Abstract
A liposome-based amplified detection system is presented for the cancer cell secreted pathogenic enzyme matrix metalloproteinase-9 which does not require the use of biological antibodies.
Matrix metalloproteinases (MMPs) are a family of 26 Zn2+ and Ca2+ dependent enzymes capable of hydrolyzing the extracellular matrix under physiological pH.1 These enzymes are involved in the progression and metastasis of a large number of cancers, in cardiovascular diseases, stroke, periodontal diseases, etc.2–4 Amongst the isozymes, MMP-2 and -9 are overexpressed in metastatic cancers of breast, prostate, lungs, pancreas, ovary etc.2 Levels of these enzymes are prognostic markers for the disease.5
The expression of MMP-2 and -9 in diseased tissues is usually detected by ELISA, Western Blot analysis and zymography.6–8 Due to the built-in amplification strategy, ELISA can detect MMP-2 or -9 at a concentration of ng mL−1 or less.9 Recently a few other protocols for detecting MMPs have been reported which do not use anitibodies. Bioluminescence resonance energy transfer (BRET) between quantum dots and substrate conjugated luciferase was used to detect MMP-2 in low concentration as isolated enzymes, in mouse serum and in cell culture media (without the added fetal bovine serum, FBS).9–11 Changes in the spin–spin relaxation times of appropriately-functionalized superparamagnetic nanoparticles have been monitored to detect MMP-2 activity in cell culture media (without added FBS).12 These methods, although sensitive, are not continuous assays for the enzyme. An electrochemical detection method based on proteolytic beacon was shown to detect isolated, purified MMP-7 in low concentration.13
Liposomes have been extensively used as drug delivery vehicles.14–16 We have recently demonstrated that when liposomes incorporate triple-helical substrate lipopeptides, the encapsulated dye is released selectively in the presence of recombinant as well as cancer cell-secreted MMP-9.17,18 However, in presence of less than 100 nM MMP-9, the rate as well as the extent of dye release was low.18 We reasoned that if the enzyme horseradish peroxidase (HRP) is encapsulated in the liposomes and a chromogenic substrate for HRP is present in the buffer solution, the hydrolysis of the liposome-incorporated lipopeptides by MMP-9 will bring HRP into contact with its substrate. This will result in time-dependent enhancement of the absorption, akin to ELISA. Herein, we report our results demonstrating the proof-of-concept for this principle to detect MMP-9 employing o-phenylenediamine (OPD) as the chromogenic substrate for HRP (Scheme 1).
Scheme 1.
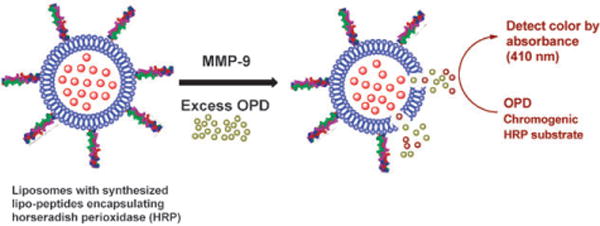
The amplified detection strategy for MMP-9.
We synthesized the triple-helical lipopeptide GPO4 [CH3(CH2)16CONH-GPQGIAGQR(GPO)4GG; O represents 4-(R)-hydroxyproline] as the substrate for MMP-9 employing a microwave-assisted, solid phase peptide synthesizer (see ESI†).18 In this lipopeptide, the MMP-9 cleavage site is the amide bond between G and I (indicated by an underline). The lipopeptide adopts a triple helical conformation in aqueous buffers and when incorporated in liposomes and serves as the recognition moiety for MMP-9.17 Since MMP-9 and -2 have very similar substrate selectivity, we anticipated that the lipopeptide substrate GPO4 will be cleaved by MMP-2 as well.19
We prepared liposomes (25 mM HEPES buffer, pH = 8.0) containing 30 mol% of GPO4 and varied the major lipid component. The enzyme HRP was encapsulated in the liposomes following a reported procedure.20 The HRP encapsulated liposomes were incubated with recombinant, human MMP-9 (2.3 μM) in 25 mM HEPES buffer (pH = 8.0) containing excess (200 μM) of the chromogenic substrate for HRP (OPD) and H2O2 (10 μM). The absorbance of the reaction was monitored at 410 nm. When the major lipid of the liposomes was 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine (POPC), the solution turned deep brown (Fig. 1, pink circles) within 5 min and a brown precipitate was observed (2,3-diaminophenazine).21 The color formation was also very rapid in the presence of 500 nM (Fig. 1, blue circles) and 200 nM (Fig. 1, olive circles) MMP-9. No increase in absorbance was observed in the absence of MMP-9 (red circles), or from the liposomes without the lipopeptide GPO4 in the presence (purple circles) and absence (green circles) of MMP-9. Changing the amount of lipopeptide in these liposomes to either 20 mol% or to 40 mol% led to the decrease in the rate of color formation. We do not have an explanation for this observation yet. However, we have previously observed that for the MMP-9 mediated release of liposome-encapsulated carboxyfluorescein, 30 mol% of GPO4 was optimal.18
Fig. 1.
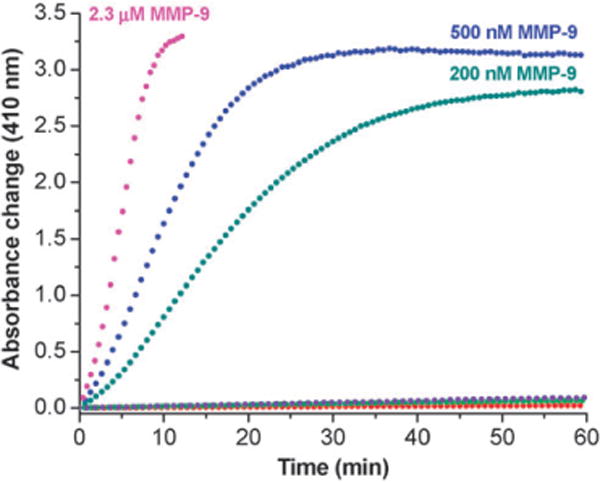
Absorbance changes (410 nm) of the assay system as a function of time. HRP encapsulated liposomes incorporating GPO4 were mixed with 2.3 μM (pink circles), 500 nM (blue circles) and 200 nm (olive circles) recombinant human MMP-9 in 25 mM HEPES buffer, pH = 8.0. No increase in absorbance was observed in the absence of MMP-9 (red circles), or from the liposomes without the lipopeptide GPO4 in the presence (purple circles) and absence (green circles) of MMP-9. All plots shown are the averages of six replicates with errors 5–10%.
We ascertained that recombinant MMP-9 is not inhibited by OPD and the enzyme activity is not impaired in the presence of 10 μM H2O2 (data not shown). Cumulatively, these results indicate that MMP-9 mediated cleavage of liposome-incorporated GPO4 destabilizes the liposomal membrane, bringing HRP in contact with its chromogenic substrate (OPD).
The concentration of active MMP-9 in normal tissues is about 10 nM.5 In some lung cancer patients, the concentration of MMP-9 in the bronchial lavage fluid was determined to be as high as 200 nM.22 In order to determine if our detection system is capable of detecting the MMP-9 concentrations found in cancerous as well as in normal tissues, we studied the time dependent enhancement of absorption at 410 nm in the presence of various concentrations of added recombinant, human MMP-9 (Fig. 2). As the concentration of the added enzyme was reduced, the maximum absorbance change progressively decreased. The initial lag phase for observing the change in absorbance also increased. For 10 nM MMP-9, no significant brown color formation was detected for the first 15 min of the assay. However, the color change was clearly discernable after 25–30 min. This time requirement is comparable to the ELISA-based protocols for the detection of MMP-9.6 For these experiments, the total assay volume was 200 μL. The recombinant MMP-9 with the catalytic and fibronectin domains has a molecular weight of 40 kDa. Hence, our method is capable of detecting the presence of 80 ng of recombinant MMP-9.
Fig. 2.
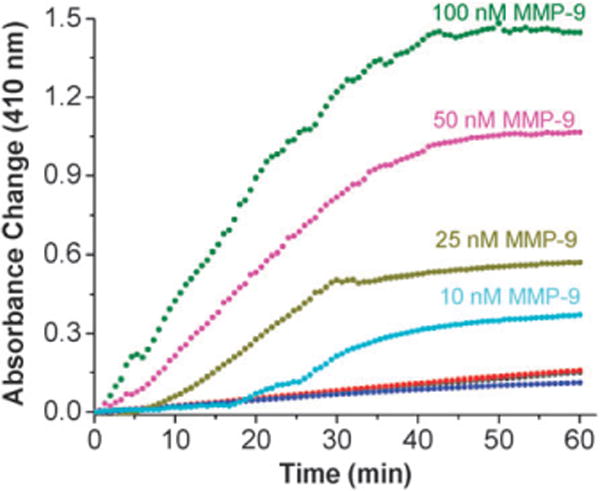
Absorbance change (410 nm) of the assay system as a function of time in the presence of different concentrations of MMP-9. HRP encapsulated liposomes incorporating GPO4 were mixed with 100 nM (olive circles), 50 nM (magenta circles), 25 nm (dark yellow circles) and 10 nM (cyan circles) recombinant human MMP-9 in 25 mM HEPES buffer, pH = 8.0. No significant increase in absorbance was observed in the presence of 50 nM recombinant, human MMP-7 (red circles), 50 nM recombinant MMP-10 (black circles overlapping with the red circles) or 50 nM trypsin (blue circles). All plots shown are the averages of six replicates with errors 5–10%.
We did not observe any significant increases in absorbance from these liposomes in the presence of 50 nM of recombinant human MMP-7 (Fig. 2, red circles), MMP-10 (Fig. 2, black circles, overlapping with red circles) or trypsin (Fig. 2, blue circles). The lipopeptide GPO4 adopts the triple helical conformation in aqueous buffer and in liposomes with POPC as the major lipid.17 The triple helical structures serve as the recognition motif for the enzyme MMP-9.18,19 The fibronectin domain of the enzyme unwinds the triple helix and, subsequently, the catalytic domain of the enzyme hydrolyzes the peptide bond between the amino acids glycine and isoleucine.19 MMP-7, -10 and trypsin do not hydrolyze triple helical peptides due their inability to unwind the triple helix.19
To prepare a calibration curve for our amplified detection system for MMP-9, the absorbance changes after 25 min were plotted as a function of added enzyme concentration (ESI, Fig. S1†). We observed that the absorbance change at 410 nm increased linearly with increasing concentration of MMP-9, up to 200 nM. Considering that the MMP-9 concentration is about 10 nM in normal tissues5 and about 100–200 nM in cancerous tissues,22 our system has the potential to serve as a quick method for the detection of MMP-9 without using any antibody.
Next, we proceeded to estimate the concentration of cell-secreted MMP-9 in conditioned media from cancer cells. For these studies, the metastatic breast adenocarcinoma cells MCF7 was selected as these cells secrete high amounts of MMP-9 and -2 in the extracellular matrix.23 We used the human colorectal adenocarcinoma cells HT-29 as control since these cells do not secrete high levels of MMP-9 and -2 in the extracellular matrix.23 When the cells achieved 70% confluency, the old culture media was replaced with fresh culture medium containing 10% added fetal bovine serum (FBS) but without phenol red. The cells were further incubated for 24 h and centrifuged. The supernatant conditioned media were collected for the detection experiments.
We observed that the addition of the media before cell culture (50 μL) containing 10% FBS to the liposomes led to a small increase in the absorbance at 410 nm (absorbance change of about 0.01 over 35 min). This trace was used as the background and subtracted from the absorbance change traces obtained with the conditioned media. Addition of the conditioned media from the MCF7 cells to the liposomes led to a rapid increase in the absorbance at 410 nm (Fig. 3, red circles). The conditioned media from the HT-29 cells also increased the absorbance, but to a lesser extent (Fig. 3, dark cyan circles). We noted the absorbance changes after 25 min and used the calibration curve (ESI, Fig. S1†) to estimate the concentration of MMP-9 secreted by the MCF7 and HT-29 cells. The concentrations of MMP-9 in the conditioned media from the MCF7 and HT-29 cells were estimated to be 80 nM and less that 10 nM, respectively. Employing commercially available ELISA kits, we estimated the concentrations of MMP-9 to be 35 nM and 6 nM and those of MMP-2 to be 30 and 2 nM in the conditioned media from the MCF7 and HT-29 cells respectively. The antibody used in the ELISA assays is selective for MMP-9 only. However, the MCF7 and HT-29 cells secrete both MMP-2 and -9 in the extracellular matrix.23 Since these two enzymes have very similar selectivity for triple-helical peptide substrates,19 it is likely that our liposomal detection system is responding to the presence of both MMP-9 and -2 in the conditioned media. We note that cancer cell secreted collagenases can locally unwind triple helix, making it susceptible to hydrolysis by trypsin.24 However, the conditioned media used in our experiments contained 10% (by volume) fetal bovine serum (see ESI†) and this will inactivate any trypsin present.25 It should be noted that the serum levels of both MMP-2 and -9 serve as prognostic markers of cancer.5
Fig. 3.
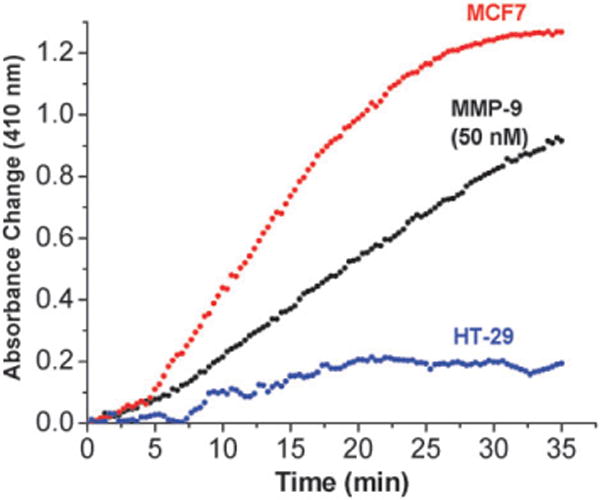
Absorbance change (410 nm) of the assay system as a function of time in the presence of conditioned media from MCF7 and HT-29 cells. HRP encapsulated liposomes incorporating GPO4 were mixed with 50 μL of conditioned media from MCF7 (red circles) and HT-29 cells (blue circles) in 25 mM HEPES buffer, pH = 8.0. The absorbance changes observed in the presence of 50 nM recombinant human MMP-9 (black circles) is included in the plot for comparison. All plots shown are the averages of six replicates with errors 5–10%.
In conclusion, we have developed a new liposome based strategy to detect MMP-9 with an ELISA-like signal amplification strategy, but without using any biological antibody. The system is capable of detecting recombinant MMP-9 in 10 nM and higher concentrations in a complex mixture of proteins. Mechanistic studies to further fine tune and enhance the sensitivity of the detection system are in progress and these results will be reported later.
Supplementary Material
Acknowledgments
This research was supported by the NIH grants 1R01 CA113746, 1 R01 CA 132034 and NSF DMR-0705767 to SM and DKS. JB was supported by the Graduate School Fellowship, North Dakota State University. The University of North Dakota Proteomics Core (JBS, WWM) is supported by NIH grant number P20 RR016741 from the INBRE program of the National Center for Research Resources.
Footnotes
Electronic supplementary information (ESI) available: Experimental details for liposome formation and MMP detection assays. See DOI: 10.1039/b926554f
Notes and references
- 1.Tu G, Xu W, Huang H, Li S. Curr Med Chem. 2008;15:1388. doi: 10.2174/092986708784567680. [DOI] [PubMed] [Google Scholar]
- 2.Rydlova M, Holubec L, Ludvikova M, Kalfert D, Franekova J, Povysil C, Ludvikova M. Anticancer Res. 2008;28:1389. [PubMed] [Google Scholar]
- 3.Newby AC. Arterioscler, Thromb, Vasc Biol. 2008;28:2108. doi: 10.1161/ATVBAHA.108.173898. [DOI] [PubMed] [Google Scholar]
- 4.Rosell A, Lo EH. Curr Opin Pharmacol. 2008;8:82. doi: 10.1016/j.coph.2007.12.001. [DOI] [PubMed] [Google Scholar]
- 5.Gorovetz M, Schwob O, Krimsky M, Yedgar S, Reich R. Front Biosci. 2008;13:1917. doi: 10.2741/2811. [DOI] [PubMed] [Google Scholar]
- 6.Wu ZS, Wu Q, Yang JH, Wang HQ, Ding XD, Yang F, Xu XC. Int J Cancer. 2008;122:2050. doi: 10.1002/ijc.23337. [DOI] [PubMed] [Google Scholar]
- 7.Karp CM, Shukla MN, Buckley DJ, Buckley AR. Oncogene. 2007;26:1780. doi: 10.1038/sj.onc.1209980. [DOI] [PubMed] [Google Scholar]
- 8.Ratnikov BI, Deryugina EI, Strongin AY. Lab Invest. 2002;82:1583. doi: 10.1097/01.lab.0000038555.67772.db. [DOI] [PubMed] [Google Scholar]
- 9.Kim YP, Daniel WL, Xia Z, Xie H, Mirkin CA, Rao J. Chem Commun. 2010;46:76. doi: 10.1039/b915612g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yao H, Zhang Y, Xiao F, Xia Z, Rao J. Angew Chem, Int Ed. 2007;46:4346. doi: 10.1002/anie.200700280. [DOI] [PubMed] [Google Scholar]
- 11.Xia Z, Xing Y, So MK, Koh AL, Sinclair R, Rao J. Anal Chem. 2008;80:8649. doi: 10.1021/ac801562f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhao M, Josephson L, Tang Y, Weissleder R. Angew Chem, Int Ed. 2003;42:1375. doi: 10.1002/anie.200390352. [DOI] [PubMed] [Google Scholar]
- 13.Liu G, Wang J, Wunschel DS, Lin YJ. J Am Chem Soc. 2006;128:12382. doi: 10.1021/ja0626638. [DOI] [PubMed] [Google Scholar]
- 14.Benson HAE. Current Drug Delivery. 2009;6:217. doi: 10.2174/156720109788680813. [DOI] [PubMed] [Google Scholar]
- 15.Ferrara KW, Borden MA, Zhang H. Acc Chem Res. 2009;42:881. doi: 10.1021/ar8002442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tseng YC, Mozumdar S, Huang L. Adv Drug Delivery Rev. 2009;61:721. doi: 10.1016/j.addr.2009.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Banerjee J, Hanson AJ, Gadam B, Elegbede AI, Tobwala S, Ganguly B, Wagh A, Muhonen WW, Law B, Shabb JB, Srivastava DK, Mallik S. Bioconjugate Chem. 2009;20:1332. doi: 10.1021/bc9000646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Elegbede AI, Banerjee J, Hanson AJ, Tobwala S, Ganguly B, Wang R, Lu X, Srivastava DK, Mallik S. J Am Chem Soc. 2008;130:10633. doi: 10.1021/ja801548g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Minond D, Lauer-Fields JL, Cudic M, Overall CM, Pei D, Brew K, Moss ML, Fields GB. Biochemistry. 2007;46:3724. doi: 10.1021/bi062199j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hwang SY, Kumada Y, Seong GH, Choo J, Katoh S, Lee EK. Anal Bioanal Chem. 2007;389:2251. doi: 10.1007/s00216-007-1614-3. [DOI] [PubMed] [Google Scholar]
- 21.Liu H, Wang Z, Liu Y, Xiao J, Wang C. Thermochim Acta. 2006;443:173. [Google Scholar]
- 22.Turpeenniemi-Hujanen T. Biochimie. 2005;87:287. doi: 10.1016/j.biochi.2005.01.014. [DOI] [PubMed] [Google Scholar]
- 23.Roomi MW, Monterrey JC, Kalinovsky T, Rath M, Niedzwiecki A. Oncol Reports. 2009;21:1323. doi: 10.3892/or_00000358. [DOI] [PubMed] [Google Scholar]
- 24.Chung L, Dinakarpandian D, Yoshida N, Lauer-Fields JL, Fields GB, Visse R, Nagase H. EMBO J. 2004;23:3020. doi: 10.1038/sj.emboj.7600318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Freshney RI. Culture of Animal Cells. 5th. John Wiley and Sons; Hoboken, New Jersey: 2005. pp. 134–135. ch. 10. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


