Abstract
Human endogenous retroviruses (ERVs) have been found to be associated with different diseases, e.g., multiple sclerosis (MS). Most human ERVs integrated in our genome are not competent to replicate and these sequences are presumably silent. However, transcription of human ERVs can be reactivated, e.g., by hypoxia. Interestingly, MS has been linked to hypoxia since decades. As some patterns of demyelination are similar to white matter ischemia, hypoxic damage is discussed. Therefore, we are interested in the association between hypoxia and ERVs. As a model, we used human SH-SY5Y neuroblastoma cells after treatment with the hypoxia-mimetic cobalt chloride and analyzed differences in the gene expression profiles in comparison to untreated cells. The vicinity of up-regulated genes was scanned for endogenous retrovirus-derived sequences. Five genes were found to be strongly up-regulated in SH-SY5Y cells after treatment with cobalt chloride: clusterin, glutathione peroxidase 3, insulin-like growth factor 2, solute carrier family 7 member 11, and neural precursor cell expressed developmentally down-regulated protein 9. In the vicinity of these genes we identified large (>1,000 bp) open reading frames (ORFs). Most of these ORFs showed only low similarities to proteins from retro-transcribing viruses. However, we found very high similarity between retrovirus envelope sequences and a sequence in the vicinity of neural precursor cell expressed developmentally down-regulated protein 9. This sequence encodes the human endogenous retrovirus group FRD member 1, the encoded protein product is called syncytin 2. Transfection of syncytin 2 into the well-characterized Ewing sarcoma cell line A673 was not able to modulate the low immunostimulatory activity of this cell line. Future research is needed to determine whether the identified genes and the human endogenous retrovirus group FRD member 1 might play a role in the etiology of MS.
Keywords: endogenous retroviruses, open reading frames, ERVFRD-1, HERV-FRD, human endogenous retrovirus group FRD member 1, hypoxia, multiple sclerosis, neural precursor cell expressed developmentally down-regulated protein 9 (NEDD9)
Introduction
Endogenous retroviruses (ERVs) are viral elements that are present in the genomes of virtually all species including human beings (Hayward and Katzourakis, 2015). At least 8% of the human genome is composed of endogenous retroviral sequences (Lander et al., 2001). These sequences were integrated into the human genome in the course of the evolution (Emerman and Malik, 2010). The great majority of ERVs are stabilized in the genome, but there is still ongoing or potential ERV genotype modification from parents to offspring through generations. Like other genes, ERVs are susceptible to mutations and proviral DNAs are predisposed to accumulate mutations as these sequences are usually not vital for the host survival and thus not under strong selective pressure. The majority of ERVs integrated in our genome is not competent to replicate and most ERV sequences are presumably silent (Jern and Coffin, 2008). Nevertheless, about one third of all ERV sequences in the genome were found to be transcriptionally active (Pérot et al., 2012). Some of these sequences still have open reading frames (ORFs) and, therefore, have the potential to code for a protein or peptide (Dupressoir et al., 2012; Wildschutte et al., 2016). ERVs can be reactivated by some herpes viruses such as Epstein–Barr virus (Mameli et al., 2012). Another possibility is the reactivation of ERV expression by hypoxia (Kewitz and Staege, 2013; Kulkarni et al., 2017). ERV-encoded superantigens might lead to hyper-stimulation of the immune system and tissue damage. In addition, fusogenic activity of ERV envelope proteins might have direct cytopathic effects which might be involved in MS pathogenesis independent on autoimmune mechanisms. Indeed, cell fusion has been detected in MS brain lesions as well as in animal models of MS (Kemp et al., 2012; Sankavaram et al., 2015). A working model for ERV reactivation and consequences is presented in Figure 1.
FIGURE 1.
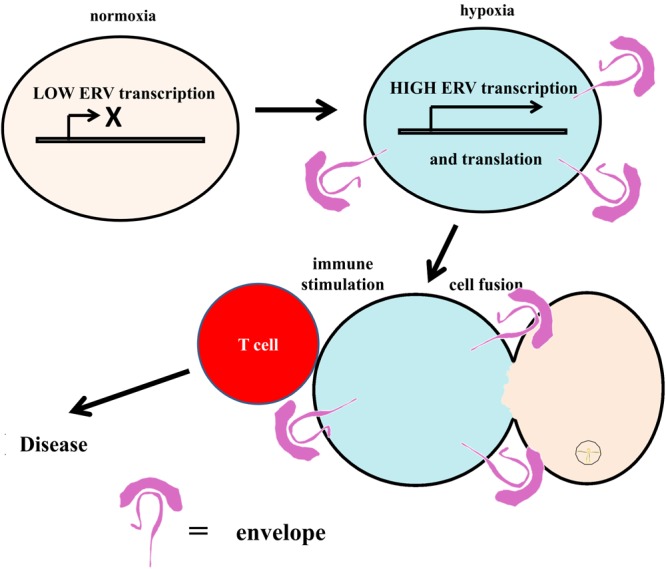
A working model for ERV reactivation. ERVs constitute an integral part of our genome. Under normal conditions, expression of ERVs is switched off epigenetically. Triggered by diverse factors like hypoxia, reactivation of ERV expression can be induced. ERV-encoded proteins can act as immunostimulatory superantigens or induce cytopathic effects, e.g., cell fusion.
Endogenous retroviruses have contributed to certain physiological genes (i.e., syncytins) through modifications (Blond et al., 1999; Mi et al., 2000; Soygur and Moore, 2016) and can sometimes probably protect the host against exogenous retrovirus infections (Malfavon-Borja and Feschotte, 2015). On the other hand, ERVs have also been found to be associated with different diseases (Dolei, 2006; Balada et al., 2009), e.g., schizophrenia and bipolar disorder (Perron et al., 2012), type 1 diabetes mellitus (Mason et al., 2014), or cancer (Goering et al., 2015) as well as multiple sclerosis (MS) (Perron and Lang, 2010; De la Hera et al., 2014). Several ERVs are considered to be associated with MS (Christensen, 2010). For example, human ERV-W envelope mRNA expression was found to be selectively up-regulated in brain tissue from individuals with MS as compared with controls (Antony et al., 2004). In addition, HERV-H Env and HERV-W Env are increased on the surface of B cells and monocytes of MS patients (Brudek et al., 2009).
Multiple sclerosis is a chronic immune-mediated inflammatory disease of the central nervous system with characteristic patchy demyelination. It is the most common chronic disabling CNS disease in young adults and affects about 2.3 million people around the world (Browne et al., 2014). The etiology of MS has not been completely decoded so far; the causes of MS are hypothesized to be multifactorial including environmental influences (Islam et al., 2007) as well as epigenetic and genetic factors (Küçükali et al., 2015; Booth and Parnell, 2017). Commonly an autoimmune attack against myelin autoantigens is considered as the main occurrence in the pathogenesis of MS (Hemmer et al., 2003; Pender and Greer, 2007). Additionally, ERVs are discussed to contribute to MS (Tselis, 2011; Emmer et al., 2014). Besides, MS has been linked to hypoxia for decades (e.g., Fischer et al., 1983; Auer et al., 1995; Trapp and Stys, 2009). Hypoxic damage is hypothesized to be a factor in MS pathogenesis, because some patterns of demyelination are similar to white matter ischemia (Lassmann, 2003).
In the present study, we analyzed the effect of hypoxia-mimetic cobalt chloride (CoCl2) on human neuronal-like SH-SY5Y neuroblastoma cells for changes in gene expression profiles in contrast to un-stimulated cells. Genes up-regulated in this model are considered to indicate transcriptionally active chromatin regions which are susceptible also for ERV reactivation. Therefore, the vicinity of up-regulated genes was scanned for endogenous retrovirus sequences in order to identify possible ERV that might be involved in the link between hypoxia and MS. In addition, we analyzed the possible immune modulatory activity of the identified syncytin 2 in the A673 cell line system. We used this system because the immunostimulatory activity of A673 cells is well-characterized (Staege et al., 2004; Max et al., 2014; Reuter et al., 2015) and they display similar gene expression and splicing features as neuronal cells (Bros et al., 2006). The immunostimulatory activity of this model cell line has been shown to be susceptible to transgenic expression of varying molecules like interleukin 2 (Staege et al., 2004), CD137 ligand (Max et al., 2014), or OX40 ligand (Reuter et al., 2015).
Materials and Methods
Cell Lines and Cell Culture
Human SH-SY5Y neuroblastoma cells (Biedler et al., 1973) were obtained from the Deutsche Sammlung von Mikroorganismen und Zellkulturen (DSMZ, Braunschweig, Germany). A673 Ewing sarcoma cells (Giard et al., 1973) were obtained from the American Type Culture Collection (Manassas, VA, United States) All cells were cultured in Dulbecco’s Modified Eagle Medium (DMEM, PAA, Pasching, Germany), supplemented with 10% fetal calf serum, 100 U/mL penicillin, and 100 μg/mL streptomycin at 37°C in a humidified atmosphere with 5% CO2. For simulation of hypoxia, a fresh stock solution (10 mM) of CoCl2 was prepared in water and added to the medium to obtain desired final concentrations. SH-SY5Y cells were treated for 24 h at a cell density of 1 × 106 cells/mL with either 0 μM CoCl2, 100 μM CoCl2, or 200 μM CoCl2. The experiment was repeated twice for the gene expression analysis with microarrays and three times for the gene expression analysis with polymerase chain reaction (PCR).
Gene Expression Analysis
RNA was isolated using GeneMatrix Universal RNA Kit (roboklon, Berlin, Germany). RNA extracted from the cells was treated with DNase (roboklon, Berlin, Germany) to remove genomic DNA. Occasionally absence of DNA contamination was proved by using isolated RNA without reverse transcription as template for PCR. Global gene expression in SH-SY5Y cells was analyzed using Affymetrix Human Exon 1.0ST arrays (Affymetrix, Santa Clara, CA, United States). Affymetrix cel files were processed with Expression Console 1.1 (Affymetrix) at gene level (core; library version: huex-1_0-st-v2.na36.1.hg19). Calculations were performed with the MAfilter software (Winkler et al., 2012). Values of cobalt (II) chloride treated samples had to be three times higher than controls and signal intensities (RMA normalized, linear values) had to be above 100 to be considered as differentially expressed. Analysis was performed separately for cells treated with 100 μM CoCl2, or 200 μM CoCl2. For further analysis we included all threefold up-regulated genes that were found in both replications. Microarray cell files have been submitted to the Gene Expression Omnibus (GEO) data base (GSE107333).
Identification of Endogenous Retrovirus Sequences
The chromosomal locations of the up-regulated genes were analyzed for the presence of putative ERV sequences essentially as described (Brütting et al., 2016). For this end, we analyzed the 2 Mbp surrounding each individual gene for the presence of ORFs with a minimal length of 1 kb by using Mobyle 1.5 (Rice et al., 2000). Identified ORFs were analyzed using BLASTP (Altschul et al., 2005) against the NCBI database of retro-transcribing viruses (taxid 35268) with the reference genome GRCh38 (primary assembly).
Polymerase Chain Reaction
One microgram of the isolated RNA was transcribed into cDNA and used as a template for PCR. Real-time quantitative reverse transcription-PCR (qRT-PCR) was performed using Go Taq pPCR master mix (Promega, Mannheim, Germany) using 10 μL Go Taq pPCR master mix, 7 μL water, 1 μL forward primer, 1 μL reverse primer (25 μM) and 1 μL cDNA. PCR conditions were: 94°C, 30 s; 60°C, 30 s; 72°C, 45 s (40 cycles). Gene expression was calculated with the 2-ΔΔCt method (Livak and Schmittgen, 2001). Conventional PCR was performed using 2 μl of the cDNA, 5 μl Green GoTaq Buffer (Promega, Mannheim, Germany), 0.5 μl of 10 mM dNTPs (Fermentas, Sankt Leon-Rot, Germany), 0.25 μl of each of the two primers (25 μM), 0.2 μl GoTaq polymerase (5 U/μl; Promega) and 16.8 μl water. All used primer sequences are listed in Table 1. The amplification protocol included an initial denaturation step at 95°C for 5 min, followed by 40 cycles with denaturation at 95°C for 60 s; primer annealing at 60°C for 60 s; amplification at 72°C for 90 s; and a final extension step at 72°C for 5 min. PCR products were subjected to agarose gel (1.5%) electrophoresis in the presence of ethidium bromide.
Table 1.
Primer combinations used in this study.
| Targeta | Primer sequences (5′–3′) |
|---|---|
| ACTB | TAC AAT GAG CTG CGT GTG GC |
| CGG ACT CGT CAT ACT CCT GC | |
| HERV-FRD | CCC TCA CCC CCT TAT TTC AT |
| TTT GAA GGA CTA CGG CTG CT | |
| HERV-FRDb | ACC ATG GGC CTG CTC CT |
| TCC TCC TTA GAA GGG TGA CTC | |
| EWSR1-FLI1 | GGC CAA GAT CAA TCC TCC AT |
| ATG GAG GAT TGA TCT TGG CC | |
| LIPI | AAC CAG CCC AAT CAG ACA AC |
| AAT CAC TGG CCA GGA CAT TC | |
| NEDD9 | CAC CGC AGT GCT TAA TGC TG |
| TCA CGG GGG TTA TCA CCT TTT T |
If not otherwise stated, primer combinations were used for qRT-PCR.
aACTB, actin beta; HERV-FRD, syncytin 1; EWSR1-FLI1, Ewing sarcoma breakpoint region 1-Friend leukemia virus integration site 1; tumor specific gene fusion; LIPI, lipase member I (cancer-testis antigen 17); NEDD9, neural precursor cell expressed developmentally down-regulated protein 9.
bUsed for conventional PCR only.
Cloning of HERV-FRD in pIRES2-AcGFP1 Vector
DNA (PCR product from SH-SY5Y cells) from the agarose gel was extracted with GeneJet Gel Extraction Kit (Thermo Fisher, Waltham, MA, United States), ligated in vector pGEM-T Easy (Promega) and transformed in Escherichia coli XL1-Blue. The DNA of one overnight colony was isolated with GeneJET Plasmid Miniprep Kit (Thermo Fisher, Waltham, MA, United States). DNA and vector pIRES2-AcGFP1 (Clontech, Mountain View, CA, United States) were digested with SacI and SacII. After agarose gel purification, ligation, and transformation into Escherichia coli XL1-Blue, individual clones were sequenced by using HERV-FRD specific primers. For sequencing, a 10 μL sequencing mix was used that contained 6.8 μL HPLC water, 0.2 μL sequence-specific sequencing primers (10 μM), 2.0 μL BigDyeTerminator v1.1 Cycle Sequencing buffer (Applied Biosystems, Foster City, CA, United States), 2.0 μL BigDyeTerminator v1.1 Cycle Sequencing Mix and 10 ng DNA. Sequence analysis was performed using ABI PrismTM 310 Genetic Analyzer (Applied Biosystems). A clone with complete HERV-FRD ORF was used for further analysis. This clone differs from the reference sequence by a silent C to T transition (corresponding to base 1,384 in reference sequence NM207582).
Transfection
For transient expression, SH-SY5Y cells and A673 cells were cultured for 24 h and then transfected with the appropriate vectors using PromoFectin (PromoKine, Heidelberg, Germany) according to the manufacturer’s protocol. For stable expression, cells were treated in the same way. After 24 h they were put under selection with the antibiotic G418.
Mixed Lymphocyte Tumor Cell Culture (MLTC) and Flow Cytometry
Peripheral blood mononuclear cells (PBMC) were prepared and mixed lymphocyte tumor cell culture (MLTC) was performed as described elsewhere (Staege et al., 2004; Foell et al., 2008). Detection of surface antigens on PBMC by flow cytometry was performed as described elsewhere (Hoennscheidt et al., 2009). The following phycoerythrin labeled antibodies have been used: anti-CD3 clone SK7, anti-CD8 clone RPA-T8, and anti-CD25 clone 2A3. All antibodies were purchased from Becton Dickinson (Heidelberg, Germany) and all samples were analyzed on a FACScan instrument (Becton Dickinson) using CellQuestPro software (Becton Dickinson).
Results and Discussion
According to our stringent filter criteria (see section “Materials and Methods”), only five genes were found to be strongly up-regulated in SH-SY5Y cells after treatment with cobalt chloride. These genes include (in alphabetical order) CLU (clusterin), GPX3 (glutathione peroxidase 3), IGF2 (insulin-like growth factor 2), NEDD9 (neural precursor cell expressed, developmentally down-regulated 9), and SLC7A11 [solute carrier family 7 (anionic amino acid transporter light chain, Xc-system), Member 11]. The up-regulated genes indicate transcriptionally active chromatin regions which might be susceptible for reactivation of other genetic elements like ERVs.
CLU (also known as apolipoprotein J, testosterone-repressed prostate message-2, or sulfated glycoprotein-2) encodes a glycoprotein which is nearly ubiquitously distributed in human tissues (Jones and Jomary, 2002). It is a 75–80 kDa heterodimer and a molecular chaperone which is normally secreted but in conditions of cellular stress, it can be transported to the cytoplasm where it can bind to BAX and inhibit neuronal apoptosis (Nuutinen et al., 2009). CLU expression has been associated with tumorigenesis of various malignancies, including tumors of the prostate, colon, and breast (Shannan et al., 2006). Variants in the clusterin gene are also associated with the risk of Alzheimer’s disease (Schrijvers et al., 2011), dementia (Weinstein et al., 2016), and stroke (Guido et al., 2015). In astrocytes of MS white matter lesions an elevated expression of clusterin was detected (van Luijn et al., 2015). All of these diseases represent states of increased oxidative stress, which in turn, promotes amorphous aggregation of target proteins, increased genomic instability and high rates of cellular death (Trougakos and Gonos, 2006).
GPX3 (also known as plasma or extracellular glutathione peroxidase) encodes a protein which functions in the detoxification of hydrogen peroxide. Most of the GPX3 mRNA is kidney-derived (Avissar et al., 1994), but it is also expressed by heart, lung, liver, brain, breast, and gastrointestinal tract (Chu et al., 1992; Tham et al., 1998). In human cancer GPX3 promotor down-regulation and hyper-methylation is rather common (Zhang et al., 2010; Chen et al., 2011). GPX3 expression and GPX3 hyper-methylation can thus be used as biomarkers for different kind of cancer (Yang et al., 2013; Zhou et al., 2015). GPX3 works as a tumor suppressor for example in colitis-associated carcinoma (Barrett et al., 2013) and in hepatocellular carcinoma (Qi et al., 2014). In initial MS lesions GPX3 was found to be downregulated (>2 log2-fold) compared to control (Fischer et al., 2012).
IGF2 encodes a protein with high homology to pro-insulin (Livingstone, 2013). IGF2 contains 10 exons and 4 promoters so that several alternatively spliced transcripts are possible (Engström et al., 1998). The IGF2 gene is imprinted: the paternal IGF2 allele is transcribed whereas the maternal allele is silent (Giannoukakis et al., 1993). As a growth factor it is especially expressed in many tissues in early stages of embryonic and fetal development (Hedborg et al., 1994). In adults, IGF2 is preferentially expressed in liver and brain (Engström et al., 1998). IGF2 regulates normal cell growth and proliferation. Moreover, it plays a role in the growth and development of tumors: epigenetic changes at this locus are for example associated with Wilms tumor, Beckwith–Wiedemann syndrome, or rhabdomyosarcoma (Bergman et al., 2013).
SLC7A11 (also known as xCT) encodes a protein that is member (together with SLC3A2) of a heterodimeric, sodium-independent, anionic amino acid transport system that is highly specific for cysteine and glutamate (Sato et al., 2000). While SLC7A11 seems to induce the transport activity, SLC3A2 leads to the surface expression of the system (Verrey et al., 2004). SLC7A11 seems to contribute to different kinds of cancer, including, e.g., malignant glioma (Robert et al., 2015) or breast cancer (Liu et al., 2011). In tumor cells, the amino acid transport system plays a critical role in regulating intracellular glutathione levels (Okuno et al., 2003) and glutathione has been broadly implicated in chemotherapy resistance (Gatti and Zunino, 2005). Besides, SLC7A11 is significantly up-regulated in post-mortem spinal cord samples from MS patients (Lieury et al., 2014). SLC1A11 is a member of the solute carrier family, a large gene family that contains several receptors for retroviruses. Interestingly, two members of this family (SLC1A4, SLC1A5) have also been suggested as receptors for ERV (Lavillette et al., 2002). A function as receptor for viruses has not been described for SLC1A11.
NEDD9 (also known as CasL and HEF1) encodes a protein which regulates diverse cellular processes that are relevant to cancer, like cell attachment, migration, invasion, apoptosis, or cell cycle regulation (Singh et al., 2007; Shagisultanova et al., 2015). Furthermore, NEDD9 seems to play a role in the nervous system as there is some association between one NEDD9 variation and the susceptibility of late-onset Alzheimer’s disease and Parkinson’s disease (Li et al., 2008). As it is involved in TGFβ-mediated differentiation into the neuronal lineage and NEDD9 possibly promotes a progenitor status that renders the cells competent to differentiation into neurons (Vogel et al., 2010). It is enriched in neural progenitor cells (Abramova et al., 2005) and its down-regulation is linked to neuronal lineage commitment (Aquino et al., 2008).
Based on our search strategy (see section “Materials and Methods”), we found in the vicinity of the up-regulated genes large (>1,000 bp) ORFs (from 11 in the vicinity of NEDD9 to 169 in the vicinity of IGF2). For all genes, these ORFs included candidates that passed the default threshold of the NCBI BLASTP implementation [expect (E) value < 10] against the database of retro-transcribing viruses. For four of the genes (all with the exception of SLC7A11) these BLASTP hits include envelope sequences from retro-transcribing viruses. The E-values for nearly all of these hits were higher than 0.01 and, therefore, are not convincing retroviral (ERV) sequences. However, we found one hit with very high similarity to retroviral envelope proteins in the vicinity of NEDD9 (see Supplementary Figure 1).
We validated up-regulation of NEDD9 in CoCl2 treated SH-SY5Y cells by qRT-PCR (Figure 2). Our results are in agreement with observations from other groups also demonstrating that NEDD9 is induced by hypoxia (Martin-Rendon et al., 2007; Kim et al., 2010).
FIGURE 2.
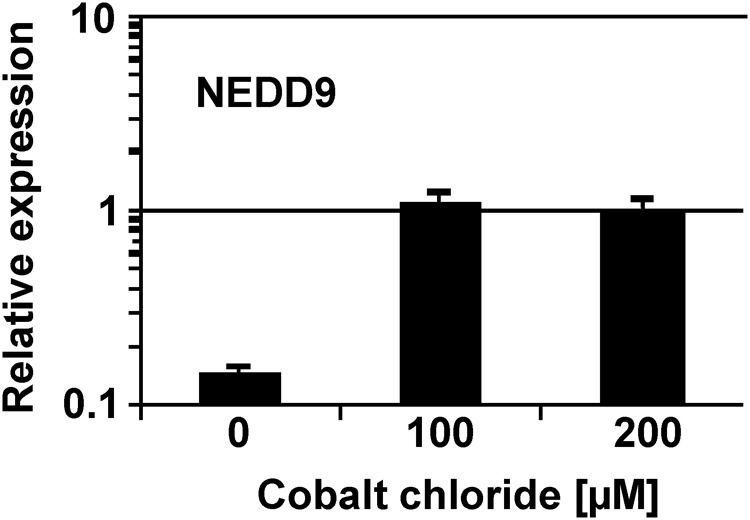
Expression of NEDD9 in SH-SY5Y cells. Expression of NEDD9 was analyzed in SH-SY5Y cells under different culture conditions by qRT-PCR. Cells were cultured in absence of CoCl2 (water control), with 100 μM of CoCl2 or with 200 μM of CoCl2. Presented are means and standard deviations from three independent experiments. For comparative analysis, beta actin was used as housekeeping control and the median expression of all samples was set as one.
The BLASTP hit in the vicinity of NEDD9 (accession number CAB94192.1; see Supplementary Figure 1) represents a sequence (“HERV-H/env62”) of the human HERV-H family. With about 1,000 elements the HERV-H family is one of the largest HERV families in the human genome (Wilkinson et al., 1994). Analyzes showed that there are three envelopes with large ORFs corresponding to potential 59-, 60-, and 62-kDa translational products (de Parseval et al., 2001). Moreover, the higher HERV seroreactivity in patients with active MS correlates with the higher levels of HERV-H Env expression on B cells and monocytes (Brudek et al., 2009).
The sequence in the vicinity of NEDD9 is identical to the human endogenous retrovirus group FRD, member 1 (HERV-FRD). HERV-FRD is located in an intron of the small integral membrane protein 13 (SMIM13). The close association between NEDD9 and SMIM13 is highly conserved in vertebrates. However, in non-primate vertebrates, HERV-FRD is absent (Figure 3). HERV-FRD entered the primate genomes more than 40 million years ago (de Parseval and Heidmann, 2005). It has inactivating mutations in the gag and pol genes whereas the envelope glycoprotein gene is preserved (Renard et al., 2005). The encoded protein product is called syncytin 2 (Blaise et al., 2003) which plays a major role in placental development and trophoblast fusion (Malassiné et al., 2007; Vargas et al., 2009). The protein has the characteristics of a typical retroviral envelope protein, including a cleavage site that separates the surface and transmembrane units which together form a heterodimer of the mature syncytin 2 (Renard et al., 2005). Syncytin 2 can induce cell-cell fusion (Blaise et al., 2003).
FIGURE 3.
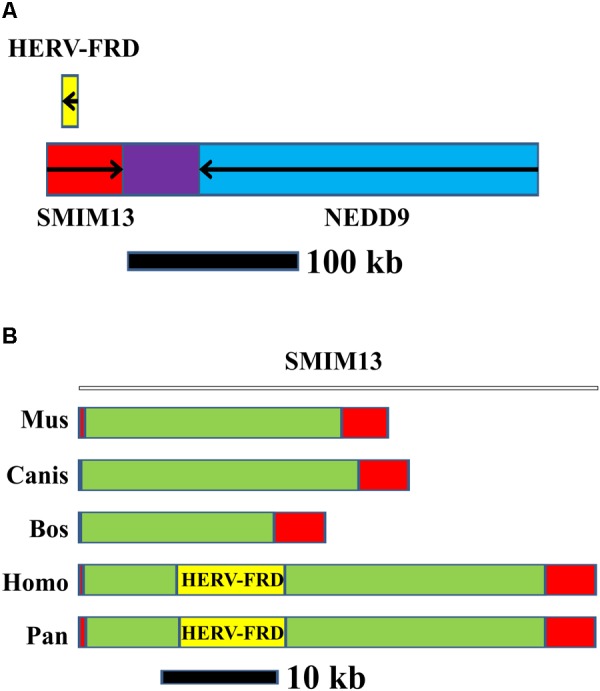
Organization of the NEDD9/SMIM13/HERV-FRD region in humans and other species. (A) HERV-FRD is located in the intron of SMIM13. SMIM13 and NEDD9 are located tail-to-tail orientated on human chromosome 6. (B) Comparison of the SMIM13/HERV-FRD region in different vertebrate species. Red: exons of SMIM13; green: SMIM13 intron; yellow: HERV-FRD.
In our model we found up-regulation only for the mentioned five genes and not for the associated ERVs and we have no evidence that ERVs are functionally involved in up-regulation of the genes or vice versa. From our data we only found HERV-FRD to be a candidate for a possible association between hypoxia and ERVs in MS. Other factors (e.g., patient specific polymorphisms) might be necessary to induce expression of the ERVs and subsequent effects. Under such conditions, it seems possible that over-expression of syncytin 2 in the brain, e.g., as a consequence of local hypoxia, elicits an immunomodulating activity. Therefore, we tested whether syncytin 2 overexpression lead to altered immunostimulatory activity in the well-characterized A673 model system (Staege et al., 2004; Reuter et al., 2015). HERV-FRD transfected A673 cells retained the expression of tumor associated antigens (Figure 4A). However, we were not able to find altered immunostimulatory activity of transfected cells (Figure 4B) in this system. Further investigations are needed to analyze possible immunomodulatory properties.
FIGURE 4.
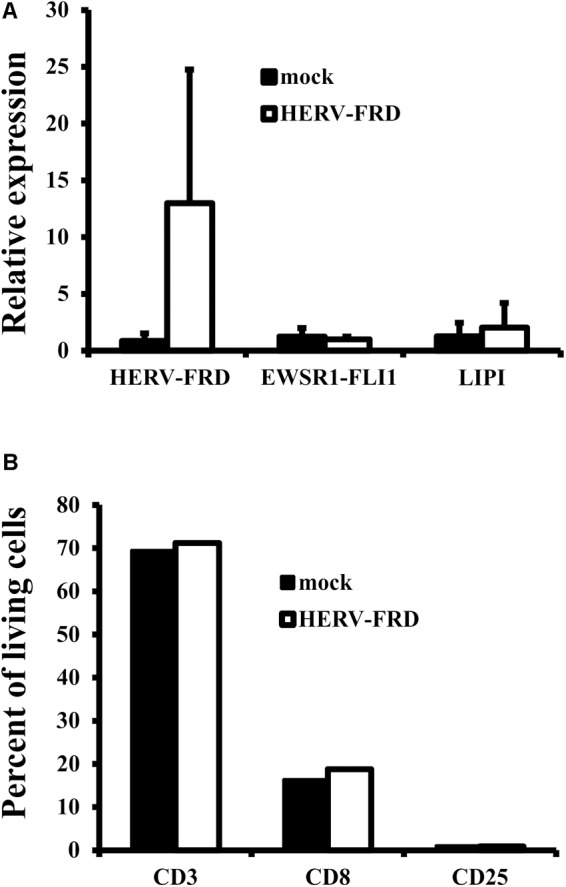
Absence of immunomodulatory effects of HERV-FRD transfected A673 cells. A673 cells were transfected with HERV-FRD or with empty vector (mock). (A) RNA from transfected cells were analyzed for presence of HERV-FRD and the Ewing sarcoma specific transcripts EWSR1-FLI1 and LIPI. (B) Transfected cells were used as stimulatory cells in mixed lymphocyte/tumor cell cultures. Stimulated cells were analyzed for presence of T cells (CD3), cytotoxic T cells (CD8), and activated lymphocytes (CD25). Presented are means and standard deviations from three independent experiments.
Taking together, our study shows changes in gene expression profiles of hypoxia-mimetic CoCl2 treated human neuronal-like SH-SY5Y cells in contrast to untreated cells. Five genes were found to be strongly up-regulated: CLU, GPX3, IGF2, NEDD9, and SLC7A11. Three of them (CLU, GPX3, and SLC7A11) showed in the past some associations to MS. The identified ERV in the vicinity of NEDD9 might thus be involved in the association between hypoxia and MS.
Author Contributions
CB: data collection, data analysis, and interpretation, generating figures, and drafting the article. HN: part of data collection. FH and MK: conception of the work. MS: conception of the work, generating figures, and critical revision of the article. AE: conception of the work and final approval of the version to be published.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank the Wilhelm Roux Program of the Medical Faculty of the Martin Luther University of Halle-Wittenberg (FKZ 28/45) for the kind support for our studies.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2018.00287/full#supplementary-material
References
- Abramova N., Charniga C., Goderie S. K., Temple S. (2005). Stage-specific changes in gene expression in acutely isolated mouse CNS progenitor cells. Dev. Biol. 283 269–281. 10.1016/j.ydbio.2005.03.040 [DOI] [PubMed] [Google Scholar]
- Altschul S. F., Wootton J. C., Gertz E. M., Agarwala R., Morgulis A., Schäffer A. A., et al. (2005). Protein database searches using compositionally adjusted substitution matrices. FEBS J. 272 5101–5109. 10.1111/j.1742-4658.2005.04945.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antony J. M., Van Marle G., Opii W., Butterfield D. A., Mallet F., Yong V. W., et al. (2004). Human endogenous retrovirus glycoprotein–mediated induction of redox reactants causes oligodendrocyte death and demyelination. Nat. Neurosci. 7 1088–1095. 10.1038/nn1319 [DOI] [PubMed] [Google Scholar]
- Aquino J. B., Marmigère F., Lallemend F., Lundgren T. K., Villar M. J., Wegner M., et al. (2008). Differential expression and dynamic changes of murine NEDD9 in progenitor cells of diverse tissues. Gene Expr. Patterns 8 217–226. 10.1016/j.gep.2008.01.001 [DOI] [PubMed] [Google Scholar]
- Auer R. N., Rowlands C. G., Perry S. F., Remmers J. E. (1995). Multiple sclerosis with medullary plaques and fatal sleep apnea (Ondine’s curse). Clin. Neuropathol. 15 101–105. [PubMed] [Google Scholar]
- Avissar N., Ornt D. B., Yagil Y., Horowitz S., Watkins R. H., Kerl E. A., et al. (1994). Human kidney proximal tubules are the main source of plasma glutathione peroxidase. Am. J. Physiol. 266 C367–C375. 10.1152/ajpcell.1994.266.2.C367 [DOI] [PubMed] [Google Scholar]
- Balada E., Ordi-Ros J., Vilardell-Tarre′s M. (2009). Molecular mechanisms mediated by human endogenous retroviruses (HERVs) in autoimmunity. Rev. Med. Virol. 19 273–286. 10.1002/rmv.622 [DOI] [PubMed] [Google Scholar]
- Barrett C. W., Ning W., Chen X., Smith J. J., Washington M. K., Hill K. E., et al. (2013). Tumor suppressor function of the plasma glutathione peroxidase gpx3 in colitis-associated carcinoma. Cancer Res. 73 1245–1255. 10.1158/0008-5472.CAN-12-3150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergman D., Halje M., Nordin M., Engström W. (2013). Insulin-like growth factor 2 in development and disease: a mini-review. Gerontology 59 240–249. 10.1159/000343995 [DOI] [PubMed] [Google Scholar]
- Biedler J. L., Helson L., Spengler B. A. (1973). Morphology and growth, tumorigenicity, and cytogenetics of human neuroblastoma cells in continuous culture. Cancer Res. 33 2643–2652. [PubMed] [Google Scholar]
- Blaise S., de Parseval N., Bénit L., Heidmann T. (2003). Genomewide screening for fusogenic human endogenous retrovirus envelopes identifies syncytin 2, a gene conserved on primate evolution. Proc. Natl. Acad. Sci. U.S.A. 100 13013–13018. 10.1073/pnas.2132646100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blond J. L., Besème F., Duret L., Bouton O., Bedin F., Perron H., et al. (1999). Molecular characterization and placental expression of HERV-W, a new human endogenous retrovirus family. J. Virol. 73 1175–1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth D. R., Parnell G. P. (2017). The multiple sclerosis genetic risk factors indicate both acquired and innate immune cell subsets contribute to MS pathogenesis and identify novel therapeutic opportunities. Front. Immunol. 8:425. 10.3389/fimmu.2017.00425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bros M., Boissel J. P., Gödtel-Armbrust U., Förstermann U. (2006). Transcription of human neuronal nitric oxide synthase mRNAs derived from different first exons is partly controlled by exon 1-specific promoter sequences. Genomics 87 463–473. 10.1016/j.ygeno.2005.11.013 [DOI] [PubMed] [Google Scholar]
- Browne P., Chandraratna D., Angood C., Tremlett H., Baker C., Taylor B. V., et al. (2014). Atlas of multiple sclerosis 2013: a growing global problem with widespread inequity. Neurology 83 1022–1024. 10.1212/WNL.0000000000000768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brudek T., Christensen T., Aagaard L., Petersen T., Hansen H. J., Møller-Larsen A. (2009). B cells and monocytes from patients with active multiple sclerosis exhibit increased surface expression of both HERV-H Env and HERV-W Env, accompanied by increased seroreactivity. Retrovirology 6:104. 10.1186/1742-4690-6-104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brütting C., Emmer A., Kornhuber M., Staege M. S. (2016). A survey of endogenous retrovirus (ERV) sequences in the vicinity of multiple sclerosis (MS)-associated single nucleotide polymorphisms (SNPs). Mol. Biol. Rep. 43 827–836. 10.1007/s11033-016-4004-0 [DOI] [PubMed] [Google Scholar]
- Chen B., Rao X., House M. G., Nephew K. P., Cullen K. J., Guo Z. (2011). GPx3 promoter hypermethylation is a frequent event in human cancer and is associated with tumorigenesis and chemotherapy response. Cancer Lett. 309 37–45. 10.1016/j.canlet.2011.05.013 [DOI] [PubMed] [Google Scholar]
- Christensen T. (2010). HERVs in neuropathogenesis. J. Neuroimmune Pharmacol. 5 326–335. 10.1007/s11481-010-9214-y [DOI] [PubMed] [Google Scholar]
- Chu F. F., Esworthy R. S., Doroshow J. H., Doan K., Liu X. F. (1992). Expression of plasma glutathione peroxidase in human liver in addition to kidney, heart, lung, and breast in humans and rodents. Blood 79 3233–3238. [PubMed] [Google Scholar]
- De la Hera B., Varadé J., García-Montojo M., Alcina A., Fedetz M., Alloza I., et al. (2014). Human endogenous retrovirus HERV-Fc1 association with multiple sclerosis susceptibility: a meta-analysis. PLoS One 9:e90182. 10.1371/journal.pone.0090182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Parseval N., Casella J. F., Gressin L., Heidmann T. (2001). Characterization of the three HERV-H proviruses with an open envelope reading frame encompassing the immunosuppressive domain and evolutionary history in primates. Virology 279 558–569. 10.1006/viro.2000.0737 [DOI] [PubMed] [Google Scholar]
- de Parseval N., Heidmann T. (2005). Human endogenous retroviruses: from infectious elements to human genes. Cytogenet. Genome Res. 110 318–332. 10.1159/000084964 [DOI] [PubMed] [Google Scholar]
- Dolei A. (2006). Endogenous retroviruses and human disease. Expert Rev. Clin. Immunol. 2 149–167. 10.1586/1744666X.2.1.149 [DOI] [PubMed] [Google Scholar]
- Dupressoir A., Lavialle C., Heidmann T. (2012). From ancestral infectious retroviruses to bona fide cellular genes: role of the captured syncytins in placentation. Placenta 33 663–671. 10.1016/j.placenta.2012.05.005 [DOI] [PubMed] [Google Scholar]
- Emerman M., Malik H. S. (2010). Paleovirology—modern consequences of ancient viruses. PLoS Biol. 8:e1000301. 10.1371/journal.pbio.1000301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emmer A., Staege M. S., Kornhuber M. E. (2014). The retrovirus/superantigen hypothesis of multiple sclerosis. Cell. Mol. Neurobiol. 34 1087–1096. 10.1007/s10571-014-0100-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engström W., Shokrai A., Otte K., Granerus M., Gessbo A., Bierke P., et al. (1998). Transcriptional regulation and biological significance of the insulin like growth factor II gene. Cell Proliferat. 31 173–189. 10.1111/j.1365-2184.1998.tb01196.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer B. H., Marks M., Reich T. (1983). Hyperbaric-oxygen treatment of multiple sclerosis: a randomized, placebo-controlled, double-blind study. N. Engl. J. Med. 308 181–186. 10.1056/NEJM198301273080402 [DOI] [PubMed] [Google Scholar]
- Fischer M. T., Sharma R., Lim J. L., Haider L., Frischer J. M., Drexhage J., et al. (2012). NADPH oxidase expression in active multiple sclerosis lesions in relation to oxidative tissue damage and mitochondrial injury. Brain 135 886–899. 10.1093/brain/aws012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foell J. L., Volkmer I., Giersberg C., Kornhuber M., Horneff G., Staege M. S. (2008). Loss of detectability of Charcot-Leyden crystal protein transcripts in blood cells after treatment with dimethyl sulfoxide. J. Immunol. Methods 339 99–103. 10.1016/j.jim.2008.08.006 [DOI] [PubMed] [Google Scholar]
- Gatti L., Zunino F. (2005). Overview of tumor cell chemoresistance mechanisms. Methods Mol. Med. 111 127–148. 10.1385/1-59259-889-7:127 [DOI] [PubMed] [Google Scholar]
- Giannoukakis N., Deal C., Paquette J., Goodyer C. G., Polychronakos C. (1993). Parental genomic imprinting of the human IGF2 gene. Nat. Genet. 4 98–101. 10.1038/ng0593-98 [DOI] [PubMed] [Google Scholar]
- Giard D. J., Aaronson S. A., Todaro G. J., Arnstein P., Kersey J. H., Dosik H., et al. (1973). In vitro cultivation of human tumors: establishment of cell lines derived from a series of solid tumors. J. Natl. Cancer Inst. 51 1417–1423. 10.1093/jnci/51.5.1417 [DOI] [PubMed] [Google Scholar]
- Goering W., Schmitt K., Dostert M., Schaal H., Deenen R., Mayer J., et al. (2015). Human endogenous retrovirus HERV-K (HML-2) activity in prostate cancer is dominated by a few loci. Prostate 75 1958–1971. 10.1002/pros.23095 [DOI] [PubMed] [Google Scholar]
- Guido V., Musaro V., San Millan D., Ghika J. A., Fishman D., Dayer E., et al. (2015). Serum and urine clusterin levels are elevated in stroke patients. J. Neurol. Sci. 357 e379–e380. 10.1016/j.jns.2015.08.1352 [DOI] [Google Scholar]
- Hayward A., Katzourakis A. (2015). Endogenous retroviruses. Curr. Biol. 25 R644–R646. 10.1016/j.cub.2015.05.041 [DOI] [PubMed] [Google Scholar]
- Hedborg F., Holmgren L., Sandstedt B., Ohlsson R. (1994). The cell type-specific IGF2 expression during early human development correlates to the pattern of overgrowth and neoplasia in the Beckwith-Wiedemann syndrome. Am. J. Pathol. 145 802–817. [PMC free article] [PubMed] [Google Scholar]
- Hemmer B., Kieseier B., Cepok S., Hartung H. P. (2003). New immunopathologic insights into multiple sclerosis. Curr. Neurol. Neurosci. 3 246–255. 10.1007/s11910-003-0085-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoennscheidt C., Max D., Richter N., Staege M. S. (2009). Expression of CD4 on Epstein–Barr virus-immortalized B cells. Scand. J. Immunol. 70 216–225. 10.1111/j.1365-3083.2009.02286.x [DOI] [PubMed] [Google Scholar]
- Islam T., Gauderman W. J., Cozen W., Mack T. M. (2007). Childhood sun exposure influences risk of multiple sclerosis in monozygotic twins. Neurology 69 381–388. 10.1212/01.wnl.0000268266.50850.48 [DOI] [PubMed] [Google Scholar]
- Jern P., Coffin J. M. (2008). Effects of retroviruses on host genome function. Annu. Rev. Genet. 42 709–732. 10.1146/annurev.genet.42.110807.091501 [DOI] [PubMed] [Google Scholar]
- Jones S. E., Jomary C. (2002). Clusterin. Int. J. Biochem. Cell Biol. 34 427–431. 10.1016/S1357-2725(01)00155-8 [DOI] [PubMed] [Google Scholar]
- Kemp K., Gray E., Wilkins A., Scolding N. (2012). Purkinje cell fusion and binucleate heterokaryon formation in multiple sclerosis cerebellum. Brain 135 2962–2972. 10.1093/brain/aws226 [DOI] [PubMed] [Google Scholar]
- Kewitz S., Staege M. S. (2013). Expression and regulation of the endogenous retrovirus 3 (ERV3) in Hodgkin’s lymphoma cells. Front. Oncol. 3:179. 10.3389/fonc.2013.00179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S. H., Xia D., Kim S. W., Holla V., Menter D. G., DuBois R. N. (2010). Human enhancer of filamentation 1 is a mediator of hypoxia-inducible factor-1α–mediated migration in colorectal carcinoma cells. Cancer Res. 70 4054–4063. 10.1158/0008-5472.CAN-09-2110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Küçükali C. Ý., Kürtüncü M., Çoban A., Çebi M., Tüzün E. (2015). Epigenetics of multiple sclerosis: an updated review. Neuromol. Med. 17 83–96. 10.1007/s12017-014-8298-6 [DOI] [PubMed] [Google Scholar]
- Kulkarni A., Mateus M., Thinnes C. C., McCullagh J. S., Schofield C. J., Taylor G. P., et al. (2017). Glucose metabolism and oxygen availability govern reactivation of the latent human retrovirus HTLV-1. Cell Chem. Biol. 24 1377.e3–1387.e3. 10.1016/j.chembiol.2017.08.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lander E. S., Linton L. M., Birren B., Nusbaum C., Zody M. C., Baldwin J., et al. (2001). Initial sequencing and analysis of the human genome. Nature 409 860–921. 10.1038/35057062 [DOI] [PubMed] [Google Scholar]
- Lassmann H. (2003). Hypoxia-like tissue injury as a component of multiple sclerosis lesions. J. Neurol. Sci. 206 187–191. 10.1016/S0022-510X(02)00421-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavillette D., Marin M., Ruggieri A., Mallet F., Cosset F. L., Kabat D. (2002). The envelope glycoprotein of human endogenous retrovirus type W uses a divergent family of amino acid transporters/cell surface receptors. J. Virol. 76 6442–6452. 10.1128/JVI.76.13.6442-6452.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Grupe A., Rowland C., Holmans P., Segurado R., Abraham R., et al. (2008). Evidence that common variation in NEDD9 is associated with susceptibility to late-onset Alzheimer’s and Parkinson’s disease. Hum. Mol. Genet. 17 759–767. 10.1093/hmg/ddm348 [DOI] [PubMed] [Google Scholar]
- Lieury A., Chanal M., Androdias G., Reynolds R., Cavagna S., Giraudon P., et al. (2014). Tissue remodeling in periplaque regions of multiple sclerosis spinal cord lesions. Glia 62 1645–1658. 10.1002/glia.22705 [DOI] [PubMed] [Google Scholar]
- Liu X. X., Li X. J., Zhang B., Liang Y. J., Zhou C. X., Cao D. X., et al. (2011). MicroRNA-26b is underexpressed in human breast cancer and induces cell apoptosis by targeting SLC7A11. FEBS Lett. 585 1363–1367. 10.1016/j.febslet.2011.04.018 [DOI] [PubMed] [Google Scholar]
- Livak K. J., Schmittgen T. D. (2001). Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT method. Methods 25 402–408. 10.1006/meth.2001.1262 [DOI] [PubMed] [Google Scholar]
- Livingstone C. (2013). IGF2 and cancer. Endocr. Relat. Cancer 20 R321–R339. 10.1530/ERC-13-0231 [DOI] [PubMed] [Google Scholar]
- Malassiné A., Blaise S., Handschuh K., Lalucque H., Dupressoir A., Evain-Brion D., et al. (2007). Expression of the fusogenic HERV-FRD Env glycoprotein (syncytin 2) in human placenta is restricted to villous cytotrophoblastic cells. Placenta 28 185–191. 10.1016/j.placenta.2006.03.001 [DOI] [PubMed] [Google Scholar]
- Malfavon-Borja R., Feschotte C. (2015). Fighting fire with fire: endogenous retrovirus envelopes as restriction factors. J. Virol. 89 4047–4050. 10.1128/JVI.03653-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mameli G., Poddighe L., Mei A., Uleri E., Sotgiu S., Serra C., et al. (2012). Expression and activation by Epstein Barr virus of human endogenous retroviruses-W in blood cells and astrocytes: inference for multiple sclerosis. PLoS One 7:e44991. 10.1371/journal.pone.0044991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Rendon E., Hale S. J., Ryan D., Baban D., Forde S. P., Roubelakis M., et al. (2007). Transcriptional profiling of human cord blood CD133+ and cultured bone marrow mesenchymal stem cells in response to hypoxia. Stem Cells 25 1003–1012. 10.1634/stemcells.2006-0398 [DOI] [PubMed] [Google Scholar]
- Mason M. J., Speake C., Gersuk V. H., Nguyen Q. A., O’Brien K. K., Odegard J. M., et al. (2014). Low HERV-K (C4) copy number is associated with type 1 diabetes. Diabetes Metab. Res. Rev. 63 1789–1795. 10.2337/db13-1382 [DOI] [PubMed] [Google Scholar]
- Max D., Kühnöl C. D., Burdach S., Niu L., Staege M. S., Föll J. L. (2014). Indoleamine-2,3-dioxygenase in an immunotherapy model for Ewing sarcoma. Anticancer Res. 34 6431–6441. [PubMed] [Google Scholar]
- Mi S., Lee X., Li X., Veldman G. M., Finnerty H., Racie L., et al. (2000). Syncytin is a captive retroviral envelope protein involved in human placental morphogenesis. Nature 403 785–789. 10.1038/35001608 [DOI] [PubMed] [Google Scholar]
- Nuutinen T., Suuronen T., Kauppinen A., Salminen A. (2009). Clusterin: a forgotten player in Alzheimer’s disease. Brain Res. Rev. 61 89–104. 10.1016/j.brainresrev.2009.05.007 [DOI] [PubMed] [Google Scholar]
- Okuno S., Sato H., Kuriyama-Matsumura K., Tamba M., Wang H., Sohda S., et al. (2003). Role of cystine transport in intracellular glutathione level and cisplatin resistance in human ovarian cancer cell lines. Br. J. Cancer 88 951–956. 10.1038/sj.bjc.6600786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pender M. P., Greer J. M. (2007). Immunology of multiple sclerosis. Curr. Allergy Asthma Rep. 7 285–292. 10.1007/s11882-007-0043-x [DOI] [PubMed] [Google Scholar]
- Pérot P., Mugnier N., Montgiraud C., Gimenez J., Jaillard M., Bonnaud B., et al. (2012). Microarray-based sketches of the HERV transcriptome landscape. PLoS One 7:e40194. 10.1371/journal.pone.0040194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perron H., Hamdani N., Faucard R., Lajnef M., Jamain S., Daban-Huard C., et al. (2012). Molecular characteristics of human endogenous retrovirus type-W in schizophrenia and bipolar disorder. Transl. Psychiatry 2:e201. 10.1038/tp.2012.125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perron H., Lang A. (2010). The human endogenous retrovirus link between genes and environment in multiple sclerosis and in multifactorial diseases associating neuroinflammation. Clin. Rev. Allergy Immunol. 39 51–61. 10.1007/s12016-009-8170-x [DOI] [PubMed] [Google Scholar]
- Qi X., Ng K. T. P., Lian Q. Z., Liu X. B., Li C. X., Geng W., et al. (2014). Clinical significance and therapeutic value of glutathione peroxidase 3 (GPx3) in hepatocellular carcinoma. Oncotarget 5 11103–11120. 10.18632/oncotarget.2549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renard M., Varela P. F., Letzelter C., Duquerroy S., Rey F. A., Heidmann T. (2005). Crystal structure of a pivotal domain of human syncytin-2, a 40 million years old endogenous retrovirus fusogenic envelope gene captured by primates. J. Mol. Biol. 352 1029–1034. 10.1016/j.jmb.2005.07.058 [DOI] [PubMed] [Google Scholar]
- Reuter D., Staege M. S., Kühnöl C. D., Föll J. (2015). Immunostimulation by OX40 ligand transgenic Ewing sarcoma cells. Front. Oncol. 5:242. 10.3389/fonc.2015.00242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice P., Longden I., Bleasby A. (2000). EMBOSS: the European molecular biology open software suite. Trends Genet. 16 276–277. 10.1016/S0168-9525(00)02024-2 [DOI] [PubMed] [Google Scholar]
- Robert S. M., Buckingham S. C., Campbell S. L., Robel S., Holt K. T., Ogunrinu-Babarinde T., et al. (2015). SLC7A11 expression is associated with seizures and predicts poor survival in patients with malignant glioma. Sci. Transl. Med. 7:289ra86. 10.1126/scitranslmed.aaa8103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sankavaram S. R., Svensson M. A., Olsson T., Brundin L., Johansson C. B. (2015). Cell fusion along the anterior-posterior neuroaxis in mice with experimental autoimmune encephalomyelitis. PLoS One 10:e0133903. 10.1371/journal.pone.0133903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato H., Tamba M., Kuriyama-Matsumura K., Okuno S., Bannai S. (2000). Molecular cloning and expression of human xCT, the light chain of amino acid transport system xc. Antioxid. Redox Signal. 2 665–671. 10.1089/ars.2000.2.4-665 [DOI] [PubMed] [Google Scholar]
- Schrijvers E. M., Koudstaal P. J., Hofman A., Breteler M. M. (2011). Plasma clusterin and the risk of Alzheimer disease. JAMA 305 1322–1326. 10.1001/jama.2011.381 [DOI] [PubMed] [Google Scholar]
- Shagisultanova E., Gaponova A. V., Gabbasov R., Nicolas E., Golemis E. A. (2015). Preclinical and clinical studies of the NEDD9 scaffold protein in cancer and other diseases. Gene 567 1–11. 10.1016/j.gene.2015.04.086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shannan B., Seifert M., Leskov K., Willis J., Boothman D., Tilgen W., et al. (2006). Challenge and promise: roles for clusterin in pathogenesis, progression and therapy of cancer. Cell Death Differ. 13 12–19. 10.1038/sj.cdd.4401779 [DOI] [PubMed] [Google Scholar]
- Singh M. K., Cowell L., Seo S., O’Neill G. M., Golemis E. A. (2007). Molecular basis for HEF1/NEDD9/Cas-L action as a multifunctional co-ordinator of invasion, apoptosis and cell cycle. Cell Biochem. Biophys. 48 54–72. 10.1007/s12013-007-0036-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soygur B., Moore H. (2016). Expression of Syncytin 1 (HERV-W), in the preimplantation human blastocyst, embryonic stem cells and trophoblast cells derived in vitro. Hum. Reprod. 31 1455–1461. 10.1093/humrep/dew097 [DOI] [PubMed] [Google Scholar]
- Staege M. S., Hansen G., Baersch G., Burdach S. (2004). Functional and molecular characterization of interleukin-2 transgenic Ewing tumor cells for in vivo immunotherapy. Pediatr. Blood Cancer 43 23–34. 10.1002/pbc.20013 [DOI] [PubMed] [Google Scholar]
- Tham D. M., Whitin J. C., Kim K. K., Zhu S. X., Cohen H. J. (1998). Expression of extracellular glutathione peroxidase in human and mouse gastrointestinal tract. Am. J. Physiol. 275 G1463–G1471. 10.1152/ajpgi.1998.275.6.G1463 [DOI] [PubMed] [Google Scholar]
- Trapp B. D., Stys P. K. (2009). Virtual hypoxia and chronic necrosis of demyelinated axons in multiple sclerosis. Lancet Neurol. 8 280–291. 10.1016/S1474-4422(09)70043-2 [DOI] [PubMed] [Google Scholar]
- Trougakos I. P., Gonos E. S. (2006). Regulation of clusterin/apolipoprotein J, a functional homologue to the small heat shock proteins, by oxidative stress in ageing and age-related diseases. Free Radic. Res. 40 1324–1334. 10.1080/10715760600902310 [DOI] [PubMed] [Google Scholar]
- Tselis A. (2011). Evidence for viral etiology of multiple sclerosis. Semin. Neurol. 31 307–316. 10.1055/s-0031-1287656 [DOI] [PubMed] [Google Scholar]
- van Luijn M. M., van Meurs M., Stoop M. P., Verbraak E., Wierenga-Wolf A. F., Melief M. J., et al. (2015). Elevated expression of the cerebrospinal fluid disease markers chromogranin A and clusterin in astrocytes of multiple sclerosis white matter lesions. J. Neuropathol. Exp. Neurol. 75 86–98. 10.1093/jnen/nlv004 [DOI] [PubMed] [Google Scholar]
- Vargas A., Moreau J., Landry S., LeBellego F., Toufaily C., Rassart E., et al. (2009). Syncytin-2 plays an important role in the fusion of human trophoblast cells. J. Mol. Biol. 392 301–318. 10.1016/j.jmb.2009.07.025 [DOI] [PubMed] [Google Scholar]
- Verrey F., Closs E. I., Wagner C. A., Palacin M., Endou H., Kanai Y. (2004). CATs and HATs: the SLC7 family of amino acid transporters. Pflugers Arch. 447 532–542. 10.1007/s00424-003-1086-z [DOI] [PubMed] [Google Scholar]
- Vogel T., Ahrens S., Büttner N., Krieglstein K. (2010). Transforming growth factor β promotes neuronal cell fate of mouse cortical and hippocampal progenitors in vitro and in vivo: identification of Nedd9 as an essential signaling component. Cereb. Cortex 20 661–671. 10.1093/cercor/bhp134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstein G., Beiser A. S., Preis S. R., Courchesne P., Chouraki V., Levy D., et al. (2016). Plasma clusterin levels and risk of dementia, Alzheimer’s disease, and stroke. Alzheimers Dement. 3 103–109. 10.1016/j.dadm.2016.06.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wildschutte J. H., Williams Z. H., Montesion M., Subramanian R. P., Kidd J. M., Coffin J. M. (2016). Discovery of unfixed endogenous retrovirus insertions in diverse human populations. Proc. Natl. Acad. Sci. U.S.A. 113 E2326–E2334. 10.1073/pnas.1602336113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson D. A., Mager D. L., Leong J.-A. C. (1994). “Endogenous human retroviruses,” in The Retroviridae ed. Levy J. A. (New York, NY: Springer; ) 465–535. [Google Scholar]
- Winkler C., Steingrube D. S., Altermann W., Schlaf G., Max D., Kewitz S., et al. (2012). Hodgkin’s lymphoma RNA-transfected dendritic cells induce cancer/testis antigen-specific immune responses. Cancer Immunol. Immunother. 61 1769–1779. 10.1007/s00262-012-1239-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z. L., Yang L., Zou Q., Yuan Y., Li J., Liang L., et al. (2013). Positive ALDH1A3 and negative GPX3 expressions are biomarkers for poor prognosis of gallbladder cancer. Dis. Markers 35 163–172. 10.1155/2013/187043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X., Yang J. J., Kim Y. S., Kim K. Y., Ahn W. S., Yang S. (2010). An 8-gene signature, including methylated and down-regulated glutathione peroxidase 3, of gastric cancer. Int. J. Oncol. 36 405–414. [PubMed] [Google Scholar]
- Zhou J. D., Yao D. M., Zhang Y. Y., Ma J. C., Wen X. M., Yang J., et al. (2015). GPX3 hypermethylation serves as an independent prognostic biomarker in non-M3 acute myeloid leukemia. Am. J. Cancer Res. 5 2047–2055. [PMC free article] [PubMed] [Google Scholar] [Retracted]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


