 2011). This work was supported by the Fond d'Intervention of the University Grenoble Alpes (to J. Govin), the Agence Nationale de la Recherche (grant ANR-11-PDOC-0011 to J. Govin), a European Union FP7 Marie Curie Action career integration grant (grant 304003 to J. Govin), the Fondation pour la Recherche Médicale (grant SPF20140129159 to E. García-Oliver), and the FINOVI program of the Région Rhone Alpes (to M. Champleboux).
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
We are grateful to Christophe d'Enfert, Murielle Chauvel, and Aaron P. Mitchell for the generous gifts of strains and a plasmid used in this study. We thank Rose-Laurence Bertini, Marie Arlotto, and Marie Courçon for technical help.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/AAC.01785-17.
REFERENCES
- 1.Brown GD, Denning DW, Gow NAR, Levitz SM, Netea MG, White TC. 2012. Hidden killers: human fungal infections. Sci Transl Med 4:165rv13. doi: 10.1126/scitranslmed.3004404. [DOI] [PubMed] [Google Scholar]
- 2.Kullberg BJ, Arendrup MC. 2015. Invasive candidiasis. N Engl J Med 373:1445–1456. doi: 10.1056/NEJMra1315399. [DOI] [PubMed] [Google Scholar]
- 3.Pappas PG, Kauffman CA, Andes DR, Clancy CJ, Marr KA, Ostrosky-Zeichner L, Reboli AC, Schuster MG, Vazquez JA, Walsh TJ, Zaoutis TE, Sobel JD. 2016. Executive summary: clinical practice guideline for the management of candidiasis: 2016 update by the Infectious Diseases Society of America. Clin Infect Dis 62:409–417. doi: 10.1093/cid/civ1194. [DOI] [PubMed] [Google Scholar]
- 4.Vallabhaneni S, Cleveland AA, Farley MM, Harrison LH, Schaffner W, Beldavs ZG, Derado G, Pham CD, Lockhart SR, Smith RM. 2015. Epidemiology and risk factors for echinocandin nonsusceptible Candida glabrata bloodstream infections: data from a large multisite population-based candidemia surveillance program, 2008–2014. Open Forum Infect Dis 2:ofv163. doi: 10.1093/ofid/ofv163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Castanheira M, Messer SA, Rhomberg PR, Pfaller MA. 2016. Antifungal susceptibility patterns of a global collection of fungal isolates: results of the SENTRY Antifungal Surveillance Program (2013). Diagn Microbiol Infect Dis 85:200–204. doi: 10.1016/j.diagmicrobio.2016.02.009. [DOI] [PubMed] [Google Scholar]
- 6.Shields RK, Nguyen MH, Clancy CJ. 2015. Clinical perspectives on echinocandin resistance among Candida species. Curr Opin Infect Dis 28:514–522. doi: 10.1097/QCO.0000000000000215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Delarze E, Ischer F, Sanglard D, Coste AT. 2015. Adaptation of a Gaussia princeps luciferase reporter system in Candida albicans for in vivo detection in the Galleria mellonella infection model. Virulence 6:684–693. doi: 10.1080/21505594.2015.1081330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Delarze E, Sanglard D. 2015. Defining the frontiers between antifungal resistance, tolerance and the concept of persistence. Drug Resist Updat 23:12–19. doi: 10.1016/j.drup.2015.10.001. [DOI] [PubMed] [Google Scholar]
- 9.Onyewu C, Blankenship JR, Del Poeta M, Heitman J. 2003. Ergosterol biosynthesis inhibitors become fungicidal when combined with calcineurin inhibitors against Candida albicans, Candida glabrata, and Candida krusei. Antimicrob Agents Chemother 47:956–964. doi: 10.1128/AAC.47.3.956-964.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sanglard D, Ischer F, Marchetti O, Entenza J, Bille J. 2003. Calcineurin A of Candida albicans: involvement in antifungal tolerance, cell morphogenesis and virulence. Mol Microbiol 48:959–976. doi: 10.1046/j.1365-2958.2003.03495.x. [DOI] [PubMed] [Google Scholar]
- 11.Singh SD, Robbins N, Zaas AK, Schell WA, Perfect JR, Cowen LE. 2009. Hsp90 governs echinocandin resistance in the pathogenic yeast Candida albicans via calcineurin. PLoS Pathog 5:e1000532. doi: 10.1371/journal.ppat.1000532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yu S-J, Chang Y-L, Chen Y-L. 2015. Calcineurin signaling: lessons from Candida species. FEMS Yeast Res 15:fov016. doi: 10.1093/femsyr/fov016. [DOI] [PubMed] [Google Scholar]
- 13.Wiederhold NP, Kontoyiannis DP, Prince RA, Lewis RE. 2005. Attenuation of the activity of caspofungin at high concentrations against Candida albicans: possible role of cell wall integrity and calcineurin pathways. Antimicrob Agents Chemother 49:5146–5148. doi: 10.1128/AAC.49.12.5146-5148.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dichtl K, Samantaray S, Wagener J. 2016. Cell wall integrity signalling in human pathogenic fungi. Cell Microbiol 18:1228–1238. doi: 10.1111/cmi.12612. [DOI] [PubMed] [Google Scholar]
- 15.Rauceo JM, Blankenship JR, Fanning S, Hamaker JJ, Deneault J-S, Smith FJ, Nantel A, Mitchell AP. 2008. Regulation of the Candida albicans cell wall damage response by transcription factor Sko1 and PAS kinase Psk1. Mol Biol Cell 19:2741–2751. doi: 10.1091/mbc.E08-02-0191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Prasad T, Saini P, Gaur NA, Vishwakarma RA, Khan LA, Haq QMR, Prasad R. 2005. Functional analysis of CaIPT1, a sphingolipid biosynthetic gene involved in multidrug resistance and morphogenesis of Candida albicans. Antimicrob Agents Chemother 49:3442. doi: 10.1128/AAC.49.8.3442-3452.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Healey KR, Challa KK, Edlind TD, Katiyar SK. 2015. Sphingolipids mediate differential echinocandin susceptibility in Candida albicans and Aspergillus nidulans. Antimicrob Agents Chemother 59:3377–3384. doi: 10.1128/AAC.04667-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.LaFayette SL, Collins C, Zaas AK, Schell WA, Betancourt-Quiroz M, Gunatilaka AAL, Perfect JR, Cowen LE. 2010. PKC signaling regulates drug resistance of the fungal pathogen Candida albicans via circuitry comprised of Mkc1, calcineurin, and Hsp90. PLoS Pathog 6:e1001069. doi: 10.1371/journal.ppat.1001069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cowen LE. 2013. The fungal Achilles' heel: targeting Hsp90 to cripple fungal pathogens. Curr Opin Microbiol 16:377–384. doi: 10.1016/j.mib.2013.03.005. [DOI] [PubMed] [Google Scholar]
- 20.Leach MD, Klipp E, Cowen LE, Brown AJP. 2012. Fungal Hsp90: a biological transistor that tunes cellular outputs to thermal inputs. Nat Rev Microbiol 10:693–704. doi: 10.1038/nrmicro2875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li X, Cai Q, Mei H, Zhou X, Shen Y, Li D, Liu W. 2015. The Rpd3/Hda1 family of histone deacetylases regulates azole resistance in Candida albicans. J Antimicrob Chemother 70:1993–2003. doi: 10.1093/jac/dkv070. [DOI] [PubMed] [Google Scholar]
- 22.Marr KA, Rustad TR, Rex JH, White TC. 1999. The trailing end point phenotype in antifungal susceptibility testing is pH dependent. Antimicrob Agents Chemother 43:1383–1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cornet M, Gaillardin C. 2014. pH signaling in human fungal pathogens: a new target for antifungal strategies. Eukaryot Cell 13:342–352. doi: 10.1128/EC.00313-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nobile CJ, Solis N, Myers CL, Fay AJ, Deneault J-S, Nantel A, Mitchell AP, Filler SG. 2008. Candida albicans transcription factor Rim101 mediates pathogenic interactions through cell wall functions. Cell Microbiol 10:2180–2196. doi: 10.1111/j.1462-5822.2008.01198.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cheng S, Clancy CJ, Xu W, Schneider F, Hao B, Mitchell AP, Nguyen MH. 2013. Profiling of Candida albicans gene expression during intra-abdominal candidiasis identifies biologic processes involved in pathogenesis. J Infect Dis 208:1529–1537. doi: 10.1093/infdis/jit335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mitchell BM, Wu TG, Jackson BE, Wilhelmus KR. 2007. Candida albicans strain-dependent virulence and Rim13p-mediated filamentation in experimental keratomycosis. Invest Ophthalmol Vis Sci 48:774–780. doi: 10.1167/iovs.06-0793. [DOI] [PubMed] [Google Scholar]
- 27.Davis DA. 2009. How human pathogenic fungi sense and adapt to pH: the link to virulence. Curr Opin Microbiol 12:365–370. doi: 10.1016/j.mib.2009.05.006. [DOI] [PubMed] [Google Scholar]
- 28.Parsons AB, Brost RL, Ding H, Li Z, Zhang C, Sheikh B, Brown GW, Kane PM, Hughes TR, Boone C. 2004. Integration of chemical-genetic and genetic interaction data links bioactive compounds to cellular target pathways. Nat Biotechnol 22:62–69. doi: 10.1038/nbt919. [DOI] [PubMed] [Google Scholar]
- 29.Cornet M, Gaillardin C, Richard ML. 2006. Deletions of the endocytic components VPS28 and VPS32 in Candida albicans lead to echinocandin and azole hypersensitivity. Antimicrob Agents Chemother 50:3492–3495. doi: 10.1128/AAC.00391-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Subcommittee on Antifungal Susceptibility Testing (AFST) of the ESCMID European Committee for Antimicrobial Susceptibility Testing (EUCAST). 2008. EUCAST definitive document EDef 7.1: method for the determination of broth dilution MICs of antifungal agents for fermentative yeasts. Clin Microbiol Infect 14:398–405. doi: 10.1111/j.1469-0691.2007.01935.x. [DOI] [PubMed] [Google Scholar]
- 31.Arendrup MC, Cuenca-Estrella M, Lass-Flörl C, Hope W, EUCAST-AFST. 2012. EUCAST technical note on the EUCAST definitive document EDef 7.2: method for the determination of broth dilution minimum inhibitory concentrations of antifungal agents for yeasts EDef 7.2 (EUCAST-AFST). Clin Microbiol Infect 18:E246–E247. doi: 10.1111/j.1469-0691.2012.03880.x. [DOI] [PubMed] [Google Scholar]
- 32.Gomez-Raja J, Davis DA. 2012. The β-arrestin-like protein Rim8 is hyperphosphorylated and complexes with Rim21 and Rim101 to promote adaptation to neutral-alkaline pH. Eukaryot Cell 11:683–693. doi: 10.1128/EC.05211-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Roe SM, Prodromou C, O'Brien R, Ladbury JE, Piper PW, Pearl LH. 1999. Structural basis for inhibition of the Hsp90 molecular chaperone by the antitumor antibiotics radicicol and geldanamycin. J Med Chem 42:260–266. doi: 10.1021/jm980403y. [DOI] [PubMed] [Google Scholar]
- 34.Whitesell L, Mimnaugh EG, De Costa B, Myers CE, Neckers LM. 1994. Inhibition of heat shock protein HSP90-pp60v-src heteroprotein complex formation by benzoquinone ansamycins: essential role for stress proteins in oncogenic transformation. Proc Natl Acad Sci U S A 91:8324–8328. doi: 10.1073/pnas.91.18.8324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhao R, Houry WA. 2005. Hsp90: a chaperone for protein folding and gene regulation. Biochem Cell Biol 83:703–710. doi: 10.1139/o05-158. [DOI] [PubMed] [Google Scholar]
- 36.Bensen ES, Martin SJ, Li M, Berman J, Davis DA. 2004. Transcriptional profiling in Candida albicans reveals new adaptive responses to extracellular pH and functions for Rim101p. Mol Microbiol 54:1335–1351. doi: 10.1111/j.1365-2958.2004.04350.x. [DOI] [PubMed] [Google Scholar]
- 37.Bruno VM, Wang Z, Marjani SL, Euskirchen GM, Martin J, Sherlock G, Snyder M. 2010. Comprehensive annotation of the transcriptome of the human fungal pathogen Candida albicans using RNA-seq. Genome Res 20:1451–1458. doi: 10.1101/gr.109553.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.O'Meara TR, Veri AO, Polvi EJ, Li X, Valaei SF, Diezmann S, Cowen LE. 2016. Mapping the Hsp90 genetic network reveals ergosterol biosynthesis and phosphatidylinositol-4-kinase signaling as core circuitry governing cellular stress. PLoS Genet 12:e1006142. doi: 10.1371/journal.pgen.1006142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.O'Meara TR, Robbins N, Cowen LE. 2017. The Hsp90 chaperone network modulates Candida virulence traits. Trends Microbiol 25:809–819. doi: 10.1016/j.tim.2017.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen G, Bradford WD, Seidel CW, Li R. 2012. Hsp90 stress potentiates rapid cellular adaptation through induction of aneuploidy. Nature 482:246–250. doi: 10.1038/nature10795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pachl J, Svoboda P, Jacobs F, Vandewoude K, van der Hoven B, Spronk P, Masterson G, Malbrain M, Aoun M, Garbino J, Takala J, Drgona L, Burnie J, Matthews R, Mycograb Invasive Candidiasis Study Group. 2006. A randomized, blinded, multicenter trial of lipid-associated amphotericin B alone versus in combination with an antibody-based inhibitor of heat shock protein 90 in patients with invasive candidiasis. Clin Infect Dis 42:1404–1413. doi: 10.1086/503428. [DOI] [PubMed] [Google Scholar]
- 42.Bolger AM, Lohse M, Usadel B. 2014. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics 30:2114–2120. doi: 10.1093/bioinformatics/btu170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Langmead B, Salzberg SL. 2012. Fast gapped-read alignment with Bowtie 2. Nat Methods 9:357–359. doi: 10.1038/nmeth.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Anders S, Pyl PT, Huber W. 2015. HTSeq—a Python framework to work with high-throughput sequencing data. Bioinformatics 31:166–169. doi: 10.1093/bioinformatics/btu638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Love MI, Huber W, Anders S. 2014. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol 15:550. doi: 10.1186/s13059-014-0550-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fonzi WA, Irwin MY. 1993. Isogenic strain construction and gene mapping in Candida albicans. Genetics 134:717–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Davis D, Edwards JE, Mitchell AP, Ibrahim AS. 2000. Candida albicans RIM101 pH response pathway is required for host-pathogen interactions. Infect Immun 68:5953–5959. doi: 10.1128/IAI.68.10.5953-5959.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Davis DA, Bruno VM, Loza L, Filler SG, Mitchell AP. 2002. Candida albicans Mds3p, a conserved regulator of pH responses and virulence identified through insertional mutagenesis. Genetics 162:1573–1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wilson RB, Davis D, Mitchell AP. 1999. Rapid hypothesis testing with Candida albicans through gene disruption with short homology regions. J Bacteriol 181:1868–1874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Davis D, Wilson RB, Mitchell AP. 2000. RIM101-dependent and -independent pathways govern pH responses in Candida albicans. Mol Cell Biol 20:971–978. doi: 10.1128/MCB.20.3.971-978.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Li M, Martin SJ, Bruno VM, Mitchell AP, Davis DA. 2004. Candida albicans Rim13p, a protease required for Rim101p processing at acidic and alkaline pHs. Eukaryot Cell 3:741–751. doi: 10.1128/EC.3.3.741-751.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gigou-Cornet M. 2006. Rôle des gènes RIM et VPS dans la signalisation du pH, la virulence et la résistance aux antifongiques chez la levure Candida albicans. PhD thesis Biochimie, INAPG (AgroParisTech), Paris, France: https://tel.archives-ouvertes.fr/file/index/docid/143611/filename/These_Murielle_Cornet.pdf. [Google Scholar]
- 53.Cornet M, Bidard F, Schwarz P, Da Costa G, Blanchin-Roland S, Dromer F, Gaillardin C. 2005. Deletions of endocytic components VPS28 and VPS32 affect growth at alkaline pH and virulence through both RIM101-dependent and RIM101-independent pathways in Candida albicans. Infect Immun 73:7977–7987. doi: 10.1128/IAI.73.12.7977-7987.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.