ABSTRACT
The activities of ceftolozane-tazobactam and comparator agents against organisms deemed to be the cause of pneumonia among patients hospitalized in the United States during 2013 to 2015 were evaluated. Organisms included 1,576 Pseudomonas aeruginosa and 2,362 Enterobacteriaceae isolates susceptibility tested using reference broth microdilution methods. Ceftolozane-tazobactam, cefepime, ceftazidime, meropenem, and piperacillin-tazobactam inhibited 96.3%, 84.8%, 83.5%, 80.0%, and 78.6%, respectively, of the P. aeruginosa isolates. Ceftolozane-tazobactam inhibited 77.5 to 85.1% of isolates nonsusceptible to antipseudomonal β-lactams and 86.6% and 71.0% of the 372 (23.6% overall) multidrug- and 155 (9.8%) extensively drug-resistant isolates tested. The activity of this combination was greater than those of other β-lactams evaluated against P. aeruginosa groups across all U.S. census divisions. Ceftolozane-tazobactam was active against 90.6% of the Enterobacteriaceae, being less active than only meropenem (95.6% susceptible) among the β-lactams evaluated. Against 145 Escherichia coli and Klebsiella pneumoniae isolates carrying extended-spectrum-β-lactamase (ESBL)-encoding genes without carbapenemases, ceftolozane-tazobactam inhibited 82.8% of these isolates and was more active than cefepime and piperacillin-tazobactam (15.2% and 74.3% susceptible, respectively). ESBL genes included in this analysis were mainly blaCTX-M-15-like (89 isolates) and blaCTX-M-14-like (22) genes but also blaSHV (31) and blaTEM (3). Ceftolozane-tazobactam also displayed activity (84.6% susceptible) against 13 isolates harboring acquired AmpC genes. All β-lactams displayed limited activity against blaKPC-carrying isolates. Ceftolozane-tazobactam was the most active β-lactam tested against P. aeruginosa isolates from isolates that were the probable cause of pneumonia and displayed in vitro activity against Enterobacteriaceae, including isolates resistant to cephalosporins and carrying ESBL genes.
KEYWORDS: P. aeruginosa, ESBL, β-lactamase inhibitor combination
INTRODUCTION
Nosocomial pneumonia infections are associated with high morbidity and mortality rates worldwide (1, 2). Patients with these infections are usually started on empirical antimicrobial therapy (3, 4). Knowledge of local antimicrobial susceptibility patterns is very important for selecting appropriate antimicrobial agents that will directly impact mortality rates (1, 3).
Among organisms causing pneumonia in hospitalized patients, Pseudomonas aeruginosa and Enterobacteriaceae species among other organisms such as Gram-positive bacteria and other Gram-negative bacteria are important causes of hospital-acquired pneumonia (HAP) and ventilator-associated pneumonia (VAP) (3, 4). P. aeruginosa isolates are intrinsically less susceptible to several antimicrobial agents, and isolates displaying resistance to antipseudomonal agents are not uncommon in the hospital setting. Furthermore, the prevalence of extended-spectrum-β-lactamase (ESBL)-producing Enterobacteriaceae is increasing worldwide; these organisms can colonize different body sites and can cause, among other diseases, pneumonia (5).
Using parenteral colistin or tigecycline monotherapy to manage nosocomial pneumonia caused by ESBL-producing Enterobacteriaceae or P. aeruginosa infections still requires cautious evaluation (1), and carbapenems are often used as last-resort agents to treat infections caused by these organisms. Noncarbapenem agents and combinations have been suggested in the literature (6, 7), and among these options, ceftolozane-tazobactam has demonstrated acceptable treatment success rates for serious infections caused by P. aeruginosa, including carbapenem-resistant isolates (8, 9) and ESBL-producing Enterobacteriaceae isolates from complicated urinary tract and intra-abdominal infections (10).
Ceftolozane-tazobactam has recently been approved by the U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMA) for the treatment of complicated intra-abdominal infections (in combination with metronidazole) and complicated urinary tract infections (11). A phase 3 clinical trial of ceftolozane-tazobactam for the treatment of hospital-acquired and ventilator-associated pneumonia is in progress (ClinicalTrials.gov registration no. NCT02070757).
In this study, we evaluated the activity of ceftolozane-tazobactam against 1,576 P. aeruginosa and 2,362 Enterobacteriaceae isolates deemed to be the probable cause of pneumonia among patients hospitalized in the United States during 2013 to 2015. This collection included multidrug- and extensively drug-resistant (MDR and XDR) P. aeruginosa, Escherichia coli, and Klebsiella pneumoniae isolates characterized for the presence of ESBL-encoding genes.
RESULTS
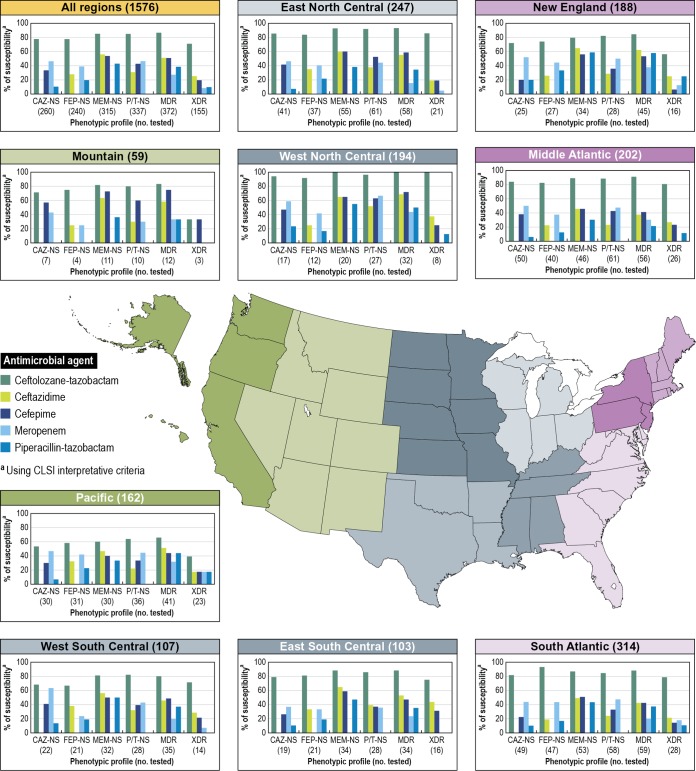
Ceftolozane-tazobactam inhibited 96.3% of the 1,576 P. aeruginosa isolates deemed to be the probable cause of pneumonia among patients hospitalized in the United States at the current CLSI breakpoint criteria (MIC, ≤4/4 μg/ml). Ceftolozane-tazobactam inhibited a greater number of isolates at its susceptible MIC breakpoint than did cefepime (84.8%), ceftazidime (83.5%), meropenem (80.0%), and piperacillin-tazobactam (78.6%) at each of their respective susceptible MIC breakpoints (Fig. 1). Additionally, ceftolozane-tazobactam displayed greater in vitro activity than those of the other β-lactams tested against isolates of P. aeruginosa that were nonsusceptible to comparator β-lactams. A total of 260 (16.5% overall isolates) ceftazidime-, 240 (15.2%) cefepime-, 315 (20.0%) meropenem-, and 337 (21.4%) piperacillin-tazobactam-nonsusceptible isolates were observed in this collection, and ceftolozane-tazobactam inhibited 77.5 to 85.1% of these isolates. In-class comparators inhibited 0.0 to 55.9% of these different groups, using CLSI breakpoints (Fig. 1).
FIG 1.
Susceptibility profiles of P. aeruginosa isolates collected from hospitalized patients with pneumonia in the United States.
MDR (372 isolates) and XDR (155) P. aeruginosa corresponded to 23.6% and 9.8% of the isolates tested, and ceftolozane-tazobactam inhibited 86.6% and 71.0% of the isolates, respectively, at the CLSI susceptible breakpoint. MDR and XDR isolates displayed reduced susceptibility rates for the comparator β-lactams that included cefepime, ceftazidime, piperacillin-tazobactam, and meropenem, ranging from 27.2 to 51.1% for MDR isolates and 8.4 to 25.2% for XDR isolates. For both groups of isolates, ceftolozane-tazobactam exhibited the highest susceptibility rates, followed by ceftazidime (51.1% and 25.2%, respectively). Meropenem had the lowest susceptibility rates for MDR (27.2% susceptible) and XDR (8.4% susceptible) isolates among the 4 comparators shown in Fig. 1.
The activity of ceftolozane-tazobactam was stable regardless of the U.S. census division and was greater than those of other β-lactams. The P. aeruginosa susceptibility rates for this combination ranged from 91.4 to 99.5%, being lower in the Pacific division and higher in the West North Central. Ceftazidime- and piperacillin-tazobactam-nonsusceptible P. aeruginosa isolates were more common in the Mid-Atlantic division (24.8% and 30.2%, respectively), whereas piperacillin-tazobactam- and meropenem-nonsusceptible isolates were more common in the East South Central division (27.2% and 33.0%, respectively). The highest MDR P. aeruginosa rate was noted in the East South Central (33.0%) division, followed by the West South Central (32.7%) and the Mid-Atlantic (27.7%) divisions. XDR isolates were more frequently observed in the East South Central (15.5%), Pacific (14.2%), and the West South Central (13.1%) divisions. As observed for ceftolozane-tazobactam, isolates from the West North Central division displayed higher susceptibility rates for comparator β-lactams agents and combinations than did other isolates from census divisions (Fig. 1).
Pan-drug-resistant (PDR) P. aeruginosa isolates were not observed among hospitalized patients with pneumonia during the study period.
Ceftolozane-tazobactam inhibited 90.6% of the 2,362 Enterobacteriaceae isolates recovered from the respiratory tracts of patients hospitalized in the United States during the study period (Table 1). Among β-lactam agents, ceftolozane-tazobactam was more active than ceftazidime (84.0% susceptible), piperacillin-tazobactam (86.1%), and cefepime (88.2%) and less active than only meropenem (95.6% susceptible [Table 2]).
TABLE 1.
Antimicrobial activity of ceftolozane-tazobactam against isolates collected from hospitalized patients with pneumonia in U.S. hospitals
| Organism or organism group (no. of isolates) | No. of isolates at MIC (μg/ml) (cumulative %) |
MIC (μg/ml) |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0.03 | 0.06 | 0.12 | 0.25 | 0.5 | 1 | 2 | 4 | 8 | 16 | 32 | >32 | 50% | 90% | |
| Pseudomonas aeruginosa (1,576) | 3 (0.2) | 4 (0.4) | 20 (1.7) | 164 (12.1) | 765 (60.7) | 385 (85.1) | 114 (92.3) | 62 (96.3) | 29 (98.1) | 9 (98.7) | 5 (99.0) | 16 (100.0) | 0.5 | 2 |
| Enterobacteriaceae (2,362) | 1 (<0.1) | 21 (0.9) | 515 (22.7) | 790 (56.2) | 535 (78.8) | 192 (87.0) | 85 (90.6) | 47 (92.5) | 40 (94.2) | 30 (95.5) | 24 (96.5) | 82 (100.0) | 0.25 | 2 |
| Escherichia coli (441) | 7 (1.6) | 161 (38.1) | 188 (80.7) | 56 (93.4) | 15 (96.8) | 5 (98.0) | 4 (98.9) | 3 (99.5) | 1 (99.8) | 0 (99.8) | 1 (100.0) | 0.25 | 0.5 | |
| Klebsiella pneumoniae (627) | 6 (1.0) | 141 (23.4) | 227 (59.6) | 101 (75.8) | 46 (83.1) | 17 (85.8) | 4 (86.4) | 5 (87.2) | 15 (89.6) | 14 (91.9) | 51 (100.0) | 0.25 | 32 | |
| Klebsiella oxytoca (190) | 3 (1.6) | 89 (48.4) | 55 (77.4) | 24 (90.0) | 8 (94.2) | 6 (97.4) | 1 (97.9) | 0 (97.9) | 0 (97.9) | 2 (98.9) | 2 (100.0) | 0.25 | 0.5 | |
| Enterobacter cloacae (270) | 30 (11.1) | 129 (58.9) | 37 (72.6) | 12 (77.0) | 17 (83.3) | 13 (88.1) | 14 (93.3) | 8 (96.3) | 6 (98.5) | 4 (100.0) | 0.25 | 8 | ||
| Enterobacter aerogenes (161) | 32 (19.9) | 67 (61.5) | 22 (75.2) | 12 (82.6) | 12 (90.1) | 8 (95.0) | 6 (98.8) | 1 (99.4) | 0 (99.4) | 1 (100.0) | 0.25 | 2 | ||
| Citrobacter koseri (42) | 2 (4.8) | 16 (42.9) | 19 (88.1) | 5 (100.0) | 0.25 | 0.5 | ||||||||
| Citrobacter freundii (32) | 4 (12.5) | 12 (50.0) | 4 (62.5) | 1 (65.6) | 1 (68.8)) | 4 (81.2) | 2 (87.5) | 3 (96.9) | 0 (96.9) | 1 (100.0) | 0.25 | 16 | ||
| Proteus mirabilis (90) | 3 (3.3) | 26 (32.2) | 53 (91.1) | 5 (96.7) | 2 (98.9) | 0 (98.9) | 0 (98.9) | 0 (98.9) | 0 (98.9) | 1 (100.0) | 0.5 | 0.5 | ||
| Indole-positive Proteeae (46) | 1 (2.2) | 6 (15.2) | 19 (6.5) | 14 (87.0) | 3 (93.5) | 1 (95.7) | 0 (95.7) | 2 (100.0) | 0.25 | 1 | ||||
| Serratia spp. (354) | 2 (0.6) | 21 (6.5) | 205 (64.4) | 85 (88.4) | 21 (94.4) | 7 (96.3) | 2 (96.9) | 0 (96.9) | 1 (97.2) | 10 (100.0) | 0.5 | 2 | ||
| Isolates carrying ESBL genes (145) | 2 (1.4) | 11 (9.0) | 37 (34.5) | 35 (58.6) | 23 (74.5) | 12 (82.8) | 7 (87.6) | 4 (90.3) | 6 (94.5) | 1 (95.2) | 7 (100.0) | 0.5 | 8 | |
| Isolates carrying blaCTX-M (111) | 1 (0.9) | 7 (7.2) | 30 (34.2) | 29 (60.4) | 22 (80.2) | 9 (88.3) | 4 (91.9) | 3 (94.6) | 3 (97.3) | 0 (97.3) | 3 (100.0) | 0.5 | 4 | |
| Isolates carrying blaCTX-M-15-like genes (89) | 1 (1.1) | 5 (6.7) | 17 (25.8) | 24 (52.8) | 20 (75.3) | 9 (85.4) | 4 (89.9) | 3 (93.3) | 3 (96.6) | 0 (96.6) | 3 (100.0) | 0.5 | 8 | |
| Isolates carrying blaCTX-M-14-like genes (22) | 2 (9.1) | 13 (68.2) | 5 (90.9) | 2 (100.0) | 0.25 | 0.5 | ||||||||
| Isolates carrying blaSHV ESBL (31) | 1 (3.2) | 3 (12.9) | 6 (32.3) | 5 (48.4) | 1 (51.6) | 3 (61.3) | 3 (71.0) | 1 (74.2) | 3 (83.9) | 1 (87.1) | 4 (100.0) | 1 | >32 | |
| Isolates carrying blaTEM ESBL (3) | 1 (33.3) | 1 (66.7) | 1 (100.0) | 0.25 | ||||||||||
| Isolates carrying blaKPC (70) | 1 (1.4) | 1 (2.9) | 2 (5.7) | 10 (20.0) | 13 (38.6) | 43 (100.0) | >32 | >32 | ||||||
| Isolates carrying transferable AmpC genes (13) | 3 (23.1) | 4 (53.8) | 2 (69.2) | 2 (84.6) | 0 (84.6) | 1 (92.3) | 0 (92.3) | 0 (92.3) | 1 (100.0) | 0.5 | 8 | |||
TABLE 2.
Susceptibility profiles of Enterobacteriaceae isolates collected from hospitalized patients with pneumonia in U.S. hospitals
| Organism or organism group (no. tested) | % susceptibility using CLSI interpretative criteria |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Ceftolozane-tazobactam | Ceftazidime | Cefepime | Meropenem | Piperacillin-tazobactam | Amikacin | Colistina | Levofloxacin | Tigecyclineb | |
| Enterobacteriaceae (2,362) | 90.6 | 84.0 | 88.2 | 95.6 | 86.1 | 98.0 | 75.1 | 84.2 | 98.5 |
| Escherichia coli (441) | 98.0 | 85.9 | 85.0 | 99.8 | 90.4 | 99.5 | 99.5 | 60.2 | 100.0 |
| Klebsiella pneumoniae (627) | 85.8 | 77.5 | 78.6 | 88.7 | 81.7 | 93.8 | 96.8 | 82.5 | 99.0 |
| Klebsiella oxytoca (190) | 97.4 | 97.4 | 97.4 | 98.9 | 88.9 | 100.0 | 100.0 | 98.9 | 100.0 |
| Enterobacter cloacae (270) | 83.3 | 74.4 | 90.0 | 98.9 | 81.0 | 99.6 | 79.6 | 96.3 | 99.6 |
| Enterobacter aerogenes (161) | 90.1 | 78.9 | 98.1 | 98.8 | 81.2 | 99.4 | 96.3 | 97.5 | 99.4 |
| Citrobacter koseri (42) | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | 92.9 | 100.0 |
| Citrobacter freundii (32) | 68.8 | 62.5 | 87.5 | 96.9 | 71.9 | 100.0 | 96.9 | 96.9 | 100.0 |
| Proteus mirabilis (90) | 98.9 | 98.9 | 96.7 | 98.9 | 100.0 | 100.0 | 0.0 | 67.8 | 75.6 |
| Indole-positive Proteeae (46) | 95.7 | 89.1 | 93.5 | 100.0 | 97.8 | 100.0 | 0.0 | 69.6 | 93.5 |
| Serratia spp. (354) | 94.4 | 95.2 | 95.2 | 96.9 | 90.7 | 99.2 | 5.1 | 95.2 | 99.7 |
| Isolates carrying ESBL genes (145) | 82.8 | 20.0 | 15.2 | 97.9 | 74.3 | 93.8 | 97.2 | 29.0 | 97.9 |
| Isolates carrying blaCTX-M (111) | 88.3 | 23.4 | 4.5 | 98.2 | 77.3 | 95.5 | 98.2 | 22.5 | 97.3 |
| Isolates carrying blaCTX-M-15-like genes (89) | 85.4 | 9.0 | 1.1 | 97.8 | 71.6 | 94.4 | 97.8 | 25.8 | 96.6 |
| Isolates carrying blaCTX-M-14-like genes (22) | 100.0 | 81.8 | 18.2 | 100.0 | 100.0 | 100.0 | 100.0 | 9.1 | 100.0 |
| Isolates carrying blaSHV ESBL (31) | 61.3 | 6.5 | 51.6 | 96.8 | 61.3 | 87.1 | 93.5 | 54.8 | 100.0 |
| Isolates carrying blaTEM ESBL (3) | 100.0 | 33.3 | 33.3 | 100.0 | 100.0 | 100.0 | 100.0 | 0.0 | 100.0 |
| Isolates carrying blaKPC (70) | 1.4 | 0.0 | 1.4 | 2.9 | 0.0 | 54.3 | 85.3 | 8.6 | 100.0 |
| Isolates carrying transferable AmpC genes (13) | 84.6 | 7.7 | 84.6 | 100.0 | 61.5 | 100.0 | 100.0 | 53.8 | 100.0 |
EUCAST breakpoints were applied for colistin.
FDA package insert breakpoints were applied for tigecycline.
Ceftolozane-tazobactam inhibited 85.8% of the 627 K. pneumoniae isolates, 98.0% of the 441 E. coli isolates, and 94.4% of the 354 Serratia spp., which were mostly S. marcescens (345 isolates) but also included 3 other species (Table 1). K. pneumoniae, E. coli, and Serratia spp. were the most common Enterobacteriaceae pathogens deemed as the probable cause of pneumonia in the United States and represented >60% of the isolates evaluated.
The lowest ceftolozane-tazobactam susceptibility rates were observed for Citrobacter freundii (32 isolates; 68.8% susceptible), but this combination demonstrated activity against all 42 Citrobacter koseri isolates tested (Table 1). Ceftazidime and piperacillin-tazobactam also displayed low susceptibility rates with C. freundii (62.5% and 71.9% susceptible, respectively). Ceftolozane-tazobactam inhibited 83.3 to 98.9% of the isolates belonging to other Enterobacteriaceae genera or species tested (Table 2).
Among 269 E. coli (n = 86) and K. pneumoniae (n = 161) isolates displaying elevated MICs for ceftazidime, ceftriaxone, and/or aztreonam (CLSI ESBL markers) that were screened for β-lactamase-encoding genes, 145 isolates carried genes encoding ESBLs without carbapenemases or transferable cephalosporinases (plasmid-mediated AmpCs). ESBL-carrying isolates comprised 77 K. pneumoniae and 68 E. coli isolates, and 111 of these carried blaCTX-M genes alone (63 E. coli and 48 K. pneumoniae isolates). The most common blaCTX-M group observed was the group including blaCTX-M-15-like genes, and 98 isolates carrying these genes were distributed in all U.S. census divisions. Isolates carrying blaCTX-M-15-like genes accounted for >36% of the E. coli (44 isolates [51.2%]) and K. pneumoniae (45 [27.9%]) isolates recovered from the respiratory tracts of hospitalized patients that displayed the CLSI ESBL screening criteria.
Isolates carrying blaCTX-M-14-like genes were also noted in all U.S. census divisions, but in smaller numbers (1 to 5 isolates) than for blaCTX-M-15-like genes (2 to 42 isolates). Fifty-two isolates carried blaSHV ESBL, but only 31 isolates did not harbor genes encoding carbapenemases or transferable AmpCs in addition to blaSHV. Five isolates yielded positive results for blaTEM ESBL; 2 of these carried blaKPC or blaCTX-M-14-like genes, and 1 isolate carried a blaCTX-M-2-like gene.
Transferable AmpC genes were detected among 13 isolates, including 11 isolates carrying blaCMY-2-like genes, 2 blaFOX, and 1 blaDHA. Additionally, 70 isolates showing elevated MICs for the CLSI ESBL markers were also resistant to 1 or more carbapenems and carried blaKPC. These isolates were mostly K. pneumoniae (69/70 isolates) and were observed mainly in the Mid-Atlantic division (47/70 isolates). Isolates carrying blaKPC were observed in 5 other U.S. census divisions, but in much smaller numbers (1 to 13 isolates).
Ceftolozane-tazobactam inhibited 82.8% of the isolates harboring ESBL encoding genes using the CLSI current breakpoint (≤2/4 μg/ml [Table 1]). The activity of this combination was greater than the activities of ceftazidime (20.0% susceptible), cefepime (15.2%), and piperacillin-tazobactam (74.3%) against ESBL-carrying E. coli and K. pneumoniae isolates. Meropenem was the only β-lactam with activity greater than that of ceftolozane-tazobactam, inhibiting 97.9% of the isolates when using the CLSI breakpoints. Amikacin, colistin, and tigecycline exhibited activities against 93.8%, 97.2%, and 97.9% of these isolates, respectively. Isolates carrying ESBL-encoding genes were considerably less susceptible (29.0%) to levofloxacin than E. coli (60.2%) and K. pneumoniae (82.5%) isolates overall (Table 2).
Against isolates harboring blaCTX-M and blaCTX-M-15-like genes, the activity of ceftolozane-tazobactam (88.3% and 85.4% susceptible) was greater than the activities of ceftazidime (23.4% and 9.0%), cefepime (4.5% and 1.1%), and piperacillin-tazobactam (77.3% and 71.6%). All isolates carrying blaCTX-M-14-like genes were susceptible to ceftolozane-tazobactam, piperacillin-tazobactam, and meropenem.
Ceftolozane-tazobactam displayed activity similar to that of cefepime against isolates carrying transferable AmpC genes, and both compounds inhibited 84.6% of these isolates, whereas piperacillin-tazobactam inhibited only 61.5%. Meropenem, amikacin, colistin, and tigecycline inhibited all isolates when the CLSI or EUCAST (colistin) breakpoints were applied.
Tigecycline and colistin were the only agents with acceptable activity against isolates harboring blaKPC.
DISCUSSION
In a review of European data on nosocomial pneumonia infections, more than 30 antimicrobial regimens were used for the initial treatment of VAP (3). Approximately one-third of the patients received monotherapy, one-third received 2 antimicrobial agents, and the last third of patients received 3 or more agents. Carbapenems (18.5%), piperacillin-tazobactam (13.1%), and fluoroquinolones (9.9%) were among the antimicrobials used. The same European study concluded that the mortality rates of nosocomial pneumonia affecting critically ill patients was greater than 35.0% and that when patients received appropriate initial antimicrobial therapy, mortality was lower than for patients who received inappropriate therapy.
The National Healthcare Safety Network (NHSN) reported that P. aeruginosa, Klebsiella spp., Enterobacter spp., and E. coli were among the top 6 pathogens most frequently causing VAP during 2011 and 2012 in U.S. acute-care hospitals, long-term-care centers, and inpatient rehabilitation facilities (4). Interestingly, these are 4 out of the 6 pathogens among the ESKAPE organisms (Enterococcus faecium, Staphylococcus aureus, Klebsiella pneumoniae, Acinetobacter baumannii, Pseudomonas aeruginosa, and Enterobacter spp.), which were highlighted as important challenges to the available antimicrobial chemotherapy armamentarium by the Infectious Diseases Society of America in 2009 (12).
Initiating appropriate antimicrobial chemotherapy is pivotal for successfully treating patients with nosocomial pneumonia. Ceftolozane-tazobactam has been shown to be an effective agent for P. aeruginosa isolates that show resistance to other antimicrobial agents and for MDR strains (8, 9). The in vitro data presented in this study support the idea that ceftolozane-tazobactam is active against P. aeruginosa isolates collected from respiratory tract infections (RTIs) that were deemed as the probable cause of pneumonia, including isolates nonsusceptible to other antipseudomonal β-lactams and MDR isolates. Additionally, this combination displayed greater activity against XDR isolates than did comparator antipseudomonal β-lactam agents and combinations.
Enterobacteriaceae species are also an important cause of nosocomial pneumonia (3, 4), and isolates producing ESBL enzymes can be associated with these infections (1, 5). Data obtained by Popejoy et al. (10) in 2 double-blinded phase 3 noninferiority clinical trials for ceftolozane-tazobactam presented a 97.4% overall clinical cure rate for ESBL-producing Enterobacteriaceae infections when the infections were treated with this combination. These trials were focused on isolates from complicated urinary tract infections and complicated intra-abdominal infections for which the therapy was a combination of ceftolozane-tazobactam and metronidazole (10).
In this study, we demonstrate that ceftolozane-tazobactam exhibited in vitro activity against Enterobacteriaceae isolates producing ESBLs that were detected among 13.4% of the E. coli and K. pneumoniae isolates collected from U.S. hospitals during 2013 to 2015. This combination was active against isolates carrying blaCTX-M, including those carrying blaCTX-M-15-like and blaCTX-M-14-like genes, which are commonly detected in U.S. hospitals (13). This combination was less active only than meropenem against blaCTX-M-carrying isolates and displayed activity greater than or equal to that of piperacillin-tazobactam and greater than those of ceftazidime and cefepime. Additionally, ceftolozane-tazobactam demonstrated acceptable activity against isolates carrying transferable AmpCs that are poorly inhibited by tazobactam. Although only a small number of isolates harboring transferable AmpCs was noted, the main representatives of this β-lactamase group observed in the United States (13) were included.
As with other β-lactams, the in vitro activity of ceftolozane-tazobactam was limited against isolates carrying blaKPC that were detected mainly among K. pneumoniae from the northeastern coast divisions.
A clinical trial is currently ongoing to evaluate the safety and efficacy of ceftolozane-tazobactam against organisms causing ventilated nosocomial pneumonia (https://clinicaltrials.gov/show/NCT02070757), and it is expected that the results will elucidate the role of this β-lactam–β-lactamase combination to treat these serious infections.
MATERIALS AND METHODS
Bacterial isolates.
A total of 1,576 P. aeruginosa and 2,362 Enterobacteriaceae clinical isolates were collected in 31 U.S. hospitals during 2013 to 2015 as part of the Program to Assess Ceftolozane-Tazobactam Susceptibility (PACTS). Participating institutions were requested to collect consecutive bacterial isolates from lower respiratory tract invasive sampling (transtracheal aspiration, bronchoalveolar lavage, protected brush samples, qualified sputum samples, etc.) considered significant by local criteria as the reported probable cause of pneumonia. Only 1 isolate per patient infection episode was included in the study. Species identification was confirmed when necessary by matrix-assisted laser desorption ionization–time of flight mass spectrometry (MALDI-TOF MS) using a Bruker Daltonics MALDI Biotyper (Billerica, MA) according to the manufacturer's instructions.
Antimicrobial susceptibility testing.
Ceftolozane-tazobactam and comparator antimicrobial agents were susceptibility tested using the reference broth microdilution method as described by CLSI (14). Tazobactam was tested at a fixed concentration of 4 μg/ml. Categorical interpretations for all antimicrobials were those found in CLSI document M100-S26 (15), EUCAST guidelines (colistin for Enterobacteriaceae only) (16), or the FDA package insert (tigecycline) (17). Quality control was performed using E. coli ATCC 25922 and 35218, K. pneumoniae ATCC 700603, and P. aeruginosa ATCC 27853 (18), and results were within the CLSI published ranges (15).
Resistant subsets.
P. aeruginosa isolates were categorized as MDR, XDR, or PDR according to criteria initially published by Magiorakos et al. (19) and adapted by Farrell et al. (20), which define MDR as nonsusceptible to ≥1 agent in ≥3 antimicrobial classes, XDR as nonsusceptible to ≥1 agent in all but ≤2 antimicrobial classes, and PDR as nonsusceptible (CLSI criteria) to all antimicrobial classes tested. The antimicrobial classes and drug representatives used in the analysis for P. aeruginosa were antipseudomonal cephalosporins (ceftazidime and cefepime), carbapenems (imipenem, meropenem, and doripenem), broad-spectrum penicillins combined with a β-lactamase inhibitor (piperacillin-tazobactam), fluoroquinolones (ciprofloxacin and levofloxacin), aminoglycosides (gentamicin, tobramycin, and amikacin), and the polymyxins (colistin).
Screening for β-lactamases.
A total of 269 E. coli and K. pneumoniae isolates displaying the CLSI ESBL screening criteria (MIC, >1 μg/ml for aztreonam, ceftazidime, and/or ceftriaxone) (18) were tested for β-lactamase-encoding genes using the microarray based assay Check-MDR CT101 kit (Check-Points, Wageningen, Netherlands). The assay was performed according to the manufacturer's instructions and has the capability to detect blaCTX-M groups 1 (blaCTX-M-15-like), 2 (blaCTX-M-2-like), 8 plus 25 and 9 (blaCTX-M-14-like), blaTEM wild type (WT) and ESBL, blaSHV WT and ESBL, blaACC, blaACT/MIR, blaCMY-2-like, blaDHA, blaFOX, blaKPC, and blaNDM-1-like genes. The most common amino acid alterations that expand the spectrum of TEM and SHV enzymes are detected by this assay, and these include E104K, R164S/H, or G238S for TEM and G238A/S and E240K for SHV. Validation of the assay against U.S. isolates was previously performed (13).
ACKNOWLEDGMENTS
We thank the U.S. hospitals participating in the Program to Assess Ceftolozane-Tazobactam Susceptibility (PACTS).
This study was performed by JMI Laboratories and supported by Merck & Co., Inc., Kenilworth, NJ, which included funding for services related to preparing the manuscript.
JMI Laboratories contracted to perform services in 2016 for Achaogen, Actelion, Allecra Therapeutics, Allergan, AmpliPhi Biosciences, API, Astellas Pharma, AstraZeneca, Basilea Pharmaceutica, Bayer AG, BD, Biomodels, Cardeas Pharma Corp., CEM-102 Pharma, Cempra, Cidara Therapeutics, Inc., CorMedix, CSA Biotech, Cutanea Life Sciences, Inc., Debiopharm Group, Dipexium Pharmaceuticals, Inc., Duke, Entasis Therapeutics, Inc., Fortress Biotech, Fox Chase Chemical Diversity Center, Inc., Geom Therapeutics, Inc., GSK, Laboratory Specialists, Inc., Medpace, Melinta Therapeutics, Inc., Merck & Co., Micromyx, MicuRx Pharmaceuticals, Inc., Motif Bio, N8 Medical, Inc., Nabriva Therapeutics, Inc., Nexcida Therapeutics, Inc., Novartis, Paratek Pharmaceuticals, Inc., Pfizer, Polyphor, Rempex, Scynexis, Shionogi, Spero Therapeutics, Symbal Therapeutics, Synlogic, TenNor Therapeutics, TGV Therapeutics, The Medicines Company, Theravance Biopharma, ThermoFisher Scientific, VenatoRx Pharmaceuticals, Inc., Wockhardt, Zavante Therapeutics, Inc. There are no speakers' bureaus or stock options to declare.
REFERENCES
- 1.Jean SS, Hsueh PR. 2011. Current review of antimicrobial treatment of nosocomial pneumonia caused by multidrug-resistant pathogens. Expert Opin Pharmacother 12:2145–2148. doi: 10.1517/14656566.2011.599320. [DOI] [PubMed] [Google Scholar]
- 2.Nair GB, Niederman MS. 2015. Ventilator-associated pneumonia: present understanding and ongoing debates. Intensive Care Med 41:34–48. doi: 10.1007/s00134-014-3564-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Koulenti D, Tsigou E, Rello J. 2017. Nosocomial pneumonia in 27 ICUs in Europe: perspectives from the EU-VAP/CAP study. Eur J Clin Microbiol Infect Dis 36:1999–2006. doi: 10.1007/s10096-016-2703-z. [DOI] [PubMed] [Google Scholar]
- 4.Weiner LM, Webb AK, Limbago B, Dudeck MA, Patel J, Kallen AJ, Edwards JR, Sievert DM. 2016. Antimicrobial-resistant pathogens associated with healthcare-associated infections: summary of data reported to the National Healthcare Safety Network at the Centers for Disease Control and Prevention, 2011–2014. Infect Control Hosp Epidemiol 37:1288–1301. doi: 10.1017/ice.2016.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Biehl LM, Schmidt-Hieber M, Liss B, Cornely OA, Vehreschild MJ. 2016. Colonization and infection with extended spectrum beta-lactamase producing Enterobacteriaceae in high-risk patients—review of the literature from a clinical perspective. Crit Rev Microbiol 42:1–16. [DOI] [PubMed] [Google Scholar]
- 6.Rodríguez-Baño J, Navarro MD, Retamar P, Picon E, Pascual A, Extended-Spectrum Beta-Lactamases-Red Espanola de Investigacion en Patologia Infecciosa/Grupo de Estudio de Infeccion Hospitalaria Group. 2012. β-Lactam/β-lactam inhibitor combinations for the treatment of bacteremia due to extended-spectrum β-lactamase-producing Escherichia coli: a post hoc analysis of prospective cohorts. Clin Infect Dis 54:167–174. doi: 10.1093/cid/cir790. [DOI] [PubMed] [Google Scholar]
- 7.Perez F, Bonomo RA. 2012. Can we really use beta-lactam/beta-lactam inhibitor combinations for the treatment of infections caused by extended-spectrum beta-lactamase-producing bacteria? Clin Infect Dis 54:175–177. doi: 10.1093/cid/cir793. [DOI] [PubMed] [Google Scholar]
- 8.Munita JM, Aitken SL, Miller WR, Perez F, Rosa R, Shimose LA, Lichtenberger PN, Abbo LM, Jain R, Nigo M, Wanger A, Araos R, Tran TT, Adachi J, Rakita R, Shelburne S, Bonomo RA, Arias CA. 2017. Multicenter evaluation of ceftolozane/tazobactam for serious infections caused by carbapenem-resistant Pseudomonas aeruginosa. Clin Infect Dis 65:158–161. doi: 10.1093/cid/cix014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dinh A, Wyplosz B, Kerneis S, Lebeaux D, Bouchand F, Duran C, Beraud G, Lazaro P, Davido B, Henard S, Canoui E, Ferry T, Wolff M. 2017. Use of ceftolozane/tazobactam as salvage therapy for infections due to extensively drug-resistant Pseudomonas aeruginosa. Int J Antimicrob Agents 49:782–783. doi: 10.1016/j.ijantimicag.2017.04.001. [DOI] [PubMed] [Google Scholar]
- 10.Popejoy MW, Paterson DL, Cloutier D, Huntington JA, Miller B, Bliss CA, Steenbergen JN, Hershberger E, Umeh O, Kaye KS. 2017. Efficacy of ceftolozane/tazobactam against urinary tract and intra-abdominal infections caused by ESBL-producing Escherichia coli and Klebsiella pneumoniae: a pooled analysis of phase 3 clinical trials. J Antimicrob Chemother 72:268–272. doi: 10.1093/jac/dkw374. [DOI] [PubMed] [Google Scholar]
- 11.van Duin D, Bonomo RA. 2016. Ceftazidime/avibactam and ceftolozane/tazobactam: second-generation beta-lactam/beta-lactamase inhibitor combinations. Clin Infect Dis 63:234–241. doi: 10.1093/cid/ciw243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boucher HW, Talbot GH, Bradley JS, Edwards JE, Gilbert D, Rice LB, Scheld M, Spellberg B, Bartlett J. 2009. Bad bugs, no drugs: no ESKAPE! An update from the Infectious Diseases Society of America. Clin Infect Dis 48:1–12. doi: 10.1086/595011. [DOI] [PubMed] [Google Scholar]
- 13.Castanheira M, Farrell SE, Deshpande LM, Mendes RE, Jones RN. 2013. Prevalence of β-lactamase-encoding genes among Enterobacteriaceae bacteremia isolates collected in 26 U.S. hospitals: report from the SENTRY Antimicrobial Surveillance Program (2010). Antimicrob Agents Chemother 57:3012–3020. doi: 10.1128/AAC.02252-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.CLSI. 2015. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically; approved standard, 10th ed M07-A10. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 15.CLSI. 2016. Performance standards for antimicrobial susceptibility testing: 26th informational supplement. M100-S26. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 16.EUCAST. 2016. Breakpoint tables for interpretation of MICs and zone diameters, version 6.0, 1 January 2016. http://www.eucast.org/clinical_breakpoints/ Accessed January 2016.
- 17.Wyeth Pharmaceuticals. 2016. Tygacil package insert. Wyeth Pharmaceuticals, Philadelphia, PA: http://www.tygacil.com Accessed 23 February 2017. [Google Scholar]
- 18.CLSI. 2015. Performance standards for antimicrobial susceptibility testing: 25th informational supplement. M100-S25. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 19.Magiorakos AP, Srinivasan A, Carey RB, Carmeli Y, Falagas ME, Giske CG, Harbarth S, Hindler JF, Kahlmeter G, Olsson-Liljequist B, Paterson DL, Rice LB, Stelling J, Struelens MJ, Vatopoulos A, Weber JT, Monnet DL. 2012. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect 18:268–281. doi: 10.1111/j.1469-0691.2011.03570.x. [DOI] [PubMed] [Google Scholar]
- 20.Farrell DJ, Flamm RK, Sader HS, Jones RN. 2013. Antimicrobial activity of ceftolozane-tazobactam tested against Enterobacteriaceae and Pseudomonas aeruginosa with various resistance patterns isolated in U.S. hospitals (2011–2012). Antimicrob Agents Chemother 57:6305–6310. doi: 10.1128/AAC.01802-13. [DOI] [PMC free article] [PubMed] [Google Scholar]