ABSTRACT
Mosaic penA alleles have caused most of the cephalosporin resistance in Neisseria gonorrhoeae, but their evolution is mostly unknown. The penA gene from Neisseria cinerea strain AM1601 (ceftriaxone MIC, 1.0 μg/ml) caused ceftriaxone resistance (MIC, 1 μg/ml) in a ceftriaxone-susceptible gonococcal strain. The 3′-terminal half of AM1601 penA was almost identical to that of the ceftriaxone-resistant gonococcal GU140106 and FC428 strains. N. cinerea can serve as a reservoir of ceftriaxone resistance-mediating penA sequences that can be transferred to gonococci.
KEYWORDS: Neisseria cinerea, Neisseria gonorrhoeae, antimicrobial resistance, cefixime, ceftriaxone, penA, penicillin-binding protein 2
TEXT
Resistance to the extended-spectrum cephalosporin (ESC) ceftriaxone in Neisseria gonorrhoeae has sporadically emerged worldwide. The main ESC resistance determinant is mosaic penA alleles, encoding penicillin-binding protein 2 (PBP2) (1–10). These gonococcal mosaic penA alleles are proposed to have evolved through transformation of partial penA sequences from commensal Neisseria species (1, 2, 8, 11, 12); however, detailed knowledge is lacking. The ceftriaxone-resistant gonococcal strains H041 (4), A8806 (6), GU140106 (7), and FC428 (8) possessed different mosaic penA alleles. However, A8806 (6), GU140106 (7), and FC428 (8) possessed identical or almost identical 3′-terminal halves of penA, although the central region of penA showed substantially less nucleotide sequence similarity (8). The conserved 3′-terminal part of penAFC428 was very different from the FA1090 wild-type penA (8), illustrating that the origin of gonococcal ceftriaxone resistance exists in other species.
Here, we investigated Neisseria cinerea AM1601, which was isolated in 2016 from a patient with bacteremia in Aichi, Japan (13), and has high MICs for ceftriaxone (1.0 μg/ml) and cefixime (2.0 μg/ml), as determined by an agar dilution method according to CLSI guidelines (14). To verify the species of N. cinerea AM1601, ribosomal multilocus sequence typing was performed. Briefly, the single-nucleotide polymorphisms (SNPs) on 53 rps alleles were extracted from the AM1601 genome sequence generated by a MiSeq sequencer (Illumina, San Diego, CA, USA) and were compared with those on 53 rps alleles from various Neisseria species (15). The results indicated that N. cinerea AM1601 clustered with six N. cinerea reference strains (see Fig. S1 in the supplemental material) (15, 16).
To show that penA of N. cinerea AM1601 is responsible for ceftriaxone resistance, a full-length AM1601 penA gene was PCR amplified using PrimeStar HS Premix (TaKaRa Bio, Shiga, Japan), genomic DNA, and the primers 5′-ATGTTGATTAAGAGCGAATATAAG-3′ and 5′-TTAAGACGGTGTTTTGACGG-3′. The primers were designed on the basis of the N. cinerea AM1601 penA sequence determined by whole-genome sequencing and confirmed by conventional Sanger sequencing, as described previously (8). After purification with the High Pure PCR product purification kit (Roche Diagnostics GmbH, Mannheim, Germany), the PCR product was transformed into NG9807, as described previously (17). The ceftriaxone-susceptible gonococcal strain NG9807 (MIC of 0.016 μg/ml, with the wild-type penA) was used as a recipient for transformation (17). NG9807 has a single-nucleotide (A) deletion in the inverted repeat of the mtrR promoter, a PBP1 L421P alteration, and penB alterations at PorB1b G120 and A121 (4). The transformation frequency was estimated as 1 in 107 recipient cells. The full-length penA gene in the NG9807 transformant was sequenced using conventional Sanger sequencing.
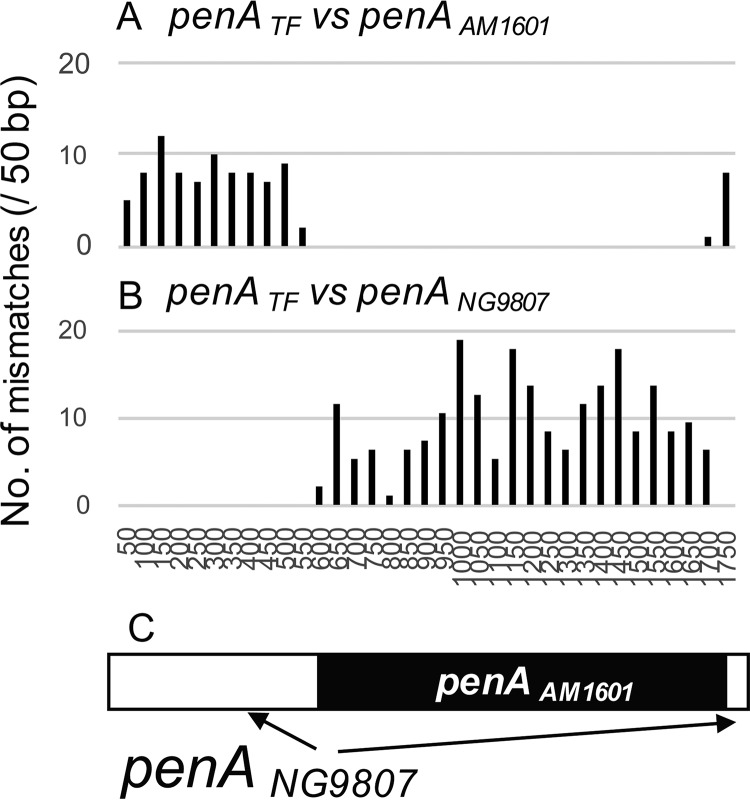
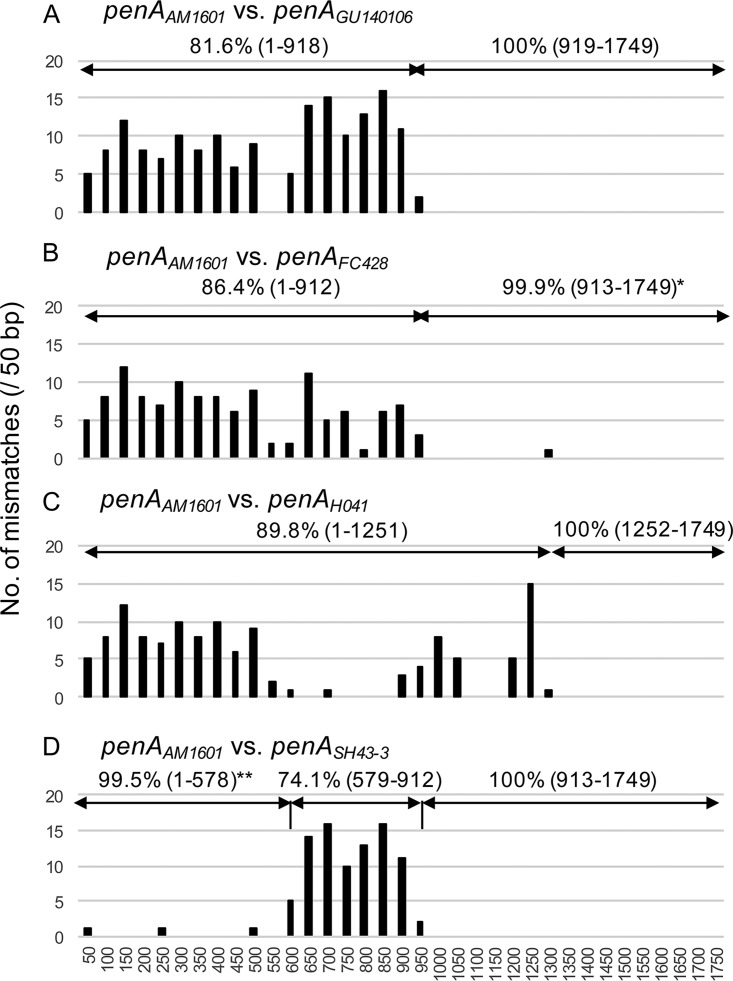
Transformation of penAAM1601 into the ceftriaxone-susceptible gonococcal strain NG9807 caused resistance to ceftriaxone (64-fold MIC increase, from 0.016 to 1 μg/ml) and cefixime (128-fold MIC increase, from 0.016 to 2 μg/ml) (14, 18). Only ∼62% of the donor penAAM1601 was incorporated into the recipient penANG9807 (Fig. 1), resulting in a mosaic penA allele. To characterize the penA sequence of N. cinerea AM1601, we compared the penA gene of AM1601 with the penA genes of ceftriaxone-resistant N. gonorrhoeae strains using ClustalW. The levels of similarity between N. cinerea penAAM1601 and ceftriaxone-resistant gonococcal penAH041 (4), penAGU140106 (7), and penAFC428 (8) were 92.7%, 90.3%, and 92.9%, respectively. The mismatches between penAAM1601 and penAGU140106 accumulated in the 5′-terminal half, whereas there were no SNPs in the 3′-terminal half, including the PBP2 β-lactam-active motifs (Fig. 2A). Similarly, the penAAM1601 3′-terminal half was highly homologous to the corresponding penAFC428 sequence (only one synonymous SNP) (Fig. 2B). Accordingly, the AM1601 PBP2 shared a trait with those of the ceftriaxone-resistant gonococcal strains GU140106 (7) and FC428 (8). Importantly, the PBP2 forms of all of these strains possessed V311 and S483, which are two of the three mutated amino acids causing high-level ceftriaxone resistance in N. gonorrhoeae H041 (19). However, the penA 3′-terminal region that was conserved between penAAM1601 and penAH041 was smaller (Fig. 2). To further characterize penAAM1601, it was compared with another penA (GenBank accession number AB904039), from a Neisseria strain that has high ESC MICs (ceftriaxone MIC, 2 μg/ml; cefixime MIC, 4 μg/ml). The strain was verified as N. cinerea by rps gene comparison in this study (Fig. S1). N. cinerea SH43-3 was isolated in 2013 from an asymptomatic female sex worker, in Kyoto, Japan, during a routine examination for sexually transmitted infections (gonococcus-negative pharyngeal specimen). The 5′-terminal and 3′-terminal parts were very similar (99.5%) and identical (100%), respectively. Nearly all of the mismatches accumulated in the central part of penA, indicating recombination event(s) (Fig. 2D).
FIG 1.
Mosaic penA allele generated by transformation of Neisseria cinerea penAAM1601 into Neisseria gonorrhoeae NG9807 (with wild-type penA). The penA allele of the NG9807 transformant (penATF) was compared with the donor allele of N. cinerea AM1601 (penAAM1601) and the recipient wild-type allele of N. gonorrhoeae NG9807 (penANG9807). (A) Number of mismatches in each 50 bp between alleles penATF and penAAM1601. (B) Number of mismatches in each 50 bp between alleles penATF and penANG9807. (C) Schematic representation of the mosaic penATF by a white rectangle (remaining from penANG9807) and a black rectangle (derived from penAAM1601).
FIG 2.
Sequence comparison of penA genes from ceftriaxone-resistant Neisseria cinerea and Neisseria gonorrhoeae strains isolated in Japan. Pairwise comparisons of penA nucleotide sequences for penA genes from N. cinerea AM1601 and N. gonorrhoeae GU140106 (7) (A), N. gonorrhoeae FC428 (8) (B), N. gonorrhoeae H041 (4) (C), and N. cinerea SH43-3 (D) are shown. The mismatched bases in each 50 bp of the penA genes were counted. The similarity of each region is indicated, with the nucleotide positions evaluated in parentheses. *, one synonymous mismatch, at nucleotide position 1296; **, three mismatches, at nucleotide positions 48, 219, and 489.
Commensal Neisseria species, including N. cinerea, are members of the human oropharyngeal microflora (20–23). These Neisseria species might be genetic reservoirs of resistance determinants for β-lactam antimicrobials (including ESCs) that can be transferred to the pathogenic species Neisseria meningitidis and gonococci (1, 11, 12, 23–26). We demonstrate that N. cinerea strains with high ceftriaxone MICs (1 to 2 μg/ml) possess ceftriaxone resistance-mediating penA sequences that can be transferred to gonococci by transformation and result in ceftriaxone and cefixime resistance. The 3′-terminal half of mosaic penA in the transformant, which was transferred from N. cinerea, has also been described in ceftriaxone-resistant clinical gonococcal strains, i.e., A8806 (6), GU140106 (7), and FC428 (8), isolated in 2013 to 2015 in Australia and Japan. Accordingly, the 3′-terminal half of N. cinerea penA has caused ceftriaxone resistance in genetically different gonococcal strains in different countries. This indicates that N. cinerea strains represent an origin of the ceftriaxone resistance-mediating penA sequences in the gonococcal strains A8806 (6), GU140106 (7), and FC428 (8). However, an unknown origin of ceftriaxone resistance-mediating penA sequences might also exist, from which genetic material has been transferred to both ceftriaxone-resistant gonococcal strains and ceftriaxone-resistant N. cinerea strains, which might be supported by the mismatches in the central region of penA in AM1601 versus SH43-3. Further investigations of commensal Neisseria species are imperative.
In conclusion, N. cinerea can serve as a reservoir of ceftriaxone resistance-mediating penA sequences that are transferred to and cause ceftriaxone and cefixime resistance in clinical gonococcal strains. Examinations of commensal Neisseria species are crucial to understand, and ideally to mitigate, the emergence and evolution of resistance to ESCs in gonococci. This will provide new insights regarding interspecies sharing and reservoirs of resistance determinants for other antimicrobials in commensal bacteria.
Accession number(s).
The N. cinerea AM1601 penA sequence was deposited in the DDBJ (accession number LC316656).
Supplementary Material
ACKNOWLEDGMENTS
We thank Shinji Hoshina for the isolation of N. cinerea SH43-3.
This work was supported by the Research Program on Emerging and Re-emerging Infectious Diseases, Japan Agency for Medical Research and Development.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/AAC.02069-17.
REFERENCES
- 1.Unemo M, Shafer WM. 2014. Antimicrobial resistance in Neisseria gonorrhoeae in the 21st century: past, evolution, and future. Clin Microbiol Rev 27:587–613. doi: 10.1128/CMR.00010-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wi T, Lahra MM, Ndowa F, Bala M, Dillon JR, Ramon-Pardo P, Eremin SR, Bolan G, Unemo M. 2017. Antimicrobial resistance in Neisseria gonorrhoeae: global surveillance and a call for international collaborative action. PLoS Med 14:e1002344. doi: 10.1371/journal.pmed.1002344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ohnishi M, Saika T, Hoshina S, Iwasaku K, Nakayama S, Watanabe H, Kitawaki J. 2011. Ceftriaxone-resistant Neisseria gonorrhoeae, Japan. Emerg Infect Dis 17:148–149. doi: 10.3201/eid1701.100397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ohnishi M, Golparian D, Shimuta K, Saika T, Hoshina S, Iwasaku K, Nakayama SI, Kitawaki J, Unemo M. 2011. Is Neisseria gonorrhoeae initiating a future era of untreatable gonorrhea? Detailed characterization of the first strain with high-level resistance to ceftriaxone. Antimicrob Agents Chemother 55:3538–3545. doi: 10.1128/AAC.00325-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Unemo M, Golparian D, Nicholas R, Ohnishi M, Gallay A, Sednaoui P. 2012. High-level cefixime- and ceftriaxone-resistant Neisseria gonorrhoeae in France: novel penA mosaic allele in a successful international clone causes treatment failure. Antimicrob Agents Chemother 56:1273–1280. doi: 10.1128/AAC.05760-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lahra MM, Ryder N, Whiley DM. 2014. A new multidrug-resistant strain of Neisseria gonorrhoeae in Australia. N Engl J Med 371:1850–1851. doi: 10.1056/NEJMc1408109. [DOI] [PubMed] [Google Scholar]
- 7.Deguchi T, Yasuda M, Hatazaki K, Kameyama K, Horie K, Kato T, Mizutani K, Seike K, Tsuchiya T, Yokoi S, Nakano M, Yoh M. 2016. New clinical strain of Neisseria gonorrhoeae with decreased susceptibility to ceftriaxone, Japan. Emerg Infect Dis 22:142–144. doi: 10.3201/eid2201.150868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nakayama SI, Shimuta K, Furubayashi KI, Kawahata T, Unemo M, Ohnishi M. 2016. New ceftriaxone- and multidrug-resistant Neisseria gonorrhoeae strain with a novel mosaic penA gene isolated in Japan. Antimicrob Agents Chemother 60:4339–4341. doi: 10.1128/AAC.00504-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cámara J, Serra J, Ayats J, Bastida T, Carnicer-Pont D, Andreu A, Ardanuy C. 2012. Molecular characterization of two high-level ceftriaxone-resistant Neisseria gonorrhoeae isolates detected in Catalonia, Spain. J Antimicrob Chemother 67:1858–1860. doi: 10.1093/jac/dks162. [DOI] [PubMed] [Google Scholar]
- 10.Fifer H, Natarajan U, Jones L, Alexander S, Hughes G, Golparian D, Unemo M. 2016. Failure of dual antimicrobial therapy in treatment of gonorrhea. N Engl J Med 374:2504–2506. doi: 10.1056/NEJMc1512757. [DOI] [PubMed] [Google Scholar]
- 11.Ameyama S, Onodera S, Takahata M, Minami S, Maki N, Endo K, Goto H, Suzuki H, Oishi Y. 2002. Mosaic-like structure of penicillin-binding protein 2 gene (penA) in clinical isolates of Neisseria gonorrhoeae with reduced susceptibility to cefixime. Antimicrob Agents Chemother 46:3744–3749. doi: 10.1128/AAC.46.12.3744-3749.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ito M, Deguchi T, Mizutani KS, Yasuda M, Yokoi S, Ito S, Takahashi Y, Ishihara S, Kawamura Y, Ezaki T. 2005. Emergence and spread of Neisseria gonorrhoeae clinical isolates harboring mosaic-like structure of penicillin-binding protein 2 in central Japan. Antimicrob Agents Chemother 49:137–143. doi: 10.1128/AAC.49.1.137-143.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kawahara-Matsumizu M, Yamagishi Y, Mikamo H. 2017. Misidentification of Neisseria cinerea as Neisseria meningitidis in matrix-assisted laser desorption/ionization time of flight mass spectrometry (MALDI-TOF MS). Jpn J Infect Dis doi: 10.7883/yoken.JJID.2017.183. [DOI] [PubMed] [Google Scholar]
- 14.Clinical and Laboratory Standards Institute. 2015. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically; approved standard—10th ed. CLSI document M07-A10 Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 15.Bennett JS, Jolley KA, Earle SG, Corton C, Bentley SD, Parkhill J, Maiden MCJ. 2012. A genomic approach to bacterial taxonomy: an examination and proposed reclassification of species within the genus Neisseria. Microbiology 158:1570–1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jolley KA, Bliss CM, Bennett JS, Bratcher HB, Brehony C, Colles FM, Wimalarathna H, Harrison OB, Sheppard SK, Cody AJ, Maiden MCJ. 2012. Ribosomal multilocus sequence typing: universal characterization of bacteria from domain to strain. Microbiology 158:1005–1015. doi: 10.1099/mic.0.055459-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ohnishi M, Watanabe Y, Ono E, Takahashi C, Oya H, Kuroki T, Shimuta K, Okazaki N, Nakayama SI, Watanabe H. 2010. Spread of a chromosomal cefixime-resistant penA gene among different Neisseria gonorrhoeae lineages. Antimicrob Agents Chemother 54:1060–1067. doi: 10.1128/AAC.01010-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.European Committee on Antimicrobial Susceptibility Testing. 2015. Breakpoint tables for interpretation of MICs and zone diameters. http://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Breakpoint_tables/v_5.0_Breakpoint_Table_01.pdf.
- 19.Tomberg J, Unemo M, Ohnishi M, Davies C, Nicholas RA. 2013. Identification of amino acids conferring high-level resistance to expanded-spectrum cephalosporins in the penA gene from Neisseria gonorrhoeae strain H041. Antimicrob Agents Chemother 57:3029–3036. doi: 10.1128/AAC.00093-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Keijser BJF, Zaura E, Huse SM, van der Vossen JM, Schuren FHJ, Montijn RC, ten Cate JM, Crielaard W. 2008. Pyrosequencing analysis of the oral microflora of healthy adults. J Dent Res 87:1016–1020. doi: 10.1177/154405910808701104. [DOI] [PubMed] [Google Scholar]
- 21.Zaura E, Keijser BJ, Huse SM, Crielaard W. 2009. Defining the healthy “core microbiome” of oral microbial communities. BMC Microbiol 9:259. doi: 10.1186/1471-2180-9-259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu G, Tang CM, Exley RM. 2015. Non-pathogenic Neisseria: members of an abundant, multi-habitat, diverse genus. Microbiology 161:1297–1312. doi: 10.1099/mic.0.000086. [DOI] [PubMed] [Google Scholar]
- 23.Knapp JS, Hook EW. 1988. Prevalence and persistence of Neisseria cinerea and other Neisseria spp. in adults. J Clin Microbiol 26:896–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Spratt BG, Bowler LD, Zhang QY, Zhou J, Smith JM. 1992. Role of interspecies transfer of chromosomal genes in the evolution of penicillin resistance in pathogenic and commensal Neisseria species. J Mol Evol 34:115–125. doi: 10.1007/BF00182388. [DOI] [PubMed] [Google Scholar]
- 25.Spratt B, Zhang Q, Jones D, Hutchison A, Brannigan J, Dowson C. 1989. Recruitment of a penicillin-binding protein gene from Neisseria flavescens during the emergence of penicillin resistance in Neisseria meningitidis. Proc Natl Acad Sci U S A 86:8988–8992. doi: 10.1073/pnas.86.22.8988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bowler LD, Zhang QY, Riou JY, Spratt BG. 1994. Interspecies recombination between the penA genes of Neisseria meningitidis and commensal Neisseria species during the emergence of penicillin resistance in N. meningitidis: natural events and laboratory simulation. J Bacteriol 176:333–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.