Abstract
In 2012, the Food and Drug Administration (FDA) approved the use of F-18 florbetapir to estimate β-amyloid neuritic plaque density when indicated. A normal scan will show increased radiotracer uptake in the white matter. Mild uptake in salivary glands, skin, muscles, and bones is considered normal. Being a new and infrequently performed study, familiarity with normal biodistribution and variants is important. We hereby present 2 cases with F-18 florbetapir uptake in lacrimal glands. Patients had no symptoms or known systemic conditions to explain this uptake. We speculate that lacrimal gland uptake of F-18 florbetapir could represent a normal variant.
Keywords: F-18 florbetapir, Amyloid, PET/CT, Lacrimal gland, Normal variation
Introduction
β-Amyloid neuritic plaques are extracellular deposits of the insoluble neurotoxic β-amyloid protein. These plaques are seen in cortical gray matter of patients with Alzheimer disease (AD) [1]. Positron emission tomography (PET) amyloid imaging allows assessment of density and distribution of amyloid neuritic plaques in cortical gray matter [2], [3].
F-18 florbetapir is 1 of 3 F-18-labeled amyloid PET radiopharmaceuticals approved by the U.S. Food and Drug Administration and the European Medicines Agency [4], [5], [6]. Distribution of F-18 florbetapir in the absence of amyloid deposition results in a clear gray matter-white matter contrast. Positive scans, on the other hand, show cortical uptake of radiotracer resulting in reduction or loss of the distinct contrast between gray and white matter [5]. A normal amyloid PET scan has a high negative predictive value, reported to be 96% with florbetaben [7], and almost excludes the diagnosis of AD in a patient with cognitive impairment [5]. A positive scan, however, is not specific and can be seen with other types of neurologic conditions as well as in older people with normal cognition [5].
The recommended dose of 18F-florbetapir is 370 MBq (10 mCi) in a maximum volume of 10 mL administered as an intravenous bolus, followed by a saline flush [3]. Recommended uptake time is 30-50 minutes [8] and images should be acquired for 10 minutes [5], [6].
As imaging findings of amyloid PET scan have prognostic value and may alter the treatment, recognition of normal variations is very important. Here, we report 2 cases of lacrimal gland uptake of 18F-florbetapir.
Case reports
Case 1
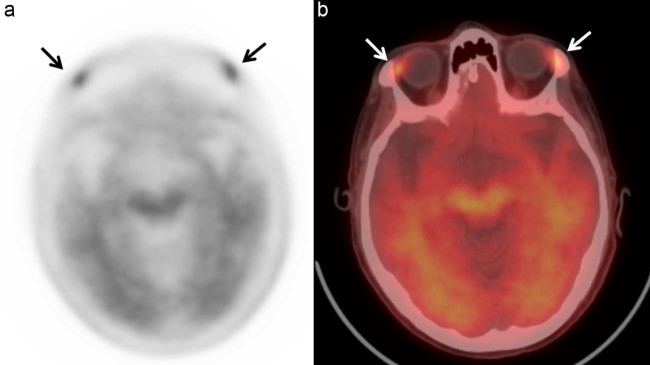
A 70-year-old woman with progressive memory and language impairment was referred for amyloid PET/CT scan. Medical history was unremarkable except for gastroesophageal reflux disease and osteopenia. F-18 florbetapir PET/CT scan showed diffuse and symmetric cortical uptake of radiotracer in the gray matter of frontal, temporal, parietal, and occipital lobes, indicating deposition of amyloid neuritic plaques. There was also moderate to intense radiotracer uptake within the lacrimal glands bilaterally (Fig. 1). The patient had no relevant clinical signs or symptoms.
Fig. 1.
Bilateral lacrimal gland uptake: (A) Axial attenuation corrected image of the brain and (B) fused PET/CT axial image acquired 36 minutes after intravenous injection of 10.7 mCi of F-18 florbetapir demonstrate intense radiotracer uptake in the lacrimal glands bilaterally (arrows). PET/CT, positron emission tomography/computed tomography.
Case 2
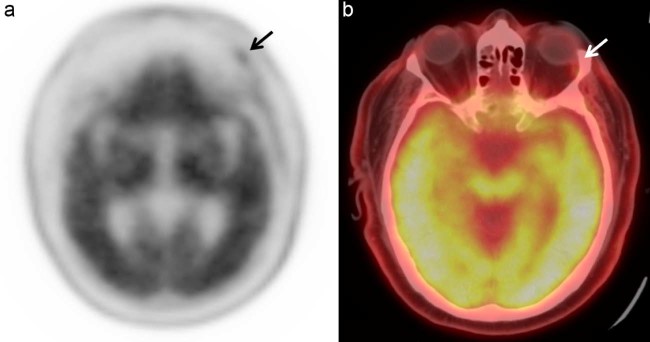
A 67-year-old man had progressive cognitive and behavioral changes. He had a past medical history of prostate cancer. F-18 florbetapir PET/CT scan showed diffuse uptake of radiopharmaceutical in the gray matter and subsequent loss of gray-white distinction, compatible with moderate to frequent β-amyloid neuritic plaque deposition. Moreover, there was mild to moderate radiotracer uptake unilaterally in the left lacrimal gland (Fig. 2). Again, the patient had no positive ophthalmologic signs or symptoms.
Fig. 2.
Unilateral lacrimal gland uptake: (A.) Axial attenuation corrected image of the brain and (B) fused PET/CT axial image acquired 31 minutes after intravenous injection of 10.7 mCi of F-18 florbetapir demonstrates diffuse cortical uptake compatible with moderate to frequent β-amyloid neuritic plaques and mild uptake in the left lacrimal gland (arrow). PET/CT, positron emission tomography/computed tomography.
Discussion
Amyloid PET/CT has revolutionized the workup and management of patients with cognitive impairment. Imaging findings have been reported to change diagnosis or management plans in up to 68% of patients [9], [10]. Amyloid imaging also has prognostic value as it helps predict the risk of subsequent cognitive decline in patients with mild cognitive impairment and cognitively normal older adults [11]. For interpretation of amyloid PET scans, knowledge of physiological distribution and normal variations is important.
In a normal scan, the highest level of F-18 florbetapir uptake is in the white matter [3]. Radiotracer uptake in the scalp, salivary glands, muscles, and bones has been described and is considered normal biodistribution [6]. β-Amyloid neuritic plaques do accumulate in the lacrimal glands in humans, and this could explain the binding of amyloid PET agents to lacrimal glands in patients with AD [12]. The exact molecular mechanism of binding of amyloid PET agents to amyloid plaques is not fully understood, but molecular dynamic simulations suggest that the agent is placed in surface grooves along the amyloid fibril axis [13]. This model provides an alternate hypothesis of nonspecific accumulation of PET amyloid agents within the salivary glands. C-11 Pittsburg compound B (C-11 PiB) has been reported to bind to lacrimal glands in a mouse model of Alzheimer's [14]. There are no human reports of lacrimal gland uptake with any of the Food and Drug Administration-approved amyloid PET agents: florbetapir, flutemetamol, or florbetaben. Other radiopharmaceuticals that have been reported or are known to show lacrimal gland uptake are gallium (Ga)-67 citrate [15], Tc99m-exametazime (HMPAO) [16], Ga-68 PSMA agents PSMA-11 (HBED-CC) [17] and DCFPyl [18], as well as sodium iodide, and pertechnetate due to presence of sodium iodide symporters in the lacrimal gland [19]. Although bilateral lacrimal gland uptake of radiotracers can be common and nonspecific, unilateral lacrimal gland uptake of radiotracers such as Ga-67 is favored to represent a focal process such as inflammation or tumor as reported in a case of lymphoma involvement of the lacrimal gland [20]. According to our literature search, F-18 florbetapir uptake has not been described previously in the lacrimal glands, neither bilaterally nor unilaterally. F-18 florbetapir uptake in the lacrimal glands could represent a normal variant. Other possible etiologies could include inflammation, neoplasm, systemic diseases such as sarcoidosis and amyloidosis, or early deposition of amyloid protein secondary to Alzheimer's disease.
Footnotes
Competing Interests: All authors declare that they have no potential conflict of interest to disclose.
References
- 1.Querfurth H.W., LaFerla F.M. Mechanisms of disease: Alzheimer's disease. N Engl J Med. 2010;362:329–344. doi: 10.1056/NEJMra0909142. [DOI] [PubMed] [Google Scholar]
- 2.Mathis C.A., Mason N.S., Lopresti B.J., Klunk W.E. Development of positron emission tomography b-amyloid plaque imaging agents. Semin Nucl Med. 2012;42:423–432. doi: 10.1053/j.semnuclmed.2012.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Trembath L., Newell M., Devous M.D., Sr Technical considerations in brain amyloid PET imaging with 18F-florbetapir. J Nucl Med Technol. 2015;43(3):175–184. doi: 10.2967/jnmt.115.156679. [DOI] [PubMed] [Google Scholar]
- 4.U.S. Food and Drug Administration, Center for Drug Evaluation and Research Amyvid (Florbetapir F 18 Injection) drug approval package summary review. 2012. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2012/202008Orig1s000SumR.pdf Approval date April 6, 2012. accessed 13.08.17.
- 5.Amyvid (florbetapir F 18 injection) for intravenous use. 2012. pi.lilly.com/us/amyvid-uspi.pdf Eli Lilly and Company website. Published; accessed 08.07.15; Revised December 2013.
- 6.EPAR summary for the public: Amyvid florbetapir (18F) 2012. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/002422/WC500137635.pdf European Medicines Agency website. accessed 08.07.15; Updated January 2013.
- 7.Seibyl J., Stephens A., Barthel H., Ishii K., Akatsu H., Murayama S. A negative florbetaben PET scan reliably excludes AD pathology as confirmed by histopathology. J Nucl Med. 2014;55(Suppl. 1):243. [Google Scholar]
- 8.Minoshima S., Drzezga A.E., Barthel H., Bohnen N., Djekidel M., Lewis D.H. SNMMI Procedure Standard/EANM Practice Guideline for Amyloid PET Imaging of the Brain 1.0. J Nucl Med. 2016;57(8):1316–1322. doi: 10.2967/jnumed.116.174615. [DOI] [PubMed] [Google Scholar]
- 9.Apostolova L.G., Haider J.M., Goukasian N., Rabinovici G.D., Chételat G., Ringman J.M. Critical review of the appropriate use criteria for amyloid imaging: effect on diagnosis and patient care. Alzheimers Dement (Amst) 2016;5:15–22. doi: 10.1016/j.dadm.2016.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Leuzy A., Chiotis K., Jelic V., Andersen P., Friman J., Lilja J. Investigating the clinical impact of [18F]flutemetamol PET in a tertiary memory clinic setting in patients with uncertain diagnosis. 2017. https://www.alz.org/aaic/releases_2017/AAIC17-Sun-PET-Scan-Release.pdf Alzheimer's Association International Conference 2017 London; Abstract 19062 / Proposal ID P1-357. accessed 13.08.17.
- 11.Doraiswamy P.M., Sperling R.A., Johnson K., Reiman E.M., Wong T.Z., Sabbagh M.N. AV45-A11 Study Group; AV45-A11 Study Group. Florbetapir F 18 amyloid PET and 36-month cognitive decline: a prospective multicenter study. Mol Psychiatry. 2014;19(9):1044–1051. doi: 10.1038/mp.2014.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bermejo-Pareja F., Antequera D., Vargas T., Molina J.A., Carro E. Saliva levels of Abeta1-42 as potential biomarker of Alzheimer's disease: a pilot study. BMC Neurol. 2010;10(1):108. doi: 10.1186/1471-2377-10-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Skeby K.K., Sørensen J., Schiøtt B. Identification of a common binding mode for imaging agents to amyloid fibrils from molecular dynamics simulations. J Am Chem Soc. 2013;135(40):15114–15128. doi: 10.1021/ja405530p. [DOI] [PubMed] [Google Scholar]
- 14.Manook A., Yousefi B.H., Willuweit A., Platzer S., Reder S., Voss A. Small-animal PET imaging of amyloid-beta plaques with [11C] PiB and its multi-modal validation in an APP/PS1 mouse model of Alzheimer's disease. PLoS ONE. 2012;7(3) doi: 10.1371/journal.pone.0031310. e31310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Love C., Palestro C.J. Altered biodistribution and incidental findings on gallium and labeled leukocyte/bone marrow scans. Semin Nucl Med. 2010;40(4):271–282. doi: 10.1053/j.semnuclmed.2010.03.004. [DOI] [PubMed] [Google Scholar]
- 16.Villanueva-Meyer J., Thompson D., Mena I., Marcus C.S. Lacrimal gland dosimetry for the brain imaging agent technetium-99m-HMPAO. J Nucl Med. 1990;31(7):1237–1239. [PubMed] [Google Scholar]
- 17.Demirci E., Sahin O.E., Ocak M., Akovali B., Nematyazar J., Kabasakal L. Normal distribution pattern and physiological variants of 68Ga-PSMA-11 PET/CT imaging. Nucl Med Commun. 2016;37(11):1169–1179. doi: 10.1097/MNM.0000000000000566. [DOI] [PubMed] [Google Scholar]
- 18.Szabo Z., Mena E., Rowe S.P., Plyku D., Nidal R., Eisenberger M.A. Initial evaluation of [18F] DCFPyL for prostate-specificmembrane antigen (PSMA)-targeted PET imaging of prostate cancer. Mol Imaging Biol. 2015;17(4):565–574. doi: 10.1007/s11307-015-0850-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Świętaszczyk C., Pilecki S.E. Enhanced accumulation of bone seekers at superior lateral orbital margin: potential origin. World J Nucl Med. 2014;13(1):3. doi: 10.4103/1450-1147.138567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Khoury J., Nassrala S., Loberant N., Jerushalmi J. vol. 33. WB Saunders; 2003. Gamut: unilateral orbital uptake on Ga-67 scintigraphy; pp. 331–333. (Seminars in nuclear medicine). No. 4. [DOI] [PubMed] [Google Scholar]